Abstract
The retina is one of the most metabolically active tissues in the body, consuming high levels of oxygen and nutrients. A well-organized ocular vascular system adapts to meet the metabolic requirements of the retina to ensure visual function. Pathological conditions affect growth of the blood vessels in the eye. Understanding the neuronal biological processes that govern retinal vascular development is ofinterest for translational researchers and clinicians to develop preventive and interventional therapeutics for vascular eye diseases that address early drivers of abnormal vascular growth. This review summarizes the current knowledge of the cellular and molecular processes governing both physiological and pathological retinal vascular development, which is dependent on the interaction among retinal cell populations, including neurons, glia, immune cells, and vascular endothelial cells. We also review animal models currently used for studying retinal vascular development.
Keywords: retina, vasculature, development, ROP, AMD, DR, animal model
1. RETINAL VASCULATURE IN DEVELOPMENT
1.1. Retinal Vessels in the Eye
The retina is supplied by two vascular systems: The central retinal artery supplies the inner retina and the choriocapillaris supplies the retinal pigment epithelium and outer retina (consisting primarily of photoreceptors) (Figure 1). The central retinal artery travels along the inferior margin of the optic nerve sheath and then enters the eye through the center of the optic nerve. The artery then branches in the inner retina to form three capillary layers (Netter 2006). The retinal vessels provide blood to the inner retinal neurons. The avascular photoreceptor layer relies on the choriocapillaris lying beneath the retinal pigment epithelium to supply oxygen by diffusion.
Figure 1:
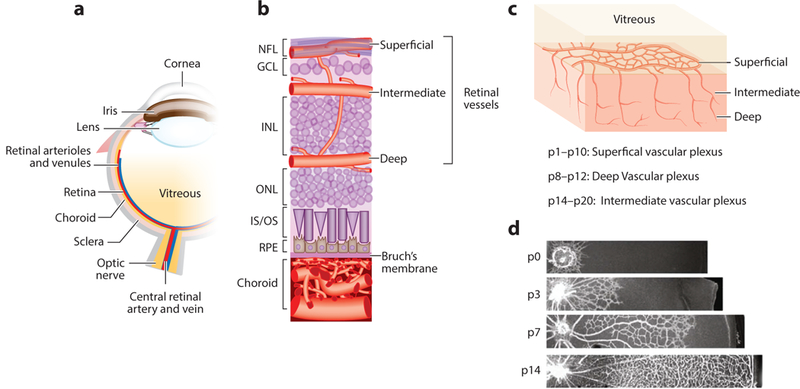
Schematic diagram of the ocular vasculature. (a) Cross-sectional image of an eye. (b) An enlarged cross-sectional view of the retinal and choroidal vasculature. Three interconnected layers of retinal vessels are embedded among inner retinal neurons: The superficial retinal vasculature lies in the NFL; the intermediate and deep retinal vascular networks lie along each side of the INL. The choroidal vessels are located beneath the RPE and Bruch’s membrane and supply oxygen and nutrients to the outer portion of the retina, which includes primarily photoreceptors in the outer nuclear layer. (c) Retinal vascular development begins with the formation of the superficial vascular plexus from pi to p10, then the deep vascular plexus from p8 to p12, and finally, the intermediate vascular plexus from p14 to p20. (d) Retinal superficial vascular plexus at different developmental stages p0, p3, p7, and p14. Panels a and b adapted from Liu et al. (2017). Panels c and d adapted from Joyal et al. (2018). Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; IS/OS, inner segment/outer segment of photoreceptor; NFL, nerve fiber layer; ONL, outer nuclear layer; P, postnatal day; RPE, retinal pigment epithelium.
The choroid, a densely pigmented vascular sinusoidal plexus, originates from two ophthalmic artery branches: the short posterior ciliary arteries, which supply the posterior portion of the choroid, and the long posterior ciliary arteries, which supply the anterior choroid, ciliary body, and iris. The choroidal vasculature has three interconnected distinct layers: the innermost choriocapillaris, the intermediate Sattler’s layer, and the outermost Haller’s layer (Hartnett 2013). It composes up to 85% of the blood volume in the eye (Anand-Apte & Hollyfield 2011).
1.2. Development of Retinal Vasculature
During embryonic development, the hyaloid vasculature supplies oxygen and nutrients to the developing inner retina and the lens during its maturation. The hyaloid artery enters the embryonic fissure, extends through the vitreous humor, and surrounds the lens (Hartnett 2013). The hyaloid vessels appear at the fourth week of gestation and reach maximum prominence at the ninth week in the human fetus. When the retinal vasculature develops during midgestation, the hyaloid vessels regress concurrently. The central hyaloid extension from the optic disk to the posterior lens surface regresses last (Hartnett 2013). Retinal vascular development is completed before birth in humans—unlike in mice and rats, where development occurs postnatally to eventually form a vascular network structurally similar to that in primates (Stone et al. 1995).
In humans, retinal vascularization starts in utero at about 16 weeks of gestation and is completed at approximately 40 weeks of gestation, just before birth. The most superficial retinal vascular layer forms first, starting from the optic nerve head and progressing toward the peripheral edge of the retina, reaching the nasal side of the ora serrata at about 36 weeks of gestation. As the superficial layer nears completion, retinal vessels dive into the retina to form the deep retinal vascular layer at the base of the outer plexiform layer. The intermediate layer then forms between the superficial and deep layers, superficial to the inner plexiform layer, forming a well-organized network to complete the three vascular layers (Dorrell et al. 2009). In other mammals, the same pattern of three retinal vascular layers forms, although over varying timescales (Gariano & Gardner 2005). In mice, the superficial plexus starts to form during the first week after birth. During the second week, angiogenic sprouts from the superficial layer grow into the retina to form the deep layer followed by the intermediate layer of the retinal capillary networks, and the entire retinal vasculature matures by three weeks after birth (Stahl et al. 2010a).
Human choriocapillaris is unusual in its development compared with other capillary beds in the body (Chirco et al. 2017), developing via hemovasculogenesis between 6 and 8 weeks of gestation, before the formation of intermediate or large vessels. At 11 to 12 weeks of gestation, the development of intermediate choroidal vessels within Sattler’s layer occurs by angiogenesis; this process allows the choriocapillaris to connect with the larger, outer choroidal vessels of Haller’s layer. Three distinct layers of choroidal vessels are seen at 21 weeks of gestation, which coincides with the beginning of photoreceptor cell differentiation. Interestingly, the three layers of the choroid (i.e., choriocapillaris, Sattler’s layer, and Haller’s layer) are found only in the posterior pole. The choroid outside the posterior pole consists of only the choriocapillaris and a layer of larger vessels (Hasegawa et al. 2007). The choriocapillaris appears fully mature, with flat, thin-walled fenestrated vessels, at 22 weeks of gestation (Baba et al. 2009, Hasegawa et al. 2007; for a review, see Lutty et al. 2010).
Retinal blood vessels in primates form a four-lobed pattern along the major vascular arcades and do not appreciably cross the horizontal raphe (Gariano 2010). Nasal-temporal retinal asymmetry is absent primarily because of the contour of perifoveal vessels. Ganglion cell axon bundles and an astrocytic plexus in the nerve fiber layer show a similar pattern. Murine retinal vessels, in contrast, are simply radially organized, without reference to a horizontal midline or macula (Gariano 2010).
2. RETINAL VASCULATURE IN DISEASES
Pathological blood vessels in the eye pose threats to normal vision. Angiogenesis, important in both physiological vascular development and pathological neovascularization, occurs as endothelial cells proliferate and form new vessels following guidance cues and angiogenic stimulators and inhibitors (Potente et al. 2011). Dysregulated angiogenesis disrupts delivery of oxygen and nutrients, resulting in unbalanced metabolic demand and supply and disturbed neural retinal function. Pathological angiogenesis is associated with many diseases, including cancers, cardiovascular diseases, neurodegeneration, and proliferative retinopathies (Folkman 1995). Pathological retinal neovascularization is characterized by leaky and tuft-like vessels, which are associated with retinal exudates and hemorrhage, leading to retinal damage, retinal detachment, or both (Al-Latayfeh et al. 2012). Abnormal ocular angiogenesis can occur in a broad spectrum of eye disorders, such as retinopathy of prematurity (ROP), diabetic retinopathy (DR), neovascular age-related macular degeneration (AMD), neovascular glaucoma, and corneal neovascularization (Al-Latayfeh et al. 2012).
2.1. Retinopathy of Prematurity
ROP is a major cause of blindness in children, affecting approximately 16,000 infants per year in the United States (Good 2004). Approximately 10% of children worldwide are born preterm (Blencowe et al. 2012), many with lifelong visual impairment. ROP contributes to 6–18% of cases of blindness in the United States (Lad et al. 2009). ROP is a biphasic disease, with an initial phase of vessel loss followed by a second phase of vessel proliferation. Because retinal blood vessels start developing in humans during the fourth month of gestation and reach the retinal periphery just before birth, infants born prematurely have incompletely vascularized retinas with a peripheral avascular zone at birth. The relative hyperoxic environment after birth compared with that in utero suppresses the normal secretion of angiogenic growth factors, leading to regression of existing vessels, the vessel loss phase of ROP. In the absence of an adequate vascular system between birth and gestational age of approximately 30–32 weeks, the retina becomes ischemic, leading to tissue hypoxia and secretion of hypoxia-driven angiogenic growth factors such as vascular endothelial growth factor (VEGF). The vessel-proliferation phase of ROP begins at about 3234 weeks of gestational age. In severe ROP, retinal neovessels can cause retinal detachment, leading to blindness.
