Abstract
Despite critical functions in cutaneous health and disease, it is unclear how resident skin microbial communities are altered by topical antimicrobial interventions commonly used in personal and clinical settings. Here we show that acute exposure to antiseptic treatments elicits rapid but short-term depletion of microbial community diversity and membership. Thirteen subjects were enrolled in a longitudinal treatment study to analyze the effects of topical treatments (ethanol, povidone-iodine, chlorhexidine, water) on the skin microbiome at two skin sites of disparate microenvironment: forearm and back. Treatment effects were highly dependent on personalized and body site-specific colonization signatures, which concealed community dynamics at the population level when not accounted for in this analysis. The magnitude of disruption was influenced by the identity and abundance of particular bacterial inhabitants. Lowly abundant members of the skin microbiota were more likely to be displaced, and subsequently replaced by the most abundant taxa prior to treatment. Members of the skin commensal family Propionibactericeae were particularly resilient to treatment, suggesting a distinct competitive advantage in the face of disturbance. These results provide insight into the stability and resilience of the skin microbiome, while establishing the impact of topical antiseptic treatment on skin bacterial dynamics and community ecology.
INTRODUCTION
Skin represents a unique habitat, colonized by an equally unique set of microorganisms (Grice and Segre, 2011). Previous studies have analyzed these residents in-depth, describing a stable community distinguished by both inter- and intrapersonal differences (Costello et al., 2009, Grice et al., 2009) and the distribution of microbial residents at distinct biogeographic regions (Oh et al., 2014). Microbial residents have important roles in skin health, including immune stimulation and tolerance, and colonization resistance to pathogenic skin microorganisms (Naik et al., 2015, Nakatsuji et al., 2017, Scharschmidt et al., 2017, Zipperer et al., 2016).
Despite these functions, humans are constantly working to disrupt skin microbial communities in personal and clinical settings (Aiello et al., 2008, Dumville et al., 2015, Hovi et al., 2017, Septimus and Schweizer, 2016). While antimicrobial agents are largely employed to reduce infection by pathogenic microorganisms (Digison, 2007, Echols et al., 2015, Lopez-Gigosos et al., 2017), these treatments can also act on resident cutaneous species (Beausoleil et al., 2012, Carty et al., 2014, Olson et al., 2012). This is especially true for antiseptics, a group of antimicrobial agents used specifically for their indiscriminate mechanisms of action (Kampf and Kramer, 2004, McDonnell and Russell, 1999). As the significance of cutaneous resident microorganisms becomes increasingly apparent, assessing the impact of these treatments on the stability and resilience of skin microbiota becomes equally important. We recently illustrated the potential for altered skin bacterial communities to impact colonization by Staphylococcus aureus in murine models, while others have expounded their importance in cutaneous diseases such as atopic dermatitis (Gao et al., 2007, Kobayashi et al., 2015, Kong et al., 2012). These studies have highlighted the significance of skin microbial residents, and necessitated further research into treatment-derived perturbations.
To expand our knowledge in this regard, we designed a longitudinal treatment study to analyze how a “pulse” disturbance generated by topical antiseptics influences skin microbial community ecology using 16S ribosomal RNA (rRNA) gene sequencing. A single treatment was sufficient to elicit a significant impact on skin communities that was personalized and body site-specific. Certain microorganisms were more likely to be perturbed than others, with both abundance and bacterial identity representing key predictors of this response. These results further our understanding of stability and resilience of cutaneous microbial communities in the face of perturbation, and outline the potential for topical treatments to disrupt skin bacterial residents.
RESULTS
Thirteen subjects were recruited to evaluate the effects of short-term antiseptic treatment on the skin microbiome. Treatments were applied to the volar forearm and the upper back to evaluate alternate skin microenvironments, and each subject received identical treatments to control for interpersonal variability. Subjects received water and alcohol (80% ethanol) on contralateral body sites during their first series of visits, and povidone-iodine and chlorhexidine during their second series of visits, with two weeks separating visit series. Swab specimens were collected at baseline, prior to treatment, and at 6 time points post-treatment (1, 6, 12, 24, 36, and 72 hours; Fig. S1a). Treated body sites were also accompanied by adjacent, untreated control sites. Specific treatment topography, timing, and subject demographics are provided in Fig. S1a and Table S1.
