Abstract
Background
Infection with the protozoan Entamoeba histolytica is common in low‐ and middle‐income countries, and up to 100,000 people with severe disease die every year. Adequate therapy for amoebic colitis is necessary to reduce illness, prevent development of complicated disease and extraintestinal spread, and decrease transmission.
Objectives
To evaluate antiamoebic drugs for treating amoebic colitis.
Search methods
We searched the available literature up to 22 March 2018. We searched the Cochrane Infectious Diseases Group Specialised Register, CENTRAL, MEDLINE, Embase, LILACS, mRCT, and conference proceedings. We contacted individual researchers, organizations, and pharmaceutical companies, and we checked reference lists.
Selection criteria
Randomized controlled trials of antiamoebic drugs given alone or in combination, compared with placebo or another antiamoebic drug, for treating adults and children with a diagnosis of amoebic colitis.
Data collection and analysis
Two review authors independently assessed the eligibility and methodological quality of trials and extracted and analysed the data. We calculated clinical and parasitological failure rates and rates of relapse and adverse events as risk ratios (RRs) with 95% confidence intervals (CIs), using a random‐effects model. We determined statistical heterogeneity and explored possible sources of heterogeneity using subgroup analyses. We carried out sensitivity analysis by using trial quality to assess the robustness of reported results.
Main results
In total, 41 trials (4999 participants) met the inclusion criteria of this review. In this update, we added four trials to the 37 trials included in the first published review version. Thirty trials were published over 20 years ago. Only one trial used adequate methods of randomization and allocation concealment, was blinded, and analysed all randomized participants. Only one trial used an E histolytica stool antigen test, and two trials used amoebic culture.
Tinidazole may be more effective than metronidazole for reducing clinical failure (RR 0.28, 95% CI 0.15 to 0.51; 477 participants, eight trials; low‐certainty evidence) and is probably associated with fewer adverse events (RR 0.65, 95% CI 0.46 to 0.92; 477 participants, 8 trials; moderate‐certainty evidence). Compared with metronidazole, combination therapy may result in fewer parasitological failures (RR 0.36, 95% CI 0.15 to 0.86; 720 participants, 3 trials; low‐certainty evidence), but we are uncertain which combination is more effective than another. Evidence is insufficient to allow conclusions regarding the efficacy of other antiamoebic drugs.
Authors' conclusions
Compared with metronidazole, tinidazole may be more effective in reducing clinical failure and may be associated with fewer adverse events. Combination drug therapy may be more effective for reducing parasitological failure compared with metronidazole alone. However, these results are based mostly on small trials conducted over 20 years ago with a variety of poorly defined outcomes. Tests that detect E histolytica more accurately are needed, particularly in countries where concomitant infection with other bacteria and parasites is common.
11 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (22 Mar, 2018) were included and two ongoing studies have been identified (see 'Characteristics of ongoing studies' section)
Plain language summary
Antiamoebic drugs for treating amoebic colitis
What is the aim of this review?
This Cochrane Review aims to determine the effectiveness and safety of drugs used to treat people with amoebic colitis, which is an infection of the large intestines caused by the parasite, Entamoeba histolytica. Cochrane researchers searched for all relevant studies to answer this question and included 41 relevant studies in this review.
Key messages
Tinidazole may be more effective than metronidazole for reducing clinical symptoms and may be associated with fewer adverse events. Combination therapy resulted in fewer parasitological failures than occurred with metronidazole alone. Evidence is insufficient to allow conclusions regarding the efficacy of other antiamoebic drugs. Better quality randomized trials using accurate diagnostic methods and standardized outcomes are needed to evaluate the efficacy of drugs for treating individuals with amoebic colitis.
What was studied in the review?
Entamoeba histolytica is distributed throughout the world and is commonly acquired by ingestion of contaminated food or water. An estimated 40 to 50 million people infected with E histolytica develop amoebic colitis or extraintestinal abscesses, resulting in up to 100,000 deaths per year.
Metronidazole is currently the standard therapy for treating adults and children with invasive amoebiasis, but it may not be sufficient to eliminate amoebic cysts from the intestine. Some unpleasant adverse effects have been associated with metronidazole, and the possibility of parasite resistance to metronidazole has led to the development of alternative drugs. Combinations of metronidazole with other drugs that eradicate surviving cysts in the intestines have been recommended, so evidence to support this approach needs to be assessed.
This review compares different drugs used against amoebic colitis, alone or in combination, and also assesses single‐dose regimens versus longer regimens.
What are the main results of the review?
This review included 41 studies, most of which were conducted in countries considered to be highly endemic for amoebiasis. Most trials were old: 30 were conducted before 1998. Trials varied in the inclusion criteria used to enrol participants and in the definition and timing of measured outcomes. Stool microscopy with direct wet saline smear was the method used most often to detect the presence of E histolytica in stools. Study participants ranged in age from seven months to 80 years. Included trials reported a variety of comparisons and involved 25 individual drugs, two herbal products, and 15 different combinations.
The review shows that in individuals with amoebic colitis, tinidazole may be better for reducing clinical symptoms (low‐certainty evidence) and probably results in fewer adverse events when compared with metronidazole (moderate‐certainty evidence). However, we do not know whether it is more effective for eradicating amoebae from the stools. Combination drug therapy may be more effective than metronidazole alone for eradicating amoebae (low‐certainty evidence), but we are uncertain which drug combination is most effective, and if combination treatment will lead to more rapid resolution of clinical symptoms or in more adverse events (very low‐certainty evidence). Evidence is insufficient to allow conclusions regarding efficacy of the other antiamoebic drugs.
How up‐to‐date is this review? The review authors searched for studies that had been published up to 22 March 2018.
Summary of findings
Background
Description of the condition
Amoebiasis is a parasitic disease caused by Entamoeba histolytica, a protozoan parasite that is found worldwide. An estimated 40 to 50 million people infected with E histolytica develop amoebic colitis or extraintestinal abscess, which results in up to 100,000 deaths annually (Bercu 2007; Choudhuri 2012). Amoebic colitis is a leading cause of severe diarrhoea worldwide, particularly in children below five years of age living in low‐ and middle‐income countries (LMICs) (Shirley 2018). The greatest burden of amoebiasis occurs in LMICs in Asia, the sub‐Saharan and tropical regions of Africa, and in Central and South America (Choudhuri 2012; Shirley 2018). In these areas, prevalence rates vary with the population studied and differ between countries and areas with different socioeconomic and sanitary conditions and with the diagnostic test used.
Seroprevalence studies have detected antibodies ranging from 12% to 65% among those living in highly endemic areas in Asia and Latin America, including asymptomatic individuals (Braga 1996; Haque 1999; Haque 2001; Barwick 2002; Gatti 2002). Antibodies that develop after invasive infection can be measured by several immunological tests, but these tests will differentiate past infection from current or active amoebiasis. Studies using more sensitive tests that can differentiate pathogenic E histolytica from non‐pathogenic species, such as enzyme‐linked immunosorbent assay (ELISA) stool antigen detection or polymerase chain reaction (PCR), reported that the incidence of intestinal amoebiasis in highly endemic areas ranged from 13% to 67% among individuals with diarrhoea (Haque 1997; Abd‐Alla 2002; Tanyuksel 2005; Rivera 2006; Samie 2006), and from 1.0% to 13.8% among asymptomatic individuals (Haque 1997; Braga 1998; Rivera 1998; Ramos 2005). A prospective study conducted in asymptomatic schoolchildren two to five years of age living in an urban slum in Bangladesh showed that 90% were infected with E histolytica at least once, as determined by stool antigen detection, and that repeat infection occurred in 68% of 162 children who completed 8.2 years of observation (Petri 2009).
Infection is commonly acquired by ingestion of food or water contaminated with cysts of E histolytica, but transmission also occurs through oral and anal sex and via contaminated enema apparatuses (Haque 2003; Stanley 2003; Shirley 2018). In high‐income countries, infection occurs primarily among returning travellers or recent immigrants from endemic regions, homosexuals engaging in oral‐anal sexual practices, immunosuppressed people, and institutionalized individuals (Salit 2009; Petri 2010; Herbinger 2011; Shirley 2018). HIV infection was shown to be a common coexisting condition with amoebiasis among USA residents who died (Gunther 2011), and E histolytica remains an important diagnostic consideration for those presenting with bloody diarrhoea (Petri 2010). Studies have documented increased prevalence of amoebiasis among HIV‐positive men who have sex with men in several Asia Pacific countries (Tsai 2006; Chen 2007; Park 2007; Hung 2008; James 2010; Nagata 2012; Zhou 2013), with higher risk of developing invasive disease reported in this population (Hung 2008; Stark 2008; Watanabe 2011).
About 90% of people infected with E histolytica have no symptoms of disease and spontaneously clear their infection, while the remaining 10% develop invasive disease (Haque 2002; Stanley 2003; Choudhuri 2012). The underlying factors responsible for variable clinical outcomes of infection by E histolytica remain largely unknown and may be determined by a complex interaction between host factors, parasite genotype, and environmental factors (Ralston 2011; Wilson 2012; Shirley 2018).
Amoebic colitis is a manifestation of intestinal amoebiasis that commonly presents as ulcers and inflammation of the colon. This results in a complete spectrum of colonic signs and symptoms ranging from non‐bloody diarrhoea to dysentery (acute diarrhoea with bloody stools), and to necrotizing colitis (severe inflammation of the colon) with intestinal perforation and peritonitis (infection of abdominal cavity membranes) (Ravdin 2005; Shirley 2018).
Based on clinical manifestation, amoebic colitis may be classified as amoebic dysentery or non‐dysenteric amoebic colitis (Bercu 2007; Petri 2010; Ximenez 2011; Choudhuri 2012). Amoebic dysentery is acute diarrhoea with visible blood and mucus in stools and the presence of haematophagous trophozoites (trophozoites with ingested red blood cells) in stools or tissues. Non‐dysenteric amoebic colitis presents as recurrent bouts of diarrhoea with or without mucus but with no visible blood and the presence of E histolytica cysts or non‐haematophagous trophozoites (trophozoites with no ingested red blood cells) in the stools. The sigmoidoscopic examination of the colon originally described in the Report of the WHO Expert Committee on Amoebiasis showed inflamed mucosa with discrete ulcers in amoebic dysentery but usually normal results in the nondysenteric type (WHO 1969). However, recent studies have documented mucosal inflammation with small colonic ulcers or erosions on colonoscopy even in those with mild or nonspecific symptoms of non‐dysenteric colitis (Okamoto 2005; Lee 2015).
The most severe complication of amoebic colitis is fulminant or necrotizing colitis, occurring in 0.5% of cases (Haque 2003; Choudhuri 2012; Shirley 2018). Necrotizing colitis occurs with profuse bloody diarrhoea, fever, and widespread abdominal pain, frequently progressing to severe injury of the bowel wall, intestinal haemorrhage, or perforation with peritonitis (Haque 2003; Stanley 2003; Choudhuri 2012; Shirley 2018). Among people with this condition, the case‐fatality rate ranges from 40% to 89% (Choudhuri 2012; Shirley 2018). Young children, malnourished individuals, pregnant women, immunocompromised individuals, and those receiving corticosteroids are at higher risk for invasive disease (Stanley 2003; Petri 2010; Shirley 2016). Extraintestinal complications of amoebic infection include abscess in various organs, empyema (accumulation of pus around the lungs), and pericarditis (inflammation of membranes surrounding the heart) (Petri 2010; Choudhuri 2012). For treatment of necrotizing colitis and extraintestinal amoebiasis, surgery and additional antibiotics may be required, aside from specific antiamoebic drugs (Petri 2010; Choudhuri 2012; Shirley 2018).
In many countries where amoebiasis is endemic, amoebic colitis is commonly diagnosed by identifying cysts or motile trophozoites in a saline wet mount of a stool specimen. Finding in the stool trophozoites that contain ingested red blood cells is considered by many to be diagnostic of invasive intestinal amoebiasis (Tanyuksel 2003; Choudhuri 2012; Talamas‐Lara 2014). Stool microscopy is incapable of differentiating E histolytica from non‐pathogenic species such as Entamoeba dispar or Entamoeba moshkovskii, and the accuracy of microscopic methods is highly dependent on the competence of the diagnostic laboratory (Haque 2003; Petri 2010). When a definitive diagnosis by microscopy is not possible, the presence of the E histolytica/E dispar complex should be reported (WHO 1997; Haque 1998; CDC 2010). Culture followed by isoenzyme analysis will differentiate E histolytica from E dispar but is technically difficult and is associated with significant false‐negative rates (Fotedar 2007). Currently, specific and sensitive means to detect E histolytica in stools include stool antigen detection testing and PCR techniques based on amplification of the target parasite RNA and DNA (Haque 1998; Nesbitt 2004; Fotedar 2007; Petri 2010; Choudhuri 2012; Shirley 2018). Ideally, stool samples positive forE histolytica on microscopy should be confirmed with stool antigen or PCR before treatment starts. Unfortunately, in resource‐limited countries, where the incidence of amoebiasis is highest, these tests are not routinely used and are not widely available for the diagnosis of amoebic colitis.
Description of the intervention
The goals of treatment for individuals with amoebic colitis are to treat invasive disease and to eradicate intestinal carriage of the organism (Haque 2003; Kappagoda 2011). E histolytica may be found in the bowel lumen, in the bowel wall, and in tissues, including the liver (Choudhuri 2012; Shirley 2018). Antiamoebic drugs vary in efficacy at the three sites where parasites commonly exist and generally are divided into two classes based on their main site of activity. Luminal amoebicides act principally in the bowel lumen, and tissue amoebicides act principally in the bowel wall and in the liver. See Table 3 for examples.
1. Amoebicide classes and examples.
| Amoebicide | Class | Examples |
| Luminal | Arsenical compounds | Carbarsone, acetarsone or acetarsol, treparsol, diphetarsone, glycobiarsol or bismuth glycolylarsanilate, stovarsol, thioarsenite, thiocarbarsone, and arsthinol |
| Hydroxyquinoline derivatives | Chiniofon or quinoxyl, clioquinol or iodochlorhydroxyquin, and iodoquinol or diiodohydroxyquin | |
| Dichloroacetamide derivatives | Diloxanide furoate or entamide furoate, clefamide, eticlordifene or ethylchlordiphene, etofamide or etophamide, and quinfamide | |
| Benzylamine derivatives | Teclozan, chlorbetamide or mantomide, and chlorphenoxamide or mebinol | |
| Antibiotic amoebicides | Tetracycline, oxytetracycline, chlortetracycline, erythromycin, paromomycin, and fumagillin | |
| Tissue | Emetine and its derivatives | Emetine hydrochloride, emetine bismuth iodide, dehydroemetine dihydrochloride, and dehydroemetine resinate |
| Aminoquinoline | Chloroquine | |
| Thiazole derivative | Niridazole | |
| Nitroimidazoles | Metronidazole, tinidazole, ornidazole, secnidazole, and nimorazole | |
| Nithrothiazole salicylamide | Nitazoxanide |
Among the antiamoebic drugs listed in the table, nitazoxanide is the most recent addition. Nitazoxanide is a nitrothiazole derivative whose structure is similar to metronidazole; however, it has greater antiparasitic activity against various intestinal protozoal and parasitic infections when compared with metronidazole (Fox 2005; Ochoa 2005; Parashar 2005). Effectiveness of nitazoxanide and its major metabolite, tizoxanide against both luminal and invasive forms have been demonstrated (Adagu 2002; Cedillo‐Rivera 2002; Petri 2003; Shirley 2018), but further studies are needed to determine if this can be recommended as treatment for amoebic colitis.
Metronidazole is considered standard therapy for treating people with invasive amoebiasis (WHO 2005; The Medical Letter 2013; AAP 2015). The recommended regimen of metronidazole for treatment of amoebic colitis is 500 to 750 mg given three times daily in adults, and 30 to 50 mg/kg/day given for five to 10 days in children (WHO 2005; The Medical Letter 2013; AAP 2015). Although this dose may have sufficient activity against both trophozoites and cysts (WHO 1994; Li 1996), the predominant belief is that metronidazole alone is not reliably effective for eliminating cysts in the colonic lumen due to its failure to reach adequate therapeutic concentrations in the large intestines (Haque 2003; Stanley 2003). This results in persistence of the parasites in the intestine in as many as 40% to 60% of patients (Haque 2003; Stanley 2003; Petri 2010). Thus, the general recommendation is that patients with invasive amoebiasis should receive a luminal amoebicide after treatment with a tissue amoebicide, to eliminate any surviving organisms in the colon (Kappagoda 2011; Choudhuri 2012; The Medical Letter 2013; AAP 2015). This recommendation is based on the assumption that drugs acting on different protozoal processes may enhance the effects of other drugs. However, evidence to support combination therapy has not been reviewed, and it is not known whether drug combinations reduce clinical symptoms or eradicate parasites more effectively than tissue amoebicides given alone. Controversy surrounds the need for cyst eradication following metronidazole or tinidazole treatment, especially in endemic areas, where re‐infection is frequent. Furthermore, the increased complexity of combination regimens, additional drug costs, and potentially increased adverse events, combined with the unavailability of luminal agents on the market, act as major deterrents to compliance with combination therapy.
Adverse effects may occur even with conventional doses of metronidazole and include headache, loss of appetite, metallic taste, nausea, and vomiting (Petri 2003; The Medical Letter 2013), the last two of which may be exacerbated by drinking alcohol. Dizziness, convulsions, poor co‐ordination, and numbness of the extremities are less common but more serious adverse effects that warrant discontinuation of metronidazole (Petri 2003). Other nitroimidazole drugs with longer half‐lives, such as tinidazole, ornidazole, and secnidazole, allow shorter periods of treatment and appear to be better tolerated than metronidazole. These drugs have been used successfully when administered in shorter courses and have been recommended as alternative antiamoebic drugs to metronidazole (Haque 2003; Stanley 2003; WHO 2005; The Medical Letter 2013; AAP 2015).
Treatment failure has been reported with metronidazole, and most of these cases have been attributed to incorrect diagnosis, selection of an unsuitable drug, or failure to observe certain principles of treatment, rather than to drug resistance (Wassman 1999; Stanley 2003). However, induction of metronidazole‐resistant E histolytica strains in the laboratory suggests that indiscriminate use of antiamoebic drugs can result in an increased minimum inhibitory concentration against E histolytica (Samarawickrema 1997; Wassman 1999; Bansal 2006; Nagpal 2012). Furthermore, continued morbidity and higher mortality seen among those who develop complicated severe disease, despite the availability of antiamoebic drugs such as metronidazole, not only imply delayed diagnosis and inappropriate treatment but also suggest that current therapeutic options may be insufficient (Haque 2003; Ralston 2011; Hayat 2016).
Why it is important to do this review
In addition to being a potentially fatal disease, invasive amoebiasis has important social and economic consequences. Amoebic colitis is a temporarily incapacitating disease that may require hospitalization for some individuals presenting with severe diarrhoea or dysentery. Amoebic colitis affecting adults in the wage‐earning group may require several weeks of hospitalization and up to two to three months for full recovery (WHO 1985; Walsh 1986). Pregnant and postpartum women appear to have increased risk of severe disease and death (Stanley 2003; Petri 2010). Persistent infection can impair physical and mental growth and can affect the nutrition and general development of children. Children with E histolytica‐associated diarrhoea during the first two years of life were three times more likely to be malnourished and were five times more prone to be stunted (Mondal 2006; Verkere 2012). Other studies have demonstrated that malnutrition and amoebic dysentery were associated with cognitive deficiencies, particularly in preschool children (Tarleton 2006; Petri 2009).
Adequate therapy for amoebic colitis is necessary to reduce severity of illness, prevent development of complicated disease and extraintestinal spread, and decrease infectiousness and transmission to others. In LMICs where amoebiasis is common and most patients are treated in private practice or as hospital outpatients, the aim of treatment should be to provide an effective, safe, and simple regimen that can be given on an outpatient basis.
A reliable summary of the evidence is needed to determine the best treatment for people with amoebic colitis. Rapid relief of diarrhoea and other gastrointestinal symptoms associated with intestinal amoebiasis is an important concern of the individual with the disease, and eradication of the parasite is important to prevent further invasion with damage to the intestinal mucosa and possible extraintestinal spread. Treatment failure and unpleasant adverse effects associated with metronidazole in some patients and the possibility of overt clinical resistance of E histolytica to metronidazole make it imperative that alternative treatments are investigated. The benefits of using combination regimens over monotherapy and single‐dose regimens over longer regimens remain to be determined. Furthermore, the effectiveness of newly discovered antiamoebic drugs must be ascertained.
Objectives
To evaluate antiamoebic drugs for treating amoebic colitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We excluded quasi‐RCTs.
Types of participants
We included trials with adults and children with clinical symptoms of amoebic colitis (as previously described) and demonstration of E histolytica cysts or trophozoites in a stool sample, or E histolytica trophozoites in a tissue biopsy or ulcer scraping by histopathology. We included individuals with positive E histolytica/E dispar on stool examination confirmed by E histolytica antigen detection testing or PCR.
We excluded trials including only individuals with asymptomatic infection and those requiring surgery or additional antibiotic therapy, such as those with fulminant or necrotizing colitis; peritonitis, intestinal perforation, or haemorrhage; or with evidence of extraintestinal amoebiasis including hepatic amoebiasis.
Types of interventions
Interventions
Antiamoebic drugs, administered alone or in combination.
Controls
Placebo or another antiamoebic drug.
Types of outcome measures
Primary outcomes
Clinical failure, defined as absence of E histolytica in stools or scrapings but with little or no relief of signs or symptoms, or with persistent rectal ulcerations on sigmoidoscopy
Parasitological failure, defined as persistence of E histolytica cysts or trophozoites in stools or colonic ulcer scrapings, with or without the presence of symptoms or rectal ulcers
Relapse, defined as reappearance of cysts or trophozoites of E histolytica after their initial disappearance, with or without recurrence of clinical signs or symptoms of amoebic colitis after completion of treatment
Serious adverse events (death, life‐threatening events, hospitalization required or duration of hospitalization prolonged, development of a persistent or significant disability or incapacity, having offspring with a congenital anomaly or birth defect, or development of cancer)
Secondary outcomes
Adverse events resulting in discontinuation of treatment
Other adverse events including gastrointestinal adverse events, systemic symptoms such as weakness or fatigue, central nervous system effects such as headache or dizziness, and dermatological effects such as skin rashes
Search methods for identification of studies
We searched for all publications that described RCTs on antiamoebic drugs for treating amoebic colitis, regardless of language or publication status.
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1: the Cochrane Infectious Diseases Group Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library (2018, Issue 1); MEDLINE (1966 to March 2018); Embase (1974 to March 2018); and Latin American Caribbean Health Sciences Literature (LILACS) (1982 to March 2018). Using ‘amoebic,' ‘amoeba', and ‘amoebiasis' as search terms, we also searched the metaRegister of Controlled Trials (mRCT; latest search February 2018), the WHO International Clinical Trials Registry Platform (ICTRP search portal; latest search February 2018), and the United Kingdom Clinical Trials Gateway (UKCTG; last searched February 2018).
Searching other resources
Conference proceedings
We searched electronic databases of the conference proceedings listed in Appendix 2 for relevant abstracts.
Organizations and pharmaceutical companies
To help identify unpublished and ongoing trials, we contacted researchers working for the organizations listed in Appendix 3, as well as the pharmaceutical companies and associated databases listed in Appendix 4.
Reference lists
We checked the reference lists and bibliographies of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Two review authors (MLMG, LFD) independently assessed results of the literature search to determine whether the title or abstract of each trial described an RCT. We retrieved full reports for all trials considered by one or both review authors to be potentially relevant, as well as for those whose relevance was unclear. We used a standard eligibility form based on the inclusion criteria to assess trials independently. We contacted trial authors for clarification if necessary and resolved disagreements through discussion or by consultation with the third review author (JSA in this update).
We included RCTs assessing the effectiveness of antiamoebic drugs given alone or in combination for treatment of amoebic colitis, and for which outcomes were measured in both experimental and control populations. We excluded quasi‐randomized trials (e.g. those utilizing alternate allocation), animal studies, duplicate publications, reviews, abstracts with no full report, and studies describing only results without providing detailed background and methods.
Data extraction and management
For this update, two review authors (MLMG, JSA) independently extracted data from study reports using pre‐tested data extraction forms. We collected details regarding inclusion and exclusion criteria for participants, treatment interventions given, total numbers randomized, number of participants in each group for all outcomes, dropouts and withdrawals, and numbers experiencing each outcome. For dichotomous data, we extracted the number of participants who experienced the event of interest and the number of participants randomized and analysed in each treatment group. We resolved disagreements by referring to the trial report and holding discussions. When data were insufficient or missing, we made attempts to contact the trial authors. Review author MLM Gonzales entered data for analysis.
For each study, we collected the following data: study methods (study design, sequence generation. allocation sequence concealment, blinding), participants (total number, age, sex, type of amoebic colitis, diagnostic method used, presence of concomitant infection with other intestinal parasites, duration of follow‐up), interventions (total number of intervention groups and specific interventions including dosage, route, and duration), setting, and funding source. For each outcome, we recorded the number of participants allocated to each intervention group, the proportion of participants with the outcome, methods or tests used to measure the outcome, and timing of outcome measurement.
Assessment of risk of bias in included studies
Two review authors (MLMG, LFD) independently assessed risk of bias in each trial using a prepared form. We resolved disagreements through discussion between review authors and with the third review author (JSA) if needed.
We assessed risk of bias for each of the included trials and evaluated sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting, and ‘other sources of bias', using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For each item, we provided a description of what was reported to have happened in the study along with a subjective judgement regarding protection from bias (‘Yes’ for low risk of bias, ‘No’ for high risk of bias, ‘Unclear’ otherwise). For sequence generation and allocation concealment, we described for each included study the method used, and we made subjective judgements on the adequacy of the procedure to protect against possible bias. For blinding, we assessed who was blinded, such as trial participants, care providers, or outcome assessors, for both clinical and parasitological outcomes and for adverse events. We prepared separate reports for outcomes evaluated 1 to 14 days after end of treatment and those evaluated 15 to 60 days after end of treatment. We stated numbers included in the analysis compared with the total number of randomized participants, whether attrition and exclusions were reported, reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. For selective reporting bias, we described for each included trial whether it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported. For ‘other sources of bias', we described for each included study any important concerns identified that could be possible sources of bias, such as study design, method of diagnosing amoebic infection, and presence of concomitant parasitic or protozoal infection.
We recorded all assessments in risk of bias tables and produced an overall pictorial summary of the risk of bias assessment.
For trials that were at high risk of bias according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact the findings. We explored the impact of the level of bias by performing sensitivity analyses.
Measures of treatment effect
We used risk ratios (RRs) with 95% confidence intervals (CIs) to compare dichotomous data. When available, we recorded continuous data, such as time until resolution of clinical symptoms and disappearance of amoeba parasites in the stools, as mean value and standard deviation or as median with range of outcome measurements.
Unit of analysis issues
For trials with more than two intervention groups (e.g. two or more experimental interventions, different doses or preparations of the same drug), we combined multiple treatment arms as appropriate into one group and compared them collectively with the standard or control group to avoid counting placebo or control participants more than once in the same meta‐analysis.
Dealing with missing data
If we noted a discrepancy between the number randomized and the number analysed, we calculated the percentage lost to follow‐up for each treatment group and reported this information. We performed an available‐case analysis, wherein only available data were analysed and no assumptions were made regarding missing data.
Assessment of heterogeneity
We calculated summary RRs from meta‐analysis using both a fixed‐effect model (Mantel‐Haenszel method), which assumes trial homogeneity, and a random‐effects model (DerSimonian and Laird method), which accounts for trial heterogeneity.
We reported results using the random‐effects model when we noted differences between trials that may potentially influence the size of the treatment effect, or when we detected significant statistical heterogeneity. We determined the presence of statistical heterogeneity among the same interventions by inspecting forest plots for overlapping confidence intervals and by applying the Chi2 test for heterogeneity (P < 0.10 considered statistically significant) and the I2 statistic to quantify inconsistency across trials (I2 > 50% used to denote substantial heterogeneity). If we detected heterogeneity but still considered it clinically meaningful to combine trial data, we explored potential sources of heterogeneity by conducting subgroup analysis. We presented subtotals for each subgroup only if pooled results showed significant heterogeneity.
Assessment of reporting biases
When at least 10 trials were included in the meta‐analysis, we determined publication bias by looking for asymmetry in a funnel plot. The presence of asymmetry in the funnel plot suggests possible publication bias but may also indicate heterogeneity or poor methodological quality of trials.
Data synthesis
We analysed data collected using Review Manager 5 (RevMan 5) (RevMan 2014). For dichotomous outcomes, we calculated risk ratios (RRs) with 95% confidence intervals (CIs). We did not perform meta‐analysis of continuous data because of inconsistency of trial reporting, but we described and summarized outcomes in a table.
The main comparisons were between any single antiamoebic drug and metronidazole (current standard therapy), any antiamoebic drug and placebo, combination regimens and monotherapy, and any single‐dose regimen and longer regimens. We included but did not pool data from other trials that compared any antiamoebic drug with another antiamoebic drug, and we did not address any particular pharmacological or clinical questions relevant to this review.
For trials reporting results at multiple or varying time points, we performed separate analyses for outcomes measured from end of treatment to 14 days and 15 to 60 days after end of treatment. For trials comparing drugs with different treatment durations, we measured the time point in relation to the last day of the longest treatment period. We did not consider outcomes that were measured during treatment or before completion of treatment. Likewise, we did not include outcomes measured beyond two months because this could indicate re‐infection rather than true failure or relapse.
