Wnt4 is a key regulator of ovary development in mammals, but its role in other vertebrates is unknown. Here, Kossack et al. show that zebrafish wnt4a is the ortholog of mammalian Wnt4 and is expressed....
Keywords: wnt4a, sex determination, sex differentiation, zebrafish, reproductive duct, Genetics of Sex
Abstract
In laboratory strains of zebrafish, sex determination occurs in the absence of a typical sex chromosome and it is not known what regulates the proportion of animals that develop as males or females. Many sex determination and gonad differentiation genes that act downstream of a sex chromosome are well conserved among vertebrates, but studies that test their contribution to this process have mostly been limited to mammalian models. In mammals, WNT4 is a signaling ligand that is essential for ovary and Müllerian duct development, where it antagonizes the male-promoting FGF9 signal. Wnt4 is well conserved across all vertebrates, but it is not known if Wnt4 plays a role in sex determination and/or the differentiation of sex organs in nonmammalian vertebrates. This question is especially interesting in teleosts, such as zebrafish, because they lack an Fgf9 ortholog. Here we show that wnt4a is the ortholog of mammalian Wnt4, and that wnt4b was present in the last common ancestor of humans and zebrafish, but was lost in mammals. We show that wnt4a loss-of-function mutants develop predominantly as males and conclude that wnt4a activity promotes female sex determination and/or differentiation in zebrafish. Additionally, both male and female wnt4a mutants are sterile due to defects in reproductive duct development. Together these results strongly argue that Wnt4a is a conserved regulator of female sex determination and reproductive duct development in mammalian and nonmammalian vertebrates.
ZEBRAFISH (Danio rerio) is a major model research organism, yet little is known about its underlying molecular mechanism of sex determination. Zebrafish that were domesticated for laboratory use do not have a single sex chromosome; instead, several loci have been identified that appear to influence sex ratios in a strain-dependent manner (Bradley et al. 2011; Anderson et al. 2012; Howe et al. 2013). In contrast, nondomesticated strains use a ZZ/ZW genetic sex determination mechanism, with the major sex locus being located on chromosome 4 (Wilson et al. 2014). Until this locus is characterized, the conserved genes involved in sex determination and/or differentiation in other vertebrates may offer insight into zebrafish sex determination.
Although domesticated zebrafish do not possess a major sex-determining locus, some progress has been made toward understanding how sex is determined. Overt sex differences are not apparent until ∼20–30 days postfertilization (dpf), when males and females tend to differ in number of oocytes, with female gonads generally having more oocytes than male gonads (Wang et al. 2007). It is therefore presumed that definitive sex determination occurs sometime between 20 and 25 dpf, though an earlier time point cannot be ruled out.
Prior to sex determination, the zebrafish gonad, like the mammalian gonad, is bipotential. Starting at ∼10 dpf, a subset of germ cells in all zebrafish enter meiosis to form early stage oocytes (Takahashi 1977) and establish the bipotential gonad. At the same time, based on gene expression analysis, the somatic gonad is a mixture of male- and female-like cells. For example, we and others have shown that during this stage, some somatic gonad cells begin to express the female-specific gene cyp19a1a (aromatase), while neighboring cells express the male-specific gene anti-Müllerian hormone (amh) (Rodríguez-Marí et al. 2005; Leerberg et al. 2017). Beginning at ∼20 dpf, oocytes in some individuals undergo apoptosis as these gonads begin the transition to testis development. In contrast, oocytes in gonads destined to become ovaries continue their maturation (Uchida et al. 2002; Maack and Segner 2003). Importantly, if all germ cells, or specifically oocytes, are ablated prior to or during the bipotential phase, all animals develop as phenotypic males (Slanchev et al. 2005; Siegfried and Nüsslein-Volhard 2008; Rodríguez-Marí et al. 2010; Dai et al. 2015). These results led to the model that oocytes produce a signal that stabilizes female development; in the absence of a threshold level of the signal, the animals develop as males. The identity of the oocyte-producing signal or how it affects sex determination of somatic gonad cells remains to be determined.
Growing evidence suggests that Wnt signaling may also play a conserved role in teleost sex determination and/or differentiation. In zebrafish, overexpression of a dominant-negative TCF transcription factor, the downstream effector of the canonical Wnt signaling pathway, increases the production of males over females (Sreenivasan et al. 2014). Thus canonical Wnt signaling appears to be involved in female sexual development in zebrafish. In mammals, WNT4 is the WNT ligand involved in sex determination (Vainio et al. 1999), but the specific Wnt ligand that functions to regulate sex determination in zebrafish remains to be determined.
WNT4 (wingless-type MMTV integration site family, member 4) is a signaling ligand that binds to the Frizzled receptor and activates the canonical Wnt signaling pathway (as reviewed in Nusse and Clevers 2017). In mammals, which use an XX/XY genetic sex determination system, Wnt4 is critical for female sex determination. In addition, the early mammalian gonad is bipotential and both sexes initially express the male-specific gene fibroblast growth factor 9 (Fgf9) in the overlying gonadal epithelium, while the underlying mesonephros expresses the female-specific gene Wnt4 (Vainio et al. 1999; Bowles et al. 2010). In the absence of Sry, the Y-linked male sex determinant, WNT4 inhibits the expression of FGF9 and the gonad develops into an ovary that continues to express WNT4. In contrast, expression of SRY in XY animals stabilizes Fgf9 expression, which in turn leads to the inhibition of Wnt4, and Fgf9 encourages Sox9 expression, which leads to testis development (Kim et al. 2006). Importantly, XX mice lacking WNT4 sex-revert to male (Vainio et al. 1999), demonstrating that WNT4 is necessary for female development. Interestingly, simultaneous loss of WNT4 and FGF9 in XY animals results in normal testis development, arguing that the main role of FGF9 in males is to antagonize the female-promoting WNT4 signal (Jameson et al. 2012). Additionally, whereas both male and female wild-type mouse embryos develop both Müllerian and Wolffian ducts, neither male nor female Wnt4 mutant mouse embryos develop Müllerian ducts, which in females form the fallopian tubes and uterus (Vainio et al., 1999). Finally, mutations in the human WNT4 gene can lead to a variety of reproductive diseases that affect ovary development, including polycystic ovary syndrome (Pellegrino et al. 2010) and female sex reversal and dysgenesis of kidneys, adrenals, and lungs, where chromosomally XX gonads lacking WNT4 function no longer develop as an ovary and instead develop testicular tissue (Mandel et al. 2008).
Here we show that Wnt4a functions to promote female sex determination and/or differentiation in zebrafish. In addition, we show that Wnt4a is required for the development of the reproductive ducts in both male and female zebrafish. These results therefore demonstrate that Wnt4 is a conserved regulator of female sex determination or differentiation and reproductive duct development in a nonmammalian vertebrate.
Materials and Methods
Phylogenetic analysis
Phylogenetic and conserved synteny analysis of Wnt4a was conducted as previously described (Vilella et al. 2009).
Zebrafish rearing
The Institutional Animal Care and Use Committees at the University of California, Davis and the University of Oregon approved all animals used in this study (protocols #18483 and #14-08R, respectively). Zebrafish husbandry was performed as previously described (Westerfield 2007) with the following modifications to the larval fish (5–30 dpf) feeding schedule: 5–12 dpf, 40 fish/250 ml in static fish water [4 parts per thousand (ppt) ocean salts] were fed rotifers (Brachionus plicatilis, L-type) twice daily ad libitum; 12–15 dpf, 40 fish/1 liter gently flowing fish water (<1 ppt ocean salts) were fed both rotifers and freshly hatched Artemia nauplii ad libitum twice daily; 15–30 dpf, 40 fish/1 liter gently flowing fish water (<1 ppt ocean salts) were fed freshly hatched Artemia nauplii ad libitum twice daily.
Fish lines
The ziwi:EGFP transgenic line and wnt4a(fh294)/+ and wnt4a(fh295/+) mutant lines were developed in an AB background. These mutant lines were created by treating adult AB zebrafish males with ENU and identifying sequence changes in the wnt4 gene (Moens and ZFIN Staff 2009). The resulting wnt4a mutation is a nucleotide substitution that creates a premature stop codon at amino acid 307 of 352 (Moens and ZFIN Staff 2009). The wnt4a(uc55) and wnt4a(uc56) alleles were produced by CRISPR/Cas9 genome editing, with the following guide RNA targeting exon two: 5′- AGCTGTCGTCGGTGGGGAGC(PAM)-3′. wnt4a(uc55) and wnt4a(uc56) are predicted to cause a translational frame shift in exon two.
Fluorescent in situ hybridization
The wnt4a in situ probe was generated by PCR (see Supplemental Material, Table S1 for primers), producing a 1987-bp fragment. This was cloned into pGEM-T Easy Vector (Promega, Madison, WI). For whole-mount studies on 10–30 dpf gonads, wnt4a was hybridized at a concentration of 1:200 at 65° to permeabilized tissue for 48 hr, after which whole-mount gonads were developed using an alkaline-phosphatase reaction with FastRed (Sigma, St. Louis, MO) for 8 hr. VASA antibody (1:1500) staining was performed after a glycine wash, as described in Draper (2012). Gonads were imaged with an Olympus FV1000 laser scanning confocal microscope. Acquired images were adjusted equally using ImageJ.
RT-PCR gene expression analysis
RNA was extracted from the gonads of three individuals at 90 dpf or 30 dpf and RNA was combined before reverse transcription. Amplification of wnt4a, wnt4b, cyp19a1a, amh, and rpl13a was performed with the following program: step 1 was 94° for 2 min; step 2 was 94° for 15 sec, 65° for 15 sec, 72° for 15 sec, repeated 28 times; and step 3 was 72° for 2 min. Primers are listed in Table S1. Products were run on a 1% agarose gel and imaged.
