Abstract
Peroxisomes are ubiquitous membrane-enclosed organelles involved in lipid processing and reactive oxygen detoxification. Mutations in human peroxisome biogenesis genes (Peroxin, PEX, or Pex) cause developmental disabilities and often early death. Pex5 and Pex7 are receptors that recognize different peroxisomal targeting signals called PTS1 and PTS2, respectively, and traffic proteins to the peroxisomal matrix. We characterized mutants of Drosophila melanogaster Pex5 and Pex7 and found that adult animals are affected in lipid processing. Pex5 mutants exhibited severe developmental defects in the embryonic nervous system and muscle, similar to what is observed in humans with PEX5 mutations, while Pex7 fly mutants were weakly affected in brain development, suggesting different roles for fly Pex7 and human PEX7. Of note, although no PTS2-containing protein has been identified in Drosophila, Pex7 from Drosophila can function as a bona fide PTS2 receptor because it can rescue targeting of the PTS2-containing protein thiolase to peroxisomes in PEX7 mutant human fibroblasts.
Keywords: peroxisome, peroxin, Pex gene, lipids, protein targeting, developmental defects
PEROXISOMES are involved in a variety of important biochemical functions, notably lipid metabolism and the detoxification of reactive species (De Duve and Baudhuin 1966; Bowers 1998; Wanders and Waterham 2006; Nguyen et al. 2008). Peroxisomes also have important roles in development, immune signaling, and viral maturation (Dixit et al. 2010; Smith and Aitchison 2013; You et al. 2015; Di Cara et al. 2017). Peroxisome biogenesis genes (Peroxin, PEX, or Pex) are, for the most part, conserved across the breadth of eukaryotes (Schrader and Fahimi 2006; Platta and Erdmann 2007). Thirteen PEX genes are required for peroxisome biogenesis in humans, and mutations in these genes cause the peroxisome biogenesis disorders, which manifest as heterogeneous syndromes with varied developmental defects (Braverman et al. 2013). PEX5 and PEX7 act as receptors that recognize signals, called peroxisome targeting signals (PTS), in soluble peroxisomal proteins to traffic them from the cytosol to the peroxisome matrix (Purdue et al. 1997; Klein et al. 2001; Ito et al. 2007; Smith and Aitchison 2013). PEX5 and PEX7 homologs are found across the eukaryota (McCollum et al. 1993; Rehling et al. 1996; Purdue et al. 1997; Kragler et al. 1998; Matsumura et al. 2000; Woodward and Bartel 2005; Lazarow 2006; Kanzawa et al. 2012). PEX5 recognizes the C-terminal PTS1 with the canonical sequence Ser-Lys-Leu (SKL), while PEX7 recognizes an N-terminal nonapeptide PTS2 with the consensus sequence (R/K)(L/V/I)X5(H/Q)(L/A) (McCollum et al. 1993; Glover et al. 1994; Rehling et al. 1996; Shimozawa et al. 1999; Ito et al. 2007). Mutation of PEX5 and PEX7 gives rise to Zellweger spectrum disorder (ZSD) and rhizomelic chondrodysplasia punctata type 1 (RCDP1) (Purdue et al. 1997), respectively.
Patients with ZSD exhibit a spectrum of clinical phenotypes, with the most severely affected usually dying within their first year with profound neurologic impairment and liver failure. Patients with RCDP1 also exhibit a spectrum of clinical phenotypes, although generally of less severity than those seen in ZSD. Central nervous system (CNS) defects are prevalent in patients with RCDP1, including brains of decreased volume and deficient in both neurons and white matter, as well as progressive cerebellar degeneration. Defects in the β-oxidation of very-long-chain fatty acids (VLCFAs) constitute a major pathology in patients with ZSD, while deficient plasmalogen (ether lipid) synthesis is a defining characteristic of patients with RCDP1 (Braverman et al. 2014).
Mutation of Drosophila Pex genes is linked to a range of phenotypes, including lethality (Pex1, Pex3, Pex19) and male sterility (Pex16) (Beard and Holtzman 1987; Chen et al. 2010; Mast et al. 2011; Nakayama et al. 2011; Faust et al. 2014; Bülow et al. 2018). In Drosophila Schneider 2 (S2) cells, knockdown of the Pex5 transcript reduces targeting of PTS1-containing proteins to peroxisomes, while depletion or overexpression of the Pex7 transcript leads to smaller or larger peroxisomes, respectively, than normal (Baron et al. 2016). However, the actual function of Drosophila Pex7 remains unclear, as no bona fide peroxisomal PTS2-containing protein has been identified in Drosophila; fly homologs of peroxisomal proteins use the PTS1/Pex5 pathway in Drosophila, e.g., peroxisomal thiolase, trafficked by the PTS2/PEX7 import pathway in other organisms (Faust et al. 2012; Baron et al. 2016).
Here, we show that Drosophila Pex5 mutants exhibit severe developmental defects in the embryonic nervous system and muscle, similar to that observed in patients with ZSD with Pex5 mutations. Pex7 fly mutants exhibited minor defects in brain development. We also show that Drosophila Pex7 can function as a bona fide PTS2 receptor because it can rescue targeting of the PTS2-containing protein thiolase to peroxisomes in Pex7 mutant human fibroblasts.
Materials and Methods
Cell culture
Human fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (ThermoFisher, Waltham, MA) supplemented with 10% fetal bovine serum, 50 units penicillin/ml, and 50 μg streptomycin sulfate/ml.
Fly husbandry, egg collection, and survival assays
Mutant lines y1Mi{y+mDint2 = MIC}Pex5MI06050 w*/FM7h) (designated as Pex5MI06050) and y1 w*; Mi{y+mDint2 = MIC}Pex7MI14471 (designated as Pex7MI14471); the Minos transposase strain y1 w*; snaSco/SM6a, P{w+mC = hsILMiT}2.4; the third chromosome deficiency line w1118; Df(3L)BSC816, P+PBac{w+mC = XP3.WH3}BSC816/TM6C, Sb1 cu1 [designated Df(3L)BSC816]; and the X chromosome balancing line FM7(GFP) Df(1)JA27/FM7c, P{w+mC = GAL4-Kr.C}DC1, P{w+mC = UAS-GFP.S65T}DC5, sn+ were from the Bloomington Drosophila Stock Center (BDSC). The y1, w* strain used as a control in all experiments and the P{GAL4::VP16-nos.UTR}MVD2, w1118 strain were from the BDSC. y1, w*; UAS-dmPex7cDNA was made by our laboratory. To make the Pex5ΔMI06050 strain, the MiMIC element was excised from the Pex5MI06050 strain as verified by PCR (Venken et al. 2011). Flies were maintained at 25° on standard BDSC corn meal medium. Pex5MI06050 mutants balanced over FM7(GFP) were allowed to lay eggs on apple juice agar plates for 2 days. On day 3, embryos were collected every 2 hr. GFP-negative embryos were incubated on apple juice agar plates at 25°. After 24 hr, hatched larvae were transferred to standard corn meal medium, and surviving animals were counted at the same time each day.