Certainty of the evidence
We assessed the certainty of the evidence for important outcomes using the GRADE approach (GRADE 2004), and we presented this information in ‘Summary of fIndings' tables.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis for the time of outcome measurements (from end of treatment to 14 days and 15 to 60 days after end of treatment) and for subgroups that may influence treatment response, such as clinical categories (amoebic dysentery, non‐dysenteric amoebic colitis, or unspecified amoebic colitis) and participant age (adults 15 years of age or older, and children younger than 15 years). We could not undertake subgroup analysis based on diagnostic tests as planned because only one trial used a stool E histolytica ELISA test.
Potential sources of heterogeneity explored for the primary outcome measures involved the methodological quality of studies. Other sources of heterogeneity included in the post hoc subgroup analysis were type of intestinal infection (E histolytica infection alone or mixed intestinal infection) and criteria for determining outcomes (based on WHO 1969 criteria or other criteria).
Sensitivity analysis
We performed sensitivity analysis to assess the robustness of overall estimates by calculating the results using all trials and then excluding trials of lower methodological quality (i.e. trials with inadequate generation of allocation sequence, allocation concealment, or blinding, or trials that analysed < 90% of randomized participants), and by excluding trials that were sponsored by pharmaceutical companies. Although pharmaceutical industry‐sponsored trials may publish only when demonstrating positive treatment effects, it is possible that pharmaceutical industry‐sponsored trials were conducted with better methodological quality because they received adequate funds. We determined the effect of the date of publication on the overall pooled effect in a sensitivity analysis when we noted large differences in the publication dates. It is unclear whether two trials reported the same results, and our attempts to contact trial authors for clarification were not successful (Misra 1977; Misra 1978). We entered these two trials as separate trials and carried out sensitivity analysis to determine whether exclusion of the latter trial would have an effect on the overall estimate.
Results
Description of studies
We have presented a summary of included studies in Table 4, and we have listed further study details in the ‘Characteristics of included studies' table.
2. Summary of included studies.
| Study ID | Year completed | Setting | Participants | Intervention | Control | Outcome measures | Test used to measure parasitological outcome |
| Various antiamoebic drugs versus placebo | |||||||
| Donckaster 1964 | 1964 | Outpatient clinic of the University of Chile in Santiago, Chile | 346 adults and children with clinical symptoms of intestinal amoebiasis and stool specimens positive for cysts and/or trophozoites of E histolytica |
|
Placebo (starch): once daily after meals for 10 days at the following oral daily doses – children 250 mg for every 2 years of age and adults 1500 mg |
|
Stool microscopy using modified Telemann concentration technique (centrifugation with saline formol and ether) for cysts; polyvinyl alcohol with fixative of Schaudinn for the trophozoites |
| Huggins 1982 | 1982 | Clinical Hospital of the Federal University of Pernambuco, Brazil | 96 adults with chronic intestinal amoebiasis and stool specimens positive for E histolytica |
|
Placebo: 300 mg daily dose orally, no information given on the frequency of administration |
|
Stool microscopy using Lugol's stain (Telemann‐Richter or Hoffman, Pons, and Janer methods) |
| Rossignol 2001 | 2001 | Outpatient clinic of the Department of Hepatology, Gastroenterology, and Infectious Diseases of the Benha University Hospital, Governorate of Kalubia, Nile Delta, Egypt | 67 adults and children with diarrhoea and stool specimens positive for cysts or trophozoites of E histolytica and/or E dispar alone or with concomitant G intestinalis | Nitazoxanide: 500 mg twice daily orally for 3 days | Placebo tablet (identical): twice daily orally for 3 days |
|
Stool microscopy using direct saline smear, concentration technique, Ziehl‐Neelsen stain, and immunofluorescent assay (MeriFluor Meridian Diagnostics) |
| Rossignol 2007 | 2005 | Outpatient clinic of the Benha University Hospital, Benha, Egypt | 100 adults and children with diarrhoea; ≥ 1 enteric symptoms; E histolytica/E dispar trophozoites identified in stool and stool‐positive for E histolytica by antigen‐based ELISA | Nitazoxanide: for 3 days; adults aged ≥ 12 years, 500‐mg tablet twice daily; children 100 mg/5 mL suspension – 1 to 3 years received 5 mL twice daily, 4 to 11 years received 10 mL twice daily | Placebo: matching placebo tablet or suspension twice daily for 3 days |
Not included in this review: time from first dose to passage of last unformed stool (survival graph) |
Stool microscopy using direct saline smear and concentration technique; E histolytica by antigen‐based ELISA |
| Tinidazole versus metronidazole | |||||||
| Awal 1979 | 1979 | Hospital in Bangladesh | 66 adults and children with clinical signs and symptoms of intestinal amoebiasis and motile haematophagous trophozoites of E histolytica in fresh stool specimens and on sigmoidoscopy |
|
Metronidazole: 2 g single dose for 2 days |
|
Stool microscopy using direct saline smear |
| Chunge 1989 | 1989 | Outpatient departments of 3 district hospitals in Kiambo, Kilifi, and Machakos in Kenya | 225 adults and children presenting with at least any 4 of the following symptoms of intestinal amoebiasis: abdominal pain, diarrhoea, constipation, mucoid stools, malaise, flatulence, nausea, fever, tenesmus, and stool specimens positive for trophozoites or cysts of E histolytica |
|
|
|
Stool microscopy using direct smear or formol‐ether concentration technique |
| Joshi 1975 | 1975 | Ahmedabad, India (location not stated) | 60 adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica | Tinidazole: 600 mg twice daily orally for 5 days Treatment period was extended to 10 days in both groups when 5 days' treatment was inadequate to relieve symptoms or clear the stools of E histolytica |
Metronidazole: 400 or 800 mg thrice daily orally for 5 days |
|
Stool microscopy using direct saline smear |
| Mathur 1976 | 1976 | India (location not stated) | 60 adults and adolescents with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica | Tinidazole: 600 mg twice daily orally for 5 days Treatment period was extended to 10 days in both groups when 5 days' treatment was inadequate to relieve symptoms or clear the stools of E histolytica |
Metronidazole: 400 mg thrice daily orally for 5 days (for acute amoebic dysentery) or 800 mg thrice daily for 5 days (for other cases) |
|
Stool microscopy using direct saline smear |
| Misra 1974 | 1974 | Medical College Hospital in Bhopal, India | 60 adults and children with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E. histolytica | Tinidazole: 600 mg twice daily orally for 5 days Treatment period was extended to 10 days in both groups when 5 days' treatment was inadequate to relieve symptoms or clear the stools of E histolytica |
Metronidazole: 400 mg thrice daily orally for 5 days (for acute amoebic dysentery) or 800 mg thrice daily orally for 5 days (for chronic intestinal amoebiases if symptoms were of more than 15 days' duration) |
|
Stool microscopy using direct saline smear or concentration method |
| Misra 1977 | 1977 | Hospital in Bhopal, India | 60 adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica | Tinidazole: 2 g single oral dose daily for 3 days | Metronidazole: 2 g single oral dose daily for 3 days |
|
Stool microscopy using direct saline smear or formol‐ether concentration technique |
| Misra 1978 | 1978 | Hospital in Bhopal, India | 60 adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica | Tinidazole: 2 g single oral dose daily for 3 days | Metronidazole: 2 g single oral dose daily for 3 days |
|
Stool microscopy using direct smear or formol‐ether concentration technique, sigmoidoscopy for colonic pathology |
| Pehrson 1984 | 1984 | Outpatient clinic in Stockholm, Sweden | 30 adults with clinical symptoms of intestinal amoebiasis but no signs of invasion (e.g. no fever or acute dysentery) and stool specimens positive for trophozoites or cysts of E histolytica | Tinidazole: 600 mg twice daily orally for 5 days | Metronidazole: 800 mg thrice daily orally for 5 days |
|
Stool microscopy using direct saline smear or formol‐ether concentration technique |
| Singh 1977 | 1977 | Medical outpatient department of the Government Medical College and Hospital, Patiala, India | 60 adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica | Tinidazole: 500 mg tablets × 4 (2 g) single dose daily for 3 days | Metronidazole: 400‐mg tablets × 5 (2 g) single dose daily for 3 days |
|
Stool microscopy using direct saline smear or formol‐ether concentration technique |
| Swami 1977 | 1977 | Visakhapatnam, India (location not stated) | 60 adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica | Tinidazole: 2 g single dose daily for 3 days Treatment was extended if E histolytica persisted in the stool on the day following the last treatment period |
Metronidazole: 2 g single dose daily for 3 days |
Not included in this review: number of participants who required extension of treatment beyond 3 days |
Stool microscopy using direct saline smear |
| Ornidazole versus metronidazole | |||||||
| Botero 1974 | 1974 | Hospital in Medellin, Colombia | 120 adult males with clinical symptoms of intestinal amoebiasis confirmed by the presence of E histolytica in the stools | Ro 7‐0207 (ornidazole): 2 × 250‐mg capsules twice daily for 10 days | Metronidazole: 2 × 250‐mg capsules twice daily for 10 days |
|
Stool microscopy using direct saline smear and Ritchie formalin‐ether concentration methods |
| Naoemar 1973 | 1973 | Outpatient clinics in Jakarta, Indonesia | 20 adults and children with bloody diarrhoea and stools positive for motile haematophagous trophozoites of E histolytica | Ro 7‐0207 (ornidazole) given as follows: 2 to 6 years of age – 125 mg daily in 3 divided doses for 7 days; 7 to 12 years of age – 250 mg daily in 3 divided doses for 7 days; adults – 1500 mg daily in 3 divided doses for 5 days | Metronidazole given as follows: 2 to 6 years of age – 125 mg daily in 3 divided doses for 7 days; 7 to 12 years of age – 250 mg daily in 3 divided doses for 7 days; adults – 1500 mg daily in 3 divided doses for 5 days |
|
Stool microscopy using direct saline smear and stained smears using eosin and iodine |
| Pudjiadi 1973 | 1973 | Hospital Department of Child Health, Medical School University of Indonesia, Jakarta, Indonesia | 20 children with bloody diarrhoea and stools positive for E histolytica | Ro 7‐0207 (ornidazole): 125‐mg capsule given as follows: up to 2 years of age – 62.5 mg, 2 to 6 years of age – 125 mg, and 6 to 12 years of age 250 mg daily, divided into 3 daily doses for 7 days | Metronidazole: 125‐mg capsule given as follows: up to 2 years of age – 62.5 mg, 2 to 6 years of age – 125 mg, and 6 to 12 years of age 250 mg daily, divided into 3 daily doses for 7 days |
|
Stool microscopy using direct saline smear and eosin and Lugol's solution |
| Secnidazole versus metronidazole | |||||||
| Karabay 1999 | 1999 | Military hospital in Erzurum, Turkey | 44 adults with acute amoebic dysentery and stool specimens positive for E histolytica cysts and/or trophozoites | Secnidazole: 2 g single oral dose | Metronidazole: 750 mg thrice daily orally for 10 days |
|
Stool microscopy using 0.85% saline water, Lugol's solution, and trichrome stain |
| Panidazole versus metronidazole | |||||||
| Botero 1977 | 1977 | Colombia (location not stated) | 100 adult males with clinical symptoms of intestinal amoebiasis and stools positive for E histolytica | Panidazole: 2 × 250‐mg tablets (500 mg), 4 times daily for 6 days | Metronidazole: 2 × 250‐mg tablets (500 mg), 4 times daily for 6 days |
Not included in this review: number of stools passed in 24 hours on day 3 and day 6 of treatment, and on days 7 and 21 after treatment; clearance of E histolytica in 14 asymptomatic carriers |
Stool microscopy using direct saline smear and Ritchie formalin‐ether concentration methods |
| Satranidazole versus metronidazole | |||||||
| Tripathi 1986 | 1986 | Hospital in Bhopal, India | 40 adults with symptoms of intestinal amoebiasis and stool specimens positive for E histolytica | GO 10213 (satranidazole): 150 mg thrice daily for 10 days | Metronidazole: 400 mg thrice daily for 10 days |
Not included in this review: frequency of loose stools/d from start of treatment |
Stool microscopy using formol‐ether concentration methods, sigmoidoscopy, colonic ulcer scrapings, and positive stool culture on NIH media |
| Praziquantel versus metronidazole | |||||||
| Mohammed 1998 | 1995 | Outpatients in Iraq | 69 adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for vegetative trophozoite forms (acute amoebic dysentery) or cysts of E histolytica | Praziquantel: 40 mg/kg body weight divided into 2 doses orally and taken 4 to 6 hours apart | Metronidazole: 800 mg thrice daily orally for 5 days |
|
Stool microscopy using direct saline smear |
| Combination versus metronidazole | |||||||
| Rubidge 1970 | 1970 | Hospital in Durban, South Africa | 39 children with amoebic dysentery presenting with acute onset of diarrhoea with blood, mucus, and actively motile haematophagous trophozoites of E histolytica in stool specimens | Dehydroemetine, tetracycline, and diloxanide furoate: dehydroemetine (2 mg/kg body weight daily by subcutaneous injection for 10 days), tetracycline (50 mg/kg body weight daily orally for 7 days), and diloxanide furoate (25 mg/kg body weight daily orally for 10 days) | Metronidazole: 50 mg per kg body weight orally for 7 days |
|
Stool microscopy using direct saline smear and zinc sulphate flotation technique |
| Asrani 1995 | 1995 | Various cities in India (not specified) | 961 male and non‐pregnant female patients > 12 years of age with clinical symptoms of intestinal amoebiasis and/or presence of trophozoites or cysts ofE histolytica in stool specimens | Metronidazole and diiodohydroxyquinoline: fixed‐drug combination of metronidazole (200 mg) plus diiodohydroxyquinoline (325 mg) (Qugyl by Sil Pharma, Bombay, India) given as 2 tablets thrice daily for 5 days | Metronidazole: 400 mg thrice daily orally for 5 days Treatment period was extended to 10 days in both groups when 5 days' treatment was inadequate to clear the stools of E histolytica |
Not included in this review: average daily frequency of stools on admission and on day 5 and day 10 of treatment; overall clinical response (rated as "poor" if < 25% relief and not tolerated, "fair" if 25% to 49% relief and not well tolerated, "poor" if 50% to 74% relief and well tolerated, or "excellent" if 75% to 100% relief and well tolerated) |
Stool microscopy using direct smear |
| Prasad 1985 | 1985 | Paediatric outpatient department of S.N. Medical College, Agra, India | 180 children with clinical symptoms of intestinal amoebiasis or giardiasis (diarrhoea, abdominal pain, dysentery, gastrocolic urgency, etc.) and whose stools were positive for amoebae or Giardia | Metronidazole plus furazolidone: fixed‐drug combination suspension of (per 5 mL) metronidazole 75 mg plus furazolidone 25 mg, given as 5 mL thrice daily for those 1 to 5 years of age and as 10 mL thrice daily for those 6 to 15 years of age for 5 or 10 days depending on severity of disease | Metronidazole: 100 mg/5 mL suspension, given as 5 mL thrice daily for those 1 to 5 years of age and as 10 mL thrice daily for those 6 to 15 years of age for 5 or 10 days depending on severity of disease |
Not included in this review: clinical and parasitological response in those with mixed amoebiasis and giardiasis infection; 12/63 from the metronidazole group and 15/101 from the fixed‐drug combination metronidazole plus furazolidone had mixed amoebiasis and giardiasis and were not included in this review |
Stool microscopy using direct saline smear |
| Combination versus aminosidine or etophamide or nimorazole | |||||||
| Pamba 1990 | 1990 | 3 district hospitals of Kiambo, Machakos, and Kilifi in Kenya, Africa | 417 adults and children with clinical symptoms of intestinal amoebiasis with stool specimens positive for E histolytica |
|
|
Not included in this review: cumulative daily clearance of E histolytica from stools during treatment, at end of treatment, and on days 15, 30, and 60 after start of treatment; evolution of mild and severe amoebic ulcers seen on rectosigmoidoscopy; and anatomical cure (healing of previous ulceration) |
Stool microscopy using direct smear and a concentration method (not specified) |
| Quinfamide and mebendazole versus nitazoxanide | |||||||
| Davila 2002 | 2002 | 3 communities in Colima, Mexico | 275 children enrolled with various helminthic and protozoal intestinal infections; 105/275 (38%) had E histolytica or E dispar infection (25 single infection and 80 mixed infection with other intestinal parasites) and were included in the review | Quinfamide: 100 mg/5 mL single oral dose; mebendazole 100 mg/5 mL twice daily orally for 3 days was added to quinfamide when another parasite other than E histolytica/E dispar was observed | Nitazoxanide: 100 mg/5 mL twice daily orally for 3 days |
Data for parasitological cure were presented separately for nitazoxanide versus quinfamide for single infections and for nitazoxanide versus quinfamide plus mebendazole for mixed infections, and were included in a separate meta‐analysis |
Stool microscopy with direct smear or Kato‐Katz technique |
| Combination tetracycline and clioquinol versus secnidazole | |||||||
| Soedin 1985 | 1983 | Outpatient in the Padang Bulan Health Centre, Medan, Indonesia | 80 children with clinical symptoms of acute intestinal amoebiasis with stool specimens positive for trophozoites or haematophagous forms of E histolytica | Tetracycline and clioquinol: tetracycline (750 mg) and clioquinol (1 g for 5 days) | Secnidazole: 2 g orally in a single dose Co‐intervention: 2 patients in secnidazole group were given spasmolytics (unspecified) for stomach cramps |
|
Stool microscopy using direct saline smear |
| Combination tinidazole and diloxanide versus tinidazole | |||||||
| Pehrson 1983 | 1983 | Hospital in Stockholm, Sweden | 41 adults and children with clinical symptoms of intestinal amoebiasis but no signs of invasion (e.g. no fever or acute dysentery) and stool specimens positive for trophozoites or cysts of E histolytic | Tinidazole plus diloxanide furoate: tinidazole 40 mg/kg body weight in a single oral dose daily for 5 days plus diloxanide furoate 20 mg/kg body weight divided into 3 daily doses for 10 days | Tinidazole: 40 mg/kg body weight in a single oral dose daily for 5 days |
|
Stool microscopy using direct smear or formol‐ether concentration technique by Ridley and Hawgood |
| Secnidazole single dose versus tinidazole for 2 days | |||||||
| Salles 1999 | 1999 | 5 different centres in Brazil | 303 children with clinical symptoms of intestinal amoebiasis with stool specimens positive for E histolytica enrolled; 275/303 (90.7%) included in evaluation for clinical efficacy; 300/303 (99%) included in evaluation for parasitological efficacy | Secnidazole: 1 mL/kg body weight orally in a single dose | Tinidazole: 0.5 mL/kg body weight once daily orally for 2 days |
|
Stool microscopy using direct smear and the Faust and Katz method and no history of intolerance to imidazole drugs |
| Ornidazole versus tinidazole | |||||||
| Panggabean 1980 | 1978 | Outpatient clinic of the sub‐department of Gastroenterology, Department of Child Health Medical School, General Hospital, Medan, Indonesia | 40 children with amoebic dysentery presenting with bloody stools and motile haematophagous trophozoites of E histolytica in stools: 25/40 (62.5%) analysed 1 week after treatment, 17/40 (42.5%) analysed 2 weeks after treatment, 11/40 (27.5%) analysed 3 weeks after treatment, and 6/40 (15%) analysed 4 weeks after treatment | Ornidazole: 50 mg/kg body weight in a single oral dose daily for 3 days Other interventions: Children with concomitant intestinal helminthic infection were given single‐dose pyrantel pamoate 10 mg/kg; those with trichuriasis were given mebendazole 1 tablet twice daily for 3 consecutive days |
Tinidazole: 50 mg/kg body weight in a single oral dose daily for 3 days |
|
Stool microscopy using direct smear and eosin 2% stain |
| Sitepu 1982 | 1979 | Outpatient clinic of the Pediatric Gastroenterology Subdivision, Department of Child Health, School of Medicine, University of North Sumatra/Dr Pirngadi Hospital, Medan, Indonesia | 50 children with amoebic dysentery presenting with bloody diarrhoea and motile haematophagous trophozoites of E histolytica in stools: 41/50 (82%) analysed on the third day or 2 days after treatment, 18/50 (36%) were analysed 1 week after treatment Losses to follow‐up: 9/51 (18%) were lost to follow‐up by the third day or 2 days after treatment ‐ 7 participants in the tinidazole group and 2 in the ornidazole group; 32/50 (64%) were lost to follow‐up 1 week after treatment ‐ 18 in the tinidazole group and 14 in the ornidazole group |
Ornidazole: 50 mg/kg body weight in a single oral dose | Tinidazole: 50 mg/kg body weight in a single oral dose |
|
Stool microscopy using direct smear and eosin 1% stain |
| Secnidazole versus quinfamide | |||||||
| Padilla 2000 | 2000 | 2 urban federal elementary schools in Celaya, Guanajuato, Mexico (Urban Federal Elementary schools ‘Carmen Serdan' and ‘Juan Jesus de los Reyes') | 239 children with clinical symptoms of non‐dysenteric amoebic colitis with at least 1 of 3 stool specimens positive for E histolytica cysts | Secnidazole: 30 mg/kg body weight orally in a single dose | Quinfamide: 4.3 mg/kg body weight orally in a single dose |
Not included in this review: acceptability of the test |
Stool microscopy using direct smear and the Faust concentration method |
| Ornidazole versus secnidazole | |||||||
| Toppare 1994 | 1994 | Medical Center Hospital, Ankara, Turkey | 102 children with gastrointestinal symptoms and stool specimens positive for haematophagous trophozoites of E histolytica | Ornidazole 15 mg/kg body weight given twice daily orally for 10 days | Secnidazole: 30 mg/kg body weight given as a single oral dose daily for 3 days |
|
Stool microscopy using direct saline smear |
| Quinfamide versus teclozan | |||||||
| Guevara 1980 | 1980 | Patients were hospitalized for 1 day, then were followed up as outpatients | 40 adults with non‐dysenteric amoebiasis with trophozoites of E histolytica in recently emitted faecal material and/or in recto‐colonic mucosal exudate; recto‐colonic lesions suggestive of amoebiasis present or not; and not presenting clinical manifestations of acute amoebic recto‐colitis | Quinfamide given at 3 doses in 1 day: 100 mg for 3 doses (300 mg), 200 mg for 3 doses (600 mg), 400 mg for 3 doses (1200 mg) | Teclozan at 3 doses in 1 day: 500 mg for 3 doses (1500 mg) |
|
Stool microscopy using direct saline smear |
| Chlorhexidine versus diiodohydroxyquinoline | |||||||
| Kapadia 1968 | 1968 | Bombay, India (location not stated) | 100 patients with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites and/or cysts of E histolytica | Chlorhydroxquinoline: 500 mg thrice daily orally for 10 days | Di‐diiodohydroxyquinoline: 500 mg thrice daily orally for 10 days |
|
Stool microscopy using direct saline smear |
| MK‐910 low dose versus high dose | |||||||
| Batra 1972 | 1972 | Hospital in New Delhi, India | 40 patients (age unspecified) with acute amoebic dysentery and stool specimens positive for trophozoites of E histolytica | 1‐Methyl‐2‐(4'fluorophenyl)‐5‐nitroimidazole (MK‐910) at low doses: 0.5 mg/kg body weight or 1.0 mg/kg body weight, given in 3 divided doses orally for 10 days | 1‐Methyl‐2‐(4'fluorophenyl)‐5‐nitroimidazole (MK‐910) at high doses: 2.0 mg/kg body weight or 3.0 mg/kg body weight, given in 3 divided doses orally for 10 days |
Not included in this review: disappearance of colonic ulcers on sigmoidoscopic examination on day 5 and at end of treatment on day 10 |
Stool microscopy using direct saline and iodine smears |
| Fixed drug combination diloxanide‐tetracycline‐chloroquine versus fixed‐drug combination diloxanide‐tetracycline | |||||||
| Nnochiri 1967 | 1966 | Yaba Military Hospital in Lagos, Nigeria | 60 military personnel and their families given diagnosis of acute amoebic dysentery and stool specimens positive for E histolytica: 60 analysed at end of treatment, and 58 (96.8%) analysed 7 weeks after end of treatment | Diloxanide furoate, tetracycline hydrochloride, and chloroquine phosphate (per capsule): diloxanide furoate (187.5 mg), tetracycline hydrochloride (125 mg), and chloroquine phosphate (50 mg) given in 3 dosage regimens of 2 capsules 4 times a day for 5 days, 2 capsules 4 times a day for 7 days, or 2 capsules 4 times a day for 10 days | Diloxanide furoate and tetracycline hydrochloride (per capsule): diloxanide furoate (187.5 mg) and tetracycline hydrochloride (125 mg) given in 3 dosage regimens of 2 capsules 4 times a day for 5 days, 2 capsules 4 times a day for 7 days, or 2 capsules 4 times a day for 10 days |
Not included in this review: results of stool examination 3, 6, and 12 months after treatment; clearance of E histolytica from stools of 36 asymptomatic cyst carriers |
Stool microscopy using direct saline and iodine‐stained smears |
| Metronidazole and S boulardii versus metronidazole | |||||||
| Savas‐Erdeve 2009 | 2007 | Outpatient in Turkey | 90 children from 1 to 15 years of age who presented with E histolytica‐ associated diarrhoea defined as presence of compatible clinical presentations (acute diarrhoea, fever, and abdominal pain) and presence of E histolytica trophozoite engulfing red blood cells in diarrhoeal stool | Metronidazole: 30 to 50 mg/kg/d orally for 10 days (maximum: 500 to 750 mg) | Metronidazole plus S boulardii (Reflor, Sanofi‐Synthelabo, France): metronidazole 30 to 50 mg/kg/d orally (maximum: 500 to 750 mg) plus lyophilized S boulardii 250 mg (includes 5,000,000 living microorganisms) orally once a day for 10 days |
Not included in this review: survival analysis graph of the number of stools per day during the 10‐day treatment period |
Stool microscopy using direct saline and trichrome stain |
| Metro‐iodoquinol versus metro‐iodoquinol + Saccharomyces | |||||||
| Mansour‐Ghanaei 2003 | 1996 | Shahid Beheshti Educational and Therapeutic Center in Shiraz, Iran | 57 adults with amoebic dysentery presenting with mucous bloody diarrhoea, fever, and abdominal pain; stool specimens positive for haematophagous trophozoites of E histolytica in the laboratory | Metronidazole, iodoquinol, and placebo: metronidazole 750 mg and iodoquinol 650 mg given thrice daily orally with placebo tablets for 10 days | Metronidazole, iodoquinol, and S boulardii: 750 mg and iodoquinol 650 mg thrice daily given orally for 10 days plus lyophilized S boulardii 250 mg orally thrice daily given for 10 days |
|
Stool microscopy using direct faecal smear and flotation technique |
| Herbal versus fixed‐drug combination metronidazole‐diloxanide | |||||||
| Siddiqui 2015 | 2009 | Outpatient department of 2 centres in Pakistan (Shifa‐Ul‐Maluk Hospital, Gadap and Zahida Medical Centre, North Karachi) | 171 patients between the ages of 5 and 60 years with symptoms of amoebiasis (abdominal pain, blood in stool, or diarrhoea) and positive for E histolytica cyst or trophozoite: 153 analysed; 18/171 were not included in the analysis | Herbal product (Endemali, Pakistan) available in 4‐g sachet containing Boswellia glabra 270.9 mg, Kaolinum ponderosum 255 mg, Ocimum pilosum 580 mg, Pistacia terebinthus 116.1 mg, Plantago ispagula 812.7 mg, and Vateria indica 232.2 mg sweetening agent q.s. Endemali was given 4 times a day for 10 days | Combination of metronidazole 400 mg + diloxanide furoate 500 mg (Entamizole DS, Pakistan) in tablet form given 3 times a day for 5 days |
|
Stool microscopy using direct smear, Lugol's iodine smear, zinc sulphate flotation preparation, or formalin‐ether sedimentation method |
| Herbal product versus metronidazole | |||||||
| Shah 2016 | 2012 | Hospital, multi‐centre (Shifa‐ul‐mulk Memorial Hospital, Hamdard University Karachi, Hakeem, Pakistan) | 184 adult patients suffering from amoebiasis infection | Herbal drug Amoebex 400‐mg tablet 2 tablets after meal thrice daily, duration not reported | Metronidazole 400 mg 2 tablets thrice daily for 5 days |
Not included in this review: improvement in intensity of symptoms |
Stool microscopy using direct saline smear |
E dispar: Entamoeba dispar; E histolytica:Entamoeba histolytica; ELISA: enzyme‐linked immunosorbent assay; G intestinalis: Giardia intestinalis; SGOT: aspartate aminotransferase; SGPT: alanine aminotransferase.
Results of the search
Thirty‐seven trials met the inclusion criteria of the first published version of this review (Gonzales 2009). We retrieved one trial previously classified under ‘Studies awaiting classification' following the initial search (Guevara 1980), and we assessed 14 additional studies identified in updated searches conducted from the time of publication of the review in 2009 until 22 March 2018. Of these, we retrieved the full‐text articles of six studies, of which we excluded three for the following reasons: one was quasi‐randomized with alternate treatment assignment (Dinleyici 2009), and two included an ineligible population: one enrolled patients with bacillary dysentery with no mention of amoebic colitis (Sharif 2017); one with asymptomatic schoolchildren (Speich 2013)). See Figure 1 and the ‘Characteristics of excluded studies' table for studies detected by the search specifications but excluded from this review.
