Abstract
Two new xanthone derivatives, named schomburgones A (1) and B (2), along with eight known compounds, including xanthones (3–8) and anthraquinones (9–10) were isolated from the bark of Garcinia schomburgkiana. Their structures were determined by spectroscopic analysis especially 1D and 2D NMR spectroscopies. All isolated compounds were evaluated for their cytotoxicity against five cancer cell lines (KB, HeLa S-3, HT-29, MCF-7 and HepG-2). Compounds 3–6 and 8 showed good cytotoxicity against all the five cancer cell lines with IC50 values in the range of 1.45–9.46 µM.
Electronic supplementary material
The online version of this article (10.1007/s11418-018-1240-8) contains supplementary material, which is available to authorized users.
Keywords: Garcinia schomburgkiana, Clusiaceae, Xanthone, Cytotoxicity
Introduction
Garcinia schomburgkiana Pierre (family Clusiaceae) is a medium-sized tree distributed in Thailand, Laos, Vietnam, and Cambodia. In folk medicine in these countries, its leaves, roots, and fruits are used for the treatment of cough, menstrual disturbances, expectorant, laxative and diabetes [1]. Previous chemical and biological studies on the chemical constituents of G. schomburgkiana showed the presence of xanthones, depsidones, biphenyls, flavonoids, triterpenoids, and phloroglucinols, some of which exhibited antimalarial activity and cytotoxicity [2], [3]. Here, we reported two new xanthone derivatives, named schomburgones A (1) and B (2), along with six known xanthones (3–8) and two known anthraquinones (9–10) from the bark of this plant. The structures of all isolated compounds were elucidated using spectroscopic methods especially 1D and 2D NMR spectroscopies and compared with their 1H and 13C NMR spectroscopic data from the literature. The cytotoxicity of all isolated compounds was evaluated using the MTT method against five cancer cell lines.
Results and discussion
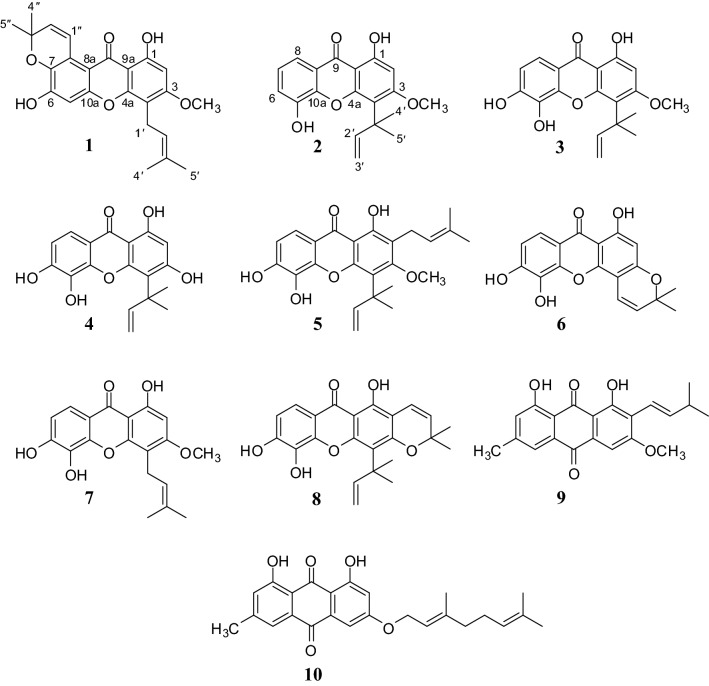
Phytochemical investigation of CH2Cl2 crude extract from the bark of G. schomburgkiana led to the isolation of two new xanthone derivatives, named schomburgones A (1) and B (2), along with eight known compounds (Fig. 1), including isocudraniaxanthone B (3) [4], gerontoxanthone I (4) [5], nigrolineaxanthone E (5) [6], isojacareubin (6) [7], dulxanthone A (7) [8], macluraxanthone (8) [9], vismiaquinone A (9) [10], and 3-geranylemodin (10) [11]. The structures of all isolated compounds were elucidated using spectroscopic methods especially NMR spectroscopies and compared with their 1H and 13C NMR spectroscopic data from the literature.