Geotaxis (climbing) assay
This assay was performed as described (Madabattula et al. 2015), using 20 flies (7 days old) per assay. Each assay had four technical replicates, and the assay was done 12 times for a total of 960 flies analyzed per genotype. Flies were transferred to a 250 ml glass graduated cylinder (ThermoFisher) sealed with wax film to prevent escape. Assays were conducted in ambient light at 22° and at the same time each day.
Lipid analysis
One thousand first-instar (L1) larvae (equivalent to 1 mg of protein extract) were homogenized in 1 ml of PBS buffer and sonicated for 5 min using a BioRuptor (Diagenode, Liège, Belgium) at low power. Lipids were extracted using chloroform:methanol (2:1) as described (Folch et al. 1957). Five micrograms of heptadecane (C17) in chloroform was used as an internal control. Isolates were centrifuged at 3400 × g, and the chloroform phase containing lipids was passed through a sodium sulfate column (GE Healthcare Chicago, IL). The eluate was dried under nitrogen and resuspended in 100 μl of HPLC-grade hexane. Ten microliters of material were injected into an Agilent 6890 gas chromatograph with a flame-ionization detector. The amounts of VLCFAs were normalized to the relative amount of protein determined using a Qubit II fluorimeter (ThermoFisher). Nonesterified fatty acids (NEFAs) were analyzed as described (Bülow et al. 2018).
Quantitative RT-PCR analysis
Samples were rinsed twice with PBS, and total RNA was extracted using the RNeasy-Micro kit (QIAGEN, Valencia, CA). Next, 0.5–1 μg of RNA was reverse-transcribed using an iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR (qRT-PCR) was performed (Realplex; Eppendorf, Hamburg, Germany) using KAPASYBR Green PCR master mix (Kapa Biosystems, Wilmington, MA). Samples were normalized to RpL23 gene expression using the 2−ΔΔCT method (Livak and Schmittgen 2001). Sequences of qRT-PCR primers are as follows:
RpL23, 5ʹ-GACAACACCGGAGCCAAGAACC, 5ʹ-GTTTGCGCTGCCGAATAACCAC.
Pex5, 5ʹ-AAATGCGAAGACATGGAACC, 5ʹ-TGTAACGCACACGGATGAAG.
Pex7, 5ʹ-TCGAAATAGCCAGGCCATCAAG, 5ʹ-AAGGAACCGAAGACAAGGACTC.
All qRT-PCR data are from three biological samples each tested in triplicate. Student’s t-test was used to calculate the significance of differences in gene expression between averaged sample pairs.
Protein analysis and western blotting
Fifty microliters of cold Ephrussi–Beadle Ringer’s solution supplemented with 10 mM EDTA, 10 mM DTT, 1× complete protease inhibitor, and 1× PhosStop phosphatase inhibitor (Roche) were added to 3 × 106 pelleted cells. Twenty five microliters of 3× SDS-PAGE Buffer (Bio-Rad) containing 10 mM DTT at 70° were added to the homogenate and boiled for 10 min. Samples were resolved by SDS-PAGE on 10% acrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% skim milk powder in TBS+Tween-20 (Tw) (150 mM NaCl, 20 mM Tris-HCl, pH 7.5, 0.05% Tween-20) for 1 hr and incubated for 16 hr with primary antibody in TBSTw. After washing three times for 5 min each with TBSTw, membranes were incubated with HRP-conjugated secondary antibody (Bio-Rad) at 1:10,000 dilution for 1 hr at 24°. Membranes were washed as above, and HRP activity was detected by enhanced chemiluminescence (Amersham, Piscataway, NJ). Primary antibodies were rabbit anti-active caspase 3 (BD Pharmingen) and mouse anti–α-tubulin (Sigma-Aldrich, St. Louis, MO).
Human PEX7 and Drosophila Pex7 complementary DNA cloning and transfection
The open-reading frame of human PEX7 complementary DNA (cDNA; Braverman et al. 1997) was cloned into pENTR/D (ThermoFisher) using hPEX7-forward (5ʹ-CACCATGAGTGCGGTGTGCGGTGG) and hPEX7-reverse (5ʹ-GGTCAAGCAGGAATAGTAAGACAAG) primers. The Drosophila Pex7 cDNA clone has been described (Baron et al. 2016). Both cDNAs were cloned into pT-Rex-DEST30 using LR Clonase (ThermoFisher). Clones were transiently transfected into immortalized human fibroblasts (Braverman et al. 1997) using the Amaxa Human Dermal Fibroblast Nucleofector kit (Lonza). Wild-type and PEX7null fibroblasts were also mock-transfected using an empty DEST30 vector. Drosophila Pex7 cDNA was inserted into pUASTattB and injected into the y1 w67c23; P{CaryP}attP40 strain to establish transgenic flies (BestGene).
Microscopy and image analysis
Human fibroblasts were fixed for 30 min in 4% paraformaldehyde in PBS, rinsed twice in PBST (PBS + 0.1% Triton X-100), and blocked for 1 hr in 5% normal goat serum (Sigma-Aldrich) before incubation for 16 hr at 4° with primary antibody. After four washes in PBST, cells were incubated with secondary antibody for 16 hr at 4°, washed four times in PBST, and mounted in Prolong-Gold (ThermoFisher). Images were captured using a C9100 camera (Hamamatsu) at 130 μm vertical spacing using a ×100 oil immersion objective (NA = 1.4) on a Zeiss AxioObserver M1 microscope coupled to an ERS spinning disk confocal microscope (PerkinElmer, Norwalk, CT). Primary antibodies were anti-mouse PMP70 (Sigma-Aldrich; Imanaka et al. 2000); anti-activated caspase 3 (BD Pharmagen), anti-phosphohistone H3 (Upstate Biotechnology), anti-SKL (Szilard et al. 1995), and anti-rat 3-ketoacyl-CoA thiolase (Bodnar and Rachubinski 1990). Secondary antibodies were Alexa Fluor 568 donkey anti-mouse, Alexa Fluor 488 donkey anti-rat, and Alexa Fluor 647 donkey anti-rabbit (Abcam, Cambridge, UK).
Embryos were maintained at 18°, collected every 16 hr, and processed for microscopy as reported (Parsons and Foley 2013). Antibodies to Futsch (22C10), raised by Seymour Benzer (California Institute of Technology), and to Even-skipped (2B8) and Repo (8D12), raised by Corey Goodman (University of California), were from the Developmental Studies Hybridoma Bank. Anti-myosin II was from Abcam (ab51098). All primary antibodies were diluted 1:20, and Alexa Fluor 568 donkey anti-mouse secondary antibody was diluted 1:1000. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed as described (Parsons and Foley 2013). The volume of DAPI-stained brain volume was measured using Imaris three-dimensional analysis software (Bitplane) and optimized standard measurement protocols applied to all control and experimental samples for a particular data set. Third-instar larval length was measured using ImageJ. The number of anti-thiolase–labeled puncta was determined using ImageJ as described (Di Cara et al. 2017).