1.
Study flow diagram.
We included four new RCTs in this review update. Guevara 1980 was previously classified as awaiting classification and compared quinfamide with teclozan for treatment of adults with non‐dysenteric amoebiasis. One trial compared a probiotic, Saccharomyces boulardii, in addition to metronidazole versus metronidazole alone (Savas‐Erdeve 2009). Two trials compared various herbal products versus a combination of metronidazole and diloxanide furoate ‐ as in Siddiqui 2015 ‐ or metronidazole alone ‐ as in Shah 2016. We identified two ongoing RCTs: one trial will determine the efficacy of auranofin, a gold‐containing chemical salt oral drug, for treating adults with amoebiasis or giardiasis (NIAID 2016), and the other is a non‐randomized trial that will determine the safety and efficacy of paromomycin for treating individuals with intestinal amoebiasis (Pfizer 2016). See Characteristics of ongoing studies.
Thus, we included 41 trials in total in this review update. All trial reports were published in English, except Huggins 1982 (Portuguese), Karabay 1999 (Turkish), and Donckaster 1964 and Guevara 1980 (Spanish). Trials included in this review were published between 1964 and 2016; 27 were conducted between 1964 and 1989, three between 1990 and 1997, and eleven between 1998 and 2016 (see the ‘Characteristics of included studies' table and Table 4).
Included studies
Locations
A total of 39 trials were conducted in 16 different countries (see details in Appendix 5), 15 of which are considered to be highly endemic for amoebiasis: India (12), Indonesia (5), Mexico (3), Turkey (3), Colombia (2), Brazil (2), Pakistan (2), Kenya (2), Egypt (2), Bangladesh (1), Nigeria (1), South Africa (1), Chile (1), Iran (1), and Iraq (1). The remaining two trials were conducted in Sweden.
Trials were conducted in a variety of settings (see details in Appendix 6): hospital (14), outpatient clinic (15), community (1), and school (1). Eight trials did not state the study setting. One trial treated most participants as outpatients but treated a few with severe symptoms in the hospital (Toppare 1994). In another trial, patients were initially hospitalized for one day, then were followed up as outpatients (Guevara 1980).
Source of funding
Twenty‐one trials did not state the source of funding. Seventeen trials reported that a pharmaceutical company provided funding (Nnochiri 1967; Batra 1972; Naoemar 1973; Pudjiadi 1973; Panggabean 1980; Sitepu 1982; Tripathi 1986; Chunge 1989; Pamba 1990; Rossignol 2001; Rossignol 2007), or supplied study drugs (Kapadia 1968; Rubidge 1970; Misra 1974; Joshi 1975; Singh 1977; Davila 2002). Two trials reported that at least one trial author was connected with the pharmaceutical company manufacturing the study drug (Asrani 1995; Salles 1999), although study authors did not describe the level of involvement of the company. One trial was funded by the university at which study authors were affiliated (Siddiqui 2015).
Participants
A total of 4999 participants were enrolled in the trials; 17 trials included 1200 adults, 11 trials included 1185 children, 11 trials included 2474 children and adults, and two trials did not mention the age of participants. Included trials used different inclusion criteria for study participants.
Acute amoebic dysentery in 12 trials (Nnochiri 1967; Rubidge 1970; Batra 1972; Naoemar 1973; Pudjiadi 1973; Panggabean 1980; Sitepu 1982; Soedin 1985; Mohammed 1998; Karabay 1999; Mansour‐Ghanaei 2003; Savas‐Erdeve 2009).
Chronic or vague abdominal symptoms compatible with non‐dysenteric amoebic colitis, without bloody diarrhoea or other signs of intestinal invasion, in five trials (Guevara 1980; Huggins 1982; Pehrson 1983; Pehrson 1984; Padilla 2000).
-
Acute amoebic dysentery and non‐dysenteric amoebic colitis among enrolled participants and analysed separately in five trials.
Three trials stratified participants during the analysis of outcomes into those with acute amoebic dysentery and those with non‐dysenteric amoebic colitis (Botero 1974; Botero 1977; Swami 1977).
Two trials classified participants as having invasive trophozoite forms and non‐invasive cyst forms based on stool microscopy findings and analysed the two groups separately (Kapadia 1968; Pamba 1990).
-
Clinical symptoms of intestinal amoebiasis, with no differentiation between amoebic dysentery and non‐dysenteric amoebic colitis in 19 trials.
Two trials categorized participants as having acute amoebic dysentery, subacute amoebiasis, or chronic amoebiasis based on severity of symptoms and whether trophozoites or cysts of E histolytica were present but analysed participants as one group (Joshi 1975; Mathur 1976).
Two trials classified participants as having acute or chronic amoebiasis based on duration of symptoms but analysed study participants as one group (Misra 1974; Tripathi 1986).
Fifteen trials recruited and analysed participants with symptoms of intestinal amoebiasis or amoebic colitis, regardless of whether or not they presented with dysentery.
Participant age ranged from seven months to 80 years; see Appendix 7 for details. Seventeen trials enrolled only adults, and 11 trials recruited only children. The remaining 11 trials recruited both adults and children. Two trials did not state participant age (Kapadia 1968; Batra 1972).
Methods used to diagnose amoebic colitis
Trials used stool microscopy with direct wet saline smear as the predominant method for determining the presence of E histolytica cysts or trophozoites in stools (details in Appendix 8). In addition to direct smears, researchers used other methods ‐ various staining methods (10 trials), concentration methods such as formalin or formol‐ether (12 trials), zinc sulphate centrifugal flotation technique (four trials), or an unspecified concentration method (four trials) ‐ for better detection of cysts; one trial used polyvinyl alcohol fixative for detection of trophozoites. Two trials used National Institute of Health (NIH) media to culture stools for E histolytica, in addition to stool microscopy to evaluate parasitological response (Batra 1972; Tripathi 1986), but one trial did not use this as an inclusion criterion (Batra 1972). In addition to stool examination, 11 trials performed rectosigmoidoscopy whenever possible to determine the appearance of the bowel mucosa and the presence of ulcers but did not use this as the sole criterion for enrolling participants or evaluating outcomes. Only one trial used stool antigen‐based ELISA testing (Rossignol 2007). One trial used antibody detection testing in addition to stool microscopy to confirm amoebiasis infection (Shah 2016).
Concomitant infection with other intestinal parasites
Aside from E histolytica, 10 trials identified concomitant infection with other intestinal parasites: giardiasis (Singh 1977; Prasad 1985; Tripathi 1986; Rossignol 2001); intestinal helminth infection (Pudjiadi 1973; Panggabean 1980; Sitepu 1982); and other intestinal protozoal and helminth infections (Pehrson 1983; Salles 1999; Davila 2002). Six trials explicitly stated that stool bacterial culture was done before enrolment; five trials included only those found to be negative for pathogenic bacteria (Toppare 1994; Karabay 1999; Rossignol 2001; Rossignol 2007; Savas‐Erdeve 2009), and one trial analysed those found to be positive for Shigella separately from those positive for E histolytica (Nnochiri 1967). The remaining trials did not examine or mention concomitant infection with other intestinal pathogens or bacteria. Because clinical symptoms may not have been exclusively caused by amoebiasis in those with concomitant intestinal parasites, and given that the effect of concomitant infection on eradication of E histolytica by antiamoebic drugs is not known, we used data for E histolytica infection alone in assessing outcomes, except for trials that did not separate the data for those with single infection from those with mixed infection. Three trials performed separate analyses for clinical outcomes among those with E histolytica alone and those with concomitant infection with Giardia and E histolytica (Prasad 1985; Rossignol 2001; Davila 2002).
Drug comparisons
Included trials reported a variety of comparisons that involved over 30 individual drugs and combinations. As shown in Appendix 9, we grouped trials into the following categories (some trials are included in more than one category).
Single‐agent alternative versus metronidazole (17 trials): 10 trials on tinidazole versus metronidazole; three on ornidazole versus metronidazole; and one each on secnidazole versus metronidazole, panidazole versus metronidazole, satranidazole versus metronidazole, and praziquantel versus metronidazole.
Any antiamoebic drug versus placebo (four trials): two trials on nitazoxanide versus placebo; one on quinfamide versus placebo; and one on 10 different drugs belonging to six drug classes versus placebo.
Combination regimen versus monotherapy (seven trials): three trials on various combinations (dehydroemetine plus oral tetracycline and diloxanide furoate, metronidazole and diiodohydroxyquinolone, metronidazole and furazolidone) versus metronidazole alone; one on nimorazole and aminosidine or nimorazole and etofamide or etofamide and aminosidine versus nimorazole or aminosidine or etofamide monotherapy; and one each on tetracycline and clioquinol versus secnidazole, quinfamide and mebendazole versus nitazoxanide, and tinidazole and diloxanide furoate versus tinidazole.
Single‐dose regimens versus longer regimens (five trials): one trial each on quinfamide (one dose) versus quinfamide (two or three doses); secnidazole (one dose) versus tetracycline and clioquinol (five days); secnidazole (one dose) versus tinidazole (two days); quinfamide (one dose) versus nitazoxanide (three days); and secnidazole (one dose) versus metronidazole (10 days).
Other amoebic drug comparisons (13 trials): two trials on ornidazole versus tinidazole; 11 trials using different drug comparisons, with one trial reporting on each of the following: ornidazole versus secnidazole, chlorhydroxyquinoline versus diiodohydroxyquinoline, MK‐910 low dose (0.5 mg/kg and 1 mg/kg) versus MK‐910 high dose (2 mg/kg and 3 mg/kg), quinfamide versus secnidazole, quinfamide versus teclozan, quinfamide versus nitazoxanide, metronidazole and iodoquinol with Saccharomyces boulardii versus metronidazole and iodoquinol, metronidazole and iodoquinol with Saccharomyces boulardii versus metronidazole alone, herbal drug versus metronidazole, fixed‐drug combination of metronidazole and diloxanide furoate versus herbal product, and fixed‐drug combination of diloxanide furoate and tetracycline with chloroquine versus fixed‐drug combination of diloxanide furoate and tetracycline without chloroquine.
Six trials compared more than two interventions. Four trials compared different doses of the same drug using standard or control groups: three dosages of quinfamide with teclozan (Guevara 1980); three dosages of quinfamide with placebo (Huggins 1982); two treatment durations of tinidazole with metronidazole (Awal 1979); and four dosages of MK‐910 (Batra 1972). Donckaster 1964 compared 10 different treatment groups with placebo, and Pamba 1990 compared three drugs used alone or in three different combinations. One trial compared two brands of tinidazole and two brands of metronidazole (Chunge 1989). For trials with more than two intervention groups, we combined multiple treatment arms as appropriate into one group and compared them collectively with the standard or control group. This is the recommended approach to avoid a unit of analysis error by not counting placebo or control participants more than once in the same meta‐analysis (Higgins 2008). For the trial comparing two brands of tinidazole and two brands of metronidazole (Chunge 1989), the two brands of tinidazole were combined as one group and were compared with the two brands of metronidazole used in the other group.
Duration of follow‐up
The follow‐up period varied considerably between trials. Seven trials followed participants only until the end of the treatment period (Kapadia 1968; Batra 1972; Pudjiadi 1973; Prasad 1985; Chunge 1989; Asrani 1995; Shah 2016). Duration of follow‐up was less than 15 days and ranged from 7 to 14 days in 10 trials (Huggins 1982; Sitepu 1982; Toppare 1994; Mohammed 1998; Padilla 2000; Rossignol 2001; Davila 2002; Rossignol 2007; Savas‐Erdeve 2009; Siddiqui 2015). Seventeen trials had a duration of follow‐up of about four weeks, and two of about three weeks. Five trials had a follow‐up period longer than four weeks and ranging from 40 days to 12 months after treatment (Donckaster 1964; Nnochiri 1967; Rubidge 1970; Panggabean 1980; Pamba 1990).
Outcome measures
The primary outcomes in this review were clinical failure, parasitological failure, and relapse. Thirty‐three trials evaluated both clinical and parasitological outcomes, and six evaluated parasitological outcomes only (Donckaster 1964; Nnochiri 1967; Pehrson 1983; Pehrson 1984; Padilla 2000; Davila 2002). One trial based the final evaluation on parasitological outcomes (Guevara 1980), and it is unclear whether clinical outcomes were evaluated after treatment. The definition of clinical and parasitological cure or failure varied between trials. Nine trials ‐ Misra 1974; Joshi 1975; Mathur 1976; Misra 1977; Singh 1977; Swami 1977; Misra 1978; Awal 1979; Tripathi 1986 ‐ used the definitions set by the WHO Expert Committee on Amoebiasis (WHO 1969), which defined ‘cure' as symptom‐free, ulcers healed, stools negative for E histolytica; ‘probable failure' as persistent symptoms and rectal ulcerations despite disappearance of E histolytica from stools or ulcer scrapings; and ‘failure' as positive E histolytica with or without symptoms and rectal ulcers. For this review, review authors interpreted ‘probable failure' as clinical failure, and ‘failure' as parasitological failure, based on the definitions given. Most trials presented data for clinical and parasitological outcomes as dichotomous data.
Nine trials presented the duration of time from start of treatment until resolution of diarrhoea and other clinical symptoms but measured this in a variety of ways: range in hours (Batra 1972), number of days (Naoemar 1973; Pudjiadi 1973; Karabay 1999), mean duration in days and standard deviation (Mansour‐Ghanaei 2003), median and range in days (Toppare 1994; Savas‐Erdeve 2009), median time in days (Rossignol 2001), and survival analysis of time from first dose to passage of last unformed stools (Rossignol 2007). Two trials reported the duration of time from start of treatment to disappearance of E histolytica from stools (Naoemar 1973; Pudjiadi 1973). Four trials reported on the number of stools passed at different periods: during treatment (Savas‐Erdeve 2009); after treatment (Pudjiadi 1973); and during treatment and on follow‐up after treatment (Botero 1977; Tripathi 1986), while another reported average daily frequency of stools on admission and at the end of days 5 and 10 of treatment (Asrani 1995). One trial assessed clinical and parasitological outcomes jointly as ‘cure' (Prasad 1985); only dichotomous outcomes were included in the analysis because of inconsistency in reporting continuous data (see Table 5).
3. Time‐to‐event in trials using various antiamoebic drugs.
| Outcome | Trial | Intervention | Control | Comments |
| Time to resolution of diarrhoea | Batra 1972 |
MK‐910 low dose (≤ 1 mg/kg/d ) Range (h) = 24 to 72, n = 20 |
MK‐910 high dose (≥ 2 mg/kg/d) Range (h) = 24 to 48, n = 20 |
Mean (SD) and median not reported |
| Karabay 1999 |
Secnidazole Mean (d) = 1; n = 23 |
Metronidazole Mean (d) = 2, n = 21 |
SD not reported P > 0.05 |
|
| Mansour‐Ghanaei 2003 |
Metronidazole, iodoquinol and S boulardii Mean (h) = 12 ± 3.7 (SD), n = 28 |
Metronidazole, iodoquinol and placebo Mean (h) = 48 ± 18.5 (SD), n = 29 |
P < 0.0001 | |
| Rossignol 2001 |
Nitazoxanide Median (d) = 3, n = 36 |
Placebo No median presented because 60% still had diarrhoea at end of follow‐up period, n = 31 |
Mean (SD) and range not reported | |
| Rossignol 2007 |
Nitazoxanide Mean or median and range not presented, n = 50 |
Placebo Mean or median and range not presented, n = 50 |
Results presented as survival analysis graph of time from first dose to passage of last unformed stools | |
| Savas‐Erdeve 2009 |
Metronidazole and S boulardii Median (range, days) = 4.5 (1 to 10), n = 40 |
Metronidazole Median (range, days) = 5 (1 to 10), n = 45 |
Mean (SD) not reported | |
| Toppare 1994 |
Ornidazole Mean (d) = 2 to 3, range (d) = 1 to 5, n = 42 |
Secnidazole Mean (d) = 5, range (d) = 1 to 29, n = 60 |
SD of mean and median not reported | |
| Time to resolution of bloody stools | Batra 1972 |
MK‐910 low dose, ≤ 1 mg/kg/d Range = 48 to 72 hours, n = 20 |
MK‐910 high dose, ≥ 2 mg/kg/d Range = 48 to 72, n = 20 |
Mean (SD) and median not reported |
| Karabay 1999 |
Secnidazole Mean (d) = 1, n = 23 |
Metronidazole Mean (d) = 1, n = 21 |
SD not reported P > 0.05 |
|
| Naoemar 1973 |
Ornidazole Range (h) = 48 to 72, n = 10 |
Metronidazole Range (h) = 48 to 72, n = 10 |
Mean (SD) and median not reported | |
| Pudjiadi 1973 |
Ornidazole Range (d) = 3 to 7, n = 10 |
Metronidazole Range (d) = 3 to 7, n = 10 |
Mean (SD) and median not reported | |
| Savas‐Erdeve 2009 |
Metronidazole and S boulardii Median (range, days) = 2 (1 to 5), n = 40 |
Metronidazole Median (range, days) = 2 (1 to 3), n = 45 |
Mean (SD) not reported | |
| Time to resolution of abdominal pain | Karabay 1999 |
Secnidazole Mean (d) = 2, n = 23 |
Metronidazole Mean (d) = 3, n = 21 |
SD not reported P > 0.05 |
| Mansour‐Ghanaei 2003 |
Metronidazole, iodoquinol, and S boulardii Mean (h) = 12 ± 3.2 (SD), n = 28 |
Metronidazole, iodoquinol, and placebo Mean (h) = 24 ± 7.3 (SD), n = 29 |
P < 0.0001 | |
| Savas‐Erdeve 2009 |
Metronidazole and S boulardii Median (range, days) = 3 (1 to 10), n = 40 |
Metronidazole Median (range, days) = 2 (1 to 10), n = 45 |
Mean (SD) not reported | |
| Time to disappearance of E. histolytica in stools | Naoemar 1973 |
Ornidazole Range (d) = 2 to 3, n = 8 |
Metronidazole Range (d) = 2 to 3, n = 7 |
Mean (SD) and median not reported |
| Pudjiadi 1973 |
Ornidazole Range (d) = 2 to 4, n = 10 |
Metronidazole Range (d) = 2 to 4, n = 10 |
Mean (SD) and median not reported |
E histolytica: Entamoeba histolytica; S boulardii:Saccharomyces boulardii; SD: standard deviation.
Two trials reported relapse or recurrence; both compared ornidazole with metronidazole (Naoemar 1973; Botero 1974). Another trial reported the proportion of participants who developed recurrence, but we could not include the data because researchers did not report the actual number of participants followed up (Pamba 1990).
Measurements of clinical and parasitological outcomes were made at different time points. Fifteen trials reported outcomes between end of treatment and 14 days, and 16 trials reported outcomes from 18 to 30 days after end of treatment. Nine trials measured outcomes repeatedly, and six trials reported outcomes measured at two time points (Donckaster 1964; Nnochiri 1967; Naoemar 1973; Joshi 1975; Soedin 1985; Karabay 1999). Three trials reported results at only one time point because of high dropout rates during the other follow‐up periods (Panggabean 1980; Sitepu 1982; Pamba 1990).
A total of 37 trials reported adverse events, and four trials did not ascertain adverse events (Sitepu 1982; Chunge 1989; Karabay 1999; Mansour‐Ghanaei 2003). Seventeen trials provided incomplete data: Five reported specific adverse events but not the number of participants who developed any adverse event (Batra 1972; Pamba 1990; Asrani 1995; Padilla 2000; Rossignol 2007); two reported only the number of participants with adverse events severe enough to cause discontinuation of drug treatment (Pehrson 1983; Pehrson 1984); five did not report the actual number of participants who developed any adverse event (Kapadia 1968; Prasad 1985; Soedin 1985; Toppare 1994; Davila 2002); two mentioned that one or more adverse events were reported but did not specify the treatment groups affected (Nnochiri 1967; Rossignol 2001); two reported adverse events only for the experimental group (Mohammed 1998; Savas‐Erdeve 2009); and one reported serious adverse events and allergic reactions severe enough to result in discontinuation of treatment but did not specify the treatment groups affected (Shah 2016).
Excluded studies
We have described in the ‘Characteristics of excluded studies' table trials identified by specifications from initial and updated searches but excluded from the review.
Risk of bias in included studies
Review authors prepared a risk of bias assessment for each trial with clinical and parasitological outcomes as outcome measures. Only one trial reported using appropriate procedures to minimize or eliminate bias in allocation concealment; generation of the allocation sequence; blinding of care providers, participants, and outcome assessors; and inclusion of all randomized participants (Rossignol 2007). Many trials provided little information on which to make any assessment other than ‘unclear' for most criteria.
We assessed eight trials as having low risk of bias for at least three criteria (Nnochiri 1967; Naoemar 1973; Pudjiadi 1973; Misra 1974; Awal 1979; Padilla 2000; Rossignol 2001; Rossignol 2007). Many trials had high risk for bias for one or more criteria, most commonly lack of blinding and selective outcome reporting. Most trials had unclear risk of bias for random sequence generation and allocation concealment. Many trials also had the potential risk of misclassification of amoebic colitis because the diagnosis of amoebiasis was based solely on stool microscopy in most trials, except in one that used E histolytica stool antigen testing to confirm the diagnosis (Rossignol 2007), and in two trials that used NIH stool culture for E histolytica to monitor response (Batra 1972; Tripathi 1986).
We have provided an overall pictorial summary of the risk of bias assessment in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of allocation sequence
Only seven trials reported adequate generation of the allocation sequence: Four trials used a random numbers table (Donckaster 1964; Awal 1979; Sitepu 1982; Mohammed 1998), and one trial each used computer‐generated randomization (Rossignol 2007), coin toss (Padilla 2000), and random selection of papers marked with the treatment assignment (Siddiqui 2015). Other trials did not describe the method used.
Allocation concealment
Four trials reported adequate allocation concealment: Two trials used sequentially numbered coded drug containers prepared independently by a person or at a site remote from the study site (Pudjiadi 1973; Rossignol 2007); one trial used sealed opaque envelopes prepared by another person (Savas‐Erdeve 2009); and another trial used random selection of papers marked with the treatment assignment by another person independent of the study team (Siddiqui 2015). Two trials had inadequate allocation concealment as communicated by the primary author (Pehrson 1983; Pehrson 1984). The remaining 35 trials did not report on this.
Blinding
Only eight trials reported blinding of participants, care providers, and outcome assessors (Nnochiri 1967; Naoemar 1973; Pudjiadi 1973; Prasad 1985; Padilla 2000; Rossignol 2001; Mansour‐Ghanaei 2003; Rossignol 2007). One trial reported blinding of participants and the microscopist assessing stool specimens but did not mention blinding of the outcome assessor for clinical outcomes (Chunge 1989), and another reported blinding only of the microscopist assessing stool specimens but not of care providers or outcome assessors for clinical outcomes (Pamba 1990). Eleven trials were reported to be ‘double‐blind', but most of these (nine trials) did not describe the procedure for blinding, the person(s) blinded, similarity of the appearance of drugs, or the use of placebo (Donckaster 1964; Botero 1974; Botero 1977; Guevara 1980; Huggins 1982; Sitepu 1982; Tripathi 1986; Davila 2002; Shah 2016). One trial mentioned blinding only of participants and care providers but was unclear about blinding of outcome assessors for clinical and parasitological outcomes (Panggabean 1980), and one trial mentioned blinding only of laboratory personnel assessing the stool specimens (Siddiqui 2015). One trial was reported as ‘single‐blind', but it is unclear who was blinded (Misra 1974). Four trials were open trials (Pehrson 1984; Asrani 1995; Salles 1999; Savas‐Erdeve 2009), and three were unclear regarding blinding (Kapadia 1968; Misra 1977; Misra 1978). We assessed the other 12 trials as being at high risk of performance and detection bias because researchers used different dosages and regimens of study drugs and did not mention blinding procedures.
Incomplete outcome data
The number of participants followed up was adequate (≥ 90%) for at least one outcome (clinical or parasitological failure) in 34 trials. Of these 34 trials with adequate follow‐up, three trials had missing data owing to incomplete follow‐up of participants and lack of reporting of the treatment group to which participants were randomized (Botero 1974; Prasad 1985; Asrani 1995), and another trial did not mention the reason for incomplete data (Salles 1999). Four trials reported loss of participants greater than 10% (Panggabean 1980; Sitepu 1982; Pamba 1990; Mohammed 1998), and three trials reported only the number included in the final analysis and did not report the actual number initially randomized (Donckaster 1964; Chunge 1989; Davila 2002).
Selective reporting
Fourteen trials reported all relevant outcomes, 17 were at high risk for selective outcome reporting, and 10 were at unclear risk for selective reporting bias. Selective outcome reporting was noted in the following 17 trials: Five trials assessed parasitological outcomes but not clinical outcomes (Donckaster 1964; Guevara 1980; Pehrson 1983; Pehrson 1984; Davila 2002); four trials provided incomplete clinical assessment for some patients (Botero 1974; Botero 1977; Sitepu 1982; Soedin 1985); one trial reported only the "average days of clearance of symptoms" but did not report the number of participants analysed for clinical outcomes (Karabay 1999); three trials did not pre‐specify the method or timing used for outcome assessment or criteria for clinical cure (Rubidge 1970; Prasad 1985; Toppare 1994); one trial did not mention the timing of assessment of clinical and parasitological outcomes (Mohammed 1998); one trial did not report the number of participants remaining in the study at specified time points and reported parasitological cure as cumulative clearance of amoebic forms from stools, which was not pre‐specified (Pamba 1990); one trial included only specific adverse effects but did not mention the number of participants who showed clinical improvement (Padilla 2000); and one trial incompletely reported on adverse events (Shah 2016). Three trials did not report the number of participants who developed adverse events (Sitepu 1982; Chunge 1989; Karabay 1999), and five trials incompletely reported adverse events (Pehrson 1984; Pamba 1990; Mohammed 1998; Davila 2002; Shah 2016). The presence of selective reporting bias was unclear in 10 trials owing to the following: Three trials did not report results of rectosigmoidoscopy, even if this was pre‐specified as a criterion for enrolment and/or clinical cure (Joshi 1975; Mathur 1976; Misra 1977); one trial reported outcomes only as duration from start of treatment until disappearance of blood or parasites from the stools (Batra 1972); and six trials provided incomplete reporting of adverse events (Nnochiri 1967; Huggins 1982; Tripathi 1986; Chunge 1989; Asrani 1995; Mansour‐Ghanaei 2003). In addition, two trials included an analysis that was not pre‐specified: frequency of loose stools per day and rate of disappearance of parasites in stools (Tripathi 1986); and time from first dose to passage of last unformed stools shown on a survival analysis graph (Rossignol 2007).
Other potential sources of bias
Duration of treatment was variable in six trials and could be extended up to 10 days if there was persistence of clinical symptoms or E histolytica in stools at the end of five‐day treatment (Misra 1974; Joshi 1975; Mathur 1976; Prasad 1985; Asrani 1995), or at the end of three‐day treatment (Swami 1977). In two trials, the number of participants for whom treatment was extended was greater among those given metronidazole than among those given tinidazole (Joshi 1975; Swami 1977). In both trials, clinical and parasitological cure was greater in the tinidazole group, despite the longer treatment duration reported in more patients given metronidazole. The effect could be greater if the outcome was assessed before treatment was extended. Two other trials did not report the number of participants in each group for which treatment was extended (Prasad 1985; Asrani 1995), and bias could favour those given longer treatment. One trial studied 10 different antiamoebic drugs and one placebo and randomized participants to another treatment after poor response to the first treatment but did not mention who among the participants received additional drugs (Donckaster 1964). Another trial compared various treatment regimens (Davila 2002): For those randomized to the nitazoxanide group, nitazoxanide alone was given regardless of the type of parasitosis, while for those in the second group, participants could receive quinfamide alone, mebendazole alone, or both quinfamide and mebendazole depending on the types of parasites seen. Treatment types received by the two groups were very different, and this may represent a potential source of bias. One group stopped recruitment early owing to adverse events (Pamba 1990). Another trial administered different dosages and duration of treatment for adults (five days) and for children (seven days) but analysed these data together (Naoemar 1973).
Exept for Rossignol 2007, which used E histolytica stool antigen testing to confirm the diagnosis of intestinal amoebiasis, diagnosis of amoebiasis in the included trials was based on stool microscopy, and non‐pathogenic Entamoeba species were not differentiated by more sensitive tests such as PCR and stool antigen testing. Two trials used amoebic stool culture (Batra 1972; Tripathi 1986), but one of these did not mention whether all patients had a positive stool culture on admission (Batra 1972). Most trials did not identify E histolytica as the true cause of colitis or diarrhoea; this could lead to overestimation of the treatment effect if infection is due to non‐pathogenicEntamoeba species and resolves spontaneously. In addition, many studies did not mention whether concomitant infection with other protozoa, such as giardiasis or other helminth parasites, was determined. Many of the symptoms of giardiasis and intestinal parasites may be seen in intestinal amoebiasis, and not all trials identified E histolytica as the single cause for the intestinal symptoms; therefore, assessment of clinical outcomes may be biased if persistent symptoms after treatment were caused by these other infections.
