Abstract
Early secure fixation of total joint replacements is crucial for long-term survival. Antiresorptive agents such as bisphosphonates have been shown to increase implant fixation. We investigated whether local delivery of zoledronate from poly-D, L-lactide (PDLLA)-coated implants could improve implant fixation and osseointegration. Experimental titanium implants were bilaterally inserted press-fit into the proximal tibiae of 10 dogs. On one side the implant was coated with PDLLA containing zoledronate. The contralateral implant was uncoated and used as control. Observation period was 12 weeks. Implant fixation was evaluated with histomorphometry and biomechanical push-out test. We found an approximately twofold increase in all biomechanical parameters when comparing data from the zoledronate group with their respective controls. Histomorphometry showed increased amount of preserved bone and increased bone formation around the zoledronate implants. This study indicates that local delivery of zoledronate from a PDDLA coating has the potential to increase implant fixation.
Keywords: bisphosphonate, PDDLA, implant fixation
Longevity of total joint replacement relies upon early secure mechanical stability and sustained osseointegration in order to prevent migration and implant loosening.1,2 Early mechanical implant stability depends in part upon a supportive bone bed that is not compromised due to the surgical trauma of implant insertion.3 Implant osseointegration depends upon an osteogenic environment and a supportive bony matrix.3–5
Bisphosphonates are strong inhibitors of bone resorption.6 Clinical studies have shown that systemic and local treatment with bisphosphonate reduce implant migration measured with radiostereometric analysis.7,8 Experimental animal studies have shown that systemic and local bisphosphonate treatment can increase implant osseointegration and mechanical implant fixation.9–13 Furthermore, experimental studies, have shown that allograft can be preserved with local application of bisphosphonates while concomitantly accelerating formation of new bone.14–16 Two clinical trials have investigated the effect of coating implants with bisphosphonate.17,18 One study investigated the effect of bisphosphonate coating on titanium dental implants and found improved fixation.17 Another study investigated the effect of bisphosphonate coating on external fixation pins used when performing proximal tibial correction osteotomy and found that bisphosphonate coated pins were similar to HA-coated pins in removal torque.18
Bisphosphonate can be administrated locally or systemically. Local bisphosphonate delivery has the potential to reach high concentrations without systemic adverse effects. We have previously shown that soaking bone in bisphosphonate before implantation can increase osseointegration and implant fixation.12,13 However, we know that soaking bone in bisphosphonate and not rinsing unbound bisphosphonate away can impair implant fixation.19 We also know that soaking bone in a too high dose of bisphosphonate and rinsing away unbound bisphosphonate can inhibit new bone formation in a dose-dependent manner14 Another way to locally deliver bisphosphonate to the implant-bone interface is by using the implant surface as a drug carrier. Different coatings, including bisphospho-nate immobilized in a cross-linked fibrinogen layer and hydroxyapatite-adsorbed bisphosphonate, have been tested experimentally9,20 A general finding is increased peri-implant bone density. Another way to locally deliver bisphosphonate could be with the use of a poly(D, L-lactide) (PDLLA) implant coating.21 Bisphosphonate eluted from PDLLA coatings has shown promising results in vitro and in rodent models of fracture healing.22,23 The PDLLA coating is used as a carrier for gentamicin on a commercially available intramedullary tibia nail (UTN PROtect®, Synthes GmbH, Oberdorf, Switzerland).24 Results on osseointegration of intra-medullar k-wires in a rodent model are however less promising.25 We have previously shown that TGF-beta and IGF-1 eluted from a PDLLA coating has the ability to increase fixation of titanium implants in a canine model.26 No large animal studies have investigated the in vivo release kinetics of bisphosphonate from PDLLA, but an in vitro study has shown that 90% of the zoledronate is eluted from the PDDLA coating within the first 24 h followed by a slow release of the remaining zoledronate.25 Large animal studies investigating the effect on implant osseointegration and biomechanical fixation of bisphosphonate in a PDDLA coating are missing.
Zoledronate is a third-generation nitrogen-containing bisphosphonate. It is clinically used in the treatment of osteoporosis, bone complications of cancer, and Paget’s disease. We have previously shown that soaking allograft in zoledronate and rinsing away unbound zoledronate away can improve implant osseointegration.14
The aim of this large animal study was to investigate the effect of PDDLA releasing zoledronate on implant osseointegration and fixation. We hypothesized that zoledronate eluted from a PDLLA coating would increase biomechanical fixation and implant osseointegration in a canine model using porouscoating titanium implant after 12 weeks of observation.
