Abstract
Phenotypic plasticity is a critical component of an organism’s ability to thrive in a changing environment. The free-living nematode Caenorhabditis elegans adapts to unfavorable environmental conditions by pausing reproductive development and entering a stress-resistant larval stage known as dauer. The transition into dauer is marked by vast morphological changes, including remodeling of epidermis, neurons, and muscle. Although many of these dauer-specific traits have been described, the molecular basis of dauer-specific remodeling is still poorly understood. Here we show that the nidogen domain-containing protein DEX-1 facilitates stage-specific tissue remodeling during dauer morphogenesis. DEX-1 was previously shown to regulate sensory dendrite formation during embryogenesis. We find that DEX-1 is also required for proper remodeling of the stem cell-like epidermal seam cells. dex-1 mutant dauers lack distinct lateral cuticular alae during dauer and have increased sensitivity to sodium dodecyl sulfate. Furthermore, we find that DEX-1 is required for proper dauer mobility. We show that DEX-1 is secreted from the seam cells during dauer, but acts locally in a cell-autonomous manner. We find that dex-1 expression during dauer is regulated through DAF-16/FOXO–mediated transcriptional activation. Finally, we show that dex-1 acts with a family of zona pellucida domain-encoding genes to regulate dauer-specific epidermal remodeling. Taken together, our data indicate that DEX-1 is an extracellular matrix component that plays a central role in C. elegans epidermal remodeling during dauer.
Keywords: polyphenism, cuticlin, SNED1, tectorin, DYF-7, extracellular matrix
TO survive changing environments, organisms modify their phenotype (i.e., phenotypic plasticity). Tissue remodeling is an important component of stress-induced phenotypic plasticity. For example, desert locusts are capable of altering their morphology between distinct “gregarious” and “solitarious” phases based on population density (Pener and Simpson 2009), and certain species of butterfly change their body morphology and wing color based on seasonal cues (Windig 1994). While these large-scale displays of phenotypic plasticity are readily observed, the molecular basis of tissue remodeling in response to environmental inputs is often unclear.
Caenorhabditis elegans is a useful animal to investigate the molecular mechanisms that facilitate stress-induced remodeling. Under favorable growth conditions, C. elegans develops continuously through four larval stages (L1–L4), into a reproductive adult. However, under unfavorable environmental conditions, C. elegans larvae arrest their development at the second larval molt and enter the stress-resistant dauer stage (Cassada and Russell 1975; Golden and Riddle 1984). Dauers are specialized, nonfeeding larvae capable of withstanding extended periods of adverse environmental conditions. Dauer-specific stress resistance is likely facilitated by several morphological changes that occur during dauer formation. For example, dauers have both structural and biochemical differences in their epidermis and cuticle compared with nondauers (Cox et al. 1981; Blaxter 1993). Two morphological features characteristic of dauer formation are a general radial shrinkage of the body and the formation of longitudinal cuticular ridges called alae (Figure 1A).
Figure 1.
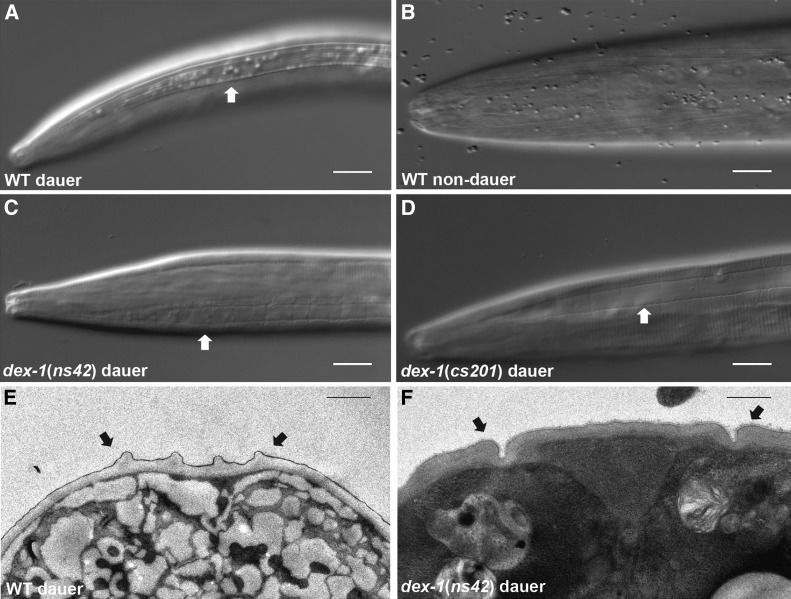
dex-1 mutants have defects in lateral alae formation. (A) Wild-type dauers have prominent lateral alae (arrows) that are not present in the comparable nondauer L3 stage (B). (C) dex-1(ns42) and (D) dex-1(cs201) mutant dauer alae are indistinct. All animals are lying laterally. Bar, 10 μm. (E and F) Transmission electron micrograph showing lateral alae (arrows) of a wild-type dauer (E) and dex-1(ns42) mutant dauer (F). Bar, 1 μm.
Radial shrinkage and alae formation are regulated by a set of lateral hypodermal seam cells (Singh and Sulston 1978; Melendez et al. 2003). Seam cell function and remodeling are critical for proper dauer morphology and increased environmental resistance. The seam cells also have stem cell-like properties; during nondauer development, the seam cells undergo asymmetrical divisions at larval molts to produce an anterior differentiated cell and a posterior seam cell (Sulston and Horvitz 1977). Alternatively, if the animal enters dauer diapause, the seam cells shrink and stop dividing (Melendez et al. 2003; Karp and Ambros 2012)
The decision to enter dauer typically results in an all or none phenotype where dauers have every dauer morphological characteristic. However, mutations in some genes necessary for dauer formation result in an intermediate dauer phenotype, called partial dauers, in which the animals will display some, but not all, dauer characteristics (Albert and Riddle 1988). Here, we characterize the role of DEX-1, a protein similar to mammalian tectorin and SNED1, in seam cell remodeling during dauer formation. DEX-1 is a transmembrane protein with two extracellular nidogen domains, and is required for proper sensory neuron dendrite formation during embryogenesis (Heiman and Shaham 2009). We find that the dex-1 mutant dauers have alae and cuticle defects that are not suppressed by dauer-constitutive (daf-c) mutants. Furthermore, we show that DEX-1 can function as a secreted protein and localizes in or near dauer alae. Finally, we find that dex-1 is upregulated in seam cells during dauer in a DAF-16–dependent manner. DEX-1 was previously shown to function with a zona pellucida (ZP) domain-containing protein, DYF-7, to mediate dendrite extension (Heiman and Shaham 2009). Our data suggest that DEX-1 acts along with additional ZP-domain proteins to regulate seam cell remodeling. Combined with previous data demonstrating a role for DEX-1 in sensory dendrite adhesion (Heiman and Shaham 2009), and data in the accompanying article showing roles for DEX-1 in epithelial shaping in the embryo (Cohen et al. 2018), our data suggest that DEX-1 is an extracellular matrix (ECM) component that plays a role in modulating cell shape of several cell types throughout development.
Materials and Methods
Strains and plasmids
All strains were grown under standard conditions unless otherwise noted (Brenner 1974). The wild-type Bristol N2 strain and the following mutant strains were used: DR27 daf-16(m27) I, FX01126 cut-1(tm1126) II, CHB27 dex-1(ns42) III, UP2571 dex-1(cs201)III; csEx402[dex-1p::dex-1a + unc-199p::gfp], CB1372 daf-7(e1372) III, DR129 daf-2(e1370) unc-32(e189) III, RB1574 cut-6(ok1919) III, RB1629 cut-5(ok2005) X, SP1735 dyf-7(m537) X. All mutant strains were backcrossed at least twice. dex-1(cs201) was generated using standard EMS mutagenesis protocols (Brenner 1974; Flibotte et al. 2010) and identified based on balancer mapping and whole genome sequencing (Cohen et al. 2018). dex-1(ns42) was a gift from Dr. Maxwell Heiman (Department of Genetics, Harvard University, Boston, MA). cut-1(tm1126) was provided by the Mitani Consortium (Department of Physiology, Tokyo Women’s Medical University School of Medicine, Japan). The following transgenic strains were used: ST65 ncIs13[ajm-1::gfp] was used to observe the apical junctions of the seam cells (Köppen et al. 2001). The IL2 neurons were observed using PT2660 myIs13[klp-6p::gfp+pBx] III; PT2762 myIs14[klp-6p::gfp+pBx] V and JK2868 qIs56[lag-2p::gfp] V (Blelloch et al. 1999; Ouellet et al. 2008; Schroeder et al. 2013). The deirid neurons were observed using TG2435 vtIs1[dat-1p::gfp+rol-6(su1006)] V (Nass et al. 2002).
The following plasmids were a generous gift from Dr. Maxwell Heiman: pMH7 dex-1p::dex-1, pMH8 pha-4p::dex-1, pMH111 dex-1p(5.7 kb)::gfp, pMH125 dex-1p(2.1 kb)::gfp (Heiman and Shaham 2009). The sur-5::gfp construct used for mosaic analysis was a generous gift from Dr. Trent Gu (Gu et al. 1998; Yochem et al. 1998). Plasmids were constructed using Gibson Assembly (E2611S; New England Biolabs, Beverly, MA) and restriction enzyme cloning (for a complete list of primers and plasmids, see Supplemental Material, Table S1). The seam cell-specific expression plasmid was built by replacing the dex-1 promoter from pMH7 with a 1.21 kb cut-5 promoter region. The hypodermal-specific dex-1 plasmid was constructed by replacing the dex-1 promoter in pMH7 with the cut-6 promoter (Muriel et al. 2003; Sapio et al. 2005). The insulin response sequence was deleted from pMH111 using the Q5 Site Directed Mutagenesis Kit (E05525; New England Biolabs). Additionally, translational reporters dex-1p::sfgfp::dex-1 (pJC24) and dex-1p::dex-1(ecto)::sfgfp (pJC15) contain the 2.1 kb dex-1 promoter and dex-1 (isoform a) complementary (cDNA) from pMH7 [see Cohen et al. (2018)]. sfgfp was inserted either at an internal endogenous BglII restriction site to generate a full-length fusion tagged upstream of the first nidogen domain, or inserted at the 3′ end of a cDNA truncated before the transmembrane domain. See accompanying article (Cohen et al. 2018) for further cloning details.