Fig. 1.
Chemical structures of 1–10
Schomburgone A (1) was obtained as yellow oil. Its molecular formula was determined as C24H24O6 by the negative HRESIMS measurement through the ion peak at m/z 407.1527 [M–H]+ (calcd. for C24H23O6, 407.1495). The UV spectrum displayed absorption bands at λmax 395, 315 and 243 nm, which is typical of the xanthone chromophore [12]. The IR spectrum showed phenolic hydroxyl groups and a hydrogen bonded carbonyl group at 3422 and 1632 cm−1. The 1H NMR spectrum showed the presence of a 3,3-dimethylallyl substituent, which was confirmed by two singlets at δH 1.67 (3H, s, H-4′) and 1.85 (3H, s, H-5′) for the vinyl methyls, a triplet at δH 5.20 (1H, t, J = 7.23 Hz, H-2′) for the vinylic proton and a doublet at δH 3.44 (2H, d, J = 7.23 Hz, H-1′) for the allylic proton of prenyl group. In addition, the methoxy signal, a hydroxyl signal, two aromatic proton signals and a hydrogen bonded hydroxyl signal appeared as five singlets at δH 3.89 (3H, s, OCH3-3), 6.26 (1H, s, OH-6), 6.33 (1H, s, H-2), 6.85 (1H, s, H-5) and 13.38 (1H, s, OH-1), respectively. The signals at δH 1.50 (6H, s, H-4″ and H-5″), 5.83 (1H, d, J = 10.23 Hz, H-2″) and 8.02 (1H, d, J = 10.23 Hz, H-1″) in the spectrum were indicative of a dimethylchromene ring. The angular fusion of the chromene ring at C-8 was deduced from the low field shift of H-1″ (δH 8.02), which was located in the deshielding area of the carbonyl group. The 1H and 13C NMR spectroscopic data (Table 1) were shown to be similar to those of the known xanthone, paxanthone B [13], except that the hydroxyl group at C-3 of paxanthone B was replaced by a methoxy group. In the HMBC correlations of 1 (Fig. 2), the methoxy proton at δH 3.89 showed a cross-peak with δC 163.5 (C-3). In addition, a methine proton at δH 8.02 showed cross-peaks with δC 77.1 (C-3″), 108.5 (C-8a), and 136.9 (C-7), confirming that a dimethylchromene ring was located at C-8, and a methylene proton at δH 3.44 showed cross-peaks with δC 131.7 (C-3′), 153.7 (C-4a), and 163.5 (C-3), indicating that a prenyl group was attached to C-4. Thus, the complete assignment of schomburgone A was determined as 1.
Table 1.
NMR spectroscopic data (400 MHz, CDCl3) for 1 and 2
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 162.0 | 163.0 | ||
| 2 | 6.33 (s) | 94.1 | 6.43 (s) | 96.2 |
| 3 | 163.5 | 166.1 | ||
| 4 | 107.2 | 113.9 | ||
| 4a | 153.7 | 154.0 | ||
| 5 | 6.85 (s) | 102.6 | 145.9 | |
| 6 | 151.1 | 7.26 (d, 7.62) | 120.3 | |
| 7 | 136.9 | 7.23 (t, 7.62) | 124.6 | |
| 8 | 119.9 | 7.71 (d, 7.62) | 116.6 | |
| 8a | 108.5 | 121.0 | ||
| 9 | 183.0 | 182.0 | ||
| 9a | 103.9 | 104.1 | ||
| 10a | 153.5 | 144.8 | ||
| 1′ | 3.44 (d, 7.23) | 21.7 | 42.0 | |
| 2′ | 5.20 (t, 7.23) | 122.4 | 6.70 (dd, 10.63,17.67) | 156.3 |
| 3′ | 131.7 | 5.22 (d, 17.67), 5.07 (d, 10.63) | 104.5 | |
| 4′ | 1.67 (s) | 25.9 | 1.61 (s) | 28.4 |
| 5′ | 1.85 (s) | 18.0 | 1.61 (s) | 28.4 |
| 1″ | 8.02 (d, 10.23) | 121.2 | ||
| 2″ | 5.83 (d, 10.23) | 132.5 | ||
| 3″ | 77.1 | |||
| 4″ | 1.50 (s) | 27.5 | ||
| 5″ | 1.50 (s) | 27.5 | ||
| 1-OH | 13.38 (s) | 13.25 (s) | ||
| 5-OH | 6.42 (s) | |||
| 6-OH | 6.26 (s) | |||
| 3-OCH3 | 3.89 (s) | 56.1 | 3.91 (s) | 56.2 |
Fig. 2.