Effects of interventions
Summary of findings for the main comparison. Summary of findings table 1.
| Tinidazole compared with metronidazole as treatment for amoebic colitis | |||||||
|
Patient or population: adults and children with amoebic colitis Settings: low‐ and middle‐income countries Intervention: tinidazole Comparison: metronidazole | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Metronidazole | Tinidazole | ||||||
| Clinical failure | 1 to 14 days after end of treatment | 5 per 100 |
1 per 100 (< 1 to 7) |
RR 0.17 (0.02 to 1.30) |
285 (2 studies) | ⊕⊕⊝⊝ LOWa‐d due to risk of bias and imprecision |
Tinidazole may be more effective than metronidazole for reducing clinical failure |
| 15 to 60 days after end of treatment | 21 per 100 |
6 per 100 (3 to 11) |
RR 0.28 (0.15 to 0.51) |
477 (8 studies) | ⊕⊕⊝⊝
LOWe‐h due to risk of bias |
Tinidazole may be more effective than metronidazole for reducing clinical failure | |
|
Parasitological failure Method: stool microscopy demonstrating E histolytica cysts or trophozoites |
1 to 14 days after end of treatment | 48 per 100 |
48 per 100 (28 to 84) |
RR 1.01 (0.58 to 1.74) | 285 (2 studies) | ⊕⊕⊝⊝
LOWa,c,d,i due to risk of bias and imprecision |
Comparing tinidazole and metronidazole treatment, there may be little or no difference in number of parasitological failures |
| 15 to 60 days after end of treatment | 14 per 100 |
9 per 100 (4 to 23) |
RR 0.64 (0.25 to 1.64) |
507 (9 studies) | ⊕⊝⊝⊝
VERY LOWd,e,g,j due to imprecision, risk of bias, and inconsistency |
It is uncertain whether the number of parasitological failures differs comparing tinidazole or metronidazole treatment | |
| Adverse events | Until 30 days after start of treatment | 45 per 1000 |
29 per 100 (21 to 41) |
RR 0.65 (0.46 to 0.92) |
477 (8 studies) | ⊕⊕⊕⊝
MODERATEg,k‐m due to risk of bias |
Tinidazole is probably associated with fewer adverse events than metronidazole |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; E histolytica:Entamoeba histolytica; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence. High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aDowngraded by 1 for serious risk of bias: the two trials that assessed outcomes 1 to 14 days after end of treatment were unclear regarding randomization, allocation concealment, and blinding. Both trials used only stool microscopy to diagnose and assess parasitological outcomes, and misclassification of diagnosis and eradication of E histolytica in stools is possible. bHeterogeneity could not be assessed because only one trial contributed data. cNo serious indirectness: studies were conducted in countries endemic for amoebiasis: India (Joshi 1975) and Kenya (Chunge 1989). Trials included participants with unspecified intestinal amoebiasis or amoebic colitis, and results could be applied to other populations for whom amoebic colitis is endemic and who have similar clinical presentation. dDowngraded by 1 for imprecision: total sample size and number of events are small. The 95% confidence interval around pooled estimates includes both no effect and appreciable benefit or appreciable harm for tinidazole. eDowngraded by 2 for very serious risk of bias: trials were at high risk of selection bias because of unclear randomization and allocation concealment and inadequate blinding of outcome assessors. In four trials (Misra 1974; Joshi 1975; Mathur 1976; Swami 1977), treatment was extended to 10 days if there was persistence of clinical symptoms or presence of E histolytica in stools at the end of the planned treatment duration, but outcomes were analysed regardless of duration of treatment. It is also possible that Misra 1978 is a duplicate of the study Misra 1977. All trials used only stool microscopy to diagnose and assess parasitological outcomes, and misclassification of diagnosis and eradication of E histolytica in stools is possible. fNo serious inconsistency: there was no statistical heterogeneity (I2 is 0% and the P value for heterogeneity is greater than 0.10). Effect sizes in these trials all seem to favour tinidazole. gNo serious indirectness: eight trials were conducted in endemic areas (seven trials in India and one trial in Bangladesh), and one trial was conducted in an industrialized country (Sweden). All trials included patients with unspecified intestinal amoebiasis or amoebic colitis, and study results could be applied to other populations for whom amoebic colitis is endemic and who have similar clinical presentation. hNo serious imprecision: these studies are adequately powered to detect 50% reductions in clinical and parasitological failure. The result is statistically significant. iNo serious inconsistency: there was no statistical heterogeneity (I2 is 10% and the P value for heterogeneity is greater than 0.10). Confidence intervals in trials overlap, and the point estimate indicates both benefit and harm for tinidazole. jDowngraded by 1 for inconsistency: statistical heterogeneity was high (I2 is 64% and the P value for heterogeneity is less than 0.10), which could be explained by differences in populations. All studies indicate that tinidazole is comparable with metronidazole, except Pehrson 1984, which favours metronidazole. kDowngraded by 1 for serious risk of bias: trials had inadequate or unclear blinding of outcome assessors for adverse events. Procedures for reporting adverse events and for monitoring laboratory test results were not standardized and were inadequately reported. lNo serious inconsistency: statistical heterogeneity was not significant (I2 is 48% and the P value for heterogeneity is 0.10), except for one trial (Swami 1977); all trials consistently show lower risk of adverse events among those given tinidazole compared with those given metronidazole. Adverse effects reported were predominantly gastrointestinal, such as nausea, vomiting, anorexia, bitter or metallic taste, and abdominal discomfort. mNo serious imprecision: studies are adequately powered to detect 50% difference in adverse events between the two groups. The result is statistically significant.
Summary of findings 2. Summary of findings table 2.
| Combination therapy compared with metronidazole alone as treatment for amoebic colitis | ||||||
|
Patient or population: adults and children with amoebic colitis Settings: low‐ and middle‐income countries Intervention: combination therapy Comparison: metronidazole alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Combination therapy | Metronidazole alone | |||||
| Clinical failure 1 to 14 days after end of treatment | 71 per 100 |
23 per 100 (8 to 70) |
RR 0.33 (0.11 to 0.98) |
1025 (3 studies) | ⊕⊝⊝⊝
VERY LOWa‐d due to risk of bias, inconsistency, and indirectness |
It is uncertain whether clinical failure differs between combination therapy or metronidazole treatment |
|
Parasitological failure 1 to 14 days after end of treatment Method: stool microscopy demonstrating E histolytica cysts or trophozoites |
13 per 100 |
5 per 100 (2 to 11) |
RR 0.36 (0.15 to 0.86) |
720 (3 studies) | ⊕⊕⊝⊝
LOWa,c‐e due to risk of bias and indirectness |
Combination therapy may result in fewer parasitological failures compared with metronidazole |
| Adverse events | Adverse events were incompletely reported and could not be combined in a meta‐analysis | 1025 (3 studies) |
⊕⊝⊝⊝
VERY LOWc,f due to indirectness and risk of bias |
It is uncertain whether the number of adverse events differs with combination therapy or metronidazole treatment | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; E histolytica:Entamoeba histolytica; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by 1 for serious risk of bias: all three trials included for this outcome had unclear randomization and allocation concealment, and two trials had inadequate blinding. Prasad 1985 was at high risk of selective reporting bias because of inadequate reporting of the method used for outcome evaluation and variable treatment duration ranging from 5 to 10 days. All trials used only stool microscopy to diagnose and assess parasitological outcomes, and misclassification of diagnosis and eradication of E histolytica in stools is possible. bDowngraded by 1 for inconsistency: heterogeneity was statistically significant (I2 is 71% and the P value for heterogeneity is less than 0.05). Heterogeneity could be explained by differences in severity of illness and variable drug combinations used. cDowngraded by 1 for indirectness: trials were conducted in countries that are endemic for amoebiasis (India ‐ Asrani 1995 and Prasad 1985 ‐ and South Africa ‐ Rubidge 1970) but used various drug combinations. Studies using different combination of drugs would need to be studied. Some of these drugs are no longer marketed, and it is not known whether the results could be applied to other combinations. dNo serious imprecision: these studies are adequately powered to detect 50% reductions in clinical and parasitological failure 15 to 60 days after end of treatment. The result is statistically significant. eNo serious inconsistency: statistical heterogeneity was moderate with I2 of 42% and P value for heterogeneity of 0.18). The CIs overlap, and the pooled estimate shows significant benefit favouring combination therapy. fDowngraded by 2 for very serious risk of bias: blinding was inadequate, and reporting of the frequency and type of adverse events in trials was incomplete.
We have shown in Appendix 9 details of the comparisons and interventions included in this review. We have presented ‘Summary of findings' tables for two important outcomes: tinidazole compared with metronidazole as treatment for amoebic colitis (Table 1); and combination therapy compared with metronidazole alone as treatment for amoebic colitis (Table 2).
1. Single alternative drug versus metronidazole
Sixteen trials compared alternative nitroimidazoles versus metronidazole, and one trial compared praziquantel versus metronidazole (Mohammed 1998).
1.1. Tinidazole versus metronidazole
Ten trials compared tinidazole versus metronidazole, with two trials evaluating clinical and parasitological failure 1 to 14 days after end of treatment (Joshi 1975; Chunge 1989); eight trials evaluating clinical failure 15 to 60 days after end of treatment (Misra 1974; Joshi 1975; Mathur 1976; Misra 1977; Singh 1977; Swami 1977; Misra 1978; Awal 1979); and nine trials evaluating parasitological failure 15 to 60 days after end of treatment (Misra 1974; Joshi 1975; Mathur 1976; Misra 1977; Singh 1977; Swami 1977; Misra 1978; Awal 1979; Pehrson 1984). We graded the overall certainty of evidence as low because of serious risk of bias (see Table 1): All trials had unclear allocation concealment and randomization except Awal 1979; five trials were not blinded for clinical outcomes and were unclear on blinding for parasitological outcomes (Joshi 1975; Mathur 1976; Singh 1977; Awal 1979; Pehrson 1984); and four trials had variable duration of treatment with treatment extended to 10 days for persistence of clinical symptoms or E histolytica in the stools at the end of planned treatment (Misra 1974; Joshi 1975; Mathur 1976; Swami 1977). In addition, all trials used only stool microscopy for diagnosis and assessment of parasitological outcomes, hence misclassification of diagnosis and eradication of E histolytica in stools is possible. Nine trials were conducted in countries endemic for amoebiasis (eight in India, one in Bangladesh). For clinical failure 1 to 14 days after end of treatment, results showed imprecision probably due to small sample sizes and few events (RR 0.17, 95% CI 0.02 to 1.30; 285 participants, 2 trials; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.
Comparison 1 Alternative drug versus metronidazole, Outcome 1 Clinical failure: 1 to 14 days after end of treatment.
For clinical failure 15 to 60 days after end of treatment, tinidazole reduced clinical failure by 72% compared with metronidazole (RR 0.28, 95% CI 0.15 to 0.51; 477 participants, 8 trials; low‐certainty evidence; Analysis 1.2 and Figure 3). A sensitivity analysis evaluating quality in relation to allocation concealment and blinding was not possible. We noted no significant change in the overall result when we excluded Misra 1978, which may be a duplicate publication of an earlier trial ‐ Misra 1977 (RR 0.31, 95% CI 0.16 to 0.61; 418 participants, 7 trials; low‐certainty evidence; Analysis 12.1). Excluding four trials funded by pharmaceutical companies also did not affect the overall result (RR 0.24, 95% CI 0.11 to 0.50; 241 participants, 4 trials; low‐certainty evidence; Analysis 12.2) (Misra 1974; Joshi 1975; Mathur 1976; Singh 1977).
1.2. Analysis.
Comparison 1 Alternative drug versus metronidazole, Outcome 2 Clinical failure: 15 to 60 days after end of treatment.
3.
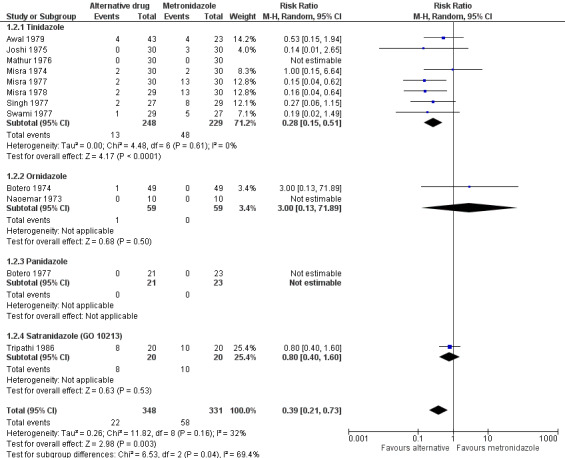
Alternative drug versus metronidazole: clinical failure 15 to 60 days after end of treatment.
12.1. Analysis.
Comparison 12 Sensitivity analysis: tinidazole versus metronidazole 15 to 60 days after end of treatment, Outcome 1 Clinical failure: 15 to 60 days after end of treatment, excluding Misra 1978.
12.2. Analysis.
Comparison 12 Sensitivity analysis: tinidazole versus metronidazole 15 to 60 days after end of treatment, Outcome 2 Clinical failure: 15 to 60 days after end of treatment, excluding trials sponsored by pharmaceutical companies.
Results for parasitological failure did not show that tinidazole was more effective than metronidazole in eradicating E histolytica 1 to 14 days after end of treatment (RR 1.01, 95% CI 0.58 to 1.74; 285 participants, 2 trials; low‐certainty evidence; Analysis 1.3) or 15 to 60 days after end of treatment (RR 0.64, 95% CI 0.25 to 1.64; 507 participants, 9 trials; very low‐certainty evidence; Analysis 1.4 and Figure 4). Heterogeneity was significant in trials that evaluated parasitological failure 15 to 60 days after end of treatment. Subgroup analysis conducted to investigate possible sources of heterogeneity showed reduced heterogeneity in trials with non‐dysenteric amoebic colitis and unspecified amoebic colitis (Analysis 6.1), as well as in trials that used the WHO criteria (Analysis 6.4). Age and the presence or absence of other concomitant intestinal infection did not explain heterogeneity (Analysis 6.2 and Analysis 6.3). Subgroup analysis showed greater treatment effects of tinidazole in those given the higher dose of 2 grams in a single dose for three days compared with lower doses of tinidazole at 600 mg twice daily for five days. although this was significant only for clinical improvement (RR 0.24, 95% CI 0.13 to 0.47; 297 participants, 5 trials; low‐certainty evidence; Analysis 11.1) ‐ not for parasitological response (Analysis 11.2).
1.3. Analysis.
Comparison 1 Alternative drug versus metronidazole, Outcome 3 Parasitological failure: 1 to 14 days after end of treatment.
1.4. Analysis.
Comparison 1 Alternative drug versus metronidazole, Outcome 4 Parasitological failure: 15 to 60 days after end of treatment.
4.
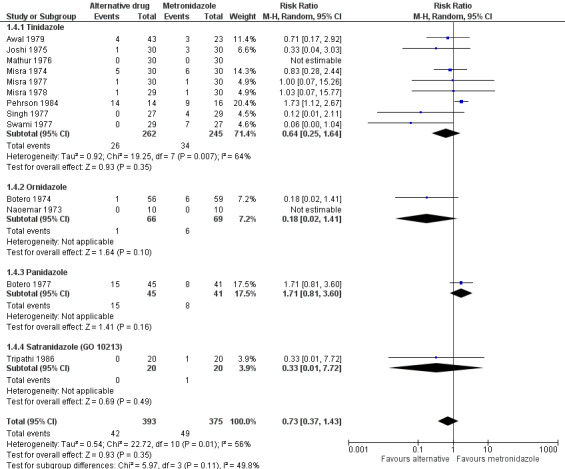
Alternative drug versus metronidazole: parasitological failure 15 to 60 days after end of treatment.
6.1. Analysis.
Comparison 6 Subgroup analyses: alternative drug versus metronidazole, Outcome 1 Parasitological failure 15 to 60 days after end of treatment, by clinical category.
6.4. Analysis.
Comparison 6 Subgroup analyses: alternative drug versus metronidazole, Outcome 4 Parasitological failure 15 to 60 days after end of treatment, by criteria.
6.2. Analysis.
Comparison 6 Subgroup analyses: alternative drug versus metronidazole, Outcome 2 Parasitological failure 15 to 60 days after end of treatment, by age group.
6.3. Analysis.
Comparison 6 Subgroup analyses: alternative drug versus metronidazole, Outcome 3 Parasitological failure 15 to 60 days after end of treatment, single or mixed intestinal infection.
11.1. Analysis.
Comparison 11 Subgroup analysis: tinidazole versus metronidazole 15 to 60 days after end of treatment, based on tinidazole dose, Outcome 1 Clinical failure: 15 to 60 days after end of treatment.
11.2. Analysis.
Comparison 11 Subgroup analysis: tinidazole versus metronidazole 15 to 60 days after end of treatment, based on tinidazole dose, Outcome 2 Parasitological failure: 15 to 60 days after end of treatment.
Researchers reported no data on relapse.
Eight trials reported adverse events (Misra 1974; Joshi 1975; Mathur 1976; Misra 1977; Singh 1977; Swami 1977; Misra 1978; Awal 1979). We graded the certainty of evidence for this outcome as moderate because of serious risk of bias due to lack of blinding or unclear blinding in all trials, and lack of standardization in reporting of both clinical and laboratory adverse events. Four trails reported no blinding of outcome assessors for adverse events (Joshi 1975; Mathur 1976; Singh 1977; Awal 1979), and in the other four trials, this was unclear (Misra 1974; Misra 1977; Swami 1977; Misra 1978). Participants in seven trials voluntarily reported adverse events, but one trial did not specify the method used to solicit adverse events (Misra 1974). Five trials monitored adverse events for 30 days from start of treatment, but two trials did not mention the duration of monitoring (Misra 1974; Misra 1978). All eight trials reported no abnormalities seen on haematological, biochemical, and urine analyses, and two trials reported no abnormalities on electrocardiographic studies (Misra 1974; Misra 1977). All trials conducted laboratory tests before treatment, but trials repeated testing at different time points during and after treatment. No trials reported that serious adverse events or adverse events necessitated drug withdrawal. Other non‐serious adverse events appeared to be less common among those given tinidazole than among those given metronidazole (RR 0.65, 95% CI 0.46 to 0.92; 477 participants, 8 trials; moderate‐certainty evidence; Analysis 1.6); nausea, vomiting, decreased appetite, and altered taste or metallic taste were the most common (see Appendix 10 for other details).
1.6. Analysis.
Comparison 1 Alternative drug versus metronidazole, Outcome 6 Adverse events.
1.2. Other nitroimidazole drugs versus metronidazole
Other alternative drugs tested were ornidazole (155 participants, 3 trials; Naoemar 1973;Pudjiadi 1973;Botero 1974), secnidazole (44 participants, 1 trial; Karabay 1999), panidazole (86 participants, 1 trial; Botero 1977), and satranidazole (40 participants, 1 trial; Tripathi 1986). The number of participants in these trials comparing other nitroimidazoles versus metronidazole was inadequate to allow detection of any significant difference in clinical failure or parasitological failure 1 to 14 days after end of treatment (Analysis 1.1 and Analysis 1.3), or 15 to 60 days after end of treatment (Analysis 1.2 and Analysis 1.4). Researchers reported no differences in time to resolution of clinical symptoms and eradication of E histolytica in stools between intervention and control groups (see Table 5).
For relapse, the data reported in two small trials, both comparing ornidazole versus metronidazole (Naoemar 1973; Botero 1974), were of very low certainty because of inadequate description of the randomization process and allocation concealment, and additionally in one trial for unclear blinding procedures (Botero 1974). In these trials, more relapses were evident among those given ornidazole than among those given metronidazole (RR 4.74, 95% CI 1.07 to 20.99; 135 participants, 2 trials; very low‐certainty evidence; Analysis 1.5), but data are insufficient to allow definitive conclusions because of the small numbers of events reported.
1.5. Analysis.
Comparison 1 Alternative drug versus metronidazole, Outcome 5 Relapse (ornidazole).
Three trials comparing ornidazole versus metronidazole reported adverse events (Naoemar 1973; Pudjiadi 1973; Botero 1974), as did one trial comparing panidazole with metronidazole ‐ Botero 1977 ‐ and another trial comparing satranidazole with metronidazole ‐ Tripathi 1986. No trials reported serious adverse events or withdrawals resulting from adverse events, except one ‐ Botero 1974 ‐ in which one participant given ornidazole developed temporary numbness of the hands and tongue with difficulty speaking that disappeared after treatment was stopped. In another trial (Naoemar 1973), the dosage of two participants each in the ornidazole group and the metronidazole group had to be reduced because of dizziness or nausea. No abnormalities in laboratory tests were seen in trials that conducted these tests (see Appendix 10 for other details). There seems to be no difference in adverse events among those given ornidazole, panidazole, and satranidazole compared with metronidazole (Analysis 1.6).
2. Any antiamoebic drug versus placebo
Four studies involved comparison of any antiamoebic drug versus placebo: nitazoxanide (167 participants, 2 trials; Rossignol 2001; Rossignol 2007) and quinfamide (96 participants, 1 trial; Huggins 1982); and versus 10 different drugs belonging to six drug classes (367 participants, 1 trial; Donckaster 1964).
Compared with placebo, both quinfamide and nitazoxanide reduced clinical and parasitological failure rates 1 to 14 days after end of treatment (Analysis 2.1 and Analysis 2.2). However, heterogeneity among trials was significant, even in the two trials that evaluated nitazoxanide. Subgroup analysis using clinical categories did not explain heterogeneity (Analysis 7.1), but such heterogeneity was reduced in trials that included adult participants only (Analysis 7.2 and Analysis 7.3). Excluding the single trial that used stool antigen‐based ELISA testing to confirm E histolytica ‐ Rossignol 2007 ‐ also reduced heterogeneity in the remaining trials (Analysis 7.4 and Analysis 7.5). Sensitivity analysis using concealment and blinding was not possible because only one trial was concealed ‐ Rossignol 2007 ‐ and only two trials were blinded ‐ Rossignol 2001 and Rossignol 2007.
2.1. Analysis.
Comparison 2 Any antiamoebic drug versus placebo, Outcome 1 Clinical failure: 1 to 14 days after end of treatment.
2.2. Analysis.
Comparison 2 Any antiamoebic drug versus placebo, Outcome 2 Parasitological failure: 1 to 14 days after end of treatment.
7.1. Analysis.
Comparison 7 Subgroup analyses: any antiamoebic drug versus placebo, Outcome 1 Parasitological failure 1 to 14 days after end of treatment, by clinical category.
7.2. Analysis.
Comparison 7 Subgroup analyses: any antiamoebic drug versus placebo, Outcome 2 Clinical failure 1 to 14 days after end of treatment, by age group.
7.3. Analysis.
Comparison 7 Subgroup analyses: any antiamoebic drug versus placebo, Outcome 3 Parasitological failure 1 to 14 days after end of treatment, by age group.
7.4. Analysis.
Comparison 7 Subgroup analyses: any antiamoebic drug versus placebo, Outcome 4 Clinical failure 1 to 14 days after end of treatment, by diagnostic method.
7.5. Analysis.
Comparison 7 Subgroup analyses: any antiamoebic drug versus placebo, Outcome 5 Parasitological failure 1 to 14 days after end of treatment, by diagnostic method.
Researchers reported no data on relapse.
No trial reported serious adverse events or withdrawals due to adverse events. Also no trials reported differences in adverse events among those given antiamoebic drugs compared with placebo (530 participants, 3 trials; Analysis 2.3), although the results could be biased because of a great imbalance in the numbers of those given active drugs versus placebo. The most common adverse events were mild gastrointestinal symptoms, such as nausea, vomiting, abdominal pain, and headache. One individual given diiodohydroxyquinoline presented with severe intestinal colic (see Appendix 11 for details).
2.3. Analysis.
Comparison 2 Any antiamoebic drug versus placebo, Outcome 3 Adverse events.
3. Combination regimen versus monotherapy
Three trials compared various combination regimens versus metronidazole alone (Rubidge 1970; Prasad 1985; Asrani 1995), and four trials compared other combination regimens versus alternative single drugs (Pehrson 1983; Soedin 1985; Pamba 1990; Davila 2002).
3.1. Combination regimen versus metronidazole alone
We graded the overall certainty of evidence as very low for the outcome of clinical failure 1 to 14 days after end of treatment (Table 2). All three trials did not describe the randomization process and allocation concealment, and blinding was lacking in two trials (Rubidge 1970; Asrani 1995). Prasad 1985 was at high risk of selective reporting bias because researchers did not adequately describe the method used for outcome evaluation and researchers analysed participants after different treatment durations ranging from 5 to 10 days, depending on severity of disease and response to therapy. All three trials were conducted in countries endemic for amoebiasis and used only stool microscopy to assess parasitological outcomes, hence misclassification of eradication of E histolytica from stools is possible. The pooled result shows that compared with metronidazole alone, combination therapy reduced clinical failure 1 to 14 days after end of treatment by 67% (RR 0.33, 95% CI 0.11 to 0.98; 1025 participants, 3 trials; very low‐certainty evidence; Analysis 8.1). However, significant heterogeneity seen in these trials could be due to the various combination regimens used: a combination of dehydroemetine, tetracycline, and diloxanide furoate (Rubidge 1970); a fixed‐drug combination suspension of metronidazole and furazolidone (Prasad 1985); and a fixed‐drug combination tablet of metronidazole and diiodohydroxyquinoline (Asrani 1995). Heterogeneity could also be explained by differences in clinical disease, because exclusion of the trial that included only children with amoebic dysentery resulted in greater effect favouring combination therapy in patients with unspecified intestinal amoebiasis (RR 0.17, 95% CI 0.12 to 0.25; 986 participants, 2 trials; very low‐certainty evidence; Analysis 9.1) (Rubidge 1970). This could be attributed to additional luminal drugs (diiodohydroxyquinoline in Asrani 1995 and furazolidone in Prasad 1985) that may be more effective against cyst forms in patients with unspecified intestinal amoebiasis.
8.1. Analysis.
Comparison 8 Subgroup analyses: combination regimen versus monotherapy, Outcome 1 Clinical failure: 1 to 14 days after end of treatment, by intervention.
9.1. Analysis.
Comparison 9 Subgroup analyses: combination regimen versus metronidazole, Outcome 1 Clinical failure: 1 to 14 days after end of treatment, by clinical diagnosis.
For parasitological failure 1 to 14 days after end of treatment, we graded the overall certainty of evidence as low because of lack of allocation concealment and blinding, selective reporting, and indirectness as described above. Results showed a 64% reduction in parasitological failure 1 to 14 days after end of treatment among those given the combination compared with those given metronidazole alone (RR 0.36, 95% CI 0.15 to 0.86; 720 participants, 3 trials; low‐certainty evidence; Analysis 8.2). We noted no significant heterogeneity among trials (Figure 5). Subgroup analysis showed that excluding the trial on children with amoebic dysentery showed greater benefit for those with unspecified intestinal amoebiasis (RR 0.25, 95% CI 0.13 to 0.46; 681 participants, 2 trials; low‐certainty evidence; Analysis 9.2).
8.2. Analysis.
Comparison 8 Subgroup analyses: combination regimen versus monotherapy, Outcome 2 Parasitological failure: 1 to 14 days after end of treatment, by intervention.
5.
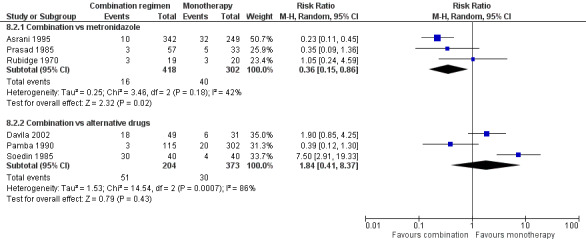
Combination regimen versus monotherapy: parasitological failure 1 to 14 days after end of treatment, subgrouped by intervention.
9.2. Analysis.
Comparison 9 Subgroup analyses: combination regimen versus metronidazole, Outcome 2 Parasitological failure: 1 to 14 days after end of treatment, by clinical diagnosis.
For both clinical and parasitological failure, the overall results were driven by one trial, which analysed a large number of participants (896 participants analysed for clinical failure; 591 participants analysed for parasitological failure) compared with the other two trials (Asrani 1995). This was an open‐label trial with unclear allocation concealment and method of randomization, hence the high possibility of bias. This trial also may have been funded by a pharmaceutical company because one of the study authors is connected with the company that provided the study drug ‐ a fixed‐drug combination of metronidazole and diiodohydroxyquinoline. A sensitivity analysis performed while excluding this trial reduced heterogeneity and significantly changed the overall results for both clinical and parasitological outcomes (i.e. no benefit in giving combination therapy compared with giving metronidazole alone) (RR 0.58, 95% CI 0.20 to 1.73; 129 participants, 2 trials; low‐certainty evidence; Analysis 13.1; Analysis 13.2).
13.1. Analysis.
Comparison 13 Sensitivity analyses: combination regimen versus metronidazole alone, excluding pharmaceutical company‐sponsored study (Asrani 1995), Outcome 1 Clinical failure: 1 to 14 days after end of treatment.
13.2. Analysis.
Comparison 13 Sensitivity analyses: combination regimen versus metronidazole alone, excluding pharmaceutical company‐sponsored study (Asrani 1995), Outcome 2 Parasitological failure: 1 to 14 days after end of treatment, by intervention.
Researchers reported no data on relapse.