Animals containing extrachromosomal arrays were generated using standard microinjection techniques (Mello et al. 1991), and genotypes confirmed using PCR analysis and observation of co-injection markers. dex-1(ns42) animals were injected with 20 ng/μl of plasmid and 80 ng/μl of unc-122p::gfp, unc-122p::rfp or sur-5::gfp as the co-injection marker. dex-1(cs201) animals were injected with 30 ng/μl of pJC15 or pJC24 and 50 ng/μl of co-injection marker pHS4 (lin-48p::mrfp).
Domain schematics were constructed using the wormweb.org Exon-Intron Graphic Maker. Domain locations were determined using the Simple Modular Architecture Research Tool domain prediction software (Schultz et al. 1998). The additional low complexity area with similarity to zonadhesin was assigned based on previous work (Heiman and Shaham 2009).
Dauer formation assays
Dauers were induced by one of two methods. For nontemperature-sensitive strains, we used plates containing crude dauer pheromone extracted by previously established procedures (Vowels and Thomas 1992; Schroeder and Flatt 2014). Dauers were induced in daf-16(m27) mutants using plates with high pheromone concentrations (EC90) (Gottlieb and Ruvkun 1994; Schroeder and Flatt 2014). For temperature-sensitive strains with mutations in daf-7(e1372) or daf-2(e1370), dauers were induced using the restrictive temperature of 25° (Riddle et al. 1981).
Microscopy and rescue analysis
Unless otherwise specified, animals were mounted onto 4% agarose pads and immobilized with 0.1 or 0.01 M levamisole for dauers and nondauer or partial dauers, respectively. In our hands, dauers frequently lay in a dorsal-ventral position following anesthesia. Therefore, to image the lateral side, dauers were immobilized by mounting on 4% agarose pads with Polybead Polystyrene 0.10 μm microspheres (Polysciences Inc., #00876) (Kim et al. 2013). A Zeiss AxioImager microscope equipped with DIC and fluorescence optics was used to collect images. For radial constriction experiments, Z-stack images were taken and Z-projections were made using FIJI. Diameter measurements were taken near the center of the terminal pharyngeal bulb. Measurement data were analyzed using a one-way ANOVA with Bonferroni’s multiple comparisons test using GraphPad Prism 6 software. The resulting Z-projections were used to measure body diameter. For seam cell area analysis, area was measured for V2pap, V2ppp, and V3pap and averaged to give one measurement per animal (Sulston and Horvitz 1977). Seam cell measurement data were analyzed by an unpaired t-test. For confocal microscopy of the IL2 neurons, dauers were mounted on 10% agarose pads and anesthetized with 0.1 or 0.01 M levamisole. Animals were imaged using a Zeiss LSM 880 confocal microscope.
For transmission electron microscopy of dex-1(ns42) and N2 animals, dauer larvae were induced using pheromone plates and processed for high-pressure freezing and freeze substitution modified from previously established methods (Hall et al. 2012; Manning and Richmond 2015). Using OP50 Escherichia coli as a substrate and 1% propylene phenoxetol in M9 buffer as an anesthetic, animals were loaded into a metal specimen carrier coated with 1-hexadecane and frozen in an HPM 010 high-pressure freezer. Freeze substitution was performed in an FS-8500 freeze substitution system using 2% OsO4 (Electron Microscopy Sciences), 0.1% uranyl acetate (Polysciences) in 2% H20, and 100% acetone. Samples were held at −90° for 110 hr, then warmed to −20° at the rate of 5° per hour (14 hr). Samples were then held at −20° for 16 hr, then warmed to 0° at the rate of 5° per hour (4 hr). Samples were washed three times in prechilled (0°) 100% acetone and incubated at 0° for 1 hr after the final wash. Samples were then warmed to room temperature and washed an additional two times with 100% acetone. Samples were infiltrated with 1:1 Polybed812 (Polysciences) resin:acetone for 6 hr, 2:1 resin:acetone for 14 hr, and 100% resin for 72 hr. All infiltration steps were incubated on an orbital shaker at room temperature. Samples were then embedded in molds in 100% resin plus DMP-30 hardener (Polysciences) and baked at 60° for 48 hr, then 70 nm sections were cut with a diamond knife using a PowerTome PC ultramicrotome and collected onto formvar-coated copper slot grids. Samples were imaged with a Philips CM200 transmission electron microscope.
Sodium dodecyl sulfate sensitivity assays
Sodium dodecyl sulfate (SDS) dose-response assays were performed using 12-well culture dishes containing M9 buffer and specified concentrations of SDS. Dauers were exposed to SDS for 30 min and scored as alive if movement was observed following stimulation with an eyelash. Each concentration was tested in triplicate with each experiment containing a separate wild-type (N2) control. Dose-response curves and LD50 values were determined by testing 20 dauers per treatment at each concentration, with three independent experiments. The LD50 and 95% confidence interval of each concentration was calculated using probit analysis in Minitab 18. LD50 values were considered significantly different if the 95% confidence intervals did not overlap. Significant difference was denoted with a single asterisk. Single concentration assays were conducted at 0.1% SDS with 20 dauers for each genotype and three independent experiments. Data were analyzed using a one proportion exact method analysis in Minitab 18 and considered significantly different if the 95% confidence intervals did not overlap. Significant difference was denoted with a single asterisk.
Fluorescent bead and behavioral assays
Fluorescent bead assays were carried out using established methods (Nika et al. 2016). Fluorescent beads (L3280; Sigma) were added to a ×10 concentrated OP50 E. coli overnight culture. Fresh NGM plates were seeded with 65 μl of the bead/bacteria suspension and allowed to dry. Twenty nematodes were added to the plate and incubated at 20° for 40 min. Worms were observed for the presence of fluorescent beads in the intestinal tract. The experiment was performed twice.
Pharyngeal pumping assays were modified from previous established methods (Keane and Avery 2003). dex-1(ns42) and wild-type dauers were transferred to seeded NGM plates and allowed to recover for 10 min. After recovery, each animal was scored individually for 2 min, and then removed from the plate. Data were analyzed by an unpaired t-test, using GraphPad Prism 6 software.
For movement assays, animals were transferred to unseeded NGM plates and allowed to sit at room temperature for 10 min before being assayed. Animals were stimulated near the anus with an eyelash and the number of body bends was scored. Counting was stopped if the animal did not complete another body bend within 5 sec of stopping, or if the animal reversed direction. Each animal was scored twice and then removed from the plate. Counts were averaged and then analyzed using a nonparametric Kruskal–Wallis test, with Dunn’s multiple comparisons test for dauers and the Mann–Whitney U test for adult animals, using GraphPad Prism 6 software.
DEX-1 expression analysis
To analyze dex-1p::gfp expression in the seam, images of dauer animals were taken using identical fluorescence settings and exposure times (10 ms). The fluorescence intensities of the V2pap, V2ppp, and V3pap seam cells were measured using established methods (McCloy et al. 2014). Each cell was outlined and the area, integrated density, and mean gray value were measured. Measurements were also taken for areas without fluorescence surrounding the cell. The total corrected cell fluorescence [TCCF = integrated density − (area of selected cell × mean fluorescence of background reading)] was calculated for each cell. The intensities of the three cells from each worm were averaged such that each nematode comprised a single data point. Variability in copy number between dex-1p::gfp and daf-16(m27); dex-1p::gfp was controlled by using the same transgene in both wild-type and daf-16(m27) backgrounds. Multiple independent lines were examined. To control for potential variation in copy number between the dex-1p(IRS∆)::gfp strain and dex-1p::gfp, we examined multiple independent lines. The data were analyzed using one-way ANOVA and Bonferroni’s multiple comparisons test. Ten animals were measured for each genotype.
Mosaic analysis was conducted using dex-1(ns42) dauer animals expressing extrachromosomal dex-1p::dex-1 and sur-5::gfp (Yochem et al. 1998). Lateral view micrographs were taken of transgenic animals and seam cells were scored for the presence of sur-5::gfp in the nucleus. Areas of lateral alae adjacent to seam cell nuclei positive for sur-5::gfp expression were scored as either full, partial, or no rescue. Seam cells not expressing sur-5::gfp were also counted and the adjacent alae scored. Twelve animals were observed for mosaicism, with one set of seam cells being scored for each animal.
Data availability statement
All strains and plasmids are available upon request. Table S1 contains all plasmid constructs used in this study. Figure S1 shows the dex-1(ns42) body diameter data for dauers and L3s. Figure S2 shows the results of fluorescent bead feeding and pharyngeal pumping assays. Figure S3 shows that dauer-specific lag-2p::gfp is present in dex-1(ns42) dauers. Figure S4 shows that starved and pheromone-induced dauers are morphologically similar. Figure S5 describes dyf-7(m537) dauer morphology. Figure S6 contains a dose-response curve of SDS resistance of wild type and single- and double-mutants. Figure S7 contains gene schematics of cuticlin (CUT) mutants used in this study. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7077146.
Results
DEX-1 is required for proper dauer morphology and behavior
Wild-type C. elegans dauers have a distinctive morphology due to radial shrinkage that leads to a thin appearance compared with nondauers (Figure 1, A and B). We found that dex-1(ns42) mutants produce dauers that are defective in radial shrinkage, leading to a “dumpy dauer” phenotype (Figures 1C and 2A). The defect in body size appears specific to the dauer stage, as comparable nondauer dex-1(ns42) mutant L3s show no differences in body size compared with wild-type L3s (Figure S1). Radial shrinkage in dauers is correlated with the formation of longitudinal cuticular ridges on the lateral sides of the animal, called the alae (Cassada and Russell 1975). The lateral alae of dex-1(ns42) mutant dauers are indistinct compared with wild-type dauers (Figure 1, C, E, and F).