Genotyping
wnt4a(fh294) PCR:
The primers used for genotyping the wnt4a(fh294) mutant line are listed in Table S1, using the following PCR conditions: 94° for 1 min; 35 cycles of 94° for 30 sec, 60° for 30 sec, 72° for 1 min and 30 sec; followed by 15° until program was ended. Resulting amplicons were digested with DdeI at 37° overnight (Moens and ZFIN Staff 2009). The sizes of bands after DdeI digest were 384 bp for wild type and 270 and 114 bp for the mutant.
wnt4a(fh295) PCR:
Genomic DNA was extracted and fh295 mutant fish were identified by high-resolution melt analysis (Dahlem et al. 2012) using primers listed in Table S1. The program was as follows: step 1 was 95° for 1 min; step 2 was 39 cycles of 94° for 10 sec and 69° for 15 sec; step 3 was 94° for 20 sec; step 4 was 72° for 20 sec; followed by a melt profile from 80 to 92° with increments of 0.2°.
wnt4a(uc55 and uc56) PCR:
The primer pairs used for PCR genotyping are listed in Table S1, using the following PCR protocol: step 1 was 94° for 1 min; step 2 was 34 cycles of 94° for 10 sec and 55° for 10 sec; and step 3 was 72° for 15 sec. The PCR products were separated as follows on a 3% agarose gel: 123 bp for wild type, 140 bp for wnt4a(uc55), and 149 bp for wnt4a(uc56).
Sex ratios and characterization of mutant phenotypes
At 90 dpf or more, fish were genotyped and killed. Secondary sexual characteristics were examined, and the gonad of each fish was dissected to confirm gonadal sex. A subset from animals of each genotype was randomly measured for standard length. Characterization of mutant development was performed by anti-Vasa antibody staining, as described previously (Draper 2012).
Mutant fertility assessment
wnt4a(uc55) and wnt4a(fh294) heterozygous fish were set up in a crossing cage with either heterozygous or mutant counterparts. Eggs were collected and counted for percent fertilization. Following mating tests, wnt4a(uc55) and wnt4a(fh294) heterozygous or mutant males and females were squeezed for sperm or eggs following techniques described in Walker and Streisinger (2007). If eggs or sperm were not released, gonads were dissected and fertilized with the heterozygous counterpart in vitro. Percent of eggs successfully fertilized and embryo survivability were tracked to 5 dpf.
Hematoxylin and eosin staining
At 90 dpf, wnt4a(uc55) and wnt4a(fh295) mutant and wild-type fish (n = 3) were identified by PCR genotyping, then killed and fixed in Bouin’s fixative for 24 hr. Samples were then embedded in paraffin, cut into 7 µm sections, and stained with hematoxylin and eosin (H&E). Reproductive ducts were examined and representative images were taken at 5× on a Zeiss Axiophot microscope.
Microcomputed tomography
At 90 dpf, fish were genotyped and confirmed to be wild type or wnt4a(uc55) mutant (n = 3). Fish were anesthetized with MS22 for 5 min and exsanguinated by cutting off the tail and placing the fish head up in a filter column in a 1.5-ml microcentrifuge tube, followed by centrifuging at 40 relative centrifugal force (RFC) at room temperature for 5 min. The blood clot was then removed and the fish was centrifuged again at 40 RFC. Fish were then fixed in 4% paraformaldehyde in 1× phosphate buffered saline (PBS) for 24 hr. Before imaging, fish were placed in 2.75% Lugol’s iodine solution (14 ml/fish) for 24 hr and then washed in 1× PBS for 1 hr.
Zebrafish were imaged at the Center for Molecular and Genomic Imaging (University of California, Davis) with X-ray computed tomography (CT). Fish (90 dpf) were embedded in 1% agar gel and positioned in a 15-mm diameter conical tube. The straw was mounted on an aluminum post for placing in the CT scanner. X-ray tomographic images were obtained on the Center’s MicroXCT-200 specimen CT scanner (X-ray Microscopy; Carl Zeiss, Thornwood, NY). Samples were mounted on the scanner’s sample stage, which can be positioned to the submicron level. Scan parameters were adjusted based on the manufacturer’s recommended guidelines. The 4× objective of the MicroXCT was chosen for optimal spatial resolution of reproductive ducts. The source and detector distances were set at 30 and 10 mm, respectively. Once the source and detector settings were established, the optimal X-ray filtration was determined by selecting 1 of 12 proprietary filters; in this case, no filtration was necessary. Following this procedure, the optimal voltage and power settings were determined for optimal contrast (80 kV and 100 µA). A total of 1600 projections over 360° were obtained with 0.75 sec per projection. The camera pixels were binned by two and the source-detector configuration resulted in a voxel size of 5.0693 µm. Tomographic images were reconstructed with a center shift (7.11 pixels) and beam-hardening parameter value of 0.2 to obtain optimized images. A smoothing filter of kernel size 0.7 was applied during reconstruction. Images were reconstructed into 16-bit values.
In situ hybridization on sections
Animals were collected at multiple stages of zebrafish male reproductive duct development. Animals were then killed, fixed, and cryosectioned as previously described (Rodríguez-Marí et al. 2005). The probe for wnt4a was created using primers listed in Table S1. The wnt4a complementary DNA was cloned using the TOPO vector and used to synthesize DIG-labeled probes. For in situ hybridization experiments, two 25 dpf, two 35 dpf male, and two 55 dpf male zebrafish were used.
Data availability
All fish lines are available upon request, and will be deposited at the Zebrafish International Stock Center. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7098779.
Results
wnt4a is the ortholog of mammalian Wnt4
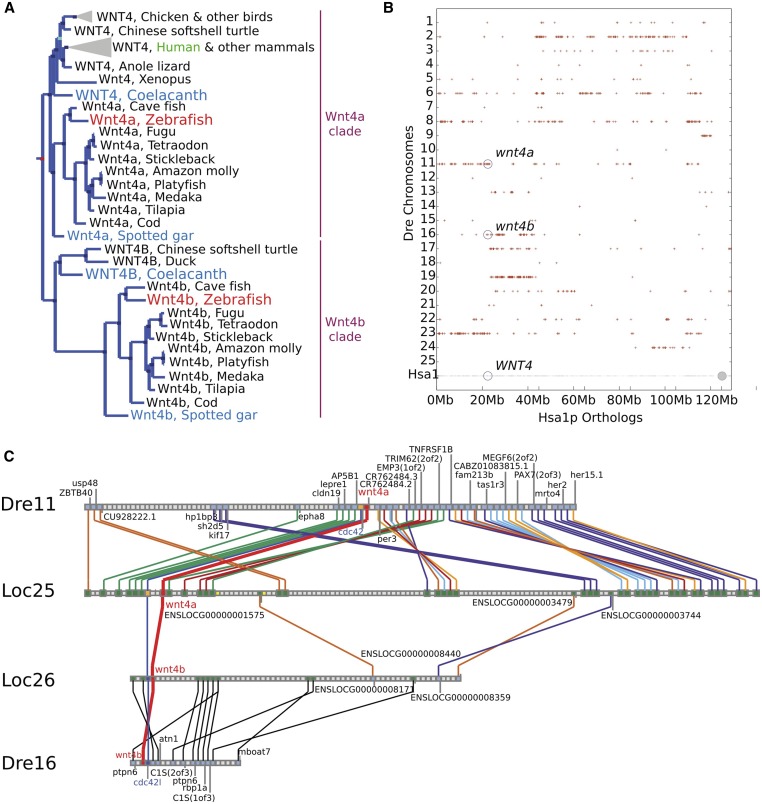
Mammalian genomes contain a single WNT4 gene but most teleost genomes have two Wnt4-related genes called wnt4a and wnt4b (Ungar et al. 1995; Liu et al. 2000). Connectivity of teleost genomes to the human genome requires accurate designation of orthologs, which necessitates an understanding of gene histories. The two teleost wnt4-related genes could have resulted from either: (1) gene duplication after the divergence of mammalian and teleost lineages, for example, in the teleost genome duplication event (Amores et al. 1998; Postlethwait et al. 1998; Jaillon et al. 2004) or by tandem duplication; or (2) duplication before the divergence of the human and zebrafish lineages followed by loss of either Wnt4a or Wnt4b in the mammalian lineage, if this hypothesis were true, then it would be important to know whether teleost wnt4a or wnt4b is the ortholog of mammalian Wnt4. To test these models, we studied gene phylogenies and conserved syntenies. Phylogenetic analysis showed that ancestral lobe-finned vertebrates had two wnt4-related genes, the wnt4a and wnt4b clades, because several lobe-finned vertebrates (birds, reptiles, and coelacanth) have both of these genes today (Figure 1A). Ancestral ray-finned vertebrates also had both wnt4 clades because orthologs of both genes appear in genomes of spotted gar and teleost fishes (Figure 1A). This evidence shows that the last common ancestor of human and zebrafish had both wnt4a and wnt4b, ruling out the hypothesis that wnt4a and wnt4b arose in the teleost genome duplication and supporting the loss of wnt4b in the origin of mammals.
Figure 1.