2.2. Diabetic Retinopathy
DR, the leading cause of blindness in adults 24–70 years of age, eventually occurs in approximately one-third of patients with diabetes (Cheung et al. 2010). The prevalence of DR is higher inpatients with type 1 diabetes than in those with type 2 diabetes (Yau et al. 2012) perhaps because of the longer duration of disease. Hyperglycemia and other metabolic dysregulation lead to the development of microangiopathy, including microaneurysms, hemorrhages, and basement membrane thickening (Nentwich & Ulbig 2015). This results in increased vascular permeability of vessels, causing leakage and macular edema (Cheung et al. 2010). Increased capillary occlusion results in retinal ischemia, triggering an increase in the levels of VEGF, which then promotes pathological neovascularization (Cheung et al. 2010, Nentwich & Ulbig 2015).
Classification of DR as either nonproliferative or proliferative is based on the presence of neovascularization. Nonproliferative DR, which can progress to preproliferative DR, exhibits microaneurysms, dot and blot hemorrhages, cotton-wool spots, and capillary nonperfusion owing to microvascular damage and pericyte loss. Microglial changes and diabetic macular edema can also occur in nonproliferative DR. In proliferative DR, neovascularization can result in retinal and vitreous hemorrhages and can lead to retinal detachment (Cheung et al. 2010, Olivares et al. 2017).
2.3. Age-Related Macular Degeneration
Choroidal neovascularization (CNV) and retinal angiomatous proliferation (RAP) are features of neovascular AMD (Campochiaro 2015). CNV lesions refer to the abnormal new vessel growth from the choriocapillaris, extending through Bruch’s membrane into the subretinal pigment epithelium or subretinal spaces. Left untreated, CNV may advance to a stage that forms fibrotic scars underlying the retina, leading to vision loss (Grossniklaus et al. 2010). The progression of CNV is as variable as its response to certain stimuli, depending on the underlying diseases and the stage of CNV development (Grossniklaus et al. 2010). Disruption or degradation of Bruch’s membrane is one of the early stages of CNV and is followed by proliferating choroidal neovascular tissue invading the subretinal space. Pathological subretinal vessel formation can originate not only from the choroid but also sometimes from the retinal vasculature. Abnormal intraretinal angiogenesis invading the subretinal space with formation of a retinal-choroidal anastomosis is characteristic of a distinct variant of neovascular AMD known as RAP, which is present in approximately 12–15% of patients with neovascular AMD (Bottoni et al. 2005).
2.4. Other Vascular Eye Diseases
Macular telangiectasia type 2 is a disease of unknown cause with characteristic alterations of the macular capillary network and neurosensory retina. The disease manifests initially temporal to the foveal center but may later encompass an oval area centered on the foveola (Charbel Issa et al. 2013). In several rare human eye diseases, including familial exudative vitreoretinopathy and Norrie disease, incomplete peripheral retinal vasculature and lack of deep retinal vascular layers are observed (Criswick & Schepens 1969). There are many other eye diseases with abnormal angiogenesis, and they have been reviewed elsewhere (Bek 2013, Usui et al. 2015).
3. ANIMAL MODELS OF RETINAL VASCULAR DISEASES
The past several decades of angiogenesis research produced several animal models of ocular angiogenesis that mimic various aspects of neovascular eye diseases with retinal and choroidal angiogenesis. Mouse models of oxygen-induced retinopathy (OIR) and laser-induced CNV are two commonly used models for investigating pathological neovascularization in the retina and choroid, respectively. Additionally, transgenic knockout or knockin mice with intraretinal or subretinal neovascularization were developed as experimental tools for studying retinal vascular diseases.
3.1. Animal Models of Retinopathy of Prematurity
The OIR model was established to mimic ROP in humans. It reproduces the two phases of the disease: an initial vaso-obliteration phase with oxygen exposure and a subsequent hypoxiainduced neovascularization phase. The OIR models were established in several vertebrate animals, including cat (Ashton et al. 1954), mouse (Smith et al. 1994), rat (Penn et al. 2001), dog (McLeod et al. 1996), and fish (Cao et al. 2008). These animal models share the basic principle that exposure to high oxygen induces loss of existing retinal vasculature (Stahl et al. 2010a). After being returned to normoxic conditions with inadequate vessels to support retinal neurons, retinas become hypoxic and signal new (abnormal) blood vessel formation.
The cat model of ROP, developed in the 1950s by Ashton et al. (1954), was the first model to use high oxygen concentration to suppress retinal vascular development. Kittens are exposed to 70–80% oxygen for 4 days, causing retinal vascular obliteration, and then returned to normal room air exposure (21% oxygen), resulting in hypoxia-induced vasoproliferation (Ashton et al. 1954). In the less commonly used dog model of OIR, puppies are exposed to 95–100% oxygen for 4 days and returned to normoxic conditions. The pattern and severity of the vascular reaction to hyperoxia in this model are similar to those observed in the kitten model and in human infants (McLeod et al. 1996).
The advantage of genetic manipulation in mice makes the mouse model of OIR (Smith et al. 1994) one of the most commonly used ROP models. In this model, neonatal mice are exposed to 75% oxygen for 5 days, from postnatal day 7 to 12, and subsequent normal room air exposure from postnatal day 12 to 17. With hyperoxia exposure, the formed retinal vessels regress in the central zone, leading to vaso-obliteration, and vascular growth ceases in the periphery, mimicking the initial phase of ROP. After the pups are returned to normoxic conditions at postnatal day 12, the vaso-obliterated area becomes ischemic and hypoxic, resulting in upregulation of proangiogenic pathways, such as hypoxia-inducible factor (HIF)-dependent pathways (Pierce et al. 1995, Stahl et al. 2010a), including VEGF and erythropoietin (Aiello et al. 1995, Chen et al. 2008, Pierce et al. 1995, Robinson et al. 1996). This leads to both physiological revascularization of the vasoobliterated area and pathological neovascularization (Smith et al. 1994). In the mouse model of OIR, pathological neovessel growth reaches the maximal severity at postnatal day 17. After postnatal day 17, the abnormal neovascularization gradually regresses and completely disappears by about postnatal day 25 (Connor et al. 2009). Vaso-obliteration and neovascularization can be quantified as a percentage of the total retinal area in retinal whole mounts stained with isolectin IB4 to visualize blood vessels (Connor et al. 2009, Stahl et al. 2009). Vascular leakage can be visualized by fluorescein angiography and quantified by the Miles assay measuring permeability (J. Chen et al. 2011a, Pierce et al. 1995, Stahl et al. 2009, Vahatupa et al. 2016). The mouse model of OIR was used in early work investigating the role of VEGF in ROP (Aiello et al. 1995; Pierce et al. 1995, 1996; Robinson et al. 1996) as well as those factors critical in the third trimester and missing after preterm birth, such as insulin-like growth factor (Hellstrom et al. 2001; Smith et al. 1997, 1999) and omega-3 polyunsaturated fatty acids (Connor et al. 2007; Fu et al. 2015; Sapieha et al. 2011, 2012; Shao et al. 2014; Smith et al. 1999; Stahl et al. 2010b).
Penn et al. (2001) developed a rat model of OIR utilizing alternating hyperoxia-hypoxia cycles in which the oxygen levels are cycled between 50% and 10% every 24 h from birth to postnatal day 14, and then the pup is exposed to room air through postnatal day 20. The cycling levels of oxygen slow retinal vascular development and lead to a peripheral avascular zone. After returning to room air, pathological neovessels grow at the boundary between vascularized and avascular areas in the midperipheral retina. In the rat model of OIR, fluctuations in oxygen concentrations reflect variable oxygen levels measured in preterm human infants with severe ROP (Hartnett & Penn 2012). Vaso-obliteration in the peripheral retina is geographically similar to the peripheral avascular zone observed in human ROP (Grossniklaus et al. 2010). Overall, the rat model shows many clinically relevant features of ROP. However, it is difficult to genetically manipulate rats, and the levels of neovascularization are relatively low compared with those of the mouse model of OIR, limiting its use in screening antiangiogenic compounds.
3.2. Animal Models of Diabetic Retinopathy
Several animal models have been developed to investigate the etiology and pathogenesis of DR and to create and test therapies to treat the disease. Animal models of DR are generated through surgical injury, laser or chemical damage, drugs, or diet. Genetic models are created with selective gene editing in rodents (Olivares et al. 2017).
Induced models include surgical removal of the pancreas, administration of the drug alloxan, administration of the drug streptozotocin, high-galactose diets, and laser or chemical damage to the eye (Grossniklaus et al. 2010). Of all the methods of induction, the most common is administration of streptozotocin, as it results in the fastest onset of disease (Rakieten et al. 1963). The most frequently used animal models for inducing DR are mice and rats, but dogs, cats, pigs, rabbits, monkeys, and zebrafish are also used.
