Abstract
The effect of carbon nanotube (CNT) functionalization in altering the properties of Epoxy-CNT composites is presented. The presence of functional groups effectively influenced the colloidal behavior of CNTs in the precursor epoxy resin and the hardener triethylenetetramine (TETA), which affected the synthesis process and eventually the interfacial interactions between the polymer matrix and the CNTs. The physical, thermal and electrical properties of the composites exhibited strong dependence on the nature of functionalization. At a 0.5 wt% CNTs loading, the enhancement in tensile strength was found to be 7.2, 11.2, 11.4 and 14.2 percent for raw CNTs, carboxylated CNTs, octadecyl amide functionalized CNTs and hydroxylated CNTs, respectively. Glass transition temperatures (Tg) also varied with the functionalization and composite prepared using hydroxylated CNTs showed the maximum enhancement of 34%.
Keywords: Interfacial interaction, Nanocomposites, Functionalized carbon nanotube, Physical properties, Thermal properties
1. INTRODUCTION
There has been much interest in nano-carbon based composites and in particular carbon nanotubes (CNTs) 1–10. The incorporation of small amounts of CNTs is known to improve properties such as mechanical strength, fracture toughness, thermal stability, permittivity and electrical conductivity 11–17. Epoxy has unique mechanical, thermal and electrical properties, which makes it attractive for various applications such as fiber reinforced composites, laminates, adhesives, and coatings 18–21. However, cured epoxy is brittle, rigid and shows poor crack propagation resistance and low impact strength 22,23. Researchers have been studied the mechanical, thermal and electrical properties of epoxy-CNT composites thoroughly 24–27. The application of epoxy-CNT composite onto glass fibers have been shown to reduce stress concentration 28 and enhanced electromagnetic shielding 29. The influence of different types of CNTs with varying length and graphitization on the properties of epoxy-CNT nanocomposite and the kinetics of curing of liquid-crystal epoxy with CNTs has also been investigated 30–32.
The capability to regulate the CNTs dispersion into a polymer matrix is important in applications related to nanocomposites 33. In general, the effective incorporation of nanomaterials (NMs) including CNTs into a polymer matrix depends upon the interactions with the polymer 34,35. The high specific surface area of the NMs provides desirable interface, however the uncertainty in interfacial bonding has been a concern 36. The CNTs have a high aspect ratio, but they entangle with each other and are difficult to disperse 37–39. The introduction of functional groups on the CNT surface promotes efficient adhesion to the polymer and contributes to better dispersion 40–42. While much has been reported about the epoxy-CNT composites, specific interaction based on functionalization is yet to be studied. Another important factor is that the colloidal behavior of CNT dispersions, which depends on the type of functionalization, is important in understanding their stability in the liquid phase precursors, and eventually determines the polymer-CNT interactions in the solid composite. The objective of the present work is to study the influence of various CNTs functional groups on different properties of Epoxy-CNT composites.
2. Materials and Methods
2.1. Materials synthesis
Epoxy resin (D.E.R.™ 332, epoxy equivalent weight 176) and an amine hardener (Triethylenetetramine, Tech., 60%) were used for this study. Anhydrous 1-Butanol (99.8%) and xylene (ACS Reagent, 98.5%) were used as solvent for the epoxy and the amine hardener. All of the materials were purchased from Sigma Aldrich Inc., Saint Louis, MO.
The raw CNTs and CNT-OH (30 nm average diameter and ~15-micron length) were procured from Cheap Tubes Inc., Brattleboro, VT and were purified with dilute nitric acid. Functionalization via carboxylation (CNT-COOH) and octadecyl amide (-CO-NH-C18H37) formation (CNT-ODA) were carried out in our laboratory in a Microwave Accelerated Reaction System (Mode: CEM Mars) using procedures published before by our group 43,44. CNTs were treated with a mixture of concentrated sulfuric acid and nitric acid solutions at 140°C for 20 min in a microwave reactor. Carboxylic group were grafted over the CNTs surface by this reaction. The CNT-COOH was then washed, filtered and dried under vacuum at 80°C. Octadecyl amide grafted CNTs (CNT-ODA) were prepared in two steps. First, CNTs were reacted with thionyl chloride in dimethyl formamide solution to produce –COCl functionalized CNTs in a 20 min microwave induced reaction for 20 min at 70°C. It was then washed and dried. After modification, CNT-COCl was further treated with octadecyl amine for 10 min at 120°C under microwave reaction conditions. Excess ODA was removed by washing with ethanol followed by dichloromethane. It was then dried at room temperature under vacuum.
2.2. Dispersion of CNTs and preparation of composites
Due to the agglomeration tendency of the CNTs, uniform dispersion into the polymer matrices is believed to be one of the most important issues related to composite synthesis 45,46. Ultrasonic dispersion method is considered an effective, less time consuming as compared to conventional dispersing systems, though direct ultrasonication is known to damage CNTs and reduce tube length 47–49. In this study, a mixed solvent (xylenes and butanol, 1:1 by weight), which was compatible for both CNTs and polymer was used to disperse the CNTs. After mechanically stirring for 30 min, The CNT-mixed solvent dispersion was placed in a sonication bath for 3 hours. The solvent allowed the dissipation of the local energy input, thus reducing the tube damage. Epoxy resin was then added under stirring condition to the dispersion for 1 hr. The mixed solvent was removed completely from the system under vacuum at 70°C for 12 hr. The solvent-free CNT-polymer dispersion was allowed to cool under vigorous stirring and this was followed by addition of the hardener. The resin was cured at room temperature for 24 hr., and annealed at 60oC for 6 hr.
2.3. Characterization
2.3.1. Colloidal behavior of CNTs in epoxy resin and TETA
Dynamic light scattering (Beckman Coulter N4 Plus) was used to characterize the colloidal behavior of different types of CNTs dispersed in epoxy resin and TETA. The dispersions of CNTs (250 mg-lit−1) were prepared in xylene-butanol solution under sonication for 3 hr. Polymer solution was prepared by adding 5 wt% of epoxy resin or TETA to the dispersion under stirring condition for 10 min. The dispersion of various functionalized CNTs was prepared using a similar procedure described above. The stability of the CNT dispersion was monitored for a period of 6 hr.