MATERIALS AND METHODS
Study Design
We used 10 skeletally mature female hound dogs with a median weight of 29 kg (range, 27–32 kg). This study was approved by our institution’s Animal Care and Use Committee. Institutional guidelines for treatment and care of experimental animals were followed. Two unrelated studies were conducted in this set of dogs. One study investigating the effect of different graft substitutes placed around implants inserted into the proximal part of the humerus. Another study in the medial femoral condyle investigating a surgical technique used to improve implant fixation in a revision model. None of the to studies included drugs that potentially could influence this study.
Our study was designed as a paired randomized study with 20 implants. We inserted one porous-coated titanium implant into the medial proximal aspect of each tibia. We inserted one PDLLA-zoledronate coated implant into one of the tibiae and our control implant in the contralateral tibia. Each dog served as its own control. We observed the animals for 12 weeks.
We determine sample size from power estimate based on previous studies. We assumed the standard deviation of the relative change to be 50%.13,27 Two-sided a and b were set to 5% and 20%, respectively. Two extra animals were added to the calculated sample size of eight to counteract decreased power if implants from one or two animals were lost for subsequent analysis.
Implants
Our 20 implants consisted of a titanium alloy core (Ti-6Al-4V) onto which a 0.75 mm porous coating was obtained by sintering spherical beads (commercially pure Ti). The porous-bead coating had an average pore size of 250–300 μm and porosity range of 40–50%. The implants had a nominal diameter of 6.0 mm and length of 10.0 mm. The porous-bead coating was manufactured by Depuy Orthopaedics, Inc. (Warsaw, IN) and donated as a gift.
Pure zoledronate (Novartis Pharma AG, Basel, Switzerland) was dissolved in a poly-(D, L-lactide) (PDLLA)—Resomer 203 (Boehringer Ingelheim GmbH, Germany) and ethyl acetate solution resulting in a 2% (w/w) ratio of zoledronate to PDLLA. Implants were dipped twice in the solution and air dried under sterile conditions. Based on coating experiments, where the implants where weighed before and after coating, an estimated 0.02 mg zoledronate was incorporated into the PDDLA coating on each implant.
Surgical Procedure
All surgical procedures were performed under sterile conditions with the animals under general anaesthesia. We exposed the proximal anteromedial surface of the tibia through a medial incision. Then we inserted a 2.5-mm guidewire perpendicular into the surface of the proximal part of tibia. The guidewire was inserted 10 mm distal of the tibia plateau. Over the guidewire, we used a cannulated drill with an outer diameter of 6.0 mm to make an 11 mm deep cavity. All drilling was done water-cooled and at low speed with two revolutions per second to avoid thermal trauma to the bone. After removing bone-debris and irrigating the cavity, we inserted the implant. We inserted our implant in exact-fit with light hammer blows. In order to avoid potential contamination of our control implant with zoledronate, we inserted the control implant before inserting the PDDLA-zoledronate implant in the contralateral tibia. Antibiotics (Rocephin; Sandoz GmbH, Kundl, Austria) were administered immediately before surgery and 3 days postoperatively. Analgesics (Buprenox; Hospira Inc, Lake Forest, IL) were used for the first 3 postoperative days. All dogs were euthanized 12 weeks postoperatively.
Specimen Preparation
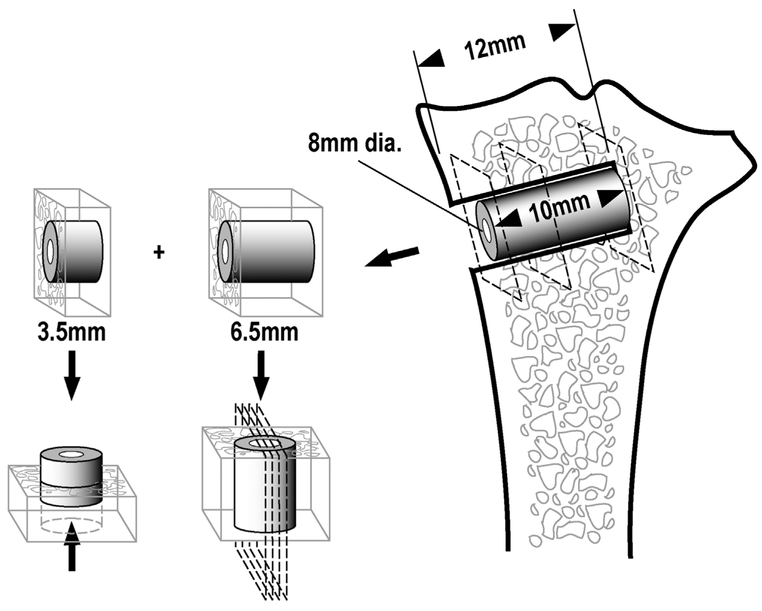
Immediately after euthanasia, we removed the proximal part of each tibia, cleaned it of soft tissue, and stored it at −20˚C. Two specimens containing a part of the implant and surrounding bone were cut from each tibia perpendicular to the long axis of the implant using a water-cooled band saw (Exact Apparatebau, Nordenstedt, Germany) (Fig. 1). The first and most superficial specimens, with a thickness of 3.5 mm, were stored at −20°C for later biomechanical testing. The second specimens, with a thickness of 6.5 mm, were fixed in 70% ethanol and used for later histomorphometrical analysis. Preparation of specimens and subsequent evaluation was performed blinded.
