Despite abundant evidence suggesting the critical involvement of inflammation in the pathogenesis of cardiac remodeling1,2, targeting inflammatory cells in heart failure patients poses major challenges. Most inflammatory cell types exert a wide range of actions that may have context-dependent protective or detrimental functional consequences in the failing heart. Moreover, immune cells do not function as stable unidimensional effectors of a specific response, but exhibit remarkable plasticity and may undergo dramatic changes in the highly dynamic microenvironment of the failing heart.
A growing body of evidence suggests that regulatory T cells (Tregs), a subset of T lymphocytes with immunosuppressive properties, may participate in repair and remodeling of the infarcted myocardium. During the inflammatory phase of cardiac repair, Tregs infiltrate the infarcted myocardium, recruited through activation of chemokine-dependent signals3. Infiltrating Tregs play an important role in repair of the infarcted heart and protect from cardiac rupture and adverse remodeling, by driving activation of reparative macrophages4 and by modulating fibroblast phenotype5. The protective reparative functions of Tregs in the infarcted heart may involve systemic anti-inflammatory actions, local secretion of anti-inflammatory cytokines and growth factors, or contact-dependent effects. Although Tregs are key orchestrators of the reparative response following acute myocardial injury, their potential role in chronic ischemic heart failure has not been studied. Under the assumption that infarct Tregs maintain a stable anti-inflammatory phenotype, one would have expected that they may exert prolonged protective effects on the remodeling myocardium, by attenuating activation of macrophages and of effector T cells. On that basis, it has been speculated that cell therapy with Tregs may be a promising approach for patients surviving acute myocardial infarction.
In the current issue of Circulation, Bansal and co-workers6 report that, in contrast to their reparative role in myocardial infarction, Tregs in mice with chronic ischemic heart failure exhibit functional perturbations and acquire a pro-inflammatory phenotype, contributing to the progression of adverse remodeling. In a mouse model of non-reperfused myocardial infarction, chronic heart failure was associated with marked and prolonged expansion of Tregs in the heart, spleen, and circulating blood. During the chronic phase of cardiac remodeling, Tregs in failing hearts exhibited pro-inflammatory properties and impaired immunoregulatory capacity. Depletion of Tregs in mice with established post-infarction heart failure, using either a genetic approach based on a diphtheria toxin system, or treatment with an antibody to CD25, protected the heart from progressive dilation and even reversed adverse remodeling, decreasing systemic inflammation, reducing interstitial myocardial fibrosis, and attenuating capillary rarefaction.
The study challenges conventional views on the phenotypic stability of Tregs following tissue injury, demonstrating that following myocardial infarction, Tregs undergo a phenotypic transition from a “reparative” phenotype to a dysfunctional pro-inflammatory cell type that contributes to the pathogenesis of heart failure. The findings also raise several intriguing questions regarding the emerging role of Tregs in myocardial injury, repair and remodeling.
Which molecular signals mediate expansion of dysfunctional Tregs in chronic heart failure?
Traditional teachings suggest that Tregs function predominantly as immunosuppressive and anti-inflammatory cells. In contrast to these established concepts, a growing body of experimental evidence in both experimental models and in human patients suggests that under inflammatory conditions, Tregs can be reprogrammed into T helper cells that secrete pro-inflammatory cytokines7. In many pathologic conditions, the functional in vivo role of these dysregulated, pro-inflammatory Tregs remains debated. The current investigation provides robust evidence, through both genetic and pharmacologic approaches, supporting the notion that perturbed Treg function may be an essential contributor to chronic myocardial injury.
The molecular basis for the heart failure-associated perturbations in Treg phenotype and function is unknown (Figure 1). It should be emphasized that similar pro-inflammatory alterations have been reported in Tregs associated with a variety of inflammatory conditions, including proriasis, inflammatory bowel disease, arthritis and multiple sclerosis7. Thus, this common process of dysregulation may not be unique to heart failure, but may involve activation of inflammatory signaling cascades. Induction and prolonged secretion of pro-inflammatory cytokines is a hallmark of chronic heart failure; chronic cytokine stimulation may promote differentiation of Tregs into Th17-like cells with pro-inflammatory properties8. Although conversion of Tregs into other T cell subsets has not been documented in the failing heart, several mechanisms may contribute to acquisition of a pro-inflammatory phenotype in heart failure-associated Tregs. Activation of Toll-Like Receptor (TLR)-2 signaling is also prominent in failing hearts, and has been suggested to stimulate Interleukin (IL)-17A expression in Tregs9. Activation of the renin-angiotensin-aldosterone system or adrenergic stimulation promote pro-inflammatory actions in both macrophages and lymphocytes10;11 whether neurohumornal stimulation of Tregs may be involved in acquisition of a dysfunctional pro-inflammatory phenotype remains unknown. Moreover, in the remodeling failing heart, Tregs are exposed to increased mechanical stress, and are enmeshed within an interstitium enriched with a wide range of specialized extracellular matrix proteins12, capable of modulating immune cell phenotype and function. Whether activation of mechanosensitive pathways, or stimulation of matricellular actions may contribute to the perturbed phenotype of Tregs in failing hearts is unknown. Regardless of the initial activating stimulus, acquisition of a pro-inflammatory phenotype by heart failure Tregs may also involve perturbations of the Nuclear Factor (NF)-κB signaling cascade, an essential pathway for maintenance of Treg function. It has been suggested that activation of non-canonical NF-κB signaling via accumulation of NF-κB-inducing kinase may promote Treg instability inducing pro-inflammatory gene synthesis13.