2.3.2. Composites Characterization
Scanning electron microscope (SEM) (LEO 1530 VP), thermogravimetric analysis (TGA, Perkin–Elmer Inc.), transmission electron microscopy (TEM, Hitachi H-7500) and differential scanning calorimetric (DSC, Perkin–Elmer Inc.) were used to characterize the composites. The colloidal behavior of the CNTs in epoxy resin solution (xylenes-butanol, 1:1) was also explored. A 3-D image of the surface was taken using digital microscope (Keyence) to study the surface roughness and the distribution of CNTs. Instron 8516 was used to study the mechanical properties of the composite materials. Tensile strength measurements were carried out using dumbbell shaped specimens at a fixed cross-head movement of 3 mm per min at room temperature. The surface hardness of the composites was measured using Leco Microindentation Hardness Testing Systems at a fixed load of 25 gf. The reproducibility of the data measured by repeating the experiments three times. The relative standard deviation was found to be less than 1%.
2.3.3. Electrical conductivity
Electrical resistivity of a material was a measure of the restriction of electric current flow through the material, usually expressed in terms of volume resistivity.
Volume resistivity (ρv) can be defined as,
Where, A = Effective area of measuring electrode in cm2, t = Thickness of the test specimen in cm, Rm = Measured resistance in ohms
The volume resistivity and surface resistance of various composites was measured by using Keithley 6715B electrometer. The specimens for the surface resistance measurements were prepared using two electrodes painted with conducting silver ink. The active area was 1×1 cm2.
3. Results and Discussion
3.1. Colloidal behavior of various CNTs
A strong interfacial physico-chemical affinity of CNTs with the polymer matrices enhances the dispersibility of the CNTs and overall performance of the composite materials. The introduction of functional groups on CNTs surfaces not only impairs the strong attractive interactions between the CNTs themselves, but also alters the wettability, and dispersibility in the liquid precursors. These lead to improved interfacial bonding with the composite 50–52.
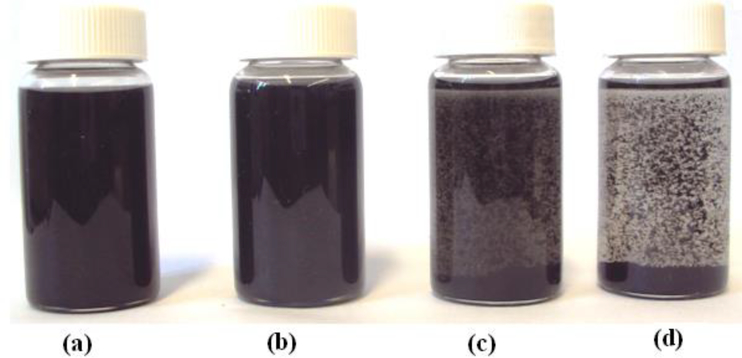
Figure 1 shows the photographs of various CNTs dispersions in dilute epoxy solution in a xylene-butanol mixture when the mixture was allowed to stand for six hours. CNTs tend to agglomerate and the large aggregates tend to settle out. It was observed from the figure that the stability of the CNTs increased with the introduction of surface functionalization. The size of the aggregates was smaller, they remained dispersed for a longer period of time. The surface functionalization reduced the strong van der Wall forces between the individual CNTs, thus decreasing the agglomeration tendency and the functional groups increased interactions with solvent molecules. While –COOH and –OH are highly polar, amide groups (-CO-NH-) and non-polar octadecyl groups (-C18H37) make CNT-ODA highly stable in the mixed polar-nonpolar (butanol-xylene) solvents. The dispersion of pure CNTs showed relatively poor stability, which was followed by CNT-COOH, while the other two were very stable for the six-hour period shown in Figure 1.
Figure 1.
Photographs of various CNTs dispersion in dilute epoxy solution (xylene-butanol (1:1) mixture) when the mixture was allowed to settle for 6 hours; a. CNT-ODA; b. CNT-OH; c. CNT-COOH; d. CNT-Raw.
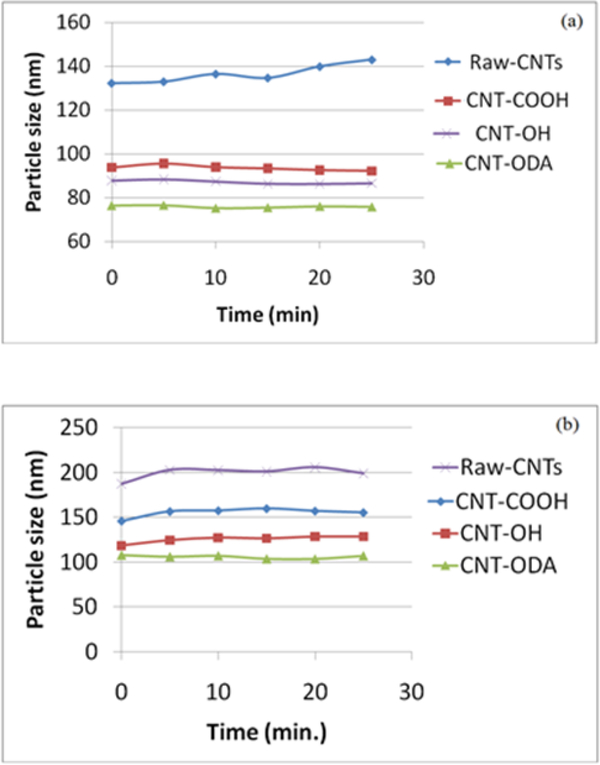
The particle size of various CNTs in the mixed solvent as well as in the presence of epoxy and TETA was measured by dynamic light scattering. The interfacial modification of CNTs via functionalization has been critical to the distribution of CNTs in the polymer matrix and has significant effects on the final properties of the CNT epoxy nanocomposites. Figures 2a and b show the stability of various CNTs agglomerates in the presence of epoxy and TETA. These figures represent those particles that were in the suspension and had not settled out. The increase in particle size indicates the aggregation tendency of the corresponding nanotubes, which may lead to poor dispersibility into the polymer matrix during fabrication. Among all of the CNTs, the particle size of CNT-ODA was found to be the smallest followed by CNT-OH, CNT-COOH and raw CNT. The larger particle size was attributed to the higher agglomeration tendency 53.
Figure 2.
Particle size of various f-CNTs in (a) Epoxy resin solution and (b) TETA solution from 0–25 min (5 wt% in xylene-butanol 1:1).
