Abstract
Background:
Frequent wheezing in original Asthma Predictive Index (API) was defined by parental report of recurrent wheezing within 1 year during the first 3 years of life. The nature of frequent wheezing in children, particularly aged over 3 years has not been studied. We aimed to assess the frequency and interval of wheezing to define frequent wheezing in ascertaining asthma for children using medical records.
Methods:
Among children who participated in a previous study (n=427), all wheezing episodes documented in medical records were collected for children who had ≥2wheezing episodes PLUS met one major criteria or two minor criteria of API. We compared the distribution of known risk factors for asthma between subjects having two consecutive wheezing episodes with shorter interval (≤1year) compared to those with longer interval (1to3 years).
Results:
62 children met API at median age of 2.3 years. During follow-up period (median age: 11.3 years), a total of 198 wheezing episodes were observed. 81% of wheezing intervals were within 3 years from the earlier wheezing episode, including 60% within 1 year. Children who met API based on 1 year interval (n=40) vs. 1to3 year interval (n=13) appeared to be similar in regard to the known risk factors for asthma.
Conclusions:
Our exploratory study finding suggests that children who had frequent wheezing episodes with longer interval (<3 years) need to be considered to be determined as asthma cases when API is applied to retrospective medical records. Prospective studies with a larger sample size need to replicate this finding.
Keywords: Asthma Predictive Index (API), Heterogeneity, Medical records, Retrospective studies, Wheezing
Introduction
Asthma Predictive Index (API) was developed to predict asthma at school age using factors that were found during the first 3 years of life1 and suggested to ascertain asthma status for a timely treatment by NAEPP guidelines.2 The original API study did not define the specific number of wheezing episodes for “frequent wheezer” assessed by annual questionnaire, but was based on parent’s response to a five-item Likert-scale to measure the frequency of wheezing episodes (i.e., “frequent wheezing” which is a prerequisite for API was defined by a value of ≥3 on a scale of 1 (“very rarely”) to 5 (“on most days”)).1 Other modified API such as mAPI, m2API, and ucAPI required frequent wheezing defined by the number of wheezing episodes (e.g., 2 or 4) within the past 12 months, although they used wheezing frequency during the child’s first 3 years of life.3-5 For example, modified API (mAPI) required 4 or more wheezing episodes in the past 1 year, whereas m2API and university of Cincinnati API (ucAPI) are based on 2 or more episodes of wheezing during the same period.3-5 The original API is the only algorithm to predict asthma that was done in a general population. Its major strength is its good positive likelihood ratio (LR) ~7.4 (the effect on posttest probability of disease improved significantly), but because its sensitivity is modest, it is limited to identify asthma6,7 and has not been applied to retrospective studies based on medical records until recently by Wi et al.8,9
Given that there is no gold standard for a diagnosis of asthma, it is challenging to define 1) how many wheezing episodes, and 2) over what period of time at any given time point constitutes ‘frequent wheezing’ in terms of ascertaining asthma status, particularly if API is applied to retrospective studies using medical records (e.g., 2 vs. 3 or more wheezing episodes within 1 year vs. 3 year interval between wheezing episodes). As the needs and trends of utilizing retrospective data based on medical records for asthma research grow, a suitable operational definition of API for retrospective studies based on medical records is warranted.
Specifically, it is unclear whether a one-year period (a prior year) is a suitable interval for counting the number of wheezing episodes documented in medical records to define “frequent wheezing” to apply API. Addressing this question is important when one applies API to retrospective studies using medical records of children, particularly aged over 3 years. Given that wheezing intervals among children with asthma may increase with age and the frequency of wheezing decreases with age, wheezing intervals need to reflect this age-dependent change and be broadened. To address this question, we conducted a retrospective cohort study, which followed a random sample of the 2002-2006 population-based birth cohort. We hypothesize that subjects who met API with shorter interval (<1 year) vs. longer interval (1 to <3 years) share similar distribution of risk factors for asthma.
Methods
Study design:
This is a retrospective study nested in a 2002-2006 population-based birth cohort study, limiting to children who were born at Mayo Clinic, Rochester, Minnesota. The study identified all wheezing episodes via manual chart review up to the last follow-up date before 12/31/2015 and assessed wheezing intervals and their corresponding number of frequency of wheezing episodes among children who met API (i.e., at least two wheezing episodes with an interval of at least 3 weeks10-12 (but, no limit for maximum interval) PLUS one of the major criteria or two of the minor criteria). We analyzed the frequency of wheezing episodes and time intervals between wheezing episodes in relation to age.