There are also several genetic modes of DR in mouse, rat, and zebrafish. These models include spontaneous, strain-specific, and genetically edited mutations. Several inbred mouse strains—for example, nonobese diabetic (NOD) and db/db (Leprdb)—exhibit hyperglycemia, one of the main characteristics of diabetes. Genetic mouse models of DR are relatively easy to maintain, have well-characterized genetic backgrounds, and can be manipulated to generate knockout or transgenic models. Genetic models exist for both type 1 and type 2 diabetes, and models for type 2 diabetes can be either obese or nonobese (Weir et al. 2009).
Five commonly used genetic mouse models of DR are Ins2Akita, NOD, db/db (Leprdb), Kimba, and Akimba (Olivares et al. 2017). The Ins2Akita mouse is a model of type 1 diabetes that harbors a missense mutation in the gene Ins2, which leads to a conformational change in the insulin protein. Ins2 accumulates in pancreatic β cells, leading to β cell death (Izumi et al. 2003). The NOD mouse model of type 1 diabetes exhibits an autoimmune response in which CD4+ and CD8+ cells attack pancreatic β cells (Serreze et al. 1997). The db/db (Leprdb) mouse was developed to study type 2 diabetes. db/db mice harbor a mutation in the leptin receptor and develop hyperglycemia and obesity after 4–8 weeks (Chen et al. 1996, Hummel et al. 1966), and disease progression continues for 10 months. A mouse model of DR with hyperglycemia-induced background vascular changes (such as pericyte loss) but with non-hyperglycemia-induced neovascularization can be obtained by breeding two mutant mouse strains. The Akimba mouse was generated by crossing Kimba mice, a nondiabetic model of proliferative retinopathy resulting from overexpression of Vegf driven by the rhodopsin promoter (Tee et al. 2008), with the diabetic Ins2Akita mice to create a model of diabetes with retinal neovascularization secondary to non-diabetes-driven expression of Vegf in photoreceptors.
Genetic rat models of DR include Zucker diabetic fatty (ZDF), Otsuka Long-Evans Tokushima fatty (OLETF), biobreeding (BB), WBN/Kob, spontaneously diabetic Torii (SDT), and Goto-Kakizaki (GK). ZDF, OLETF, and BB are monogenic models of DR with independent mutations that perturb different nodes of the DR disease pathway. SDT, WBN/Kob, and GK, in contrast, are polygenic models. These models demonstrate the genetic complexity of DR (Olivares et al. 2017).
3.3. Animal Models of Neovascular Age-Related Macular Degeneration
Ideal animal models of CNV should be stable, efficient, and reproducible and exhibit pathological features similar to those observed in CNV lesions in AMD patients. Taking into account the complex conditions that lead to CNV in humans, three main types of animal models of CNV have been developed: laser-induced CNV, surgical-incision-induced CNV, and transgenic animal models. Laser-induced CNV is one of the most widely used models recapitulating CNV seen in neovascular AMD. However, the CNV produced is in response to injury and does not reflect age-driven disease. The laser-induced CNV model is relatively rapid.
Laser-induced CNV models have been used extensively in the rat and mouse to study key pathways involved in CNV formation and to evaluate pharmacologic antiangiogenic intervention (Takahashi et al. 2000). In the mouse model, laser photocoagulation spots are placed through the dilated pupil, avoiding the major retinal vessels, around an area 2-disc diameters from the optic nerve. CNV lesions reach maximal volume and area on day 7 after the laser burn, forming diffuse subretinal fibrovascular tissue, which can be visualized and quantitatively analyzed for leakage and lesion size by means of fluorescein fundus angiography and immunohistochemical staining (Gong et al. 2015). After day 7 post laser, CNV starts to regress and CNV completely disappears approximately one month post laser. Subretinal fibrosis at the lesion sites can also be observed in this model and this continues to increase through day 35 post laser (Ishikawa et al. 2016). This fibrotic aspect of CNV can be used to study subretinal fibrosis, and the progression of which is often considered a contributing factor to the lack of responsiveness to anti-VEGF therapies.
Although the laser-induced CNV model is frequently used as a neovascular AMD model (Lambert et al. 2013), as noted above, it is an acute injury model and does not recapitulate the aging aspect of AMD. Nor does it mimic macula-specific aspects of AMD, as rodents do not have a macula. In small-rodent eyes, considerable variations in lesion size, even among several lesions within the same eye, are likely due to variations among laser applications. Therefore, sufficient sample size and rigorous application of preestablished inclusion and exclusion criteria are necessary to obtain reliable results (Gong et al. 2015, Poor et al. 2014). Nevertheless, the laser-induced CNV model, together with OIR models (Aiello et al. 1995, Robinson et al. 1996), has been used to evaluate the therapeutic value of anti-VEGF therapies (Krzystolik et al. 2002). In addition to laser-induced CNV, subretinal injection of matrigel is used to induce CNV in rats and mice (Li et al. 2011). This method can be more convenient for researchers without access to laser photocoagulation.
3.4. Genetic Models for Pathological Retinal Vessel Growth
The advantage of genetic manipulation in mice makes them the most commonly used animal model in laboratories for studying retinal vessel growth. Genetic models include transgenic mouse models with intraretinal angiogenesis, subretinal angiogenesis, and deficient intraretinal angiogenesis.
RAP is a distinct form of neovascular AMD. Animal models of RAP exhibit intraretinal, sub-retinal, and choroidal angiogenic features that mimic the vascular changes seen in human patients. Loss of very-low-density lipoprotein receptor (Vldlr) and rhodopsin-driven Vegf mutations result in RAP-like lesions (Heckenlively et al. 2003, Hu et al. 2008, Joyal et al. 2016, Okamoto et al. 1997, Sun et al. 2017, Tobe et al. 1998).
Mice lacking Vldlr were discovered as a unique spontaneous model of RAP and CNV, both of which are features of neovascular AMD (Heckenlively et al. 2003). Genetic variations such as single-nucleotide polymorphisms in VLDLR are associated with AMD in human patients (Haines et al. 2006). In 1995, Herz and colleagues (Frykman et al. 1995) generated Vldlr germ-line knockout (Vldlr−/−) mice by targeted disruption of exon 5 of the Vldlr gene on chromosome 19. These mice were first characterized as a model of subretinal neovascularization and choroidal anastomosis (Heckenlively et al. 2003). The abnormal neovessels in Vldlr−/− retinas sprout from the deep vascular layer in the outer plexiform layer toward normally avascular photoreceptors and invade the subretinal space as early as postnatal day 15 and continue growth through postnatal day 20 (Heckenlively et al. 2003). Subretinal hemorrhages and choroidal anastomosis are evident in Vldlr−/− eyes by about 2 months of age (Heckenlively et al. 2003). Moreover, Vldlr−/− mice not only recapitulate the pathological vascular features of human RAP, but also exhibit secondary photoreceptor degeneration and subretinal fibrosis (Hu et al. 2008).
VEGF-overexpressing transgenic mice were developed in the late 1990s by overexpressing VEGF in photoreceptor cells by incorporating a full-length complementary DNA of human VEGF under the bovine rhodopsin promoter (Okamoto et al. 1997, Tobe et al. 1998). This rhodopsin/VEGF transgenic mouse model (rho/VEGF mouse) shows focal areas of intraretinal and subretinal neovascularization that correspond to areas of vascular leakage on fluorescein angiograms (Okamoto et al. 1997). In adult rho/VEGF retinas, bloodvessels extend from the inner nuclear layer toward the retinal pigment epithelium to form a large plexus of blood vessels in the subretinal space. This model presents features similar to those of RAP and induces intraretinal and subretinal neovascularization rather than CNV. This model is useful for investigating early VEGF-induced changes that occur in the retina that ultimately lead to neovascularization and for testing the effects of potential angiogenesis inhibitors (Liu et al. 2017).
The neoretinal vascularization 2 (NRV2, or JR5558) mouse harbors currently unknown genetic mutations (Hasegawa et al. 2014) and has spontaneous intraretinal and subretinal neovascularization (Won et al. 2011). The mouse strain NRV2 was discovered through the Jackson Laboratory Eye Mutant Screening Program (Won et al. 2011). Retinal neovascularization in JR5558 mice originates from the retinal vascular plexus and grows toward the subretinal space, forming neovascular structures at the retinal pigment epithelium and Bruch’s membrane interface that mimic the early clinical presentation of RAP in humans (Liu et al. 2017). This genetic model of intraretinal neovascularization provides a useful tool that will help researchers understand the molecular causes of RAP and AMD and evaluate preclinical therapeutics for both diseases (Doyle et al. 2015, Hasegawa et al. 2014, Nagai et al. 2015, Paneghetti & Ng 2016).
There are also genetic mouse models with deficient intraretinal angiogenesis, and they are usually based on mutations in the WNTpathway. Familial exudative vitreoretinopathy and Norrie disease are modeled in Lrp5 knockout and Norrin knockout mice. Mice with genetic deficiency in Lrp5 (Lrp5−/−) and Norrin (Ndpy/-) show similar ocular vascular abnormalities that resemble human pathologies in familial exudative vitreoretinopathy and Norrie disease, respectively (Wang et al. 2016). Lrp5−/− mice have delayed primary retinal angiogenesis, with less dense vascular coverage and fewer branching points in the superficial vascular plexus (J. Chen et al. 2011b, Wang et al. 2016). Similar severe retinal vascular defects are observed in Ndpy/- mice, including delayed development of superficial retinal blood vessels, absence of the intermediate and deep layers of retinal vessels flanking the inner nuclear layer, delayed regression of the hyaloid vasculature, and, in some cases, intraocular hemorrhage (Luhmann et al. 2005).