Measurement of NEFAs
NEFAs were measured using the copper-triethanolamine method. Tissue was homogenized in 20 μl chloroform + 1% Triton-X 100 per mg of tissue, and subjected to centrifugation at 13,000 × g for 10 min. The supernatant was removed and evaporated at 60°. Lipids were taken up in the same volume of phosphate buffer, and 25 μl of sample were transferred to a glass vial with 500 μl of chloroform/heptane (4:3). Vials were shaken for 2 min and subjected to centrifugation for 5 min at 2,000 × g. Three hundred microliters of the organic phase were transferred to a glass vial containing 250 μl of copper-triethanolamine, shaken for 2 min, and subjected to centrifugation for 5 min at 2,000 × g. One hundred fifty microliters of the organic phase were removed and evaporated at 60°. Lipids were taken up in 150 μl of ethanol, and vials were shaken for 15 min at 37°. Copper was detected by complexation with a mixture of dicarbazone–dicarbazide, and color intensity was measured in a 96-well plate at 550 nm in a microplate reader (TECAN, Männedorf, Switzerland).
Data availability
Strains and plasmids are available upon request. The authors state that all data necessary for confirming the conclusions presented are represented within the manuscript. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7221503.
Results and Discussion
Drosophila Pex5 is required for development
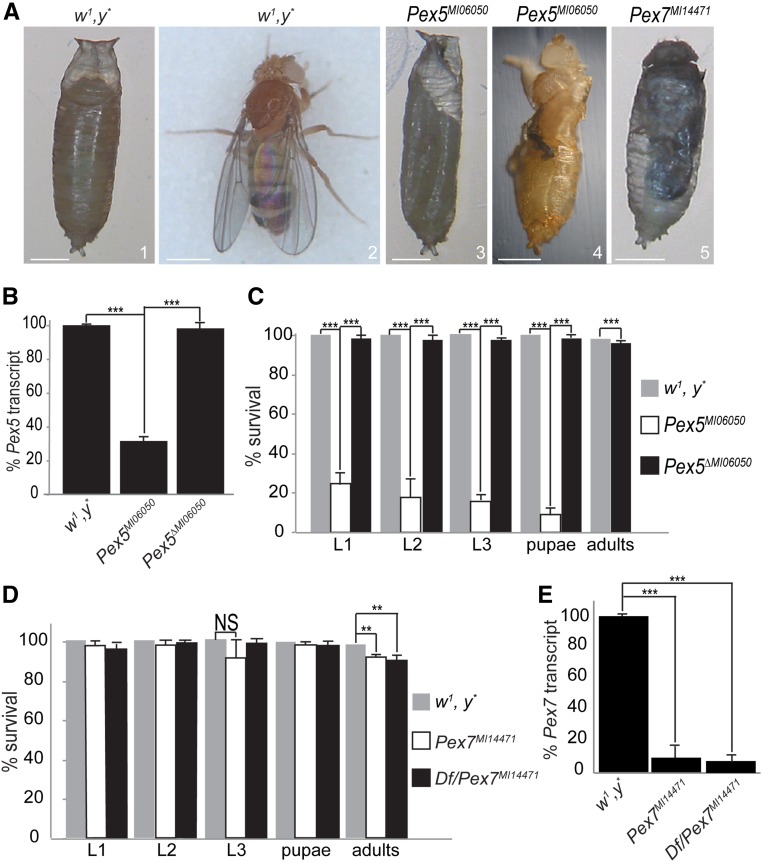
The Pex5MI06050 mutation resulted from a MiMIC insertion that disrupted the second exon of the coding region of Pex5-RA or the one exon of Pex5-RB (Venken et al. 2011). Pex5 is on the X chromosome, and Pex5MI06050 mutants were lethal when homozygous or as hemizygous males with only 20% of embryos hatching (Figure 1, A–C). A further 15% of Pex5MI06050/Pex5MI06050 mutants died as larvae, with only 5% pupating (Figure 1, A and C, images 1–3). The 2% of pupae that survived died at eclosion (Figure 1, A and C, images 1–4). Pex5MI06050/Pex5MI06050 embryos had 35% of Pex5 messenger RNA compared to controls (Figure 1B); maternally provided Pex5 messenger RNA likely caused the phenotypic variability observed. Finally, the Pex5ΔMI06050 strain was viable and exhibited Pex5 transcript levels comparable to those of the control w1, y* strain, supporting the hypothesis that the phenotypes observed in the mutant strains were due to Pex5 disruption (Figure 1, B and C).
Figure 1.
Pex5 and Pex7 mutants present developmental defects. (A) 1: Control (w1, y*) pupa 7 days after egg laying (AEL). 2: Control adults eclosed at day 9 AEL. 3: Most Pex5MI06050/Pex5MI06050 mutants arrested at the pseudo-pupal stage (day 11 AEL). 4: Some Pex5MI06050/Pex5MI06050 mutants die during eclosion (day 10 AEL). 5: Some Pex7MI14471/Pex7MI14471 mutants arrested at the pupa stage (day 11 AEL). Bar, 0.5 mm. (B) qRT-PCR measurement of Pex5 messenger RNA levels in Pex5MI06050/Pex5MI06050 embryos and Pex5ΔMI06050/Pex5ΔMI06050 embryos, relative to control (w1, y*) embryos. Values are the averages of four independent experiments ± SD. (C) Most Pex5MI06050/Pex5MI06050 mutants die at the embryo stage, and none eclose as adults. Pex5ΔMI06050/Pex5ΔMI06050 mutants can eclose as adults like control flies. Values are the averages of four independent experiments ± SD 200 embryos were analyzed for each genotype. (D) Most Pex7MI14471/Pex7MI14471 mutants eclose as adults resembling the control (w1, y*) strain; however, ∼5% of Pex7MI14471/Pex7MI14471 embryos and Df(3L)BSC816/Pex7MI14471 embryos arrest at the pupal stage. Values are the averages of four independent experiments ± SD; 200 embryos were analyzed for each genotype. (E) qRT-PCR confirms a reduction in Pex7 transcript levels in Pex7MI14471/Pex7MI14471 and Df(3L)BSC816/Pex7MI14471 (Df/Pex7MI14471) embryos, relative to control (w1, y*) embryos. Values are the averages of four independent experiments ± SD. In B–E, significance was determined using Student’s t-test; *** P < 0.001; ** P < 0.01; NS, not significant.