Baseline characteristics of skin microbiota in study cohort
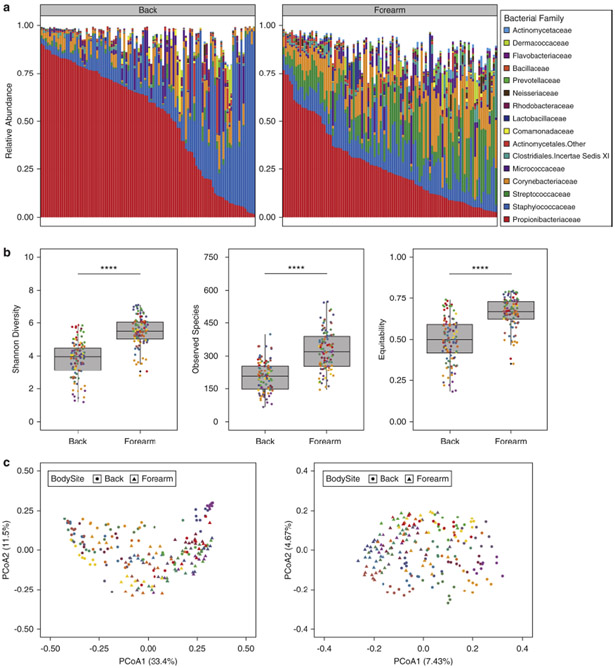
As previously reported (Grice et al., 2009, Oh et al., 2014), we observed a strong impact of biogeography on the skin microbiota. Back communities were dominated by Propionibacteriaceae and Staphylococcaceae (Fig. 1a). By contrast, forearm communities were more permissive, hosting increased proportions of additional taxa including Streptococcaceae and Corynebacteriaceae. Alpha diversity was significantly higher on the forearm compared to back by multiple metrics, including Shannon diversity, observed species, and equitability (Fig. 1b). At the population-level, prominent clustering of subjects and body sites was observed by both weighted and unweighted UniFrac metrics (Fig. 1c). Interpersonal variability and site-specificity were the most significant contributors to variation, followed by time and body symmetry respectively (Fig. S1b-c).
Fig. 1.
Skin bacterial communities exhibit site-specificity and interpersonal variability at baseline. (A) Family-level relative abundances of baseline communities for subjects at the forearm and back. Each bar represents an individual sample with eight samples per subject based on controls at adjacent and contralateral body sites for each visit series. (B) Alpha diversity of baseline communities at the forearm and back. Shannon diversity, observed species, and equitability are illustrated separately. Each point is colored by subject. (C) Weighted (left) and unweighted (right) UniFrac principal coordinates analyses of baseline samples. Each point is colored by subject and shaped by body site. **** P < 0.0001 by Wilcoxon rank sum test (Mann-Whitney U test).
Treatment elicits personalized and site-specific shifts to skin bacterial community structure
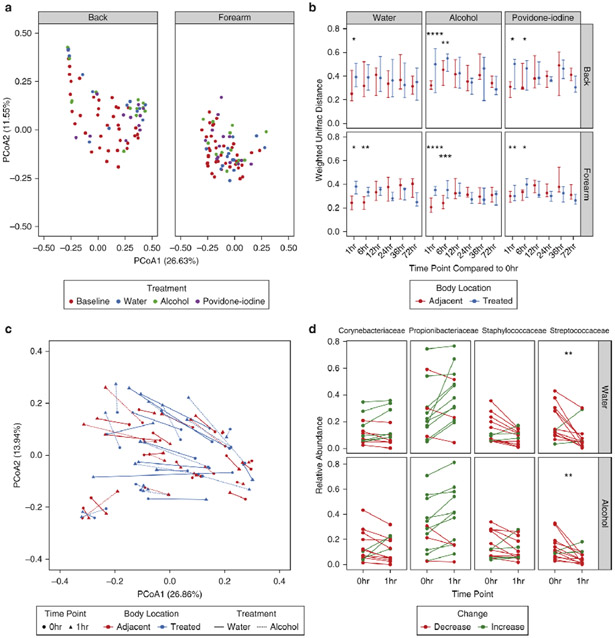
In our initial analyses we observed that chlorhexidine had very minimal effects on the skin microbiota, which was surprising given its proven efficacy against pathogenic microorganisms in hospital settings (Milstone et al., 2008). We performed a series of experiments to conclude that chlorhexidine treatment confounds DNA-based metrics and their interpretation (See Supplemental Results). We therefore focused additional investigations on water, alcohol, and povidone-iodine treatments. We first compared baseline microbial communities to posttreatment communities at the 1 hour timepoint. By the weighted UniFrac metric, treatment was unable to elicit a significant shift in bacterial community structure (Fig. 2a). Because interpersonal differences were the strongest contributors to variability in baseline samples and could thus mask more subtle effects of treatment, we further controlled for interpersonal variation. This method revealed a significant effect of both water and alcohol at the forearm, but not the back, for 6 hours post-treatment (Fig. 2b). While both treatments caused a more robust shift in forearm communities than that seen in adjacent controls, neither shifted bacterial communities to a state outside that of the broader study cohort (Fig. 2c). Comparisons of alpha diversity and bacterial burden also confirmed these effects with alcohol eliciting significant decreases in diversity at the forearm, but not the back. However, water and alcohol were found to decrease overall bacterial load at each body site (Fig. S2a-b).