Figure 2.
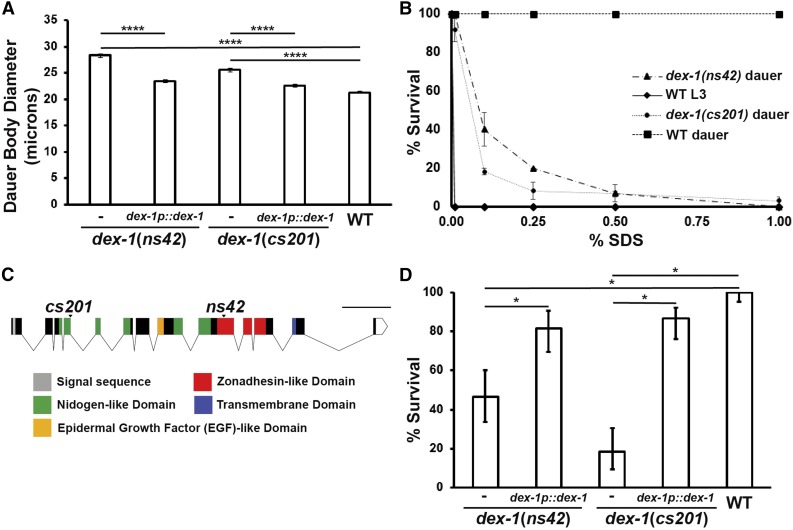
dex-1 mutants form partial dauers. (A) dex-1 mutant dauers are defective for dauer-specific radial shrinkage. The radial shrinkage defect can be rescued with dex-1 cDNA driven by its endogenous promoter (n = 60 per genotype pooled from three trials). **** indicates statistical significance at P < 0.0001. Error bars indicate SEM. (B) Dose-response survival assay to SDS. dex-1 dauers are sensitive to SDS compared to wild-type dauers, but are able to survive low levels of SDS exposure. Nondauer animals are sensitive at all tested SDS concentrations (n = 60 per treatment and dose pooled from three independent trials). Error bars indicate SEM from three independent trials. (C) dex-1 gene schematic. DEX-1 contains two nidogen-like domains, a single epidermal growth factor-like domain, a low-complexity domain previously predicted to have similarity to zonadhesin, and a transmembrane domain. The cs201 mutant allele is a point mutation at the exon 4 splice donor. The previously isolated ns42 mutation truncates the DEX-1 protein in its predicted zonadhesin-like domain. Bar, 1 kb. (D) Percent survival of dauers at 0.1% SDS. The dex-1 SDS sensitivity phenotype is partially rescued by the endogenous dex-1 promoter and DEX-1 cDNA (pMH7) (n = 60 per genotype pooled from three independent trials). Error bars indicate 95% confidence intervals. Nonoverlapping confidence intervals were considered significantly different (*).
In addition to radial shrinkage, dauers have an ability to survive high concentrations of SDS (Cassada and Russell 1975; Androwski et al. 2017). Wild-type dauers survive for hours in 1% SDS (Cassada and Russell 1975). We found that while dex-1(ns42) mutant dauers were able to survive significantly higher concentrations of SDS than wild-type nondauer animals, they were sensitive to 1% SDS (Figure 2B and Table 1).
Table 1. % SDS concentration necessary to kill 50% of animals tested for each genotype.
| Strain | LD50a |
|---|---|
| Wild-type dauer | >10% ± — |
| Wild-type L3 | <0.01 ± — |
| dex-1(ns42) | 0.15 ± 0.03 |
| cut-1(tm1126) | 0.28 ± 0.05 |
| cut-6(ok1919) | 2.02 ± 0.15 |
| cut-5(ok2005) | 0.25 ± 0.06 |
| cut-1; dex-1 | 0.20 ± 0.03 |
| dex-1 cut-6 | 0.71 ± 0.10 |
| dex-1; cut-5 | —b |
| cut-1; cut-5 | 0.08 ± 0.02 |
| cut-1; cut-6 | 1.85 ± 0.10 |
| cut-6; cut-5 | 0.01 ± —c |
In all cases “-” indicates no variation. For WT dauer and L3 the LD50 was above or below the limits of the doses tested, respectively. For b (dex-1; cut-5), the LD50 was not determined due to the early larval lethality.
For c, (cut-6; cut-5) survival was exactly 50% at the lowest dose (0.01%) and there were no survivors at higher concentrations. Thus, no 95% CI is available using the Probit analysis.
50% lethal dose ± 95% confidence interval as determined by probit analysis. Genotypes with nonoverlapping confidence intervals are considered statistically different.
Synthetic lethal at L1.
Synthetic dumpy at all stages.
The previously isolated dex-1(ns42) allele results in a premature stop codon in exon 9, encoding the predicted zonadhesin-like functional domain (Heiman and Shaham 2009) (Figure 2C). In an unrelated screen for mutants with embryonic and early larval lethality, we isolated dex-1(cs201), which introduces a point mutation at the splice donor site of intron 4 (Figure 2C; also see Cohen et al. (2018). Analysis of dex-1(cs201) cDNA suggests this results in multiple transcripts with read through into intron 4, with all introducing a new stop codon 10 bp into this intron (Cohen et al. 2018). dex-1(cs201) mutants have a high rate of early larval lethality (96% dead L1s, n = 170). Similar to dex-1(ns42), dex-1(cs201) dauers are defective in radial shrinkage, alae formation, and SDS resistance (Figures 1D and 2, A and B). To confirm dex-1 as regulating these phenotypes, we rescued the radial shrinkage phenotype and SDS sensitivity of both dex-1 mutants with dex-1 cDNA under the control of its endogenous promoter (Figure 2, A and D). Together, these data suggest that dex-1 mutants form partial dauers with defects in epidermal remodeling. The enhanced sensitivity of dex-1(cs201) to SDS compared with dex-1(ns42) (Figure 2D) combined with our molecular data suggest that dex-1(ns42) is a hypomorphic allele; however, due to the early larval lethality of dex-1(cs201), all further experiments were done using the dex-1(ns42) background, unless otherwise noted.
We characterized additional dauer-specific phenotypes in dex-1(ns42) mutants. Dauers suppress pharyngeal pumping and have a buccal plug that prevents feeding (Cassada and Russell 1975; Popham and Webster 1979). Nondauers will readily ingest fluorescent beads and show fluorescence throughout the digestive system (Nika et al. 2016). We did not find fluorescent beads in dex-1(ns42) mutant dauer intestines; however, we occasionally observed them in the buccal cavity (Figure S2, A–C). Additionally, we found no difference in the rate of pharyngeal pumping between dex-1(ns42) and wild-type dauers (Figure S2D). These data suggest that while pharyngeal pumping is efficiently suppressed, dex-1(ns42) dauers have low-penetrance defects in buccal plug formation. Finally, we examined dex-1(ns42) dauers for the presence of dauer-specific gene expression of lag-2p::gfp in the IL2 neurons during dauer (Ouellet et al. 2008). Similar to wild-type dauers, dex-1(ns42) dauers showed appropriate dauer-specific expression (Figure S3).
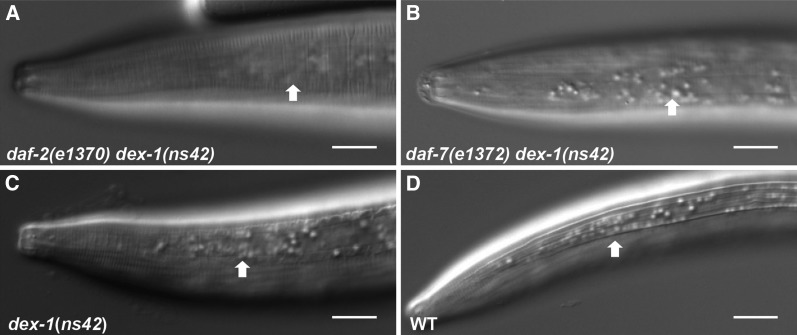
We sought to determine whether the dex-1(ns42) mutation would affect the initial decision to enter dauer. The dauer formation decision is based on the ratio of population density to food availability during early larval development (Golden and Riddle 1984). C. elegans constitutively secrete a pheromone mixture that is sensed by conspecific animals and, at high levels, triggers dauer formation (Golden and Riddle 1982). Dauers can be picked from old culture plates (starved) or can be induced using purified dauer pheromone. We found no difference in the dauer morphology phenotype between starved or pheromone-induced dex-1(ns42) mutant dauers (Figure S4). The C. elegans insulin/IGF-like and TGF-β signaling pathways function in parallel with regulate dauer formation (Thomas et al. 1993; Gottlieb and Ruvkun 1994). Reduced insulin and TGF-β signaling induced by overcrowding and scarce food promotes dauer formation (Riddle and Albert 1997). Disruption of either the insulin-receptor homolog DAF-2 or the TGF-β homolog DAF-7 results in a daf-c phenotype. The dex-1(ns42) alae formation defects were not suppressed in the daf-7(e1372) or daf-2(e1370) mutant backgrounds. While nonnull alleles were used in these crosses, these data may suggest that DEX-1 acts outside of the dauer formation decision to generate dauer alae (Figure 3).
Figure 3.
dex-1 functions outside of the dauer decision pathway. (A–D) Lateral view micrographs of (A) daf-2(e1370) dex-1(ns42), (B) daf-7(e1372) dex-1(ns42), (C) dex-1(ns42), and (D) wild-type dauers. daf-c mutations in the dauer decision pathway did not suppress the dex-1(ns42) alae phenotype. Arrows point to the lateral alae. Bar, 10 μm.
