Abstract
Background:
L-arginine is an important precursor for the formation of nitric oxide (NO). According to previous studies, NO function is related to gender. Likewise, chronic renal diseases have lower prevalence in female. Gentamicin (GM) is an aminoglycoside antibiotic. According to some studies, males are more sensitive to GM renal nephrotoxicity. This study attempts to find protective effects of L-arginine on GM nephrotoxicity in male and female rats.
Methods:
Male and female rats were divided into eight groups: Rats were randomly assigned to 8 groups each including both male and female rats. The first and second groups received vehicle (saline), the third and fourth groups received gentamicin (80 mg/kg), the fifth and sixth groups received L-arginine (150 mg/kg), and finally, seventh and eighth groups received gentamicin+ L- arginine. Next, 9 days after administering drugs, blood samples were collected from the heart. After making sacrifices, the level of blood urea, creatinine (Cr), nitrite, and malondialdehyde (MDA) was measured in serums. Likewise, nitrite and MDA were measured in the homogenized kidney tissue.
Results:
GM significantly increased serum level of urea and Cr in male and female rats (P < 0.05). However, co-administration of GM + L-arginine significantly did not decrease urea and Cr level in male rats, whereas, in female rats, they significantly reduced (P < 0.05). In response to GM, renal MDA level increased in male and female rats (P < 0.05), and in the presence of GM + L-arginine, the level of MDA significantly decreased in both genders (P < 0.05).
Conclusions:
L-arginine demonstrated some protective effects in female rats but did not protect against GM nephrotoxicity in male rats for unknown reasons, probably related to the effects of sex hormones which needs further studies to be confirmed.
Keywords: Gentamicin, L-arginine, Nephrotoxicity, Sex difference
Introduction
L-arginine as an amino acid is an important precursor for the formation of nitric oxide (NO).[1,2] NO is a soluble gas. This gas plays important roles in endothelial functions.[3] According to previous studies, NO function is related to gender.[4] It was reported that L-arginine has some protective roles against gentamicin (GM) nephrotoxicity;[5] however, the role of gender is not clear. Some reports indicate that chronic renal diseases have lower prevalence in female.[6,7,8] On the other hand, they have a lower risk for renal end-stage diseases.[6,7,8] NO is a potent vasodilator factor in kidney and female sex hormones increase NO production.[9,10,11] GM is an aminoglycoside antibiotic.[12,13] It is an effective drug against Gram-negative infections.[13,14,15] Its clinical application is limited because of renal nephrotoxicity in about 15%–30% of patients.[16,17] The exact impact of gender on GM nephrotoxicity is not well known. There are reports that male rats are more sensitive to GM nephrotoxicity.[18,19] Human studies indicate that the prevalence of GM nephrotoxicity in men is more than that of women.[19] Moreover, NO has some pivotal roles in endothelial functions, and L-arginine as an endogenous donor has also important roles in endothelial function.[20,21] Based on these facts, the aim of this study was finding protective effects of L-arginine on GM nephrotoxicity in male and female rats.
Methods
Animals
Sixty adult female rats (weight: 162.0 ± 4.1 g) and male rats (weight: 184.4 ± 7.2 g) were the subjects of this study. In this research, the Wistar rats were used from the Animal Centre of Zahedan University of Medical Sciences. The rats were housed at a temperature of 23°C–25°C. The rats had free access to water and rat chow. The rats were acclimatized to this diet for at least 1 week before the experiment. The experimental procedure was approved in advance by the Zahedan University Medical Sciences Ethics Committee.
In accordance with the experimental protocol, the rats were randomly assigned to eight groups each including both male and female rats. The first and second groups received vehicle (saline) (n = 9). Similarly, the third and the fourth groups (n = 6–8) received a regular dose of gentamicin (GM) (80 mg/kg) for 9 days.[22] The fifth and the sixth groups (n = 7) received a continuous dose of L-arginine (150 mg/kg, ip)[23] for 9 days. The seventh and the eighth groups (n = 6–8) received a dose of GM (80 mg/kg) + L-arginine (150 mg/kg, ip) constantly for 9 days.