The three trials reported no serious adverse events but indicated that one participant given a fixed‐drug combination tablet of metronidazole and diiodohydroxyquinoline developed an unspecified allergic reaction on the first day, necessitating withdrawal from the trial. Two trials did not blind outcome assessors for adverse events (Asrani 1995; Rubidge 1970). One trial reported that tolerance of both regimens was excellent and noted no toxicity (Rubidge 1970); another trial reported no difference in the overall incidence of side effects between the two groups but did not report on the number who developed adverse events (Asrani 1995). The most frequently reported adverse events in both groups were metallic taste, abdominal pain, and nausea. Only one trial reported a higher incidence of side effects with metronidazole compared with the fixed‐drug combination suspension of furazolidone and metronidazole but did not report the specific adverse events and the number who developed adverse events (Prasad 1985). See Appendix 12 for details.
3.2. Combination regimen versus other single‐drug regimens
Four trials studied the efficacy of combination regimens compared with other single‐drug regimens. Two trials compared combination regimens with other nitroimidazoles: a combination of tetracycline and clioquinol versus secnidazole alone (80 participants, 1 trial; Soedin 1985); and a combination of tinidazole and diloxanide furoate versus tinidazole alone (41 participants, 1 trial; Pehrson 1983). The third trial compared three different combinations (nimorazole and aminosidine, nimorazole and etophamide, and etophamide and aminosidine) versus the same drugs given as monotherapy (400 participants, 1 trial; Pamba 1990). The fourth trial compared quinfamide and mebendazole versus nitazoxanide (80 participants, 1 trial; Davila 2002).
Trials could not be pooled because they performed different drug comparisons, but we have presented the data for clinical failure (Analysis 3.1) and parasitological failure (Analysis 3.2). Trials did not show any difference in clinical or parasitological failure rates between combination regimens and single‐drug regimens, except in two comparisons. Soedin 1985 showed that secnidazole alone resulted in greater resolution of clinical symptoms and greater eradication of E histolytica when compared with the combination of tetracycline and clioquinol on day 28 of treatment (80 participants, 1 trial; Analysis 3.1; Analysis 3.2). Pehrson 1983, another small trial, showed that the combination of tinidazole and diloxanide furoate resulted in greater eradication of E histolytica compared with tinidazole alone one month after end of treatment (41 participants, 1 trial). Both trials reported wide confidence intervals; thus no definitive conclusions regarding these regimens can be made.
3.1. Analysis.
Comparison 3 Combination regimen versus monotherapy, Outcome 1 Clinical failure: 1 to 14 days after end of treatment.
3.2. Analysis.
Comparison 3 Combination regimen versus monotherapy, Outcome 2 Parasitological failure: 1 to 14 days after end of treatment.
Researchers reported no data on relapse.
Trials incompletely reported adverse events. Pamba 1990 discontinued recruitment of participants in the combination etophamide‐aminosidine group because of the high incidence of severe diarrhoea. Soedin 1985 and Davila 2002 reported that both treatment regimens were well tolerated with only a few side effects but did not report the specific adverse events and the number of participants who developed any adverse events. Pehrson 1983 reported that no serious adverse events necessitated discontinuation of treatment but provided no details. See Appendix 12 for details.
4. Single‐dose regimen versus longer or multiple‐dose regimens
Five trials compared a single‐dose regimen versus longer duration of therapy or multiple‐dose regimens. Three trials compared longer duration of other drugs versus single‐dose secnidazole (Soedin 1985; Karabay 1999; Salles 1999), and two trials compared longer duration of other drugs versus single‐dose quinfamide (Huggins 1982; Davila 2002).
4.1. Single‐dose secnidazole versus longer or multiple‐dose regimens
Salles 1999 compared single‐dose secnidazole versus tinidazole for two days (303 participants, 1 trial), Karabay 1999 compared single‐dose secnidazole versus metronidazole for 10 days (44 participants, 1 trial), and Soedin 1985 compared single‐dose secnidazole versus a combination of tetracycline and clioquinol for five days (80 participants, 1 trial). These trials were unclear on allocation concealment and were not blinded.
Soedin 1985 showed that single‐dose secnidazole resulted in greater resolution of clinical symptoms at end of treatment compared with five days of tetracycline and clioquinol (RR 0.12, 95% CI 0.03 to 0.48; 80 participants, 1 trial; low certainty evidence; Analysis 4.1). Salles 1999 did not show any difference in clinical failure 19 days after end of treatment between single‐dose secnidazole and two‐day tinidazole treatment (275 participants, 1 trial; Analysis 4.2). We could not pool results for clinical failure because of a difference between the two trials in the time of evaluation of clinical outcomes (Analysis 4.1 and Analysis 4.2).
4.1. Analysis.
Comparison 4 Single‐dose regimen versus longer regimen, Outcome 1 Clinical failure: 1 to 14 days after end of treatment.
4.2. Analysis.
Comparison 4 Single‐dose regimen versus longer regimen, Outcome 2 Clinical failure: 15 to 60 days after end of treatment.
Single‐dose secnidazole may result in lower parasitological failure 1 to 14 days after end of treatment compared with multi‐dose regimens (Soedin 1985; Karabay 1999) (RR 0.14, 95% CI 0.06 to 0.35; 124 participants, 2 trials; low‐certainty evidence; Analysis 4.3 and Analysis 10.1). Although no heterogeneity was evident, the antiamoebic drugs compared with secnidazole were different; secnidazole was compared with metronidazole in one trial (Karabay 1999), and with a combination of tetracycline and clioquinol in another trial (Soedin 1985). Both trials were small with unclear allocation concealment and blinding of the microscopist examining the stools. Another trial compared single‐dose secnidazole versus tinidazole and suggested that secnidazole may be more effective than tinidazole for eradication of amoebae from the stools 19 days after end of treatment (RR 0.61, 95% CI 0.43 to 0.88; 300 participants, 1 trial; low‐certainty evidence; Analysis 4.4) (Salles 1999). As this was an open trial, Salles 1999 appears to be at high risk of bias.
4.3. Analysis.
Comparison 4 Single‐dose regimen versus longer regimen, Outcome 3 Parasitological failure: 1 to 14 days after end of treatment.
10.1. Analysis.
Comparison 10 Subgroup analyses: any single‐dose regimen versus longer regimen, Outcome 1 Parasitological failure: 1 to 14 days after end of treatment, by intervention.
4.4. Analysis.
Comparison 4 Single‐dose regimen versus longer regimen, Outcome 4 Parasitological failure: 15 to 60 days after end of treatment.
Researchers reported no data on relapse.
Only Salles 1999 reported on adverse events. Researchers reported no serious adverse events or withdrawals for adverse events. Adverse events most commonly reported were bitter taste, nausea, vomiting, and abdominal pain, with no difference in frequency between those given single‐dose secnidazole compared with tinidazole for two days. Soedin 1985 did not report the proportion of participants who developed adverse events but mentioned that side effects were few and treatment was well tolerated regardless of the regimen received (see Appendix 12 for details).
4.2. Single‐dose quinfamide versus multiple doses of quinfamide or longer duration of another drug
Investigators compared single‐dose quinfamide versus two or three doses of quinfamide (72 participants; Huggins 1982), and they compared single‐dose quinfamide versus nitazoxanide for three days (25 participants; Analysis 4.1; Analysis 4.3) (Davila 2002).
Huggins 1982 showed no difference in clinical failure between those given one dose compared with two or three doses of quinfamide (72 participants; Analysis 4.1).
For parasitological failure 1 to 14 days after end of treatment, pooling of results from two trials revealed a trend favouring more doses compared with single‐dose quinfamide for eradicating E histolytica (RR 2.13, 95% CI 1.02 to 4.46; 97 participants; two trials; low‐certainty evidence; Analysis 10.1) (Huggins 1982; Davila 2002). Both trials were unclear regarding generation of the allocation sequence, concealment, and blinding. Results were not heterogeneous, but numbers of trials and participants were too small to permit any definitive conclusions.
Researchers reported no data on relapse.
Only Huggins 1982 reported on adverse events; these reports were based on only two symptoms ‐ nausea and headache. None of those given single‐dose quinfamide developed adverse effects, but 12 among those who received two or three doses of quinfamide developed nausea and headache. Davila 2002 reported that both quinfamide and nitazoxanide were well‐tolerated but mentioned no specific adverse effects (see Appendix 12 for details).
5. Other antiamoebic drug comparisons
Thirteen trials studied different drug comparisons (see Appendix 9 for details). Only two trials were adequately concealed (Savas‐Erdeve 2009; Siddiqui 2015). Blinding was not done or was unclear in all except two trials (Nnochiri 1967; Padilla 2000). Dropout rates were high in two trials, with one trial analysing only 62.5% of those initially randomized (Panggabean 1980), and the other trial analysing 82% (Sitepu 1982).
Eight trials assessed clinical failure 1 to 14 days after end of treatment (Kapadia 1968; Batra 1972; Panggabean 1980; Sitepu 1982; Toppare 1994; Savas‐Erdeve 2009; Siddiqui 2015; Shah 2016). Kapadia 1968 showed chlorhydroxyquinoline to be probably more effective than diiodohydroxyquinoline in reducing clinical failure (RR 0.24, 95% CI 0.11 to 0.53; 100 participants, 1 trial). Two trials reported no difference in clinical failure rates when comparing the other antiamoebic drugs: ornidazole versus tinidazole (66 participants, 2 trials; Panggabean 1980; Sitepu 1982). Other trials reported no difference in clinical failure rates when comparing ornidazole versus secnidazole (102 participants, 1 trial; Toppare 1994), a fixed combination of metronidazole and diloxanide furoate versus an herbal product composed of several different natural products (153 participants, 1 trial; Siddiqui 2015), and metronidazole versus an herbal product (184 participants, 1 trial; Shah 2016). Two trials reported no clinical failures when comparing respectively four dosage regimens of MK‐910 (40 participants, 1 trial) and Saccharomyces boulardii probiotic added to metronidazole versus metronidazole alone (85 participants, 1 trial; Analysis 5.1) (Batra 1972; Savas‐Erdeve 2009). Two trials evaluated the effect of added S boulardii on duration of clinical symptoms; one trial reported this outcome as the mean (Mansour‐Ghanaei 2003), and the other trial as median and range (Savas‐Erdeve 2009). One trial evaluating resolution of diarrhoea and abdominal pain showed significantly shorter mean duration among those given S boulardii in addition to metronidazole and iodoquinol (Mansour‐Ghanaei 2003), but another trial did not show a difference in median and range for this outcome when S boulardii was added to metronidazole (Savas‐Erdeve 2009). See Table 5.
5.1. Analysis.
Comparison 5 Other antiamoebic drug comparisons, Outcome 1 Clinical failure: 1 to 14 days after end of treatment.
Ten trials assessed parasitological failure one to 14 days after end of treatment (Kapadia 1968; Batra 1972; Panggabean 1980; Sitepu 1982; Toppare 1994; Padilla 2000; Davila 2002; Savas‐Erdeve 2009; Siddiqui 2015; Shah 2016). Two trials assessed parasitological failure approximately one month after treatment (Guevara 1980; Mansour‐Ghanaei 2003), and another trial assessed parasitological failure during two time periods ‐ 1 to 14 days and 15 to 60 days after treatment (Nnochiri 1967). One trial showed that chlorhydroxyquinoline probably was more effective than diiodohydroxyquinoline in reducing parasitological failure 1 to 14 days after end of treatment (RR 0.53, 95% CI 0.35 to 0.80; 100 participants, 1 trial; low‐certainty evidence; Analysis 5.3) (Kapadia 1968). Researchers reported no difference in eradication of amoebae from the stools in trials comparing ornidazole versus other nitroimidazoles: ornidazole versus tinidazole (74 participants, 2 trials; Panggabean 1980; Sitepu 1982); and ornidazole versus secnidazole (102 participants, 1 trial; Toppare 1994). Single‐dose quinfamide appeared to result in better parasitological eradication when compared with single‐dose secnidazole in one trial (RR 0.57, 95% CI 0.34, 0.96; 239 participants, 1 trial ‐ Padilla 2000; low‐certainty evidence; Analysis 5.3) but not when compared with nitazoxanide in another trial (25 participants, 1 trial ‐ Davila 2002;Analysis 5.3). Another trial comparing three doses of quinfamide versus teclozan reported no differences between the two groups (37 participants, 1 trial ‐ Guevara 1980; Analysis 5.4). Batra 1972 noted no difference in parasitological failure when comparing low‐dosage regimens of MK‐910 versus higher dosages (≥ 2 mg/kg/d) of the same drug (40 participants, 1 trial). Two trials evaluated the efficacy of adding the probiotic S boulardii to metronidazole and found a trend toward increased parasitological eradication in the group given S boulardii in addition to metronidazole and iodoquinol (54 participants, 1 trial ‐ Mansour‐Ghanaei 2003;Analysis 5.3), and in addition to metronidazole alone (85 participants, 1 trial ‐ Savas‐Erdeve 2009;Analysis 5.2), but the results were not statistically significant. Another trial showed a non‐significant increase in both clinical and parasitological failure at end of treatment among those given an herbal product compared with those given a fixed‐drug combination of metronidazole and diloxanide furoate (154 participants, 1 trial ‐ Siddiqui 2015; Analysis 5.1 and Analysis 5.2). One trial showed no significant difference in parasitological failure at end of treatment between an herbal drug product and metronidazole (184 participants, 1 trial; Shah 2016). A small trial that compared a fixed‐drug combination of diloxanide furoate, tetracycline, and chloroquine versus the fixed‐drug combination without chloroquine showed no difference in parasitological failure between the two groups at end of treatment (59 participants, 1 trial; Analysis 5.2) but showed a significant advantage for the combination containing chloroquine on follow‐up seven weeks after end of treatment (RR 0.29, 95% CI 0.09 to 0.92; 58 participants, 1 trial; low‐certainty evidence; Analysis 5.3) (Nnochiri 1967).
5.3. Analysis.
Comparison 5 Other antiamoebic drug comparisons, Outcome 3 Parasitological failure: 15 to 60 days after end of treatment.
5.4. Analysis.
Comparison 5 Other antiamoebic drug comparisons, Outcome 4 Adverse events.
5.2. Analysis.
Comparison 5 Other antiamoebic drug comparisons, Outcome 2 Parasitological failure: 1 to 14 days after end of treatment.
Researchers reported no data on relapse.
One trial reported that the higher dosage regimen of MK‐910 resulted in nausea, vomiting, and abdominal pain severe enough to require withdrawal from treatment for two participants (Batra 1972). Gastrointestinal adverse effects occurred more frequently in the secnidazole group than in the quinfamide group (Padilla 2000). Mild vomiting occurred in one participant given ornidazole, but none occurred among those given tinidazole (Panggabean 1980). Those given a fixed‐drug combination of metronidazole and diloxanide furoate had significantly greater gastrointestinal adverse effects compared with those given the herbal product (Siddiqui 2015). One trial reported no difference in adverse effects between those given quinfamide or teclozan (Guevara 1980). Three trials mentioned that participants reported no side effects but provided no further details (Toppare 1994; Davila 2002; Savas‐Erdeve 2009). One trial reported that 57.4% of those given metronidazole developed mild side effects, including nausea and vomiting, but did not report any adverse effects of the herbal drug (Shah 2016). Two trials reported only on specific adverse events ‐ not on the number of participants with adverse events (Nnochiri 1967; Batra 1972); and three trials did not report on clinical adverse effects (Kapadia 1968; Sitepu 1982; Mansour‐Ghanaei 2003). See Appendix 12 for details.
Funnel plot
We constructed a funnel plot with 10 trials for one outcome measure and examined it visually for possible bias or heterogeneity: any antiamoebic drug versus metronidazole and measuring parasitological failure 15 to 60 days after end of treatment (13 trials; Figure 6). This included nine trials that compared tinidazole with metronidazole. Asymmetry in the funnel plot may indicate the presence of publication bias but may also indicate inadequate trial methodological quality or heterogeneity resulting from differences in study populations, interventions, outcome measurements, and trial design.
6.
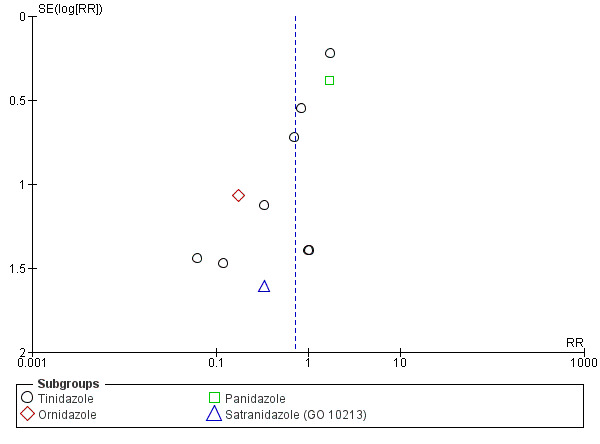
Funnel plot. Alternative drug versus metronidazole: parasitological failure 15 to 60 days after end of treatment.
Discussion
Tinidazole versus metronidazole
In patients with amoebic colitis, treatment with tinidazole reduced clinical failure by 72% compared with treatment with metronidazole for outcomes evaluated 15 to 60 days after end of treatment and may be as effective as metronidazole in eradicating Entamoeba histolytica from stools. The incidence of mild to moderate gastrointestinal complaints also appeared to be lower among those given tinidazole. These results must be interpreted with caution because most trials are old (8 of the 10 trials were conducted between 1974 and 1978), the overall certainty of trial evidence is very low, and standardization in enrolment, diagnosis, treatment, and outcome assessment is lacking. None of the trials used E histolytica antigen detection or culture for diagnosis, and none determined the presence of infection with other pathogenic organisms, so uncertainty surrounds the diagnosis of amoebic colitis and the decision of whether clinical symptoms are due to amoebic colitis alone. Differences in clinical responses could also be due to lack of standardization of dosage, interval, and duration of drug treatments given. Other studies have shown that tinidazole is better when given as a single dose than in divided doses because of its longer half‐life of approximately 12 to 14 hours, resulting in longer concentrations in the body (Monro 1974; Looke 1987), whereas metronidazole has a shorter half‐life of about 6 to 10 hours and is better given in divided doses. Also, longer courses may lead to re‐excretion through the bile, resulting in higher concentrations within the bowel lumen (Tracy 2001). This is supported by the summary report of nine trials conducted in India, which reported that tinidazole given as a single dose daily was more effective than divided doses, and was more effective and was associated with fewer gastrointestinal adverse events when compared with metronidazole given once daily (Bakshi 1978).
The risk difference for clinical failure among those given tinidazole and those given metronidazole is 0.16, yielding a number needed to treat for an additional beneficial outcome (NNTB) of 6.25. Thus, seven people will have to be treated with tinidazole for clinical failure to be reduced in one more individual. However, this finding cannot be applied to parasitological failure, as no significant difference in eradication of E histolytica is apparent between those given tinidazole and those given metronidazole.
Other nitroimidazole drugs versus metronidazole
Ornidazole and secnidazole are promising alternative antiamoebic drugs because they share the same mechanism of action as metronidazole against amoebae but remain longer in the blood. Compared with metronidazole, ornidazole remains in the blood around 1.7 times longer (with half‐life ranging from 11 to 14 hours), and secnidazole remains in the blood around three times longer (with half‐life ranging from 17 to 28 hours) (Lamp 1999). This review shows that evidence is insufficient at the moment to demonstrate advantage of these drugs over metronidazole for treating individuals with amoebic colitis. More high‐quality trials in larger populations will be needed to determine whether or not these other nitroimidazole agents will be significantly more effective than metronidazole in reducing clinical signs of amoebiasis and in preventing persistence of amoebae in the stools.
Antiamoebic drugs versus placebo
The general recommendation is to give antiamoebic treatment to all individuals with definitive E histolytica infection, including those who have no symptoms of disease (WHO 1997; The Medical Letter 2013; AAP 2015). Approximately 3% to 10% of infected individuals may develop symptoms of invasive amoebiasis if left untreated (Haque 2001; Haque 2002; Blessman 2003). However, it is not known who among these asymptomatic individuals with E histolytica infection will develop symptomatic disease. Therefore, unless the diagnosis of E histolytica infection is uncertain for an asymptomatic individual, use of placebo as a comparison drug, particularly in patients with symptoms of invasive disease, is not appropriate. This review shows that antiamoebic drugs were more effective than placebo in reducing clinical symptoms of amoebic colitis and in eradicating E histolytica from the stools, although trials were of very low quality and heterogeneity was significant. Heterogeneity could be attributed to differences in participant characteristics or to the varied antiamoebic drugs used. The disappearance of parasites in 50 out of 133 (38%) individuals taking placebo may be explained by spontaneous eradication of E histolytica or infection with non‐pathogenic amoebae. Studies have shown that up to 90% of individuals with untreated E histolytica infection spontaneously clear their infection within one year (Gathiram 1987; Haque 2001; Haque 2002; Blessman 2003; Stanley 2003; Choudhuri 2012). It may also be possible that patients were actually infected with non‐pathogenic amoebae because stool microscopy was the only diagnostic test utilized.
Combination regimen versus metronidazole alone
For all forms of invasive disease, including amoebic colitis, the standard recommendation is to give a tissue amoebicide followed by a luminal amoebicide to eliminate surviving cysts in the bowel lumen (WHO 1995; WHO 1997; The Medical Letter 2013; AAP 2015). Compared with metronidazole alone, combination therapy resulted in a reduction of about 60% for both clinical and parasitological failure. The advantage of combination therapy is attributed to the distinct activities of different drugs against cysts and trophozoites found at the different sites (WHO 1995; Tracy 2001; The Medical Letter 2013). This was consistent with the greater effect of combination therapy for those with unspecified intestinal amoebiasis when both invasive and cyst forms could be present compared with individuals with amoebic dysentery alone. However, interpretation of these results is complicated because trials used different combinations of drugs in comparison with metronidazole: fixed‐drug combination of diiodohydroxyquinoline and metronidazole (Asrani 1995); fixed‐drug combination suspension of furazolidone and metronidazole (Prasad 1985); and combination of subcutaneous dehydroemetine plus oral tetracycline and diloxanide furoate (Rubidge 1970). No conclusions can be drawn regarding the most effective combination antiamoebic drug regimen because none of the included trials were of sufficient size to reveal this. Some of these drugs are no longer marketed, and it is not known whether these results could be applied to other combinations. It is also not known whether combination therapy would lead to increased adverse events, because this information was incompletely reported.
Single‐dose regimen compared with longer‐duration or other single‐dose regimens
The advantages of single‐dose regimens are numerous, including ease of administration, convenience, better patient compliance, and reduced cost with no evidence of increased adverse effects. Two antiamoebic drugs ‐ secnidazole and quinfamide ‐ were evaluated as single‐dose therapy: Results were inconclusive owing to the small sample size and the low methodological quality of trials. More trials are needed to determine the clinical and parasitological efficacy of single‐dose regimens of secnidazole or quinfamide and of other antiamoebic drugs that can be given for a shorter duration than other drugs, including the current standard antiamoebic drug, metronidazole.
Other antiamoebic drug comparisons
Available data are insufficient to establish the efficacy and safety of the other antiamoebic drugs for treating amoebic colitis. More recently, interest in the effect of non‐traditional therapy against amoebiasis has been increasing. Two trials evaluated the effect of adding Saccharomyces boulardii, a probiotic fungal organism, to metronidazole therapy. Probiotics are live micro‐organisms that confer a health benefit on the host, including prevention and treatment of diarrhoea. Reviews on the efficacy of probiotics support clinical benefit in preventing Clostridium difficile‐associated diarrhoea (Goldenberg 2013), as well as in reducing the duration and severity of acute infectious diarrhoea in children (Allen 2010). In general, this beneficial effect has been shown to be dose‐dependent and strain‐dependent. Probiotics may have the potential to restore the normal gut flora, although the exact mechanism of the antiamoebic effect of S boulardii remains to be elucidated. Two studies included in this review reported conflicting results: In one study, the addition of S boulardii to the combination of metronidazole and iodoquinol reduced stool frequency and duration of illness in adults with acute amoebic colitis (Mansour‐Ghanaei 2003), whereas the second study, which enrolled children, did not show a significant decrease in symptoms nor in eradication of amoebae from stools when S boulardii was added to metronidazole (Savas‐Erdeve 2009). Two other studies evaluated the effects of herbal products and suggested that herbal products may be as effective as or superior to conventional antiamoebic therapy with fewer adverse effects (Siddiqui 2015; Shah 2016). Potential use of probiotics or herbal products in combination with antiamoebic drugs includes situations in which single‐drug therapy does not result in satisfactory clinical and parasitological cure rates, additional antiamoebic drugs such as luminal antiamoebic drugs are warranted but are not available, and adverse reactions to additional or higher doses of antiamoebic drugs may arise. Further studies are needed to determine the role of these natural products in treating people with amoebic colitis.
Summary of main results
This review shows that for individuals with amoebic colitis, tinidazole may be better in reducing clinical symptoms and may result in fewer adverse events compared with metronidazole, but we do not know if it will be more effective in eradicating amoebae from the stools. Combination drug therapy may be more effective than metronidazole alone for eradicating amoebae, but we do not know which drug combination will be most effective, and if this will lead to more rapid resolution of clinical symptoms or to an increase in adverse events. Evidence is insufficient to allow conclusions regarding the efficacy of other antiamoebic drugs. Two trials comparing ornidazole versus metronidazole evaluated relapse and showed higher occurrence of relapse among those given ornidazole, but we are uncertain about this result. Randomized controlled trials of better quality and using standardized outcomes are needed to evaluate the efficacy of drugs for treating patients with amoebic colitis.
Overall completeness and applicability of evidence
This review was limited to symptomatic individuals with uncomplicated amoebic colitis. The effects of antiamoebic drugs on those with severe amoebic colitis, complicated disease, or extraintestinal amoebiasis were not studied. The potential effect of malnutrition, immune suppression, or AIDS on treatment is not known. Studies have demonstrated that severity of disease outcomes following E histolytica infection are determined by host susceptibility, which can be dependent on genetic factors or on environmental factors, such as malnutrition, and therefore may vary among different populations and geographical locations (Morfl 2012). Although asymptomatic infection with E histolytica is more common than symptomatic disease, treatment of these individuals remains controversial because most will clear their infection within one year, and only about 3% to 10% will manifest invasive disease (Gathiram 1987; Haque 2001; Haque 2002; Blessman 2003).
The limited availability of many antiamoebic drugs must be addressed in the light of reports that newer nitroimidazole drugs may be as effective as, and better tolerated than, metronidazole, and that clinical and parasitological failures may be fewer when luminal agents are given in conjunction with tissue amoebicides. Metronidazole is widely used and may be the only available antiamoebic drug in many countries. Tinidazole and the other nitroimidazole drugs, such as ornidazole and secnidazole, and luminal agents, such as diloxanide furoate, iodoquinol, and paromomycin, are not widely available and may be purchased only from certain pharmaceutical companies or requested from government agencies. Although tinidazole was shown in this review as probably more effective and better tolerated than metronidazole, the limitations of currently available evidence and the limited availability of tinidazole in many regions would make a widespread recommendation for its use impractical. Similarly, evidence by which combination therapy can be recommended is inadequate, and the limited availability of luminal agents in the market poses a major deterrent to compliance with the recommendation for combination therapy.
Certainty of the evidence
We used the GRADE approach in assessing the certainty of trial evidence. Limitations in study quality, imprecise or sparse data for some outcomes, important inconsistencies across trials, and a high probability of reporting or publication bias decrease the certainty of evidence. Therefore the conclusions of this review should be interpreted with caution. More than half of the included studies were conducted before 1990, and the very low quality of trials included for primary outcomes implies uncertainty in the results. Inaccurate diagnosis of E histolytica infection by stool microscopy, absence of standardized classification of the various categories of amoebic colitis (particularly non‐dysenteric amoebic colitis), and variable timing and definitions of outcome measurements would lead to inaccuracy in assessing treatment effects. In areas highly endemic for amoebiasis, true treatment failure or relapse would be difficult to differentiate from re‐infection without the benefit of finger typing or genotyping. Incomplete reporting may lead to an inaccurate assessment of adverse events.
Potential biases in the review process
This systematic review included data from a large number of small, randomized, low‐quality trials comparing all eligible treatments, making it difficult to draw an overall conclusion about the best treatment for amoebic colitis. Asymmetry in the funnel plot for an outcome with a sufficient number of studies indicates the presence of publication bias, as well as possible overestimation of intervention effects in smaller trials of poor methodological quality.
An advanced approach to meta‐analysis of multiple treatments, such as a network meta‐analysis, may be conducted in the future to incorporate information from a combination of all relevant direct and indirect treatment comparisons and to generate a ranking scheme of different drugs according to best treatment outcomes (Caldwell 2005; Catalla‐Lopez 2014).
Agreements and disagreements with other studies or reviews
A systematic review published in Clinical Evidence summarized the effects of different drug treatments for amoebic dysentery in endemic areas (Dans 2006). This systematic review included 12 randomized controlled trials and concluded that ornidazole, secnidazole, and tinidazole were likely to be beneficial in treating amoebic dysentery, but that metronidazole was unlikely to be beneficial. Trial results were not combined, and no formal statistical methods were performed to determine summary measures of drug effectiveness. Updates to the Clinical Evidence review ‐ Mackey‐Lawrence 2011 and Marie 2013 ‐ mainly summarized findings from the previous version of this current Cochrane review on antiamoebic drugs and performed GRADE evaluation of the certainty of evidence for applied interventions (Gonzales 2009). Authors of the Clinical Evidence reviews recognized the generally poor quality of the included trials, largely due to methodological flaws and limitations of diagnostic tests for amoebic infection.