Study setting:
Rochester, Minnesota, is centrally located in Olmsted County. During the study period, characteristics of the City of Rochester and Olmsted County populations were similar to those of the U.S. White population, with the exception of a higher proportion of the working population employed in the health care industry.13-15 Health care in Olmsted County, Minnesota, is virtually self-contained within the community, and when patients register with any health care providers in the community for the first time, they are asked whether they authorize using their medical records for research. If one grants the authorization (95%) for using medical record for research, each patient is assigned a unique identifier under the auspices of the Rochester Epidemiology Project (REP), which links all inpatient and outpatient clinical diagnoses and information from every episode of care to each patient and health care provider.16 St. Sauver et al reported that about 95% of children living in Olmsted County who participated in the REP were followed during their study period (2000-2010).17 This unique longitudinal population-based resource has been continuously funded by the NIH since 1966 and has been the source of over 2000 publications on the epidemiology of disease.18-21 Our previous study showed that parents sought medical evaluation for 1 of 3 mild acute illnesses of their children in our study setting,22 while about 1 out of 4 (217/800) children and adults with illnesses or injuries visited a clinician’s office nationally.23
Study subjects:
The study utilized a part of the 2002-2006 population-based birth cohort born at Mayo Clinic in Rochester, Minnesota, who had been enrolled in a previous asthma study.24 Study subjects of this present study were obtained from the original study designed to examine the relationship between late-preterm infancy and the risk of asthma. Briefly, the original study enrolled 579 subjects comprised of 282 late-preterm infants (34 0/7 to 36 6/7 weeks of gestation) and 297 gender- and birth year-matched term infants (37 0/7 to 40 6/7 weeks of gestation) randomly selected from the 2002-2006 birth cohort born in Olmsted County, Minnesota. In this present study, a total of 152 subjects were excluded due to the following reasons; 1) change in research authorization status (n=17), 2) non-Mayo Clinic patients (n=132), and 3) adopted children (n=3; API requires checking biological parental history for asthma as one of major criteria), leaving 427 study subjects eligible for this present study. The children excluded (n=152), compared to those not excluded (n=427), had similar proportion of female, family history of asthma, eczema, and allergic rhinitis, but tend to be non-White and late-preterm (data not shown). Among them, 62 subjects met the API criteria without regard to wheezing interval (i.e., no interval limit) and were included in the present study.
Asthma ascertainment by Asthma Predictive Index (API):
We operationalized the API criteria for a retrospective study and the details were previously reported.8,9 It is delineated in Table 1. Briefly, we identified all wheezing episodes with an interval of at least 3 weeks10-12 between recurrent wheezing episodes as well as the minimal wheezing interval (in years) for recurrent (2 or more) wheezing episodes.25-27 If the subject had recurrent (i.e., 2 or more) wheezing episodes, major and minor criteria of API were applied. A physician diagnosis of atopic dermatitis (or eczema) and allergic rhinitis (or hay fever) of patients and parental history of asthma were obtained from their medical records, which includes information on present family history provided by parents on a regular basis at each clinic visit. As for the item of ‘wheezing apart from cold’, ‘cold’ was defined by upper respiratory infection or cold documented, OR fever (≥100.4°F) plus runny or stuffy nose documented in medical records. Eosinophilia was considered to be present if eosinophils were ≥ 4% of the total white blood cells in the test performed during study period for any purpose.
Table 1.
Operationalization of the original Asthma Predictive Index (API)
| Original Asthma Predictive Index | Operationalized Asthma Predictive Index |
|
|---|---|---|
| Early frequent wheezer | Parental report - a value ≥3 in the scale (scale: 1 to 5, from “very rarely” to “on most days”) |
≥2 wheezing episodes at any given time on history or examination with at least 3 weeks of interval |
| Parental asthma a | Parental report (diagnosed by a physician) | Parental history of asthma documented |
| Eczema | Parental report (diagnosed by a physician) | Physician-diagnosed atopic dermatitis or eczema documented |
| Allergic rhinitis | Parental report (diagnosed by a physician) - Hay fever or any other condition that made a child’s nose stuffy, itchy, or runny apart from colds during the first 3 years of life and whether a doctor had said that these symptoms were due to allergies. |
Physician-diagnosed allergic rhinitis or hay fever documented |
| Wheezing apart from cold | Parental report | Wheezing documented without any symptom of cold b |
| Eosinophilia (≥4%) | Blood specimens obtained around at 1 year of age (10.9±0.6 month) | Blood specimen obtained up to the last follow-up date |
| Asthma Index date | N/A | The date when operationalized API was all met. |
Parental history of asthma was reviewed up to the last follow-up date.