Teleost wnt4a is the ortholog of tetrapod WNT4. (A) Phylogenetic analysis shows that vertebrates have two Wnt4-related clades (designated Wnt4a and Wnt4b). The Wnt4a clade includes teleosts and gar as ray-finned fish and coelacanth, birds, and mammals as lobe-finned fish. The Wnt4b clade also includes teleosts and gar as ray-finned fish as well as coelacanth and birds, but not mammals, as lobe-finned fish. This result shows that the duplication event that produced the wnt4a and wnt4b clades predated the divergence of ray-finned (e.g., gar, teleost) and lobed-finned (e.g., coelacanth, bird, mammal) lineages. (B) A dot plot comparing zebrafish orthologs and paralogs of genes on the short arm of human chromosome 1 (Hsa1p) shows conserved syntenies along zebrafish chromosome Dre11 (wnt4a) and Dre16 (wnt4b). (C) Conserved synteny analysis shows that the zebrafish chromosome segments containing wnt4a and wnt4b are orthologous to regions on different spotted gar chromosomes, and that these two spotted gar chromosomes show ancient paralogy. Based on phylogenetic and conserved synteny analyses, wnt4a and wnt4b were both in the last common ancestor of zebrafish and humans but mammals lost the ortholog of wnt4b, and wnt4a in teleosts is the ortholog of WNT4 in mammals.
Several lines of evidence argue that wnt4a and wnt4b have their origin in the two rounds of vertebrate genome duplication (VGD1 and VGD2), but not from retrotransposition, a simple one-gene duplication event, or in the teleost genome duplication event. First, the presence of introns in orthologous locations in both wnt4 genes rules out the origin of either gene by retrotransposition. Second, analysis of conserved syntenies shows that wnt4a is located on zebrafish (D. rerio) chromosome Dre11 adjacent to cdc42, while wnt4b is adjacent to cdc42l on Dre16, arguing that wnt4a and wnt4b arose from a genomic event more complicated than a simple one-gene tandem duplication (Figure 1B; Amores et al. 1998). Third, the teleost duplication ohnolog of wnt4a-containing chromosome Dre11 is Dre23, while that of wnt4b-containing Dre16 is Dre19 (Figure 1B; Amores et al. 1998). Finally, the portion of Dre11 that contains wnt4a is orthologous to spotted gar (Lepisosteus oculatus) chromosome Loc25, which contains gar wnt4a, while the portion of Dre16 that contains wnt4b is orthologous to Loc26, which contains gar wnt4b (Figure 1C), as expected from whole genome duplication but not by tandem duplication. The finding that Loc25 and Loc26 are at least in part paralogous (Figure 1C) and that the gar lineage did not experience a genome duplication event after the divergence of ray-finned and lobe-finned vertebrates (Amores et al. 2011; Braasch et al. 2016) are as predicted by the hypothesis that wnt4a and wnt4b arose in one of the two genome duplication events at the base of the vertebrate radiation (Dehal and Boore 2005; Smith and Keinath 2015) and the WNT4B gene was lost in the mammalian lineage after it split from the bird lineage. We therefore conclude that the zebrafish wnt4a gene is the ortholog of the human gene WNT4.
Early gonadal somatic cells express wnt4a
In mice, both XX and XY individuals express Wnt4 in early bipotential gonads (9 days postconception); thereafter, male gonads suppress Wnt4 expression but female gonads maintain Wnt4 expression (Vainio et al. 1999). We therefore used RT-PCR to determine if the expression of either wnt4a or wnt4b were sexually dimorphic in adult and juvenile zebrafish gonads. Experiments detected wnt4a but not wnt4b in the ovary and wnt4b but not wnt4a in the testis in both adult and juvenile gonads (Figure S1, A and B). Thus, like mammalian WNT4, wnt4a in zebrafish appears to be associated with ovarian development.
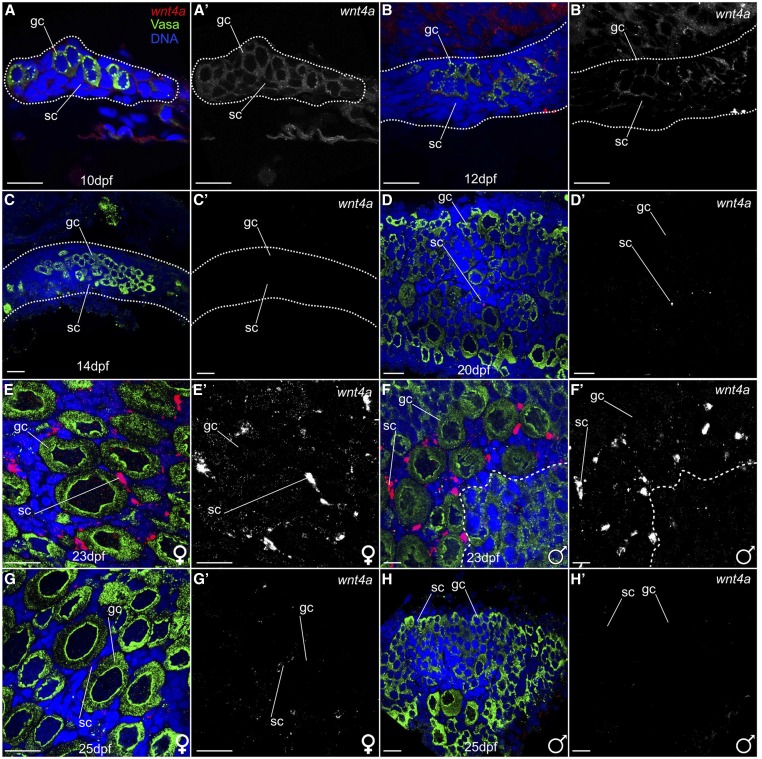
We next asked which cells express wnt4a in larval zebrafish gonads, bracketing the sex determination and early sex differentiation period between 10 and 25 dpf. Fluorescent RNA in situ hybridization experiments on wild-type gonads detected wnt4a expression in germ cells and in somatic gonad cells in all 17 individuals examined at 10 dpf and 12 dpf, though levels appeared to be higher in 10 dpf gonads (Figure 2, A and A′; n = 8) relative to 12 dpf wild-type gonads (Figure 2, B and B′; n = 9). In contrast, we were unable to detect wnt4a expression in any gonads in 14 dpf individuals (Figure 2, C and C′; n = 6). At 20 dpf, wnt4a expression was no longer detected in germ cells and appeared in only a subset of somatic cells (Figure 2, D and D′; n = 4). Somatic cell-specific expression of wnt4a increased from 20 dpf until 23 dpf when wnt4a was highly expressed in all gonads (n = 10), specifically in the somatic cells surrounding larger oocytes, indicating a presumptive ovary (Figure 2, E and E′). Less expression was found surrounding smaller oocytes or cyst-like divisions of a presumptive testis (Figure 2, F and F′). At 25 dpf, when gonads had committed to the ovary or testis fate, which can be distinguished based on the numbers of oocytes present (Uchida et al. 2002), wnt4a expression was detected only in female gonads (Figure 2, G and G′; n = 6) and was no longer detected in developing male gonads (Figure 2, H and H′; n = 10). This sexually dimorphic expression of wnt4a continued throughout adulthood (Figure S1A). We conclude that wnt4a is expressed in a dynamic, sex-nonspecific pattern in early gonads, and that by 25 dpf onward its expression is limited to somatic cells in ovaries.
Figure 2.
wnt4a is expressed in early zebrafish gonads. Confocal images of isolated gonads stained for wnt4a mRNA (red), Vasa to label germ cells (green), nuclei (blue) (A–H), or wnt4a RNA only (A′–H′), and gonadal tissue outlined by a dotted line (A–C). (A and A′) At 10 dpf (n = 8) and (B and B′) 12 dpf (n = 9), wnt4a RNA was detected ubiquitously in germ cells (gc) and somatic cells (sc). (C and C′) In contrast, wnt4a RNA was not detected in gonadal cells at 14 dpf (n = 6). (D and D′) At 20 dpf, wnt4a RNA was detected in a small subset of somatic cells (n = 4). At 23 dpf (n = 10), wnt4a expression was detected in somatic cells both in presumptive ovaries (E and E′) and in gonads that are transitioning to testes, with cyst-like divisions to the right of the dashed line (F and F′). At 25 dpf, wnt4a mRNA was detected (G and G′) in the developing ovary (n = 6), (H and H′) but not in gonads that appeared to be transitioning to testes (n = 10). Bar, 20 µm.
We next asked if we could identify the somatic cell type that expresses wnt4a at 23 dpf. At this stage, wnt4a-expressing cells associated closely with stage-IB oocytes (20–140 µm) and were therefore likely to be either theca cells or granulosa cells. To distinguish between these possibilities, we used the transgenic reporter line Tg(cyp19a1a:egfp), which expresses GFP in theca cells that surround stage-IB oocytes (Dranow et al. 2016). Results showed that wnt4a-expressing cells did not coexpress GFP (n = 3; Figure S2). We conclude that wnt4a is not expressed in theca cells, but rather in another component of the gonadal soma, likely granulosa cells, although another gonadal cell type cannot be excluded.
