Abstract
Background:
Diabetes mellitus (DM) is a prime risk factor for cardiovascular disease. The convincing experimental and clinical evidence indicated that the onset of DM is closely associated with oxidative stress and that the generation of reactive oxygen species increases in both the types of diabetes. The aim of the present study was to evaluate the effect of Teucrium polium (TP) hydroalcoholic extract on the blood glucose, cholesterol, triglyceride, and oxidative stress markers of the heart and aorta in streptozotocin (STZ)-induced diabetic rats.
Methods:
The male Wistar rats assigned into six groups (n = 8 in each group): Control, diabetic, and diabetic rats treated with TP extract (100, 200, and 400 mg/kg) or met and metformin (300 mg/kg) formin (300 mg/kg) group, by daily gavage for 6 weeks. Diabetes was induced by injection of STZ (60 mg/kg, i.p). Serum lipids and glucose, malondialdehyde (MDA) level, total thiol level, and also the activities of Cu, Zn-superoxide dismutase (SOD) in the cardiac and aortic tissues were assessed.
Results:
TP extract reduced serum glucose, triglyceride and cholesterol. The MDA levels were reduced significantly in all TP-treated groups and metformin. Total thiol levels were improved in the heart and aorta of TP extract-treated groups and metformin compared to the diabetic rats. The activity of SOD in the cardiac and aortic tissues of TP extract- and metformin-treated groups was higher than diabetic group.
Conclusions:
The results showed that chronic administration of TP in STZ-induced diabetic rats could decrease blood glucose, cholesterol, and triglyceride and also attenuate the oxidative stress in the aortic and cardiac tissues.
Keywords: Aorta, diabetes mellitus, heart, oxidative stress, Teucrium polium
Introduction
Diabetes mellitus (DM) is a risk factor for cardiovascular disease. Mounting evidence has established the role of free radicals and oxidative stress in pathogenesis and the development of DM and its associated complications, including retinopathy, nephropathy, neuropathy, accelerated coronary artery disease, and heart failure.[1] Although reactive oxygen species (ROS) are generated under physiological conditions and are known mediators of intracellular signaling cascades, excessive ROS formation results in oxidative stress and could cause oxidative damage to biological macromolecules, especially the plasma membrane.[2] A variety of mechanisms are considered to be responsible for oxidative stress in diabetes including overproduction of oxygen radicals from glucose autoxidation,[3] glycated proteins,[4] and glycation of antioxidative enzymes, which restrict their capacity to detoxify oxygen radicals.[5] There is growing acknowledgment of the potential role for nutraceuticals to diminish health risks and improve well-being quality.
Lamiaceae or Labiatae also known as the mint family comprises about 210 genera and 7000 species. Teucrium polium L. (TP) (Lamiaceae) is a popular species of this family which is native to the Mediterranean region and the Middle East. The phytochemistry and medicinal properties of TP which are reviewed by Bahramikia and Yazdanparast[6] TP contain chemical compounds such as flavonoids (rutin, luteolin, apigenin, cirsiliol, salvigenin, and cirsiliol),[7] monoterpenes (α- and β-pinene, sabinene, and myrcene), and sesquiterpenes (germacrene D, β-caryophyllene, and spathulenol).[8,9] TP has been used in traditional medicine mainly for its antidiabetic, antipyretic, and anti-inflammatory.[10] TP has been reported to have antibacterial,[11] anticancer,[12] neuroprotective,[13] and antidiabetic[14,15] activity. TP also has cardiovascular effects such as decreasing of blood pressure[16,17] and antihyperlipidemic effects.[15] There is also surmounting evidence demonstrating the antioxidant properties of TP in vivo[14,18,19] and in vitro studies.[11,20] Thus, this study was designed to investigate the antioxidant properties of TP within the aorta and hearts of diabetic rats.
Methods
Animals and induction of diabetes
The male Wistar rats (250–280 g, 10 weeks old) were housed on a 12 h light/12 h dark cycle, under constant temperature (22°C ± 1°C) and were allowed free access to standard rat chow and drinking water. All experiments were performed under license from the Animal Experimentation Ethics Committee of Mashhad University of Medical Sciences (approval No. 900910).