4. KEY FACTORS THAT INFLUENCE RETINAL VASCULAR DEVELOPMENT
4.1. Oxygen in Retinal Vascular Development
Oxygen plays a major role in retinal vascular development. In ROP and DR, an initial lack of vessels or loss of retinal vessels resulting in retinal ischemia precedes pathological vessel growth, supporting the idea of hypoxia as a critical stimulator of new blood vessel growth (Gariano & Gardner 2005). During development, as retinal neurons and glial cells differentiate and mature and their metabolic demands increase, physiological hypoxia leads to the development of new vessels from the center toward the periphery of the retina (Chan-Ling et al. 1995). After new vessels have formed, bringing oxygen and alleviating hypoxia, vascular growth continues radially toward the peripheral retina (Liu et al. 2017).
4.2. Angiogenic Regulators in Retinal Vascular Development
The effect of oxygen on vascular growth is mediated in large part by VEGF, a hypoxia-induced growth factor, which is expressed in the developing retina in a pattern that coincides with retinal blood vessel development (Pierce et al. 1995, 1996; Stone et al. 1995). VEGF and other hypoxiaregulated growth factors are controlled by HIF (Wang & Semenza 1993). A gradient of VEGF precedes the radial growth of the superficial vascular plexus in the retina, and it is postulated that the relative hypoxia of the deeper retinal layers during development also results in a VEGF gradient that favors sprouting from the superficial layer downward, resulting in the formation of first the deep layer and then the intermediate layer of capillary networks (Pierce et al. 1995, Stone et al. 1995). In addition to VEGF, erythropoietin is a hypoxia-induced growth factor that plays an important role during retinal blood vessel homeostasis (Chen et al. 2008). Erythropoietin also has potent neuroprotective and proangiogenic effects (Caprara & Grimm 2012) and was suggested to be an important factor in the formation of the intermediate plexus of retinal vessels in an HIF-dependent manner (Caprara et al. 2011). These oxygen-regulated hypoxia-responsive factors are therefore considered some of the master regulators of both normal retinal vessel development and proliferative retinopathies (Aiello et al. 1995, Smith et al. 1997, Stone et al. 1995). Besides these hypoxia-regulated growth factors, other non-oxygen-regulated growth factors important for retinal vascular development, partly through modulation of the VEGF response, include the Tie1-Tie2 receptors (Sato et al. 1995), the Tie2 ligand angiopoietin 2 (Hackett et al. 2002), and insulin-like growth factor-1 (IGF-1). IGF-1 is required for maximum VEGF activation of vascular endothelial cell proliferation and survival pathways (Smith et al. 1997,1999). In premature infants, low IGF-1 levels are associated with an increased risk of ROP (Hellstrom et al. 2001, 2002, 2003; Lofqvist et al. 2006; Smith 2005), characterized by slowed, deficient development of retinal vessels.
4.3. Neurovascular Crosstalk in Retinal Vascular Development
Neurovascular interactions are important in the maintenance of the nervous system, and defects in this relationship can lead to disease. The retina, which is part of the central nervous system, requires a steady supply of nutrients and oxygen to ensure appropriate neuronal function and sensory transmission. Consequently, neural and vascular systems must be adequately paired so that oxygen and nutrient needs are matched with vascular supply. It has become clear that neurons play an important role in instigating, promoting, and steering angiogenesis within nervous tissue and specifically in the retina (Edwards et al. 2012, Fukushima et al. 2011, Sapieha et al. 2008, Sun et al. 2015a).
4.3.1. Neuronal guidance cues.
Endothelial tip cells are enriched in both receptors for angiogenic factors (such as VEGFR) and other receptors that were initially described as neuronal guidance cues but are now understood to function as vascular guidance cues as well as coordinate neural demands with vascular growth (Huber et al. 2003). Tip cells probe the tissue environment through their projecting filopodia and guide the nascent vessel toward gradients of growth factors or guidance cues to its appropriate destination. These neuronal guidance receptors include neuropilins and plexins (for semaphorins), Unc5b (uncoordinated-5 homolog b), neogenin and DCC (deleted in colorectal carcinoma) (for netrins), Eph receptors (for ephrins), and roundabouts (for slits) (Wilson et al. 2006). Tip cells respond to a given cue by advancing, stalling, turning, or retracting depending on the overall intracellular environment of the tip cells (Larrivée et al. 2009). The role of neuronal guidance cues in vascular growth has been comprehensively reviewed elsewhere (De Smet et al. 2009, Gelfand et al. 2009, Larrivée et al. 2009).
4.3.2. Influence of retinal ganglion cells on retinal vascular growth.
Among retinal neuron cell types, retinal ganglion cells (RGCs) are the most anatomically coupled with the superficial retinal vascular plexus. A role for RGCs in vascular development was established in mouse models of genetic ablation of RGCs (Edwards et al. 2012, Sapieha et al. 2008). For example, in transgenic mice that express a toxin in newly formed RGCs to eliminate RGCs as they form (Mu et al. 2005), astrocytic networks remain largely intact, yet these mice are completely devoid of a retinal vascular plexus (Sapieha et al. 2008). Similarly, Math 5−/− mice, which lack 95% of RGCs, do not form a functional retinal vascular layer (Edwards et al. 2012). However, whether astrocytes or retinal neurons such as RGCs are primary drivers of developmental retinal vascular growth is under debate, as evidence has been provided for both. One possible explanation for the contribution of each cell population during retinal vascular plexus formation is that astrocytes contribute to trophic support of vessels by providing a template for growth (Fruttiger et al. 1996, Uemura et al. 2006) and retinal neurons drive vascular growth to ensure their own metabolic support (Sapieha 2012). In addition, cell-specific depletion of VEGF, HIF-1α, or HIF-2α in astrocytes has no effect on developmental retinal vascularization (Weidemann et al. 2010), whereas neuron-specific knockout of HIF-1 a disturbs retinal vascular development (Caprara et al. 2011), suggesting that a neuronal cell population such as RGCs, rather than astrocytes, provides angiogenic factors during retinal development.
In addition, neurovascular crosstalk governs the progression of retinopathy via SOCS3 (suppressor of cytokine signaling 3) (Sun et al. 2015a). Specifically, SOCS3 deficiency in neuronal and glial cells promotes pathological retinal angiogenesis in retinopathy via titrating VEGF signaling. A novel way to suppress retinal angiogenesis may be by inhibiting through SOC3 only the excess VEGF, which is the major cause of proliferative retinopathy. This method may avoid the problems in current treatments that inhibit all VEGF, which is also important for normal vessel growth and maintenance (Figure 2).
Figure 2:
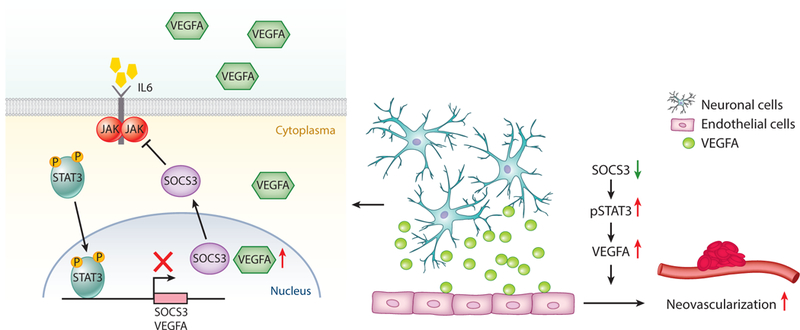
Neuronal and glial cell regulation of pathological neovascularization in the eye via STAT3 and SOCS3 signaling pathways. SOCS3 deficiency in neuronal or glial cells promotes pathological retinal angiogenesis in retinopathy via titrating VEGF signaling controlled by activated transcription factor STAT3. A red arrow indicates upregulation, and a green arrow indicates downregulation. Abbreviations: IL-6, interleukin-6; JAK, Janus kinase; SOCS3, suppressor of cytokine signaling 3; STAT3, Signal transducer and activator of transcription 3; VEGF, vascular endothelial growth factor. Figure adapted from Sun & Smith (2015), with permission from Atlas of Science.
4.3.3. Influence of photoreceptors on retinal vascular growth: inflammatory signals in photoreceptors.
In addition to RGCs, photoreceptors may play a significant role in determining vascular growth. Photoreceptors are the most abundant neuronal cell population in the retina and have the highest oxygen demand (Wangsa-Wirawan & Linsenmeier 2003). Photoreceptors (rods and cones) have an important role in the pathogenesis of ROP (Akula et al. 2007, Hansen et al. 2017). Previous studies of the role of photoreceptors in ROP focused mainly on photoreceptor function during ROP development. Cone photoreceptors develop early before birth with approximately the same birthdate as the other early-born neurons such as RGCs and horizontal cells. Rod photoreceptors develop later than cones (Cepko 2015). The onset of neovascularization in ROP is coincident with the development of the rod outer segment (Fulton et al. 1999). Clinical studies found that ROP has less effect on cone photoreceptors than on rod photoreceptors (Hansen & Fulton 2005, Fulton et al. 2008). Photoresponses suggest that cone photoreceptors are more resistant to the ROP disease process. The similar shape of the b-wave stimulus-response functions in preterm infants and controls implies that ROP does not alter the balance of ON and OFF signals in the cone photoreceptor pathway, suggesting that ROP pathology likely originates from rods instead of cones.