Figure 1.
Schematic diagram showing the specimen preparation. Each bone-implant specimen is cut into two pieces: A 6.5 mm for histomorphometrical analysis, and a 3.5 mm for biomechanical push-out test.
Biomechanical Testing
We tested implants to failure by axial push-out test on an MTS Bionics Test Machine (MTS, Eden Prairie, MN). We placed the specimens on a metal support jig with a 7.4-mm diameter central opening. Implants were pushed from the peripheral side towards the inside of the bone. A preload of 2 N defined the start of the test. We used a displacement rate of 5 mm/min, and load versus displacement data was continuously recorded. Maximum shear strength (MPa) was determined from the maximum force applied until failure of the bone-implant interface. Failure was defined as the maximum force measured on a load versus displacement curve. Maximum shear stiffness (MPa/mm) was obtained from the slope of the linear section of the load versus displacement curve. We calculated total energy absorption (J/m2) as the area under the load displacement curve until failure. We normalized all push-out parameters by the cylindrical surface area of the transverse implant section, as determined from the measured thickness of the individual section tested.
Histomorphometry
Specimens were dehydrated gradually in ethanol (70–100%) containing basic fuchsin, and embedded in methylmethacry-late. Four vertical uniform random sections were cut with a hard tissue microtome (KDG-95, MeProTech, Heerhugowaard, The Netherlands) around the central part of each implant (Fig. 1). Before making the sections, the implant was randomly rotated around its long axis. The sections were cut parallel to this axis. The 25-μm thick sections were cut with a distance of 400 μm, and counterstained with 2% light-green (BDH Laboratory Supplies, Poole, England). With this protocol, bone was stained green and non-mineralized tissue red.
We performed quantitative histomorphometry using the stereological software newCAST (Visiopharm A/S, Horsholm, Denmark). Histomorphometrical specimens were during preparation given a unique identification number by a person not related to specimen preparation. The unique identification number enabled us to do blinded histomorphometry. Bone-to-implant contact was defined as the implant surface covered with woven or lamellar bone and was estimated by manually counting intercepts between sine-weighted lines and surface covered with bone. Bone volume fractions were estimated by manual point counting to determine the fraction of woven and lamellar bone in two zones around the implants: Zone 1 from the middle of the porous-bead coating and 500 mm into surrounding bone, and Zone 2 in the volume 500–1000 mm from the middle of the porous-bead coating.28 Bone was surface-stained green, and therefore could clearly be distinguished from the other tissues. Newly formed woven bone was identified by the lack of organization and large, round osteocyte lacunae. Lamellar bone was identified by its highly organized lamellas and lamella-oriented long, oval cell lacunae.
Statistical Analysis
We used Intercooled Stata 9.0 (Stata Inc., College Station, TX) for statistical analysis. Statistical analyses were done on ratios between paired data, which were not normally distributed. All variables were log-transformed and Student’s paired t-test was performed on absolute differences between normally distributed log-transformed paired data. An absolute difference between the logarithms of a pair of data equals the logarithm of the ratio within the pair.29 Two tailed p-values below 0.05 were considered statistically significant. Results are presented as medians of relative differences between the paired data. The 95% confidence intervals were obtained by back transformation of log-transformed data unless otherwise stated.
Correlation analyses were done between relative increases in biomechanical and histomorphometrical parameters. All assumptions for correlation analysis were met.