Fig. 2.
Treatment elicits personalized shifts in weighted comparisons of skin bacterial populations. (A) Principal coordinates analysis of weighted UniFrac distances for treated body sites at baseline and 1hr post-treatment. Each point represents a single sample, colored by treatment and shaped by body site. (B) Weighted UniFrac distances of subjects’ longitudinal time points compared to their individual baseline communities at treated and adjacent body sites. Points represent the median of participants. Error bars designate interquartile regions. (C) Subanalysis of weighted UniFrac distances visualized by principal coordinates analysis in subjects treated with water and alcohol at the forearm. Lines connect baseline and 1hr post-treatment samples for individual subjects, and line types designate treatment regimen. Line colors refer to treated body sites or their respective adjacent controls. (D) Comparison of relative abundances for the top 4 taxa at baseline and 1hr post-treatment with water or alcohol. Each line represents an individual subject colored by an increase or decrease in relative abundance following treatment. * P < 0.05, ** P < 0.01 by Wilcoxon rank sum test (Mann-Whitney U test).
To determine the taxa most responsible for these shifts, we focused our analyses on bacterial families with the greatest abundances prior to treatment. Corynebacteriaceae, Propionibacteriaceae, Streptococcaceae, and Staphylococcaceae were selected, representing a mean relative abundance of ~70% in pre-treatment samples. Most taxa did not significantly change with treatment, with only Streptococcaceae significantly decreased in response to treatment at the forearm (Fig. 2d).
Treatment depletes skin bacterial community membership and richness
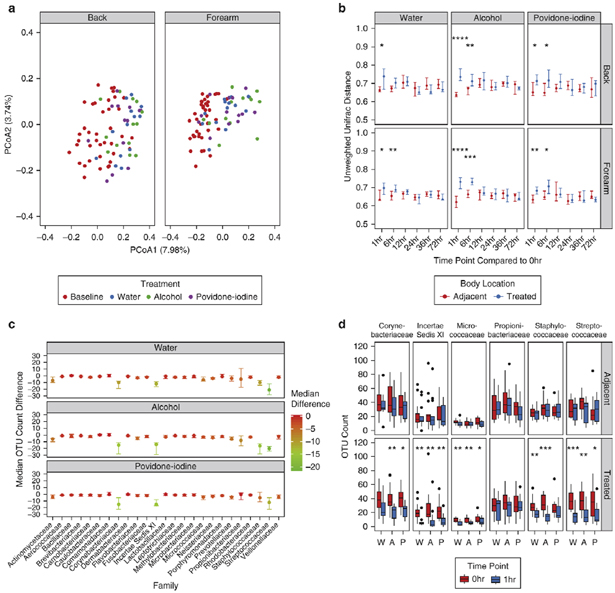
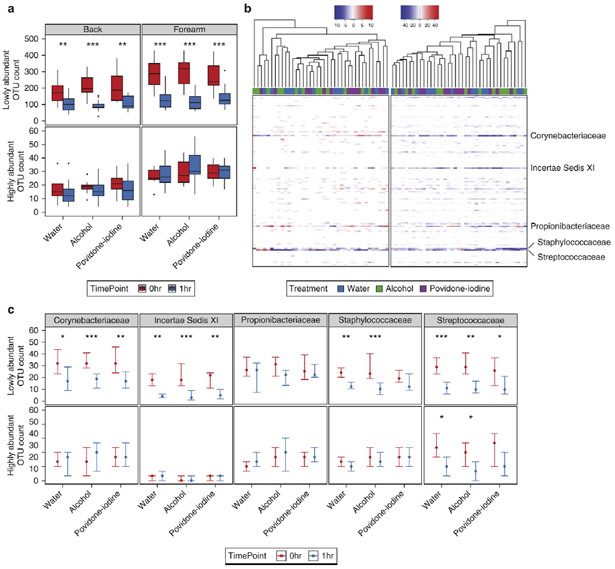
To determine whether treatment could elicit more significant changes to bacterial community membership, we used unweighted metrics which are agnostic to the relative proportions of bacterial taxa. Unweighted UniFrac revealed a prominent shift in bacterial communities following treatment at both body sites (Fig. 3a). Moreover, when comparing treated communities to their baseline controls, both the back and forearm were significantly disrupted by water, alcohol, and povidone-iodine compared to adjacent controls (Fig. 3b). To evaluate the underlying cause of this shift, we analyzed the effect of treatment on the total number of observed species. Water, alcohol, and povidone-iodine all significantly reduced the number of observed species compared to adjacent controls on the forearm (Fig. S3a). A similar effect was seen with alcohol on the back.