“Cold” was defined by upper respiratory infection or Cold documented, OR fever (≥100.4◦F) plus runny/stuffy nose.
Statistical analysis:
We summarized characteristics of study subjects. The main aim of the analysis was to assess the intervals of wheezing episodes and characterize the intervals with regard to pertinent sociodemographic and clinical characteristics. Wheezing intervals of subjects who had at least two wheezing episodes with at least a 3-week interval were analyzed. For those with multiple wheezing episodes, we collected the information on intervals for two separate wheezing episodes. For example, if a patient had three wheezing episodes during the study period, two intervals (i.e., between 1st and 2nd episode and between 2nd and 3rd episode) were recorded. At the same time, we also collected data on the frequency of wheezing episodes within a one-, two-, and three-year interval at any given time since the first wheezing episode. The means and medians of intervals between wheezing episodes were calculated and then analyzed in relation to age. For the relationship between other characteristics and intervals, we used t-test for continuous variable and chi-square test for categorical variables. All analyses were performed using JMP statistical software package (Ver 10; SAS Institute, Inc, Cary, NC).
Results
Characteristics of study subjects:
Characteristics of study subjects are summarized in Table 2. Among 62 eligible children, 58% were males, 77% Whites, and the median age at the time of asthma onset by API and at last follow-up date were 2.3 years (IQR: 1.3, 5.5) and 11.3 years (IQR: 9.5, 12.1), respectively. A total of 198 wheezing episodes were observed among 62 subjects during the study period (median (IQR) per subject: 3 (2, 5)). The prevalence of asthma in this original study cohort was 14% (62/427) (95%CI: 11-17%) by API without regard to wheezing interval. About half of children included were preterm born infants, and a total of 42 (68%) children ended up having physician diagnosis of asthma during study period (~ up to 11 years).
Table 2.
Characteristics of eligible subjects who had two or more wheezing episodes and met either one of major or two of minor criteria of API
| Variables | (Total N=62) |
|---|---|
| Age, years, median (IQR) At API index date At last follow-up date (ie, follow-up duration) |
2.3 (1.3, 5.5) 11.3 (9.5, 12.1) |
| Male, n (%) | 36 (58) |
| White, n (%) | 48 (77) |
| Number of wheezing episodes, median (IQR) | 3 (2, 5) |
| Prevalence of asthma by API (+),n (%) Using 1 year of wheezing interval Using 3 year of wheezing interval |
40 (65) 53 (85) |
| Physician diagnosis of asthma, n (%) | 42 (68) |
| Asthma medication, n (%) No medication Rescue medication only Controller |
27 (44) 23 (37) 12 (19) |
| Family history of asthma, n (%) | 35 (56) |
| Maternal smoking history during pregnancy, n (%) | 11 (18) |
| History of breastfeeding, n (%) | 51 (85) |
| Eczema, n (%) | 30 (48) |
| Allergic rhinitis, n (%) | 14 (23) |
| Birth weight, kg, mean (±SD) | 3.1 (±0.7) |
| C-section, n (%) | 16 (25) |
| Premature birth, n (%) | 32 (52%) |
Prevalence of asthma by different intervals of wheezing episodes (within 1 year vs. within 3 years):
Prevalence of asthma by applying the different intervals between wheezing episodes (within 1 year vs. within 3 years) were 9% and 12%, respectively. Children who met the API criteria for asthma based on a 1-year interval between wheezing episodes (n=40) were similar to those who met the API criteria based on a 1- to 3-year interval between wheezing episodes (n=13) with regard to gender, race, API major and minor criteria (i.e., parental history of asthma, eczema, and allergic rhinitis), and other known risk factors for asthma such as smoking history during pregnancy, breastfeeding history, C-section, and birth weight (Table 3). Some API minor criteria such as wheezing apart from cold (72% (within 1 year) vs. 46% (1 to 3 years)), eosinophilia (46% (within 1 year) vs. 0% (1 to 3 years)), and physician diagnosis of asthma (80% (within 1 year) vs. 54% (1 to 3 years), appear to be different, but only approached statistical significance (e.g., Odds ratio (OR) of physician diagnosis of asthma based on a 1 to 3 year interval [95%CI]: 0.29 [0.07-1.11]; p=0.06). At the last follow-up date, 62% of children who met the API criteria for asthma based on a 1 to 3 year interval were on any asthma medication (vs. 52% among those based on a 1 year interval; OR [95%CI]: 1.45 [0.40-5.2]; p=0.57).