RESULTS
All dogs completed the 12-weeks observation period. No clinical sign of infection were present at time of euthanasia. Implants from one animal for biomechanical testing was excluded as a result of technical error during cutting procedure for one of the two implants.
Biomechanical Testing
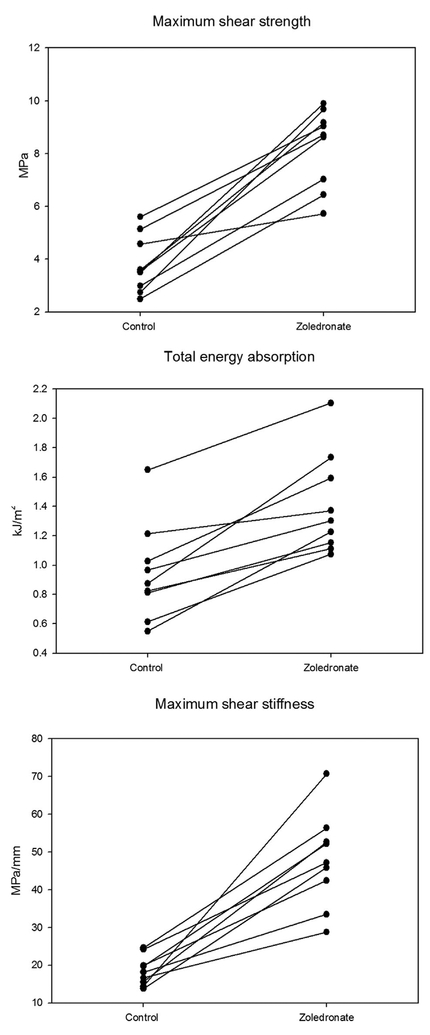
Press-fit implants coated with PDDLA-containing zoledronate had better biomechanical fixation compared with those in the control group (Fig. 2 and Table 1). The improvement in biomechanical fixation was consistent for all pairs of implants.
Figure 2.
Biomechanical push-out data. Paired data are connected by line.
Table 1.
Biomechanical Results
| Max shear strength, MPa | Max shear stiffness, MPa/mm | Total energy absorption, kJ/m2 | |
|---|---|---|---|
| Control | 3.80 (2.97;4.63) | 0.95 (0,69; 1,20) | 18.5 (15.5;21.6) |
| Zoledronate | 8.26 (7.11;9.40) | 1.41 (1,14;1,67) | 47.7 (38.2;57.3) |
| Zoledronate/control | 2.21 (1.73;2.84)* | 1.52 (1.29;1.81)** | 2.54 (1.95;3.32)*** |
Data are presented as mean for each treatment group (Control or Zoledronate) or median for the relative paired increases (Zoledronate/Control). 95%CI in parentheses.
p = 0.0001.
p = 0.0004.
p = 0.000.
Histomorphometrical Analysis
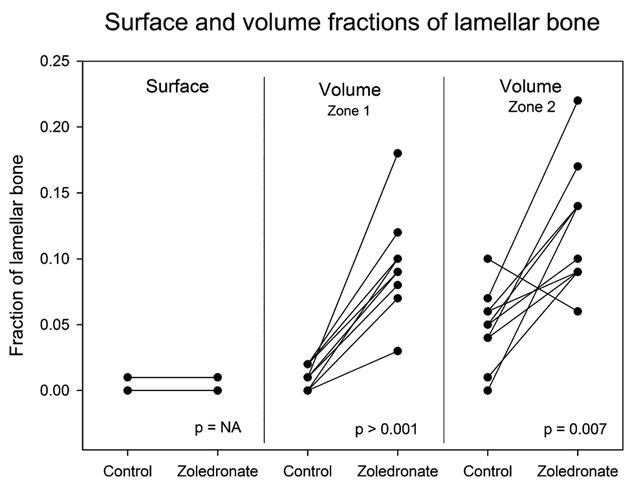
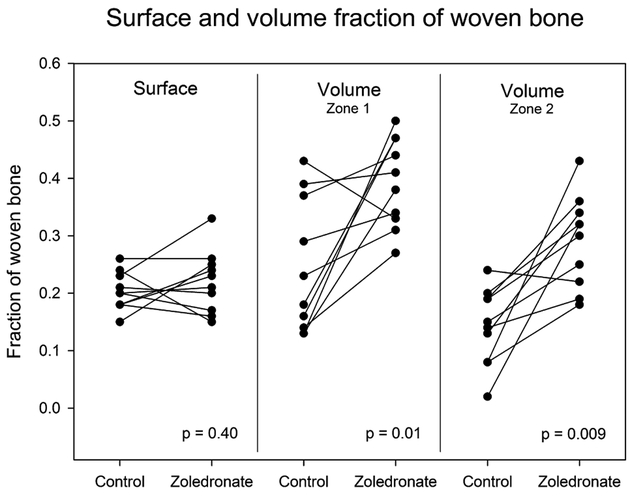
We found that local zoledronate was able to preserve lamellar bone and increase formation of woven bone in both zone 1 and 2 around the implants (Figs. 3 and 4). In zone 1, local treatment with zoledronate resulted in an increase in lamellar bone from a mean of 1% (95% CI: 5–17%) in the control group to a mean of 10% (95% CI: 7–12%) in the zoledronate group (p = 0.0001). In zone 2, lamellar bone increased from a mean of 5% (95%CI: 3–7%) in the control group to 12% (95%CI: 9–16%) in the zoledronate group (p = 0.0065). In zone 1, the volume fraction of woven bone increased from a mean of 25% (95%CI: 16–33%) in the control group to a mean of 39% (95%CI: 34–45%) in the zoledronate group (p = 0.01). The same trend was observed in zone 2, were the volume fraction of woven bone increased from a mean of 14% (95%CI: 9–19%) in the control group to a mean of 29% (95%CI: 23–35%) in the zoledronate group (p = 0.0094).