Diabetes was induced by a single intraperitoneal (i.p.) injection of streptozotocin (STZ) 60 mg/kg (freshly dissolved in 10 mM sodium citrate, pH 4.5, with 0.9% NaCl). Nondiabetic control animals were received sodium citrate buffer (i.p.). Blood glucose concentrations were determined 3 days after STZ injection. Rats with blood glucose level ≥250 mg/dl following 12 h fasting were regarded as to be diabetic.[21]
Preparation of the extract
TP was purchased from local herbal shop in Mashhad, Khorasan Province, Iran and identified by botanists in the herbarium of Ferdowsi University of Mashhad. Aerial parts of the plant were milled into fine powder. The powder was soaked in hydroalcoholic solution (50% ethanol, 50% water) for 48 h at room temperature. The extract solution was subsequently filtered and subjected to evaporation under vacuum at 40°C until the solvent was evaporated. The extract was preserved at 20°C until use. The dried extract was dissolved in distilled water to obtain the doses of 100, 200, and 400 mg/kg.
Chemicals and drugs
All chemicals were of analytical grade (Merck). STZ and metformin hydrochloride were obtained from Sigma (Germany). Serum cholesterol and triglyceride concentrations were determined by Pars Azmoon kits (Tehran, Iran).
Experimental protocol
Rats were randomly assigned to six groups (n = 8 in each group): Control (C), diabetic (D), diabetic-extract (DE), and diabetic-metformin (DM). Normal saline was administered orally by daily gavage to the C and D groups, and TP extract (100, 200, and 400 mg/kg) and metformin (300 mg/kg) administered daily by gavage to DE and DM groups for 6 weeks.
Preparation and analysis of the samples
After 12 h deprivation of food but free access to water, the animals were anesthetized with ether, and then blood samples were obtained from the orbital sinus. Plasma glucose concentrations were measured in four different periods of the experiment: before STZ injection, 3 days after STZ injection (to confirm STZ had induced diabetes), 21 days, and finally 6 weeks after STZ injection (day 42) by the glucose oxidase method with a glucose analyzer (Glucometer EasyGluco, infopia Co., Korea). Serum cholesterol and triglyceride levels were measured in three different periods of the experiment: before STZ injection, 21 days, and finally 42 days by Pars Azmoon kits.
At the end of experiment, rats were sacrificed under deep anesthesia with ether ventilation. The heart ventricles and thoracic aorta were quickly excised, cleared of adhering fat and rinsed in a cool normal saline. The tissues were homogenized in 10% (w/v) homogenizing buffer (100 mmol KH2 PO4·K2 HPO4, pH 7.4, plus 0.1% (w/v) digitonin). The supernatant was obtained by the centrifugation of the homogenates in 5000 g, 4°C for 10 min. The supernatant was used for the measurement of the biochemical markers of oxidative stress.
The animals were weighed at the beginning of experiment (before induction of diabetes), 3 weeks, and 6 weeks later.
Determination of malondialdehyde
Lipid peroxidation was quantified as malondialdehyde (MDA) concentration. MDA was measured using the thiobarbituric acid reactive substances method. In brief, 1 ml of supernatant was added to 2 ml of a complex solution containing thiobarbituric acid/trichloroacetic acid/hydrochloric acid reagent, and the solution was incubated in boiling water bath for 40 min. After reaching room temperature, the solution was centrifuged at 1000 g for 10 min. The absorbance was read at 535 nm.[21] The MDA level was expressed as μmol/g of tissue.
Determination of total thiol
The thiol level was determined in cardiac and aortic tissues using a spectrophotometric method based on the use of Ellman's reagent. 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB), which reacts with the thiol group (SH), was used to determine the total thiol groups. The resulting yellow complex has a peak absorbance at 412 nm. In brief, 50 μl of supernatant of homogenized tissue was added to 1 ml Tris-ethylenediaminetetraacetic acid (EDTA) buffer (pH 8.6), and the absorbance was read at 412 nm against Tris-EDTA buffer alone (A1). Then, 20 μl of DTNB (10 mM in methanol) was add with the solution and was stored at room temperature for 15 min, and the absorbance was read again (A2). The absorbance of DTNB reagent was also read as blank (B). Total thiol was expressed as mmol/g of tissue.[21]
Assay of Cu, Zn-superoxide dismutase activity
Superoxide dismutase (SOD) activity was measured using the procedure of Madesh and Balasubramanian.[22] This colorimetric assay is based on the production of superoxide pyrogallol autoxidation. The superoxide anion can reduce the tetrazolium dye 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) to its formazan. The Cu, Zn-SOD inhibits the later reaction. Cu, Zn-SOD activity was measured at 570 nm. One unit of Cu, Zn-SOD activity was defined as the amount of enzyme causing 50% inhibition in the MTT reduction rate. The SOD activity was expressed as unit/g tissue.