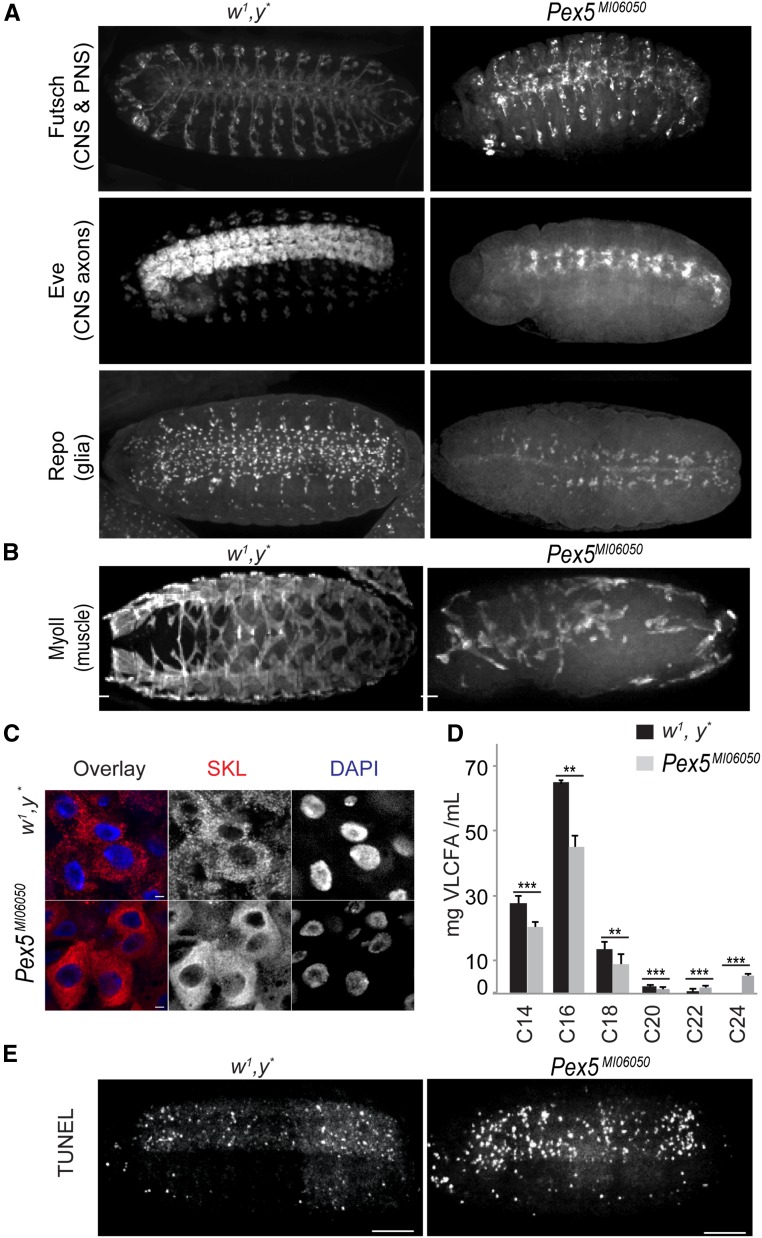
Mutations in human PEX5 cause CNS, peripheral nervous system (PNS), and musculature defects (Steinberg et al. 2006; Braverman et al. 2013). We therefore evaluated CNS and PNS organization in Pex5MI06050 mutant embryos compared to age-matched controls. In Pex5MI06050/Pex5MI06050 embryos, both the PNS and ventral nerve cord were disorganized (Figure 2A). Patients with peroxisome biogenesis disorders often show axonal demyelination (Braverman et al. 2013), and while Drosophila neurons are unmyelinated, wrapping glia play a role analogous to that of myelin (Freeman and Doherty 2006; Matzat et al. 2015). Glial cells were disorganized in Pex5MI06050/Pex5MI06050 embryos compared to controls (Figure 2A). Finally, the developing longitudinal and oblique musculature in late-stage Pex5MI06050/Pex5MI06050 embryos was also disorganized (Figure 2B).
Figure 2.
The Pex5MI06050 mutation affects CNS, PNS, and muscle structure. (A) The repeated segmental patterns of neurons of the CNS and PNS marked by anti-Futsch, CNS axons marked by anti-Even-skipped, and glial cells (except midline glia) marked by anti-Repo in control embryos (stage 15) were disrupted in Pex5MI06050/Pex5MI06050 embryos. Bar, 10 μm. (B) The repeated segmental pattern of developing muscles marked by anti-myosin II (MyoII) in control embryos was disrupted in Pex5MI06050/Pex5MI06050 embryos (stage 15). Bar, 10 μm. (C) Mature peroxisomes (punctate anti-SKL signal, red) are observed in the larval midgut. In Pex5MI06050/Pex5MI060 mutants, diffuse cytosolic anti-SKL staining and anti-SKL–positive aggregates indicate PTS1 import was impaired. DAPI-labeled nuclei are in blue. Bar, 2 μm. (D) Pex5MI06050/Pex5MI06050 mutants have lower amounts of C14, C16, C18, and C20 fatty acids and greater amounts of C22 and C24 fatty acids compared to control animals. Values are averages of four independent experiments ± SD; 1000 larvae per sample per each genotype were used in each replicate (4000 larvae total). Significance was determined using Student’s t-test; *** P < 0.001; ** P < 0.01. (E) Pex5MI06050/Pex5MI06050 embryos exhibit greater numbers of TUNEL-positive cells than control embryos. Images are representative of five independent experiments. N = 20 per experiment per genotype. Bar, 10 μm.
Drosophila Pex5 is required for peroxisome biogenesis
We localized PTS1 (SKL)-containing proteins in third-instar larvae midgut cells to determine if the Pex5MI06050 mutation affects PTS1-mediated peroxisome import. A punctate signal corresponding to peroxisomes with active PTS1 import was observed only in control w1, y* midgut cells, while an anti-SKL antibody cytosolic signal and anti-SKL–positive aggregates were observed in Pex5MI06050/Pex5MI06005 midgut cells, indicating compromised PTS1 import (Figure 2C). We profiled the spectrum of fatty acids in flies to examine the effects of Pex5MI06050 mutation on systemic peroxisome function. Pex5MI06050/Pex5MI06050 embryos accumulated C22 and C24 VLCFAs and relatively lower levels of C14, C16, C18, and C20 fatty acids compared to control embryos (Figure 2D). Increased apoptosis was seen in Pex5MI06050/Pex5MI06050 embryos as evidenced by their increased TUNEL staining compared to control embryos (Figure 2E).
Two transcript variants have been reported for Drosophila Pex5 (http://flybase.org/reports/FBgn0023516). Humans have long and short transcripts for PEX5, with the long transcript encoding a PEX5 isoform that interacts with PEX7 and functions as a chaperone in the import of PTS2-targeted proteins into the peroxisome (Otera et al. 2000). It would be interesting to characterize the roles of the Pex5 isoforms arising from the two Pex5 transcripts in Drosophila, which does not have a PTS2 import pathway into peroxisomes (Faust et al. 2012; Baron et al. 2016).