wnt4a mutants develop predominantly as males
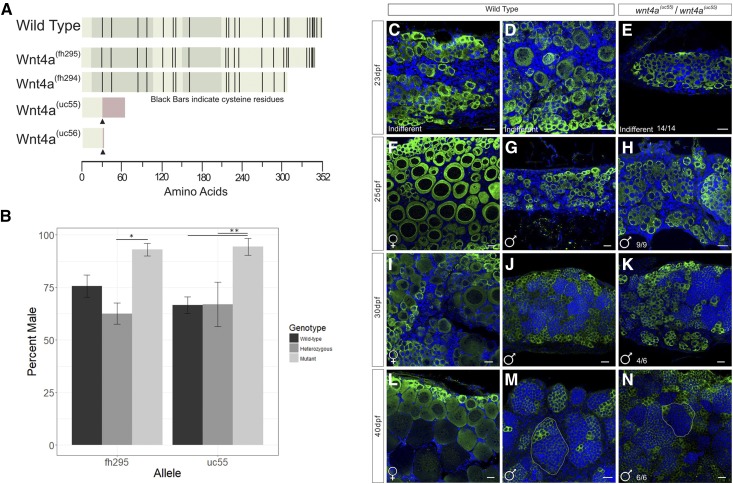
Results so far indicated that wnt4a is predominantly expressed in female somatic gonad cells. This finding is consistent with the hypothesis that Wnt4a plays a role in female sex determination and/or sex differentiation in zebrafish. To test this possibility, we analyzed the phenotype of two ENU-induced alleles of wnt4a: wnt4a(fh294) and wnt4a(fh295), which were identified by targeting-induced local lesions in genomes (Moens and ZFIN Staff 2009; Choe et al. 2013). The wnt4(fh294) and wnt4a(fh295) alleles are nonsense point mutations that are predicted to truncate the C terminus of Wnt4a protein (Figure 3A). The wnt4a(fh294) and wnt4a(fh295) mutations result in the deletion of 10 or 1 of the conserved cysteines, respectively, that are present in the C terminus of the Wnt4a protein and are necessary for proper folding of WNT proteins (Miller 2002). Without these residues, the binding of Wnts to the Frizzled receptor is likely to be disrupted (Janda et al. 2012). Importantly, deletion of the C-terminal half of the Xenopus Xwnt-8 gene results in a partial protein that has dominant-negative, cell-nonautonomous activity, perhaps because it interferes with productive interactions between the wild-type XWnt8 ligand and its receptor (Hoppler et al. 1996); given the high sequence conservation of Wnt ligands, it was therefore possible that C-terminal deletions of Wnt4a may behave similarly. To investigate this possibility, we used CRISPR/Cas9 to generate additional mutations targeted to the N terminus. The wnt4a(uc55) and wnt4a(uc56) alleles resulted from a 17- and 23-bp insertion in exon two, respectively, and are therefore predicted to cause translational frame shifts, the loss of all conserved cysteine residues (Figure 3A and Figure S3), and hence to be strong loss-of-function alleles. In support of this prediction, we could not detect any wild-type wnt4a messenger RNA (mRNA) by RT-PCR in wnt4a(uc55) mutants, suggesting that the mutant transcript is subject to nonsense-mediated decay (data not shown).
Figure 3.
Mutant wnt4a alleles result in male-biased populations. (A) Wnt4a is a 352 amino acid protein with five exons, indicated by the alternating shaded regions. Protein structures predicted to arise from each allele are indicated by truncation, wnt4a(fh295/fh294) (ENU-induced mutation), or insertion (CRISPR, ▴) resulting in missense protein coding (red bar) in uc55/uc56. (B) Sex ratios in populations of homozygous wnt4a(fh295) and wnt4a(uc55) mutants were significantly male biased by ANOVA. * P < 0.05, ** P < 0.01, n = 3 replicates. Comparison of (C, D, F, G, I, J, L, and M) representative wild-type and (E, H, K, and N) wnt4a mutant gonads stained for Vasa, to identify germ cells (green), and DAPI, to label nuclei (blue), at various ages postfertilization (dpf). At 23 dpf, (E) wnt4a mutants and (C and D) wild types both have indifferent gonad morphology. In contrast, the majority of wnt4a mutant gonads from 25 dpf animals and older (H, K and N) had a morphology that is indistinguishable of wild-type testes (G, J and M), but not wild-type ovaries (F, I and L). Bar, 20µm.
We first asked if the CRISPR-induced wnt4a mutants were viable. We crossed parents that were heterozygous for each mutant allele, genotyped the resulting offspring at 3 months of age, and determined their phenotypic sex. For all four alleles, we found the expected Mendellian 1:2:1 ratio of the three possible genotypes (wnt4a+/+: wnt4a+/−: wnt4a−/−; Table S2; Chi-squared test). In contrast to mammals, where Wnt4 mutants are embryonic lethal, homozygous wnt4a loss-of-function zebrafish mutants are viable (Figure S4). We next determined if loss of Wnt4a function affected sex ratios (Figure 3B). In the wnt4(fh295) in-cross population, wnt4(fh295) wild-type fish were 75.7% male, wnt4(fh295) heterozygous fish were 62.6% male, and the wnt4(fh295) homozygous mutant fish were 93% male (n = 3, P < 0.05, two-way ANOVA with Tukey’s post hoc analysis, compounded for multiple comparisons). The wnt4a(uc55) mutation resulted in similar ratios, with the wild-type, heterozygous, and homozygous mutant fish populations being 66.6, 76, and 94% male, respectively (n = 3, P < 0.01, two-way ANOVA with Tukey’s post hoc analysis, compounded for multiple comparisons). In addition, wnt4a(fh294) and wnt4a(uc56) mutants also had male sex bias (fh294+/+ 56% male, n = 171 vs. fh294−/− 98.6% male, n = 143; uc56+/+ 49% male, n = 102 vs. uc56−/− 100% male, n = 78). Finally, wnt4a(uc55)/wnt4a(fh295) trans-heterozygous fish had a male bias (fh295+/− 40% male, n = 20 vs. uc55/fh295 96% male, n = 23) like the homozygous single mutants. These data support two main conclusions: First, these results indicate that Wnt4a promotes—but is not absolutely required for—ovary development. Second, because wnt4a(fh295) mutants had the same magnitude of effect on sex ratios as the loss-of-function allele wnt4(uc55), we conclude that the ENU-induced alleles, wnt4a(fh294) and wnt4a(fh295), are also loss-of-function alleles.
Wnt4a is involved in primary sex determination and/or differentiation
In mammals, WNT4 is required during female primary sex determination (Vainio et al. 1999). In zebrafish, it is not known with certainty when definitive primary sex determination occurs, but it likely occurs prior to 20 dpf, because this is the time at which oocytes present in the bipotential gonad begin to die by apoptosis in presumptive males (Takahashi 1977; Uchida et al. 2002). The hypothesis that wnt4a is required for primary sex determination and/or differentiation in zebrafish predicts that oocyte apoptosis will initiate in the majority of mutants at about the same time as it does in wild-type males, but in a greater proportion of the population because the end result is more males in the mutant population. Alternatively, the hypothesis that wnt4a is instead required to maintain female sex differentiation predicts that many animals should begin to develop as females, but then revert to male phenotype during the early juvenile stage, as occurs in bmp15 mutants (Dranow et al. 2016). We therefore compared gonad development between wild-type and wnt4a mutants between 23 and 40 dpf (Figure 3, C–N). Results showed that the majority of wnt4a mutant gonads were morphologically similar to wild-type males at all stages analyzed (compare Figure 3, G, J, and M, to Figure 3, H, K, and N), but not to wild-type females (compare Figure 3, F, I, and L, to Figure 3, H, K, and N). In particular, early stage oocytes were present in all gonads at 23 dpf regardless of genotype but, by 25 dpf, mutant gonads appeared to contain predominantly premeiotic germ cells, which have nuclei containing a single large nucleolus, similar to those found in presumed wild-type males (Figure 3H). By 40 dpf, all mutant gonads had a morphology that was indistinguishable from a wild-type testis, where germ cells are organized into tubules (compare Figure 3M to Figure 3N). These data thus argue that Wnt4a is involved in primary sex determination and/or differentiation rather than in the maintenance of a female phenotype.
Wnt4a mutants are unable to release gametes
The ovaries and testes of wnt4a mutant adults are morphologically indistinguishable from those of their wild-type siblings (Figure S5). It was therefore surprising that neither mutant males nor mutant females produced progeny when mated to each other or to wild-type fish. For example, wnt4a(uc55) mutant males stimulated wild-type females to lay eggs, but no eggs were fertilized (n = 155 eggs). For wnt4a(fh294), nine homozygous wild-type male siblings and nine homozygous mutant males were individually crossed to two to three AB wild-type females, resulting in wild-type males producing 349/504 (69.2%) viable offspring, while those with mutant males producing only unfertilized eggs (n = 471).
Because our histological analysis showed that mature sperm were present in the testes of mutant males (Figure S5B), we next attempted to expel sperm from mutants by gentle squeezing. We found that wnt4a(uc55) and wnt4a(fh294) wild-type control males released sperm (n = 8/9 and 16/21, respectively), but that mutant males did not (n = 0/10 and 0/16, respectively). Finally, we used an in vitro fertilization assay to compare fertilization rates of mutant and wild-type sperm isolated from dissected and macerated testes. We found that, consistent with our histological analysis, dissection-isolated mutant sperm had fertilization rates similar to sperm isolated from heterozygous or homozygous wild-type males [for wnt4a(uc55): 56.1 ± 35.6% for mutants vs. 79.3 ± 7.2%, for wild types, P = 0.46, Student’s t-test; for wnt4a(fh294): 72.7% for mutant males and 52.2% for wild types, P = 0.71].
Similarly, histological analysis showed that ovaries in the mutant females contained all stages of oocytes, including mature eggs (Figure S5D), yet wnt4a(uc55) mutant females failed to release eggs when mated to wild-type males (0/5 mating pairs). In contrast, two of three heterozygous control females released eggs when mated to a wild-type male. We next tested if we could recover eggs by gentle squeezing and found that, although two of three control females released eggs, no wnt4a(uc55) mutant females released eggs (n = 0/5). Finally, we tested if we could recover mature eggs from dissected ovaries. We found that eggs dissected from wnt4a(uc55) mutant females yielded viable zygotes when fertilized by wild-type sperm, though at a lower rate than those isolated from heterozygous females (16.4 ± 6.2%, n = 3 mutants females vs. 61.4 ± 38.1%, n = 4 heterozygotes females, P = 0.41, Student’s t-test). These results show that the infertility of wnt4a mutant males and females is not due to a defect in gametogenesis, and we hypothesized that it was due instead to an inability of mutants to release their gametes.
