Abstract
Background
Cystic hypersecretory carcinoma is a rare subtype of breast cancer. It is a member of cystic hypersecretory lesions, which include a series of pathological disease lineages: cystic hypersecretory hyperplasia (CHH), CHH with atypia, cystic hypersecretory carcinoma (CHC) and invasive CHC. It was found that most cystic hypersecretion lesions were in situ carcinoma, and only 19 cases of invasive cystic hypersecretion carcinoma were reported.
Case presentation
We are reporting a case of a 63-year-old female who had a lump in her left breast for 3 years. A modified radical mastectomy was done and morphological diagnosis of invasive CHC with axillary node metastasis was made.
Conclusions
Owing to a smaller number of reported cases, little is known about the biological behavior, prognosis and molecular study of cystic hypersecretion lesions. Therefore, more cases with follow-up data are needed to reveal the biological behavior of this rare tumor.
Keywords: Breast cancer, Cystic hypersecretory carcinoma, Cystic hypersecretory hyperplasia
Introduction
Cystic hypersecretory lesions of the breast have a spectrum of morphological features ranging from clearly benign (CHH), CHH with atypia, cystic hypersecretory carcinoma (CHC) to invasive CHC [1]. CHC was first described by Rosen PP and Scott M in 1984 [2]. The gross and microscopic features of this entity are unique. Gross detection shows numerous cysts of varying sizes. The contents of the cysts are gelatinous material. Microscopically, dilated ducts with marked secretory activity (the secretion is thyroid colloid–like substance) and lining by pseudostratified to micropapillary epithelium [1, 2]. Among the reported cases of cystic hypersecretory breast lesion, most cases are of in situ CHC, with only a few cases of invasive CHC. Invasion is suggested by solid nests structures and are usually poorly differentiated with no secretory characteristic. Extravasation of cyst material into the stroma does not indicate invasion [1, 2]. This rare subtype of breast cancer was not included in 2012 WHO Classification of Tumors of the Breast,but its unique gross and microscopic features triggered us to report this rare tumor. Until now, only 19 cases of invasive CHC have been reported in the literature [1–11]. Our case is the twentieth case and the relevant literature is reviewed (Table 1).
Table 1.
Review of invasive CHCs in the literature
| Soure, y | Age, y | Type of Disease Present | Lymph Node Status | ER/PRa |
|---|---|---|---|---|
| Rosen and Scott [2], 1984 | 52 | Invasive | c | Pos/NA |
| 47 | Invasive | N1 | NA | |
| 62 | invasive | N0 | NA | |
| Guerry et al. [1], 1988 | NA | Invasive | N1 | NA |
| NA | Invasive | c | NA | |
| Adams and Lacey [12], 1990 | 70 | Microinvasive | N0 | Neg/pos |
| Kim et al. [3], 1997 | 37 | Invasive | N0 | NA |
| Herrmann et al. [4], 1999 | 49 | Invasive | N0 | Pos/pos |
| Lee JS [5], 2004 | 45 | Invasive | N0 | Neg/neg |
| Shin SJ [6], 2004 | 42b | Invasive | N (micro) | NA |
| Skalova A [7], 2005 (two cases) | 66.8b | Invasive | NA | One Case Pos/pos |
| Chen DB [13], 2010 | 44 | Microinvasive | NA | Pos/pos |
| Song SW [8], 2011 | 43 | Invasive | NA | NA |
| D’Alfonso TM [14], 2014 | 62.8b | Microinvasive | NA | Pos/pos |
| Bi R [9], 2014 | 37 | Invasive | N1 | NA |
| 46 | Invasive | NA | NA | |
| Gupta P [10], 2014 | 57 | Invasive | N0 | Neg/neg |
| Sahoo N [11], 2017 | 32 | Invasive | N1 | Neg/neg |
| Present case | 63 | Invasive | N1 | Neg/neg |
aER/PR indicates estrogen receptor/progesterone receptor; NA: not available;
pos: positive; neg: negative;
N (micro): lymph node micrometastasis
bMean age
cIndicates cases with distal metastatic disease
Case presentation
A 63-year-old female presented with a palpable mass in her left breast for 3 years. The lump was gradually progressive in size for the past 3 years. Physical examination revealed a painless, ill-defined, hard, large mass with no nipple discharge in the upper outer quadrant of the left breast. Skin dimpling and ulceration were also seen. The patient had no past or family history of a breast disease. A modified radical mastectomy was performed. The CAF chemotherapy was administered after surgery.