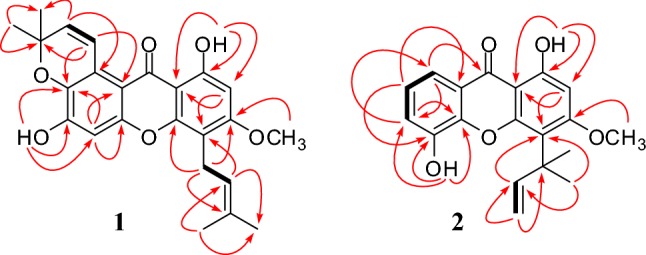
Key HMBC (arrow curves) and COSY (bold lines) correlations of 1 and 2
Schomburgone B (2) was obtained as yellow oil. Its molecular formula was determined as C19H18O5 by the negative HRESIMS measurement through the ion peak at m/z 325.1098 [M–H]+ (calcd. for C19H17O5, 325.1076). The UV spectrum displayed absorption bands at λmax 394, 315 and 244 nm. The IR spectrum showed phenolic hydroxyl groups and a hydrogen bonded carbonyl group at 3432 and 1642 cm−1. The 1H NMR spectrum showed the presence of a 1,1-dimethylallyl group, which was confirmed by a singlets at δH 1.61 (6H, s, H-4′ and H-5′) for two methyls, a doublet of doublet at δH 6.70 (1H, dd, J = 10.63 Hz, 17.67, H-2′) for the methine proton and two doublets at δH 5.07 (1H, d, J = 10.63 Hz, H-3′) and 5.22 (1H, d, J = 17.67 Hz, H-3′) for the methylene protons. Moreover, the methoxy signal, hydroxyl signal, aromatic proton signal and hydrogen bonded hydroxyl signal appeared as four singlets at δH 3.91 (3H, s, OCH3-3), 6.42 (1H, s, OH-5), 6.43 (1H, s, H-2) and 13.25 (1H, s, OH-1), respectively. The ABC-type aromatic protons were assigned at δH 7.23 (1H, t, J = 7.62 Hz, H-7), 7.26 (1H, d, J = 7.62 Hz, H-6) and 7.71 (1H, d, J = 7.62 Hz, H-8). The 1H and 13C NMR spectroscopic data (Table 1) were shown to be similar to those of the known xanthone, pancixanthone A [14], except that the hydroxyl group at C-3 of pancixanthone-A was substituted by a methoxy group. In the HMBC correlations of 2 (Fig. 2), the methoxy proton at δH 3.91 showed a cross-peak with δC 166.1 (C-3). Moreover, two methyl protons at δH 1.61 showed cross-peaks with δC 113.9 (C-4) and 156.3 (C-2′), confirming that a 1,1-dimethylallyl group was connected at C-4. Thus, the completed assignment of schomburgone B was determined as 2.
In previous research many xanthones showed cytotoxicity [15]. Therefore, all isolated compounds were evaluated in vitro for their cytotoxicity against five cancer cell lines (KB, HeLa S-3, HT-29, MCF-7 and HepG-2) (Table 2). Compounds 3–6 and 8 showed good cytotoxicity against all five cancer cell lines with IC50 values in the range of 1.45–9.46 µM. Compounds 1 and 7 showed weak cytotoxicity against all five cancer cell lines with IC50 values in the range of 34.69–73.10 µM. Compounds 2, 9 and 10 showed inactive cytotoxicity against all five cancer cell lines with IC50 values >100 µM. The SAR studied data (Fig. 1; Table 2) of xanthones suggest that the ortho hydroxy group at C-5 and C-6 and the 1,1-dimethylallyl group at C-4 might improve the cytotoxicity as inferred from the comparison of their cytotoxicity of compounds 1–10.