Fig. 3.
Treatment results in distinct alterations to skin bacterial residents by unweighted metrics. (A) Visualization of unweighted UniFrac distances by principal coordinates analysis for treated body sites at baseline and 1hr post-treatment. Each point represents a single sample, colored by treatment and shaped by body site. (B) Comparison of unweighted UniFrac distances for baseline and post-treatment communities in response to treatment at the forearm and back. Points represent the median of participants. Error bars designate interquartile regions. (C) Difference between OTU counts for the top 25 families at the forearm for baseline and 1hr post-treatment samples in response to water, alcohol, and povidone-iodine treatment. Points represent the median of participants and are colored by scaled differences in total count. Error bars designate interquartile regions. (D) Box and whisker plots of OTU counts for major taxa at adjacent and treated body sites of the forearm for water (W), alcohol (A), and povidone-iodine (P) treatments between baseline and 1hr time points. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 by Wilcoxon rank sum test (Mann-Whitney U test).
To further investigate these results, we tested the effect of treatment on the membership of individual bacterial families. Corynebacteriaceae, Incertae Sedis XI, Micrococcaceae, Staphylococcaceae, and Streptococcaceae all were depleted of observed species with treatment (Fig. 3c; Fig. S3b). Moreover, when comparing the richness of these taxa at treated and adjacent control sites, each of these families were significantly decreased at treated, but not untreated, areas of the skin (Fig. 3d; Fig. S3c). This effect did not extend to all highly abundant families, as Propionibacteriaceae remained largely unchanged regardless of treatment or body site.
Skin microbiome converges at distinct community types following treatment
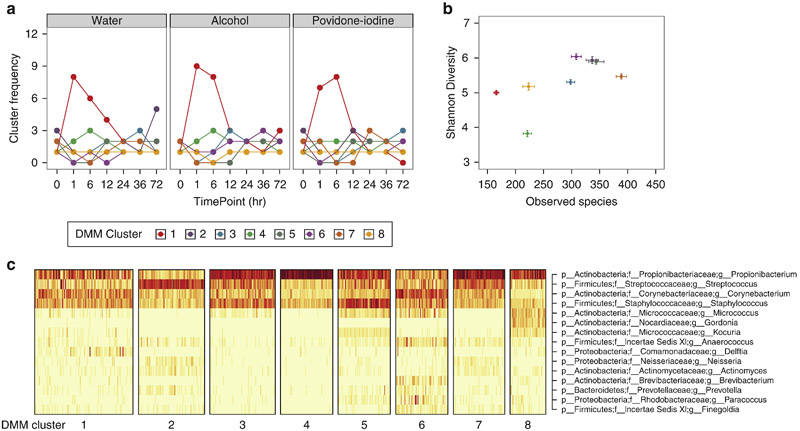
To determine if a conserved microbial signature defined post-treatment microbial communities, we used an unsupervised approach, Dirichlet multinomial mixture (DMM) models, which identified 8 distinct clusters, or microbial “community types” at the forearm. Individual subjects were often dominated by a single community type (Fig. S4a-b), but prominently converged to DMM cluster 1 in response to all treatments, an effect that was not observed at adjacent untreated body sites (Fig. 4a; Fig. S4c). DMM cluster 1 was differentiated by decreased bacterial diversity, specifically richness (Fig. 4b) and fewer taxon-specific attributes, suggesting a normalization of bacterial residents in response to treatment (Fig. 4c). In contrast to the forearm, back communities did not converge following treatment (Fig. S4d-e).
Fig. 4.
Dirichlet multinomial modeling identifies convergence at distinct forearm community types following treatment. (A) Longitudinal frequencies of DMM clusters in response to treatment with water, alcohol, and povidone-iodine. (B) Shannon diversity and observed species counts of individual DMM clusters. Data are presented as mean ± s.e.m. (C) Heat map of square root counts for the top bacterial taxa contributing to cluster identity. Dark bars correspond to greater counts.
Highly abundant bacterial families contribute most to treatment-derived changes in skin microbiome
Our initial analyses suggested that certain bacterial taxa were disrupted more significantly by treatment than others. To assess this hypothesis, we tested characteristics shown to influence variation in untreated settings. We reasoned that the most variable taxa in the absence of treatment were also the most likely to be altered in response to topical intervention. As previous analyses have identified intermediately abundant taxa as the most susceptible to temporal fluctuation (Oh et al., 2016), we assessed the baseline variance of these taxa in our study cohort. Similar to previous findings, we observed a distinct second-order, power-law relationship, with intermediately abundant members varying the most in untreated, baseline communities (Fig. S5a).