3.2. Characterization of CNT-composite
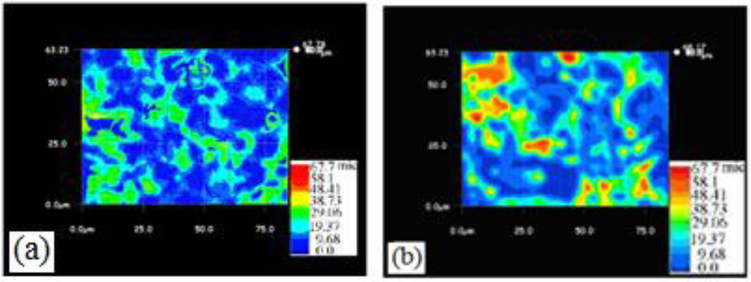
Figure 3a and b show three-dimensional optical images of the nanocomposites surfaces. The images provide nanometer level profile and roughness. It is clear from the images that Epoxy-CNT composites have relatively less planer surface in comparison to pure epoxy. The presence of CNTs in the polymer matrix hindered the flow of the binder, hence showed poor planarity of the composites.
Figure 3.
3-D optical images of (a) Pure epoxy surface, (b) Epoxy-CNTs-Raw composite surface from digital microscope (Keyence) (scale bar: red 67.7, yellow 38.7, green 29.06, and dark blue 0.0 lm).
Figure 4 shows the SEM images of the cross-section of the fractured surface. These show the distribution of CNTs within the matrix. Figure 4a shows the original CNTs and the images of the f-CNTs were similar to what has been reported before and are not included for brevity 44,54. SEM images of cross-sectional view of different Epoxy-CNT composites are show in Figures 4b to f. The raw CNTs did not appear to disperse uniformly in the composite matrix and better dispersion was observed for the f-CNTs. The dispersion of the CNTs into the polymer matrix exhibited similar type of behavior as observed in the liquid phase. The functionalization of CNTs reduced the agglomeration tendency and promoted uniform distribution in the polymer matrix.
Figure 4.
SEM images of (a) CNT-raw; the cross-section image of (b) Pure epoxy, (c) Epoxy-CNTs-Raw composite, (d) Epoxy-CNTs-COOH composite, (e) Epoxy-CNTs-OH composite, (f) Epoxy-CNTs-ODA composite
It has been reported that fatigue crack growth suppression in epoxy nanocomposites strongly depends on CNT properties and can be reduced by improving nanotube dispersibility 55. It is clear from the cross-sectional images that the nature of fracture was quite different in the presence of CNTs. The fracture surface of pure epoxy was also flatter and smoother. The cleavage planes in the pure epoxy were in a radiating pattern indicating a brittle fracture associated with low impact resistance and fracture toughness 56. The fracture surfaces of the composites were quite different where the CNTs served as reinforcing fibers. The surface of the composite was significantly rougher indicating improved polymer chain interaction leading to enhanced interface crack resistance and toughness 57.
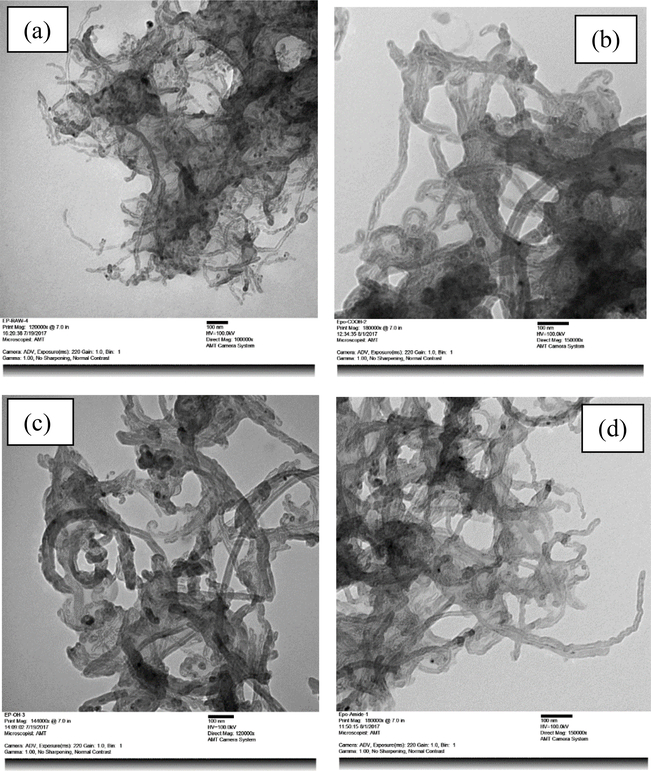
The TEM images of various CNTs in polymer matrix are shown in Figure 5. A high energy ultrasonic probe was used to disperse various CNTs in toluene-xylene mixed solvent and then mixed the dispersed suspension with a dilute solution of epoxy in similar solvent mixtures, again with ultrasonic agitation. The low viscosity of the dilute polymer solution permitted the CNTs to allocate freely through the polymer matrix. The drops of the mixed solution were placed on copper TEM grids. The thin film specimen of various epoxy-CNT composites were prepared by evaporating the mixed solvents.
Figure 5.
TEM images of (a) Epoxy-CNTs-Raw, (b) Epoxy-CNTs-COOH, (c) Epoxy-CNTs-OH, (d) Epoxy-CNTs-ODA
TEM images of (a) Epoxy-CNTs-Raw, (b) Epoxy-CNTs-COOH, (c) Epoxy-CNTs-OH, (d) Epoxy-CNTs-ODA are shown in Figure 5. a, b, c and d, respectively. As can be seen from all of the figures, the raw nanotubes contained some disordered carbon impurities. The length of the multiwalled nanotubes varied tens of nanometers to several micrometers, and in outer diameter from about 15 to 30 nm. From the figures, it was observed that the CNTs were embedded in the epoxy matrix, although the difference in agglomeration tendency was not obvious from these images.
3.3. Mechanical properties
The mechanical performances of the composites materials are strongly influenced by the phase morphology developed during mixing of CNTs and the polymers, and the interfacial adhesion between the phases. Good dispersion and strong interactions of CNTs is important for enhancing the mechanical properties of composites 58. The implementation of surface functionalization was expected to enhance the performance of the epoxy composites through better interfacial bonding as well as better dispersion. The colloidal behaviors of various CNT forms were expected to be an indicator of dispersibility in the final composites.