Table 3.
Comparison of characteristics between those who had two wheezing episodes within 1 year vs. within 1-3 years*
| Variables | Minimum interval of two wheezing episodes |
P- value |
|
|---|---|---|---|
| <1 year (n=40) | 1-3 years (n=13) | ||
| Age, years, median (IQR) At API index date At last follow-up date (ie, follow-up duration) |
1.9 (1.0, 2.8) 11.2 (9.1, 11.8) |
2.9 (2.1, 5.6) 11.5 (10.1, 13.2) |
0.01 0.18 |
| Male, n (%) | 24 (60) | 7 (53) | 0.69 |
| White, n (%) | 30 (75) | 12 (92) | 0.18 |
| Items of API (Major Criteria) Parental history of asthma, n (%) Eczema, n (%) (Minor Criteria) Allergic rhinitis, n (%) Wheezing apart from cold, n (%) Eosinophilia (≥4%), n (%) |
18 (45) 18 (45) 9 (22) 29 (72) 12/26 (46) |
7 (53) 6 (46) 3 (23) 6 (46) 0/4 (0) |
0.57 0.94 0.96 0.08 0.12 |
| Physician diagnosis of asthma, n (%) | 32 (80) | 7 (54) | 0.06 |
| Any asthma medication, n (%) | 21 (52) | 8 (62) | 0.57 |
| Maternal smoking history during pregnancy, n (%) | 8 (20) | 2 (15) | 0.68 |
| History of breastfeeding, n (%) | 32 (84) | 12 (92) | 0.46 |
| Birth weight, kg, median (IQR) | 2.8 (2.4, 3.5) | 3.1 (2.7, 3.8) | 0.20 |
| C-section, n (%) | 10 (25) | 3 (23) | 0.88 |
9 children who met API, but had minimal gap of wheezing episodes greater than 3 years were excluded.
Proportion of children with wheezing episodes in various ranges of intervals of wheezing episodes:
Only 60% among total wheezing episodes had an interval within 1 year between the first and subsequent wheezing episodes, but 73% within 2 years, and 81% within 3 years. The median interval of wheezing episodes was 0.71 years (IQR, 0.26, 1.92; range, 0.05, 11.6).
Characterization of intervals between wheezing episodes by age:
The relationship between wheezing interval (i.e., minimal wheezing interval for each patient) and age at the API index date is summarized in Figure 1. The results showed that intervals between wheezing episodes increased with age (p<.001 for trends, i.e., heteroskedasticity), possibly due to different biologic patterns of wheezing for a greater range of interval with age. Interestingly, there seems to be two clusters in patterns of wheezing intervals based on the timing of asthma onset (i.e., API index date) which occurred after about 6 years of age (i.e., Cluster A and B in Figure 1). Seven subjects in Cluster A with minimal wheezing intervals < 3 years had a range of 2-4 wheezing episodes, while only one of the seven subjects (14%) had a physician diagnosis of asthma and three subjects (43%) showed favorable response to albuterol. On the other hand, all eight subjects in Cluster B with minimal wheezing interval > 3 years had a total of two wheezing episodes, but 50% of subjects had a physician diagnosis of asthma with 50% showing favorable response to albuterol, suggesting later-onset of asthma may be associated with longer wheezing interval and less frequent wheezing episodes documented in the medical records.
Figure 1.