All animals were sacrificed 9 days after GM administration, and blood samples were taken from each animal. The levels of blood urea, creatinine (Cr), malondialdehyde (MDA), and nitrite were measured. During the study, the weights of rats were recorded daily. At the end of the experiment, their left kidneys were subjected to histopathological investigations, and their right kidneys were homogenized and centrifuged to determine the supernatant MDA and nitrite levels. GM was obtained from Caspian Company in Iran, and L-arginine was bought from Fluka-Garatie.
Measurements
The levels of serum urea and Cr were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The level of nitrite in serum and supernatant (stable NO metabolite) was measured using a colorimetric assay kit (Zelbio, Germany) that involves the Griess reaction.
MDA levels of serum and the supernatant from the homogenized tissue were quantified according to the manual methodology. Briefly, 500 μL of the sample was mixed with 1000 μL 10% trichloroacetic acid. The mixture was vigorously shaken and centrifuged at 2000 g for 10 min; 500 μL of the supernatant was added to 500 μL 0.67% thiobarbituric acid. The solution was then incubated in hot water bath at the temperature of 100°C for 10 min. After cooling, the absorbance was measured at 532 nm. The concentration of MDA was reported as μmol/L for the serum and as nanomol/100g of tissue for kidney.[22]
The removed kidney was fixed in 10% formalin solution, embedded in paraffin for histopathological staining. The hematoxylin and eosin stains were applied to examine the tubular damage. The presence of acute tubular injuries such as tubular dilation and simplification, tubular cells swelling, necrosis, tubular casts, and intraluminal cell debris with inflammatory cells infiltration were considered. As mentioned above, based on the intensity of tubular lesions, they were scored from 0 to 4, while the score of zero was assigned to the normal tissue without damage.[22] The assessment was conducted by two pathologists blindly. The pathologic changes of the kidneys were recorded using a grading scale of 0–4 which was based on subjective impression of the extent of cortical changes as follows:
0 = indistinguishable from control
1 = minimal, ≤25% cortex affected
2 = Mild > 25% and ≤ 50% cortex affected
3 = Moderate > 50% and ≤ 75% cortex affected
4 = Sever ≥ 75% cortex affected.
This grading scale is adapted from Goering and coworkers[24] with minor modification
Statistical analysis
Data are expressed as mean ± SEM. The levels of urea, Cr, MDA, and SOD and kidney weights were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey test. These parameters were compared in each group between male and female by t-Student test. The groups were compared by the Kruskal-Wallis or Mann-Whitney U tests with regard to the KTDS. P values less than 0.05 were considered statistically significant using SPSS version 16 (Chicago, IL, USA) for the data.
Results
The results will be discussed under three subsections including (1) the effects of GM on kidney weight, (2) the effects of GM on serum urea, Cr, nitrite, and MDA level and KTDS, and (3) the effects of GM on kidney nitrite and MDA level.
Effect of gentamicin on kidney weight
In both genders, the use of GM significantly increased kidney weight (P < 0.05), but co-administration of L-arginine did not have any effects on kidney weight [Figure 1].
Figure 1.
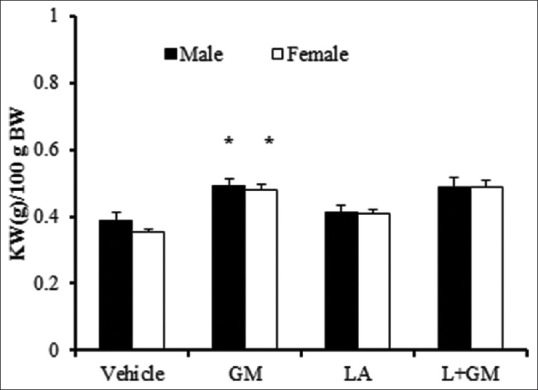
Kidney weight per 100 gram of body weight (KW/100 (g) BW). The groups received, Vehicle, Gentamicin 80 mg/kg(GM), L-arginine 150 mg/kg (LA) and L-arginine +Gentamicin (L+GM) for 9 days; *Indicate significant difference from vehicle group in the same gender (P < 0.05)
Effect of gentamicin on serum urea, creatinine, nitrite, and malondialdehyde level and kidney tissue damage score
The present study showed that the administration of GM significantly increased serum level of urea and Cr in male and female rats similarly (P < 0.05). However, co-administration of GM and L-arginine in male gender, contrary to the expectation, did not decrease urea nor Cr level. Similarly, this co-administration increased urea and Cr levels significantly in comparison with GM group in male rats (P < 0.05). On the other hand, L-arginine attenuates urea and Cr level in female rats when compared with GM group at the significant level (P < 0.05). These findings are compatible with the renal pathologic examination [Figures 2–4]. In female rats, L-arginine has a stronger effect in reducing urea and Cr levels.