Figure 3.
Fractions of lameller bone in contact with the implant surface and in a 0–1000 μm zone around the implant. Paired data are connected by a line.
Figure 4.
Fractions of woven bone in contact with the implant surface and in a 0–1000 μm zone around the implant. Paired data are connected by a line.
The mean surface fraction of woven bone in both the control and zoledronate group was 20% (95%CI (control): 18–23% / 95%CI (zoledronate): 18–26%) (p = 0.40). No lamellar bone was observed in contact with the implant surfaces in either group.
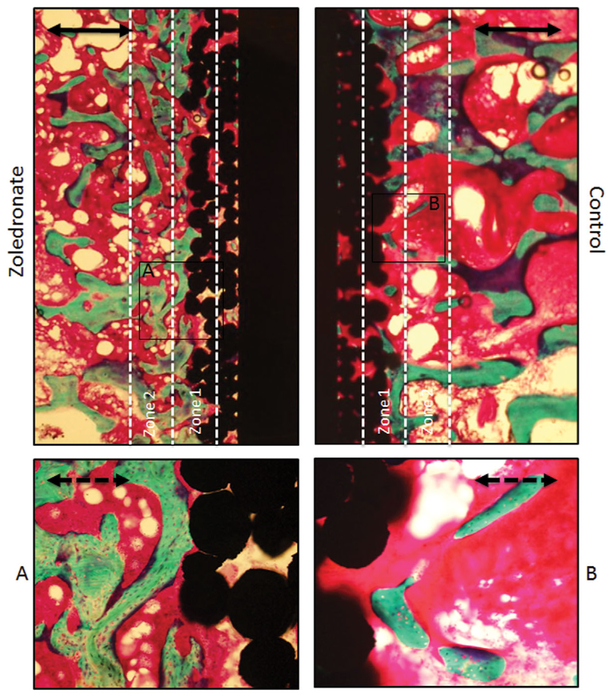
The histomorphometrical findings are reflected in a histological evaluation of the implants. The most striking histological difference between the two treatment groups is a relatively dense zone of cancellous bone around the zoledronate implants. Further away from the implant surface no histological difference was observed between the two treatment groups. No remnants of the PDDLA coating were observed (Fig. 5).
Figure 5.
Representative photomicrographs of samples from the same animal. The samples were stained with basic fuchsin and counter-stained with 2% light green. Implant appears as black, marrow as red, and bone as green. Note the increased amount of bone around the zoledronate implant. No remnants of the PDDLA coating were seen. Solid bar = 1.0 mm. Dotted bar = 0.3 mm.
DISCUSSION
The purpose of this study was to investigate whether zoledronate delivered locally from a PDDLA coating on a Ti-coated implant could improve osseointegration and biomechanical implant fixation. We found that zoledronate increased both the amount of new woven bone and old lamellar bone around the implants and improved the biomechanical implant fixation.