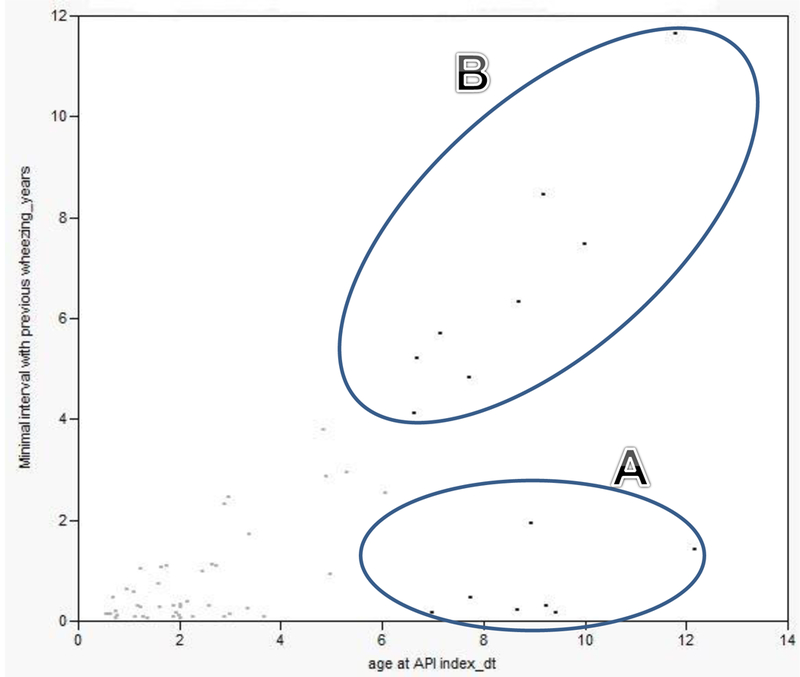
Correlation between minimal wheezing interval (in years) and the age at the API index date (i.e., one wheezing interval per each subject included)
Age at the first wheezing episode (≤ 3 vs. > 3 years of age) and minimal wheezing interval:
We assessed whether or not age at the first wheezing episode is associated with minimal wheezing intervals. The majority of children [n=55 (89%)] had their first wheezing episode at the age of 3 years or less (Figure 2). Although the minimal wheezing interval of children who had the first wheezing episode before 3 years of life (Cluster 1) tended to be longer than those of children who had the first wheezing episode after 3 years of life (Cluster 2), it was not statistically significant, possibly due to a small sample size [median (IQR): 0.31 years (0.13-1.7) vs. 0.93 years (0.17-1.9); p=0.76].
Figure 2.
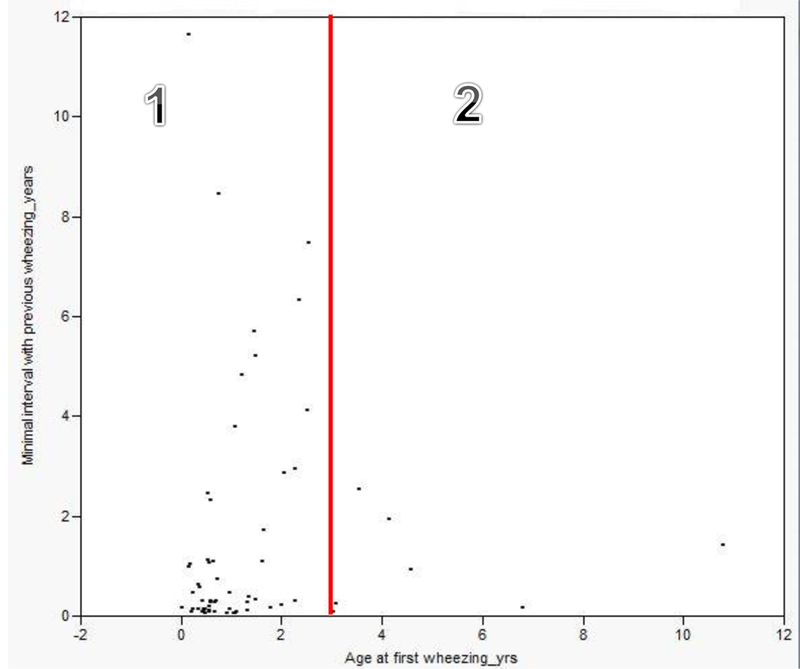
Pattern of minimal wheezing interval for each subject by age at the time of first wheezing episode (i.e., one wheezing interval per each subject included)
Discussion
To our knowledge, this is the first study that assessed the wheezing intervals and frequency in relation to age among children who met the criteria for Asthma Predictive Index (API) based on medical records. Recurrent (2 or more) wheezing episodes within an interval of 3 years documented in medical records (i.e., clinically more significant than parent-reported wheezing episodes in a survey questionnaire) as a prerequisite of API appear to be suitable for API to be applied to medical record-based retrospective studies in children (81% of wheezing episodes occur within a 3-year interval).