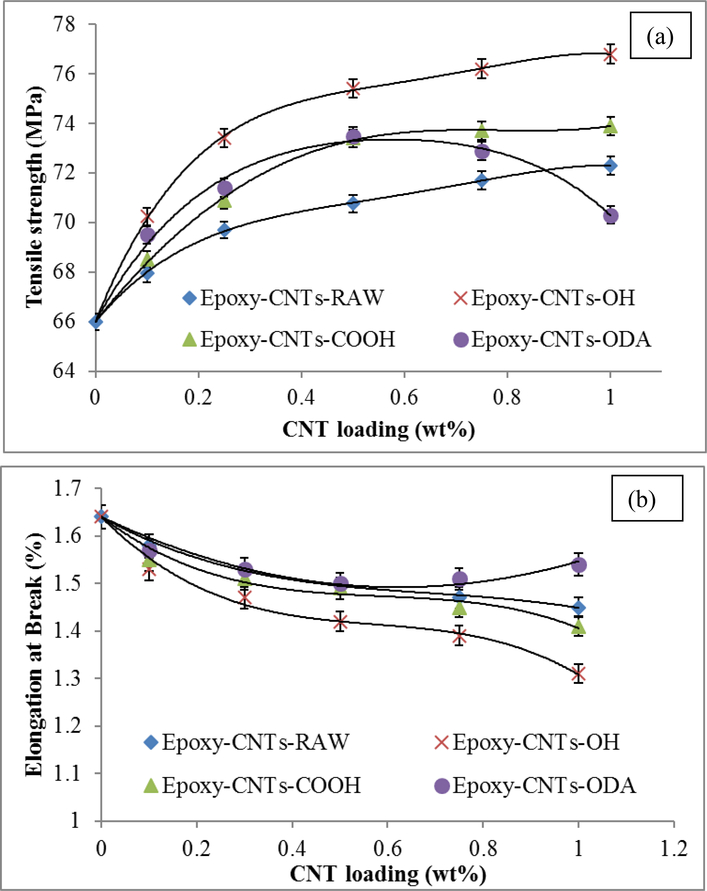
The tensile strength and elongation at break of various Epoxy-CNT composites at different concentrations of CNTs into the polymer matrix are compared in Figure 6a and b. The zero CNT concentration represents the pure epoxy. Figure 6a clearly demonstrates significant enhancement in tensile strength at relatively low CNT loadings. It is also clear from the figure that the f-CNTs exhibited better tensile strength than the raw CNTs. This was attributed to the superior dispersion of f-CNTs as well as stronger interfacial interactions with the polymer matrix.
Figure 6.
Tensile properties of Pure Epoxy and Epoxy-nanocomposites at different CNTs loading (a) Tensile strength (b) Elongation at break (%)
It was observed from Figure 6a that all of the f-CNTs exhibited better tensile properties at low CNTs loadings. The rate of enhancement at low CNTs loading was found to be higher compared to what was observed at higher CNTs loading. Compared to the pure epoxy, at 0.5 weight percent loading the enhancement in tensile strength were found 7.2, 11.2, 11.4 and 14.2 for raw CNTs, CNTs-COOH, CNTs-ODA and CNTs-OH composites respectively. This was quite higher than what has been reported (3 to 8.5% enhancement) for high CNT and CNT-COOH loadings 53. The tensile strength of Epoxy-CNTs-ODA showed a different trend compared than the other composites. It increased up to 0.5 wt% and then began to decrease. At lower concentrations, the enhanced dispersion of CNTs-ODA led to higher tensile strength compared to CNTs-COOH and the raw CNTs. However, the presence of long octadecyl (-C18H37) at high concentration may influence the crosslinking and close packing of the polymer chains, which negatively affected the mechanical properties.
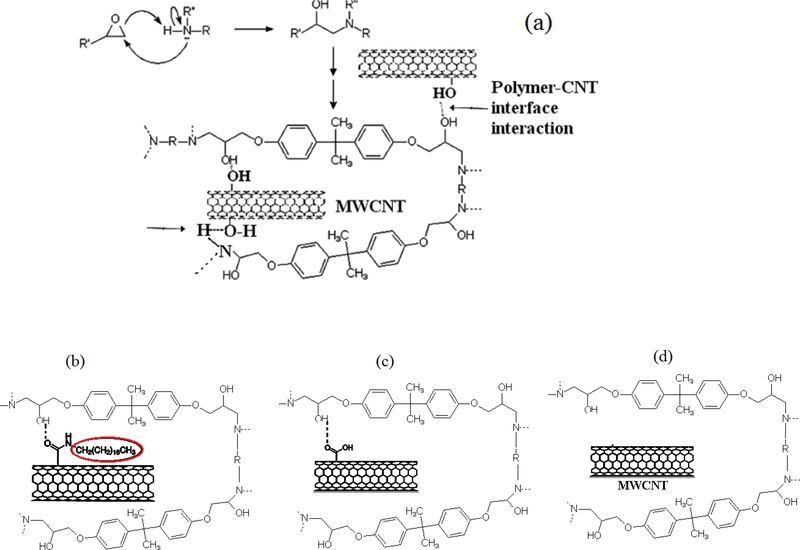
It is interesting to observe that among the f-CNTs, Epoxy-CNTs-OH exhibited higher tensile strength compared to the others, followed by Epoxy-CNTs-ODA and Epoxy-CNTs-COOH up to 0.5 wt% of CNTs. This trend was slightly different from what we observe in particle size distribution analysis where the CNT-ODA exhibited better dispersibility than that of CNT-OH. Therefore the enhancement in tensile strength was attributed to specific interactions between the hydroxyl groups (-OH) of the CNTs and the polymer matrix. This is shown in Figure 7a. Similar types of interactions also take place with CNT-ODA and or CNT-COOH and are shown in Figure 7b and c. The presence of large octadecyl groups may hinder the close packing of the polymer chains and poor dispersibility of CNT-COOH may affect the overall tensile strength of the composites.
Figure 7.
Interactions of the CNTs with Epoxy and TETA; a) CNT-OH; b) CNT-ODA; c) CNT-COOH and d) raw CNTs (no specific interactions).