Table 2.
In vitro cytotoxicity of compounds 1–10 against five cancer cell lines
| Compounds | IC50 (μM) ± SD | ||||
|---|---|---|---|---|---|
| KB | HeLa S-3 | HT-29 | MCF-7 | HepG-2 | |
| 1 | 45.05 ± 2.08 | 69.22 ± 4.02 | 61.92 ± 2.40 | 52.21 ± 1.71 | 73.19 ± 1.14 |
| 2 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 3 | 5.23 ± 0.19 | 7.95 ± 0.25 | 7.87 ± 0.30 | 6.70 ± 0.81 | 5.93 ± 0.94 |
| 4 | 4.69 ± 0.21 | 7.57 ± 0.26 | 9.18 ± 0.38 | 5.26 ± 0.55 | 4.89 ± 0.83 |
| 5 | 5.08 ± 0.36 | 5.82 ± 0.15 | 4.17 ± 0.07 | 7.19 ± 0.36 | 9.46 ± 0.45 |
| 6 | 4.30 ± 0.12 | 6.60 ± 0.24 | 5.92 ± 0.40 | 3.21 ± 0.71 | 3.19 ± 0.14 |
| 7 | 38.17 ± 6.83 | 65.26 ± 3.89 | 34.69 ± 2.29 | 46.03 ± 1.29 | 54.80 ± 1.18 |
| 8 | 1.45 ± 0.09 | 1.62 ± 0.20 | 1.87 ± 0.30 | 1.70 ± 0.81 | 1.93 ± 0.94 |
| 9 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 10 | > 100 | > 100 | > 100 | > 100 | > 100 |
| Doxorubicin | 0.13 ± 0.006 | 0.03 ± 0.001 | 0.31 ± 0.07 | 0.42 ± 0.14 | 1.23 ± 0.02 |
IC50 ≤ 10 = good activity, 10 < IC50 ≤ 30 = moderate activity, IC50 > 100 = inactive
Experimental
General experimental procedures
NMR spectra were recorded on Bruker 400 AVANCE spectrometer. HRESIMS spectra were obtained using a Bruker MICROTOF model mass spectrometer. The UV–visible absorption spectra were recorded on a UV-2550 UV–Vis spectrometer (Shimadzu, Kyoto, Japan). The IR spectra were measured on a Nicolet 6700 FT-IR spectrometer using KBr discs.
Plant material
The bark of G. schomburgkiana was collected from Bang Ramat Road, Khwaeng Bang Ramat, Khet Taling Chan, Bangkok Thailand (13°45′42″N, 100°24′56″E), in June 2017. The plant material was identified by Dr. Suttira Sedlak, a botanist at the Walai Rukhavej Botanical Research Institute, Mahasarakham University, and a specimen retained as a reference (Khumkratok no. 92-08).
Extraction and isolation
The air-dried bark of G. schomburgkiana (2.0 kg) was extracted with CH2Cl2 at room temperature for 7 days (2 × 25 L). The CH2Cl2 crude extract (91.0 g) was further separated by column chromatography (CC) over silica gel CC and eluted with a gradient of Hexane–EtOAc (90, 70, 50 and 30% Hexane–EtOAc each 5 L) to give six fractions (A-F). Fraction A (4.0 g) was purified by Sephadex LH-20 column eluted with 80% CH2Cl2–MeOH (2 L) and further applied to a radial chromatography (chromatotron) with 95% hexane–EtOAc (200 mL) to afford compound 9 (3.2 mg). Fraction B (10.5 g) was purified by Sephadex LH-20 column eluted with 80% CH2Cl2–MeOH (2 L) and further applied to a chromatotron with 50% hexane–CH2Cl2 (200 mL) to obtain compounds 2 (4.2 mg), 4 (2.5 mg) and 7 (2.3 mg). Compound 1 (7.2 mg) was separated by Sephadex LH-20 column eluted with 50% CH2Cl2–MeOH (2 L) from fraction C (2.0 g). Fraction D (6.5 g) was purified by Sephadex LH-20 column eluted with 50% CH2Cl2–MeOH (2 L) to give compounds 5 (8.5 mg) and 8 (4.6 mg). Fraction E (8.5 g) was purified by Sephadex LH-20 column eluted with 50% CH2Cl2–MeOH (2 L) and further applied to a chromatotron with 70% hexane–EtOAc (200 mL) to obtain compounds 3 (5.2 mg) and 10 (5.5 mg). Finally, fraction F (1.2 g) was subjected to silica gel CC eluted with 100% CH2Cl2 and further purified by Sephadex LH-20 column eluted with 80% CH2Cl2–MeOH (2 L) to yield compound 6 (6.5 mg).