Male and female infertility is caused by reproductive duct malformation
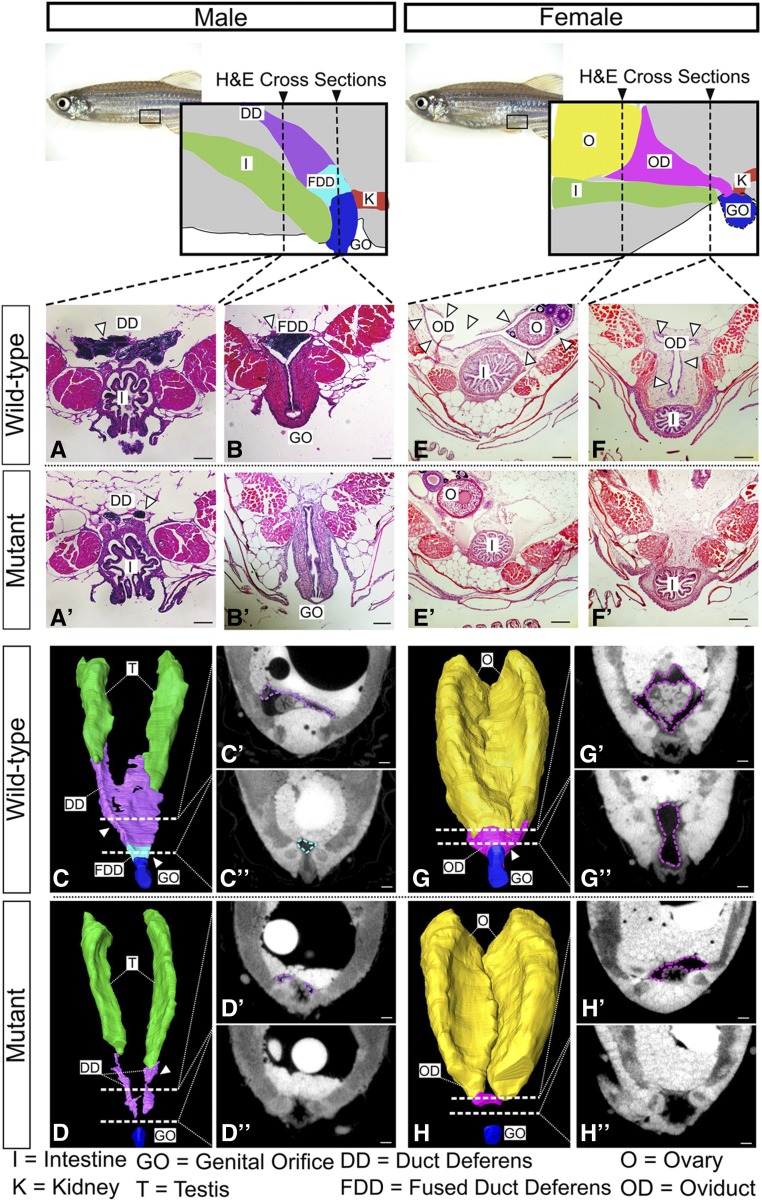
Given that wnt4a mutant zebrafish cannot expel their gametes, we asked if they had defects in the formation of the reproductive ducts. In wild-type males, each testis connects to the genital orifice by the duct deferens, which extends posteriorly from the testis and fuses with the genital orifice to form the fused duct deferens (Figure 4, A and B). We analyzed duct formation first by histology using H&E-stained paraffin sections. We found that although the duct deferens initiated development in mutant males, their extension was variable and the ducts failed to fuse (n = 3; Figure 4, A′ and B′).
Figure 4.
Duct morphology in wnt4a mutant and wild-type males. From the posterior end of each testis, wild-type males developed duct deferens (A) that joined to form the fused duct deferens (B). Mutant males, however, failed to form a full duct deferens (A′) or a fused duct deferens (B′). Three-dimensional renderings built from individual traces of sections (C′, C″, D′, and D″) of (C) the wild-type ducts and (D) mutant ducts show that the mutant ducts never fully connected to the genital orifice. H&E-stained sections of wild-type females showed an oviduct that wrapped around the posterior of the ovaries (E, G, and G′) and extended ventroposteriorly (F, G, and G″) out to the genital orifice. Mutant females, however, failed to organize an oviduct around the ovary (E′, H, and H′) and did not form a connective duct to the genital orifice (F′, H, and H″). See movies in Files S1 and S2. Bar, 100 µm. I, intestine; K, kidney; T, testis, O, ovary; OD, oviduct; GO, genital orifice; DD, duct deferens; FDD fused duct deferens.
To increase the resolution of assessing duct development in mutants, we next used microcomputed tomography (microCT) to render three-dimensional (3D) representations of the reproductive ducts in both wild-type and mutant adults. For this analysis, we traced the structures of interest in individual slices (Figure 4, C′ and C″) to build a 3D model of the complete duct structure (Figure 4C). In wild-type animals, as expected, we could identify all parts of the reproductive ducts (Figure 4, C, C′, and C″). By contrast, in wnt4a(uc55) mutant males, we could identify the duct deferens but no duct fusion or connection to the genital orifice (Figure 4, D and D′, and File S1).
In females, histology and microCT analysis revealed a similar defect in reproductive duct development in wnt4a mutants. In wild-type females, the oviduct wrapped around the posterior end of the ovary and extended ventroposteriorly until it connected to the genital orifice (Figure 4, E–G). In mutant females, however, the small amount of oviduct tissue present did not fully envelop the posterior end of the ovaries (Figure 4, E′, H, and H′, and File S2), and failed to extend toward the genital orifice (Figure 4, F′, H, and H″, and File S2). Together, these analyses explain mutant sterility and show that Wnt4a is required not for the specification of reproductive duct development, but is likely required for the growth and/or extension of the reproductive duct primordium in both male and female zebrafish.
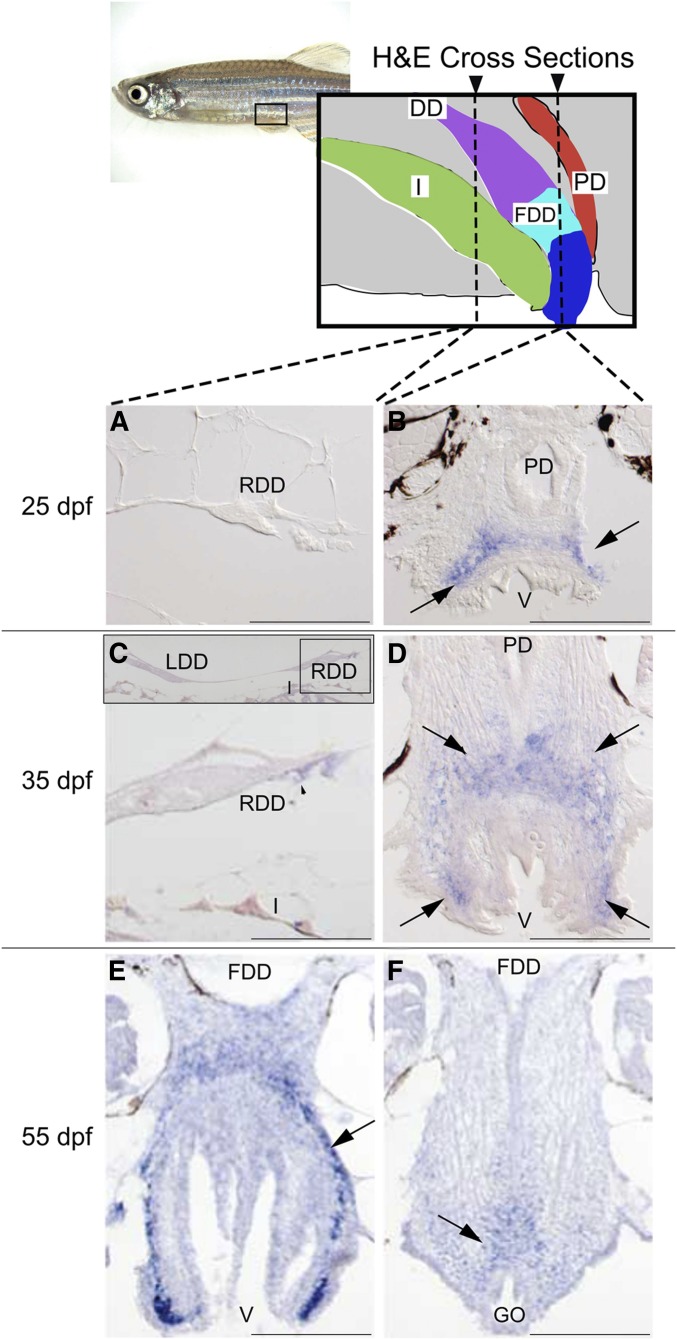
To further understand how Wnt4a regulates duct development, we first determined when the ducts form during larval development. We scored duct formation in wild-type males using serial H&E sections. We evaluated males at four ages from 25 to 55 dpf and discovered that the ducts appear to originate at the posterior end of the testis before 25 dpf and elongate toward the genital orifice. Reproductive ducts of wild-type males had reached and fused to the genital orifice by 55 dpf (Figure S6).