Our experimental model was intended to represent the portion of a cementless human joint replacement placed in cancellous bone. The canine cancellous bone was chosen because it resembles human bone in terms of composition, density, and quality.30 The paired design of this study allowed us to eliminate the biological difference between individuals.
This study has limitations. This canine implant model is unloaded and thereby limited, as the effects of loading are not addressed. We choose to use a control implant not coated with PDDLA in order to imitate the clinical setting with an uncemented Ti-implant. Thus, in the context of this study any positive effect cannot solely be attributed to zoledronate, but to the combination of PDLLA and zoledronate. We know from a previous study that implants coated with pure PDDLA do not stimulate bone formation in a similar implant model.31 Furthermore, another study has shown that PDDLA can impair osseointegration of Ti-coated coated implants compared to Ti-coated implants not coated with PDDLA.32 The experimental model in this previous study used identical implants to this current model, but had the implants placed in 1 mm gap-fit in the proximal part of the humerus for only 4 weeks.32 In each animal, we inserted our control implant before our zoledronate implant. This was done in order not to contaminate the control implant with zoledronate. The surgeon was thereby not blinded and a potential bias could be introduced. Only one time point was investigated and long-term should be done with caution.
In this study, we were also limited to use of a single dose of zoledronate. We used zoledronate in a 2% w/w ratio of the PDDLA coating. Our dose was chosen based on a study where the same w/w ratio was able to accelerate fracture healing in a rodent model.22,23 An in vitro study has shown that 90% of the zoledronate is eluted from the PDDLA coating within the first 24 h followed by a slow release of the remaining zoledro-nate.25 The release kinetics in vivo of this particular zoledronate/PDLLA coating has not been investigated, but a separate model demonstrated that release of zoledronate has been found to remain highly localized.33
With a 2% w/w ratio of zoledronate in a PDDLA coating, we are able to consistently increase all three biomechanical parameters representing implant fixation (strength, stiffness, energy). This is in accordance with previous studies where the bone bed was soaked in bisphosphonate before insertion of experimental implants.13,34 One explanation for the improved bio-mechanical fixation is the increased amount of woven and lamellar bone observed in a 1 mm zone around the implants coated with PDDLA and zoledronate. In spite of improved biomechanical fixation of the zoledronate implants, it is of interest to note that no significant differences with respect to fractions of lamellar or woven bone were observed on the implant surfaces themselves. This could indicate that the weakest link in the chain fixating the implant to bone might be the peri-implant bone and not the bone in contact with the implant surface. We have previously found it difficult to improve fixation of experimental implants inserted press-fit.35,36 The observed improvement in biomechanical fixation in this study indicates that coating implants with PDDLA containing zoledro-nate might have a clinical advantage.
With the dose of zoledronate used in this study we were able to increase formation of woven bone in a 1 mm zone around the implants. One explanation for this increased formation of woven bone could be the preserving effect of zoledronate on the lamellar bone. The surface of the preserved lamellar bone could act as a scaffold that by means of osteoconduction stimulate formation of new bone. Similar effect has been observed in others studies.14,15
We did not find any difference in the amount of woven bone in contact with the implant surface between the two groups. The implants used in this study were porous bead coated. As a consequence, most of the implant surface itself was located within the porosity of the implant. We did not find any lamellar bone within the porosity in either the zoledronate or control implants. If zoledronate increases new bone formation by preserving lamellar bone, then the lack of lamellar bone with in implant porosity could explain the lack in difference in woven bone between the two groups. Another explanation for the absence of a difference would be that the potential positive effect of zoledronate is balanced by a potential negative effect of PDDLA.
We chose to administer zoledronate locally by elution from a PDDLA coating. Other studies have shown that implant fixation and osseointegration can be increased by systemic administration or local treatment of the bone bed.7,11,27,37 Restricting the zoledronate exposure to the implantation site will limit potential systemic effects while assuring a high enough dose to be effective. Delivering the zoledronate by elution from a PDDLA coating will ensure a reproducible method with a controlled target dose compared to soaking the bone bed in a zoledronate solution and rinsing away excess unbound zoledronate. We know from previous studies that soaking the bone bed in a bisphosphonate solution can increase new bone formation at least 1 mm away from the implant surface.13 However, we also know that soaking bone in bisphosphonate can inhibit implant fixation in a dose dependent manner.14,19 One concern when delivering zoledronate with the use of PDDLA coating is the potentially limited exposure of the bone bed. Zoledronate eluted from the PDDLA coating has to be transported into the bone bed in order to be effective. With this study, we are able to demonstrate preservation of lamellar bone and increased formation of woven bone up to 1 mm away from the implant surface, when PDLLA is used.