Drosophila Pex7 is required for neuronal development
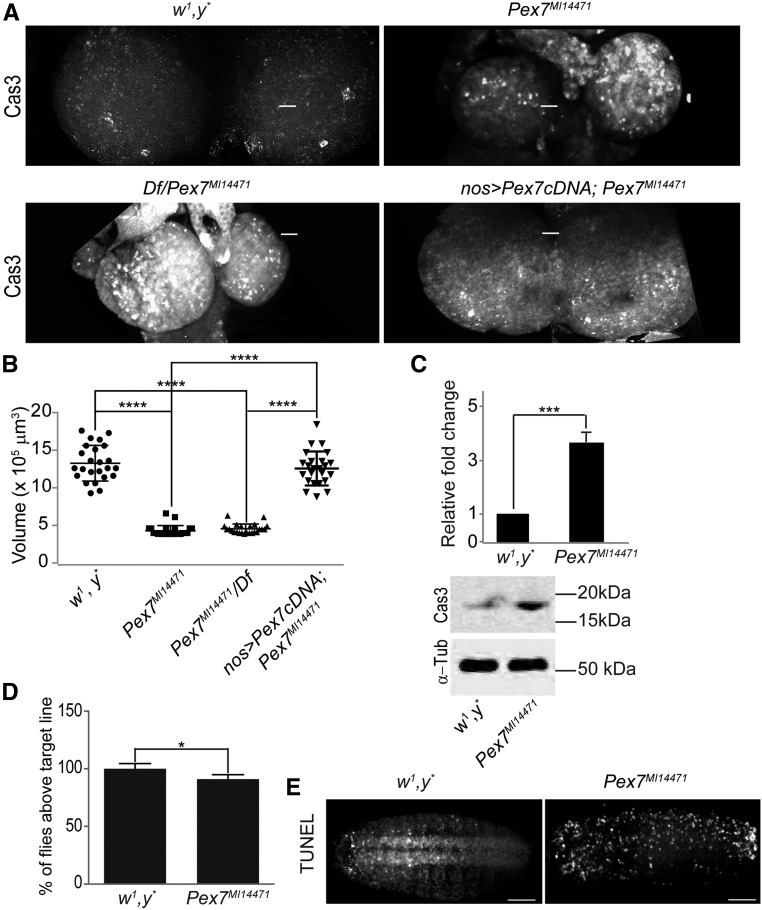
Pex7MI14471 arose by MiMIC insertion into the Pex7 coding region (exon II) (Venken et al. 2011). Unlike Pex5MI06050, Pex7MI14471/Pex7MI14471 mutants were viable, with only 5% arresting at the pupal stage (Figures 1, A and D, images 1, 2, and 5). Similarly, animals carrying the Pex7MI14471 allele over a deletion covering the region Df(3L)BSC816 (designated as Df/Pex7MI14471) also showed ∼5% arrest at the pupal stage (Figure 1D). Arrested pupae were the same size as control pupae but exhibited developmental abnormalities (Figure 1A, images 1, 2, and 5). qRT-PCR analysis showed Pex7MI14471/Pex7MI14471 and Df/Pex7MI14471 embryos had ∼10% of the levels of Pex7 transcript relative to controls (Figure 1E). Because patients with RCDP1 exhibit defects in neurogenesis (Steinberg et al. 2006; Braverman et al. 2013), we analyzed the brain morphology of Pex7MI14471/Pex7MI14471 third-instar larvae. Compared to the brains of control w1, y* larvae, the brains of Pex7MI14471/Pex7MI14471 larvae were smaller and of less volume (Figure 3, A and B). Although Pex7MI14471/Pex7MI14471 animals were slightly larger than control animals at the same stage (Supplemental Material, Figure S1, A and B), the mutation did not appear to affect developmental timing as most animals reached the adult stage at the same time as control animals. This observation is unusual in that small brain together with enlarged body size is usually associated with developmental arrest (Colombani et al. 2005; Mirth et al. 2005).
Figure 3.
Pex7 mutation causes defects in CNS development. (A) Pex7MI14471/Pex7MI14471 and Df/Pex7MI14471 animals have smaller brains than animals of the control strain w1, y*. Overexpression of Drosophila Pex7 cDNA using nanos-GAl4 in Df/Pex7MI14471 animals restores brain size to that of control animals. The number of apoptotic cells marked by activated caspase 3 (Cas3) was greater in brains of Pex7MI14471/Pex7MI14471 and Df/Pex7MI14471 animals than in brains of control animals. Overexpression of Drosophila Pex7 cDNA in Df/Pex7MI14471 animals reduces the number of Cas3-positive cells in the brain to that observed in the brain of control animals. Bar, 10 μm (B) Loss of Pex7 in Pex7MI14471/Pex7MI14471 and Df/Pex7MI14471 animals results in reduced L3 brain volume compared to control L3 brain volume. Overexpression of Drosophila Pex7 cDNA in Df/Pex7MI14471 animals restores brain volume to that of brains of control animals. N = 15 per genotype. Significance was determined using one-way ANOVA; **** P < 0.0001. (C) Representative western blot and quantification showing activated Cas3 amounts are higher in Pex7MI14471/Pex7MI14471 L2-L3 brains than in control L2–L3 brains. α-Tubulin (α-Tub) served as a control for protein loading. Values represent the averages of four independent experiments ± SD. Significance was determined using Student’s t-test; *** P < 0.001. (D) Pex7MI14471/Pex7MI14471 animals show reduced performance in a climbing assay that tests coordinated locomotion than do control animals. Values represent the averages of 12 independent experiments ± SD. N = 960 for each genotype. Significance was determined using Student’s t-test; * P < 0.05. (E) Pex7MI14471/Pex7MI14471 embryos exhibit greater numbers of TUNEL-positive cells than control embryos. Images are representative of five independent experiments. N = 20 per experiment per genotype. Bar, 10 μm.
Reduced brain size could be due to either reduced cell proliferation or excess cell death (apoptosis) (Shklyar et al. 2014). The number of mitotic cells marked by the presence of phospho-Ser10-histone 3 (Wei et al. 1999) was similar in brains of control animals and Pex7MI14471/Pex7MI14471 animals (Figure S1C). Extracts of brain from Pex7MI14471/Pex7MI14471 animals had greater amounts of active caspase 3 compared to control extracts (Figure 3C). Also, there were more activated Caspase 3–positive cells in developing Pex7MI14471/Pex7MI14471 and Df/Pex7MI14471 brains compared to control brain (Figure 3A). Overexpression of Pex7 cDNA using the nanos-Gal4 driver in Pex7MI14471/Pex7MI14471 animals rescued brain size and reduced activated caspase 3 staining to values observed in control animals (Figure 3, A and B), showing that these abnormal phenotypes exhibited by Pex7MI14471/Pex7MI14471 animals were due to dysfunctional Pex7. Increased apoptosis was observed in Pex7MI14471/Pex7MI14471 embryos as evidenced by their increased TUNEL staining compared to control embryos (Figure 3E), similar to Pex5MI06050/Pex5MI06050 embryos (Figure 2E).
We assayed neural and muscular function in flies by analyzing the ability of adults to exhibit a negative geotaxis response (Feany and Bender 2000; Madabattula et al. 2015). Control flies were more successful in the climbing assay than Pex7MI14471/Pex7MI14471 flies (Figures 3D and S1D).
Drosophila Pex7 has a role in peroxisome fatty acid processing
Lack of Drosophila PTS2 import calls into question if Pex7MI14471/Pex7MI14471-associated defects are linked to peroxisome dysfunction. Circulating NEFAs are increased in amount in central obesity, insulin resistance, and diabetes and serve as a biomarker for these conditions (Boden 1998; Stich and Berlan 2004). Flies with dysfunctional peroxisomes have been shown to exhibit impaired lipid metabolism (Bülow et al. 2018; Di Cara et al. 2018). Similarly, we observed increased amounts of NEFAs in Pex7MI14471/Pex7MI14471 larvae compared to control larvae (Figure S1E).