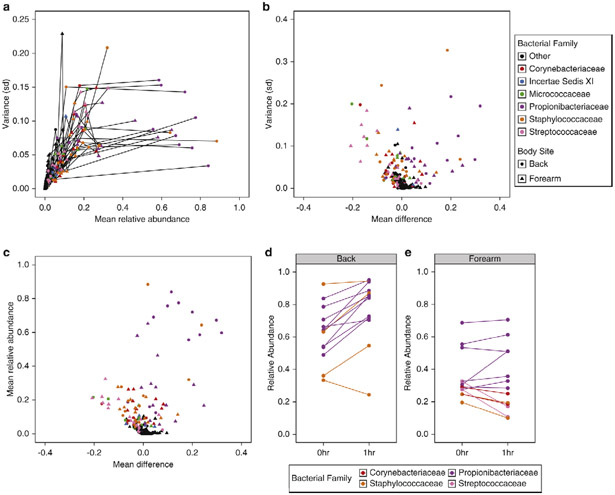
To test which taxa were specifically responsible for these shifts, we assessed baseline variance at the family level for each subject at each body site. Propionibacteriaceae, Streptococcaceae, Staphylococcaceae, Corynebacteriaceae, Micrococcaceae, and Incertae Sedis XI constituted the most variable groups in baseline communities (Fig. S5b-c). Rather than representing intermediately abundant taxa, however, these families were often the most abundant residents in our study cohort, and also the most likely to vary in response to treatment. To investigate this discrepancy more directly, we again compared the variance of baseline taxa in our study cohort to their mean relative abundances, but further controlling for both inter-individual differences and body site-specificity. Stratification resulted in a more nuanced effect than the previously observed second-order relationship, with the variance of taxa frequently plateauing when plotted against their mean relative abundances (Fig. 5a).
Fig. 5.
Baseline variance and abundance are indicators of treatment-derived alterations to the skin microbiota. (A) Family-level comparison of the baseline variances (standard deviation) and mean relative abundances for subjects at the forearm and back. Each point represents the values for bacterial families of an individual subject, shaped by body site and colored by family. Lines connect families of an individual subject and body site. “Other” designations refer to any bacterial family different from the listed members (B) Baseline variance of bacterial families plotted against their mean treatment effect in response to water, alcohol, and povidone-iodine treatment at the forearm and back. (C) Mean relative abundance of bacterial families at baseline compared to mean treatment effects at the forearm and back. (D, E) Mean difference in relative abundance of the most dominant taxon per subject following treatment at the back (D) and forearm (E). Each point represents a single subject colored by bacterial family identity.
We next tested whether taxonomic variation at baseline predicts post-treatment effects. Specifically, we compared the baseline variance of bacterial families to their response following treatment. The most variable taxa in the absence of treatment were also the most variable with treatment, with decreases in the relative proportions of most taxa being offset by increases in Propionibacteriaceae (Fig. 5b). Interpersonal variability strongly contributed to this trend, as subjects with low variation of a given bacterial family were also less likely to exhibit shifts by those residents following treatment. This trend was recapitulated when comparing the mean relative abundances of taxa to their mean treatment response as well. Once again, the greatest differences were observed within the Propionibacteriaceae family, which was both the most abundant bacterial family and the most likely to increase following treatment (Fig. 5c).
Body site specificity informs fluctuations of the most abundant bacterial taxa
Unlike other taxa, we noted that Propionibacteriaceae often increased in relative abundance following treatment of the back. A subset of subjects exhibited similar dynamics when Staphylococcaceae was their most abundant taxon, which together suggested a personalized response in which the most abundant taxon was also the most likely to persist following treatment. To test this hypothesis, we compared the levels of each subject’s most abundant taxon at baseline to its mean relative abundance following treatment. In all cases but one, the most abundant taxon at the back increased in relative proportion following treatment regardless of identity, indicating a distinct competitive advantage (Fig. 5d).
In contrast to the back, only three subjects had taxa at the forearm with >50% relative abundance. Though not absolute, relative proportions of Propionibacteriaceae increased in multiple subjects following treatment (Fig. 5e). This trend did not extend to all skin residents, as Corynebacteriaceae, Staphylococcaceae, and Streptococcocaceae all decreased in abundance at the forearm, regardless of status. These results thus verify that abundance can be used to predict treatment effects, but also highlights the importance of body site to these particular outcomes.