Our finding suggests that there may be a clinical advantage in coating an implant with PDDLA containing zoledronate. The PDDLA coating allows a reproducible and targeted delivery of zoledronate. No clinically serious adverse events have been observed when using the PDDLA coating as a carrier for gentamicin on a commercially available intramedullary tibia nail (UTN PROtect®, Synthes GmbH, Oberdorf, Switzerland).24
In conclusion, this study demonstrated that zoledro-nate in a PDDLA coating has the potential to improve osseointegration and implant fixation in a canine model. Local delivery of zoledronate to the bone bed from a PDLLA coating appears to be a targeted and reproducible method that might have the potential to increase early fixation and longevity of total joint replacements. Studies investigating dose-response relationships and longer observation periods are needed.
ACKNOWLEDGMENTS
Zoledronate was donated by Novartis (Novartis Pharma AG, Basel, Switzerland). Implants was donated by Depuy (Depuy Orthopaedics, Inc.).
Grant sponsor: NIH; Grant number: AR 42051.
REFERENCES
- 1.Ryd L, Albrektsson E, Carlsson L, et al. 1995. Roentgen of knee of mechanical analysis loosening as a predictor continues. J Bone Jt Surg Br 77:377–383. [PubMed] [Google Scholar]
- 2.Kärrholm J, Borss en B, Löwenhielm G, et al. 1994. Does early micromotion matter? 4–7 year stereoradiographic follow-up of 84 cemented prostheses. J Bone Jt Surg Br 76:912–917. [PubMed] [Google Scholar]
- 3.Bauer TW, Schils J. 1999. The pathology of total joint arthroplasty. I. Mechanisms of implant fixation. Skeletal Radiol 28:423–432. [DOI] [PubMed] [Google Scholar]
- 4.Aspenberg P 2013. Special Review: Accelerating fracture repair in humans: a reading of old experiments and recent clinical trials. Bonekey Rep 2:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannoudis PV, Einhorn TA, Marsh D. 2007. Fracture healing: the diamond concept. Injury 38:3–6. [DOI] [PubMed] [Google Scholar]
- 6.Fleisch H 2002. Development of bisphosphonates. Breast Cancer Res 4:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilding M, Aspenberg P. 2007. Local peroperative treatment with a bisphosphonate improves the fixation of total knee prostheses: a randomized, double-blind radiostereometric study of 50 patients. Acta Orthop 78:795–799. [DOI] [PubMed] [Google Scholar]
- 8.Hilding M, Aspenberg P. 2006. Postoperative clodronate decreases prosthetic migration: 4-year follow-up of a randomized radiostereometric study of 50 total knee patients. Acta Orthop 77:912–916. [DOI] [PubMed] [Google Scholar]
- 9.Andersson T, Agholme F, Aspenberg P, et al. 2010. Surface immobilized zoledronate improves screw fixation in rat bone: a new method for the coating of metal implants. J Mater Sci Mater Med 21:3029–3037. [DOI] [PubMed] [Google Scholar]
- 10.Bobyn JD, McKenzie K, Karabasz D, et al. 2009. Locally delivered bisphosphonate for enhancement of bone formation and implant fixation. J Bone Joint Surg Am 91:23–31. [DOI] [PubMed] [Google Scholar]
- 11.Tanzer M, Karabasz D, Krygier JJ, et al. 2005. The otto aufranc award: bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res 441:30–39. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsen T, Kold S, Bechtold JE, et al. 2006. Effect of topical alendronate treatment on fixation of implants inserted with bone compaction. Clin Orthop Relat Res 444:229–234. [DOI] [PubMed] [Google Scholar]
- 13.Jakobsen T, Baas J, Kold S, et al. 2009. Local bisphospho-nate treatment increases fixation of hydroxyapatite-coated implants inserted with bone compaction. J Orthop Res 27:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakobsen T, Baas J, Bechtold JE, et al. 2010. The effect of soaking allograft in bisphosphonate: a pilot dose-response study. Clin Orthop Relat Res 468:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aspenberg P, Astrand J. 2002. Bone allografts pretreated with a bisphosphonate are not resorbed. Acta Orthop Scand 73:20–23. [DOI] [PubMed] [Google Scholar]
- 16.Tägil M, Åstrand J, Westman L, et al. 2004. Alendronate prevents collapse in mechanically loaded osteochondral grafts A bone chamber study in rats. Acta Orthop 75:756–761. [DOI] [PubMed] [Google Scholar]