Figure 6b shows the effect of various CNTs content on elongation at break (EAB). It is clear from the figure that an increase in CNTs content led to the reduction of EAB in all composites. Epoxy-CNTs-OH exhibited higher reduction in EAB and this was attributed to the better interaction with the epoxy matrix. Epoxy-CNTs-ODA showed a different trend where EAB first decreased up to 0.5 wt% and then remained unchanged or increased slightly. This was attributed to the presence of large octadecyl groups into the matrix, which may prevent the interactions of the amide group with the functional groups present in the epoxy. Both tensile strength and EAB results indicate an optimum amount of CNTs-ODA is needed for optimization of the nanocomposite mechanical properties.
The surface hardness was measured using Vickers Microhardness and the value for pure epoxy was found to be 17.2 HV. The results are presented in Table 1. An enhancement by ~18% was observed for all other CNT composites. It is clear that a small amount of CNTs can significantly improve the microhardness.
Table 1.
Different properties of pure epoxy and epoxy nanocomposites
| Sample | Tg (°C) | Microhardness (HV) | Volume resistivity (ohm.cm) | Surface Resistance of 1 cm2 area (ohm) |
|---|---|---|---|---|
| Epoxy-Pure | 77.8 | 17.2 | 1.31 × 1011 | 7.1 × 109 |
| Epoxy-CNTs-COOH | 86.9 | 20.4 | 1.64 × 109 | 1.04 × 109 |
| Epoxy-CNTs-OH | 103.2 | 20.2 | 1.81 × 109 | 1.17 × 109 |
| Epoxy-CNTs-ODA | 84.9 | 19.8 | 1.12 × 109 | 1.23 × 109 |
| Epoxy-CNTs-Raw | 95.5 | 20.8 | 0.8 × 109 | 0.9 × 109 |
3.4. Thermal properties of Epoxy-CNT composites
The thermal properties of the nanocomposites typically depend upon the nature of the polymer matrix, orientation and dispersion of the NMs, and the interfacial thermal boundary resistance between the matrix material and the nanoparticles 59. The change in thermal properties of different nanocomposites are reflected in their glass transition temperatures (Tg) and thermogravimetric analysis profiles.
The glass transition temperature (Tg) depends upon the motion of polymer chains and a sharp decrease of the free volume in the presence of the secondary elements (CNTs). The presence of CNTs which are dimensionally similar to the polymer chain building units are known to influence the alignment of polymer chains and thereby alter the Tg. The presence of CNTs has been reported to affect the structure of the cured epoxy thereby affecting Tg 60. Jin et al. reported improved glass transition temperature (11oC increment) of dodecylamine treated CNT epoxy nanocomposites 61. However, the reduction in Tg in the presence of nanomaterials has also been reported 40,41. Khare et. al. studied the Tg of various CNTs based epoxy nanocomposites and observed that the weak matrix−filler interactions cause the interphase region in the nanocomposite to be more compressible 62,63. Simulation have also shown that dynamic heterogeneity and the fraction of the immobile domains in crossed linked structures increase rapidly below Tg 64.
Table 1 shows the Tgof composites with different f-CNTs studied here. Composite prepared by using CNT-OH showed enhancement as high as 34%. This is attributed to strong binding through –OH interactions of the polymer molecules to the CNTs surface, thereby decreasing their mobility (as shown in Figure 7a). The low enhancement in CNT-ODA composite was attributed to the presence of bulky octadecyl (C18H37) groups in the CNTs surface which restricted the close packing of the polymer chains. The relatively low enhancement of the CNT-COOH was attributed to the preferential adsorption of basic curing agent on acidic CNTs leading to non-stoichiometric balance in overall curing system, which would result in decrease in cross-link density to reduce Tg 41,65.
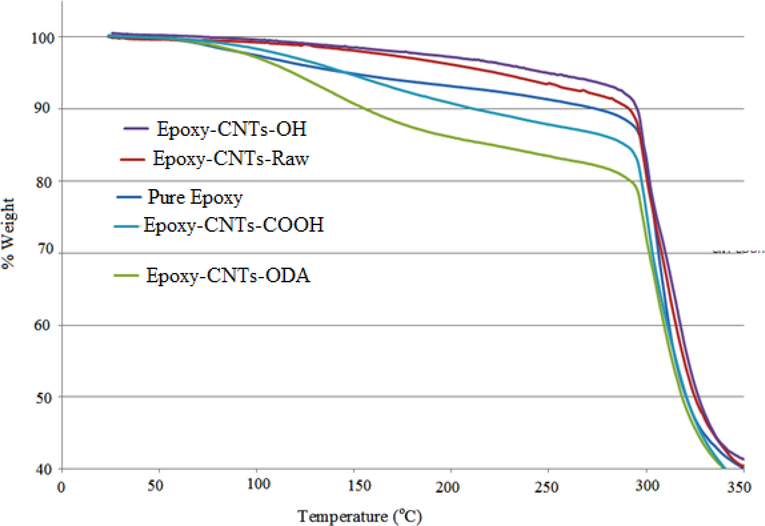
TGA was carried out in a flow of nitrogen at a temperature ramp rate of 10 °C/min. The results are shown in Figure 8. The trend in the initial degradation up to 300°C was different for the different composites. Composite prepared from CNT-OH showed better initial thermal stability. The ODA group in CNT-ODA began to degrade at low temperature leading to rapid drop in mass.
Figure 8.
TGA curve of pure epoxy and various CNT-composites
3.5. Electrical conductivity
Typical epoxy is nonconductive and the incorporation of CNTs improved the conductivity 66. Table 1 presents the volume resistivity and surface resistance of pure epoxy and the various CNT based composites at 0.5 wt% loading.