Apart from our previous studies, no study has assessed whether API can be used to ascertain asthma status in medical record-based retrospective studies. Identifying a suitable wheezing interval for API documented in medical records for children is important and warranted when one applies API to retrospective studies using medical records given the growing needs and trends of utilizing retrospective data based on medical records for asthma research. Furthermore, capturing wheezing episodes in preschoolers via parental report is challenging as parentally reported wheezing may be unreliable,28 other conditions (e.g., snoring, upper airway secretions, rattling sounds reflective of airway secretions or noisy breathing) could be misinterpreted as a wheeze,29 and conventional pulmonary function testing is unavailable for children under the age of 5-6 years. In our ongoing study based on an independent study cohort from a birth cohort who met API (n=64, mean age: 12.1 years (±2.9)), we also observed similar findings that only 54% of wheezing intervals between wheezing episodes were within an interval of 1 year, but 81% were within 3 years. Thus, findings from our present and ongoing studies suggest that counting wheezing episodes within one year in ascertaining asthma status based on wheezing episodes documented in medical records appears to be too stringent as almost 40% of recurrent wheezing episodes occur beyond a one-year interval and wheezing episodes captured in medical records are likely to be clinically more significant or severe. A three-yearinterval to count the number of wheezing episodes to apply API to medical records needs to be considered regardless of the purpose of application of API (e.g., asthma prediction during the first 3 years of life and asthma ascertainment beyond the first 3 years of life).
In support of this interpretation, our previous study extended the wheezing interval to three years and assessed asthma prevalence.8 We found that the mean number of wheezing episodes per year documented for children with a physician diagnosis of asthma was 2 (95%CI: 1-3) during the first 3 years of life. 8,9 Based on two or more wheezing episodes as a prerequisite for asthma ascertainment by API, 14% of children met the criteria for asthma, compared to 6% with 3 or more wheezing episodes. Yawn et al reported that the prevalence of current asthma defined by a physician diagnosis and asthma-related visit within the past 2 years was 12.9% and that of asthma ever defined by a physician diagnosis ever was 17.9% in our community.30 In this present study, based on 2 or more wheezing episodes as a prerequisite for API, asthma prevalence is 14% consistent with the prevalence estimate of the study by Yawn et al and our previous study. Thus asthma prevalence based on two or more wheezing episodes appears to be consistent with asthma prevalence in our community.
For the rationale for the 3-year interval, the literature typically defines remission of asthma as lack of asthma-related events (symptoms, medication, or medical visits) for 2 or 3 consecutive years.31,32 It implies that if one has active asthma, asthma symptoms (e.g., wheezing episodes) are likely to occur (or recur) within a period of 2-3 years. Thus, the 3-year interval for wheezing episodes for the onset of asthma is conceptually consistent with the prognosis of asthma. In this regard, although our data showed that children with frequent wheezing episodes with an interval of more than 3 years (Cluster B, Figure 1) are likely to have similar (or higher) clinical asthma features (e.g., physician diagnosis of asthma, favorable response to albuterol) as those with wheezing intervals of less than 3 years (Cluster A, Figure 1). Caution is needed given the small number of children within clusters and that observed differences between clusters could be modified by other factors. For example, if asthma treatment tended to be given more to children in either cluster before the second wheezing episode for some reason, minimal interval between wheezing episodes would be possibly extended by asthma treatment and/or reporting bias of wheezing episodes (mild episodes could not be reported in medical records). While this is plausible, we observed similar proportions of children in Cluster A vs. B who had any asthma medications at the last follow-up date (57% vs. 63%). The nature of clusters of children with regard to wheezing interval and age for meeting API needs to be further studied in a prospective study with a larger sample size. In addition, we demonstrated similarities between children who meet the API criteria based on a 1-year interval vs. a 1- to 3-year interval in demographic and clinical characteristics (i.e., known risk factors for asthma). However, the frequency of wheezing apart from cold and eosinophilia were different, potentially suggesting that extension of the interval for wheezing episodes might encompass heterogeneous subgroups of asthmatics in terms of biological and behavioral factors (e.g., early onset vs. later onset, atopic vs. non-atopic asthma or greater vs. less medical care/test seeking behavior by parents and clinicians) (Table 3).
The main implication of this study is to make API criteria available to retrospective studies based on medical records as the electronic medical record is increasingly utilized for clinical studies for asthma. In addition, retrospective API criteria can be utilized as a tool for clinical practice to identify children with asthma given the poor sensitivity of ICD codes. In addition to Predetermined Asthma Criteria (PAC), another existing asthma criteria for retrospective studies developed by the renowned asthma researchers, Drs. John Yunginger and Charles Reed,15 proper use of retrospective API criteria in conjunction with PAC will potentially enhance asthma research and care.
The main strength of our study is the unique epidemiological advantages of our study setting including a self-contained health care environment and medical record linkage system under the auspices of the REP enabling us to capture all asthma-related events in medical records since birth. Another strength is the use of a population-based sampling frame including medical records of young children since birth, instead of a convenience sample. With these strengths, our present study was able to show study findings consistent with those of our previous study, which operationalized API for retrospective studies suggesting consistency.