- 17.Abtahi J, Tengvall P, Aspenberg P. 2012. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 50:1148–1151. [DOI] [PubMed] [Google Scholar]
- 18.Toksvig-Larsen S, Aspenberg P. 2013. Bisphosphonate-coated external fixation pins appear similar to hydroxyapatite-coated pins in the tibial metaphysis and to uncoated pins in the shaft. Acta Orthop 84:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakobsen T, Baas J, Bechtold JE, et al. 2007. Soaking morselized allograft in bisphosphonate can impair implant fixation. Clin Orthop Relat Res 463:195–201. [DOI] [PubMed] [Google Scholar]
- 20.Bobyn JD, Thompson R, Lim L, et al. 2014. Local alendronic acid elution increases net periimplant bone formation: a micro-CT analysis. Clin Orthop Relat Res 472:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidmaier G, Wildemann B, Stemberger A, et al. 2001. Biodegradable poly(D,L-lactide) coating of implants for continuous release of growth factors. J Biomed Mater Res 58:449–455. [DOI] [PubMed] [Google Scholar]
- 22.Greiner S, Kadow-Romacker A, Lübberstedt M, et al. 2007. The effect of zoledronic acid incorporated in a poly(D,L-lactide) implant coating on osteoblasts in vitro. J Biomed Mater Res A 80:769–775. [DOI] [PubMed] [Google Scholar]
- 23.Greiner SH, Wildemann B, Back a D, et al. 2008. Local application of zoledronic acid incorporated in a poly(D,L-lactide)-coated implant accelerates fracture healing in rats. Acta Orthop 79:717–725. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs T, Stange R, Schmidmaier G, et al. 2011. The use of gentamicin-coated nails in the tibia: preliminary results of a prospective study. Arch Orthop Trauma Surg 131:1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Back a D, Pauly S, Rommel L, et al. 2012. Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet. Disord 13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamberg A, Schmidmaier G, Søballe K, et al. 2006. Locally delivered TGF-beta1 and IGF-1 enhance the fixation of titanium implants: a study in dogs. Acta Orthop 77:799–805. [DOI] [PubMed] [Google Scholar]
- 27.Jakobsen T, Kold S, Bechtold JE, et al. 2007. Local alendronate increases fixation of implants inserted with bone compaction: 12-week canine study. J Orthop Res 25:432–441. [DOI] [PubMed] [Google Scholar]
- 28.Baas J 2008. Adjuvant therapies of bone graft around non-cemented experimental orthopaedic implants. Acta Orthop 79:3, 2–43. [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. 2006. 1996. Statistics Notes. The use of transformation when comparing two means. (May 2006).
- 30.Aerssens J, Boonen S, Lowet G, et al. 1998. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 139:663–670. [DOI] [PubMed] [Google Scholar]
- 31.Jensen T, Jakobsen T, Baas J, et al. 2010. Hydroxyapatite nanoparticles in poly-D,L-lactic acid coatings on porous titanium implants conducts bone formation. J Biomed Mater Res A 95:665–672. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen M, Baas J, Vestermark MT, et al. 2011. PDLLA delivery of local simvastatin? Effect on implant fixation. 2011 Annual Meeting of Orthopaedic research Society. Abstract.
- 33.McKenzie K, Dennis Bobyn J, Roberts J, et al. 2011. Bisphosphonate remains highly localized after elution from porous implants. Clin Orthop Relat Res 469:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobsen T, Kold S, Bechtold JE, et al. 2006. Effect of topical alendronate treatment on fixation of implants inserted with bone compaction. Clin Orthop Relat Res 444:229–234. [DOI] [PubMed] [Google Scholar]
- 35.Saksø H, Jakobsen T, Saksø M, et al. 2013. No positive effect of Acid etching or plasma cleaning on osseointegration of titanium implants in a canine femoral condyle press-fit model. Open Orthop J 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saksø M, Jakobsen SS, Saksø H, et al. 2012. Acid etching and plasma sterilization fail to improve osseointegration of grit blasted titanium implants. Open Orthop J 6:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen TB, Bechtold JE, Chen X, et al. 2007. Systemic alendronate treatment improves fixation of press-fit implants: a canine study using nonloaded implants. J Orthop Res 25:772–778. [DOI] [PubMed] [Google Scholar]