An earlier systematic review on amoebic dysentery published in Clinical Evidence concluded that metronidazole was "unlikely to be beneficial" in that some trials demonstrated ineffectiveness or associated harm, and that ornidazole, secnidazole, and tinidazole were "likely to be beneficial" because other trials demonstrated effectiveness of these drugs with no increased harm (Dans 2006). This review used the Clinical Evidence search strategy and included 12 randomized controlled trials, defined therapeutic failure as persistence of symptoms or persistence of parasites or both, analysed outcomes reported together for different time points, and did not pool data to generate an overall summary measure. Subsequent updates of this Clinical Evidence review ‐ Mackey‐Lawrence 2011 and Marie 2013 ‐ mainly summarized findings of the previous version of the earlier published Cochrane review on antiamoebic drugs and included a GRADE evaluation of the certainty of evidence for interventions (Gonzales 2009). Review authors concluded that compared with placebo, ornidazole may be more effective in clearing parasites, and that secnidazole, tinidazole, and metronidazole may be as effective as ornidazole in curing amoebic dysentery. They also concluded that metronidazole may be less effective than tinidazole in reducing clinical symptoms but may be as effective in clearing parasites. For the other antiamoebic drugs, nitazoxanide was found more effective than placebo for reducing clinical failure but not for preventing parasitological failure. As described in this Cochrane review, the authors of the Clinical Evidence review recognized the generally poor quality of trials included in the systematic review, largely due to methodological flaws such as lack of blinding, sparse data, and lack of directness due to uncertainty of the diagnosis of amoebic dysentery.
We have made no changes to the conclusions of this updated version of the earlier review (Gonzales 2009). We conclude that although tinidazole may be more effective than metronidazole in reducing clinical failure and was probably associated with fewer adverse effects, it did not show any significant advantage over metronidazole in reducing parasitological failure. Data were also insufficient to show the efficacy of other antiamoebic drugs compared with metronidazole or other drugs. Compared with metronidazole, combination therapy may result in fewer parasitological failures, although the optimal combination of antiamoebic drugs cannot be determined by this review. More high‐quality trials including sufficient numbers of participants and using more accurate diagnostic tests are needed to determine the most effective antiamoebic drug or combination of drugs for treating amoebic colitis.
Authors' conclusions
Implications for practice.
Antiamoebic drugs are indicated for treating individuals with amoebic colitis. Metronidazole has been the standard therapy for treating amoebic colitis owing to its history of long use and availability. Compared with metronidazole, tinidazole may be more effective in reducing clinical failure and probably has fewer adverse effects, but evidence is insufficient to show whether it is more effective in eradicating amoebic parasites from the stools. Combination drug therapy may be more effective than metronidazole alone in reducing parasitological failure, but data are insufficient for recommendation of a specific combination or to show whether this will lead to more rapid resolution of clinical symptoms or to increased adverse effects. Trials were generally inadequate or unclear in the key components measuring methodological quality, and most used stool microscopy alone for diagnosis and evaluation of parasitological outcomes. Thus, evidence is insufficient for review authors to be certain about study results. No definitive conclusions can be drawn regarding the efficacy of other antiamoebic drugs when compared with metronidazole or other drugs. Many antiamoebic drugs are not available in all countries; therefore, the choice of antiamoebic drugs for treatment would depend largely on availability of, and accessibility to, drugs for treatment.
Implications for research.
More randomized controlled trials on the efficacy of drugs for treating amoebic colitis, reporting better methodological quality and using standardized definitions for evaluating outcomes, are needed. The diagnosis of amoebic colitis should not rely solely on stool microscopy but should be confirmed by a reliable test that differentiates E histolytica from non‐pathogenic amoebae. The most cost‐effective and accurate diagnostic test that can be used in LMICs must be identified. Investigations on possible interactions of other intestinal pathogens affecting treatment response for E histolytica are needed, especially in areas where mixed infections along with other intestinal pathogens and helminths are common. Randomized controlled trials are also needed to determine which luminal agent would be most effective when used in conjunction with metronidazole, or another nitroimidazole, for eradicating E histolytica from the intestine and for decreasing relapse. Finally, additional trials are needed to compare single‐dose or shorter regimens versus multiple‐dose or longer duration of therapy. A network meta‐analysis to compare multiple treatments may reveal the best treatment for all or for a subgroup of patients with amoebic colitis.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2019 | New citation required but conclusions have not changed | Four new trials met the inclusion criteria. We assessed the certainty of the evidence using the GRADE approach. |
| 7 January 2019 | New search has been performed | This is an update of a review published in 2009. We included four new trials to the previously published review version |
Acknowledgements
The Academic Editor of this review is Dr Hellen Gelband.
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of LMICs (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
We thank Professor Paul Garner and Dr David Sinclair for their input. We are grateful to Dr Vittoria Lutje (Information Specialist) for developing the search strategy and searching for studies, and Christianne Esparza for retrieving copies of published studies. We thank Anne‐Marie Stephani (former CIDG Managing Editor) and Philomena Hinds (Editorial Assistant and Administrator) for valuable logistical support provided during preparation and completion of Gonzales 2009.
We are grateful to Dr Elizabeth G Martinez, who, in the initial published version of this review, extracted data from the included trials and served as third review author to resolve differences in the assessment of papers between the other two review authors. Dr Martinez and Dr Leonila F Dans conducted the systematic review on drug therapy for amoebic dysentery (Dans 2006), which set the groundwork for this Cochrane Review.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | Embaseb | LILACSb |
| 1 | amoeb* | amoeb* | amoebiasis | amoebiasis | amoeb* |
| 2 | Entamoeba | Entamoeba histolytica | DYSENTERY, AMEBIC/DRUG THERAPY | NITROIMIDAZOLE‐DERIVATIVE | Entamoeba |
| 3 | 1 or 2 | 1 or 2 | 1 OR 2 | EMETINE | 1 or 2 |
| 4 | nitroimidazoles | amoebicides | AMEBICIDES/THERAPEUTIC USE | DILOXANIDE FUROATE | nitroimidazoles |
| 5 | emetine | NITROIMIDAZOLES | NITROIMIDAZOLES | carbarsone | emetine |
| 6 | diloxanide furoate | emetine | EMETINE | acetarsone | diloxanide furoate |
| 7 | quinfamide | diloxanide furoate | carbarsone | acetarsol | quinfamide |
| 8 | etofamide | quinfamide | acetarsone | diphetarsone | etofamide |
| 9 | etophamide | etofamide | acetarsol | glycobiarsol | etophamide |
| 10 | HYDROXYQUINOLINES | etophamide | diphetarsone | stovarsol | HYDROXYQUINOLINES |
| 11 | chloroquine | HYDROXYQUINOLINES | glycobiarsol | thioarsenite | chloroquine |
| 12 | tetracycline | ARSENICALS | stovarsol | diloxanide furoate | tetracycline |
| 13 | erythromycin | chloroquine | thioarsenite | quinfamide | erythromycin |
| 14 | niridazole | tetracycline | diloxanide furoate | etofamide | niridazole |
| 15 | nitazoxanide | oxytetracycline | quinfamide | etophamide | nitazoxanide |
| 16 | 4‐15/OR | chlortetracycline | etofamide | chiniofon | 4‐15/OR |
| 17 | 3 AND 16 | erythromycin | etophamide | clioquinol | 3 AND 16 |
| 18 | — | niridazole | HYDROXYQUINOLINES | dichloroacetamide | — |
| 19 | — | nitazoxanide | chiniofon | chlorbetamide | — |
| 20 | — | 4‐19/OR | clioquinol | chlorphenoxamide | — |
| 21 | — | 3 AND 20 | dichloroacetamide | chloroquine | — |
| 22 | — | — | chlorbetamide | tetracycline | — |
| 23 | — | — | chlorphenoxamide | erythromycin | — |
| 24 | — | — | chloroquine | oxytetracycline | — |
| 25 | — | — | tetracycline | chlortetracycline | — |
| 26 | — | — | erythromycin | niridazole | — |
| 27 | — | — | oxytetracycline | nitazoxanide | — |
| 28 | — | — | chlortetracycline | nimorazole | — |
| 29 | — | — | niridazole | nitrimidazine | — |
| 30 | — | — | nitazoxanide | 2‐29/OR | — |
| 31 | — | — | nimorazole | 1 AND 30 | — |
| 32 | — | — | nitrimidazine | Limit 31 to human | — |
| 33 | — | — | 4‐32/OR | — | — |
| 34 | — | — | 3 AND 33 | — | — |
| 35 | — | — | Limit 34 to human | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by Cochrane (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Search methods: conference proceedings searched
| Conference proceedings | Date and location of conference |
| Annual Meeting of the American Society of Tropical Medicine and Hygiene | 52nd: 3‐7 December 2003, Philadelphia, PA, USA
53rd: 7‐11 November 2004, Florida, USA
54th: 11‐15 December 2005, Washington, DC, USA
55th: 12‐16 November 2006, Atlanta, GA, USA 57th: 7‐11 December 2008, New Orleans, LA, USA 58th: 18‐22 November 2009, Washington, DC, USA 59th: 3‐7 November 2010, Atlanta, GA, USA 60th: 4‐8 December 2011, Philadelphia, PA, USA 62nd: 13‐17 November 2013, Washington, DC, USA 63rd: 2‐6 November 2014, New Orleans, LA, USA 64th: 25‐29 October 2015, Philadelphia, PA, USA 65th: 13‐17 November 2016, Atlanta, GA, USA 66th: 5‐9 November 2017, Baltimore, MD, USA |
| Annual Scientific Conference (ASCON) of the ICCDRB | 11th: 4‐6 March 2007, ICDDRB, Dhaka, Bangladesh 12th: 9‐12 February 2009, ICDDRB, Dhaka,Bangladesh 13th: 14‐17 March 2011, ICDDRB, Dhaka, Bangladesh |
| Asian Conference on Diarrheal Disease and Nutrition | 13th: 10 to 12 January 2012, Tagaytay City, Philippines |
| Asian Congress of Pediatric Infectious Diseases | 4th (in conjunction with 14th Indonesian Congress of Pediatrics, Konika): 5‐9 July 2008, Surabaya, Indonesia 5th: 23‐26 September 2010, Taipei, Taiwan 6th: 28 November‐01 December 2012, Colombo, Sri Lanka 7th: 12‐15 October 2014, Beijing, China 8th: 8‐10 November 2016, Bangkok, Thailand |
| ASM Microbe (starting in 2016, American Society for Microbiology General Meeting and ICAAC were combined into one meeting ‐ "ASM Microbe") | ASM 2017/ICAAC 2017: 1‐5 June 2017, New Orleans, LA, USA |
| Commonwealth Association of Paediatric Gastroenterology & Nutrition (CAPGAN) Commonwealth Congress on Diarrhoea and Malnutrition | 7th (part of 2nd World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition): 3‐7 July 2004, Paris, France
8th: 6‐8 February 2006, International Centre for Diarrhoeal Diseases Research in Bangladesh (ICCDRB), Dhaka, Bangladesh 10th: 12‐16 August 2009, Blantyre, Malawi 11th: 21‐23 July 2011, London, United Kingdom 14th: 2‐4 October 2015, New Delhi, India |
| European Congress of Clinical Microbiology and Infectious Diseases | 15th: 2‐5 April 2005, Copenhagen, Denmark
16th: 1‐4 April 2006, Nice, France
17th (joint conference with 25th International Congress of Chemotherapy): 31 March‐3 April 2007, Munich, Germany 18th: 19–22 April 2008, Barcelona, Spain 19th: 17‐19 May 2009, Helsinki, Finland 20th: 10‐13 April 2010, Vienna, Austria 21st: 7‐10 May 2011, Milan, Italy 22nd: 31 March‐03 April 2012; London, United Kingdom 23rd: 27‐30 April 2013, Berlin, Germany 24th: 10‐13 May 2014, Barcelona, Spain 25th: 25‐28 April 2015, Copenhagen, Denmark 26th: 9‐12 April 2016, Amsterdam, Netherlands 27th: 22‐25 April 2017, Vienna, Austria |
| European Congress on Tropical Medicine and International Health | 5th: 24‐28 May 2007, Amsterdam, the Netherlands (Workshop on Amoebiasis, Side Meeting, 24 to 25 May 2007) 6th: 6‐10 September 2009, Verona, Italy 7th: 3‐6 October 2011, Barcelona, Spain 8th: 10‐13 September 2013, Copenhagen, Denmark 9th: 6‐10 September 2015, Basel, Switzerland 10th: 16‐20 October 2017, Antwerp, Belgium |
| European Society for Paediatric Infectious Diseases Annual Meeting | 25th: 2‐4 May 2007, Porto, Portugal 26th: 13‐17 May 2008. Graz, Austria 27th: 9‐13 June 2009, Brussels, Belgium 28th: 4‐8 May 2010, Nice, France 29th: 7‐11 June 2011, The Hague, The Netherlands 30th: 8‐12 May 2012, Thessaloniki, Greece 32nd: 12‐15 May 2014, Dublin, Ireland 33rd: 12‐16 May 2015, Leipzig, Germany 34th: 10‐14 April 2016, Brighton, United Kingdom 35th: 23‐27 May 2017, Madrid, Spain |
| ID Week Meeting (Joint Conference of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society) | 1st: 17‐20 October 2012, San Diego, CA, USA 2nd: 2‐6 October 2013, San Francisco, CA, USA 3rd: 8‐12 October 2014, Philadelphia, PA, USA 4th: 7‐11 October 2015, San Diego, CA, USA 5th: 26‐30 October 2016, New Orleans, LA, USA 6th: 4‐8 October 2015, San Diego, CA, USA |
| Infectious Disease Society of America Annual Meeting | 47th: 29 October‐1 November 2009, Philadelphia, PA, USA 48th: 21‐24 October 2010, Vancouver, BC, Canada 49th: 20‐23 October 2011, Boston, MA, USA (last meeting as IDSA Annual Meeting, changed to ID week from 2012 onwards) |
| International Congress of Chemotherapy | 24th: 4‐6 June 2005, Manila, Philippines
25th (Joint Conference With 17th European Congress of Clinical Microbiology and Infectious Diseases): 31 March to 3 April 2007, Munich, Germany 26th: 18‐21 June 2009, Toronto, ON, Canada 27th (held in conjunction with the 21st European Congress of Clinical Microbiology and Infectious Diseases): 7‐10 May 2011, Milan, Italy 28th: 5‐8 June 2013, Yokohama, Japan 29th (Joint With the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy), 17 to 21 September 2015, San Diego, CA, USA 30th:4‐7 November 2017, Taipei, Taiwan |
| International Congress on Infectious Diseases | 11th: 4‐7 March 2004, Cancun, Mexico
12th: 15‐18 June 2006, Lisbon, Portugal 13th: 19‐22 June 2008, Kuala Lumpur, Malaysia 14th: 9‐12 March 2010 Miami, FL, USA 15th: 13‐16 June 2012, Bangkok, Thailand 16th: 2‐5 April 2014, Capetown, South Africa 17th: 2‐5 March 2016, Hyderabad, India |
| International Society for Infectious Diseases‐Neglected Tropical Diseases Meeting | 1st: 8‐10 July 2011, Boston, MA, USA |
| Interscience Conference on Antimicrobial Agents and Chemotherapy | 44th: 30 October‐2 November 2004, Washington, DC, USA
45th: 16‐19 December 2005, Washington, DC, USA
46th: 27‐30 September 2006, San Francisco, CA, USA 48th (Joint Conference With 46th Annual Meeting of the Infectious Diseases Society of America): 25‐28 October 2008, Washington, DC, USA 49th: 12‐15 September 2009, San Francisco, CA, USA 50th: 12‐15 September 2010, Boston. MA, USA 51st: 17‐20 September 2011, Chicago, IL, California, USA 52nd: 9‐12 September 2012, San Francisco, CA, USA 53rd: 10‐13 September 2013, Denver, CO, USA 55th (Joint With the 28th International Congress of Chemotherapy Meeting): 17‐21 September 2015, San Diego, CA, USA 56th (starting in 2016, General Meeting and ICAAC were combined into 1 meeting ‐ "ASM Microbe": 16‐20 June 2016, Boston, MA, USA |
| Seminars in Amebiasis | 14th: 27‐30 November 2000, Mexico City, Mexico EMBO Global Lecture Course and Symposium on Amebiasis: 4‐7 March, 2012, Khajuraho, India |
Appendix 3. Search methods: organizations or institutions contacted for trials on amoebic colitis
| Organization | Date contacted |
| Department of Parasitology, College of Public Health, University of the Philippines, Manila, Philippines | 5 July 2005; 3 September 2012; 01 February 2018 |
| Tropical Medicine, Mahidol University, Bangkok, Thailand | 7 July 2005; 4 September 2012; 01 February 2018 |
| National Institute of Health, Manila, Philippines | 22 July 2005; 3 September 2012; 01 February 2018 |
| South East Asian Ministers Education Organization (SEAMEO) TROPMED Network | 27 July 2005; 4 September 2012; 01 February 2018 |
| Research Institute for Tropical Medicine, Alabang, Muntinglupa, Philippines | 5 September 2006; 10 August 2012; 01 February 2018 |
| Waterborne and Parasitic Diseases, World Health Organization Regional Office for the Western Pacific, Manila, Philippines (now Malaria, Vector‐borne and Parasitic Diseases, World Health Organization Regional Office for the Western Pacific, Manila, Philippines) |
5 September 2006; 6 September 2012 |
| Communicable Disease Research, Eastern Mediterranean Regional Office, World Health Organization | 23 August 2012 |
| National Institute of Cholera and Enteric Diseases, Calcutta, India | 24 September 2006; 14 August 2012; 01 February 2018 |
| South African Medical Research Council, South Africa | 17 October 2006; 14 August 2012; 01 February 2018 |
| Department of Medicine, University of Minnesota, Minneapolis,MN, USA | 5 June 2006; 16 January 2008 |
| International Centre for Diarrhoeal Diseases Research in Bangladesh (ICCDRB), Dhaka, Bangladesh | 7 July 2005; 3 February 2008; 21 August 2012 |
| Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, England | 1 February 2008; 10 August 2012; 01 February 2018 |
| University of Guanajuato, Celaya, Mexico | 3 February 2008; 01 February 2018 |
| Laboratory of Parasitic Diseases, NIAID, National Institutes of Health, Bethesda, MD, USA | 3 February 2008; 01 February 2018 |
| Department of Medicine, Washington University School of Medicine, St. Louis, MN, USA | 3 February 2008; 01 February 2018 |
| Department of Infectious Diseases, Tokai University School of Medicine, Bohseidai, Isehara, Kanagawa, Japan | 3 February 2008; 01 February 2018 |
| Division of Infectious Diseases and International Health, University of Virginia Health System, VA, USA | 10 August 2012; 01 February 2018 |
| Department of Biotechnology, Indian Institute of Technology, Roorkee, India | 5 February 2008; 01 February 2018 |
| Department of Pathology, Center for Discovery and Innovation in Parasitic Diseases, University of California, San Francisco, CA, USA | 11 August 2012; 01 February 2018 |
| Infectious Diseases, Departments of Medicine Microbiology and Immunology, Stanford University, Stanford, CA, USA | 6 February 2008; 01 February 2018 |
| Department of Molecular Biology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany | 11 February 2008; 10 August 2012; 01 February 2018 |
| Microbiology Laboratory, University of California San Diego Medical Center, San Diego, CA, USA | 17 August 2012; 01 February 2018 |
| Department of Experimental Medicine, National Autonomous University of Mexico, Mexico City, Mexico | 15 August 2012; 01 February 2018 |
Appendix 4. Search methods: pharmaceutical companies
| Company | Relevant drug(s)a | Date(s) contacted/database searched |
| Abbott India Ltd, Mumbai, India | Diloxanide furoate (Furamide); Ornidazole (ZIL) DIloxanide plus metronidazole (Entamizole) | 4 September 2012; 30 December 2014; 01 February 2018 (no results found for diloxanide furoate (Furamide); Ornidazole (ZIL); dIloxanide plus metronidazole (Entamizole)) |
| Abbott Laboratories (Pakistan) Limited | Diloxanide plus metronidazole (Entamizole) | 30 December 2014; 01 February 2018 (no results found for diloxanide plus metronidazole (Entamizole)) |
| AHPL (Astamed Healthcare Pvt Ltd) | Secnidazole (Secnil, Secnil Forte) | 4 September 2012; 30 December 2014; 01 February 2018 (no results found for secnidazole) |
| Boots Company Pharmaceuticals | Diloxanide furoate (Furamide) | 22 September 2006; 01 February 2018 (no results found for diloxanide furoate (Furamide)) |
| CIBA Pharmaceutical Company (merged with Sandoz to form Novartis) | Niridazole (Ambilhar) | 22 September 2006; 3 February 2008 |
| Glenmark Pharmaceuticals Ltd (Majesta) | Nitazoxanide (Nitazet) | 4 September 2012; 31 December 2014; 01 February 2018 |
| Glenwood LLC | Iodoquinol (Yodoxin) | 22 September 2006; 3 February 2008; 4 September 2012; 31 December 2014 |
| Hoffmann‐La Roche & Co Ltd | Oral and injectable dehydroemetine | 22 September 2006; Yodoxin discontinued 1 December 2014 |
| International Federation of Pharmaceutical Manufacturers and Associationb | — | 3 June 2006; 22 September 2006; 3 February 2008; 4 September 2012; 01 February 2018h |
| King Pharmaceuticals, Inc (now part of Pfizer) | Paromomycin (Humatin) | 31 May 2006; 3 February 2008; 4 September 2012 |
| Lupin Laboratories Ltd (Pinnacle) | Nitazoxanide (Nizonide) | 4 September 2012; 31 December 2014; 01 February 2018 |
| Medopharm | Ornidazole (Orizole) | 4 September 2012; 31 December 2014; 01 February 2018 |
| Mission Pharmacal Company | Tinidazole (Tindamax) | 4 September 2012; 01 February 2018 |
| Nicholas Piramal India Ltd | Ornidazole (Zil); Secnidazole (Secnil, Secnil Forte) | 30 December 2014; 01 February 2018 |
| Novartis: Clinical Trial Results Databasesc | — | 3 June 2006; 22 September 2006; 3 February 2008; 04 September 2012; 30 December 2014; 01 February 2018h |
| Presutti Laboratories | Tinidazole (Tindamax) ‐ recently divested to Mission Pharmaceutical | 3 June 2006 |
| Pfizerd | Metronidazole (Flagyl) Tinidazole (Fasigyn) Etofamide (Kitnos) Paromomycin (Humatin) Quinfamide (Finalam; Amefin) |
22 September 2006; 3 February 2008; 4 September 2012; 30 December 2014; 01 Febuary 2018 |
| Roche | Ornidazole (Tiberal) – transferred to Laboratoires SERB | 22 September 2006; 01 February 2018 |
| Laboratoires SERB | Ornidazole (Tiberal) | 4 September 2012; 01 February 2018 |
| Roche: Clinical Trial Registry and Results Databasee | — | 3 June 2006; 22 September 2006; 3 February 2008; 4 September 2012; 30 December 2014; 01 February 2018h |
| Romark Laboratories, LCf | Nitazoxanide (Alinia) | 22 September 2006; 3 February 2008; 4 September 2012; 30 December 2014; 01 February 2018 |
| Sandoz (merged with Ciba Geigy to form Novartis) | Metronidazole (Servizol) | 22 September 2006; 3 February 2008 |
| Sanofi Aventisg | Secnidazole (Flagentyl, Secnidal); metronidazole, (Flagyl); quinfamide (Amenox) | 22 September 2006; 3 February 2008; 4 September 2012; 30 December 2014; 01 February 2018 |
| Sanvin Laboratories Pvt Ltd | Quinfamide | 22 September 2006; 4 September 2012; 30 December 2014; 01 February 2018 |
aTrade name in brackets. bwww.ifpma.org/tag/clinical‐trials/ . cwww.novartisclinicaltrials.com. dwww.pfizer.com/science/clinical‐trials ewww.roche‐trials.com (now provided through independent registries such as ClinicalTrials.gov fwww.romark.com/research gwww.sanofi.com/en/science‐and‐innovation/clinical‐trials‐and‐results/ hSearch terms: ‘amoebiasis or amebiasis', ‘amoebic dysentery or amebic dysentery', and ‘amoebic colitis or amebic colitis'.
Appendix 5. Region and country of trial
| Region | Country | Trial(s) |
| Asia | Bangladesh | Awal 1979 |
| India | Kapadia 1968; Batra 1972; Misra 1974; Joshi 1975; Mathur 1976; Misra 1977; Singh 1977; Swami 1977; Misra 1978; Prasad 1985; Tripathi 1986; Asrani 1995 | |
| Indonesia | Naoemar 1973; Pudjiadi 1973; Panggabean 1980; Sitepu 1982; Soedin 1985 | |
| Pakistan | Siddiqui 2015; Shah 2016 | |
| Africa | Egypt | Rossignol 2001; Rossignol 2007 |
| Kenya | Chunge 1989; Pamba 1990 | |
| Nigeria | Nnochiri 1967 | |
| South Africa | Rubidge 1970 | |
| South and Central America | Brazil | Huggins 1982; Salles 1999 |
| Chile | Donckaster 1964 | |
| Colombia | Botero 1974; Botero 1977 | |
| Mexico | Guevara 1980; Padilla 2000; Davila 2002 | |
| Middle East | Iran | Mansour‐Ghanaei 2003 |
| Iraq | Mohammed 1998 | |
| Europe and Euroasia | Sweden | Pehrson 1983; Pehrson 1984 |
| Turkey | Toppare 1994; Karabay 1999: Savas‐Erdeve 2009 |
Appendix 6. Trial setting
| Setting | Trial(s) |
| Hospital | Rubidge 1970; Batra 1972; Pudjiadi 1973; Botero 1974; Misra 1974; Misra 1977; Misra 1978; Awal 1979; Huggins 1982; Pehrson 1983; Tripathi 1986; Pamba 1990; Karabay 1999; Shah 2016 |
| Outpatient clinic | Donckaster 1964; Nnochiri 1967; Naoemar 1973; Singh 1977; Panggabean 1980; Sitepu 1982; Pehrson 1984; Prasad 1985; Soedin 1985; Chunge 1989; Mohammed 1998; Rossignol 2001; Rossignol 2007; Savas‐Erdeve 2009; Siddiqui 2015 |
| Community | Davila 2002 |
| School | Padilla 2000 |
| Not stated | Kapadia 1968; Joshi 1975; Mathur 1976; Botero 1977; Swami 1977; Asrani 1995; Salles 1999; Mansour‐Ghanaei 2003 |
| Other ‐ most participants treated as outpatients, but a few with severe symptoms treated in hospital | Toppare 1994 |
| Other ‐ patients hospitalized for 1 day, then followed up as outpatients | Guevara 1980 |
Appendix 7. Participant age in included trials
| Age | Number of trials | Trial ID |
| Adults only (≥ 15 years) | 17 | Nnochiri 1967; Botero 1974; Joshi 1975; Mathur 1976; Botero 1977; Misra 1977; Singh 1977; Swami 1977; Misra 1978; Guevara 1980; Huggins 1982; Pehrson 1984; Tripathi 1986; Asrani 1995; Mohammed 1998; Karabay 1999; Mansour‐Ghanaei 2003; Shah 2016 |
| Children only (< 15 years) | 11 | Rubidge 1970; Pudjiadi 1973; Panggabean 1980; Sitepu 1982; Prasad 1985; Soedin 1985; Toppare 1994; Salles 1999; Padilla 2000; Davila 2002; Savas‐Erdeve 2009 |
| Adults and children | 11 | Donckaster 1964; Naoemar 1973; Misra 1974; Awal 1979; Pehrson 1983; Chunge 1989; Pamba 1990; Asrani 1995; Rossignol 2001; Rossignol 2007; Siddiqui 2015 |
| Not stated | 2 | Kapadia 1968; Batra 1972 |
Appendix 8. Methods used to diagnose amoebic colitis
| Method | Technique | Number of trialsa | Trials |
| Stool microscopy only | Direct saline wet mount smear | 13 | Kapadia 1968; Joshi 1975c; Mathur 1976; Swami 1977c; Awal 1979c; Guevara 1980c; Prasad 1985; Soedin 1985; Toppare 1994; Asrani 1995; Mohammed 1998; Salles 1999; Davila 2002 |
| Stool microscopy plus | Stained smears (Lugol's iodine, eosin, trichrome stain alone or in combination) | 10 | Nnochiri 1967a; Batra 1972a,c; Naoemar 1973; Pudjiadi 1973; Panggabean 1980; Huggins 1982; Sitepu 1982; Karabay 1999; Savas‐Erdeve 2009; Siddiqui 2015 |
| Formalin or formol‐ether concentration methods | 12 | Donckaster 1964a; Nnochiri 1967a; Botero 1974; Botero 1977; Misra 1977c; Singh 1977c; Misra 1978c; Pehrson 1983; Pehrson 1984; Tripathi 1986a,c; Chunge 1989; Siddiqui 2015a | |
| Zinc sulphate centrifugal flotation technique | 4 | Rubidge 1970; Padilla 2000; Mansour‐Ghanaei 2003; Siddiqui 2015a | |
| Other concentration method (not specified) | 4 | Misra 1974c; Pamba 1990c; Rossignol 2001; Rossignol 2007 | |
| Polvinyl alcohol fixative for detection of trophozoites | 1 | Donckaster 1964 | |
| Stool microscopy plus stool amoebic culture | NIH culture media for xenic cultivation of E histolyticab | 2 | Batra 1972; Tripathi 1986 |
| Stool microscopy plus antibody detection test | — | 1 | Shah 2016 |
| Stool microscopy plus stool antigen‐based ELISA test | — | 1 | Rossignol 2007 |
aCombination of methods in addition to direct stool microscopy: Nnochiri 1967 used iodine‐stained smears and formalin‐ether concentration technique; Donckaster 1964 used the formalin‐ether concentration method for cysts and polvinyl alcohol for trophozoites; Siddiqui 2015 used the zinc sulphate flotation method primarily but also used the formalin‐ether sedimentation method when fatty substances in stools interfered with the zinc sulphate flotation method; Batra 1972 used stool microscopy with saline and iodine smears with stool culture for E histolytica on NIH media. bBatra 1972 and Tripathi 1986 used NIH media to culture for E histolytica in addition to stool microscopy to evaluate parasitological response, but one trial did not use this as an inclusion criterion to enrol participants with amoebic dysentery (Batra 1972). cIn addition to stool examination, rectosigmoidoscopy was performed whenever possible in 11 trials to determine the appearance of the bowel mucosa and the presence of ulcers, but it was not used as a sole criterion for enroling participants or evaluating outcome (Batra 1972; Misra 1974; Joshi 1975; Misra 1977; Singh 1977; Swami 1977; Misra 1978; Awal 1979; Guevara 1980; Tripathi 1986; Pamba 1990).