Schomburgone A (1): yellow oil; UV (CHCl3) λmax (log ε): 395 (3.6), 315 (4.2) and 243 (4.4) nm,. IR νmax (KBr): 3422 and 1632 cm−1; 1H (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectroscopic data, see Table 1; HRESIMS m/z 407.1527 [M–H]+ (calcd. for C24H23O6, 407.1495).
Schomburgone B (2): yellow oil; UV (CHCl3) λmax (log ε): 394 (3.5), 315 (4.0) and 244 (4.2) nm,. IR νmax (KBr): 3432 and 1642 cm−1; 1H (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectroscopic data, see Table 1; HRESIMS m/z 325.1098 [M–H]+ (calcd. for C19H17O5, 325.1076).
Cytotoxicity assay
All isolated compounds (1–10) were subjected to cytotoxic evaluation against KB (human epidermoid carcinoma), HeLa S-3 (human cervical carcinoma), HT-29 (human colon adenocarcinoma), MCF-7 (human breast adenocarcinoma) and HepG-2 (human liver carcinoma) cell lines employing the colorimetric method [16]. Doxorubicin was used as the reference substance which exhibits activity against five cancer cell lines. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (Sigma Chemical Co., USA) was dissolved in saline to make a 5 mg/mL stock solution. Cancer cells (3 × 103 cells) suspended in 100 μg/wells of MEM medium containing 10% fetal calf serum (Gibco BRL, Life Technologies, NY, USA) were seeded onto a 96-well culture plate (Costar, Corning Incorporated, NY, USA). After 24 h pre-incubation at 37 °C in a humidified atmosphere of 5% CO2/95% air to allow cellular attachment, various concentrations of test solution (0.1, 0.3, 1.0, 3.0, 10.0, 30.0, and 100.0 μM, each 10 μL/well) were added and these were then incubated for 48 h under the above conditions. At the end of the incubation, 10 μL of tetrazolium reagent was added into each well followed by further incubation at 37 °C for 4 h. The supernatant was decanted, and DMSO (100 μL/well) was added to allow formosan solubilization. The optical density of each well was detected using a Microplate reader at 550 nm and for correction at 595 nm. Each determination represented the average mean of six replicates. The 50% inhibition concentration (IC50 value) was determined by curve fitting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors are grateful to the Graduate School of Chulalongkorn University for a Postdoctoral Fellowship (Ratchadaphiseksomphot Endowment Fund) to SK. We also thank Dr. Suttira Sedlak, Walai Rukhavej Botanical Research Institute, Mahasarakham University, Mahasarakham 44000, Thailand for identification and deposition of the plant material.
Contributor Information
Sutin Kaennakam, Email: n-s-k-@hotmail.com.
Santi Tip-pyang, Email: santi.Ti@chula.ac.th.