Data analysis
The results are expressed as mean ± standard error of the mean (SEM). Statistical analyses were made using one-way ANOVA followed by the Tukey's test. Statistical significance was defined as P < 0.05.
Results
Effect of Teucrium polium on weight
The body weight of diabetic rats reduced significantly during the experiment (P < 0.001). The body weight of rats in TP extract- and metformin-treated groups also reduced significantly although the weight loss was lower in TP 100 group [Figure 1].
Figure 1.
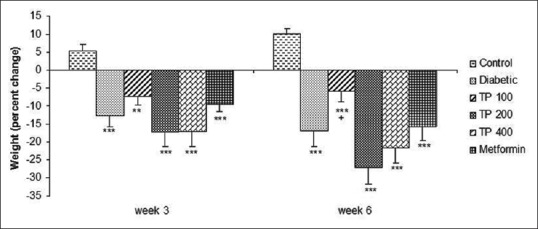
Percent weight changes in control, diabetic, diabetic-metformin, and Teucrium polium extract (TP 100, TP 200, TP 400)-treated groups. **P < 0.01, ***P < 0.001 versus control group and + P < 0.05 versus diabetic group. Statistical analyses were made using the one-way ANOVA followed by the Tukey's test. TP: Teucrium polium
Effect of Teucrium polium on plasma glucose, cholesterol, and triglyceride concentrations
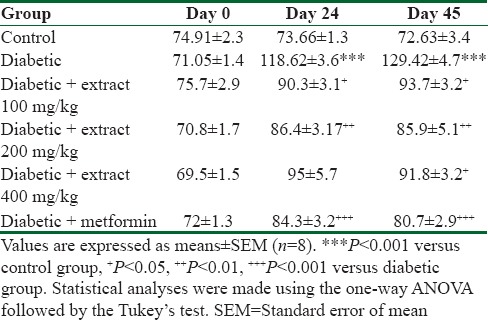
The chronic administration of TP extract reduced blood glucose levels which were significant in TP100 and TP 400 groups (P < 0.001 and P < 0.05, respectively) compared with the diabetic group [Table 1]. Metformin also reduced glucose levels compared with the diabetic group (P < 0.05).
Table 1.
The effect of Teucrium polium extract on serum glucose level (mg/dl) in streptozotocin-induced diabetic rats

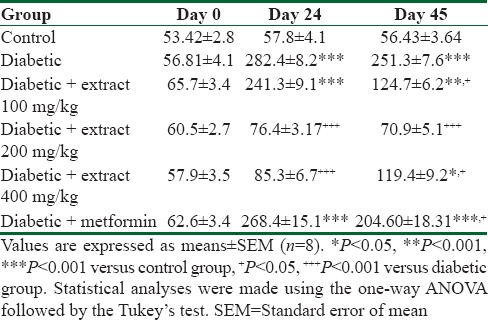
Serum cholesterol level significantly increased at 24 and 42 days in diabetic group compared with the control group (P < 0.001) [Table 2]. TP extract and metformin significantly reduced serum cholesterol level (P < 0.05 to P < 0.001) [Table 2].
Table 2.
The effect of Teucrium polium extract on serum cholesterol level (mg/dl) in streptozotocin-induced diabetic rats
The results showed significant elevation of serum triglyceride level of diabetic group compared with the control group at 21 and 42 days (P < 0.001) [Table 3]. In TP extract-treated groups, the serum triglyceride level was significantly decreased in TP 200 group at 21 and 42 days (P < 0.0001). The triglyceride level was decreased significantly in TP100 and metformin-treated groups at day 42 and in TP 400 at day 21 [Table 3].
Table 3.
The effect of Teucrium polium extract on serum triglyceride levels (mg/dl) in streptozotocin-induced diabetic rats
Effect of Teucrium polium on malondialdehyde
As Figure 2 shows in the aortic and cardiac tissues of diabetic rats, MDA level was increased considerably compared with the control group (P < 0.001). The chronic administration of TP extract and metformin significantly reduced the MDA level in the cardiac and aortic tissues (P < 0.01 to P < 0.001) [Figure 2].