Having established the schedule of duct development, we wanted to learn in which tissues wnt4a acts to cause duct elongation: Does Wnt4a act in the extending duct, or in the space through which it grows, or in the target at the vent? To find out, we analyzed serial transverse sections of wild-type males, starting anterior to the gonad and ending posterior to the genital orifice, with alternate sections taken for H&E histology (see Figure S6) and expression analysis of wnt4a by in situ hybridization. At 25 and 35 dpf, wnt4a expression appeared not in the extending duct, but around the vent and developing genital orifice (Figure 5, B and D). While no wnt4a expression was detected in cells of the duct deferens primordium at 25 dpf (Figure 5A), by 35 dpf wnt4a expression appeared in cells located ventral to the developing duct deferens (Figure 5C). Importantly, the domain of wnt4a expression preceded the arrival of the ducts to this region.
Figure 5.
Expression of wnt4a during reproductive duct development in zebrafish wild-type AB strain males. Alternate serial cross sections of wild-type males were stained by H&E to follow duct growth (see Figure S6) or prepared for in situ hybridization to reveal wnt4a expression, shown here. (A) Expression analysis showed that at 25 dpf, the duct deferens (cross section of the right duct shown here) lacked wnt4a expression. (B) At 25 dpf, however, wnt4a expression (→’s) appeared dorsal to the posterior vent, just anterior to the eventual connection of the fused duct deferens and the genital orifice. (C) At 35 dpf, the duct deferens continued to show little wnt4a expression. Insert shows the left and right duct deferens dorsal to the intestine. (D) At 35 dpf, wnt4a expression (→’s) appeared dorsal and lateral to the posterior vent, just anterior to the eventual connection of the fused ducti deferens and the genital orifice. (E) At 55 dpf, in a section just anterior to the fusion of the duct deferens to the vent, wnt4a expression (→) appeared dorsal to the posterior vent, just anterior to the connection of the fused ducti deferens and genital orifice. (F) At 55 dpf, in a section at the level of the connection of the fused duct and the vent, wnt4a expression (→) appeared just dorsal to the genital orifice. DD, ducti deferens; FDD, fused ducti deferens; GO, genital orifice; I, intestine; LDD, left duct deferens; PD, pronephric duct; RDD, right duct deferens; V, vent. Bar, 100 µm.
Serial sections showed that the wild-type duct deferens connected to the genital orifice between 45 and 55 dpf (Figure S6). Concurrent analysis of alternating sections showed wnt4a expression persisted in tissue surrounding the vent and genital orifice (Figure 5, E and F). We conclude that in wild-type male zebrafish, wnt4a expression occurs in the developing genital orifice but not in the extending duct deferens, raising the hypothesis that Wnt4a might act as a diffusible signal that encourages duct deferens outgrowth.
To further characterize the role of Wnt4a in duct development, we examined duct elongation in wnt4a mutants over time. Results showed that the duct deferens elongated more slowly in wnt4a mutant males than wild type. In mutants, the fused duct deferens did not connect to the genital orifice by 55 dpf, nor was a connection found even in elderly 2-year-old fish (Figure S6). These results suggest that wnt4a expression at the genital orifice is essential for reproductive duct growth and/or elongation and for formation of the fused duct deferens. The failure of ducts to connect to the genital orifice explains our earlier observations that wnt4a mutants are sterile despite their ability to make fertile eggs and sperm. The wnt4a expression domain anterior to the eventual connection of the fused duct deferens and the genital orifice supports the hypothesis that tissues around the genital orifice likely secrete Wnt4a protein and thus signal duct growth, elongation, and connection to the exterior. Thus, as in mammals, Wnt4a may coordinate directional cell migration and extension of the reproductive duct (Prunskaite-Hyyryläinen et al. 2015).
Discussion
After more than four decades of use as a major model organism, the mechanism of sex determination in domesticated zebrafish is still unclear. While a major sex chromosome has been identified in wild zebrafish, this sex-determining locus appears to have been lost during the domestication of zebrafish strains that are widely used in the laboratory (Wilson et al. 2014). Regardless, mounting evidence shows that many, if not most, genes that play key roles in sex determination in mammals play similar roles in zebrafish. As an example, the double-sex and mab3-related transcription factor Dmrt1, a highly conserved regulator of male development across metazoans, is required for normal male development in zebrafish (Lin et al. 2017; Webster et al. 2017). Similarly, in vertebrates, WNT4 signaling plays a key role in female sex determination and accumulating evidence argues that canonical Wnt signaling is also required for female sex determination or differentiation in zebrafish, though the specific Wnt ligand had not been previously identified (Zhang et al. 2011; Sreenivasan et al. 2014). Experiments reported here show that the zebrafish ortholog of mammalian Wnt4, wnt4a, is required for normal female sex ratios, strongly suggesting that it plays a role in, but is not required for, female sex determination and/or differentiation because a small percentage of wnt4a mutants develop as females. In addition, while WNT4 in mammals is required for the development of reproductive ducts in the female, but not the male (Vainio et al. 1999), we have shown here that in zebrafish, Wnt4a is required for reproductive duct development in both females and males. Together, these results provide further evidence that the underlying molecular genetic mechanisms for sex determination and/or differentiation are well conserved between teleosts and tetrapods.
The zebrafish genome contains two Wnt4-related genes, wnt4a and wnt4b, while the mammalian genome contains a single Wnt4 gene (Ungar et al. 1995; Liu et al. 2000). Although many gene duplicates in teleosts are the result of an additional whole-genome duplication event that occurred after the teleost and tetrapod lineages diverged (Amores et al. 1998; Postlethwait et al. 1998; Jaillon et al. 2004), our phylogenetic analysis argues that the duplication event that produced wnt4a and wnt4b predated the teleost–tetrapod divergence. Specifically, while mammals have only a single copy of Wnt4, coelacanth and birds (among basally diverging lobe-finned vertebrates) as well as spotted gar (among basally diverging ray-finned vertebrates) contain two orthologs of Wnt4. For interpreting the connectivity of our investigations to mammalian sex development, it is important to know whether the single mammalian Wnt4 gene is the ortholog of the teleost wnt4a or wnt4b gene. Based on sequence comparisons and analysis of conserved syntenies, it is clear that the single Wnt4 copy that remains in mammals is the ortholog of the teleost wnt4a gene, indicating that the ortholog of wnt4b was lost at some point after the mammalian linage diverged from the turtle and bird lineages. Thus, although we do not propose a name change for practical reasons, in principle the application of nomenclature conventions (https://wiki.zfin.org/display/general/ZFIN+Zebrafish+Nomenclature+Conventions) would result in either calling the human gene “WNT4A” to match its teleost ortholog or calling the teleost gene simply “wnt4” to match its mammalian ortholog.
The early gonad in mammals is bipotential and expresses Wnt4 initially in the mesonephros underlying the Fgf9-expressing gonadal epithelium (Vainio et al. 1999). Mutational analysis has shown that Wnt4 and Fgf9 are mutually antagonistic during mammalian sex determination: loss of Wnt4 function in XX mammals leads to upregulation of Fgf9 and partial female-to-male sex reversal (Vainio et al. 1999), whereas loss of Fgf9 in XY individuals results in stabilized expression of Wnt4 and partial male-to-female sex reversal (Kim et al. 2006). During normal development, Sox9 expression in the gonad, which is initiated by the mammalian Y-linked male sex-determinant SRY, leads to upregulation of Fgf9, which in turn downregulates Wnt4. In contrast, in the absence of Sox9 expression, as occurs normally in XX mammals, Wnt4 represses Fgf9, thus promoting female development (Kim et al. 2006).
We have shown here that the phenotypes caused by wnt4a mutations in zebrafish, such as masculinization of the gonad and disturbed sex duct development, parallel those of Wnt4 mutant mammals, yet it is not clear whether the developmental and cellular mechanisms by which Wnt4a promotes ovarian and gonadal duct development are conserved. Our results clearly show that, as in mammals, wnt4a is expressed in somatic gonadal cells during the bipotential phase of gonad development. However, while the genome of a basal ray-finned vertebrate, the spotted gar, contains an ortholog of Fgf9 (Braasch et al. 2016), orthologs of Fgf9 have not been found in the genomes of teleosts, included zebrafish (Itoh and Konishi 2007), suggesting that this gene was lost during early teleost evolution. It therefore remains to be determined if another Fgf ligand plays a similar role in teleosts to that of mammalian Fgf9 in opposing the action of Wnt4a during sex determination.
Three noteworthy features differ between the phenotypes of zebrafish and mammalian Wnt4 mutants. First, loss-of-function Wnt4 mutants in mice and humans are lethal (Vainio et al. 1999; Mandel et al. 2008), whereas zebrafish wnt4a mutants are viable. It is likely that in mammals the lethal phenotype of Wnt4 mutants is due to an additional and essential function of Wnt4 during the development of nongonadal tissues. If so, then the viability of wnt4a mutant zebrafish may be the result of Wnt4b function in nongonadal tissue development. For example, in mouse and zebrafish, Wnt4 and wnt4b, respectively, are expressed in the floor plate of the spinal cord and brain (Parr et al. 1993; Liu et al. 2000; Agalliu et al. 2009; Duncan et al. 2015). In addition, lethality of Wnt4 mutant mice and humans is likely due to kidney failure (Vainio et al. 1999; Mandel et al. 2008). To date, however, the expression of neither wnt4a nor wnt4b has been reported in the pronephros, the zebrafish functional equivalent to the mammalian mature kidney. Regardless, it remains to be determined if loss of wnt4b or the simultaneous loss of wnt4a and wnt4b in zebrafish will result in embryonic lethality.
Second, in mammals, XX Wnt4 mutants are partially sex reversed to males and germ cells undergo apoptosis; whereas in zebrafish, all wnt4a mutants produce functional gametes, including the 4–6% of Wnt4a mutants that develop as females. It is likely that this difference results from the observation that, in mammals, gametes do not survive if the gonadal sex is opposite to the somatic sex, regardless of the direction of sex reversal (Uhlenhaut et al. 2009; Matson et al. 2011). In contrast, ample evidence shows that in many teleosts, including zebrafish and medaka, the gamete type produced by premeiotic germ cells can readily switch to match the sexual phenotype of the somatic gonad, regardless of whether the phenotype matches the genetic sex of the animal (Yamamoto 1958; Dranow et al. 2013, 2016; Wong and Collodi 2013).