Lowly abundant members of predominant bacterial families are the most likely to vary in response to treatment
Because our previous investigations outlined the importance of abundance and bacterial identity to treatment-derived alterations, we further hypothesized that relative abundance could be used to predict the fluctuations of all taxa. To test this, we partitioned OTUs into highly or lowly abundant groups based on an abundance threshold of 0.5%, chosen from the inflection point of OTU counts at baseline (Fig. S6a). We observed a significant decrease in the number of lowly abundant OTUs following treatment at both the forearm and back (Fig. 6a), an effect largely due to decreases in Corynebacteriaceae, Incertae Sedis XI, Staphylococcaceae, and Streptococcaceae (Fig. 6b-c; Fig. S6b-c). By contrast, when evaluating highly abundant OTUs, only Streptococcaceae at the forearm and Corynebacteriaceae at the back were significantly reduced, a result which did not significantly decrease the total number of highly abundant OTUs. Similar to previous results, we also observed no significant differences in the membership of Propionibacteriaceae, regardless of abundance or body site. These findings confirm that bacterial identity represents a critical factor when evaluating skin resident stability, and underscores the importance of abundance to predictions of treatment response.
Fig. 6.
Lowly abundant members of prominent taxa are the greatest contributors to treatment effects at the skin surface. (A) Box and whisker plots of lowly and highly abundant OTU counts as defined by a 0.5% relative abundance threshold following treatment at the forearm and back. (B) Heat map of differences in forearm OTU counts between baseline and 1hr post treatment with water and antiseptics. Each column represents the difference measured for a single subject and treatment, and each row represents a bacterial family. Samples are clustered by the Unweighted Pair Group Method with Arithmetic means (UPGMA). Color-coded bars above the graph designate treatments for each sample. (C) Comparison of lowly and highly abundant OTU counts at the forearm in major taxonomic families at baseline and 1hr post-treatment. Points represent the median of the study cohort. Error bars designate interquartile regions. * P < 0.05, ** P < 0.01, *** P < 0.001 by Wilcoxon rank sum test (Mann-Whitney U test).
DISCUSSION
Despite important functions in cutaneous health and disease, few studies have assessed the impact of disrupting the skin microbiota or dynamics following antimicrobial stress. Herein, we present the impact of topical antiseptics on human skin bacterial populations, and outline the importance of key variables to this response.
When evaluating treatments at a comparative level, water, alcohol, and povidone-iodine had similar effects on skin bacterial residents, underscoring the generalized nature of topical interventions to reduce inhabitance by mechanical cleansing (Kampf and Kramer, 2004). This result has been well-established in culture-based systems, where reports have outlined the potential for certain topical treatments to both kill, and remove, pathogenic microorganisms, with each feature playing an important role in infection control (Bloomfield et al., 2007, Larson, 1999). Mild, non-antibacterial soaps are also used with the sole purpose of clearance, further emphasizing the importance of this mechanism to skin hygiene and community disruption (Amin et al., 2014, Kim et al., 2015).
While no study to date has investigated the impact of antiseptics on human skin microbiota by sequencing, others have assessed the effects of hand-sanitizers and soaps (Two et al., 2016, Zapka et al., 2017). These studies have largely supported culture-based tests, outlining the importance of conserved mechanisms to topical treatment response. For example, a recent study by Zapka, et al. found that water and hand washing often elicited similar alterations to the skin microbiota as alcohol-based hand sanitizers (Zapka et al., 2017). A recent comparison of mild and antibacterial soaps has further confirmed these results, showing minimal differences when comparing their impact on colonizing levels of S. epidermidis (Two et al., 2016).
Like the abovementioned studies, our initial analyses suggested a relatively minor impact of treatment on resident microbiota. However, after controlling for personalization and body site-specificity we observed the true impact of our treatment regimens on community diversity and resilience, including the finding that treatment elicited the strongest effects in low-level skin inhabitants. Highly abundant species likely exist at a given skin niche due to an ability to resist acute host-derived and external stressors. As the skin is often colonized by particular strains with temporal stability for years in length (Oh et al., 2016, Sakwinska et al., 2010), this outlines a system by which multiple taxa may exist on the skin surface at a given time, while only a subset are uniquely adapted for long-term colonization.
We found that bacterial identity influenced treatment response, with predominant skin taxa often more significantly disrupted than other residents. Treatment-derived alterations were also dependent upon body site, with the back representing a more stable habitat than the forearm in most tests. Notwithstanding, both body sites were susceptible to a loss of lowly abundant OTUs in many predominant skin residents. This result did not extend to all major taxa, as members of the Propionibacteriaceae family persisted regardless of body site. We believe this particular effect could be due to an inherent resilience of Propionibacteriaceae, or its increased abundance at deeper, newly exposed layers of the skin. Readily disrupted members of the community, such as lowly abundant members of Staphylococcaceae and Streptococcaceae, may localize to the skin surface where they are more vulnerable to removal by both physical and chemical perturbations. Imaging studies such as RNA-fluorescent in situ hybridization (FISH) to localize individual members of the skin microbiota would shed light on the differential distribution of skin commensals and the effect of niche specificity on stability and resilience of individual members.