DEX-1 is secreted, but acts locally to regulate seam cell remodeling during dauer morphogenesis
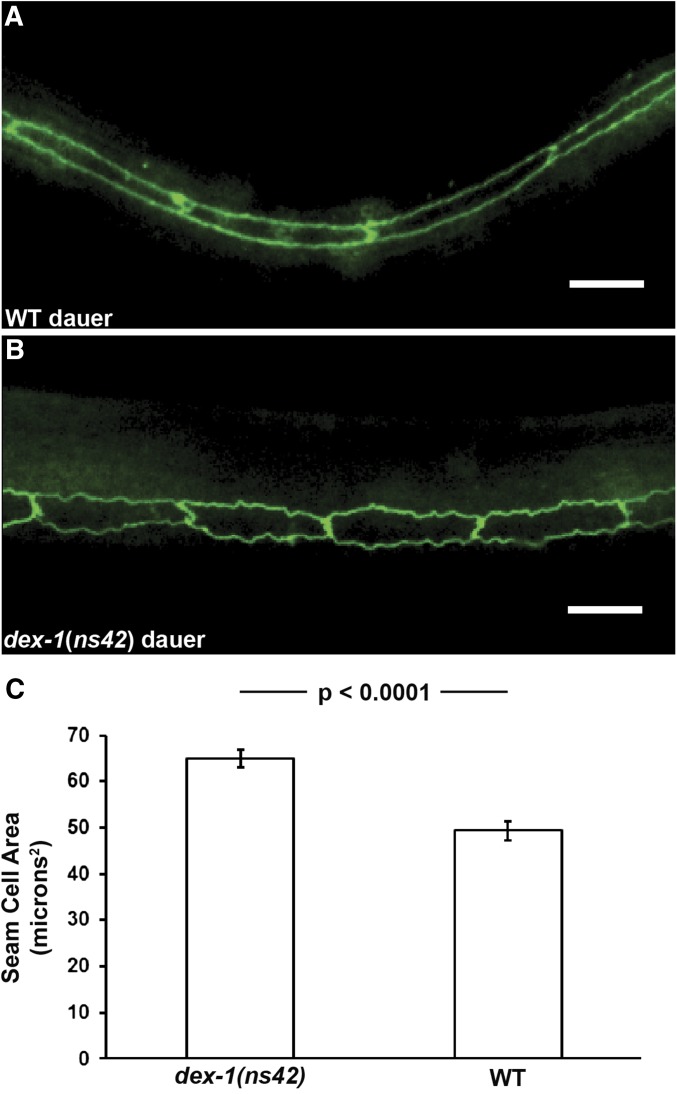
Dauer-specific radial shrinkage and subsequent lateral alae formation are facilitated by shrinkage of the seam cells (Singh and Sulston 1978; Melendez et al. 2003). Using the ajm-1::gfp apical junction marker (Köppen et al. 2001), we found that dex-1(ns42) mutant dauer seam cells are larger and have jagged, rectangular edges, unlike the smooth, elongated seam cells of wild-type dauers (Figure 4). These data suggest that DEX-1 is required for seam cell remodeling.
Figure 4.
dex-1 mutant dauers have defects in seam cell shrinkage. Lateral view micrographs of (A) wild-type (WT) and (B) dex-1(ns42) dauers expressing the apical junction marker ncIs13 [ajm-1::gfp]. The seam cells of wild-type dauers are elongated and smooth, while dex-1(ns42) mutant seam cells are wider with jagged edges. Bar, 10 μm. (C) Quantification of seam cell area as measured with the ajm-1::gfp reporter. Data were analyzed by an unpaired t-test (dex-1(ns42) dauer n = 14, WT dauer n = 15). Error bars indicate SEM.
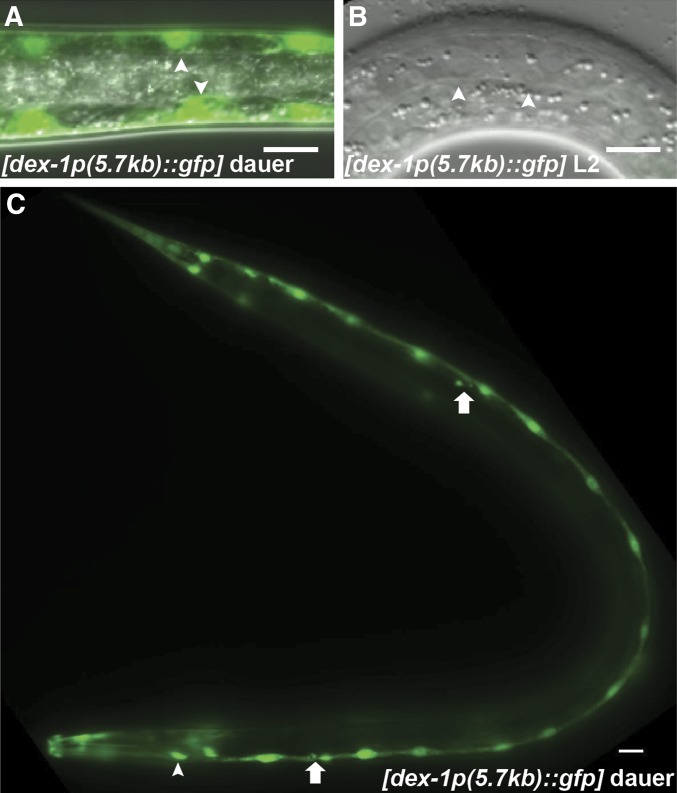
To determine the location of dex-1 expression, we generated transgenic animals expressing green fluorescent protein (GFP) driven by a 5.7 kb 5′ dex-1 upstream promoter. We observed bright fluorescence in the seam cells and glia socket cells of the anterior and posterior deirid neurons starting in the predauer L2 (L2d) stage. Expression of dex-1p::gfp in the seam cells and deirid socket cells persisted throughout dauer (Figure 5). We also observed dex-1p::gfp expression in unidentified pharyngeal cells during all larval stages (Figure 5).
Figure 5.
dex-1 is expressed in the seam cells during dauer. (A) Dorsoventral view of a dauer animal expressing dex-1p::gfp from a 5.7 kb promoter. (B) GFP is seen in the seam cells (arrowheads) during dauer and is not expressed during L3. Bar, 10 μm. (C) Lateral view of a dauer animal expressing dex-1p::gfp from a 5.7 kb dex-1 promoter region. In addition to the seam cells along the length of the animal, dex-1 is expressed in the socket glial cells of the anterior and posterior deirid neurons (arrows) during dauer, and in unidentified pharyngeal cells (arrowhead) during all larval stages. Bar, 10 μm.
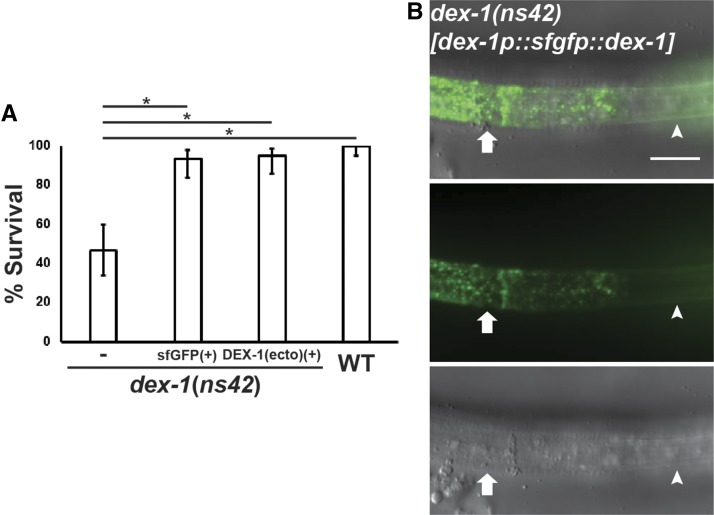
To determine the subcellular localization of DEX-1 during dauer remodeling, we expressed dex-1 cDNA (isoform A) tagged with super-folder GFP (sfGFP) under the control of its endogenous promoter. This dex-1p::sfgfp::dex-1 construct rescued the SDS phenotype in dex-1(ns42) mutant dauers, suggesting it is functional (Figure 6A). During dauer, we observed sfGFP along the length of the animal above seam cells in a mosaic pattern alternating between alae with diffuse sfGFP expression immediately under the outer ridges and alae with bright and punctate expression immediately below the lateral ridge (Figure 6B). Interestingly, regions with diffuse GFP expression correlated with proper radial constriction and intact lateral alae, whereas regions with bright, punctate expression did not undergo proper dauer shrinkage and lacked alae (Figure 6B). The mosaicism in alae formation was observed in both wild-type and dex-1(ns42) dauers expressing the dex-1p::sfgfp::dex-1 construct, indicative of dominant negative effects of the transgene.
Figure 6.
DEX-1 is localized to the outer ridges of the lateral alae. (A) Functional full-length dex-1p::sfgfp::dex-1 and truncated dex-1p::dex-1(ecto)::sfgfp constructs rescue the dex-1(ns42) SDS sensitivity phenotype to wild-type levels. Error bars indicate 95% confidence intervals. Nonoverlapping confidence intervals were considered significantly different (*) (n = 60 per genotype pooled from three independent trials). (B) Lateral view overlay (top), fluorescence (middle), and DIC (bottom) micrograph of a dex-1(ns42) dauer expressing a full-length dex-1p::sfgfp::dex-1 construct. The full-length dex-1p::sfgfp::dex-1 construct rescues the lateral alae phenotype in a mosaic pattern. dex-1::sfgfp::dex-1 localizes in a diffuse pattern to the areas immediately below the outer ridges of rescued, intact lateral alae (arrowheads). In contrast, in areas where lateral alae are not rescued, DEX-1 expression is bright and punctate and localizes throughout the lateral ridge (arrows). Bar, 10 μm.