Drosophila Pex7 can functionally substitute for human PEX7 in PTS2 protein import
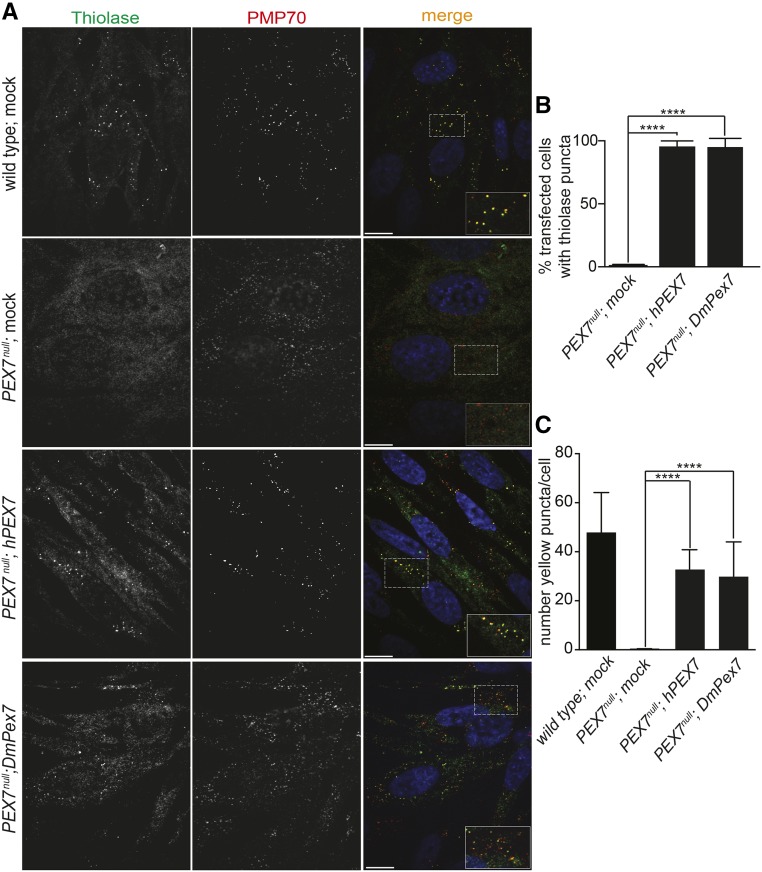
Human peroxisomal 3-ketoacyl-CoA thiolase, hereafter called thiolase, is imported into peroxisomes via the PTS2/PEX7 import pathway (Braverman et al. 1997). To determine if Drosophila Pex7 could function in PTS2 import into peroxisomes, we evaluated if expression of Drosophila Pex7 cDNA (DmPex7) could reestablish thiolase import into peroxisomes in fibroblasts from a patient with RCDP1, which contain mutations in both copies of the PEX7 gene (PEX7null) (Braverman et al. 1997; Purdue et al. 1997). Transfection of the PEX7null fibroblasts with a human PEX7 cDNA (hPEX7) restored thiolase import into peroxisomes (Figure 4, A–C). Transfection of the PEX7null fibroblasts with DmPex7 cDNA also restored thiolase import into peroxisomes (Figure 4, A–C), indicating that Drosophila Pex7 is competent to mediate PTS2 import into peroxisomes.
Figure 4.
Drosophila Pex7 can restore PTS2 import in human cells. (A) In wild-type, mock-transfected human fibroblasts, anti-thiolase antibodies (Thiolase) decorate punctate structures characteristic of peroxisomes that also label with antibodies to the peroxisomal membrane protein PMP70. Mock-transfected PEX7null fibroblasts do not exhibit punctate structures decorated by anti-thiolase antibodies, indicative of a failure to import thiolase into peroxisomes, but anti-PMP70 puncta remain, indicative of so-called “peroxisomal ghosts.” Transfection of PEX7null fibroblasts with human PEX7 cDNA (hPEX7) restores thiolase import into peroxisomes. Transfection of PEX7null fibroblasts with Drosophila Pex7 cDNA (DmPex7) also restores thiolase import into peroxisomes. Dashed boxes highlight regions expanded at the bottom right of the corresponding merged view. Bar, 10 μm. (B) Quantification of the percentage of cells exhibiting thiolase puncta in PEX7null, Pex7null transfected with hPEX7, and Pex7null transfected with DmPex7 fibroblasts. Values are the averages of four independent experiments ± SD. Each replicate used 20 cells per genotype (100 cells total). Significance was determined using one-way ANOVA; **** P < 0.0001. (C) Number of yellow puncta resulting from thiolase (green) and PMP70 (red) colocalization in wild-type, PEX7null, PEX7null transfected with hPEX7, and PEX7null transfected with DmPex7 fibroblasts. Values are the averages of four independent experiments ± SD. Each replicate used 20 cells per genotype (100 cells total). Significance was determined using one-way ANOVA; **** P < 0.0001.
The role of Drosophila Pex7 is divergent from the role of Pex7 proteins in other organisms
The localization of Pex7 to peroxisomes in Drosophila S2 cells (Baron et al. 2016), the changes in peroxisome size in S2 cells when Pex7 transcript levels are reduced (Mast et al. 2011), and the altered lipid processing and changes in brain development we have now observed in Pex7MI14471/Pex7MI14471 mutant flies clearly demonstrate a role for Pex7 in peroxisome biogenesis and/or function in Drosophila. However, the lack of a canonical PTS2 trafficking pathway in Drosophila calls into question how Pex7 functions in peroxisome biology in flies. Drosophila homologs of known yeast and human peroxisomal proteins with a PTS2 have instead a PTS1 (Faust et al. 2012; Baron et al. 2016). It is therefore likely that the canonical PTS2 import pathway into peroxisomes is not present in Drosophila, as evidenced by the failure of S2 cells to import a canonical PTS2-mCherry reporter into peroxisomes (Faust et al. 2012). Nevertheless, the extensive similarity in the primary structures of Pex7 proteins of D. melanogaster, Saccharomyces cerevisiae, Arabidopsis thaliana, Danio rerio, and Homo sapiens suggests conservation of function of Drosophila Pex7 with Pex7 proteins from these other organisms (Figure S2). In contrast, the worm Caenorhabditis elegans does not have a PTS2 import pathway, but neither does it have a Pex7 homolog (Motley et al. 2000). It is possible that Drosophila has a divergent PTS2, but its identification and mechanism of recognition by Pex7 remain areas for future study.