In all, this study furthers our understanding of skin bacterial dynamics and elucidates the effects of topical treatments on cutaneous resident populations. While we observed a similar impact of water and the antiseptics alcohol and povidone-iodine on skin inhabitants, we note that our studies were designed to assess the totality of skin residents in healthy individuals. As such, we caution against the application of these findings to clinical settings in which the dynamics of pathogens and commensals are highly skewed. Indeed, previous studies have described, in-depth, the utility of antiseptics in these particular environments (Al Maqbali, 2013, Darouiche et al., 2010, Mimoz et al., 2015). As our study assesses only the effect of acute stressors, or a “pulse” disturbance, we also advocate for further research into long-term treatment regimens more characteristic of a “press” disturbance. The potential exists that more lasting perturbations may elicit even greater shifts to human skin bacterial communities, an important consideration when evaluating the nexus of host-microbial interactions.
MATERIALS & METHODS
Human subjects and sample collection.
All protocols were approved by the Institutional Review Board of the University of Pennsylvania, and written informed consent was obtained for all study participants prior to sampling. Thirteen healthy subjects aged 23-30 (median:27, 6 females) and without chronic skin disorders were recruited to participate in a controlled skin antiseptic study (Table S1). Subjects were required to be >21 years of age, and free of oral or topical antibiotics within 6 months of their first visit. Subjects were asked to 1) refrain from showering for 24 hours prior to the first visit and until after their 36-hour visit, and 2) refrain from use of soaps or topical products containing antimicrobials 1 week prior to sampling and during the entire study. Demographic data were collected as well as usage of topical products, medications, and personal care routines. Subjects were swabbed at baseline, using a Catch-All Collection swab (Epicentre) moistened in water (UltraPure Distilled Water, Invitrogen). A 1 inch2 area was swabbed vigorously with 10 swipes followed by 10 additional swipes in the perpendicular direction. Subjects were then administered one of four treatments for 1.5 minutes, using gentle swiping with a cotton pad soaked in 5 mL of the test agent. Cotton pads and test agents were treated with UV for 20 minutes prior to use. Each participant received water (UltraPure Distilled Water, Invitrogen) and alcohol (80% ethanol) on contralateral forearm or back body sites during their first visit series, and povidone-iodine (10%), and chlorhexidine (chlorhexidine-gluconate 4%) during their second visit series (Fig. S1a). Visit series were separated by at least two weeks to allow for microbial equilibration. Swabbed regions were delineated by a skin marker to ensure that the same body site was swabbed at longitudinal time points. Subjects were instructed to refrain from showering for >12 hours prior to each time point.
DNA isolation, 16S rRNA gene sequencing, and qPCR.
Bacterial DNA was extracted as described previously (Meisel et al., 2016) using the Invitrogen PureLink kit. PCR and sequencing of the V1V3 hypervariable region was performed using 300-bp paired end chemistry and barcoded primers (27F, 534R) on the Illumina MiSeq platform. Accuprime High Fidelity Taq polymerase was used for PCR cycling conditions: 94 °C for 3 min; 35 cycles of 94 °C for 45 sec, 50 °C for 60 sec, 72 °C for 90 sec; 72 °C for 10 min. PCR products were purified using the SequalPrep kit (Invitrogen), according to manufacturer’s instructions, and pooled in equal amounts for sequencing. For bacterial load comparisons, 16S rRNA genes were amplified by qPCR using Fast SYBR Green Master Mix and the optimized primers 533F, 902R. Samples were compared to standard curves generated from known concentrations of serially diluted bacterial DNA to calculate burden.
Microbiome analysis.
The datasets generated and analyzed during the current study are available in the NCBI Short Read Archive under BioProject: PRJNA395539. Quality control, processing, and analysis are detailed in the Supplemental Methods.