There is evidence that photoreceptors control vascular development. The onset of neovascularization in ROP is found at approximately 32 weeks of gestational age (Hansen et al. 2017) no matter the gestational age at birth, which is coincident with rod maturation and outer segment development (Fulton et al. 1999). In addition, patients who have proliferative DR and then develop retinitis pigmentosa with progressive photoreceptor loss show considerably less pathological retinal angiogenesis than diabetic patients with healthy photoreceptors (Sternberg et al. 1984). Similarly, mice with genetically ablated photoreceptors (Pdebrd1 mutant mice) fail to develop retinal neovascularization in the mouse model of OIR (Lahdenranta et al. 2001).
Inflammatory mediators are involved in retinopathy (M. Chen et al. 2011, Hong et al. 2014, Lee & Dammann 2012). Photoreceptors produce soluble inflammatory factors, which in turn can induce inflammatory changes in nearby cells in diabetes (Tonade et al. 2016), suggesting that photoreceptors might play a role in diabetes-induced degeneration of retinal capillaries. Although inflammation is often thought to come from infiltrating inflammatory cells, including macrophages and neutrophils, photoreceptors also signal for blood vessel growth through inflammatory proteins (Huang et al. 2011, Sun et al. 2017, Tonade et al. 2016). In photoreceptors, transcription factor c-Fos, an immediate early gene and pro-oncogene, acting as a master regulator of many inflammatory factors, controls retinal angiogenesis by modulating photoreceptor-derived inflammatory signals in a retinal angiogenesis model, Vldlr−/− mice (Sun et al. 2017). Inflammatory signaling pathways such as c-Fos can be used by stressed photoreceptors to convey a need for blood vessels and are potential upstream targets to control the development of neovascularization (Figure 3).
Figure 3:
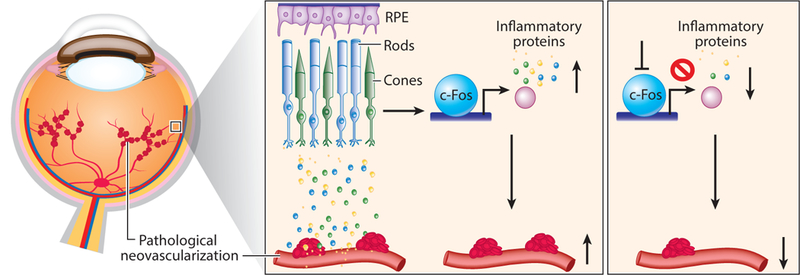
Photoreceptor regulation of pathological neovascularization in the eye via control of inflammatory signals in photoreceptors. Targeting inflammatory regulator c-Fos in photoreceptors to control inflammatory proteins may provide a novel approach to suppress pathological ocular neovascularization, which leads to blindness. Abbreviation: RPE, retinal pigment epithelium.
4.3.4. Influence of lipid and glucose metabolism on retinal angiogenesis.
Energy metabolism needs of retinal neurons may modulate vascular network development (Joyal et al. 2018). In retinal neurons, the cellular signaling events triggered by energy metabolites such as lactate (Ahmed et al. 2010), α-ketoglutarate, and succinate (He et al. 2004) have been proposed as contributors to retinal vascularization (Joyal et al. 2016, Sapieha et al. 2008). We find that glucose metabolism of photoreceptors controls normal retinal vascular development through adiponectin (Fu et al. 2018).
Importantly we have shown that lipids (as well as glucose) are used as fuel in photoreceptors (Joyal et al. 2016). Dyslipidemia is associated with AMD (Haines et al. 2006, Lim et al. 2012). Poor lipid uptake in Vldlr−/− mice (Goudriaan et al. 2004) with AMD-like RAP and CNV results in high levels of circulating triglycerides and fatty acids (Goudriaan et al. 2004). Vldlr is expressed in tissues with a high metabolic rate, including photoreceptors (Dorrell et al. 2009, Joyal et al. 2016), and facilitates the uptake of triglyceride-derived fatty acids (Lopaschuk et al. 2010). High levels of circulating lipids in Vldlr−/− mouse retinas signal through GPR40 to suppress expression of the glucose transporter Glut1. Because loss of Vldlr also results in poor lipid uptake in photoreceptors, GPR40-mediated suppression of Glut1 and glucose entry into photoreceptors in Vldlr-−/− mice result in a dual (lipid and glucose) fuel shortage and a reduction in the levels of the tricarboxylic acid cycle intermediate α-ketoglutarate. Low levels of α-ketoglutarate promote the stabilization of HIF1 a and the secretion of Vegfa by starved Vldlr−/− photoreceptors, leading to neovascularization (Joyal et al. 2016). Dysregulated lipid and glucose photoreceptor energy metabolism might be a driving force in neovascular AMD and other retinal diseases.
The retina is rich in lipids derived from essential omega-3 and omega-6 long-chain polyunsaturated fatty acids, which are critical for many retinal functions. However, they are unlikely to be used as fuel because they are essential fatty acids that must be obtained through diet, as humans lack key enzymes to synthesize a-linolenic acid (omega-3) and linoleic acid (omega-6) (SanGiovanni & Chew 2005). These essential dietary lipids and their metabolites are structural constituents of membranes and can regulate retinal neovascularization and CNV (Connor et al. 2007, Fu et al. 2015, Gong et al. 2016, Gong et al. 2017, Sapieha et al. 2011, Stahl et al. 2010b).
Development of pathological neovascularization in vascular eye diseases is linked to dysregulation of both lipid metabolism (Chew et al. 2010, Keech et al. 2007) and altered inflammation and macrophage function or polarization (Ferguson & Apte 2008). Lipid-induced alteration in inflammatory response is mediated in part by macrophages, which are phenotypically plastic with different polarization states (M1-and M2-like), playing critical roles in regulating ocular angiogenesis during development and in pathologies (Combadière et al. 2007). RORα (retinoic-acid-receptor-related orphan receptor alpha), a lipid-sensing nuclear receptor and transcription factor (Solt & Burris 2012) that regulates lipid homeostasis and inflammatory cell differentiation and cytokine production (Jetten 2009), was reported to control pathological angiogenesis through direct transcriptional control of Socs3, suggesting the role of lipid-metabolism-driven inflammation in ocular angiogenesis (Sun et al. 2015b) (Figure 4).
Figure 4:
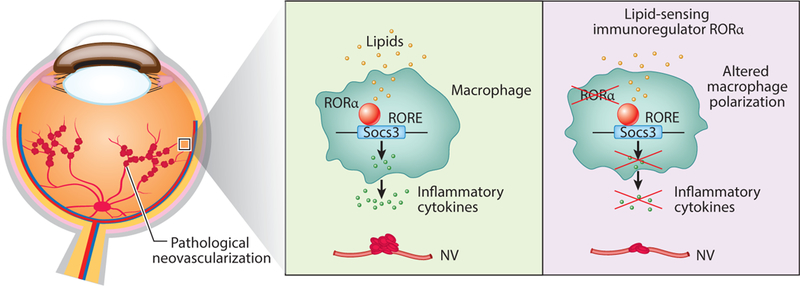
Regulation of lipid-metabolism-driven inflammation of pathological neovascularization in the eye via the RORα-SOCS3 pathway. RORα senses lipid metabolites and regulates target gene expression through binding to RORE elements in their promoter region. RORα regulates pathological neovascularization (NV) in the eye via control of tissue inflammation and macrophage polarization. Additional abbreviations: RORa retinoic-acid-receptor-related orphan receptor alpha; RORE, RORα responsive element; SOCS3, suppressor of cytokine signaling 3.
4.4. Influence of Immune Response and Inflammation on Retinal Vessel Development
Blood vessels develop accompanied by microglia, the primary resident immune cells of the retina. Microglia are monocyte-derived tissue macrophages of the central nervous system. Retinal microglia populate the mammalian retina before it becomes vascularized (Dejda et al. 2014) and are rapidly activated after an inflammatory insult (Santos et al. 2008). There is evidence that microglia associate with nascent vessels to modulate normal angiogenesis (Kubota et al. 2009, Stefater et al. 2011).
The immune response and inflammation are also associated with pathological angiogenesis. There may be an increased risk for ROP in the premature neonate because of an immature immune system (M. Chen et al. 2011). There are higher rates of ROP in infants born to mothers having evidence of chorioamnionitis than in those born to mothers without signs of inflammation (Moscuzza et al. 2011). On the basis of current knowledge about ROP etiology and pathogenesis, it seems likely that O2/VEGF-related ROP risk is modified by systemic inflammation. The role of anti-inflammatory drugs in preventing severe ROP in the presence of systemic inflammation, and possibly even without systemic inflammation, should be investigated (Lee & Dammann 2012). Recent work suggests a strong link between the SOCS3 signaling pathway and ROP (Stahl et al. 2012; Sun et al. 2015a,b). SOCS proteins are key physiological regulators of both innate and adaptive immunity and inflammatory responses. SOCS3 positively and negatively regulates macrophage and dendritic cell activation, respectively, and is essential for T cell development and differentiation. Evidence is also emerging of the involvement of SOCS proteins in diseases of the immune system (Yoshimura et al. 2007) and the involvement of the immune system in ocular neovascularization.