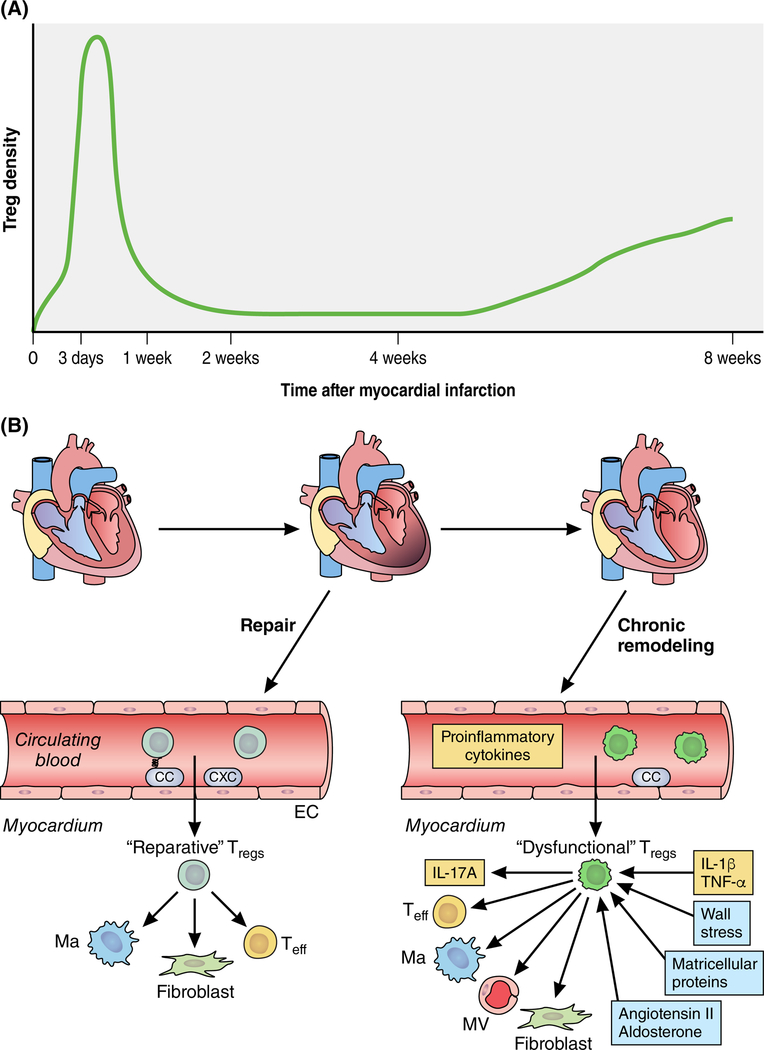
FIGURE 1: Dynamic alterations of regulatory T cell (Treg) phenotype may play an important role in repair and remodeling of the infarcted heart.
A. Tregs are recruited in the infarcted myocardium, exhibiting 2 “waves” of infiltration. The marked expansion of cardiac Tregs during the inflammatory and proliferative phase of infarct healing (3–7 days after coronary occlusion) is followed by a late, less striking increase in Treg density during the phase of chronic remodeling (8 weeks after infarction). B. Early recruitment of Tregs in the infarct is mediated through chemokine-dependent pathways3. “Reparative Tregs” play an important role in suppression of post-infarction inflammation and in repair of the infarcted heart by dowmodulating pro-inflammatory activity in macrophages (Ma) and effector T cells (Teff) and by activating fibroblasts (F)5,4. During the chronic phase of cardiac remodeling, Tregs have functional perturbations, exhibiting defective immunosuppressive and anti-inflammatory activity, producing pro-inflammatory cytokines, and exerting fibrogenic and angiostatic actions. The molecular signals promoting expansion of dysfunctional Tregs are poorly understood, but may involve chronic stimulation with pro-inflammatory cytokines, neurohumoral activation, and stimulation of Toll-like receptor signaling. The study by Bansal and co-workers6 demonstrates that dysfunctional Tregs play an important role in progression of adverse remodeling in chronic ischemic cardiomyopathy (see text). Additional symbols: EC, endothelial cell; MV, microvessel; CC, CC chemokine; CXC, CXC chemokine.