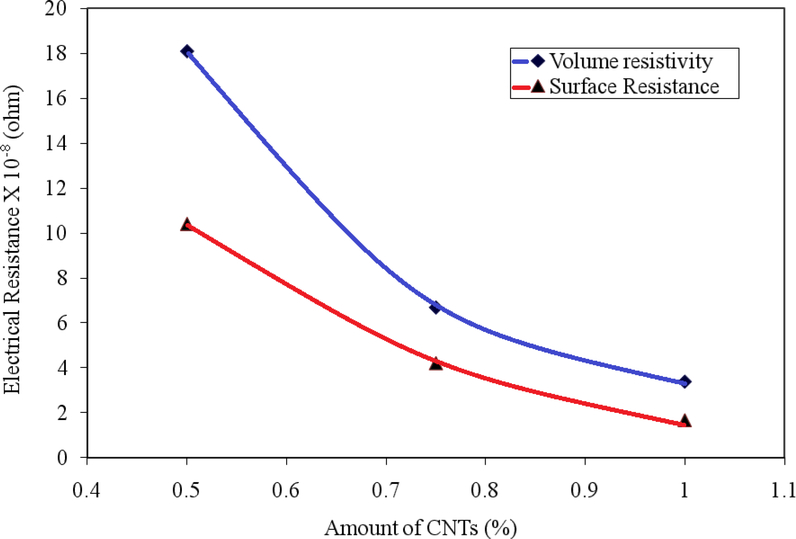
It is clear from the Table that the conductivity of the composites improved with incorporation of CNTs in the polymer matrices. Among all of the composites, raw CNTs showed the highest improvement in conductivity because the CNTs were undamaged 67. CNTs agglomeration could also favor the formation of percolating network for current flow 68–70. This was attributed to the fact that the colloidal behavior of the raw CNTs was relatively poor and led to surface agglomeration. Figure 9 shows the surface resistance and volume resistivity of CNT-OH composite as a function of CNT loading. It is clear from the figure that with increase in CNTs concentrations, there was a sharp decrease in surface as well as volume resistivity.
Figure 9.
Electrical resistance of Epoxy-CNTs-OH composite with the variation of CNTs
4. Conclusions
Various functionalized CNTs were synthesized and their colloidal behavior in the presence of epoxy resin and TETA was studied. The influence of interfacial interaction between the CNT surface and the polymer matrix has been discussed. The nature of functionalization and their specific interactions with the polymer chains affected the physical, thermal and electrical properties of the composites. For example, the enhancement in tensile strength was found to be 7.2, 11.2, 11.4 and 14.2 percent for raw CNTs, carboxylated CNTs, octadecyl amide functionalized CNTs and hydroxylated CNTs, respectively at 0.5 wt% CNTs loading. Functionalization of CNTs improved the glass transition temperature of the epoxy composites, and CNTs-OH showed highest enhancement (34%) in Tg. Addition of CNTs in polymer matrix also reduced the electrical resistance reasonably. The variation in re-aggregation behavior of CNTs in the presence of polymer and TETA indicates specific interactions between the CNTs and polymer matrix system. A direct comparison with other data is difficult because of the wide variation of epoxy resin, hardener, CNTs, degree of functionalization and processing techniques.
Acknowledgements
This work was funded by a grant from the National Institute of Environmental Health Sciences (NIEHS) under Grant No. R01ES023209. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NIEHS.
REFERENCES:
- 1.Ghosh B, Gogoi S, Thakur S, Karak N. Bio-based waterborne polyurethane/carbon dot nanocomposite as a surface coating material. Progress in Organic Coatings 2016;90:324–30. [Google Scholar]
- 2.Riquelme J, Garzón C, Bergmann C, Geshev J, Quijada R. Development of multifunctional polymer nanocomposites with carbon-based hybrid nanostructures synthesized from ferrocene. European Polymer Journal 2016;75:200–9. [Google Scholar]
- 3.Roy S, Hussain CM, Mitra S. Carbon nanotube-immobilized super-absorbent membrane for harvesting water from the atmosphere. Environmental Science: Water Research & Technology 2015;1:753–60. [Google Scholar]
- 4.Roy S, Ntim SA, Mitra S, Sirkar KK. Facile fabrication of superior nanofiltration membranes from interfacially polymerized CNT-polymer composites. Journal of membrane science 2011;375:81–7. [Google Scholar]
- 5.Zhou H, Han G, Xiao Y, Chang Y, Zhai H-J. A comparative study on long and short carbon nanotubes-incorporated polypyrrole/poly (sodium 4-styrenesulfonate) nanocomposites as high-performance supercapacitor electrodes. Synthetic Metals 2015;209:405–11. [Google Scholar]
- 6.Ates M, Eker AA, Eker B. Carbon nanotube-based nanocomposites and their applications. Journal of adhesion science and Technology 2017;31:1977–97. [Google Scholar]
- 7.Chandrasekhar P CNT Applications in Drug and Biomolecule Delivery Conducting Polymers, Fundamentals and Applications: Springer; 2018:61–4. [Google Scholar]
- 8.Wang Z, Bramnik N, Roy S, Di Benedetto G, Zunino JL, Mitra S. Flexible zinc–carbon batteries with multiwalled carbon nanotube/conductive polymer cathode matrix. Journal of Power Sources 2013;237:210–4. [Google Scholar]
- 9.Roy S, Bhadra M, Mitra S. Enhanced desalination via functionalized carbon nanotube immobilized membrane in direct contact membrane distillation. Separation and Purification Technology 2014;136:58–65. [Google Scholar]
- 10.Sharma S, Singh BP, Chauhan SS, et al. Enhanced thermomechanical and electrical properties of multiwalled carbon nanotube paper reinforced epoxy laminar composites. Composites Part A: Applied Science and Manufacturing 2018;104:129–38. [Google Scholar]
- 11.Mittal G, Dhand V, Rhee KY, Park S-J, Lee WR. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. Journal of Industrial and Engineering Chemistry 2015;21:11–25. [Google Scholar]
- 12.Bhattacharya M Polymer nanocomposites—a comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016;9:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdalla M, Dean D, Adibempe D, Nyairo E, Robinson P, Thompson G. The effect of interfacial chemistry on molecular mobility and morphology of multiwalled carbon nanotubes epoxy nanocomposite. Polymer 2007;48:5662–70. [Google Scholar]
- 14.Hong S-k, Kim D, Lee S, Kim B-W, Theilmann P, Park S-H. Enhanced thermal and mechanical properties of carbon nanotube composites through the use of functionalized CNT-reactive polymer linkages and three-roll milling. Composites Part A: Applied Science and Manufacturing 2015;77:142–6. [Google Scholar]
- 15.Spitalsky Z, Tasis D, Papagelis K, Galiotis C. Carbon nanotube–polymer composites: chemistry, processing, mechanical and electrical properties. Progress in polymer science 2010;35:357–401. [Google Scholar]
- 16.Rafiee MA, Rafiee J, Wang Z, Song H, Yu Z-Z, Koratkar N. Enhanced mechanical properties of nanocomposites at low graphene content. ACS nano 2009;3:3884–90. [DOI] [PubMed] [Google Scholar]
- 17.Niranjan K Naik KSP, Kavala Venkateswara R, Zhang Wei, Koratkar Nikhil A. High-strain rate compressive behavior of multi-walled carbon nanotube dispersed thermoset epoxy resin. Journal of Composite Materials 2015;49 903–10 [Google Scholar]
- 18.Muskopf JW, McCollister SB. Epoxy resins. Ullmann’s encyclopedia of industrial chemistry 1987. [Google Scholar]
- 19.Pascault J-P, Williams RJ. Epoxy polymers: new materials and innovations: John Wiley & Sons; 2009. [Google Scholar]
- 20.Karger‐Kocsis J Epoxy Polymers New Materials and Innovations. Macromolecular Chemistry and Physics 2010;211:1836-. [Google Scholar]
- 21.Tan C, Sun H, Fung BM, Grady BP. Properties of liquid crystal epoxy thermosets cured in a magnetic field. Macromolecules 2000;33:6249–54. [Google Scholar]
- 22.Lee H, Neville K. Epoxy resins: their applications and technology. 1957.