In addition, inflammatory pathways, including Toll-like receptors (Imai et al. 2008), and the transcriptional activator nuclear factor kappa B (de Oliveira-Marques et al. 2007) may also be modulated by oxidative stress (Vento et al. 2009). Increases in oxidized lipids and hydrogen peroxide in preterm infants are likely because of supplemental oxygen at birth. Activation of inflammatory pathways by oxygen by-products can potentiate inflammatory responses and promote further endothelial injury, resulting in generalized systemic inflammation (Volpe 2008). Prolonged exposure to supplemental oxygen and systemic inflammation [plasma interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNFα)] are strongly associated with severe ROP (Silveira et al. 2011). Inflammatory processes might interfere with normal retinal vascularization in preterm retinas (Dammann 2010). Loss of TNFα in the OIR mouse model appears to be protective (Gardiner et al. 2005). Omega-3-polyunsaturated fatty acids decrease the size of the avascular area, and this protective effect may be mediated in part via suppression of TNFa (Connor et al. 2007). Markers of inflammation also appear to be associated with ROP inhuman studies (Lavoie et al. 2010). Over the first three postnatal weeks, the systemic levels ofeight cytokines and chemokines were different in infants with ROP compared with controls, and co-occurrence of immaturity, proinflammatory cytokines, and neonatal systemic inflammation is associated with increased risk for ROP. Besides the OIR model, the expression levels of inflammatory cytokine (IL-6 and TNFα) modulated by key inflammatory factor c-Fos in photoreceptors are increased in the retinas of the Vldlr−/− mouse model (Sun et al. 2017).
5. CONCLUDING REMARKS
Visual function in the eye is supported by retinal and choroidal vessels. Pathologies in either vascular bed can cause significant visual impairment: Retinal neovascularization occurs in proliferative retinopathy (including DR and ROP), and CNV is seen in AMD. DR, ROP, and neovascular AMD are associated with rapid deterioration of visual function (Lim et al. 2012, Zhang et al. 2012). Although most patients with DR or ROP do not advance to the proliferative stage, once this progression occurs, it can cause profound vision loss. Similarly, although CNV occurs in only 10% of AMD patients, it accounts for up to 90% of vision loss associated with AMD (Apte et al. 2006). Pathological neovascularization continues to be the leading cause of vision loss, and advances in understanding the underlying mechanisms of angiogenesis have led to the development of therapeutics effective in decreasing visual morbidity. This article summarizes the current knowledge of the cellular and molecular processes that govern both physiological and pathological retinal vascular development, which is dependent on the interactions among retinal cell populations, including neurons, glia, immune cells, and vascular endothelial cells. We also review the animal models currently used for studying retinal vascular development and the key factors that influence vascular growth. We anticipate that the identification of key regulators that globally influence pathological neovascularization will lead to the development of new targeted therapies for preventing and treating these debilitating neurovascular eye diseases.
SUMMARY POINTS
The retina is supplied by two blood sources: the central retinal artery for the inner retina and the choriocapillaris for the retinal pigment epithelium and outer retina. Pathological neovascularization, which threatens vision, can arise from either vascular bed.
Both oxygen and fuel deficits in neurons play a major role in pathological neovascularization. Neuronal and vascular interactions are important in modulating angiogenesis in the retina.
Immune response and inflammation influence retinal vessel development.
Genetic and injury mouse models of intraretinal and subretinal angiogenesis, deficient intraretinal angiogenesis, and CNV are available.
FUTURE ISSUES
Photoreceptor energy demands may play a significant role in determining vascular growth.
Lipid metabolism in photoreceptors is an important aspect of energy metabolism modulating the vascular network.
Pathological neovascularization driven by inflammatory signals may originate from photoreceptors and may be linked to photoreceptor metabolism.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health/National Eye Institute (EY024864, EY017017, P01 HD18655), the Lowy Medical Research Institute, the European Commission FP7 project 305485 PREVENTROP, and the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center (BCH IDDRC, 1U54HD090255) for L.E.H.S.; and by the Boston Children’s Hospital Office of Faculty Development/Basic Translational Executive Committee/Clinical and Translational Research Executive Committee Faculty Career Development grant for Y.S.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.Annu. Rev. Vis. Sci. 2018. 4:101–22
LITERATURE CITED
- Ahmed K, Tunaru S, Tang C, Müller M, Gille A, et al. 2010. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. CellMetab. 11:311–19 [DOI] [PubMed] [Google Scholar]
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, et al. 1995. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. PNAS 92:10457–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. 2007. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Investig. Ophthalmol Vis. Sci. 48:4351–59 [DOI] [PubMed] [Google Scholar]
- Al-Latayfeh M, Silva PS, Sun JK, Aiello LP. 2012. Antiangiogenic therapy for ischemic retinopathies. Cold Spring Harb. Perspect. Med. 2:a006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Apte B, Hollyfield JG. 2011. Developmental anatomy of the retinal and choroidal vasculature In The Retina and Its Disorders, ed. Besharse J, pp. 9–15. Oxford, UK: Academic [Google Scholar]
- Apte RS, Richter J, Herndon J, Ferguson TA. 2006. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLOS Med. 3:e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N, Ward B, Serpell G. 1954. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br. J. Ophthalmol. 38:397–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Grebe R, Hasegawa T, Bhutto I, Merges C, et al. 2009. Maturation of the fetal human choriocapillaris. Investig. Ophthalmol. Vis. Sci. 50:3503–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bek T 2013. Regional morphology and pathophysiology of retinal vascular disease. Prog. Retin. Eye Res. 36:247–59 [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, et al. 2012. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379:2162–72 [DOI] [PubMed] [Google Scholar]
- Bottoni F, Massacesi A, Cigada M, Viola F, Musicco I, Staurenghi G. 2005. Treatment ofretinal angiomatous proliferation in age-related macular degeneration: a series of 104 cases of retinal angiomatous proliferation. Arch. Ophthalmol. 123:1644–50 [DOI] [PubMed] [Google Scholar]
- Campochiaro PA. 2015. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 49:67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Jensen LD, Soll I, Hauptmann G, Cao Y. 2008. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLOS ONE 3:e2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara C, Grimm C. 2012. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog. Retin. Eye Res. 31:89–119 [DOI] [PubMed] [Google Scholar]
- Caprara C, Thiersch M, Lange C, Joly S, Samardzija M, Grimm C. 2011. HIF1Ais essential for the development of the intermediate plexus of the retinal vasculature. Investig. Ophthalmol. Vis. Sci. 52:2109–17 [DOI] [PubMed] [Google Scholar]
- Cepko CL. 2015. The determination of rod and cone photoreceptor fate. Annu. Rev. Vis. Sci. 1:211–34 [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. 1995. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Investig. Ophthalmol Vis. Sci. 36:1201–14 [PubMed] [Google Scholar]
- Charbel Issa P, Gillies MC, Chew EY, Bird AC, Heeren TF, et al. 2013. Macular telangiectasia type 2. Prog. Retin. Eye Res. 34:49–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, et al. 1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–95 [DOI] [PubMed] [Google Scholar]
- Chen J, Connor KM, Aderman CM, Smith LE. 2008. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Investig. 118:526–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Stahl A, Hellstrom A, Smith LE. 2011a. Current update on retinopathy of prematurity: screening and treatment. Curr. Opin. Pediatr. 23:173–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, et al. 2011b. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation 124:1871–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Citil A, McCabe F, Leicht KM, Fiascone J, et al. 2011. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology 99:125–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, Wong TY. 2010. Diabetic retinopathy. Lancet 376:124–36 [DOI] [PubMed] [Google Scholar]
- Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, et al. 2010. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 363:233–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirco KR, Sohn EH, Stone EM, Tucker BA, Mullins RF. 2017. Structural and molecular changes in the aging choroid: implications for age-related macular degeneration. Eye 31:10–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadière C, Feumi C, Raoul W, Keller N, Rodero M, et al. 2007. CX3CR1-dependentsubretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 117:2920–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, et al. 2009. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4:1565–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, et al. 2007. Increased dietary intake of ω−3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 13:868–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswick VG, Schepens CL. 1969. Familial exudative vitreoretinopathy. Am.J. Ophthalmol. 68:578–94 [DOI] [PubMed] [Google Scholar]
- Dammann O 2010. Inflammation and retinopathy of prematurity. Acta Paediatr. 99:975–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Marques V, Cyrne L, Marinho HS, Antunes F. 2007. A quantitative study of NF-кB activation by H2O2: relevance in inflammation and synergy with TNF-α. J. Immunol. 178:3893–902 [DOI] [PubMed] [Google Scholar]
- De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. 2009. Mechanisms of vessel branching: Filopodia on endothelial tip cells lead the way. Arterioscler. Thromb. Vasc. Biol. 29:639–49 [DOI] [PubMed] [Google Scholar]