Figure 2.
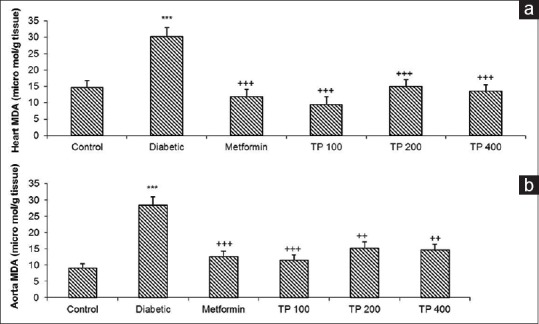
The malondialdehyde level in the cardiac (a) and aortic (b) tissues. Values are expressed as means ± standard error of the mean (n = 8). ***P < 0.001 versus control group and ++P < 0.001, +++P < 0.001 versus diabetic group. Statistical analyses were made using the one-way ANOVA followed by the Tukey's test. TP: Teucrium polium
Effect of Teucrium polium on total thiol
Total thiol levels were decreased in the aortic and cardiac tissues of diabetic rats compared with the control group (P < 0.01) while in TP extract- and metformin-treated groups, total thiol level increased in aortic and cardiac tissues (P < 0.05 to P < 0.01) [Figure 3].
Figure 3.
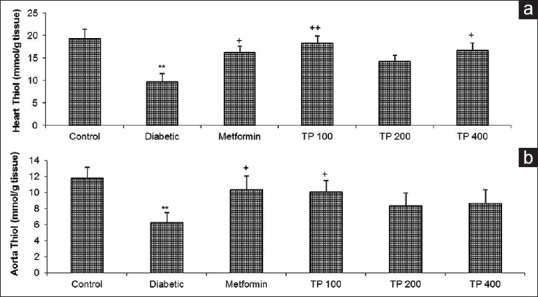
The total thiol level in the cardiac (a) and aortic (b) tissues in control, diabetic, diabetic-metformin, and Teucrium polium extract (TP 100, TP 200, TP 400)-treated groups. Values are expressed as means ± standard error of the mean (n = 8). +P < 0.05, ++P < 0.01 versus diabetic group and **P < 0.01 versus control group. Statistical analyses were made using the one-way ANOVA followed by the Tukey's test. TP: Teucrium polium
Effect of Teucrium polium on Cu, Zn-superoxide dismutase activity
The activity of Cu, Zn-SOD was decreased in diabetic group compared to the control group (P < 0.01) while TP extract- and metformin-treated groups demonstrated a significant increase in SOD activity in the aortic and cardiac tissues (P < 0.05 to P < 0.01) [Figure 4].
Figure 4.
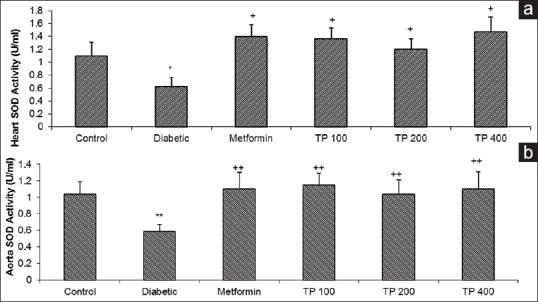
The superoxide dismutase activity (U/100 mg tissue) of the cardiac (a) and aortic (b) tissues in control, diabetic, diabetic-metformin, and Teucrium polium extract (TP 100, TP 200, TP 400)-treated groups. Values are expressed as means ± standard error of the mean (n = 8). +P < 0.05, ++P < 0.01 versus diabetic group and *P < 0.05, **P < 0.01 versus control group. Statistical analyses were made using the one-way NOVA followed by the Tukey's test. TP: Teucrium polium
Discussion
The results of this study indicate that chronic administration of TP extract in STZ-induced diabetic rats has hypoglycemic effect. All doses of TP extract reduced blood glucose, but this reduction was significant for 100 and 400 mg/kg doses although no significant differences between 200 and 400 mg/kg doses were seen. Previous studies also reported the hypoglycemic effect of TP.[14,15] Hyperglycemia causes tissue damage through five major mechanisms: increased flux of glucose and other sugars through the polyol pathway, increased intracellular formation of advanced glycation end-products (AGEs), increased expression of the receptor for AGEs and its activating ligands, activation of protein kinase C isoforms and over activity of the hexosamine pathway. Several lines of evidence indicate that all five mechanisms are activated by a single upstream event: mitochondrial overproduction of ROS.[2]
The data of this study have shown that total thiol significantly decreased in the heart and aorta of diabetic rats while the chronic administration of TP extract increased total thiol level in the aortic and cardiac tissues of diabetic rats. Total thiol groups are very susceptible to oxidation and considered as one of the most important body sacrificial antioxidants. When the cells are exposed to oxidative stress, thiol groups are the first antioxidants that are consumed.[23] Thus, the increase of total thiol in TP- and metformin-treated groups indicate the improvement of antioxidant status. Previous studies also showed total thiol reduced in type 1 diabetes.[21,24]