Figure 2.
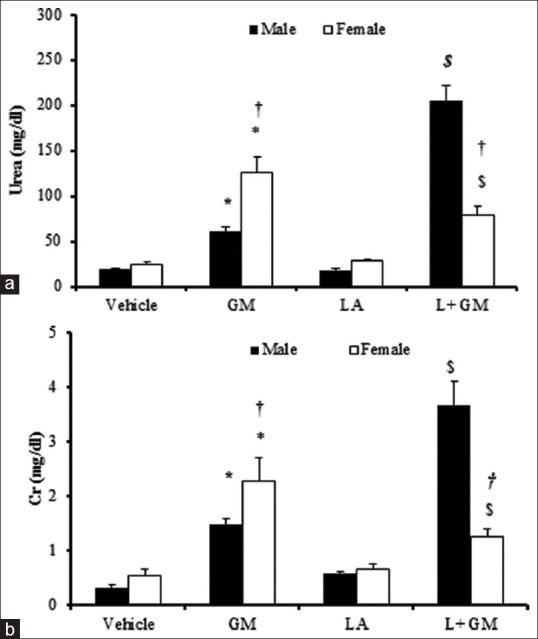
Blood urea (a) and Cr(b). The groups received, Vehicle, Gentamicin 80 mg/kg(GM), L-arginine 150 mg/kg (LA) and L-arginine +Gentamicin (L+GM) for 9 days. The symbols indicate significant difference;* from vehicle group, $ from GM group in the same gender (P <0.05). The symbol † indicates significant difference from male rats in the same group (P <0.05)
Figure 4.
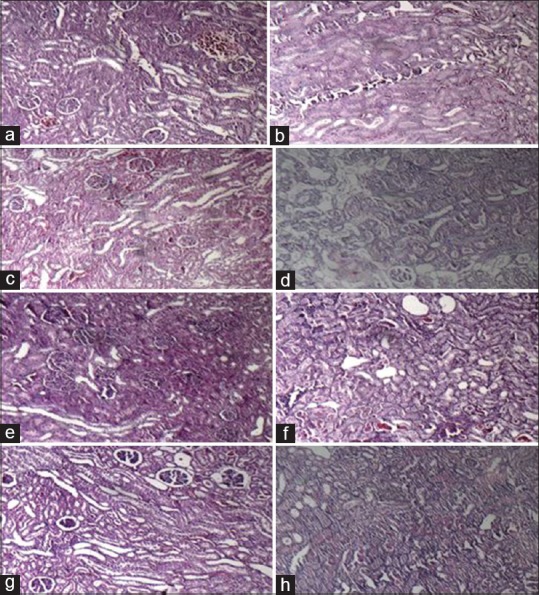
The pathology images (magnification: 100X) of kidney tissue in experimental groups. a-d, male and e-h, female rats, the groups received Vehicle, Gentamicin 80 mg/kg, L-arginine (150mg/kg) and L-arginine+ gentamicin for 9 days, respectively
Administration of GM significantly increased nitrite serum level in male rat and co- treatment with L-arginine did not have any effect on serum nitrite level. Evaluation of MDA level showed no meaningful differences between the groups in both sexes [Table 1]. The administration of GM induced renal damage in male and female rats although intensity of the damage was lower in male rats than female ones. The simultaneous presence of L-arginine with GM, unexpectedly and significantly, increased kidney damage (P < 0.05). However, the intensity of the damage was lower in female rats although the level of kidney damage was not significant in comparison with GM group [Figure 3].