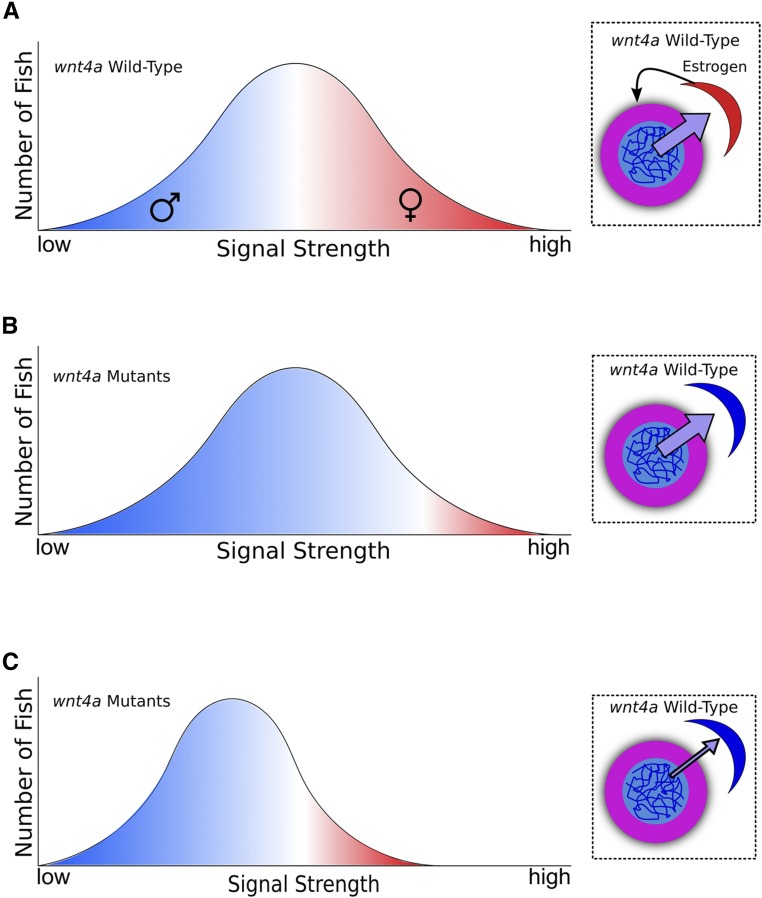
Third, unlike mammals, Wnt4a in zebrafish appears to facilitate, but is not essential for, female development, because a small percentage of wnt4a mutants develop normal ovaries. Two models could explain this difference. First, it is possible that Wnt4b can partially compensate for loss of Wnt4a during female sex determination or differentiation in zebrafish. Alternatively, it is possible that female development of wnt4a mutants is related to the numbers of oocytes that these individuals possess during the critical sex-determining window. During this time period (10–20 dpf), all zebrafish juveniles produce several early stage oocytes and mounting evidence shows that the number of oocytes an individual produces during the bipotential phase correlates with the eventual sex of the animal: animals that produce few or no oocytes become male, whereas those that produce many oocytes can become female (Uchida et al. 2002; Rodríguez-Marí et al. 2010; Dai et al. 2015). While it is not known for certain, it is likely that oocytes produce a signal that acts on the somatic gonad to promote female sex determination and, absent a threshold amount, animals develop as males (Figure 6A). We therefore propose two general models for the role of Wnt4a during normal sex determination. First, it is possible that Wnt4a may regulate the sensitivity of the somatic gonad to the oocyte-produced signal such that, in wild-type gonads, fewer oocytes are required to reach the critical threshold necessary to stabilize female sex determination relative to wnt4a mutant gonads (Figure 6B). Alternatively, Wnt4a may act on germ cells to regulate the level of signal produced (Figure 6C), either by directly regulating the amount of signal each oocyte produces or by affecting the number of oocytes produced per animal. Regardless, our observation that some wnt4a mutants can develop as females suggests that, above a certain level of signal, Wnt4a function is not required for female development. Our model favors a role for Wnt4a in sex determination, though at present we cannot rule out that Wnt4a acts instead to promote female sex differentiation.
Figure 6.
Models for how Wnt4a functions to promote female development. (A) In wild-type animals, high concentrations of a signal produced by early stage oocytes during the bipotential gonad stage (purple →) likely cause the gonadal soma to maintain production of estrogen (black →), which inhibits oocyte death and drives female sex determination. If the oocyte signal is too low, a male develops; if the signal exceeds a threshold, a female develops. In a wild-type population, this threshold and signal gradient produces about half males and half females. In A–C, the x-axis depicts the strength of the signal while the y-axis plots the numbers of animals that produce a certain amount of signal. For simplicity, signal strength vs. fish number is assumed to follow a normal distribution. Color intensity reflects the probability an individual develops as a male (blue) or female (red). (B) In this model, lack of Wnt4a desensitizes somatic gonad cells to the female-promoting oocyte signal, thereby raising the female-development threshold such that only those few wnt4a mutant animals that produce the highest signal (perhaps stochastically) can develop as females, allowing most to become males. (C) Alternatively, lack of Wnt4a causes oocytes to decrease the amount of female-promoting signal that they produce, such that fewer wnt4a mutants achieve the level required to sustain female development. Insets in B and C are graphical representations of the two models (oocyte in pink, somatic gonad cell in red or blue).
Finally, we have shown that both male and female wnt4a mutants are unable to release their gametes due to defects in reproductive duct development. In mammals, the reproductive ducts of males and females develop from separate embryonic structures, the Müllerian duct in females and the Wolffian duct in males. Both of these reproductive duct anlagen initially develop in both males and females during early embryogenesis, but after definitive sex determination, the Müllerian ducts degenerate in males, while the Wolffian ducts degenerate in females. Loss of Wnt4 function in mice inhibits Müllerian duct formation in both males and females, but does not affect the development of the Wolffian ducts (Vainio et al. 1999). This finding suggests that the reproductive ducts in both male and female zebrafish are developmentally similar to the Müllerian ducts in mammals and may therefore share a common evolutionary origin; a conclusion that suggests that the Wolffian duct is either a mammalian novelty or an ancestral feature lost in teleost evolution. Recent studies in mice have shown that WNT4 regulates the direction of Müllerian duct precursor cell migration (Prunskaite-Hyyryläinen et al. 2015). How Wnt4a regulates ductal development in zebrafish remains to be determined, although its expression around the genital orifice provides some clues.
In conclusion, results presented here establish that Wnt4 was likely a regulator of female sex determination, gonad differentiation, and reproductive duct development in the last common ancestor of humans and zebrafish 450 MYA. As such, these results provide further evidence that the core pathway for sex determination and differentiation in tetrapod vertebrates appears to be largely conserved in the teleost lineage.
Acknowledgments
We thank the members of the Draper and Postlethwait laboratories especially Matt McFaul, Anastasia Utkina, Becky Wong, and Trevor Enright for their contributions. This work was made possible by generous funding from the following sources: T32 ES-0070599 (M.E.K.), T32 HD-008348 (S.K.H.), R01 GM-085318 (J.H.P.), and IOS-1456737 (B.W.D.). The Olympus FV1000 confocal used in this study was purchased using National Institutes of Health Shared Instrumentation Grant 1S10RR019266-01. We thank the MCB Light Microscopy Imaging Facility, which is a University of California, Davis Campus Core Research Facility, for the use of this microscope. Microcomputed tomography was supported by a pilot grant from the University of California, Davis Center for Molecular and Genomic Imaging.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7098779.