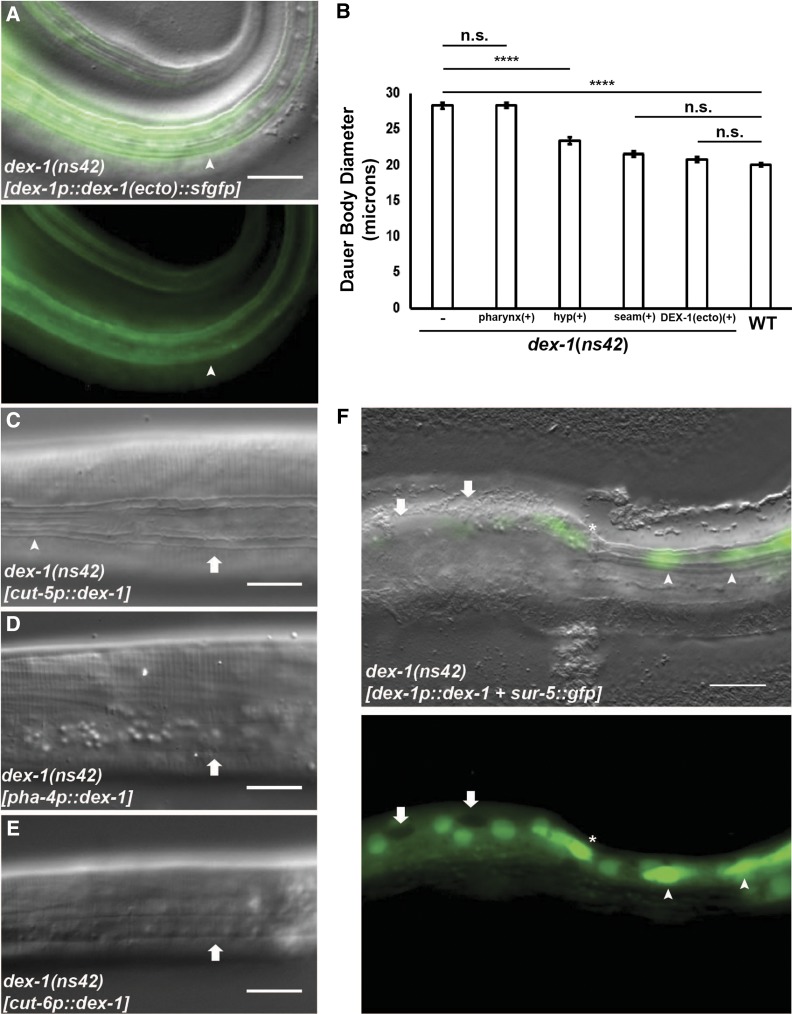
DEX-1 contains a putative transmembrane domain, but was previously suggested to be secreted through cleavage of the large extracellular domain (Heiman and Shaham 2009). To test if DEX-1 can function as a secreted protein during dauer, we expressed a dex-1p::dex-1(ecto)::sfgfp construct that truncates the C-terminal transmembrane domain. Consistent with a role as a secreted protein, the truncated DEX-1 construct rescued the SDS resistance (Figure 6A), radial shrinkage, and alae formation phenotypes (Figure 7, A and B).
Figure 7.
DEX-1 functions as a secreted protein in a tightly localized manner. (A) Lateral view DIC overlay (top) and fluorescence (bottom) images of a dex-1(ns42) dauer expressing dex-1p::dex-1(ecto)::sfgfp, which lacks the C-terminal transmembrane domain. dex-1p::dex-1(ecto)::sfgfp localizes to the outer edges of the lateral ridge and completely rescues the dex-1(ns42) alae phenotype. Arrowheads point to areas with distinct lateral alae. (B) Cell-specific rescue of body diameter in dex-1(ns42) suggests DEX-1 functions cell-autonomously. Error bars indicate SEM. **** indicates statistical significance at P < 0.0001 (n = 15). (C) dex-1 expression from the cut-5 seam cell-specific promoter rescues the dex-1(ns42) alae formation phenotype in a mosaic pattern. Arrowhead points to areas with rescued alae, arrow points to areas with partially rescued alae. dex-1 expression from a (D) pha-4 pharyngeal and (E) cut-6 hypodermal promoter fail to rescue alae formation in dex-1(ns42) dauers. Arrows point to the indistinct lateral alae. (F) DIC overlay (top) and fluorescence (bottom) image of a dex-1(ns42) dauer expressing a dex-1p::dex-1 construct with sur-5::gfp. sur-5::gfp expression in seam cell nuclei was correlated with rescue of the lateral alae (arrowheads), while the absence of sur-5::gfp correlated with indistinct lateral alae and a larger body diameter (arrows). Occasionally, sur-5::gfp was expressed in seam cell nuclei that did not show lateral alae rescue (*). Bar, 10 μm.
To further examine where DEX-1 acts to regulate seam cell remodeling, we expressed dex-1 cDNA under the control of cell-specific promoters. First, we expressed dex-1 in the seam cells using the cut-5 promoter. cut-5 was previously shown to be expressed specifically in the seam cells during L1 and dauer (Sapio et al. 2005). Seam cell-specific expression of dex-1 rescued the dex-1(ns42) radial shrinkage in a mosaic pattern similar to that seen with the full-length sfgfp::dex-1 construct (Figure 7, B and C).
Expression of dex-1 under a pharyngeal promoter failed to rescue the dex-1 seam cell phenotype, suggesting that dex-1 expression is necessary near the seam cells (Figure 7, B and D). The basolateral membranes of the seam cells are surrounded by a syncytial hypodermis. We hypothesized that the close proximity of the surrounding hypodermis to the seam cells would be sufficient for secreted DEX-1 to function during seam cell remodeling. We therefore expressed dex-1 under the control of a cut-6 promoter that was previously shown to drive expression in the hypodermis, but not in the seam cells, during dauer (Muriel et al. 2003). While dex-1 expression in the surrounding hypodermis failed to rescue the dex-1 dauer-specific alae phenotype (Figure 7E), hypodermal expression of dex-1 resulted in partial rescue of the radial shrinkage phenotype, suggesting a limited ability for DEX-1 to translocate in vivo (Figure 7B).
Finally, to verify that DEX-1 is functioning in a cell-autonomous manner, we performed a mosaic analysis using dex-1p::dex-1 and sur-5::gfp as the co-injection marker (Yochem et al. 1998). We found that in cells expressing sur-5::gfp: 56% showed full rescue of the lateral alae, 29% showed at least partial alae rescue, and 16% showed no alae rescue (n = 12 animals, 191 cells). We did not observe alae rescue in cells that did not express nuclear sur-5::gfp (Figure 7F). Together, these data indicate that dex-1 acts cell-autonomously to regulate seam cell remodeling during dauer.
DEX-1 regulates locomotion during dauer
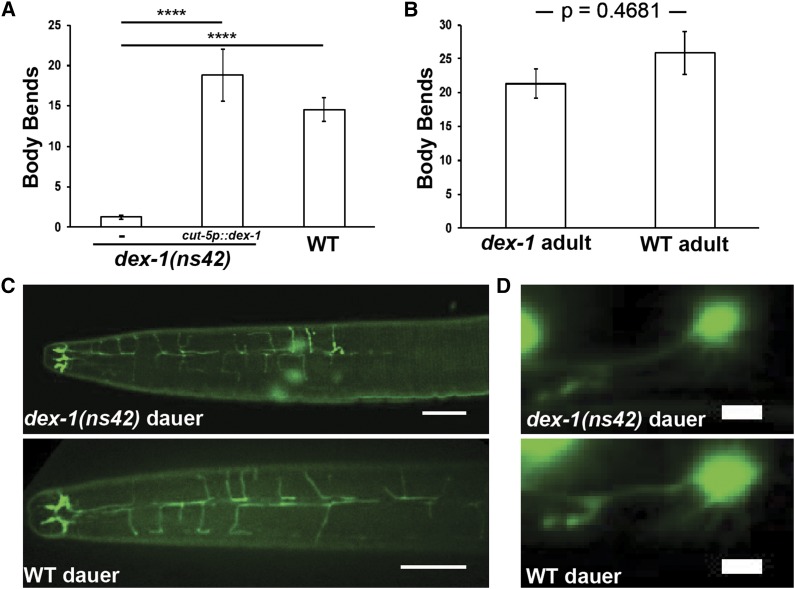
Morphological changes during dauer are accompanied by changes in behavior. Wild-type dauer animals are often quiescent, but move rapidly when mechanically stimulated (Cassada and Russell 1975). Anecdotally, we noticed a higher percentage of quiescent dex-1 dauers than wild-type dauers. To quantify this behavior, we developed a behavioral assay to measure movement following mechanical stimulation (see Materials and Methods). Although both dex-1(ns42) mutant and wild-type dauers initially respond to mechanical stimulation, the dex-1 mutant dauers have significantly reduced locomotion and display slightly uncoordinated body movements (Figure 8A). This locomotion defect was dauer-specific, as nondauer dex-1 animals moved at wild-type levels following mechanical stimulation (Figure 8B).
Figure 8.
dex-1(ns42) dauers have defects in locomotion. (A) dex-1(ns42) dauers are less responsive when mechanically stimulated compared to wild-type dauers. Seam cell-specific expression of dex-1 rescues the locomotion phenotype to wild-type levels. **** indicates statistical significance at P < 0.0001 (n = 40). (B) The locomotion defect in dex-1(ns42) dauers is dauer-specific, as nondauer dex-1(ns42) adults move at wild-type levels when mechanically stimulated (n = 40). Error bars indicate SEM. (C) Dorsoventral confocal images of the inner labial 2 (IL2) neurons in dex-1(ns42) (top) and wild-type (bottom) dauers. Bar, 10 μm. (D) Lateral view micrographs of the anterior deirid (ADE) neurons in dex-1(ns42) (top) and wild-type (bottom) dauers. We did not observe any structural differences in either the IL2 or deirid neurons. Bar, 1 μm.