Which Treg-mediated signals exacerbate chronic cardiac remodeling?
Several cellular mechanisms may explain the adverse consequences of Treg perturbations on myocardial function. First, loss of immunosuppressive functions of Tregs may result in unrestrained activation of immune cells, leading to progressive cardiomyocyte death. Second, unopposed systemic and/or myocardial activation of effector T cells (Teff) may accentuate pro-inflammatory signaling promoting cytokine-driven myocardial dysfunction, and activating fibrogenic cascades. Third, dysfunctional Tregs may promote a pro-inflammatory phenotype in macrophages, thus amplifying the consequences of perturbed Treg function on the inflammatory milieu. Fourth, as suggested by the current study, heart failure Tregs may exert contact-dependent angiostatic effects, leading to capillary rarefaction in the non-infarcted remodeling myocardium, and accentuating ventricular dysfunction. Fifth, Tregs in failing hearts may acquire a matrix-degrading phenotype, leading to release of matrix fragments and disruption of pro-survival effects of the intact matrix on cardiomyocytes. Considering the relatively low myocardial density of Tregs in failing mouse hearts, a combination of direct actions of Tregs infiltrating the myocardium, and effects on systemic inflammatory responses may account for the striking consequences of Treg-mediated actions in heart failure
Does perturbed Treg function play a role in the pathogenesis of human heart failure?
Despite growing experimental evidence on the role of immune cells in the pathogenesis of heart failure in animal models, robust evidence on the phenotypic changes in inflammatory cell populations in human heart failure patients is scarce. Defective immunosuppressive function of circulating Tregs has been reported in a population of heart failure patients; similar patterns of dysfunction were noted in subgroups with ischemic or non-ischemic etiology14. Whether Treg dysfunction in human heart failure patents is associated with inflammatory conversion remains unknown. Deep phenotyping and characterization of Treg profiles in human patients 15 is needed to explore the potential significance of Treg dysfunction in the pathophysiologically heterogeneous human heart failure populations. Such investigations may identify heart failure patient subsets that could be considered for experimental cell therapy using healthy Tregs.
Acknowledgments
SOURCES OF FUNDING: Dr Frangogiannis’ laboratory is supported by NIH grants R01 HL76246 and R01 HL85440, and by Department of Defense grants PR151134 and PR151029.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: None.
REFERENCES
- 1.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res 2016; 119:91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 2015; 15:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol 2010; 176:2177–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 2014; 115:55–67. [DOI] [PubMed] [Google Scholar]
- 5.Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, Frangogiannis NG. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol 2014; 307:H1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and pro-inflammatory regulatory T-lymphocytes are essentual for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 2018. (in press). [DOI] [PMC free article] [PubMed]
- 7.Pandiyan P, Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine 2015; 76:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008; 112:2340–2352. [DOI] [PubMed] [Google Scholar]
- 9.Bhaskaran N, Cohen S, Zhang Y, Weinberg A, Pandiyan P. TLR-2 Signaling Promotes IL-17A Production in CD4+CD25+Foxp3+ Regulatory Cells during Oropharyngeal Candidiasis. Pathogens 2015; 4:90–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 2009; 296:R208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caillon A, Mian MOR, Fraulob-Aquino JC, Huo KG, Barhoumi T, Ouerd S, Sinnaeve PR, Paradis P, Schiffrin EL. gammadelta T Cells Mediate Angiotensin II-Induced Hypertension and Vascular Injury. Circulation 2017; 135:2155–2162. [DOI] [PubMed] [Google Scholar]
- 12.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 2017; 127:1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polesso F, Sarker M, Anderson A, Parker DC, Murray SE. Constitutive expression of NF-kappaB inducing kinase in regulatory T cells impairs suppressive function and promotes instability and pro-inflammatory cytokine production. Sci Rep 2017; 7:14779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang TT, Ding YJ, Liao YH, Yu X, Xiao H, Xie JJ, Yuan J, Zhou ZH, Liao MY, Yao R, Cheng Y, Cheng X. Defective circulating CD4CD25+Foxp3+CD127(low) regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem 2010; 25:451–458. [DOI] [PubMed] [Google Scholar]
- 15.Kordasti S, Costantini B, Seidl T, Perez Abellan P, Martinez Llordella M, McLornan D, Diggins KE, Kulasekararaj A, Benfatto C, Feng X, Smith A, Mian SA, Melchiotti R, de Rinaldis E, Ellis R, Petrov N, Povoleri GA, Chung SS, Thomas NS, Farzaneh F, Irish JM, Heck S, Young NS, Marsh JC, Mufti GJ. Deep phenotyping of Tregs identifies an immune signature for idiopathic aplastic anemia and predicts response to treatment. Blood 2016; 128:1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]