Our results showed MDA level, as a lipid peroxidation marker, increased in diabetic rats and chronic administration of TP extract reduced the MDA level in the heart and aorta of diabetic rats. MDA has been documented as a primary biomarker of free radical-mediated lipid damage and oxidative stress. Increased the level of MDA in diabetes suggests that peroxidative injury may be involved in the development of diabetic complications. The increase in lipid peroxidation is also an indication of decline in defense mechanisms of enzymatic and nonenzymatic antioxidants.[25] In the present study, the SOD activity reduced in the heart and aorta of diabetic rats. TP extract increased SOD activity in the heart and aorta of diabetic rats. SOD plays important protective roles against cellular and histological damages that are produced by ROS. Decline in the level of SOD in diabetic tissue and blood has been reported in many studies.[5,26] Ardestani et al. reported chronic administration of TP extract in STZ-induced diabetic rats increased GSH level and SOD activity in the pancreatic tissue while MDA level decreased.[18] Mousavi et al. also showed TP extract reduced MDA level in brain of diabetic rats,[27] so regarding above-mentioned former studies, it could be concluded that our results are another confirmation for the preventive antioxidant role of TP extract in this model of DM.
In general, our results showed that TP hydroalcoholic extract significantly reduced blood glucose and lipids and also ameliorated the oxidative stress in the cardiac and aortic tissues of diabetic rats.
Financial support and sponsorship
This study was financially supported by the Research Affairs of Mashhad University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the Research Affairs of Mashhad University of Medical Sciences for their financial support.
References
- 1.Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016;118:1771–85. doi: 10.1161/CIRCRESAHA.115.306884. [DOI] [PubMed] [Google Scholar]
- 2.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB, et al. Impact of diabetes on cardiovascular disease: An update. Int J Hypertens 2013. 2013 doi: 10.1155/2013/653789. 653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T. Advanced glycation end products: A molecular target for vascular complications in diabetes. Mol Med. 2015;21(Suppl 1):S32–40. doi: 10.2119/molmed.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita H, Fujishima H, Chida S, Takahashi K, Qi Z, Kanetsuna Y, et al. Reduction of renal superoxide dismutase in progressive diabetic nephropathy. J Am Soc Nephrol. 2009;20:1303–13. doi: 10.1681/ASN.2008080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahramikia S, Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L.Lamiaceae. Phytother Res. 2012;26:1581–93. doi: 10.1002/ptr.4617. [DOI] [PubMed] [Google Scholar]
- 7.Rizk AM, Hammouda FM, Rimpler H, Kamel A. Iridoids and flavonoids of Teucrium polium herb. Planta Med. 1986;2:87–8. [PubMed] [Google Scholar]
- 8.De Martino L, Formisano C, Mancini E, De Feo V, Piozzi F, Rigano D, et al. Chemical composition and phytotoxic effects of essential oils from four Teucrium species. Nat Prod Commun. 2010;5:1969–76. [PubMed] [Google Scholar]
- 9.Vokou D, Bessiere JM. Volatile constituents of Teucrium polium. J Nat Prod. 1985;48:498–9. [Google Scholar]
- 10.Mir Heidar H. Encyclopedia of Medicinal Plant of Iran. 5th ed. Tehran: Islamic Culture Press; 2004. pp. 220–2. [Google Scholar]