References
- 1.Mungmee C, Sitthigool S, Suttisri R, Buakeaw A. Xanthones and biphenyls from Garcinia schomburgkiana wood and their cytotoxicity. Thai J Pharm Sci. 2012;36:6–9. [Google Scholar]
- 2.Le DH, Nishimura K, Takenaka Y, Mizushina Y, Tanahashi T. Polyprenylated Benzoylphloroglucinols with DNA polymerase inhibitory activity from the fruits of Garcinia schomburgkiana. J Nat Prod. 2016;79:1798–1807. doi: 10.1021/acs.jnatprod.6b00255. [DOI] [PubMed] [Google Scholar]
- 3.Sukandar ER, Siripong P, Khumkratok S, Tip-Pyang S. New depsidones and xanthone from the roots of Garcinia schomburgkiana. Fitoterapia. 2016;111:73–77. doi: 10.1016/j.fitote.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi M, Mahmud T, Yoshioka N, Shibuya H, Kitagawa I. Indonesian medicinal plants. XXI. Inhibitors of Na+/H+ exchanger from the bark of Erythrina variegata and the roots of Maclura cochinchinensis. Chem Pharm Bull. 1997;45:1615–1619. doi: 10.1248/cpb.45.1615. [DOI] [PubMed] [Google Scholar]
- 5.Chang CH, Lin CC, Kawata Y, Hattori M, Namba T. Prenylated xanthones from Cudrania cochinchinensis. Phytochemistry. 1989;28:2823–2826. doi: 10.1016/S0031-9422(00)98098-1. [DOI] [Google Scholar]
- 6.Rukachaisirikul V, Ritthiwigrom T, Pinsa A, Sawangchote P, Taylor WC. Xanthones from the stem bark of Garcinia nigrolineata. Phytochemistry. 2003;64:1149–1156. doi: 10.1016/S0031-9422(03)00502-8. [DOI] [PubMed] [Google Scholar]
- 7.Helboe P, Arends P. Xanthone studies. VI. Synthesis of jacareubin, isojacareubin, and some hydroxyxanthones with allylic substituents. Arch Pharm Chemi Sci Ed. 1973;1:549–555. [Google Scholar]
- 8.Ito C, Miyamoto Y, Nakayama M, Kawai Y, Rao KS, Furukawa H. A novel depsidone and some new xanthones from Garcinia species. Chem Pharm Bull. 1997;45:1403–1413. doi: 10.1248/cpb.45.1403. [DOI] [Google Scholar]
- 9.Wolfrom ML, Komitsky FJ, Fraenkel G, Looker JH, Dickey EE, McWain P. Macluraxanthone and two accompanying pigments from the root bark of the osage orange. Tetrahedron Lett. 1963;4:749–755. doi: 10.1016/S0040-4039(01)90710-5. [DOI] [Google Scholar]
- 10.Delle Monache F, Ferrari F, Marini-Bettolo GB, Maxfield P, Cerrini S, Fedeli W. Vismiones from Vismia baccifera var. dealdata (H. B. K.): chemistry and X-ray structure determination. Gazz Chim Ital. 1979;109:301–310. [Google Scholar]
- 11.Botta B, Delle Monache F, Delle Monache G, Marini Bettolo GB, Oguakwa JU. 3-Geranyloxy-6-methyl-1,8-dihydroxyanthraquinone and vismiones C, D, and E, from Psorospermum febrifugum. Phytochemistry. 1983;22:539–542. doi: 10.1016/0031-9422(83)83041-6. [DOI] [Google Scholar]
- 12.Kaennakam S, Siripong P, Tip-Pyang S. Kaennacowanols A-C, three new xanthones and their cytotoxicity from the roots of Garcinia cowa. Fitoterapia. 2015;102:171–176. doi: 10.1016/j.fitote.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro K, Nakajima M, Fukumoto H, Isoi K. Co-occurrence of prenylated xanthones and their cyclization products in cell suspension cultures of Hypericum patulum. Phytochemistry. 1995;38:867–869. doi: 10.1016/0031-9422(94)00664-F. [DOI] [Google Scholar]
- 14.Ito C, Miyamoto Y, Rao KS, Furukawa H. A novel dibenzofuran and two new xanthones from Calophyllum panciflorum. Chem Pharm Bull. 1996;44:441–443. doi: 10.1248/cpb.44.441. [DOI] [Google Scholar]
- 15.Vo HT, Nguyen NTT, Nguyen HT, Do KQ, Connolly JD, Maas G. Cytotoxic tetraoxygenated xanthones from the bark of Garcinia schomburgkiana. Phytochem Lett. 2012;5:553–557. doi: 10.1016/j.phytol.2012.05.012. [DOI] [Google Scholar]
- 16.Kongkathip N, Kongkathip B, Siripong P, Sangma C, Luangkamin S, Niyomdecha M. Potent antitumor activity of synthetic 1,2-naphthoquinones and 1,4-naphthoquinones. Bioorganic Med Chem. 2003;11:3179–3191. doi: 10.1016/S0968-0896(03)00226-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.