Table 1.
The nitrite and malondialdehyde serum level in each group
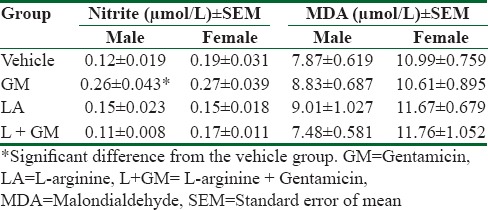
Figure 3.
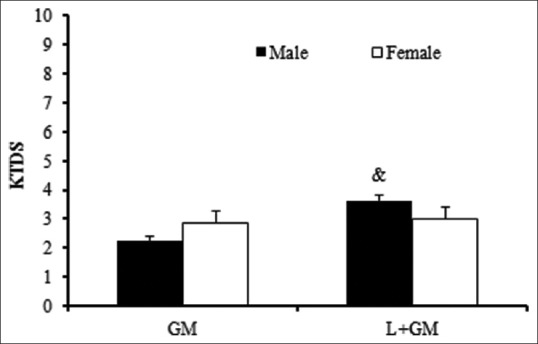
Kidney tissue damage score (KTDS), The groups received, Gentamicin 80 mg/kg(GM) and L-arginine +Gentamicin (L+GM) for 9 days; & indicate significant difference from GM group in the same gender (P < 0.05)
Effect of gentamicin on kidney nitrite and malondialdehyde level
GM significantly decreased nitrite level in male and female rats compared to vehicle group (P < 0.05). On the other hand, co-administration of L-arginine does not have any significant effect on kidney nitrite level in both gender [Figure 5a].
Figure 5.
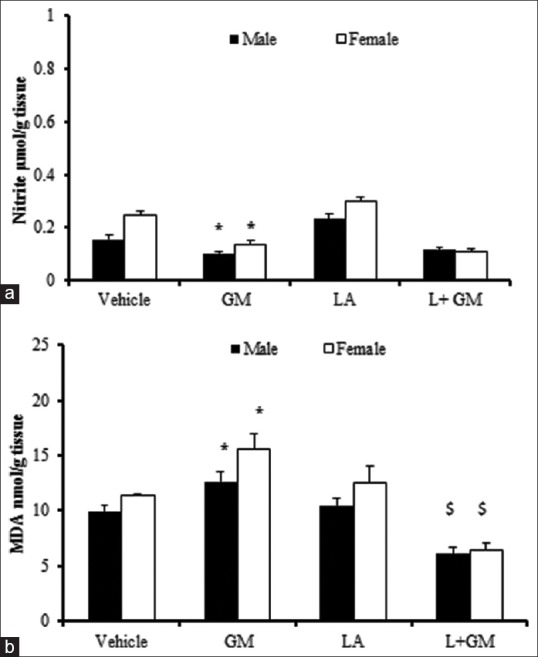
Kidney level of nitrite(a) and MDA(b). The groups received, Vehicle, Gentamicin 80 mg/kg(GM), L-arginine 150 mg/kg (LA) and L-arginine +Gentamicin (L+GM) for 9 days;. The symbols indicate significant difference; * from vehicle group, $ from GM group in the same gender (P<0.05).
According to the result of this study, treatment with GM significantly increased MDA level in both genders (P < 0.05) and co-treatment with L-arginine significantly decreased significantly, in both sexes [Figure 5b].
Discussion
Here, the most noteworthy findings of the study are presented in the order of importance. The most vital finding was the gender-related difference in the protective role of L-arginine in GM-induced nephrotoxicity. The administration of GM increased serum level of urea and Cr in male and female rats, but treatment with L-arginine only in female rats showed attenuation of the effect of GM. In this study, after the administration of GM, serum level of nitrite increased in both genders, whereas the kidney level reduced. Concurrently, L-arginine with GM had no effect on serum level of nitrite. On the contrary, kidney level of nitrite significantly decreased in female rats. Treatment with GM significantly increased MDA level in both genders, and the co-administration of GM and L-arginine could significantly decrease MDA level in both sexes.