Communicating editor: D. Parichy
Literature Cited
- Agalliu D., Takada S., Agalliu I., McMahon A. P., Jessell T. M., 2009. Motor neurons with axial muscle projections specified by wnt4/5 signaling. Neuron 61: 708–720. 10.1016/j.neuron.2008.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A., Force A., Yan Y. L., Joly L., Amemiya C., et al. , 1998. Zebrafish hox clusters and vertebrate genome evolution. Science 282: 1711–1714. 10.1126/science.282.5394.1711 [DOI] [PubMed] [Google Scholar]
- Amores A., Catchen J., Ferrara A., Fontenot Q., Postlethwait J. H., 2011. Genome evolution and meiotic maps by massively parallel DNA sequencing: spotted gar, an outgroup for the teleost genome duplication. Genetics 188: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L., Marí A., Braasch I., Amores A., Hohenlohe P., et al. , 2012. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One 7: e40701 10.1371/journal.pone.0040701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J., Feng C. W., Spiller C., Davidson T. L., Jackson A., et al. , 2010. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev. Cell 19: 440–449. 10.1016/j.devcel.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Braasch I., Gehrke A. R., Smith J. J., Kawasaki K., Manousaki T., et al. , 2016. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 48: 427–437 (erratum: Nat. Genet. 48:700) 10.1038/ng.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K. M., Breyer J. P., Melville D. B., Broman K. W., Knapik E. W., et al. , 2011. An SNP-based linkage map for zebrafish reveals sex determination loci. G3 (Bethesda) 1: 3–9. 10.1534/g3.111.000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe C. P., Collazo A., Trinh L. A., Pan L., Moens C. B., et al. , 2013. Wnt-dependent epithelial transitions drive pharyngeal pouch formation. Dev. Cell 24: 296–309. 10.1016/j.devcel.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem T. J., Hoshijima K., Jurynec M. J., Gunther D., Starker C. G., et al. , 2012. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 8: e1002861 10.1371/journal.pgen.1002861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Jin X., Chen X., He J., Yin Z., 2015. Sufficient numbers of early germ cells are essential for female sex development in zebrafish. PLoS One 10: e0117824 10.1371/journal.pone.0117824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehal P., Boore J. L., 2005. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 3: e314 10.1371/journal.pbio.0030314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranow D. B., Tucker R. P., Draper B. W., 2013. Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev. Biol. 376: 43–50. 10.1016/j.ydbio.2013.01.016 [DOI] [PubMed] [Google Scholar]
- Dranow D. B., Hu K., Bird A. M., Lawry S. T., Adams M. T., et al. , 2016. Bmp15 is an oocyte-produced signal required for maintenance of the adult female sexual phenotype in zebrafish. PLoS Genet. 12: e1006323 10.1371/journal.pgen.1006323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper B. W., 2012. Identification of oocyte progenitor cells in the zebrafish ovary, pp. 157–165 in Progenitor Cells: Methods and Protocols, edited by Mace A. K., Braun M. K. Humana Press, Totowa, NJ: 10.1007/978-1-61779-980-8_12 [DOI] [PubMed] [Google Scholar]
- Duncan R. N., Panahi S., Piotrowski T., Dorsky R. I., 2015. Identification of wnt genes expressed in neural progenitor zones during zebrafish brain development. PLoS One 10: e0145810 10.1371/journal.pone.0145810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppler S., Brown J. D., Moon R. T., 1996. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 10: 2805–2817. 10.1101/gad.10.21.2805 [DOI] [PubMed] [Google Scholar]
- Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., et al. , 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498–503 [corrigenda: Nature 505:248 (2014)] 10.1038/nature12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Konishi M., 2007. The zebrafish fgf family. Zebrafish 4: 179–186. 10.1089/zeb.2007.0509 [DOI] [PubMed] [Google Scholar]
- Jaillon O., Aury J. M., Brunet F., Petit J. L., Stange-Thomann N., et al. , 2004. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 431: 946–957. 10.1038/nature03025 [DOI] [PubMed] [Google Scholar]
- Jameson S. A., Lin Y. T., Capel B., 2012. Testis development requires the repression of Wnt4 by Fgf signaling. Dev. Biol. 370: 24–32. 10.1016/j.ydbio.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda C. Y., Waghray D., Levin A. M., Thomas C., Garcia K. C., 2012. Structural basis of wnt recognition by frizzled. Science 337: 59–64. 10.1126/science.1222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Kobayashi A., Sekido R., DiNapoli L., Brennan J., et al. , 2006. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 4: e187 10.1371/journal.pbio.0040187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerberg D. M., Sano K., Draper B. W., 2017. Fibroblast growth factor signaling is required for early somatic gonad development in zebrafish. PLoS Genet. 13: e1006993 10.1371/journal.pgen.1006993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Mei J., Li Z., Zhang X., Zhou L., et al. , 2017. Distinct and cooperative roles of amh and dmrt1 in self-renewal and differentiation of male germ cells in zebrafish. Genetics 207: 1007–1022. 10.1534/genetics.117.300274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Majumdar A., Schauerte H. E., Haffter P., Drummond I. A., 2000. Zebrafish wnt4b expression in the floor plate is altered in sonic hedgehog and gli-2 mutants. Mech. Dev. 91: 409–413. 10.1016/S0925-4773(99)00308-1 [DOI] [PubMed] [Google Scholar]
- Maack G., Segner H., 2003. Morphological development of the gonads in zebrafish. J. Fish Biol. 62: 895–906. 10.1046/j.1095-8649.2003.00074.x [DOI] [Google Scholar]
- Mandel H., Shemer R., Borochowitz Z. U., Okopnik M., Knopf C., et al. , 2008. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am. J. Hum. Genet. 82: 39–47. 10.1016/j.ajhg.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson C. K., Murphy M. W., Sarver A. L., Griswold M. D., Bardwell V. J., et al. , 2011. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476: 101–104 (erratum: Nature 477: 238) 10.1038/nature10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R., 2002. The Wnts. Genome Biol. 3: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens, C. B., and ZFIN Staff, 2009 Curation of TILLING database links. ZFIN Direct Data Submission (http://zfin.org). [Google Scholar]
- Nusse R., Clevers H., 2017. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169: 985–999. 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Parr B. A., Shea M. J., Vassileva G., McMahon A. P., 1993. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119: 247–261. [DOI] [PubMed] [Google Scholar]
- Pellegrino M., Maiorino R., Schonauer S., 2010. WNT4 signaling in female gonadal development. Endocr. Metab. Immune Disord. Drug Targets 10: 168–174. 10.2174/187153010791213074 [DOI] [PubMed] [Google Scholar]
- Postlethwait, J., A. Amores, A. Force, and Y. L. Yan, 1998 Chapter 8 The Zebrafish Genome, pp. 149–163 in The Zebrafish: Genetics and Genomics (Methods in Cell Biology, Vol. 60), edited by H. W. Detrich III, M. Westerfield, and L. I. Zon. Academic Press, San Diego, CA. [Google Scholar]
- Prunskaite-Hyyryläinen R., Skovorodkin I., Xu Q., Miinalainen I., Shan J., et al. , 2015. Wnt4 coordinates directional cell migration and extension of the müllerian duct essential for ontogenesis of the female reproductive tract. Hum. Mol. Genet. 25: 1059–1073. 10.1093/hmg/ddv621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Marí A., Yan Y. L., BreMiller R. A., Wilson C., Cañestro C., et al. , 2005. Characterization and expression pattern of zebrafish anti-Müllerian hormone (amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr. Patterns 5: 655–667. 10.1016/j.modgep.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Marí A., Cañestro C., Bremiller R. A., Nguyen-Johnson A., Asakawa K., et al. , 2010. Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 6: e1001034 10.1371/journal.pgen.1001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried K. R., Nüsslein-Volhard C., 2008. Germ line control of female sex determination in zebrafish. Dev. Biol. 324: 277–287. 10.1016/j.ydbio.2008.09.025 [DOI] [PubMed] [Google Scholar]
- Slanchev K., Stebler J., de la Cueva-Méndez G., Raz E., 2005. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc. Natl. Acad. Sci. USA 102: 4074–4079. 10.1073/pnas.0407475102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. J., Keinath M. C., 2015. The sea lamprey meiotic map improves resolution of ancient vertebrate genome duplications. Genome Res. 25: 1081–1090. 10.1101/gr.184135.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan R., Jiang J., Wang X., Bártfai R., Kwan H. Y., et al. , 2014. Gonad differentiation in zebrafish is regulated by the canonical Wnt signaling pathway. Biol. Reprod. 90: 1–10, 45. 10.1095/biolreprod.113.110874 [DOI] [PubMed] [Google Scholar]
- Takahashi H., 1977. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull. Fac. Fish. Hokkaido Univ. 28: 57–65. [Google Scholar]
- Uchida D., Yamashita M., Kitano T., Iguchi T., 2002. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J. Exp. Biol. 205: 711–718. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut N. H., Jakob S., Anlag K., Eisenberger T., Sekido R., et al. , 2009. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139: 1130–1142. 10.1016/j.cell.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Ungar A. R., Kelly G. M., Moon R. T., 1995. Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mech. Dev. 52: 153–164. 10.1016/0925-4773(95)00386-F [DOI] [PubMed] [Google Scholar]
- Vainio S., Heikkila M., Kispert A., Chin N., McMahon A. P., et al. , 1999. Female development in mammals is regulated by Wnt-4 signalling. Nature 397: 405–409. 10.1038/17068 [DOI] [PubMed] [Google Scholar]
- Vilella A. J., Severin J., Ureta-Vidal A., Heng L., Durbin R., et al. , 2009. EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 19: 327–335. 10.1101/gr.073585.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C., Streisinger G., 2007. Embryo Production by In Vitro Fertilization, in The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio), edited by Westerfield M. Unitersity of Oregon Press, Eugene, OR. [Google Scholar]
- Wang X. G., Bartfai R., Sleptsova‐Freidrich I., Orban L., 2007. The timing and extent of ‘juvenile ovary’phase are highly variable during zebrafish testis differentiation. J. Fish Biol. 70: 33–44. 10.1111/j.1095-8649.2007.01363.x [DOI] [Google Scholar]
- Webster K. A., Schach U., Ordaz A., Steinfeld J. S., Draper B. W., et al. , 2017. Dmrt1 is necessary for male sexual development in zebrafish. Dev. Biol. 422: 33–46. 10.1016/j.ydbio.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M., 2007. The zebrafish book: A guide for the laboratory use of zebrafish Danio (Brachydanio) rerio, Ed. 5. University of Oregon Press, Eugene, OR. [Google Scholar]
- Wilson C. A., High S. K., McCluskey B. M., Amores A., Yan Y. L., et al. , 2014. Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics 198: 1291–1308. 10.1534/genetics.114.169284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. T., Collodi P., 2013. Dorsomorphin promotes survival and germline competence of zebrafish spermatogonial stem cells in culture. PLoS One 8: e71332 10.1371/journal.pone.0071332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T.-O., 1958. Artificial induction of functional sex-reversal in genotypic females of the medaka (Oryzias latipes). J. Exp. Zool. 137: 227–263. 10.1002/jez.1401370203 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li F., Sun D., Liu J., Liu N., et al. , 2011. Molecular analysis shows differential expression of R-spondin1 in zebrafish (Danio rerio) gonads. Mol. Biol. Rep. 38: 275–282. 10.1007/s11033-010-0105-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All fish lines are available upon request, and will be deposited at the Zebrafish International Stock Center. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7098779.