- Dejda A, Mawambo G, Cerani A, Miloudi K, Shao Z, et al. 2014. Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J. Clin. Investig. 124:4807–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Jacobson R, Yanes O, Gariano R, et al. 2009. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J. Clin. Investig. 119:611–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SL, Lopez FJ, Celkova L, Brennan K, Mulfaul K, et al. 2015. IL-18 immunotherapy for neovascular AMD: tolerability and efficacy in nonhuman primates. Investig. Ophthalmol. Vis. Sci. 56:5424–30 [DOI] [PubMed] [Google Scholar]
- Edwards MM, McLeod DS, Li R, Grebe R, Bhutto I, et al. 2012. The deletion of Math5 disrupts retinal blood vessel and glial development in mice. Exp. Eye Res. 96:147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson TA, Apte RS. 2008. Angiogenesis in eye disease: immunity gained or immunity lost? Semin. Immunopathol. 30:111–19 [DOI] [PubMed] [Google Scholar]
- Folkman J 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1:27–31 [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Calver AR, Krüger WH, Mudhar HS, Michalovich D, et al. 1996. PDGF mediates a neuronastrocyte interaction in the developing retina. Neuron 17:1117–31 [DOI] [PubMed] [Google Scholar]
- Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. 1995. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. PNAS 92:8453–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Löfqvist CA, Liegl R, Wang Z, Sun Y, et al. 2018. Photoreceptor glucose metabolism determines normal retinal vascular growth. EMBO Mol. Med. 10:76–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Löfqvist CA, Shao Z, Sun Y, Joyal JS, et al. 2015. Dietary ω−3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am. J. Clin. Nutr. 101:879–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, et al. 2011. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Investig. 121:1974–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton AB, Dodge J, Hansen RM, Williams TP. 1999. The rhodopsin content of human eyes. Investig. Ophthalmol. Vis. Sci. 40:1878–83 [PubMed] [Google Scholar]
- Fulton AB, Hansen RM, Moskowitz A. 2008. The cone electroretinogram in retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 49:814–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW. 2005. Inhibition of tumor necrosis factor- α improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am. J. Pathol. 166:637–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF. 2010. Special features of human retinal angiogenesis. Eye 24:401–7 [DOI] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. 2005. Retinal angiogenesis in development and disease. Nature 438:960–66 [DOI] [PubMed] [Google Scholar]
- Gelfand MV, Hong S, Gu C. 2009. Guidance from above: Common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol. 19:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Fu Z, Edin ML, Liu CH, Wang Z, et al. 2016. Cytochrome P450 oxidase 2C inhibition adds to ω−3 long-chain polyunsaturated fatty acids protection against retinal and choroidal neovascularization. Arterioscler. Thromb. Vasc. Biol. 36:1919–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Fu Z, Liegl R, Chen J, Hellstrom A, Smith LE. 2017. ω−3 and ω−6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am. J. Clin. Nutr. 106:16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Li J, Sun Y, Fu Z, Liu CH, et al. 2015. Optimization of an image-guided laser-induced choroidal neovascularization model in mice. PLOS ONE 10:e0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good WV. 2004. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. 102:233–48 [PMC free article] [PubMed] [Google Scholar]
- Goudriaan JR, Espirito Santo SMS, Voshol PJ, Teusink B, van Dijk KW, et al. 2004. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J. Lipid. Res. 45:1475–81 [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Kang SJ, Berglin L. 2010. Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 29:500–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. 2002. Angiopoietin-2 plays an important role in retinal angiogenesis. J. Cell Physiol. 192:182–87 [DOI] [PubMed] [Google Scholar]
- Haines JL, Schnetz-Boutaud N, Schmidt S, Scott WK, Agarwal A, et al. 2006. Functional candidate genes in age-related macular degeneration: significant association with VEGF, VLDLR, and LRP6. Investig. Ophthalmol. Vis. Sci. 47:329–35 [DOI] [PubMed] [Google Scholar]
- Hansen RM, Moskowitz A, Akula JD, Fulton AB. 2017. The neural retina in retinopathy of prematurity. Prog. Retin. Eye Res. 56:32–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RM, Fulton AB. 2005. Development of the cone ERG in infants. Investig. Ophthalmol. Vis Sci. 46:3458–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett ME. 2013. Pediatric Retina. Philadelphia: Lippincott Williams & Wilkins [Google Scholar]
- Hartnett ME, Penn JS. 2012. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 367:2515–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E, Sweigard H, Husain D, Olivares AM, Chang B, et al. 2014. Characterization of a spontaneous retinal neovascular mouse model. PLOS ONE 9:e106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, McLeod DS, Bhutto IA, Prow T, Merges CA, et al. 2007. The embryonic human choriocapillaris develops by hemo-vasculogenesis. Dev. Dyn. 236:2089–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, et al. 2004. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429:188–93 [DOI] [PubMed] [Google Scholar]
- Heckenlively JR, Hawes NL, Friedlander M, Nusinowitz S, Hurd R, et al. 2003. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina 23:518–22 [DOI] [PubMed] [Google Scholar]
- Hellström A, Carlsson B, Niklasson A, Segnestam K, Boguszewski M, et al. 2002. IGF-I is critical for normal vascularization of the human retina. J. Clin. Endocrinol. Metab. 87:3413–16 [DOI] [PubMed] [Google Scholar]
- Hellström A, Engström E, Hård AL, Albertsson-Wikland K, Carlsson B, et al. 2003. Postnatal serum insulinlike growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 112:1016–20 [DOI] [PubMed] [Google Scholar]
- Hellström A, Perruzzi C, Ju M, Engström E, Hård AL, et al. 2001. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. PNAS 98:5804–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HK, Lee HJ, Ko JH, Park JH, Park JY, et al. 2014. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J. Neuroinflamm. 11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Jiang A, Liang J, Meng H, Chang B, et al. 2008. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model’s retinal angiomatous proliferation. Investig. Ophthalmol. Vis. Sci. 49:407–15 [DOI] [PubMed] [Google Scholar]
- Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, et al. 2011. TNFα is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investig. Ophthalmol. Vis. Sci. 52:1336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. 2003. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 26:509–63 [DOI] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. 1966. Diabetes, a new mutation in the mouse. Science 153:1127–28 [DOI] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, et al. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133:235–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Kannan R, Hinton DR. 2016. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp. Eye Res. 142:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. 2003. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes 52:409–16 [DOI] [PubMed] [Google Scholar]
- Jetten AM. 2009. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 7:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal J-S, Gantner ML, Smith LEH. 2018. Retinal energy demands control vascular supply of the retina in development and disease: the role of neuronal lipid and glucose metabolism. Prog. Retin. Eye Res. 64:131–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal J-S, Sun Y, Gantner ML, Shao Z, Evans LP, et al. 2016. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 22:439–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, et al. 2007. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370:1687–97 [DOI] [PubMed] [Google Scholar]
- Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, et al. 2002. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch. Ophthalmol. 120:338–46 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, et al. 2009. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis J. Exp. Med. 206:1089–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM. 2009. Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am.J. Ophthalmol. 148:451–58 [DOI] [PubMed] [Google Scholar]
- Lahdenranta J, Pasqualini R, Schlingemann RO, Hagedorn M, Stallcup WB, et al. 2001. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. PNAS 98:10368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, et al. 2013. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 8:2197–211 [DOI] [PubMed] [Google Scholar]
- Larrivée B, Freitas C, Suchting S, Brunet I, Eichmann A. 2009. Guidance of vascular development: lessons from the nervous system. Circ. Res. 104:428–41 [DOI] [PubMed] [Google Scholar]
- Lavoie PM, Lavoie JC, Watson C, Rouleau T, Chang BA, Chessex P. 2010. Inflammatory response in preterm infants is induced early in life by oxygen and modulated by total parenteral nutrition. Pediatr. Res. 68:248–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Dammann O. 2012. Perinatal infection, inflammation, and retinopathy of prematurity. Semin. Fetal. Neonatal. Med. 17:26–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang D, Xia X, Wang Z, Luo L, Wen R. 2011. CCR3 and choroidal neovascularization. PLOS ONE 6:e17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. 2012. Age-related macular degeneration. Lancet 379:1728–38 [DOI] [PubMed] [Google Scholar]
- Liu CH, Wang Z, Sun Y, Chen J. 2017. Animal models ofocular angiogenesis: from development to pathologies. FASEB J. 31:4665–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfqvist C, Engström E, Sigurdsson J, Hard AL, Niklasson A, et al. 2006. Postnatal head growth deficit among premature infants parallels retinopathy of prematurity and insulin-like growth factor-1 deficit. Pediatrics 117:1930–38 [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. 2010. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90:207–58 [DOI] [PubMed] [Google Scholar]
- Luhmann UF, Lin J, Acar N, Lammel S, Feil S, et al. 2005. Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Investig. Ophthalmol. Vis. Sci. 46:3372–82 [DOI] [PubMed] [Google Scholar]
- Lutty GA, Hasegawa T, Baba T, Grebe R, Bhutto I, McLeod DS. 2010. Development of the human chorio- capillaris. Eye 24:408–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Brownstein R, Lutty GA. 1996. Vaso-obliteration in the canine model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 37:300–11 [PubMed] [Google Scholar]