- 23.Lee H, Neville K. Handbook of epoxy resins 1967. ISBN-13:978–0070369979.
- 24.Roy S, Mitra K, Desai C, Petrova R, Mitra S. Detonation nanodiamonds and carbon nanotubes as reinforcements in epoxy composites—a comparative study. Journal of Nanotechnology in Engineering and Medicine 2013;4:011008. [Google Scholar]
- 25.Ma C, Liu H-Y, Du X, Mach L, Xu F, Mai Y-W. Fracture resistance, thermal and electrical properties of epoxy composites containing aligned carbon nanotubes by low magnetic field. Composites Science and Technology 2015;114:126–35. [Google Scholar]
- 26.Vahedi F, Shahverdi H, Shokrieh M, Esmkhani M. Effects of carbon nanotube content on the mechanical and electrical properties of epoxy-based composites. New Carbon Materials 2014;29:419–25. [Google Scholar]
- 27.Mahesh V, Muralidhara B, George R. Studies Of Influence on Multiwalled Carbon Nanotubes (MWCNT’s) Reinforced Epoxy Based Composites. International Journal Of Modern Engineering Research (IJMER) 2014;4:58. [Google Scholar]
- 28.Siddiqui NA, Sham M-L, Tang BZ, Munir A, Kim J-K. Tensile strength of glass fibres with carbon nanotube–epoxy nanocomposite coating. Composites Part A: Applied Science and Manufacturing 2009;40:1606–14. [Google Scholar]
- 29.Nam I, Lee H-K, Jang J. Electromagnetic interference shielding/absorbing characteristics of CNT-embedded epoxy composites. Composites Part A: Applied Science and Manufacturing 2011;42:1110–8. [Google Scholar]
- 30.Bae J, Jang J, Yoon SH. Cure Behavior of the Liquid‐Crystalline Epoxy/Carbon Nanotube System and the Effect of Surface Treatment of Carbon Fillers on Cure Reaction. Macromolecular Chemistry and Physics 2002;203:2196–204. [Google Scholar]
- 31.Saeb MR, Najafi F, Bakhshandeh E, et al. Highly curable epoxy/MWCNTs nanocomposites: an effective approach to functionalization of carbon nanotubes. Chemical engineering journal 2015;259:117–25. [Google Scholar]
- 32.Esmizadeh E, Yousefi AA, Naderi G. Effect of type and aspect ratio of different carbon nanotubes on cure behavior of epoxy-based nanocomposites. Iranian Polymer Journal 2015;24:1–12. [Google Scholar]
- 33.Grady BP. Recent developments concerning the dispersion of carbon nanotubes in polymers. Macromolecular rapid communications 2010;31:247–57. [DOI] [PubMed] [Google Scholar]
- 34.Thostenson ET, Chou T-W. Processing-structure-multi-functional property relationship in carbon nanotube/epoxy composites. Carbon 2006;44:3022–9. [Google Scholar]
- 35.Ci L, Bai J. The reinforcement role of carbon nanotubes in epoxy composites with different matrix stiffness. Composites Science and Technology 2006;66:599–603. [Google Scholar]
- 36.Li X-F, Lau K-T, Yin Y-S. Mechanical properties of epoxy-based composites using coiled carbon nanotubes. Composites science and technology 2008;68:2876–81. [Google Scholar]
- 37.Tian ZQ, Jiang SP, Liang YM, Shen PK. Synthesis and characterization of platinum catalysts on multiwalled carbon nanotubes by intermittent microwave irradiation for fuel cell applications. The Journal of Physical Chemistry B 2006;110:5343–50. [DOI] [PubMed] [Google Scholar]
- 38.Rastogi R, Kaushal R, Tripathi S, Sharma AL, Kaur I, Bharadwaj LM. Comparative study of carbon nanotube dispersion using surfactants. Journal of colloid and interface science 2008;328:421–8. [DOI] [PubMed] [Google Scholar]
- 39.Krause B, Petzold G, Pegel S, Pötschke P. Correlation of carbon nanotube dispersability in aqueous surfactant solutions and polymers. Carbon 2009;47:602–12. [Google Scholar]
- 40.Miyagawa H, Drzal LT. Thermo-physical and impact properties of epoxy nanocomposites reinforced by single-wall carbon nanotubes. Polymer 2004;45:5163–70. [Google Scholar]
- 41.Liao Y-H, Marietta-Tondin O, Liang Z, Zhang C, Wang B. Investigation of the dispersion process of SWNTs/SC-15 epoxy resin nanocomposites. Materials Science and Engineering: A 2004;385:175–81. [Google Scholar]
- 42.Tasis D, Tagmatarchis N, Bianco A, Prato M. Chemistry of carbon nanotubes. Chemical reviews 2006;106:1105–36. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Iqbal Z, Mitra S. Microwave‐Induced Controlled Purification of Single‐Walled Carbon Nanotubes without Sidewall Functionalization. Advanced Functional Materials 2007;17:3946–51. [Google Scholar]
- 44.Chen Y, Mitra S. Fast microwave-assisted purification, functionalization and dispersion of multi-walled carbon nanotubes. Journal of Nanoscience and Nanotechnology 2008;8:5770–5. [DOI] [PubMed] [Google Scholar]
- 45.Gojny FH, Wichmann MH, Fiedler B, Schulte K. Influence of different carbon nanotubes on the mechanical properties of epoxy matrix composites–a comparative study. Composites Science and Technology 2005;65:2300–13. [Google Scholar]
- 46.Carpenter C, Shipway P, Zhu Y, Weston D. Effective dispersal of CNTs in the fabrication of electrodeposited nanocomposites. Surface and Coatings Technology 2011;205:4832–7. [Google Scholar]
- 47.Zhang M, Yudasaka M, Koshio A, Iijima S. Effect of polymer and solvent on purification and cutting of single-wall carbon nanotubes. Chemical physics letters 2001;349:25–30. [Google Scholar]