Our study finding needs to be carefully interpreted because our study did not capture all wheezing episodes as parents whose children have mild respiratory illnesses including wheezing episodes typically do not seek medical evaluations. For example, our previous study showed 1 of 3 parents whose children had mild acute illnesses sought medical evaluation in our study setting (or 1 of 4 illnesses at a national level seeks medical care) (i.e., differential health care access or care seeking behavior).22,23 Since this study did not capture all wheezing episodes, the nature of the systematic differences between wheezing associated with medical evaluation and those without medical evaluations are unknown. This needs to be studied in the future through a long-term prospective cohort study capturing all wheezing episodes regardless of clinic visits. For validation purpose (internal validity), we limited our study subjects to children who were born in and received medical care from Mayo Clinic to minimize the influence of heterogeneity of health care access on our study findings. Although the parent study of this study was based on a population-based birth cohort, the pattern of their wheezing episodes may not be generalizable to other populations as this cohort includes late preterm children. However, as shown in the original study,33 late preterm itself was not an independent risk factor for asthma. 33-38 Also, our study sample size was relatively small. Thus, our study findings need to be replicated in an independent study with a larger sample size to support our conclusion.
In conclusion, the interval of wheezing episodes increases with age in children. To capture all childhood asthma including late-onset asthma, we suggest an interval of 3 years between wheezing episodes to define the frequency of wheezing episodes (2 or more) for application of API to retrospective research using medical records. Our results need to be replicated and further studied in future studies with a larger sample size for prospective estimation and clinical use.
Acknowledgement
We thank the original project staff and Mrs. Kelly Okeson for her administrative assistance. This was supported by NIH-funded R01 grant (R01 HL126667).
Acknowledgement of Funding
This was supported by NIH-funded R01 grant “Asthma Ascertainment and Characterization through Electronic Health Records” (R01 HL126667). Also, this study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The funding agency was not involved in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This study was approved by the Institutional Review Board for Human Subject Research at the Mayo Clinic and the Olmsted Medical Center.
Footnotes
Conflicts of interest
All authors declare that no conflict of interest exists.
References
- 1.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403–6. [DOI] [PubMed] [Google Scholar]
- 2.Khabbaz RF, Moseley RR, Steiner RJ, Levitt AM, Bell BP. Challenges of infectious diseases in the USA. Lancet 2014;384:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilbert TW, Morgan WJ, Krawiec M, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials 2004;25:286–310. [DOI] [PubMed] [Google Scholar]
- 4.Chang TS, Lemanske RF Jr., Guilbert TW, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract 2013;1:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amin P, Levin L, Epstein T, et al. Optimum predictors of childhood asthma: persistent wheeze or the Asthma Predictive Index? J Allergy Clin Immunol Pract 2014;2:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro-Rodriguez JA, Rodriguez-Martinez CE, Custovic A. Pediatric Asthma Infantile and Preschool Asthma. Carlsen K-H, Gerritsen J ed: European Respiratory Society; 2012. [Google Scholar]
- 7.Huffaker MF, Phipatanakul W. Utility of the Asthma Predictive Index in predicting childhood asthma and identifying disease-modifying interventions. Ann Allergy Asthma Immunol 2014;112:188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wi CI, Park MA, Juhn YJ. Development and initial testing of Asthma Predictive Index for a retrospective study: an exploratory study. J Asthma 2015;52:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wi CI, Kim BS, Mehra S, Yawn BP, Park MA, Juhn YJ. Risk of herpes zoster in children with asthma. Allergy Asthma Proc 2015;36:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulet LP, Milot J, Turcotte H. Relationship between Changes in Diurnal-Variation of Expiratory Flows, Lung-Volumes and Respiratory Symptoms after Acute Asthma. Respir Med 1991;85:487–93. [DOI] [PubMed] [Google Scholar]
- 11.Whyte MKB, Choudry NB, Ind PW. Bronchial Hyperresponsiveness in Patients Recovering from Acute Severe Asthma. Respir Med 1993;87:29–35. [DOI] [PubMed] [Google Scholar]
- 12.Emerman CL, Woodruff PG, Cydulka RK, et al. Prospective multicenter study of relapse following treatment for acute asthma among adults presenting to the emergency department. Chest 1999;115:919–27. [DOI] [PubMed] [Google Scholar]
- 13.Census Bureau, 1980 and 1990 Census of Population and Housing. Washington D.C.1983 and 1993.