Appendix 9. Interventions and comparisons included in the trials
| Comparison | A | B | Trial(s) |
| Alternative drug (A) versus metronidazole (B) |
Ornidazole (a nitroimidazole) | Metronidazole | Naoemar 1973; Pudjiadi 1973; Botero 1974 |
| Praziquantel | Metronidazole | Mohammed 1998 | |
| Tinidazole (a nitroimidazole) | Metronidazole | Misra 1974; Joshi 1975; Mathur 1976; Misra 1977; Singh 1977; Swami 1977; Misra 1978; Awal 1979; Pehrson 1984; Chunge 1989 | |
| Secnidazole (a nitroimidazole) | Metronidazole | Karabay 1999 | |
| Panidazole (a nitroimidazole) | Metronidazole | Botero 1977 | |
| Satranidazole (GO 10213) (a nitroimidazole) | Metronidazole | Tripathi 1986 | |
| Any antiamoebic drug (A) versus placebo (B) | Quinfamide (all 3 doses combined) | Placebo | Huggins 1982 |
| Nitazoxanide | Placebo | Rossignol 2001; Rossignol 2007 | |
| 10 different drugs belonging to 6 drug classes (dimethyl chlortetracycline, oxytetracycline, tetracycline, chlorphenoxamide, chlorbetamide, dehydroemetine, diiodohydroxyquinoline, iodohydroxyquinoline, phenanthridinone, bismuth glycoarsanilate) | Placebo | Donckaster 1964 | |
| Combination regimen (A) versus monotherapy (B) |
Dehydroemetine and oral tetracycline and diloxanide furoate | Metronidazole | Rubidge 1970 |
| Metronidazole and diiodohydroxyquinolone | Metronidazole | Asrani 1995 | |
| Metronidazole and furazolidone | Metronidazole | Prasad 1985 | |
| Nimorazole and aminosidine, nimorazole and etofamide, etofamide and aminosidine | Nimorazole or aminosidine or etofamide | Pamba 1990 | |
| Tetracycline and clioquinol | Secnidazole | Soedin 1985 | |
| Quinfamide and mebendazole | Nitazoxanide | Davila 2002a (mixed infections only) | |
| Tinidazole and diloxanide furoate | Tinidazole | Pehrson 1983 | |
| Single‐dose regimen versus longer regimen |
Quinfamide (1 dose) | Quinfamide (2 or 3 doses) | Huggins 1982 |
| Secnidazole (1 dose) | Tetracycline and clioquinol (5 days) | Soedin 1985 | |
| Secnidazole (1 dose) | Tinidazole (2 days) | Salles 1999 | |
| Quinfamide (1 dose) | Nitazoxanide (3 days) | Davila 2002a (Entamoeba infection only) | |
| Secnidazole (1 dose) | Metronidazole (10 days) | Karabay 1999 | |
| Other antiamoebic drug comparisons |
Ornidazole | Tinidazole | Panggabean 1980; Sitepu 1982 |
| Ornidazole | Secnidazole | Toppare 1994 | |
| Chlorhydroxyquinoline | Diiodohydroxyquinoline | Kapadia 1968 | |
| MK‐910 low dose (0.5 mg/kg and 1 mg/kg) | MK‐910 high dose (2 mg/kg and 3 mg/kg) | Batra 1972 | |
| Quinfamide | Secnidazole | Padilla 2000 | |
| Quinfamide | Teclozan | Guevara 1980 | |
| Quinfamide | Nitazoxanide | Davila 2002a (Entamoeba infection only) | |
| Metronidazole and iodoquinol with Saccharomyces boulardii | Metronidazole and iodoquinol with placebo | Mansour‐Ghanaei 2003 | |
| Metronidazole and Saccharomyces boulardii | Metronidazole | Savas‐Erdeve 2009 | |
| Herbal drug | Metronidazole | Shah 2016 | |
| Fixed‐drug combination of metronidazole and diloxanide furoate | Herbal product | Siddiqui 2015 | |
| Fixed‐drug combination of diloxanide furoate and tetracycline with chloroquine | Fixed‐drug combination of diloxanide furoate and tetracycline without chloroquine | Nnochiri 1967 | |
| Not used but mentioned in Description of studies | Quinfamide (3 doses) | Placebo | Huggins 1982b |
| Tinidazole (2 durations) | Metronidazole | Awal 1979c | |
| Tinidazole (2 brands) | Metronidazole (2 brands) | Chunge 1989d |
aDifferent interventions for single and mixed infections. bTrial included in comparison ‘single dose regimen versus longer regimen'. cTrial included in comparison ‘alternative drug versus metronidazole'. dTwo brands of tinidazole compared with two brands of metronidazole and included in comparison ‘alternative drug versus metronidazole'.
Appendix 10. Adverse events: alternative drug versus metronidazole
| Alternative drug | Trial | General/systemic | Gastrointestinal | Dermatological | Central nervous system | Other | Laboratory abnormal | Remarks |
| Tinidazole | Awal 1979 | — | Anorexia, nausea, vomiting, metallic taste in the mouth reported in both groups, but exact numbers not stated | — | Vertigo: metronidazole ‐ 2 participants | — | No abnormalities in complete blood count, serum bilirubin, alkaline phosphatase, and aspartate aminotransferase noted after treatment in both groups | More adverse effects reported in the metronidazole group (14/23, 61%) compared with the tinidazole group (10/43, 23%). All were mild and transient |
| Joshi 1975 | — | — | — | — | — | No abnormalities in complete blood count, urinalysis, serum bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and blood urea noted during and after treatment in both groups | Mild adverse effects such as general malaise, nausea, and vertigo not requiring any treatment or change in drug treatment: metronidazole ‐ 7 participants; tinidazole ‐ 6 participants | |
| Mathur 1976 | — | — | — | — | — | No abnormalities in complete blood count, urinalysis, serum bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and blood urea noted during or after treatment in both groups | Mild adverse effects such as metallic taste, anorexia, nausea, and giddiness, which did not require treatment or discontinuation of drug treatment: 9 participants in each group | |
| Misra 1974 | Malaise: tinidazole (1 participant); metronidazole (0 participants) | Loss of appetite, nausea, and vomiting: tinidazole ‐ 1 participant); Loss of appetite and nausea: tinidazole ‐ 2 participants, metronidazole ‐ 2 participants; Vomiting: metronidazole ‐ 1 participant; Altered taste: tinidazole ‐ 2 participants, metronidazole ‐ 2 participants |
No skin rashes noted in either group | Vertigo: metronidazole ‐ 5 participants, tinidazole ‐ 2 participants Headache: metronidazole ‐ 1 participant; Sleep disturbance: metronidazole ‐ 2 participants |
Blurring of vision and dysuria: metronidazole ‐ 1 participant | No abnormalities seen in complete blood count, urinalysis, serum bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, blood urea, and electrocardiography after treatment in both groups | Tinidazole better tolerated than metronidazole; Tinidazole group: 2 participants developed a total of 8 adverse effects; Metronidazole group: 9 participants developed a total of 17 adverse effects |
|
| Misra 1977 | — | — | — | — | — | No abnormalities seen in complete blood count, urinalysis, serum bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, blood urea, and electrocardiography after treatment in both groups | Significantly more adverse effects reported in participants on metronidazole (16/30, 53.3%) compared with those on tinidazole (8/30, 26.7%) (P < 0.05); 40% of adverse effects in the metronidazole group moderate in intensity, and all side effects in the tinidazole group mild; Most adverse effects were gastrointestinal complaints: nausea, anorexia, vomiting, abdominal discomfort |
|
| Misra 1978 | — | Nausea: tinidazole ‐ 3 participants, metronidazole ‐ 15 participants; Bitter taste: tinidazole ‐ 3 participants, metronidazole ‐ 1 participant; Vomiting: tinidazole ‐ 1 participant; Anorexia: metronidazole ‐ 8 participants; Abdominal pain: metronidazole ‐ 1 participant; Furry tongue: metronidazole ‐ 4 participants; Diarrhoea: metronidazole ‐ 1 participant |
— | — | Dark urine: tinidazole ‐ 2 participants, metronidazole ‐ 2 participants | No abnormalities seen in complete blood count, urinalysis, and blood chemistry after treatment in both groups | Significantly more adverse effects reported in participants on metronidazole (16/30, 53.3%) versus tinidazole (8/29, 27.6.%) (P < 0.01); 40% of adverse effects in the metronidazole group moderate in intensity, and all side effects in the tinidazole group mild |
|
| Pehrson 1984 | — | — | — | — | — | Not monitored | No participant had any adverse effects severe enough to cause cessation of treatment; Specific adverse effects not reported |
|
| Singh 1977 | — | — | — | — | — | No abnormalities seen in complete blood count, urinalysis, alkaline phosphatase, transaminases, and blood urea after treatment in both groups | Adverse effects reported in 14/27 (51.9%) participants in the tinidazole group and in 22/29 (75.9%) participants in the metronidazole group; Adverse effects referable to the gastrointestinal tract consisting of anorexia, nausea, bitter taste, and vomiting; Adverse effects mild in the tinidazole group and of mild to moderate intensity in the metronidazole group |
|
| Swami 1977 | General malaise: metronidazole ‐ 1 participant | Metallic taste: tinidazole ‐ 9 participants; Bitter taste: tinidazole ‐ 4 participants; Anorexia: tinidazole ‐ 2 participants, metronidazole ‐ 3 participants; Abdominal pain: tinidazole ‐ 2 participants, metronidazole ‐ 4 participants; Nausea: tinidazole ‐ 1 participant, metronidazole ‐ 7 participants; Vomiting: tinidazole ‐ 1 participant, metronidazole ‐ 3 participants; Diarrhoea: metronidazole ‐ 2 participants; Excessive salivation: metronidazole ‐ 2 participants |
Pruritus: metronidazole ‐3 participants; Skin rash: metronidazole ‐ 1 participant |
Vertigo: tinidazole ‐ 1 participant, metronidazole ‐ 2 participants | Dark‐coloured urine: tinidazole ‐ 2 participants, metronidazole ‐ 4 participants | No abnormalities seen in complete blood count, urinalysis, serum bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and blood urea during or after treatment in both groups | 22 adverse effects reported in 15/29 (51.7%) participants in the tinidazole group, 33 adverse effects reported in 10/27 (37%) participants in the metronidazole group; Adverse effects moderate in intensity in 2 participants on tinidazole and in 8 participants on metronidazole | |
| Ornidazole | Botero 1974 | — | Nausea or vomiting with or without dizziness: ornidazole ‐ 2 participants, metronidazole ‐ 5 participants | — | Dizziness with or without headache: ornidazole ‐ 8 participants, metronidazole ‐ 4 participants; Numbness of the hands and tongue, difficulty in speaking, and headache on day 6 of treatment, which disappeared after treatment was terminated: ornidazole ‐ 1 participant |
Joint and muscle pains: ornidazole ‐ 4 participants, metronidazole ‐ 6 participants | Not reported | The first 20 participants were given complete cardiovascular, neurological, and laboratory workup, but these were not specified or reported in detail |
| Naoemar 1973 | — | Severe nausea: metronidazole ‐ 1 participant; Nausea associated with hypersalivation, anorexia, and dizziness: metronidazole ‐ 1 participant; Both improved with rest and reduction in metronidazole dosage from 1500 mg to 1000 mg |
— | Dizziness, which disappeared after the dose was reduced from 1500 mg to 1000 mg daily: ornidazole ‐ 2 participants; Slight dizziness, which disappeared with rest: metronidazole ‐ 1 participant |
— | No abnormalities seen in complete blood count, urinalysis, alanine aminotransferase, alkaline phosphatase, blood urea, and electrocardiography after treatment in both groups | No significant difference observed in adverse effects of the 2 drugs | |
| Pudjiadi 1973 | — | — | — | — | — | No abnormalities seen in the complete blood count, urinalysis, alanine aminotransferase, alkaline phosphatase, and electrocardiography during and after treatment in both groups | No clinical adverse effects (e.g. nausea, loss of appetite, neurological signs) observed | |
| Panidazole | Botero 1977 | — | — | — | — | — | No significant changes from pre‐treatment results seen after treatment in complete blood count, urinalysis, transaminases, blood urea, and electrocardiography in both groups | 37/50 (74%) participants on panidazole presented with ≥ 1 of following adverse effects in order of frequency: dizziness, nausea, headache, vomiting, epigastric pain, cutaneous rash, numbness of mouth, and weakness; 33/50 (66%) participants on metronidazole presented with ≥ 1 of following adverse effects in order of frequency: nausea, dizziness, headache, epigastric pain, vomiting, poor appetite, and metallic taste in the mouth; All symptoms were of low to medium intensity and disappeared after treatment was terminated |
| Praziquantel | Mohammed 1998 | — | — | — | — | — | Not monitored | Main adverse effects reported by participants on praziquantel were nausea and vomiting (5.3%) and dizziness (5.3%); Other adverse effects encountered occasionally included mild fever, joint pain, sore throat, dysuria, retention of urine, and severe apprehension; No adverse events were reported for metronidazole |
| Satranidazole (GO 10213) | Tripathi 1986 | — | — | — | — | — | Complete blood count, urinalysis, serum bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, blood urea, and electrocardiography were done after treatment, but results were not presented | 7 participants in the metronidazole group and 5 participants in the satranidazole group presented with ≥ 1 of following adverse effects: nausea, vomiting, burning in the epigastrium, headache, abdominal distension, and generalized itching;. None were serious or necessitated withdrawal from treatment |
Appendix 11. Adverse events: any antiamoebic versus placebo
| Trial | General/systemic | Gastrointestinal | Dermatologic | Central nervous system | Others | Laboratory abnormal | Remarks |
| Donckaster 1964 | General adverse effects (headache, asthenia, vertigo, anorexia): antiamoebic drugs (34/339 participants, 10%); placebo (0) Breakdown in general adverse effects in antiamoebic drugs: dimethylchlortetracycline ‐ 7 participants; oxytetracycline ‐ 1 participant; tetracycline ‐ 4 participants; chlorphenoxamide ‐ 6 participants; chlorbetamide ‐ 2 participants; dehydroemetine ‐ 9 participants; diiodohydroxyquinoline ‐ 1 participant; phenanthridinone ‐ 2 participants; bismuth glycoarsanilate ‐ 2 participants |
Gastrointestinal symptoms (nausea and vomiting, meteorism, hyperacidity, epigastric pain, intestinal colic, diarrhoea): antiamoebic drugs (114/339 participants, 34%); placebo (5/28 participants, 18%) Breakdown in antiamoebic drugs: dimethylchlortetracycline ‐ 18 participants; oxytetracycline ‐ 7 participants; tetracycline ‐ 9 participants; chlorphenoxamide ‐ 18 participants; chlorbetamide ‐ 16 participants; dehydroemetine ‐ 27 participants; diiodohydroxyquinoline ‐ 5 participants; phenanthridinone ‐ 4 participants; bismuth glycoarsanilate ‐ 5 participants; iodochlorhydroxyquinoline ‐ 5 participants |
Cutaneous symptoms (anal pruritis, erythema): antiamoebic drugs (21/339, 6%); placebo (0) Breakdown in antiamoebic drugs: dimethylchlortetracycline ‐ 5 participants; oxytetracycline ‐ 1 participant; tetracycline ‐ 2 participants; chlorphenoxamide ‐ 2 participants; chlorbetamide ‐ 2 participants; dehydroemetine ‐ 5 participants; phenanthridinone ‐ 3 participants; iodochlorhydroxyquinolone ‐ 1 participant |
— | Not monitored | Not monitored | Tolerance was classified as good, fair, or bad according to the number of symptoms presented and their intensity; Tolerance was rated as bad in 27% of participants given dehydroemetine, 23% of participants given dimethylchlortetracycline, and 0% of those given placebo; 1 participant given diiodochlorydroxyquinoline presented with intense and frequent intestinal colic |
| Huggins 1982 | — | Nausea: quinfamide (6/72 participants, 8%); placebo (1/24 participants, 4%) | — | Headache: quinfamide (1/72 participants, 1%); placebo (2/24 participants, 8%) | — | Complete blood count, urinalysis, total cholesterol, blood sugar, bilirubin, urea, creatinine, alkaline phophatase, transaminases, and serum calcium were examined, but results were not presented before or after treatment | Adverse effects were based on participants' complaints, consisting of only 2 symptoms ‐ nausea and headache |
| Rossignol 2001 | — | Abdominal pain: nitazoxanide ‐ 1 participant; placebo ‐ 1 participant; Nausea: nitazoxanide ‐ 1 participant; Dyspepsia: nitazoxanide ‐ 2 participants; Worsening diarrhoea: placebo ‐ 1 participant |
— | Headache: nitazoxanide ‐ 1 participant; Dizziness: nitazoxanide ‐ 1 participant, placebo ‐ 10 participants; Drowsiness: nitazoxanide ‐ 2 participants, placebo ‐ 1 participant |
Dysuria: nitazoxanide ‐ 1 participant | Not monitored | 9 adverse effects were reported in 6 participants in the nitazoxanide group, and 4 adverse effects were reported in 4 participants in the placebo group; All adverse effects were mild and transient and none resulted in discontinuation of therapy |
| Rossignol 2007 | Drowsiness: nitazoxanide ‐ 4 participants; Fatigue: nitazoxanide ‐ 1 participant; placebo ‐ 1 participant |
Abdominal pain: nitazoxanide ‐ participants, placebo ‐ 1 participant; Dyspepsia: nitazoxanide ‐ 1 participant; Nausea: placebo ‐ 1 participant; Vomiting: placebo ‐ 1 participant |
— | Headache: nitazoxanide ‐ 2 participants, placebo ‐ 1 participant | Yellowish urine: nitazoxanide ‐ 1 participant, placebo ‐ 1 participant | Not monitored | All adverse effects were mild and transient and none required discontinuation of treatment |
Appendix 12. Adverse events: other comparisons
| Comparison | Trial | General/systemic | Gastrointestinal | Dermatologic | Central nervous system | Others | Laboratory abnormal | Remarks |
| Ornidazole versus tinidazole | Panggabean 1980 | — | Vomiting: ornidazole ‐ 1 participant | — | — | — | Not monitored | Adverse effects with both drugs were minimal; no specific details were provided |
| Secnidazole versus tinidazole | Salles 1999 | Fever: secnidazole ‐ 1 participant | Bitter taste: secnidazole ‐ 4 participants, tinidazole ‐ 8 participants; Nausea: secnidazole ‐ 4 participants, tinidazole ‐ 7 participants; Vomiting: secnidazole ‐ 4 participants, tinidazole ‐ 1 participant; Abdominal pain: secnidazole ‐ 1 participant, tinidazole ‐ 1 participant; Flatulence: secnidazole ‐ 1 participant; Soft stools: secnidazole ‐ 1 participant; Diarrhoea: tinidazole ‐ 1 participant |
— | Headache: secnidazole ‐ 2 participants, tinidazole ‐ 1 participant; Dizziness: tinidazole ‐ 1 participant |
Pharyngeal erythema: secnidazole ‐ 1 participant | Not monitored | Adverse effects were reported in 12/156 (7.7%) participants on secnidazole and in 15/147 (10.2%) participants on tinidazole; all were mild to moderate in intensity' No statistically significant difference in frequency of adverse effects was noted between the 2 groups |
| Secnidazole versus quinfamide | Padilla 2000 | — | Abdominal pain: secnidazole ‐ 18 participants, quinfamide ‐ 4 participants (P < 0.05); Nausea: secnidazole ‐ 20 participants, quinfamide ‐ 1 participant (P < 0.05); Unpleasant taste in the mouth: secnidazole ‐ 18 participants, quinfamide ‐ 0 (P < 0.0001); Vomiting: secnidazole ‐ 3 participants, quinfamide ‐ 0; Diarrhoea: secnidazole ‐ 3 participants, quinfamide ‐ 0 |
— | Headache: secnidazole ‐ 2 participants, quinfamide ‐ 0 | — | Not monitored | Adverse effects were significantly higher in the secnidazole group than in the quinfamide group as determined by Chi2 test (P ≤ 0.05 considered statistically significant) |
| Ornidazole versus secnidazole | Toppare 1994 | — | — | — | — | — | Not monitored | No adverse effects were seen; no further details were provided |
| Quinfamide versus nitazoxanide | Davila 2002 | — | — | — | — | — | Not monitored | Both treatments were well tolerated by participants; no further details were given |
| Quinfamide versus teclozan | Guevara 1980 | Mild malaise: quinfamide ‐ none reported; teclozan ‐ 1 participant; Serious adverse events and adverse events necessitating withdrawal: None were reported in both treatment groups |
Nausea: quinfamide ‐ 3 participants with moderate nausea, teclozan ‐ 2 participants with mild nausea, 1 with moderate nausea; Vomiting: quinfamide ‐ 3 participants with mild vomiting, 4 with moderate vomiting, teclozan ‐ no vomiting reported; Abdominal pain: quinfamide ‐ 3 participants with mild abdominal pain, 3 with moderate abdominal pain, teclozan ‐ none with abdominal pain; Flatulence: quinfamide ‐ 1 participant with mild flatulence, teclozan ‐ none with flatulence; Burning sensation in the stomach: quinfamide ‐ 1 participant, teclozan ‐ none with burning sensation |
Headache: teclozan ‐ 1 participant, quinfamide ‐ 0; Dizziness: quinfamide ‐ 1 participant, teclozan ‐ 0 |
Haemocytology, serum bilirubin, transaminases, alkaline phosphatase, and urinalysis were determined at baseline, then at 8 and 30 days after treatment, but results were not reported | Gastrointestinal adverse effects such as vomiting and abdominal pain were more common in those given the intermediate dose of quinfamide (200 mg 3 times a day) than in those given 100 mg 3 times a day and 400 mg 3 times a day | ||
| Etophamide versus quinfamide | Olaeta 1996 | — | Meteorism (developed during treatment period): etophamide ‐ 1 infant | — | — | — | Not monitored | No participant needed to stop treatment because of adverse events; no further details were given |
| Chlorhydroxyquinoline versus diiodohydroxyquinoline | Kapadia 1968 | — | Nausea: chlorhydroxyquinoline ‐ 1 participant; Epigastric discomfort with vomiting: chlorhydroxyquinoline ‐ 6 participants, diiodohydroxyquinoline ‐ 0 |
Mild rash: chlorhydroxyquinoline ‐ 1 participant, diiodohydroxyquinoline ‐ 1 participant | — | Coryza: diiodohydroxyquinoline ‐ 2 participants; Conjunctivitis: diiodohydroxyquinoline ‐ 1 participant |
Liver function test before and after treatment remained within the normal range in both groups | — |
| Combination dehydroemetine, tetracycline, and diloxanide furoate versus metronidazole | Rubidge 1970 | — | — | — | — | — | Not monitored | Tolerance of both regimens was reported to be "excellent", and no toxicity was encountered; tolerance was not defined, and no further details were given |
| Combination metronidazole and diiodohydroxyquinoline versus metronidazole | Asrani 1995 | — | Metallic taste: metronidazole alone ‐ 225 participants, metronidazole plus diiodohydroxyquinoline ‐ 224 participants; Abdominal pain: metronidazole alone ‐ 45 participants, metronidazole plus diiodohydroxyquinoline ‐ 46 participants; Vomiting: metronidazole alone ‐ 45 participants, metronidazole plus diiodohydroxyquinoline ‐ 36 participants; Nausea: metronidazole alone ‐ 121 participants, metronidazole plus diiodohydroxyquinoline ‐ 125 participants; Diarrhoea: metronidazole alone ‐ 5 participants, metronidazole plus diiodohydroxyquinoline ‐ 5 participants |
— | Headache: metronidazole alone ‐ 29 participants, metronidazole plus diiodohydroxyquinoline ‐ 26 participants; Drowsiness: metronidazole alone ‐ 3 participants, metronidazole plus diiodohydroxyquinoline ‐ 11 participants |
Unspecified allergic reaction (and had to be withdrawn from trial): metronidazole plus diiodohydroxyquinoline ‐ 1 participant | Not monitored | Overall incidence of adverse effects was not statistically significantly different between the 2 groups |
| Metronidazole and S boulardii versus metronidazole | Savas‐Erdeve 2009 | No adverse effects reported for all patients enrolled in the study | — | — | — | — | Not monitored | S boulardii was well tolerated |
| Fixed drug combination metronidazole and furazolidone versus metronidazole | Prasad 1985 | — | — | — | — | — | Not monitored | Both regimens were well tolerated; adverse effects were usually mild in the form of distaste, flatulence, and nausea; Incidence of adverse effects was reported to be greater with metronidazole suspension than with the combination, but no specific details were reported |
| Combination tetracycline and clioquinol versus secnidazole | Soedin 1985 | — | — | — | — | — | Not monitored | Both treatment regimens were reasonably well tolerated and few adverse effects were reported; no further details were given |
| Combination tinidazole and diloxanide furoate versus tinidazole | Pehrson 1983 | — | — | — | — | — | Not monitored | No adverse effects were severe enough to cause cessation of treatment; no further details were given |
| Fixed drug combination diloxanide furoate, tetracycline with chloroquine versus fixed drug combination diloxanide furoate and tetracycline without chloroquine | Nnochiri 1967 | — | Flatulence and abdominal discomfort: 8 participants in both groups (unclear whether adverse effects were seen in 8 participants in each of the two groups, or in a total of 8 participants in both groups) | — | — | — | No abnormalities were noted in complete blood count and urinalysis during or after treatment | — |
| Aminosidine, etophamide, nimorazole alone or in combination | Pamba 1990 | — | — | — | — | — | Not monitored | Drug tolerance was rated as poor in 1.0% of patients given aminosidine, 2.0% of patients given combination nimorazole and aminosidine, and 76.5% of patients given etophamide and aminosidine; Recruitment of participants in the etophamide‐aminosidine group was discontinued because of the high incidence of severe diarrhoea; no other details of adverse events were given |
| MK‐910 low dose (0.5 mg/kg and 1 mg/kg) versus MK‐910 high dose (2 mg/kg and 3 mg/kg) | Batra 1972 | — | Vague abdominal pain: 1 participant each in the low dosage groups (total of 2 participants), 3 participants each in the higher dosage groups (total of 6 participants); Nausea and vomiting: 4 participants each in the higher dosage groups (total of 8 participants), 2 participants, 1 in each of the higher dosage groups had to be removed from the trial because of the severity of gastrointestinal symptoms |
— | — | — | Not monitored | — |
| Herbal versus fixed drug combination metronidazole‐diloxanide | Siddiqui 2015 | Serious adverse events and adverse events necessitating withdrawal: none reported in both treatment groups; Headache: herbal ‐ 1 participant, metrodiloxanide combination ‐ 5 participants |
Anorexia: herbal ‐ 2 participants, metrodiloxanide combination ‐ 14 participants; Metallic taste: herbal ‐ 2 participants, metrodiloxanide combination ‐ 7 participants; Flatulence: herbal ‐ 0, metrodiloxanide combination ‐ 5 participants; Abdominal pain: herbal ‐ 1 participant, metrodiloxanide combination ‐ 4 participants |
Others (not specified) : herbal ‐ 1 participant, metrodiloxanide combination ‐ 2 participants | Not monitored | Significantly more side effects were reported in those given metronidazole‐diloxanide than in those given herbal (P < 0.00) |
Abbreviations:S boulardii:Saccharomyces boulardii.