In addition to seam cell remodeling, the IL2 and deirid sensory neurons remodel during dauer formation (Albert and Riddle 1983; Schroeder et al. 2013). The IL2s regulate dauer-specific behaviors (Lee et al. 2011; Schroeder et al. 2013), while the deirids respond to specific mechanical cues (Sawin et al. 2000). We therefore examined these neuron classes using fluorescent reporters; however, we observed no obvious difference in neuronal structure between dex-1(ns42) and wild type (Figure 8, C and D). Given that dex-1 was primarily expressed in the seam cells during dauer, we tested if seam cell-specific expression could rescue the behavioral phenotype. Surprisingly, seam cell-specific dex-1 expression completely rescued the dex-1(ns42) dauer locomotion defects (Figure 8A).
dex-1 expression in dauers is regulated by DAF-16
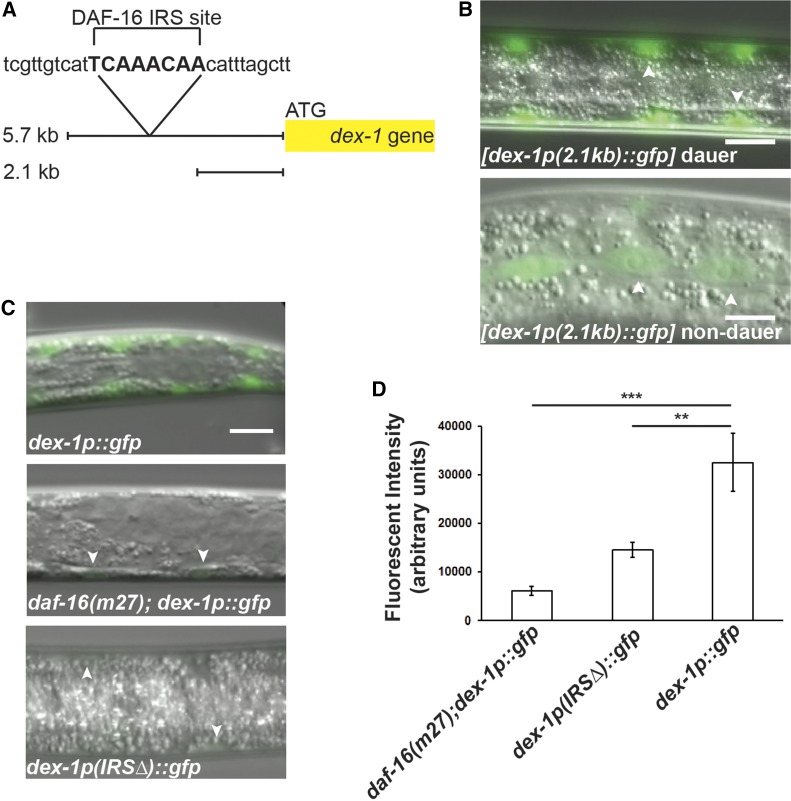
To understand how dex-1 expression is regulated, we examined the 5′ upstream region of dex-1 for potential regulatory sites. Previous chromatin immunoprecipitation sequencing data identified a putative DAF-16 binding site ∼3 kb upstream of the dex-1 coding region (Figure 9A) (Celniker et al. 2009). DAF-16 is the sole C. elegans ortholog of the human Forkhead Box O-type transcription factor and a major regulator of the dauer decision (Lin et al. 1997; Ogg et al. 1997). To examine whether this region affects expression of dex-1, we first expressed GFP from a truncated 2.1 kb dex-1 promoter that does not include the putative DAF-16 binding site. Unlike the 5.7 kb dex-1p::gfp promoter fusion, which resulted in GFP expression in the seam cells exclusively during dauer (Figure 5, A and B), the shorter dex-1 promoter drove GFP expression in the seam cells during all larval stages (Figure 9B). This suggests that dex-1 is repressed during nondauer stages and activated by DAF-16 during dauer.
Figure 9.
dex-1 expression in the seam cells is regulated by DAF-16. (A) We identified a putative DAF-16 IRS binding site (in capitals) ∼3 kb upstream from the dex-1 ATG start site. (B) Expression of dex-1p::gfp from a truncated 2.1 kb dex-1 promoter drives fluorescence in the seam cells (arrowheads) in both dauer (top) and nondauer (bottom) stages. Bar, 10 μm. (C) Dorsoventral view micrographs of GFP expression from the 5.7 kb dex-1 promoter in a wild-type background (top) produces bright fluorescence in the seam cells during dauer (also see Figure 5A). Fluorescence intensity is reduced in a daf-16(m27) partial dauer mutant background (middle). Deletion of the DAF-16 IRS sequence (bottom) from the 5.7 kb promoter region also significantly reduces GFP expression in the seam cells during dauer. Arrowheads indicate seam cells. Bar, 10 μm. (D) Quantification of GFP expression driven by the 5.7 kb dex-1 promoter in dauers. ** and *** indicate statistical significance at P < 0.01 and P < 0.001, respectively. Error bars indicate SEM.
To further examine if DAF-16 is regulating dex-1 expression during dauer, we examined the expression of the 5.7 kb dex-1p::gfp reporter in a daf-16(m27) mutant background. While mutations in daf-16 result in animals incapable of forming dauers, under high-pheromone concentrations, daf-16(m27) mutants can enter into a partial dauer state with some dauer morphological characteristics (Vowels and Thomas 1992; Gottlieb and Ruvkun 1994). daf-16(m27) partial dauers are identifiable by body morphology and the presence of indistinct lateral alae (Vowels and Thomas 1992). We found that the fluorescence intensity of dex-1p::gfp was significantly reduced in daf-16(m27) partial dauers compared to wild type, suggesting that DAF-16 regulates dex-1 seam cell expression during dauer (Figure 9, C and D).
FOXO/DAF-16 binds to canonical DAF-16 binding elements and insulin response sequences (IRS) (Paradis and Ruvkun 1998; Obsil and Obsilova 2008). Within the chromatin immunoprecipitation sequencing–identified region (Celniker et al. 2009), we identified a putative IRS binding site (Figure 9A). To determine whether the identified DAF-16 IRS site directly regulates dex-1 expression, we deleted the IRS sequence in the 5.7 kb dex-1 promoter region used to drive GFP. We found that deleting the IRS sequence results in reduced GFP expression in the seam cells during dauer, similar to the levels observed in daf-16 partial dauers (Figure 9, C and D). Taken together, these results indicate the 2.1-kb region proximal to the dex-1 start codon drives expression in the seam cells, and that an unidentified element within the 3.6-kb region upstream from the 2.1-kb activation site represses expression outside of dauer. This repression is counteracted by DAF-16 binding to the IRS during dauer formation.
dex-1 interacts with genes encoding ZP-domain proteins
DEX-1 acts with the ZP-domain protein DYF-7 to regulate primary dendrite extension during embryogenesis (Heiman and Shaham 2009). We therefore examined the dyf-7(m537) mutant for defects in dauer morphogenesis. Unlike dex-1 mutants, dyf-7 mutants are unable to enter dauer under typical dauer-inducing environmental conditions (Starich et al. 1995), and so we examined dyf-7 mutants in a daf-c mutant background. daf-7; dyf-7 double mutants had normal dauer-specific radial shrinkage and IL2 dendrite arborization (Figure S5).
The cuticlin (CUT) proteins are a family of ZP-domain proteins originally isolated from nematode cuticles (Sebastiano et al. 1991). cut-1 and cut-5 are expressed in the seam cells, while cut-6 is expressed in the surrounding hypodermis (Muriel et al. 2003; Sapio et al. 2005). Similar to dex-1, disruption of cut-1, cut-5, and cut-6 results in dauers with incomplete radial shrinkage and defective alae formation (Sebastiano et al. 1991; Muriel et al. 2003; Sapio et al. 2005). We asked whether these defects in CUT mutant larvae were due to seam cell remodeling. We found that, similar to dex-1(ns42), the seam cells of the CUT mutant dauers were enlarged with jagged edges (Figure 10). Also similar to dex-1(ns42) mutants, we found that cut-1(tm1126) and cut-5(ok2005) dauers were more sensitive to SDS compared with wild-type dauers (Figure S6 and Table 1). Interestingly, while cut-6(ok1919) mutant dauers were resistant to the standard 1% SDS treatment (Muriel et al. 2003), we found that the cut-6 mutant dauers were substantially more sensitive to SDS than wild-type dauers (Figure S6 and Table 1). Taken together, these data indicate similar roles for DEX-1 and CUTs during dauer remodeling.
Figure 10.
Cuticlin mutants phenocopy the dex-1 mutant seam cell phenotype during dauer. Lateral view of wild-type, dex-1(ns42), and cuticlin mutant dauers expressing the apical junction marker ajm-1::gfp. The seam cells in cuticlin mutants are jagged and wider, closely resembling those of dex-1(ns42) mutant dauers. Bar, 10 μm.
We hypothesized that, similar to its interaction with DYF-7 during embryogenesis, DEX-1 may genetically interact with the CUT proteins during dauer. We therefore examined double mutants of dex-1(ns42) with cut-1(tm1126), cut-5(ok2005), and cut-6(ok1919). It is worth noting that, while the cut mutations are all deletion alleles (Figure S7), these data should be interpreted with caution as dex-1(ns42) is a hypomorphic allele. The dex-1; cut-1 double mutant did not enhance SDS sensitivity beyond the dex-1 single mutant, suggesting that they may act in the same pathway to regulate dauer remodeling (Figure S6 and Table 1). The dex-1; cut-5 double mutant was synthetically lethal during embryogenesis or early L1. This is similar to the dex-1(ns42); dyf-7 double mutant (Heiman and Shaham 2009) and the severe loss-of-function mutant dex-1(cs201) (Cohen et al. 2018), suggesting that in addition to roles in dauer remodeling, CUT-5 has additional roles during early development. Interestingly, the dex-1cut-6 double mutant was intermediate in SDS sensitivity between the dex-1 and cut-6 single mutants (Figure S6 and Table 1).