Grossly, the left breast specimen showed an ill-defined, red gray, multiple nodular, 14 × 12 cm tumor with surface skin ulceration [Fig. 1]. The cut surface revealed multiple cystic spaces filled with thick, gelatinous secretions and gray-white solid areas. The individual cysts varied from 0.2 cm to 2.5 cm in dimension with cysts wall thickness from 0.1 cm to 0.5 cm. Hemorrhage and necrosis was evident.
Fig. 1.
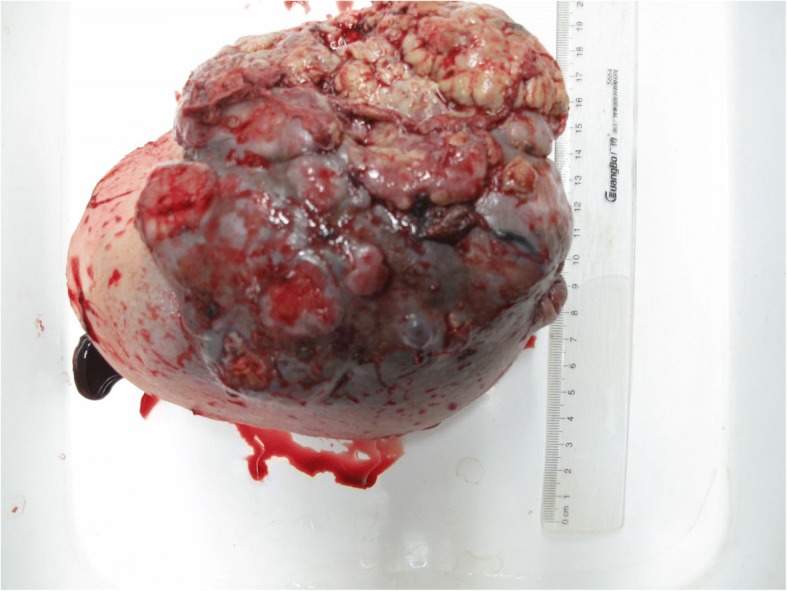
The surface of the mass showing an ill-defined, red gray, multiple nodular, 14 × 12 cm tumour with obvious skin ulceration
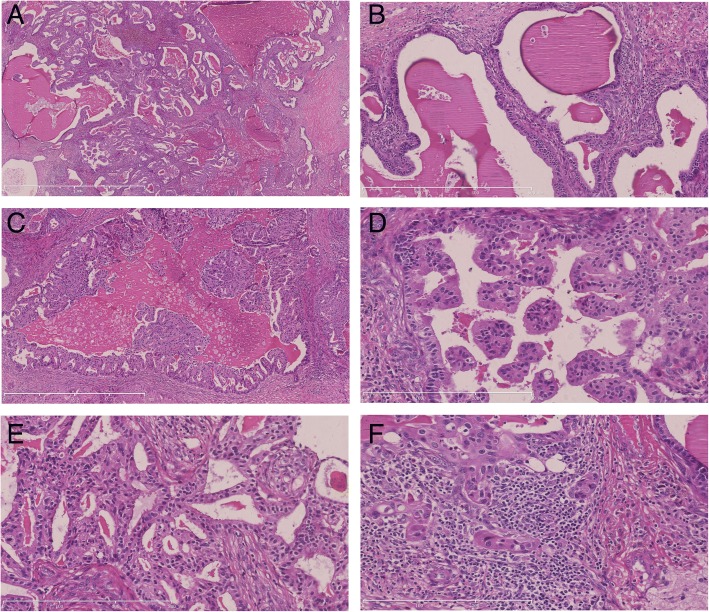
Microscopically, multiple variable-sized cystic spaces filled with thyroid colloid-like eosinophilic secretions [Fig. 2a] which was diastase resistant PAS positive and thyroglobulin negative. The eosinophilic secretions were retracted from the surrounding epithelia, producing scalloped margins. The cyst lining epithelium exhibited a variable pattern. In some areas the lining was flat to cuboidal epithelium and devoid of cellular atypia [Fig. 2b]. In other areas the epithelium showed a proliferative change in the form of pseudo stratification, knobby tufts [Fig. 2c], micropapillary [Fig. 2d] and cribriform [Fig. 2e]. An invasive component comprising of irregular neoplastic glands or nests was seen [Fig. 2f]. Eight axillary lymph nodes showed macro metastasis and cystic areas were also seen in the lymph node metastases [Fig. 3a]. Immunohistochemistry shows, the cystic contents were negative for thyroglobulin. Prognostic markers were ER negative, PR negative and HER2 3+. Ki67 was 30% positive. A diagnosis of invasive CHC with axillary node metastasis was made.
Fig. 2.