In fact, the role of NO in renal function is controversial. In this regard, our findings are compatible with Christo et al.'s report who found that after 10 days of GM administration, serum Cr and urea level increased. In the same way, nitrite serum level increased and its urinary level reduced.[25] However, in contrast with our findings, two studies showed that the protective properties of L-arginine have been observed in male albino rats in GM-induced renal failure.[5,26] Similarly, Schneider et al. showed that the administration of L-arginine improved the expression of NO and recovery phase ischemic acute renal failure in males gender.[27] Another study discovered that, in the presence of GM, nitrite production increases, and co-incubation mesangial cells with L-NAME decreases its level.[28] Other studies have demonstrated that NO and its metabolite, for example, peroxynitrite, probably has a substantial role in kidney damage in vitro[29,30] and in vivo.[31,32]
Our findings in female gender are approved by other studies which show that either L-arginine or exogenous NO donors attenuate ischemia-induced tubular injury in vivo.[33,34]
On the other hand, there are conflicting results about the effects of gender on GM-induced nephrotoxicity. Some studies on animals and human beings have reported controversial results about the effects of gender on GM nephrotoxicity. For example, male Fisher 344 rats are more sensitive to GM nephrotoxicity than female rats.[35] In contrast, among isolated perfused rats, kidney of the Fisher 344 strain, no significant difference was found between male and female rats.[36] Interestingly, gender differences of GM or tobramycin nephrotoxicity were not observed in Sprague-Dawley (SD) rats.[35,37] However, Ali et al. reported that there were partial sex-related differences in SD rats in response to GM and the administration of testosterone and estradiol did not significantly alter the nephrotoxicity.[38] In human studies, it was reported that induced GM nephrotoxicity is observed more among females than males.[19] Other studies have demonstrated that men are more susceptible to the morphologic and functional effects of GM nephrotoxicity than women.[18] Investigations performed by Sweileh have shown that gender differences did not exist with GM but amikacin-induced nephrotoxicity which is, in its own turn, a sex-related phenomenon.[39]
The main mechanism influencing gender differences is not clearly known yet. However, the differences in sex hormones and pharmacokinetics are involved. On the other hand, there are reports that testosterone is not involved in GM nephrotoxicity but estrogen exacerbates.[40] Sex hormones may be engaged in this dissimilarity caused by differences of NO synthase expression or the role of NO on the renal system.[41,42] The production of NO and activity of NO synthase enzyme are increased by estradiol.[43] In turn, estradiol enhances the release of NO among females more than males.[44,45] Reckelhoff et al. found that although levels of endothelial nitric oxide synthase messenger RNA and protein are higher in females than in males. On the other hand, in males, the renal vasculature is more responsive to NO synthase inhibition. Therefore, they suggest that renal vascular is probably more dependent on NO in male than in female rats.[46] In this study, after the administration of GM, the higher injuries in male rats may be related to more vascular responses in these animals.
In addition, it was suggested that extrarenal factors are involved in GM nephrotoxicity.[36] Hepatic metabolism may have a role in aminoglycosides nephrotoxicity and gender differences in this metabolism.[47] These findings suggest that renal membrane-binding affinity drugs, for example, GM, are closely linked to the nephrotoxic potential. In this way, several correlations have been mentioned between renal brush border membrane-binding affinity of aminoglycoside and aminoglycoside nephrotoxicity, including the greater binding affinity in male versus female rats.[48] Therefore, the results support other findings in the literature who have previously reported the higher susceptibility of nephrotoxicity in males. In sum, further investigations are recommended to study more detailed impacts of gender on GM-induced nephrotoxicity.
Conclusions
The results of this study confirmed that L-arginine has some protective effects on GM-induced nephrotoxicity in female rats. This phenomenon may probably be interrelated to the effects of sex hormones. However, its mechanisms need to be further investigated to achieve more accurate findings.
Financial support and sponsorship
The research was supported by Zahedan University of Medical Sciences (grant no. 6965).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Mohamad Hashemi of the Department of Biochemistry for his help in the biochemistry study in this work. The research was supported by Zahedan University of Medical Sciences (grant no. 6965).