We further tested the cut mutant phenotypes by generating double mutants between each of the cut mutants (Figure S6 and Table 1). The cut-1; cut-5 double mutant showed a significant reduction of SDS resistance compared to single mutants alone. Interestingly, the cut-1; cut-6 double mutants retained the cut-6 SDS sensitivity phenotype. The cut-6; cut-5 dauers showed a drastic increase in sensitivity to SDS compared to single mutants. In addition, the cut-6; cut-5 double mutant showed a severe dumpy phenotype in all developmental stages. These results suggest that CUT-5 and CUT-6, like DEX-1 (Cohen et al. 2018), also play broader roles during development.
Discussion
The dauer stage of C. elegans is an excellent example of a polyphenism, where distinct phenotypes are produced by the same genotype via environmental regulation (Simpson et al. 2011). Compared to the decision to enter dauer, little is known about the molecular mechanisms controlling remodeling of dauer morphology. DEX-1 was previously characterized for its role in embryonic neuronal development (Heiman and Shaham 2009), and in the accompanying article we show that it shapes multiple embryonic epithelia (Cohen et al. 2018). Our data show that DEX-1 also functions during dauer-specific remodeling of the stem cell-like seam cells.
We demonstrate that DEX-1 is secreted, but acts locally in a cell-autonomous manner to regulate seam cell remodeling during dauer morphogenesis. DEX-1 is similar in sequence to the human ECM protein SNED1 (Sushi, Nidogen, EGF-like domains 1). High levels of SNED1 expression promote invasiveness during breast cancer metastasis (Naba et al. 2014), suggesting a possible mechanical role in tissue remodeling. Interestingly, results from our full-length translational DEX-1 reporter indicate that seam cell remodeling can also be perturbed by a dominant negative effect of DEX-1 overexpression. Seam cells with bright, aggregated sfGFP::DEX-1 expression were correlated with indistinct lateral alae and large body diameter, whereas diffuse sfGFP::DEX-1 correlated with intact alae. We hypothesize that this could be the result of increased interaction of DEX-1 protein with either itself or other ZP proteins in the ECM, leading to protein aggregates that disrupt alae formation.
We also found the dex-1 mutant dauers have defects in locomotion when mechanically stimulated. We originally assumed that this could be due to the lack of lateral alae; however, our seam cell-specific rescue of dex-1 resulted in a mosaic pattern of alae formation while completely rescuing the behavior. One explanation may be that DEX-1 acts cell-nonautonomously to mediate dauer behaviors. Alternatively, our seam cell-specific promoter could drive undetectable expression in the neuromuscular system. Previous RNA interference data showed that knockdown of dex-1 results in low penetrance defects in motor neuron commissure formation (Schmitz et al. 2007). Dauers also exhibit changes in somatic muscle structure (Dixon et al. 2008). We therefore speculate that dex-1 mutant dauers may have defects in neuromuscular structure or function that results in locomotion defects.
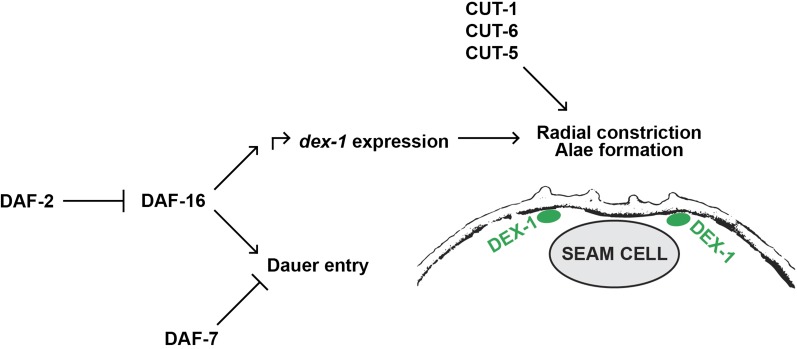
Dissection of the genetic pathways regulating the decision to enter dauer has revealed insights into TGF-β, insulin, and hormone signaling (Thomas et al. 1993; Gottlieb and Ruvkun 1994; Riddle and Albert 1997). The FOXO transcription factor DAF-16 is a well-known regulator of the dauer formation decision by acting downstream of the insulin/IGF-1 receptor DAF-2 (Gottlieb and Ruvkun 1994; Ogg et al. 1997). Dauer-inducing environmental conditions lead to a translocation of DAF-16 to the nucleus, where it activates dauer formation pathways (Lee et al. 2001; Fielenbach and Antebi 2008). We found that dex-1 expression during dauer is regulated by DAF-16. Based on our results, we propose that DEX-1 is repressed during nondauer postembryonic stages and DAF-16 serves to activate dex-1 expression via an upstream IRS (Figure 11).
Figure 11.
DEX-1 may function outside of the dauer decision pathway to facilitate dauer alae formation. A model diagram showing our proposed genetic pathway for dex-1 transcriptional regulation. We show that during dauer, dex-1(ns42) is not suppressed by daf-c mutants and that dex-1 is transcriptionally activated by DAF-16. We also show that DEX-1 functions as a secreted protein localized to the apical extracellular matrix that, along with the cuticlin proteins, facilitates dauer-specific radial constriction and alae formation.
Our data suggests that DEX-1 acts along with ZP-domain proteins to control dauer remodeling. While the cut mutants are all deletion alleles that disrupt the ZP domains and, therefore, likely functional nulls (Figure S7), the dex-1(ns42) allele is a non-sense mutation late in the coding region (Heiman and Shaham 2009). Our isolation of the larval lethal dex-1(cs201) allele suggests that dex-1(ns42) is hypomorphic. Therefore, our dex-1 genetic interaction experiments should be interpreted with caution. In addition to this complication, our double-mutant experiments do not provide a clear interaction pathway. For example, our results suggest that deletion of cut-6 abrogates loss of cut-1. One possible explanation is a compensatory mechanism in the ECM, where loss of one ECM protein leads to increased expression of other structural components. This was previously shown in cases of osteogenesis imperfecta, where mutations in type I collagen led to increases in levels of thrombospondin and fibronectin (Fedarko et al. 1995). Alternatively, while we did not observe any obvious defects in the dauer formation decision in any of the double mutants, it is possible that slight differences in the ability to form dauers bias our observations toward individuals with only mild remodeling defects.
It has been proposed that biochemical compaction of the CUTs in the extracellular space between the seam and the hypodermis causes radial constriction, and thus forms the lateral alae via a “CUT tether” (Sapio et al. 2005). We add to this by proposing that DEX-1 is another seam-specific epidermal matrix component that, along with ZP CUTs, facilitates apical constriction of the seam and formation of dauer alae (Figure 11). Our data indicate that DEX-1 acts in a cell-autonomous manner directly beneath the edges of the lateral alae. Although expression of dex-1 in the hypodermis was sufficient to partially rescue dauer-specific radial shrinkage, hypodermal expression failed to rescue the lateral alae phenotype. We therefore propose DEX-1 may act as a secreted protein during dauer with restricted localization to the cuticle or ECM immediately above the apical membrane of the seam cells. During embryogenesis, DEX-1 is secreted and localized to the dendritic tips (Heiman and Shaham 2009). DEX-1 may serve to couple physical interactions between the remodeled cuticular ECM and seam cell shape. Failure of these tissues to properly compact and thicken due to a loss of DEX-1 could lead to an overall weakening of the cuticle, and thus result in the SDS sensitivity observed in dex-1 mutant dauers. Previous research demonstrated a role for autophagy in dauer-specific seam cell remodeling (Melendez et al. 2003). It will be interesting to determine if dauer-specific changes to autophagy are influenced by DEX-1–mediated mechanical forces.
Acknowledgments
We thank Max Heiman for reagents and advice, Scott Robinson at the Beckman Institute for assistance with electron microscopy, Rebecca Androwski and Ursula Reuter-Carlson for confocal microscopy contributions, and the anonymous reviewers for providing valuable feedback. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (grant P40 OD010440). This work was supported by the National Institutes of Health (grants R01GM111566 to N.E.S., R01GM058540 to M.V.S., and T32GM008216 to J.D.C.).
Note added in proof: See Cohen et al. 2019 (pp. 185–200) in this issue for a related work.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7077146.