Microscopic findings. Multiple variable-sized cysts and ducts filled with thyroid colloid-like eosinophilic secretions (a, H&E, × 25). Some of the cysts are lined by flat to cuboidal epithelium (b, H&E, × 100). In other areas the epithelium showed a proliferative change in the form of pseudostratification, knobby tufts (c, H&E, × 50), micropapillary (d, H&E, × 200) and cribriform (e, H&E, × 100). An invasive component comprising of solid nests was seen (f, H&E, × 200)
Fig. 3.
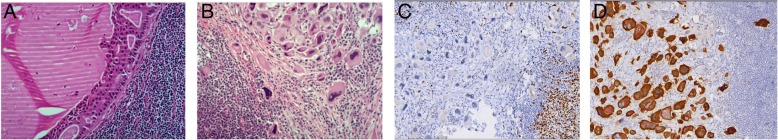
Lymph node metastases. (a) Cystic areas in the lymph node metastases (H&E, × 400). (b) Pleomorphic tumor giant cells in the lymph node metastases (H&E, × 400). (c) Negative reaction for Ki67 in pleomorphic tumor giant cells (Immunostaining, × 100). (d) Positive reaction for Her-2 in pleomorphic tumor giant cells (Immunostaining, × 100)
Discussion
CHC is an uncommon subtype of duct carcinoma. The usual clinical presentation of CHC is a palpable lump and rarely nipple discharge. Calcification may be found in some cases by molybdenum target x-ray [8]. The present case had a long onset time (3 years). The lump was gradually progressive in size with an obvious skin ulceration. Microscopically, all features of cystic hypersecretory lesions were observed, including CHH, CHC, and focal invasive carcinoma. Eight axillary lymph nodes showed macro metastasis. Metastatic foci had cystic foci that contained eosinophilic secretion. These indicate us that though CHC of the breast behaves in a low-grade fashion for many years, but, nevertheless, has a potential for invasive growth and development of distant metastasis. It is interesting that some pleomorphic tumor giant cells were seen in the lymph node metastases. The nuclear morphometry of the giant cell was bizarre, while the nucleus to cytoplasm ratio was normal and without nuclear mitosis [Fig. 3b]. The morphology of these cells was resemblance to degenerative cells. Ki67 was negative [Fig. 3c] and Her-2 3+ [Fig. 3d]. The patient was not received chemotherapy or radiation therapy before surgery, so we think that these degenerative cells may be spontaneous alternation of the tumor cells in the metastases.
The differential diagnosis of invasive CHC includes ductal carcinoma in situ (DCIS) with comedo necrosis, secretory carcinoma, mucocele-like lesion, and metastatic thyroid carcinoma. 1. DCIS with comedo necrosis: Grossly, DCIS with comedo necrosis showed an ill-defined, yellow-white, granular appearance. Comedo necrosis can be seen in the cut surface. Microscopically, dilated ducts filled with necrotic material instead of thyroid colloid-like eosinophilic secretions. 2. Secretory carcinoma: Secretory carcinoma is also known as “juvenile secretory carcinoma”. It occurs most frequently in women of childbearing age with an average onset age of 25 years old. Secretory carcinoma contains a microcystic honeycomb appearance, eosinophilic secretion and vacuolar cytoplasm, while CHC is characterized by large dilated cystoid structures; 3. mucocele-like lesion: Grossly, mucocele-like lesion is similar to CHC. It also demonstrates cystically dilated ducts containing gelatinous or mucous material. Microscopically, secretions of the lesion are pale blue, basophilic and often accompanied by gross calcification, which are not typical features of CHC. 4. Metastatic thyroid carcinoma:Metastatic follicular thyroid cancer may resemble CHC. Histological differentiation of these two lesions may require immunohistochemical staining of thyroglobulin. Negative reaction for thyroglobulin was observed in the cyst contents of our case.
Conclusion
Invasive cystic hypersecretory carcinoma is a rare subtype of breast cancer, characterized by the marked secretory activity of a thyroid colloid–like substance and cyst formation lined by pseudostratified to micropapillary epithelium along with foci of invasion. Owing to a smaller number of reported cases, little is known about the biological behavior, prognosis and molecular study of cystic hypersecretion lesions. Therefore, more cases with follow-up data are needed to reveal the biological behavior of this rare tumor.
Acknowledgements
Not applicable.
Funding
None.
Availability of data and materials
All relevant data are within the manuscript.
Abbreviations
- CHC
Cystic Hypersecretory Carcinoma
- CHH
Cystic Hypersecretory Hyperplasia
- DCIS
Ductal Carcinoma in Situ
Authors’ contributions
J S and CF W made the pathological diagnosis, as well as making a major contribution to the writing of the manuscript. X W made the pathology section and immunohistochemical staining, obtained written informed consent from the patient’s son. All authors reviewed and approved of the final manuscript.