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Palmer R, Higgs EA. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension. 1988;12:365–72. doi: 10.1161/01.hyp.12.4.365. [DOI] [PubMed] [Google Scholar]
- 3.Palmer RM, Ashton D, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 4.Huang A, Sun D, Koller A, Kaley G. Gender difference in myogenic tone of rat arterioles is due to estrogen-induced, enhanced release of NO. American J Physiology-Heart and Circulatory Physiology. 1997;272:H1804–H9. doi: 10.1152/ajpheart.1997.272.4.H1804. [DOI] [PubMed] [Google Scholar]
- 5.Can C, Şen S, Boztok N, Tuğlular I. Protective effect of oral L-arginine administration on gentamicin-induced renal failure in rats. European J Pharmacology. 2000;390:327–34. doi: 10.1016/s0014-2999(00)00025-x. [DOI] [PubMed] [Google Scholar]
- 6.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. American Journal of Kidney Diseases. 1995;25:515–33. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 7.Coggins CH, Lewis JB, Caggiula AW, Castaldo LS, Klahr S, Wang S-R. Differences between women and men with chronic renal disease. Nephrology Dialysis Transplantation. 1998;13:1430–7. doi: 10.1093/ndt/13.6.1430. [DOI] [PubMed] [Google Scholar]
- 8.Seliger SL, Davis C, Stehman-Breen C. Gender and the progression of renal disease. Current Opinion in Nephrology and Hypertension. 2001;10:219–25. doi: 10.1097/00041552-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2004;286:R233–R49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 10.Knot HJ, Lounsbury KM, Brayden JE, Nelson MT. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. American J Physiology-Heart and Circulatory Physiology. 1999;276:H961–H9. doi: 10.1152/ajpheart.1999.276.3.H961. [DOI] [PubMed] [Google Scholar]
- 11.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. American Journal of Physiology-Heart and Circulatory Physiology. 1997;272:H2765–H73. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein M, Wagman G, Oden E, Marquez J. Biological activity of the antibiotic components of the gentamicin complex. Journal of Bacteriology. 1967;94:789. doi: 10.1128/jb.94.3.789-790.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagabhushan TL, Turner WN, Daniels PJ, Morton JB. Gentamicin antibiotics. 7. Structures of the gentamicin antibiotics A1, A3, and A4. The Journal of Organic Chemistry. 1975;40:2830–4. doi: 10.1021/jo00907a028. [DOI] [PubMed] [Google Scholar]
- 14.Prat V, Bohuslav V, Hatala M. Treatment with a single daily dose of gentamicin in urinary tract infection in relation to the site of infection. Infection. 1978;6:29–31. doi: 10.1007/BF01641088. [DOI] [PubMed] [Google Scholar]
- 15.Regis D, Sandri A, Samaila E, Benini A, Bondi M, Magnan B. Release of gentamicin and vancomycin from preformed spacers in infected total hip arthroplasties: Measurement of concentrations and inhibitory activity in patients’ drainage fluids and serum. The Scientific World J. 2013;2013:752184. doi: 10.1155/2013/752184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mingeot-Leclercq M-P, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrobial Agents and Chemotherapy. 1999;43:1003–12. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hariprasad G, Kumar M, Rani K, Kaur P, Srinivasan A. Aminoglycoside induced nephrotoxicity: Molecular modeling studies of calreticulin-gentamicin complex. J Molecular Modeling. 2012;18:2645–52. doi: 10.1007/s00894-011-1289-8. [DOI] [PubMed] [Google Scholar]
- 18.Bertino JS, Booker LA, Franck PA, Jenkins PL, Franck KR, Nafziger AN. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infectious Diseases. 1993;167:173–9. doi: 10.1093/infdis/167.1.173. [DOI] [PubMed] [Google Scholar]