Acknowledgments
We thank Nancy E. Braverman (McGill University) for advice and for providing wild-type and RCDP1 human fibroblasts; and the Lipidomics Core Facility at the University of Alberta, which is supported by funding from the Faculty of Medicine & Dentistry and the Women and Children’s Health Research Institute, University of Alberta. This work was funded by a Collaborative Research Innovation Opportunities grant from Alberta Innovates ‒ Health Solutions to R.A.R. and A.J.S., a Canadian Institutes of Health Research Foundation grant 143289 to R.A.R., and charitable support from The Edgar Foundation and the Ladies Auxiliary of the Fraternal Order of Eagles 3395 to R.A.R.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7221503.
Communicating editor: H. Bellen
Literature Cited
- Baron M. N., Klinger C. M., Rachubinski R. A., Simmonds A. J., 2016. A systematic cell-based analysis of localization of predicted Drosophila peroxisomal proteins. Traffic 17: 536–553. 10.1111/tra.12384 [DOI] [PubMed] [Google Scholar]
- Beard M. E., Holtzman E., 1987. Peroxisomes in wild-type and rosy mutant Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 84: 7433–7437. 10.1073/pnas.84.21.7433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G., 1998. Free fatty acids (FFA), a link between obesity and insulin resistance. Front. Biosci. 3: d169–d175. 10.2741/A272 [DOI] [PubMed] [Google Scholar]
- Bodnar A. G., Rachubinski R. A., 1990. Cloning and sequence determination of cDNA encoding a second rat liver peroxisomal 3-ketoacyl-CoA thiolase. Gene 91: 193–199. 10.1016/0378-1119(90)90088-9 [DOI] [PubMed] [Google Scholar]
- Bowers W. E., 1998. Christian de Duve and the discovery of lysosomes and peroxisomes. Trends Cell Biol. 8: 330–333. 10.1016/S0962-8924(98)01314-2 [DOI] [PubMed] [Google Scholar]
- Braverman N., Steel G., Obie C., Moser A., Moser H., et al. , 1997. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat. Genet. 15: 369–376. 10.1038/ng0497-369 [DOI] [PubMed] [Google Scholar]
- Braverman N. E., D’Agostino M. D., Maclean G. E., 2013. Peroxisome biogenesis disorders: biological, clinical and pathophysiological perspectives. Dev. Disabil. Res. Rev. 17: 187–196. 10.1002/ddrr.1113 [DOI] [PubMed] [Google Scholar]
- Braverman N. E., Argyriou C., Moser A., 2014. Human disorders of peroxisome biogenesis: Zellweger spectrum and rhizomelic chondrodysplasia punctata, pp. 63–90 in Molecular Machines Involved in Peroxisome Biogenesis and Maintenance, edited by Brocard C., Hartig A. Springer, Vienna: 10.1007/978-3-7091-1788-0_4 [DOI] [Google Scholar]
- Bülow M. H., Wingen C., Senyilmaz D., Gosejacob D., Sociale M., et al. , 2018. Unbalanced lipolysis results in lipotoxicity and mitochondrial damage in peroxisome-deficient Pex19 mutants. Mol. Biol. Cell 29: 396–407. 10.1091/mbc.E17-08-0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cara F. D., Bülow M. H., Simmonds A. J., Rachubinski R. A., 2018. Dysfunctional peroxisomes compromise gut structure and host defense by increased cell death and Tor-dependent autophagy. Mol. Biol. Cell 29: 2766–2783. 10.1091/mbc.E18-07-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Liu Z., Huang X., 2010. Drosophila models of peroxisomal biogenesis disorder: peroxins are required for spermatogenesis and very-long-chain fatty acid metabolism. Hum. Mol. Genet. 19: 494–505. 10.1093/hmg/ddp518 [DOI] [PubMed] [Google Scholar]
- Colombani J., Bianchini L., Layalle S., Pondeville E., Dauphin-Villemant C., et al. , 2005. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310: 667–670. 10.1126/science.1119432 [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P., 1966. Peroxisomes (microbodies and related particles). Physiol. Rev. 46: 323–357. 10.1152/physrev.1966.46.2.323 [DOI] [PubMed] [Google Scholar]
- Di Cara F., Sheshachalam A., Braverman N. E., Rachubinski R. A., Simmonds A. J., 2017. Peroxisome-mediated metabolism is required for immune response to microbial infection. Immunity 47: 93–106.e7 [corrigenda: Immunity 48: 832–833 (2018)] 10.1016/j.immuni.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Dixit E. S., Boulant Y., Zhang A. S., Lee C., Odendall C., et al. , 2010. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141: 668–681. 10.1016/j.cell.2010.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust J. E., Verma A., Peng C., McNew J. A., 2012. An inventory of peroxisomal proteins and pathways in Drosophila melanogaster. Traffic 13: 1378–1392. 10.1111/j.1600-0854.2012.01393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust J. E., Manisundaram A., Ivanova P. T., Milne S. B., Summerville J. B., et al. , 2014. Peroxisomes are required for lipid metabolism and muscle function in Drosophila melanogaster. PLoS One 9: e100213 10.1371/journal.pone.0100213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany M. B., Bender W. W., 2000. A Drosophila model of Parkinson’s disease. Nature 404: 394–398. 10.1038/35006074 [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G. H., 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- Freeman M. R., Doherty J., 2006. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 29: 82–90. 10.1016/j.tins.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Glover J. R., Andrews D. W., Subramani S., Rachubinski R. A., 1994. Mutagenesis of the amino targeting signal of Saccharomyces cerevisiae 3-ketoacyl-CoA thiolase reveals conserved amino acids required for import into peroxisomes in vivo. J. Biol. Chem. 268: 7558–7563. [PubMed] [Google Scholar]
- Imanaka T., Aihara K., Suzuki Y., Yokota S., Osumi T., 2000. The 70-kDa peroxisomal membrane protein (PMP70), an ATP-binding cassette transporter. Cell Biochem. Biophys. 32: 131–138. 10.1385/CBB:32:1-3:131 [DOI] [PubMed] [Google Scholar]
- Ito T., Fujimura S., Matsufuji Y., Miyaji T., Nakagawa T., et al. , 2007. Molecular characterization of the PEX5 gene encoding peroxisomal targeting signal 1 receptor from the methylotrophic yeast Pichia methanolica. Yeast 24: 589–597. 10.1002/yea.1484 [DOI] [PubMed] [Google Scholar]
- Kanzawa N., Shimozawa N., Wanders R. J. A., Ikeda K., Murakami Y., et al. , 2012. Defective lipid remodeling of GPI anchors in peroxisomal disorders, Zellweger syndrome, and rhizomelic chondrodysplasia punctata. J. Lipid Res. 53: 653–663. 10.1194/jlr.M021204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. T., Barnett P., Bottger G., Konings D., Tabak H. F., et al. , 2001. Recognition of peroxisomal targeting signal type 1 by the import receptor Pex5p. J. Biol. Chem. 276: 15034–15041. 10.1074/jbc.M010776200 [DOI] [PubMed] [Google Scholar]