- 48.Lu K, Lago R, Chen Y, Green M, Harris P, Tsang S. Mechanical damage of carbon nanotubes by ultrasound. Carbon 1996;34:814–6. [Google Scholar]
- 49.Shelimov KB, Esenaliev RO, Rinzler AG, Huffman CB, Smalley RE. Purification of single-wall carbon nanotubes by ultrasonically assisted filtration. Chemical Physics Letters 1998;282:429–34. [Google Scholar]
- 50.Gojny F, Wichmann M, Köpke U, Fiedler B, Schulte K. Carbon nanotube-reinforced epoxy-composites: enhanced stiffness and fracture toughness at low nanotube content. Composites science and technology 2004;64:2363–71. [Google Scholar]
- 51.Bai J, Allaoui A. Effect of the length and the aggregate size of MWNTs on the improvement efficiency of the mechanical and electrical properties of nanocomposites—experimental investigation. Composites Part A: applied science and manufacturing 2003;34:689–94. [Google Scholar]
- 52.Gojny FH, Nastalczyk J, Roslaniec Z, Schulte K. Surface modified multi-walled carbon nanotubes in CNT/epoxy-composites. Chemical Physics Letters 2003;370:820–4. [Google Scholar]
- 53.Li Y, Hu N, Kojima T, et al. Experimental study on mechanical properties of epoxy/MWCNT nanocomposites—effects of acid treatment, pressured curing, and liquid rubber. Journal of Nanotechnology in Engineering and Medicine 2012;3:011004. [Google Scholar]
- 54.Ntim SA, Sae-Khow O, Witzmann FA, Mitra S. Effects of polymer wrapping and covalent functionalization on the stability of MWCNT in aqueous dispersions. Journal of colloid and interface science 2011;355:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.RCP W. Zhang, Koratkar N The effect of carbon nanotube dimensions and dispersion on the fatigue behavior of epoxy nanocomposites. Nanotechnology 2008;19 285709. [DOI] [PubMed] [Google Scholar]
- 56.Hernández-Pérez A, Avilés F, May-Pat A, Valadez-González A, Herrera-Franco P, Bartolo-Pérez P. Effective properties of multiwalled carbon nanotube/epoxy composites using two different tubes. Composites Science and Technology 2008;68:1422–31. [Google Scholar]
- 57.I-M. Low Y-WM. Properties of pure and toughened epoxy resin, Handbook of ceramics and composites In: Cheremisinoff NP, ed. Handbook of ceramics and composites. New York: Marcel Dekker, Inc.; 1992:105. [Google Scholar]
- 58.Domun N, Hadavinia H, Zhang T, Sainsbury T, Liaghat G, Vahid S. Improving the fracture toughness and the strength of epoxy using nanomaterials–a review of the current status. Nanoscale 2015;7:10294–329. [DOI] [PubMed] [Google Scholar]
- 59.Bui K, Grady BP, Saha MC, Papavassiliou DV. Effect of carbon nanotube persistence length on heat transfer in nanocomposites: A simulation approach. Applied Physics Letters 2013;102:203116. [Google Scholar]
- 60.Allaoui A, El Bounia N-E. How carbon nanotubes affect the cure kinetics and glass transition temperature of their epoxy composites?–a review. Express Polymer Letters 2009;3:588–94. [Google Scholar]
- 61.Grossiord N, Loos J, Regev O, Koning CE. Toolbox for dispersing carbon nanotubes into polymers to get conductive nanocomposites. Chemistry of materials 2006;18:1089–99. [Google Scholar]
- 62.Khare KS, Khabaz F, Khare R. Effect of carbon nanotube functionalization on mechanical and thermal properties of cross-linked epoxy–carbon nanotube nanocomposites: role of strengthening the interfacial interactions. ACS applied materials & interfaces 2014;6:6098–110. [DOI] [PubMed] [Google Scholar]
- 63.Khare KS, Khare R. Effect of carbon nanotube dispersion on glass transition in cross-linked epoxy–carbon nanotube nanocomposites: Role of interfacial interactions. The Journal of Physical Chemistry B 2013;117:7444–54. [DOI] [PubMed] [Google Scholar]
- 64.Lin P-H, Khare R. Local chain dynamics and dynamic heterogeneity in cross-linked epoxy in the vicinity of glass transition. Macromolecules 2010;43:6505–10. [Google Scholar]
- 65.Dehghan M Development of Thermomechanically Improved Epoxy Adhesives Using Carbon Nanotubes. Victoria, Australia: Swinburne University of Technology; 2015. [Google Scholar]
- 66.Jin F-L, Ma C-J, Park S-J. Thermal and mechanical interfacial properties of epoxy composites based on functionalized carbon nanotubes. Materials Science and Engineering: A 2011;528:8517–22. [Google Scholar]
- 67.Liu J, Rinzler AG, Dai H, et al. Fullerene pipes. Science 1998;280:1253–6. [DOI] [PubMed] [Google Scholar]
- 68.Seidel GD, Boehringer KL, Lagoudas DC. Analysis of Clustering and Interphase Region Effects on the Electrical Conductivity of Carbon Nanotube-Polymer Nanocomposites via Computational Micromechanics. ASME 2008 Conference on Smart Materials, Adaptive Structures and Intelligent Systems; 2008: American Society of Mechanical Engineers. p. 159–65. [Google Scholar]
- 69.Li J, Ma PC, Chow WS, To CK, Tang BZ, Kim JK. Correlations between percolation threshold, dispersion state, and aspect ratio of carbon nanotubes. Advanced Functional Materials 2007;17:3207–15. [Google Scholar]
- 70.Martin C, Sandler J, Shaffer M, et al. Formation of percolating networks in multi-wall carbon-nanotube–epoxy composites. Composites Science and Technology 2004;64:2309–16. [Google Scholar]