- 11.Samec D, Gruz J, Strnad M, Kremer D, Kosalec I, Grubesic RJ, et al. Antioxidant and antimicrobial properties of Teucrium arduini L. (Lamiaceae) flower and leaf infusions (Teucrium arduini L. antioxidant capacity) Food Chem Toxicol. 2010;48:113–9. doi: 10.1016/j.fct.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Movahedi A, Basir R, Rahmat A, Charaffedine M, Othman F. Remarkable anticancer activity of Teucrium polium on hepatocellular carcinogenic rats. Evid Based Complement Alternat Med 2014. 2014 doi: 10.1155/2014/726724. 726724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonyan KV, Chavushyan VA. Protective effects of hydroponic Teucrium polium on hippocampal neurodegeneration in ovariectomized rats. BMC Complement Altern Med. 2016;16:415. doi: 10.1186/s12906-016-1407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esmaeili MA, Zohari F, Sadeghi H. Antioxidant and protective effects of major flavonoids from Teucrium polium on beta-cell destruction in a model of streptozotocin-induced diabetes. Planta Med. 2009;75:1418–20. doi: 10.1055/s-0029-1185704. [DOI] [PubMed] [Google Scholar]
- 15.Stefkov G, Kulevanova S, Miova B, Dinevska-Kjovkarovska S, Mølgaard P, Jäger AK, et al. Effects of Teucrium polium spp.capitatum flavonoids on the lipid and carbohydrate metabolism in rats. Pharm Biol. 2011;49:885–92. doi: 10.3109/13880209.2011.552187. [DOI] [PubMed] [Google Scholar]
- 16.Mahmoudabady M, Shafei MN, Niazmand S, Khodaee E. The effects of hydroalchoholic extract of Teucrium polium L.on hypertension induced by angiotensin II in rats. Int J Prev Med. 2014;5:1255–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Niazmand S, Esparham M, Hassannia T, Derakhshan M. Cardiovascular effects of Teucrium polium L.extract in rabbit. Pharmacogn Mag. 2011;7:260–4. doi: 10.4103/0973-1296.84244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardestani A, Yazdanparast R, Jamshidi SH. Therapeutic effects of Teucrium polium extract on oxidative stress in pancreas of streptozotocin-induced diabetic rats. J Med Food. 2008;11:525–32. doi: 10.1089/jmf.2006.0230. [DOI] [PubMed] [Google Scholar]
- 19.Hasani P, Yasa N, Vosough-Ghanbari S, Mohammadirad A, Dehghan G, Abdollahi M. In vivo antioxidant potential of Teucrium polium, as compared to alpha-tocopherol. Acta Pharm. 2007;57:123–9. doi: 10.2478/v10007-007-0010-z. [DOI] [PubMed] [Google Scholar]
- 20.Ljubuncic P, Dakwar S, Portnaya I, Cogan U, Azaizeh H, Bomzon A, et al. Aqueous extracts of Teucrium polium possess remarkable antioxidant activity in vitro . Evid Based Complement Alternat Med. 2006;3:329–38. doi: 10.1093/ecam/nel028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbasnezhad A, Niazmand S, Mahmoudabady M, Soukhtanloo M, Abdolrahim Rezaee S, Mojtaba Mousavi S. Nigella sativa improve redox homeostasis in heart and aorta of diabetic rat. Curr Nutr Food Sci. 2016;12:35–41. [Google Scholar]
- 22.Madesh M, Balasubramanian KA. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35:184–8. [PubMed] [Google Scholar]
- 23.Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 24.Ates I, Kaplan M, Yuksel M, Mese D, Alisik M, Erel Ö, et al. Determination of thiol/disulphide homeostasis in type 1 diabetes mellitus and the factors associated with thiol oxidation. Endocrine. 2016;51:47–51. doi: 10.1007/s12020-015-0784-6. [DOI] [PubMed] [Google Scholar]
- 25.Davì G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–68. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 26.He K, Li X, Chen X, Ye X, Huang J, Jin Y, et al. Evaluation of antidiabetic potential of selected traditional chinese medicines in STZ-induced diabetic mice. J Ethnopharmacol. 2011;137:1135–42. doi: 10.1016/j.jep.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Mousavi SM, Niazmand S, Hosseini M, Hassanzadeh Z, Sadeghnia HR, Vafaee F, et al. Beneficial effects of Teucrium polium and metformin on diabetes-induced memory impairments and brain tissue oxidative damage in rats. Int J Alzheimers Dis 2015. 2015:493729. doi: 10.1155/2015/493729. [DOI] [PMC free article] [PubMed] [Google Scholar]