- Kragler F., Lametschwandtner G., Christmann J., Hartig A., Harada J. J., 1998. Identification and analysis of the plant peroxisomal targeting signal 1 receptor NtPEX5. Proc. Natl. Acad. Sci. USA 95: 13336–13341. 10.1073/pnas.95.22.13336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., 2006. The import receptor Pex7p and the PTS2 targeting sequence. Biochim. Biophys. Acta 1763: 1599–1604. 10.1016/j.bbamcr.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittigen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Madabattula S. T., Strautman J. C., Bysice A. M., O’Sullivan J. A., Androschuk A., et al. , 2015. Quantitative analysis of climbing defects in a Drosophila model of neurodegenerative disorders. J. Vis. Exp. (100): e52741 10.3791/52741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast F. D., Li J., Virk M. K., Hughes S. C., Simmonds A. J., et al. , 2011. A Drosophila model for the Zellweger spectrum of peroxisome biogenesis disorders. Dis. Model. Mech. 4: 659–672. 10.1242/dmm.007419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T., Otera H., Fujiki Y., 2000. Disruption of the interaction of the longer isoform of Pex5p, Pex5pL, with Pex7p abolishes peroxisome targeting signal type 2 protein import in mammals. Study with a novel Pex5-impaired Chinese hamster ovary cell mutant. J. Biol. Chem. 275: 21715–21721. 10.1074/jbc.M000721200 [DOI] [PubMed] [Google Scholar]
- Matzat T., Sieglitz F., Kottmeier R., Babatz F., Engelen D., et al. , 2015. Axonal wrapping in the Drosophila PNS is controlled by glia-derived neuregulin homolog Vein. Development 142: 1336–1345. 10.1242/dev.116616 [DOI] [PubMed] [Google Scholar]
- McCollum D., Monosov E., Subramani S., 1993. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein import deficiencies of Zellweger syndrome cells–the PAS8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal, and is a member of the TPR protein family. J. Cell Biol. 121: 761–774. 10.1083/jcb.121.4.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirth C., Truman J. W., Riddiford L. M., 2005. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr. Biol. 15: 1796–1807. 10.1016/j.cub.2005.09.017 [DOI] [PubMed] [Google Scholar]
- Motley A. M., Hettema E. H., Ketting R., Plasterk R., Tabak H. F., 2000. Caenorhabditis elegans has a single pathway to target matrix proteins to peroxisomes. EMBO Rep. 1: 40–46. 10.1093/embo-reports/kvd010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Sato H., Okuda T., Fujisawa N., Kono N., et al. , 2011. Drosophila carrying Pex3 or Pex16 mutations are models of Zellweger syndrome that reflect its symptoms associated with the absence of peroxisomes. PLoS One 6: e22984 10.1371/journal.pone.0022984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen S. D., Baes M., Van Veldhoven P. P., 2008. Degradation of very long chain dicarboxylic polyunsaturated fatty acids in mouse hepatocytes, a peroxisomal process. Biochim. Biophys. Acta 1781: 400–405. 10.1016/j.bbalip.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Otera H., Harano T., Honsho M., Ghaedi K., Mukai S., et al. , 2000. The mammalian peroxin Pex5pL, the longer isoform of the mobile peroxisome targeting signal (PTS) type 1 transporter, translocates the Pex7p.PTS2 protein complex into peroxisomes via its initial docking site, Pex14p. J. Biol. Chem. 275: 21703–21714. 10.1074/jbc.M000720200 [DOI] [PubMed] [Google Scholar]
- Parsons B., Foley E., 2013. The Drosophila platelet-derived growth factor and vascular endothelial growth factor-receptor related (Pvr) protein ligands Pvf2 and Pvf3 control hemocyte viability and invasive migration. J. Biol. Chem. 288: 20173–20183. 10.1074/jbc.M113.483818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta H. W., Erdmann R., 2007. Peroxisomal dynamics. Trends Cell Biol. 17: 474–484. 10.1016/j.tcb.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Purdue P. E., Zhang J. W., Skoneczny M., Lazarow P. B., 1997. Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat. Genet. 15: 381–384. 10.1038/ng0497-381 [DOI] [PubMed] [Google Scholar]
- Rehling P., Marzioch M., Niesen F., Wittke E., Veenhuis M., et al. , 1996. The import receptor for the peroxisomal targeting signal 2 (PTS2) in Saccharomyces cerevisiae is encoded by the PAS7 gene. EMBO J. 15: 2901–2913. 10.1002/j.1460-2075.1996.tb00653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M., Fahimi H. D., 2006. Peroxisomes and oxidative stress. Biochim. Biophys. Acta 1763: 1755–1766. 10.1016/j.bbamcr.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Shimozawa N., Zhang Z., Suzuki Y., Imamura A., Tsukamoto T., et al. , 1999. Functional heterogeneity of C-terminal peroxisome targeting signal 1 in PEX5-defective patients. Biochem. Biophys. Res. Commun. 262: 504–508. 10.1006/bbrc.1999.1232 [DOI] [PubMed] [Google Scholar]
- Shklyar B., Sellman Y., Shklover J., Mishnaevski K., Levy-Adam F., et al. , 2014. Developmental regulation of glial cell phagocytic function during Drosophila embryogenesis. Dev. Biol. 393: 255–269. 10.1016/j.ydbio.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Smith J. J., Aitchison J. D., 2013. Peroxisomes take shape. Nat. Rev. Mol. Cell Biol. 14: 803–817. 10.1038/nrm3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S. J., Dodt G., Raymond G. V., Braverman N. E., Moser A. B., et al. , 2006. Peroxisome biogenesis disorders. Biochim. Biophys. Acta 1763: 1733–1748. 10.1016/j.bbamcr.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Stich V., Berlan M., 2004. Physiological regulation of NEFA availability: lipolysis pathway. Proc. Nutr. Soc. 63: 369–374. 10.1079/PNS2004350 [DOI] [PubMed] [Google Scholar]
- Szilard R. K., Titorenko V. I., Veenhuis M., Rachubinski R. A., 1995. Pay32p of the yeast Yarrowia lipolytica is an intraperoxisomal component of the matrix protein translocation machinery. J. Cell Biol. 131: 1453–1469. 10.1083/jcb.131.6.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K. J., Schulze K. L., Haelterman N. A., Pan H., He Y., et al. , 2011. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 8: 737–743. 10.1038/nmeth.1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., Waterham H. R., 2006. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 75: 295–332. 10.1146/annurev.biochem.74.082803.133329 [DOI] [PubMed] [Google Scholar]
- Wei Y., Yu L., Bowen J., Gorovosky M. A., Allis C. D., 1999. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell 97: 99–109. 10.1016/S0092-8674(00)80718-7 [DOI] [PubMed] [Google Scholar]
- Woodward A. W., Bartel B., 2005. The Arabidopsis peroxisomal targeting signal type 2 receptor PEX7 is necessary for peroxisome function and dependent on PEX5. Mol. Biol. Cell 16: 573–583. 10.1091/mbc.e04-05-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J., Hou S., Malik-Soni N., Xu Z., Kumar A., et al. , 2015. Flavivirus infection impairs peroxisome biogenesis and early anti-viral signaling. J. Virol. 89: 12349–12361. 10.1128/JVI.01365-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors state that all data necessary for confirming the conclusions presented are represented within the manuscript. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7221503.