- 19.Moor RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Internal Med. 1984;100:352–7. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- 20.Ignarro LJ, Napoli C, Loscalzo J. Nitric Oxide Donors and Cardiovascular Agents Modulating the Bioactivity of Nitric Oxide An Overview. Circulation Research. 2002;90:21–8. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- 21.Sakuma I, Stuehr DJ, Gross SS, Nathan C, Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proceedings of the National Academy of Sciences. 1988;85:8664–7. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadeghi F, Nematbakhsh M, Noori-Diziche A, Eshraghi-Jazi F, Talebi A, Nasri H, et al. Protective effect of pomegranate flower extract against gentamicin-induced renal toxicity in male rats. J Renal Injury Prevention. 2015;4:45. doi: 10.12861/jrip.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohta F, Takagi T, Sato H, Ignarro LJ. Low-dose L-arginine administration increases microperfusion of hindlimb muscle without affecting blood pressure in rats. Proceedings of the National Academy of Sciences. 2007;104:1407–11. doi: 10.1073/pnas.0610207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goering PL, Fisher BR, Noren BT, Papaconstantinou A, Rojko JL, Marler RJ. Mercury induces regional and cell-specific stress protein expression in rat kidney. Toxicological Sci. 2000;53:447–57. doi: 10.1093/toxsci/53.2.447. [DOI] [PubMed] [Google Scholar]
- 25.Christo JS, Rodrigues AM, Mouro MG, Cenedeze MA, de Jesus Simões M, Schor N, et al. Nitric oxide (NO) is associated with gentamicin (GENTA) nephrotoxicity and the renal function recovery after suspension of GENTA treatment in rats. Nitric Oxide. 2011;24:77–83. doi: 10.1016/j.niox.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Bidadkosh A, Derakhshanfar A, Rastegar AM, Yazdani S. Antioxidant preserving effects of l-arginine at reducing the hemodynamic toxicity of gentamicin-induced rat nephrotoxicity: Pathological and biochemical findings. Comparative Clinical Pathology. 2012;21:1739–44. [Google Scholar]
- 27.Schneider R, Raff U, Vornberger N, Schmidt M, Freund R, Reber M, et al. L-Arginine counteracts nitric oxide deficiency and improves the recovery phase of ischemic acute renal failure in rats. Kidney International. 2003;64:216–25. doi: 10.1046/j.1523-1755.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 28.Rivas-Cabanero L, Rodriguez-Lopez A, Martinez-Salgado C, Saura M, Lamas S, Lopez-Novoa J. Gentamicin treatment increases mesangial cell nitric oxide production. Experimental Nephrology. 1996;5:23–30. [PubMed] [Google Scholar]
- 29.Yu L, Gengaro PE, Niederberger M, Burke TJ, Schrier RW. Nitric oxide: A mediator in rat tubular hypoxia/reoxygenation injury. Proceedings of the National Academy of Sciences. 1994;91:1691–5. doi: 10.1073/pnas.91.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wangsiripaisan A, Gengaro PE, Nemenoff RA, Ling H, Edelstein CL, Schrier RW. Effect of nitric oxide donors on renal tubular epithelial cell-matrix adhesion. Kidney International. 1999;55:2281–8. doi: 10.1046/j.1523-1755.1999.00484.x. [DOI] [PubMed] [Google Scholar]
- 31.Yanagisawa H, Nodera M, Umemori Y, Shimoguchi Y, Wada O. Role of angiotensin II, endothelin-1, and nitric oxide in HgCl 2-induced acute renal failure. Toxicology and Applied Pharmacology. 1998;152:315–26. doi: 10.1006/taap.1998.8459. [DOI] [PubMed] [Google Scholar]
- 32.Noiri E, Peresleni T, Miller F, Goligorsky MS. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clinical Investigation. 1996;97:2377. doi: 10.1172/JCI118681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumura Y, Nishiura M, Deguchi S, Hashimoto N, Ogawa T, Seo R. Protective effect of FK409, a spontaneous nitric oxide releaser, on ischemic acute renal failure in rats. Journal of Pharmacology and Experimental Therapeutics. 1998;287:1084–91. [PubMed] [Google Scholar]