Communicating editor: D. Greenstein
Literature Cited
- Albert P. S., Riddle D. L., 1983. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J. Comp. Neurol. 219: 461–481. 10.1002/cne.902190407 [DOI] [PubMed] [Google Scholar]
- Albert P. S., Riddle D. L., 1988. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev. Biol. 126: 270–293. 10.1016/0012-1606(88)90138-8 [DOI] [PubMed] [Google Scholar]
- Androwski R. J., Flatt K. M., Schroeder N. E., 2017. Phenotypic plasticity and remodeling in the stress-induced Caenorhabditis elegans dauer. Wiley Interdiscip. Rev. Dev. Biol. 6: e278 10.1002/wdev.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M. L., 1993. Cuticle surface proteins of wild type and mutant Caenorhabditis elegans. J. Biol. Chem. 268: 6600–6609. [PubMed] [Google Scholar]
- Blelloch R., Santa Anna-Arriola S., Gao D., Li Y., Hodgkin J., et al. , 1999. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev. Biol. 216: 382–393. 10.1006/dbio.1999.9491 [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada R. C., Russell R. L., 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46: 326–342. 10.1016/0012-1606(75)90109-8 [DOI] [PubMed] [Google Scholar]
- Celniker S. E., Dillon L. A. L., Gerstein M. B., Gunsalus K. C., Henikoff S., et al. , 2009. Unlocking the secrets of the genome. Nature 459: 927–930. 10.1038/459927a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Flatt K. M., Schroeder N. E., Sundaram M. V., 2018. Epithelial shaping by diverse atpical extracellular matrices requires the nidogen domain protein DEX-1 in Caenorhabditis elegans. Genetics 211: 185–200. 10.1534/genetics.118.301752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Staprans S., Edgar R. S., 1981. The cuticle of Caenorhabditis elegans. II. Stage-specific changes in ultrastructure and protein composition during postembryonic development. Dev. Biol. 86: 456–470. 10.1016/0012-1606(81)90204-9 [DOI] [PubMed] [Google Scholar]
- Dixon S. J., Alexander M., Chan K. K. M., Roy P. J., 2008. Insulin-like signaling negatively regulates muscle arm extension through DAF-12 in Caenorhabditis elegans. Dev. Biol. 318: 153–161. 10.1016/j.ydbio.2008.03.019 [DOI] [PubMed] [Google Scholar]
- Fedarko N. S., Robey P. G., Vetter U. K., 1995. Extracellular matrix stoichiometry in osteoblasts from patients with osteogenesis imperfecta. J. Bone Miner. Res. 10: 1122–1129. 10.1002/jbmr.5650100718 [DOI] [PubMed] [Google Scholar]
- Fielenbach N., Antebi A., 2008. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 22: 2149–2165. 10.1101/gad.1701508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flibotte S., Edgley M. L., Chaudhry I., Taylor J., Neil S. E., et al. , 2010. Whole-Genome profiling of mutagenesis in Caenorhabditis elegans. Genetics 185: 431–441. 10.1534/genetics.110.116616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L., 1982. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 218: 578–580. 10.1126/science.6896933 [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L., 1984. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 102: 368–378. 10.1016/0012-1606(84)90201-X [DOI] [PubMed] [Google Scholar]
- Gottlieb S., Ruvkun G., 1994. daf-2, daf-16 and daf-23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics 137: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T., Orita S., Han M., 1998. Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol. Cell. Biol. 18: 4556–4564. 10.1128/MCB.18.8.4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, D. H., E. Hartwieg, and K. C. Q. Nguyen, 2012 Modern electron microscopy methods for C. elegans. Methods Cell Biol. 107: 93–149. 10.1016/B978-0-12-394620-1.00004-7 [DOI] [PubMed] [Google Scholar]
- Heiman M. G., Shaham S., 2009. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137: 344–355. 10.1016/j.cell.2009.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp X., Ambros V., 2012. Dauer larva quiescence alters the circuitry of microRNA pathways regulating cell fate progression in C. elegans. Development 139: 2177–2186. 10.1242/dev.075986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane J., Avery L., 2003. Mechanosensory inputs influence Caenorhabditis elegans pharyngeal activity via ivermectin sensitivity genes. Genetics 164: 153–162. 10.1098/rstb.1976.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Sun L., Gabel C. V., Fang-Yen C., 2013. Long-term imaging of Caenorhabditis elegans using nanoparticle-mediated immobilization. PLoS One 8: e53419 10.1371/journal.pone.0053419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppen M., Simske J. S., Sims P. A., Firestein B. L., Hall D. H., et al. , 2001. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat. Cell Biol. 3: 983–991. 10.1038/ncb1101-983 [DOI] [PubMed] [Google Scholar]
- Lee H., Choi M., Lee D., Kim H., Hwang H., et al. , 2011. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat. Neurosci. 15: 107–112 (erratum: Nat. Neurosci. 16: 1906) 10.1038/nn.2975 [DOI] [PubMed] [Google Scholar]
- Lee R. Y. N., Hench J., Ruvkun G., 2001. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 11: 1950–1957. 10.1016/S0960-9822(01)00595-4 [DOI] [PubMed] [Google Scholar]
- Lin K., Dorman J. B., Rodan A., Kenyon C., 1997. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278: 1319–1322. 10.1126/science.278.5341.1319 [DOI] [PubMed] [Google Scholar]
- Manning, L., and J. Richmond, 2015 High-pressure freeze and freeze substitution electron microscopy in C. elegans, pp. 121–140 in C. elegans: Methods and Applications, edited by D. Biron and G. Haspel. Humana Press, Totowa, NJ 10.1007/978-1-4939-2842-2_10 [DOI] [PubMed] [Google Scholar]
- McCloy R. A., Rogers S., Caldon C. E., Lorca T., Castro A., et al. , 2014. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13: 1400–1412. 10.4161/cc.28401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez A., Talloczy Z., Seaman M., Eskelinen E.-L., Hall D. H., et al. , 2003. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301: 1387–1391. 10.1126/science.1087782 [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. 10.1002/j.1460-2075.1991.tb04966.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriel J. M., Brannan M., Taylor K., Johnstone I. L., Lithgow G. J., et al. , 2003. M142.2 (cut-6), a novel Caenorhabditis elegans matrix gene important for dauer body shape. Dev. Biol. 260: 339–351. 10.1016/S0012-1606(03)00237-9 [DOI] [PubMed] [Google Scholar]
- Naba A., Clauser K. R., Lamar J. M., Carr S. A., Hynes R. O., 2014. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife 3: e01308 10.7554/eLife.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R., Hall D. H., Miller D. M., Blakely R. D., 2002. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99: 3264–3269. 10.1073/pnas.042497999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nika L., Gibson T., Konkus R., Karp X., 2016. Fluorescent beads are a versatile tool for staging Caenorhabditis elegans in different life histories. G3 (Bethesda) 6: 1923–1933. 10.1534/g3.116.030163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obsil T., Obsilova V., 2008. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 27: 2263–2275. 10.1038/onc.2008.20 [DOI] [PubMed] [Google Scholar]
- Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., et al. , 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999. 10.1038/40194 [DOI] [PubMed] [Google Scholar]
- Ouellet J., Li S., Roy R., 2008. Notch signalling is required for both dauer maintenance and recovery in C. elegans. Development 135: 2583–2592. 10.1242/dev.012435 [DOI] [PubMed] [Google Scholar]
- Paradis S., Ruvkun G., 1998. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12: 2488–2498. 10.1101/gad.12.16.2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pener M. P., Simpson S. J., 2009. Locust phase polyphenism: An update. Adv. Insect Physiol. 36: 1–272. 10.1016/S0065-2806(08)36001-9 [DOI] [Google Scholar]
- Popham J. D., Webster J. M., 1979. Aspects of the fine structure of the dauer larva of the nematode Caenorhabditis elegans. Can. J. Zool. 57: 794–800. [DOI] [PubMed] [Google Scholar]
- Riddle D. L., Albert P. S., 1997. Genetic and Environmental Regulation of Dauer Larva Development, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- Riddle D. L., Swanson M. M., Albert P. S., 1981. Interacting genes in nematode dauer larva formation. Nature 290: 668–671. 10.1038/290668a0 [DOI] [PubMed] [Google Scholar]
- Sapio M. R., Hilliard M. A., Cermola M., Favre R., Bazzicalupo P., 2005. The Zona Pellucida domain containing proteins, CUT-1, CUT-3 and CUT-5, play essential roles in the development of the larval alae in Caenorhabditis elegans. Dev. Biol. 282: 231–245. 10.1016/j.ydbio.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Sawin E. R., Ranganathan R., Horvitz H. R., 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631. 10.1016/S0896-6273(00)81199-X [DOI] [PubMed] [Google Scholar]
- Schmitz C., Kinge P., Hutter H., 2007. Axon guidance genes identified in a large-scale RNAi screen using the RNAi-hypersensitive Caenorhabditis elegans strain nre-1(hd20) lin-15b(hd126). Proc. Natl. Acad. Sci. USA 104: 834–839. 10.1073/pnas.0510527104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder N. E., Flatt K. M., 2014. In vivo imaging of dauer-specific neuronal remodeling in C. elegans. J. Vis. Exp. (91): e51834 10.3791/51834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder N. E., Androwski R. J., Rashid A., Lee H., Lee J., et al. , 2013. Dauer-specific dendrite arborization in C. elegans is regulated by KPC-1/furin. Curr. Biol. 23: 1527–1535. 10.1016/j.cub.2013.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J., Milpetz F., Bork P., Ponting C. P., 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95: 5857–5864. 10.1073/pnas.95.11.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiano M., Lassandro F., Bazzicalupo P., 1991. cut-1 a Caenorhabditis elegans gene coding for a dauer-specific noncollagenous component of the cuticle. Dev. Biol. 146: 519–530. 10.1016/0012-1606(91)90253-Y [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Sword G. A., Lo N., 2011. Polyphenism in insects. Curr. Biol. 21: R738–R749. 10.1016/j.cub.2011.06.006 [DOI] [PubMed] [Google Scholar]
- Singh R. N., Sulston J. E., 1978. Some observations on moulting in Caenorhabditis elegans. Nematologica 24: 63–71. 10.1163/187529278X00074 [DOI] [Google Scholar]
- Starich T. A., Herman R. K., Kari C. K., Yeh W. H., Schackwitz W. S., et al. , 1995. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics 139: 171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R., 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. 10.1016/0012-1606(77)90158-0 [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Birnby D. A., Vowels J. J., 1993. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics 134: 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels J. J., Thomas J. H., 1992. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics 130: 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windig J. J., 1994. Reaction norms and the genetic basis of phenotypic plasticity in the wing pattern of the butterfly Bicyclus anynana. J. Evol. Biol. 7: 665–695. 10.1046/j.1420-9101.1994.7060665.x [DOI] [Google Scholar]
- Yochem J., Gu T., Han M., 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All strains and plasmids are available upon request. Table S1 contains all plasmid constructs used in this study. Figure S1 shows the dex-1(ns42) body diameter data for dauers and L3s. Figure S2 shows the results of fluorescent bead feeding and pharyngeal pumping assays. Figure S3 shows that dauer-specific lag-2p::gfp is present in dex-1(ns42) dauers. Figure S4 shows that starved and pheromone-induced dauers are morphologically similar. Figure S5 describes dyf-7(m537) dauer morphology. Figure S6 contains a dose-response curve of SDS resistance of wild type and single- and double-mutants. Figure S7 contains gene schematics of cuticlin (CUT) mutants used in this study. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7077146.