- 14.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Potential influence of migration bias in birth cohort studies. Mayo Clinic Proceedings 1998;73:1053–61. [DOI] [PubMed] [Google Scholar]
- 15.Yunginger JW, Reed CE, O’Connell EJ, Melton LJ 3rd, O’Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis 1992;146:888–94. [DOI] [PubMed] [Google Scholar]
- 16.Postma DS. Gender differences in asthma development and progression. Gender Med 2007;4:S133–S46. [DOI] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurland LT, Molgaard CA. The patient record in epidemiology. Scientific American 1981;245:54–63. [DOI] [PubMed] [Google Scholar]
- 19.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clinic proceedings Mayo Clinic 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Sauver Jl Fau Warner DO, Warner Do Fau Yawn BP, Yawn Bp Fau Jacobson DJ, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined american population. Mayo Clinic Proc 2013;88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocca WA, St Sauver JL, Grossardt BR, Yawn BP, Melton LJ. Use of a Medical Records Linkage System to Enumerate a Dynamic Population Over Time: The Rochester Epidemiology Project. American Journal of Epidemiology 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhn YJ, Johnson SK, Hashikawa AH, et al. The potential biases in studying the relationship between asthma and microbial infection. J Asthma 2007;44:827–32. [DOI] [PubMed] [Google Scholar]
- 23.Green LA, Fryer GE Jr., Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med 2001;344:2021–5. [DOI] [PubMed] [Google Scholar]
- 24.Voge GA, Carey WA, Ryu E, King K, Wi C, Juhn Y. What accounts for the association between late preterm births and risk of asthma? (In press). Allergy and Asthma Proceedings (Accepted on 10/14/2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emerman CL, Woodruff PG, Cydulka RK, Gibbs MA, Pollack CV Jr., Camargo CA Jr. Prospective multicenter study of relapse following treatment for acute asthma among adults presenting to the emergency department. MARC investigators. Multicenter Asthma Research Collaboration. Chest 1999;115:919–27. [DOI] [PubMed] [Google Scholar]
- 26.Whyte MK, Choudry NB, Ind PW. Bronchial hyperresponsiveness in patients recovering from acute severe asthma. Respir Med 1993;87:29–35. [DOI] [PubMed] [Google Scholar]
- 27.Boulet LP, Milot J, Turcotte H. Relationship between changes in diurnal variation of expiratory flows, lung volumes and respiratory symptoms after acute asthma. Respir Med 1991;85:487–93. [DOI] [PubMed] [Google Scholar]
- 28.Lowe L, Murray CS, Martin L, et al. Reported versus confirmed wheeze and lung function in early life. Arch Dis Child 2004;89:540–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cane RS, Ranganathan SC, McKenzie SA. What do parents of wheezy children understand by “wheeze”? Arch Dis Child 2000;82:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yawn BP, Wollan P, Kurland M, Scanlon P. A longitudinal study of the prevalence of asthma in a community population of school-age children. J Pediatr 2002;140:576–81. [DOI] [PubMed] [Google Scholar]
- 31.Javed A, Yoo KH, Agarwal K, Jacobson RM, Li X, Juhn YJ. Characteristics of children with asthma who achieved remission of asthma. J Asthma 2013;50:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair H Natural history of childhood asthma. 20-year follow-up. Arch Dis Child 1977;52:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voge GA, Carey WA, Ryu E, King KS, Wi CI, Juhn YJ. What accounts for the association between late preterm births and risk of asthma? Allergy Asthma Proc 2017;38:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dombkowski KJ, Leung SW, Gurney JG. Prematurity as a predictor of childhood asthma among low-income children. Ann Epidemiol 2008;18:290–7. [DOI] [PubMed] [Google Scholar]
- 35.Abe K, Shapiro-Mendoza CK, Hall LR, Satten GA. Late preterm birth and risk of developing asthma. J Pediatr 2010;157:74–8. [DOI] [PubMed] [Google Scholar]
- 36.Goyal NK, Fiks AG, Lorch SA. Association of late-preterm birth with asthma in young children: practice-based study. Pediatrics 2011;128:e830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Mutius E No Risk of Asthma in Late-Preterm Infants: Confounding or Misclassification? J Allergy Clin Immunol Pract 2015;3:911–2. [DOI] [PubMed] [Google Scholar]
- 38.Voge GA, Katusic SK, Qin R, Juhn YJ. Risk of Asthma in Late Preterm Infants: A Propensity Score Approach. J Allergy Clin Immunol Pract 2015;3:905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