- 34.Jerkić M, Varagić J, Jovović D, Radujković-Kuburović G, Nastić-Mirić D, Adanja-Grujić G, et al. L-arginine reduces tubular cell injury in acute post-ischaemic renal failure. Nephrology Dialysis Transplantation. 1999;14:1398–407. doi: 10.1093/ndt/14.6.1398. [DOI] [PubMed] [Google Scholar]
- 35.Goodrich J, Hottendorf G. Tobramycin gender-related nephrotoxicity in Fischer but not Sprague-Dawley rats. Toxicology letters. 1995;75:127–31. doi: 10.1016/0378-4274(94)03170-c. [DOI] [PubMed] [Google Scholar]
- 36.Miura K, Pasino DA, Goldstein RS, Hook JB. Effects of gentamicin on renal function in isolated perfused kidneys from male and female rats. Toxicology Letters. 1985;26:15–8. doi: 10.1016/0378-4274(85)90178-x. [DOI] [PubMed] [Google Scholar]
- 37.Gouvea W, Vaamonde CM, Owens B, Alpert H, Pardo V, Vaamonde CA. The protection against gentamicin nephrotoxicity in the streptozotocin-induced diabetic rat is not related to gender. Life Sciences. 1992;51:1747–58. doi: 10.1016/0024-3205(92)90304-8. [DOI] [PubMed] [Google Scholar]
- 38.Ali B, Ismail TB, Bashir A. Sex difference in the susceptibility of rats to gentamicin nephrotoxicity: Influence of gonadectomy and hormonal replacement therapy. Indian J Pharmacology. 2001;33:369–73. [Google Scholar]
- 39.Sweileh WM. Gender differences in aminoglycoside induced nephrotoxicity: A prospective, hospital-based study. Current Clinical Pharmacology. 2009;4:229–32. doi: 10.2174/157488409789375339. [DOI] [PubMed] [Google Scholar]
- 40.Carraro-Eduardo JC, Oliveira AV, Carrapatoso ME, Ornellas JF. Effect of sex hormones on gentamicin-induced nephrotoxicity in rats. Braz J Med Biol Res. 1993;26:653–62. [PubMed] [Google Scholar]
- 41.Riazi S, Madala-Halagappa VK, Dantas AP, Hu X, Ecelbarger CA. Sex differences in renal nitric oxide synthase, NAD (P) H oxidase, and blood pressure in obese Zucker rats. Gender Medicine. 2007;4:214–29. doi: 10.1016/s1550-8579(07)80042-8. [DOI] [PubMed] [Google Scholar]
- 42.Pontari MA, Ruggieri MR. Sex differences and role of nitric oxide in blood flow of canine urinary bladder. American J Physiology-Regulatory, Integrative and Comparative Physiology. 1999;276:R407–R13. doi: 10.1152/ajpregu.1999.276.2.R407. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi T, Ishikawa T, Yamada K, Kuzuya M, Naito M, Hidaka H, et al. Biphasic effect of estrogen on neuronal constitutive nitric oxide synthase via Ca 2+-calmodulin dependent mechanism. Biochemical and Biophysical Research Communications. 1994;203:1013–9. doi: 10.1006/bbrc.1994.2283. [DOI] [PubMed] [Google Scholar]
- 44.Kauser K, Rubanyi GM. Gender difference in bioassayable endothelium-derived nitric oxide from isolated rat aortae. American Journal of Physiology-Heart and Circulatory Physiology. 1994;267:H2311–H7. doi: 10.1152/ajpheart.1994.267.6.H2311. [DOI] [PubMed] [Google Scholar]
- 45.Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25:517–23. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- 46.Reckelhoff JF, Hennington BS, Moore AG, Blanchard EJ, Cameron J. Gender differences in the renal nitric oxide (NO) system. American J Hypertension. 1998;11:97–104. doi: 10.1016/s0895-7061(97)00360-9. [DOI] [PubMed] [Google Scholar]
- 47.Crann SA, Huang MY, McLaren JD, Schacht J. Formation of a toxic metabolite from gentamicin by a hepatic cytosolic fraction. Biochemical Pharmacology. 1992;43:1835–9. doi: 10.1016/0006-2952(92)90718-x. [DOI] [PubMed] [Google Scholar]
- 48.Williams P, Bennett D, Gleason C, Hottendorf G. Correlation between renal membrane binding and nephrotoxicity of aminoglycosides. Antimicrobial Agents and Chemotherapy. 1987;31:570–4. doi: 10.1128/aac.31.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]