- Moscuzza F, Belcari F, Nardini V, Bartoli A, Domenici C, et al. 2011. Correlation between placental histopathology and fetal/neonatal outcome: chorioamnionitis and funisitis are associated to intraven- tricular haemorrage and retinopathy of prematurity in preterm newborns. Gynecol. Endocrinol. 27:319–23 [DOI] [PubMed] [Google Scholar]
- Mu X, Fu X, Sun H, Liang S, Maeda H, et al. 2005. Ganglion cells are required for normal progenitor-cell proliferation but not cell-fate determination or patterning in the developing mouse retina. Curr. Biol. 15:525–30 [DOI] [PubMed] [Google Scholar]
- Nagai N, Ju M, Izumi-Nagai K, Robbie SJ, Bainbridge JW, et al. 2015. Novel CCR3 antagonists are effective mono- and combination inhibitors of choroidal neovascular growth and vascular permeability. Am. J. Pathol. 185:2534–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nentwich MM, Ulbig MW. 2015. Diabetic retinopathy—ocular complications of diabetes mellitus. World J. Diabetes 6:489–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter FH. 2006. Atlas of Human Anatomy. Philadelphia: Elsevier Health Sci. [Google Scholar]
- Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, et al. 1997. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am. J. Pathol. 151:281–91 [PMC free article] [PubMed] [Google Scholar]
- Olivares AM, Althoff K, Chen GF, Wu S, Morrisson MA, et al. 2017. Animal models of diabetic retinopathy. Curr. Diab. Rep. 17:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneghetti L, Ng YS. 2016. A novel endothelial-derived anti-inflammatory activity significantly inhibits spontaneous choroidal neovascularisation in a mouse model. Vasc. Cell 8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Rajaratnam VS, Collier RJ, Clark AF. 2001. The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 42:283–90 [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. 1995. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model ofretinal neovascularization. PNAS 92:905–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Foley ED, Smith LE. 1996. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch. Ophthalmol. 114:1219–28 [DOI] [PubMed] [Google Scholar]
- Poor SH, Qiu Y, Fassbender ES, Shen S, Woolfenden A, et al. 2014. Reliability of the mouse model of choroidal neovascularization induced by laser photocoagulation. Investig. Ophthalmol. Vis. Sci. 55:6525–34 [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. 2011. Basic and therapeutic aspects of angiogenesis. Cell 146:873–87 [DOI] [PubMed] [Google Scholar]
- Rakieten N, Rakieten ML, Nadkarni MV. 1963. Studies on the diabetogenic action of streptozotocin (NSC- 37917). Cancer Chemother. Rep. 29:91–98 [PubMed] [Google Scholar]
- Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. 1996. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. PNAS 93:4851–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. 2005. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 24:87–138 [DOI] [PubMed] [Google Scholar]
- Santos AM, Calvente R, Tassi M, Carrasco MC, Martin-Oliva D, et al. 2008. Embryonic and postnatal development of microglial cells in the mouse retina. J. Comp. Neurol. 506:224–39 [DOI] [PubMed] [Google Scholar]
- Sapieha P 2012. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood 120:2182–94 [DOI] [PubMed] [Google Scholar]
- Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, et al. 2012. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr. Diabetes 2:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal J-S, et al. 2008. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 14:1067–76 [DOI] [PubMed] [Google Scholar]
- Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, et al. 2011. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω−3 polyunsaturated fatty acids. Sci. Transl. Med. 3:69ra–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, et al. 1995. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in bloodvessel formation. Nature 376:70–74 [DOI] [PubMed] [Google Scholar]
- Serreze DV, Chapman HD, Varnum DS, Gerling I, Leiter EH, Shultz LD. 1997. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J. Immunol. 158:3978–86 [PubMed] [Google Scholar]
- Shao Z, Fu Z, Stahl A, Joyal J-S, Hatton C, et al. 2014. Cytochrome P450 2C8 cu3-long-chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization—brief report. Anerioscler. Thromb. Vasc. Biol. 34:581–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira RC, Fortes Filho JB, Procianoy RS. 2011. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Investig. Ophthalmol. Vis. Sci. 52:1297–301 [DOI] [PubMed] [Google Scholar]
- Smith LE. 2005. IGF-1 and retinopathy of prematurity in the preterm infant. Biol. Neonate 88:237–44 [DOI] [PubMed] [Google Scholar]
- Smith LE, Kopchick JJ, Chen W, Knapp J, Kinose F, et al. 1997. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science 276:1706–9 [DOI] [PubMed] [Google Scholar]
- Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, et al. 1999. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 5:1390–95 [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, et al. 1994. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 35:101–11 [PubMed] [Google Scholar]
- Solt LA, Burris TP. 2012. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol. Metab. 23:619–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, et al. 2010a. The mouse retina as an angiogenesis model. Investig. Ophthalmol. Vis. Sci. 51:2813–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Willett KL, Krah NM, et al. 2009. Computer-aided quantification of retinal neovascularization. Angiogenesis 12:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Joyal J-S, Chen J, Sapieha P, Juan AM, et al. 2012. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood 120:2925–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Sapieha P, Connor KM, SanGiovanni JP, ChenJ, et al. 2010b. Short communication: PPARγ mediates a direct antiangiogenic effect of ω3-PUFAs in proliferative retinopathy. Circ. Res. 107:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater JA 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, et al. 2011. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474:511–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg P Jr., Landers MB 3rd, Wolbarsht M. 1984. The negative coincidence of retinitis pigmentosa and proliferative diabetic retinopathy. Am. J. Ophthalmol. 97:788–89 [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, et al. 1995. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 15:4738–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ju M, Lin Z, Fredrick TW, Evans LP, et al. 2015a. SOCS3 in retinal neurons and glial cells suppresses VEGF signaling to prevent pathological neovascular growth. Sci. Signal. 8:ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Lin Z, Liu CH, Gong Y, Liegl R, et al. 2017. Inflammatory signals from photoreceptor modulate pathological retinal angiogenesis via c-Fos. J. Exp. Med. 214:1753–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu C-H, SanGiovanni JP, Evans LP, Tian KT, et al. 2015b. Nuclear receptor RORα regulates pathologic retinal angiogenesis by modulating SOCS3-dependent inflammation. PNAS 112:10401–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Smith LEH. 2015. SOCS3 in neurons halts abnormal blood vessel formation in the eye. Atlas of Science, October 15 https://atlasofscience.org/socs3-m-neurons-halts-abnormal-blood-vessel-formation-m-the-eye/ [Google Scholar]
- Takahashi T, Nakamura T, Hayashi A, Kamei M, Nakabayashi M, et al. 2000. Inhibition of experimental choroidal neovascularization by overexpression of tissue inhibitor of metalloproteinases-3 in retinal pigment epithelium cells. Am. J. Ophthalmol. 130:774–81 [DOI] [PubMed] [Google Scholar]
- Tee LB, Penrose MA, O’Shea JE, Lai CM, Rakoczy EP, Dunlop SA. 2008. VEGF-induced choroidal damage in a murine model of retinal neovascularisation. Br. J. Ophthalmol. 92:832–38 [DOI] [PubMed] [Google Scholar]
- Tobe T, Okamoto N, Vinores MA, Derevjanik NL, Vinores SA, et al. 1998. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Investig. Ophthalmol. Vis. Sci. 39:180–88 [PubMed] [Google Scholar]
- Tonade D, Liu H, Kern TS. 2016. Photoreceptor cells produce inflammatory mediators that contribute to endothelial cell death in diabetes. Investig. Ophthalmol. Vis. Sci. 57:4264–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura A, Kusuhara S, Wiegand SJ, Yu RT, Nishikawa S. 2006. Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J. Clin. Investig. 116:369–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui Y, Westenskow PD, Murinello S, Dorrell MI, Scheppke L, et al. 2015. Angiogenesis and eye disease. Annu. Rev. Vis. Sci. 1:155–84 [DOI] [PubMed] [Google Scholar]
- Vähätupa M, Prince S, Vataja S, Mertimo T, Kataja M, et al. 2016. Lack of R-Ras leads to increased vascular permeability in ischemic retinopathy. Investig. Ophthalmol. Vis. Sci. 57:4898–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento M, Moro M, Escrig R, Arruza L, Villar G, et al. 2009. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 124:e439–49 [DOI] [PubMed] [Google Scholar]
- Volpe JJ. 2008. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J. Pediatr. 153:160–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. 1993. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. PNAS 90:4304–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu CH, Sun Y, Gong Y, Favazza TL, et al. 2016. Pharmacologic activation of Wnt signaling by lithium normalizes retinal vasculature in a murine model of familial exudative vitreoretinopathy. Am. J. Pathol. 186:2588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. 2003. Retinal oxygen: fundamental and clinical aspects. Arch. Ophthalmol. 121:547–57 [DOI] [PubMed] [Google Scholar]
- Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, et al. 2010. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 58:1177–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC,Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. 2009. Towards better understanding of the contributions of overwork and glucotoxicity to the β-cell inadequacy of type 2 diabetes. Diabetes Obes. Metab. 11(Suppl. 4):82–90 [DOI] [PubMed] [Google Scholar]
- Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, et al. 2006. Netrins promote developmental and therapeutic angiogenesis. Science 313:640–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J, Shi LY, Hicks W, Wang J, Hurd R, et al. 2011. Mouse model resources for vision research. J. Ophthalmol. 2011:391384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, et al. 2012. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. 2007. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7:454–65 [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhang L, Weinreb RN. 2012. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug. Discov. 11:541–59 [DOI] [PubMed] [Google Scholar]