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Grice laboratory for their underlying contributions. Funding for this work was provided by the NIH, National Institute of Arthritis, Musculoskeletal, and Skin Diseases (R00AR060873 and R01AR066663 to EAG) and the National Institute of Nursing Research (R01NR015639 to EAG). AJS is supported by a Department of Defense National Defense Science and Engineering Graduate fellowship. JSM is supported by NIH T32HG00046, Computational Genomics Training Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health 2008;98(8):1372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Maqbali MA. Preoperative antiseptic skin preparations and reducing SSI. Br J Nurs 2013;22(21):1227–33. [DOI] [PubMed] [Google Scholar]
- Amin N, Pickering AJ, Ram PK, Unicomb L, Najnin N, Homaira N, et al. Microbiological evaluation of the efficacy of soapy water to clean hands: a randomized, non-inferiority field trial. Am J Trop Med Hyg 2014;91(2):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil CM, Paulson DS, Bogert A, Lewis GS. In vivo evaluation of the persistant and residual antimicrobial properties of three hand-scrub and hand-rub regimes in a simulated surgical environment. J Hosp Infect 2012;81(4):283–7. [DOI] [PubMed] [Google Scholar]
- Bloomfield SF, Aiello AE, Cookson B, O’Boyle C, Larson EL. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am J Infect Control 2007;35(10):S27–S64. [Google Scholar]
- Carty N, Wibaux A, Ward C, Paulson DS, Johnson P. Antimicrobial activity of a novel adhesive containing chlorhexidine gluconate (CHG) against the resident microflora in human volunteers. J Antimicrob Chemother 2014;69(8):2224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009;326(5960):1694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, et al. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. N Engl J Med 2010;362(1):18–26. [DOI] [PubMed] [Google Scholar]
- Digison MB. A review of anti-septic agents for pre-operative skin preparation. Plast Surg Nurs 2007;27(4):185–9; quiz 90-1. [DOI] [PubMed] [Google Scholar]
- Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A, Liu Z. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev 2015(4):CD003949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols K, Graves M, LeBlanc KG, Marzolf S, Yount A. Role of antiseptics in the prevention of surgical site infections. Dermatol Surg 2015;41(6):667–76. [DOI] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A 2007;104(8):2927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science 2009;324(5931):1190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011;9(4):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovi T, Ollgren J, Savolainen-Kopra C. Intensified hand-hygiene campaign including soap-and-water wash may prevent acute infections in office workers, as shown by a recognized-exposure -adjusted analysis of a randomized trial. BMC Infect Dis 2017;17(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev 2004;17(4):863–93, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SA, Moon H, Lee K, Rhee MS. Bactericidal effects of triclosan in soap both in vitro and in vivo. J Antimicrob Chemother 2015;70(12):3345–52. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity 2015;42(4):756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012;22(5):850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E Skin hygiene and infection prevention: more of the same or different approaches? Clin Infect Dis 1999;29(5):1287–94. [DOI] [PubMed] [Google Scholar]
- Lopez-Gigosos RM, Mariscal-Lopez E, Gutierrez-Bedmar M, Garcia-Rodriguez A, Mariscal A. Evaluation of antimicrobial persistent activity of alcohol-based hand antiseptics against bacterial contamination. Eur J Clin Microbiol Infect Dis 2017. [DOI] [PubMed] [Google Scholar]
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999;12(1):147–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel JS, Hannigan GD, Tyldsley AS, SanMiguel AJ, Hodkinson BP, Zheng Q, et al. Skin Microbiome Surveys Are Strongly Influenced by Experimental Design. J Invest Dermatol 2016;136(5):947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis 2008;46(2):274–81. [DOI] [PubMed] [Google Scholar]
- Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V, et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 2015;386(10008):2069–77. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015;520(7545):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Science translational medicine 2017;9(378). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Deming C, Conlan S, Program NCS, Kong HH, et al. Biogeography and individuality shape function in the human skin metagenome. Nature 2014;514(7520):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Park M, Program NCS, Kong HH, Segre JA. Temporal Stability of the Human Skin Microbiome. Cell 2016;165(4):854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LK, Morse DJ, Duley C, Savell BK. Prospective, randomized in vivo comparison of a dual-active waterless antiseptic versus two alcohol-only waterless antiseptics for surgical hand antisepsis. Am J Infect Control 2012;40(2):155–9. [DOI] [PubMed] [Google Scholar]
- Sakwinska O, Blanc DS, Lazor-Blanchet C, Moreillon M, Giddey M, Moreillon P. Ecological temporal stability of Staphylococcus aureus nasal carriage. J Clin Microbiol 2010;48(8):2724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong HA, et al. Commensal Microbes and Hair Follicle Morphogenesis Coordinately Drive Treg Migration into Neonatal Skin. Cell Host Microbe 2017;21(4):467–77 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Septimus EJ, Schweizer ML. Decolonization in Prevention of Health Care-Associated Infections. Clin Microbiol Rev 2016;29(2):201–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Two AM, Nakatsuji T, Kotol PF, Arvanitidou E, Du-Thumm L, Hata TR, et al. The Cutaneous Microbiome and Aspects of Skin Antimicrobial Defense System Resist Acute Treatment with Topical Skin Cleansers. J Invest Dermatol 2016;136(10):1950–4. [DOI] [PubMed] [Google Scholar]
- Zapka C, Leff J, Henley J, Tittl J, De Nardo E, Butler M, et al. Comparison of Standard Culture-Based Method to Culture-Independent Method for Evaluation of Hygiene Effects on the Hand Microbiome. MBio 2017;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016;535(7613):511–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.