Ethics approval and consent to participate
Ethical approval for this report was obtained from the Research Ethics Committee, Central Hospital Affiliated to Shenyang Medical College, Shenyang, China. Consent to participate was also obtained.
Consent for publication
Written informed consent for publication of this case was obtained from the patient’s son. I obtained the consent for publication from the patient’s son, rather than the patient herself because the patient had already passed away when I wrote this case report.
Competing interests
None of the authors have any competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jie Sun, Phone: +86 18002477389, Email: 1322957435@qq.com.
Xing Wang, Phone: +86 18002477403, Email: 1511441360@qq.com.
Cuifang Wang, Phone: +86 18002477400, Email: sywcf321@sohu.com.
References
- 1.Guerry P, Erlandson RA, Rosen PP. Cystic hypersecretory hyperplasia and cystic hypersecretory duct carcinoma of the breast. Cancer. 1988;61:1611–1620. doi: 10.1002/1097-0142(19880415)61:8<1611::AID-CNCR2820610819>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.Rosen PP, Scott M. Cystic hypersecretory duct carcinoma of the breast. Am J Surg Pathol. 1984;8(1):31–41. doi: 10.1097/00000478-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kim MK, Kwon GY, Gong GY. Fine needle aspiration cytology of cystic hypersecretory carcinoma of the breast. A case report. Acta Cytol. 1997;41(3):892–896. doi: 10.1159/000332724. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann ME, McClatchey KD, Siziopikou KP. Invasive cystic hypersecretory ductal carcinoma of breast: a case report and review of the literature. Arch Pathol Lab Med. 1999;123(11):1108–1110. doi: 10.1043/1543-2165-123.20.1108. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Lee YJ. Invasive cystic hypersecretory carcinoma of the breast: a case report. J Korean Med Sci. 2004;19(1):149–151. doi: 10.3346/jkms.2004.19.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin SJ, Rosen PP. Carcinoma arising from preexisting pregnancy-like and cystic hypersecretory hyperplasia lesions of the breast: a clinicopathologic study of 9 patients. Am J Surg Pathol. 2004;28(6):789–793. doi: 10.1097/01.pas.0000126060.20455.27. [DOI] [PubMed] [Google Scholar]
- 7.Skalova A, Ryska A, Kajo K, et al. Cystic hypersecretory carcinoma: rare and poorly recognized variant of intraductal carcinoma of the breast. Report of five cases. Histopathology. 2005;46(1):43–49. doi: 10.1111/j.1365-2559.2005.02055.x. [DOI] [PubMed] [Google Scholar]
- 8.Song SW, Whang IY, Chang ED. Cystic hypersecretory ductal carcinoma of the breast: a rare cause of cystic breast mass. Jpn J Radiol. 2011;29(9):660–662. doi: 10.1007/s11604-011-0601-y. [DOI] [PubMed] [Google Scholar]
- 9.Bi R, Cheng Y, Yu B, et al. Clinicopathologic features of cystic hypersecretory lesion of the breast. Zhonghua Bing Li Xue Za Zhi. 2014;43(1):25–29. [PubMed] [Google Scholar]
- 10.Gupta P, Dhingra S, Musa O, et al. Invasive cystic hypersecretory carcinoma of the breast associated with papillary pattern: a rare and poorly recognised variant of ductal carcinoma of the breast. Ecancermedcalscience. 2014;8:477. doi: 10.3332/ecancer.2014.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahoo N, Mishra P, Patra S, et al. Invasive cystic hypersecretory carcinoma of breast: a rare and under diagnosed variant of ductal carcinoma. J Clin Diagn Res. 2017;11(6):ED16–ED17. doi: 10.7860/JCDR/2017/27937.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams GD, Lacey S. Cystic hypersecretory breast carcinoma: an unusual breast cancer. Nebr Med J. 1990;75(5):104–108. [PubMed] [Google Scholar]
- 13.Chen DB, Kan X. Cystic hypersecretory carcinoma with microinvasive carcinoma and cystic hypersecretory hyperplasia of breast: report of a case. Zhonghua Bing Li Xue Za Zhi. 2010;39(1):54–55. [PubMed] [Google Scholar]
- 14.D’Alfonso TM, Ginter PS, Liu YF, et al. Cystic hypersecretory (in situ) carcinoma of the breast: a clinicopathologic and immunohistochemical characterization of 10 cases with clinical follow-up. Am J Surg Pathol. 2014;38(1):45–53. doi: 10.1097/PAS.0b013e31829fc47b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.