Data and analyses
Comparison 1. Alternative drug versus metronidazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 1 to 14 days after end of treatment | 5 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.11, 1.64] |
| 1.1 Tinidazole | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.30] |
| 1.2 Ornidazole | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Praziquantel | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.78] |
| 2 Clinical failure: 15 to 60 days after end of treatment | 12 | 679 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.21, 0.73] |
| 2.1 Tinidazole | 8 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.15, 0.51] |
| 2.2 Ornidazole | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.89] |
| 2.3 Panidazole | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Satranidazole (GO 10213) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.40, 1.60] |
| 3 Parasitological failure: 1 to 14 days after end of treatment | 6 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.85, 1.29] |
| 3.1 Tinidazole | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.58, 1.74] |
| 3.2 Ornidazole | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Praziquantel | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.78] |
| 3.4 Secnidazole | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.12] |
| 4 Parasitological failure: 15 to 60 days after end of treatment | 13 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.37, 1.43] |
| 4.1 Tinidazole | 9 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.25, 1.64] |
| 4.2 Ornidazole | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.41] |
| 4.3 Panidazole | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.81, 3.60] |
| 4.4 Satranidazole (GO 10213) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 5 Relapse (ornidazole) | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 4.74 [1.07, 20.99] |
| 6 Adverse events | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Tinidazole | 8 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.92] |
| 6.2 Ornidazole | 3 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.57, 1.73] |
| 6.3 Satranidazole (GO 10213) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.27, 1.88] |
| 6.4 Panidazole | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.87, 1.45] |
Comparison 2. Any antiamoebic drug versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 1 to 14 days after end of treatment | 3 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.13, 0.57] |
| 1.1 Quinfamide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.21, 0.60] |
| 1.2 Nitazoxanide | 2 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.81] |
| 2 Parasitological failure: 1 to 14 days after end of treatment | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.22, 0.50] |
| 2.1 Quinfamide | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.19, 0.47] |
| 2.2 Nitazoxanide | 2 | 167 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.27] |
| 2.3 10 different drugs belonging to 6 drug classes | 1 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.26, 0.53] |
| 3 Adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Quinfamide | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.22, 4.63] |
| 3.2 Nitazoxanide | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.41, 4.43] |
| 3.3 10 different drugs belonging to 6 drug classes | 1 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.97, 4.88] |
Comparison 3. Combination regimen versus monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 1 to 14 days after end of treatment | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 DHE, tetracycline, and diloxanide furoate vs metronidazole | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.24, 4.59] |
| 1.2 Metronidazole and diiodohydroxyquinoline vs metronidazole | 1 | 896 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.13, 0.21] |
| 1.3 Metronidazole‐furazolidone vs metronidazole | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.09, 1.36] |
| 1.4 Combinations vs nimorazole, aminosidine, and etofamide | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.07, 5.21] |
| 1.5 Tetracycline and clioquinol vs secnidazole | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 8.5 [2.10, 34.40] |
| 2 Parasitological failure: 1 to 14 days after end of treatment | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Dehydroemetine and tetracycline and diloxanide furoate vs metronidazole | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.24, 4.59] |
| 2.2 Metronidazole and diiodohydroxyquinoline vs metronidazole | 1 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.11, 0.45] |
| 2.3 Fixed‐drug combination metronidazole‐furazolidone vs metronidazole | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.09, 1.36] |
| 2.4 Combinations vs nimorazole or aminosidine or etofamide | 1 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.30] |
| 2.5 Quinfamide and mebendazole vs nitazoxanide (mixed infections only) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.85, 4.25] |
| 2.6 Tetracycline and clioquinol vs secnidazole | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 7.50 [2.91, 19.33] |
| 3 Parasitological failure: 15 to 60 days after end of treatment | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.63] |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 DHE, tetracycline, and diloxanide furoate vs metronidazole | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.3. Analysis.
Comparison 3 Combination regimen versus monotherapy, Outcome 3 Parasitological failure: 15 to 60 days after end of treatment.
3.4. Analysis.
Comparison 3 Combination regimen versus monotherapy, Outcome 4 Adverse events.
Comparison 4. Single‐dose regimen versus longer regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 1 to 14 days after end of treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Quinfamide: 1 dose vs 2 or 3 doses | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.92, 9.22] |
| 1.2 Secnidazole (1 dose) vs tetracycline and clioquinol (5 days) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.48] |
| 2 Clinical failure: 15 to 60 days after end of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Secnidazole (1 dose) vs tinidazole (2 days) | 1 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.37, 1.85] |
| 3 Parasitological failure: 1 to 14 days after end of treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Quinfamide (1 dose) vs nitazoxanide (3 days) | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [0.37, 33.98] |
| 3.2 Quinfamide: 1 dose vs 2 or 3 doses | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.91, 4.38] |
| 3.3 Secnidazole (1 dose) vs metronidazole (10 days) | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.12] |
| 3.4 Secnidazole (1 dose) vs tetracycline and clioquinol (5 days) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.05, 0.34] |
| 4 Parasitological failure: 15 to 60 days after end of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Secnidazole (1 dose) vs tinidazole (2 days) | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.43, 0.88] |
| 5 Adverse events | 2 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.87] |
| 5.1 Quinfamide: 1 dose vs 2 or 3 doses | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 2.57] |
| 5.2 Secnidazole (1 dose) vs tinidazole (2 days) | 1 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.37, 1.56] |
4.5. Analysis.
Comparison 4 Single‐dose regimen versus longer regimen, Outcome 5 Adverse events.
Comparison 5. Other antiamoebic drug comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 1 to 14 days after end of treatment | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Ornidazole vs tinidazole | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.07, 4.41] |
| 1.2 Ornidazole vs secnidazole | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.17, 5.45] |
| 1.3 Chlorhydroxyquinoline vs diiodohydroxyquinoline | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.53] |
| 1.4 MK‐910 (low dose, ≤ 1 mg/kg/d vs high dose, ≥ 2 mg/kg/d) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Metronidazole and Saccharomyces boulardii vs metronidazole | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Herbal product vs fixed dose combination metronidazole‐diloxanide | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.69, 1.88] |
| 1.7 Herbal drug vs metronidazole | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.34, 1.07] |
| 2 Parasitological failure: 1 to 14 days after end of treatment | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Ornidazole vs tinidazole | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 60.51] |
| 2.2 Ornidazole vs secnidazole | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.45] |
| 2.3 Chlorhydroxyquinoline vs diiodohydroxyquinoline | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.80] |
| 2.4 MK‐910 (low dose, ≤ 1 mg/kg/d vs high dose, ≥ 2 mg/kg/d) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.39, 2.58] |
| 2.5 Quinfamide vs secnidazole | 1 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.34, 0.96] |
| 2.6 Quinfamide vs nitazoxanide (Entamoeba infection only) | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [0.37, 33.98] |
| 2.7 Metronidazole and Saccharomyces boulardii vs metronidazole | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.20, 3.54] |
| 2.8 Two fixed‐drug combinations of diloxanide furoate and tetracycline with or without chloroquine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.31, 1.12] |
| 2.9 Herbal product vs fixed‐dose combination metronidazole‐diloxanide | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.67, 2.01] |
| 2.10 Herbal drug vs metronidazole | 1 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.01] |
| 3 Parasitological failure: 15 to 60 days after end of treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Quinfamide vs teclozan | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.08, 1.32] |
| 3.2 Metronidazole and iodoquinol plus Saccharomyces boulardii vs metronidazole and iodoquinol | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.57] |
| 3.3 Two fixed‐drug combinations of diloxanide furoate and tetracycline with or without chloroquine | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.20, 0.96] |
| 4 Adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Ornidazole vs tinidazole | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.12, 65.34] |
| 4.2 Quinfamide vs teclozan | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.82] |
| 4.3 MK‐910 low dose vs high dose | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.26, 98.00] |
| 4.4 Herbal vs fixed‐drug combination metronidazole‐diloxanide | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.09, 0.41] |
Comparison 6. Subgroup analyses: alternative drug versus metronidazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure 15 to 60 days after end of treatment, by clinical category | 13 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.45, 1.48] |
| 1.1 Amoebic dysentery | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.07, 8.68] |
| 1.2 Non‐dysenteric amoebic colitis | 3 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.09, 2.42] |
| 1.3 Amoebic colitis or intestinal amoebiasis, unspecified | 9 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.29, 1.10] |
| 2 Parasitological failure 15 to 60 days after end of treatment, by age group | 13 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.37, 1.43] |
| 2.1 Adults (age ≥ 15 years) | 10 | 622 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.25, 1.54] |
| 2.2 Children (age < 15 years) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Both adults and children | 3 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.34, 1.85] |
| 3 Parasitological failure 15 to 60 days after end of treatment, single or mixed intestinal infection | 13 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.37, 1.43] |
| 3.1 Amoebic infection only | 10 | 586 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.25, 1.59] |
| 3.2 Mixed intestinal infection | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.10, 3.91] |
| 4 Parasitological failure 15 to 60 days after end of treatment, by criteria | 13 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.37, 1.43] |
| 4.1 WHO criteria | 9 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.29, 1.10] |
| 4.2 Other criteria | 4 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.58, 2.94] |
Comparison 7. Subgroup analyses: any antiamoebic drug versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure 1 to 14 days after end of treatment, by clinical category | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.22, 0.50] |
| 1.1 Non‐dysenteric amoebic colitis | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.19, 0.47] |
| 1.2 Amoebic colitis or intestinal amoebiasis, unspecified | 3 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.17, 0.62] |
| 2 Clinical failure 1 to 14 days after end of treatment, by age group | 3 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.14, 0.51] |
| 2.1 Adults (age ≥ 15 years) | 3 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 2.2 Children (age < 15 years) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.56] |
| 3 Parasitological failure 1 to 14 days after end of treatment, by age group | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.23, 0.48] |
| 3.1 Adults (age ≥ 15 years) | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.20, 0.56] |
| 3.2 Children (age < 15 years) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.01, 0.54] |
| 3.3 Both adults and children | 1 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.26, 0.53] |
| 4 Clinical failure 1 to 14 days after end of treatment, by diagnostic method | 3 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.13, 0.57] |
| 4.1 Stool microscopy with staining or concentration technique | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.23, 0.56] |
| 4.2 Antigen‐based ELISA test | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.03, 0.33] |
| 5 Parasitological failure 1 to 14 days after end of treatment, by diagnostic method | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.22, 0.50] |
| 5.1 Stool microscopy with staining or concentration technique | 3 | 530 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.29, 0.47] |
| 5.2 Antigen‐based ELISA test | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.34] |
Comparison 8. Subgroup analyses: combination regimen versus monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 1 to 14 days after end of treatment, by intervention | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Combination vs metronidazole | 3 | 1025 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.11, 0.98] |
| 1.2 Combination vs alternative drugs | 2 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [0.20, 33.80] |
| 2 Parasitological failure: 1 to 14 days after end of treatment, by intervention | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Combination vs metronidazole | 3 | 720 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.15, 0.86] |
| 2.2 Combination vs alternative drugs | 3 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.41, 8.37] |
Comparison 9. Subgroup analyses: combination regimen versus metronidazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 1 to 14 days after end of treatment, by clinical diagnosis | 3 | 1025 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.11, 0.98] |
| 1.1 Intestinal amoebiasis, unspecified | 2 | 986 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.12, 0.25] |
| 1.2 Amoebic dysentery | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.24, 4.59] |
| 2 Parasitological failure: 1 to 14 days after end of treatment, by clinical diagnosis | 3 | 720 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.15, 0.86] |
| 2.1 Intestinal amoebiasis, unspecified | 2 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.13, 0.46] |
| 2.2 Amoebic dysentery | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.24, 4.59] |
Comparison 10. Subgroup analyses: any single‐dose regimen versus longer regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure: 1 to 14 days after end of treatment, by intervention | 4 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.11, 4.91] |
| 1.1 Secnidazole single dose vs longer duration | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.06, 0.35] |
| 1.2 Quinfamide single dose vs longer duration | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.02, 4.46] |
Comparison 11. Subgroup analysis: tinidazole versus metronidazole 15 to 60 days after end of treatment, based on tinidazole dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 15 to 60 days after end of treatment | 8 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.15, 0.51] |
| 1.1 High‐dose tinidazole vs metronidazole | 5 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.13, 0.47] |
| 1.2 Low‐dose tinidazole vs metronidazole | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.08, 3.31] |
| 2 Parasitological failure: 15 to 60 days after end of treatment | 9 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.25, 1.64] |
| 2.1 High‐dose tinidazole vs metronidazole | 5 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.16, 1.31] |
| 2.2 Low‐dose tinidazole vs metronidazole | 4 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.44, 2.72] |
Comparison 12. Sensitivity analysis: tinidazole versus metronidazole 15 to 60 days after end of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 15 to 60 days after end of treatment, excluding Misra 1978 | 7 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.61] |
| 2 Clinical failure: 15 to 60 days after end of treatment, excluding trials sponsored by pharmaceutical companies | 4 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.50] |
Comparison 13. Sensitivity analyses: combination regimen versus metronidazole alone, excluding pharmaceutical company‐sponsored study (Asrani 1995).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure: 1 to 14 days after end of treatment | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.20, 1.73] |
| 2 Parasitological failure: 1 to 14 days after end of treatment, by intervention | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.20, 1.73] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asrani 1995.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% for parasitological assessment; 93.4% (898/961) for clinical assessment |
|
| Participants |
Numbers: 961 enrolled, 898/961 (93.4%) included in analysis of clinical outcome; 591/591 (100%) positive for E histolytica on stool examination at baseline included in analysis of parasitological outcome Inclusion criteria: male and non‐pregnant female patients > 12 years of age with clinical symptoms of intestinal amoebiasis and/or presence of trophozoites or cysts ofE histolytica in stool specimens Exclusion criteria: history of alcohol abuse; hypersensitivity or contraindications to any of the study drugs; systemic amoebiasis; severe illness; and/or persistent vomiting |
|
| Interventions |
Treatment period was extended to 10 days in both groups when 5 days' treatment was inadequate to clear the stools of E histolytica |
|
| Outcomes |
Not included in this review: average daily frequency of stools on admission and on day 5 and day 10 of treatment; overall clinical response (rated as "poor" if < 25% relief and not tolerated, "fair" if 25% to 49% relief and not well tolerated, "poor" if 50% to 74% relief and well tolerated, or "excellent" if 75% to 100% relief and well tolerated) |
|
| Notes |
Location: various cities (not specified) in India Date: 1995 (date of publication only; actual study period not reported) Source of funding: not stated; one of the study authors (Dr SJ Phaterpekar) is connected with Searle (India) Limited, Bombay, India Several attempts were made to inquire about study methods, but no response was obtained from the primary author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomization schedule was prepared for a group of 120 patients in advance. Each co‐ordinator used the same randomization schedule" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Reported to be an open‐label study |
| Blinding (performance bias and detection bias) Parasitological outcomes | High risk | Reported to be an open‐label study |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 5 days after end of treatment (day 10): For clinical assessment, 63/961 (6.6%) lost to follow‐up or were protocol violators (33/421 in metronidazole group, 30/540 in combination therapy group); 1 participant in the combination group withdrawn from the study owing to allergic reaction on the first day of treatment. Missing patients were those lost to follow‐up, who received other antiamoebic drugs or met exclusion criteria and were not included for efficacy analysis, but actual numbers in the 2 groups were not specified. In addition, 1 participant in the combination group developed an allergic reaction on the first day of treatment and was withdrawn from the study. Total number of participants analysed overall for clinical evaluation was 93.3% (898/961). For parasitological evaluation, no data were missing among the 249 in the metronidazole group and no data were missing among the 342 in the combination group whose stools were positive forE histolytica on admission. "Patients whose stool samples could not be examined were excluded from the parasitological efficacy assessment." Total number of participants analysed overall for parasitological evaluation was 591/591 (100%) |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Unclear risk | Published report included pre‐specified outcomes, although data on adverse effects included only those with specific adverse effects and did not report the number of participants in whom adverse effects were observed in both treatment groups |
| Other bias | High risk | Diagnosis of intestinal amoebiasis was based on presence of clinical symptoms and those "suspected to be suffering from amoebiasis". Not all participants had stool exams positive for E histolytica. Stools were examined by microscopy, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA test or PCR From the report, those with persistent E histolytica at the end of 5 days' treatment were advised to continue the same treatment for another 5 days and were examined again at the end of 10 days' therapy. The number of cases that required treatment extension to 10 days was not mentioned, and there was only 1 analysis regardless of duration of treatment It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Awal 1979.
| Methods |
Generation of allocation sequence: random numbers table Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 66 enrolled and analysed Inclusion criteria: adults and children with clinical signs and symptoms of intestinal amoebiasis and motile haematophagous trophozoites of E histolytica in fresh stool specimens and on sigmoidoscopy Exclusion criteria: antiamoebic treatment during previous 4 weeks; pregnant women; dehydrated patients; those with evidence of hepatic or renal dysfunction |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: hospital in Bangladesh Date: 1979 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated to any one of the three treatment regimens by a prearranged randomization table" Comment: Randomization table probably refers to a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and regimens were used (tinidazole 2 g for 2 or 3 days; metronidazole 2 g for 2 days) and blinding of participants, study personnel, and clinical outcome assessors was not mentioned Comment: Blinding of participants, study personnel, and outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not determined |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 28 days after end of treatment (day 30): No outcome data were missing from both treatment groups; all randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes |
| Other bias | Unclear risk | Trial enrolled only those who showed haematophagous trophozoites of E histolytica in the stools, but diagnosis of intestinal amoebiasis was based only on stool microscopy and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa or helminth parasites was determined |
Batra 1972.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 40 enrolled; 40 analysed; 2 withdrawn from treatment because of severe gastrointestinal adverse effects Inclusion criteria: acute amoebic dysentery and stool specimens positive for trophozoites of E histolytica by saline and iodine smears Exclusion criteria: pregnant women; critically ill patients; those with neurological and cardiac abnormalities or disturbed renal function |
|
| Interventions | MK‐910: Each arm used 1‐methyl‐2‐(4'fluorophenyl)‐5‐nitroimidazole (MK‐910) at different daily dosages, all given in 3 divided doses orally for 10 days
|
|
| Outcomes |
Not included in this review: disappearance of colonic ulcers on sigmoidoscopic examination on day 5 and at end of treatment on day 10 |
|
| Notes |
Location: hospital in New Delhi, India Date: 1972 (date of publication only; actual study period not reported) Source of funding: Merck, Sharp, and Dohme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The allocation was randomized on the basis of a pre‐planned schedule" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages of MK‐910 were used (daily doses of 0.5 mg/kg,1.0 mg/kg, 2.0 mg/kg, and 3.0 mg/kg in 3 divided doses for 10 days), and blinding of participants, study personnel, and clinical outcome assessors was not mentioned Comment: Blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of treatment (day 10): 2/20 in the high‐dose group (1 participant each in the 2‐mg/kg and 3‐mg/kg groups) had to be dropped from the study because of severe adverse effects, but it is unclear whether they were excluded from the analysis of outcomes |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Unclear risk | Clinical outcome was measured by determining duration in hours from start of treatment until relief of symptoms, cessation of unformed stools, and disappearance of blood and mucus from stools. Parasitological outcome was reported as duration in hours from start of treatment to disappearance of E histolytica from the stools |
| Other bias | Unclear risk | Diagnosis of acute amoebic dysentery was based on stool microscopy demonstrating trophozoites of E histolytica and sigmoidoscopic examination, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR. The stool was cultured for E histolytica but only on the fifth and tenth days of treatment, not at baseline It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Botero 1974.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear; reported as "double‐blind", but blinding of participants, care providers, and outcome assessors not described Inclusion of all randomized participants: 95.8% (115/120) |
|
| Participants |
Numbers: 120 enrolled; 115 analysed; 5 lost to follow‐up; 1 participant in Ro 7‐0207 terminated treatment after day 6 because of adverse effects Inclusion criteria: adult males with clinical symptoms of intestinal amoebiasis confirmed by the presence of E histolytica in the stools examined by direct smear and Ritchie formalin‐ether concentration methods Exclusion criteria: not stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: hospital in Medellin, Colombia Date: 1974 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to one of two treatment groups..." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported as "double‐blind", but it is unclear who was blinded. Both Ro 7‐0207(ornidazole) and metronidazole were given 1 g daily, administered as two 250‐mg capsules twice daily for 10 days, but the appearance of the 2 drugs was not described Comment: It is not specifically mentioned who among the participants, study personnel, and clinical outcome assessors was blinded |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not specifically mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | At end of treatment (day 10 after onset of treatment): Total number analysed was 115/120 (95.8%). 5 out of the 120 participants enrolled in the trial left the hospital after treatment, did not complete follow‐up, and were not included in the analysis. The type of intestinal amoebiasis (acute or chronic amoebiasis), treatment groups to which the 5 were randomized, and reasons for non‐compliance with follow‐up were not specified |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | 30 days after end of treatment: Total number analysed was 115/120 (95.8%) at complete follow‐up, and they were not included in the analysis. Type of intestinal amoebiasis (acute or chronic amoebiasis), treatment groups to which the 5 were randomized, and reasons for non‐compliance with follow‐up were not reported |
| Selective reporting (reporting bias) | High risk | No clinical assessment was done for those with chronic intestinal amoebiasis, even if on enrolment, it is mentioned that all participants had symptomatic intestinal amoebiasis |
| Other bias | Unclear risk | Separate analysis was carried out for those with acute dysenteric intestinal amoebiasis and those with chronic intestinal amoebiasis, but this was not pre‐specified Diagnosis of intestinal amoebiasis was based on demonstration of E histolytica on stool microscopy (direct smear and concentration technique), but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Botero 1977.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear; reported as "double‐blind", but blinding of participants, care providers, and outcome assessors was not described Inclusion of all randomized participants: 100% |
|
| Participants |
Number: 100 enrolled and 100 analysed Inclusion criteria: adult males with clinical symptoms of intestinal amoebiasis and stools positive for E histolytica examined by direct smear and Ritchie formalin‐ether concentration methods Exclusion criteria: not stated Concomitant intestinal infection: 26 participants in panidazole group and 27 participants in metronidazole group had concomitant infection with other enteric protozoa and intestinal helminths (Entamoeba coli, Endolimax nana, Iodamoeba butschlii, Ascaris lumbricoides, Trichuris trichiura, Necator americanus, Strongyloides stercoralis) |
|
| Interventions |
|
|
| Outcomes |
Not included in this review: number of stools passed in 24 hours on day 3 and day 6 of treatment and on days 7 and 21 after treatment; clearance of E histolytica in 14 asymptomatic carriers |
|
| Notes |
Location: Colombia Date: 1977 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "study was performed in 100 adult male patients randomly assigned to receive one of the two drugs..." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported as "double‐blind trial", but it is unclear who was blinded. Both panidazole and metronidazole were administered in 250‐mg tablets at a dose of 2 grams per day (500 mg QID), but the appearance of the 2 drugs was not described Comment: It is not specifically mentioned who among participants, study personnel, and clinical outcome assessors was blinded |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not specifically mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | One to 4 weeks after therapy: no missing data from 45 in the panidazole group and 41 in the metronidazole group with diagnosis of acute dysentery and non‐dysenteric amoebiasis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 4 weeks after end of treatment: no missing data from 45 in the panidazole group and 41 in the metronidazole group with diagnosis of acute dysentery and non‐dysenteric amoebiasis |
| Selective reporting (reporting bias) | High risk | Published report did not completely report data for clinical outcomes in those with chronic non‐dysenteric amoebiasis, so data could not be included. "Most of the intestinal symptoms due to amoebiasis improved or disappeared even in cases which did not obtain a complete parasitological cure" |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based on stool microscopy demonstrating cysts or trophozoites of E histolytica, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR Number of cases with concomitant infection with other protozoa such as Entamoeba coli, Endolimax nana, and Iodamoeba butschlii was similar in the 2 groups (26 in the panidazole group and 27 in the metronidazole group). Other helminth parasites were also identified (Ascaris lumbricoides, Trichuris trichiura, Necator americanus, Strongyloides stercoralis), but exact numbers in each group were not reported |
Chunge 1989.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: Only participants and laboratory staff examining stools were blinded; unclear whether those assessing clinical outcomes were blinded Inclusion of all randomized participants: unclear; only those who completed the required stool examinations were included (225 participants), and the number initially randomized was not stated |
|
| Participants |
Numbers: number enrolled and randomized not stated, 225 analysed Inclusion criteria: adults and children presenting with at least any 4 of the following symptoms of intestinal amoebiasis: abdominal pain, diarrhoea, constipation, mucoid stools, malaise, flatulence, nausea, fever, tenesmus, and stool specimens positive for trophozoites or cysts of E histolytica by direct smear or formol‐ether concentration technique Exclusion criteria: pregnant women |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: outpatient departments of 3 district hospitals in Kiambo, Kilifi, and Machakos in Kenya Date: 1989 (date of publication only; actual study period not reported) Source of funding: Farmitalia Carlo Erba |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: Patients were "randomly allocated to 4 treatment groups receiving different treatment schedules" Comment: no information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Different dosages and regimens were used (tinidazole single dose for 3 days; metronidazole thrice daily for 5 days), and although participants were reported to be unaware of the treatment regimen used, blinding of study personnel and clinical outcome assessors was not mentioned Comment: insufficient information about blinding of study personnel and clinical outcome assessors |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Quote: "Neither the laboratory staff examining the specimens, nor the patients knew the various treatment regimens being tried" |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | 1 day after end of treatment (day 6): Only those who completed required stool examinations were included in the final analysis of results; 225 treated patients were evaluated. However, the total number initially randomized was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Unclear risk | Published report included pre‐specified outcomes. Adverse effects were not reported |
| Other bias | Unclear risk | Diagnosis of Intestinal amoebiasis was based only on stool microscopy (direct smear and concentration technique) demonstrating cysts or trophozoites of E histolytica, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Davila 2002.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear; reported as "double‐blind", but blinding of participants, care providers, and outcome assessors was not described Inclusion of all randomized participants: unclear; no mention of how many were randomized; children who did not complete treatment or did not provide post‐treatment faecal sample were not included in the final analysis |
|
| Participants |
Numbers: 275 enrolled with various helminthic and protozoal intestinal infections; 105/275 (38%) had E histolytica or E dispar infection (25 single infections and 80 mixed infections with other intestinal parasites) and were included in the review and analysed Inclusion criteria: children with stool specimens positive for E histolytica/E dispar and/or other intestinal parasites by direct smear or Kato‐Katz technique Exclusion criteria: not stated |
|
| Interventions |
Not stated whether placebo was used |
|
| Outcomes |
Data for parasitological cure were presented separately for nitazoxanide versus quinfamide for single infections and for nitazoxanide versus quinfamide plus mebendazole for mixed infections, and were included in a separate meta‐analysis |
|
| Notes |
Location: 3 communities in Colima, Mexico Date: 2002 (date of publication only; actual study period not reported) Source of funding: Instituto Mexicano del Seguro Social (IMSS); nitazoxanide was provided by Laboratories Columbia, S.A. de C.V., Mexico, D.F., Mexico Several attempts made to contact the primary author were not successful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "children were randomly assigned to one of the 2 treatment groups in a double‐bind design" Comment: no information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported to have a "double blind design", but it is unclear who was blinded. Different dosages and regimens were used (nitazoxanide 100 mg/5 mL, given as 10 mL twice daily for 3 days; quinfamide 100 mg/5 mL given as 5 mL single dose given for 3 days). Those randomized to the quinfamide group could be given quinfamide alone or both quinfamide and mebendazole when mixed parasitosis was detected Comment: insufficient information on how blinding of participants, study personnel, or clinical outcome assessors was ensured |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | 14 days after treatment: Of 105 with E histolytica/E dispar infection, 25 had E histolytica/E dispar infection alone and 80 had concomitant Giardia or helminth infection. Trial reports that children who did not complete treatment or did not provide post‐treatment faecal sample were not included in the final analysis, but no further information was provided |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | High risk | Study report did not include clinical response and adverse effects that would be expected to be reported for such a study. Only tolerance to the drugs was reported; adverse effects were not reported |
| Other bias | High risk | Study design involves giving varied treatment regimens; type of treatment received by the 2 groups is too different and may be a potential source of bias: For those randomized to the nitazoxanide group, nitazoxanide alone was given regardless of type of parasitosis, and for those in the second group, participants could receive quinfamide alone, mebendazole alone, or both quinfamide and mebendazole depending on the type of parasites seen. The trial author reported that parasite identification was exclusively morphological because only stool microscopy was used to diagnose intestinal amoebiasis, so differentiation of pathogenic E histolytica from non‐pathogenic species such as E dispar was not possible |
Donckaster 1964.
| Methods |
Generation of allocation sequence: random numbers table Allocation concealment: unclear Blinding: unclear; reported as "double‐blind", but blinding of participants, care providers, and outcome assessors not described Inclusion of all randomized participants: unclear; no mention of how many were randomized |
|
| Participants |
Number: 346 were treated initially; 21 cases who failed after administration of the primary drugs were randomized a second time to receive a different drug and were analysed twice under 2 different groups Inclusion criteria: adults and children with clinical symptoms of intestinal amoebiasis and stool specimens positive for cysts and/or trophozoites of E histolytica examined by the modified Telemann concentration technique (centrifugation with saline formol and ether) for cysts and polyvinyl alcohol with fixative of Schaudinn for trophozoites Exclusion criteria: those without a source of potable water at home; unable to dispose of their excrement properly; or with other non‐parasitic infections and taking other medications for these infections |
|
| Interventions |
Not stated which among the drugs, if any, were identical in appearance to placebo |
|
| Outcomes |
|
|
| Notes |
Location: outpatient clinic of the University of Chile in Santiago, Chile Date: 1964 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From English translation: "Randomized table of distribution" was used Comment: probably refers to a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported as "double‐blind", but it is unclear who was blinded. There were 10 treatment groups and 1 placebo group. All medications were given once daily orally but at different times and at different durations of therapy: Antibiotics with antiamoebic activity were not given with meals or with milk, and the other antiamoebic drugs and placebo were given with or after meals. Durations of therapy were different, with quinolones given for 21 days and all other antiamoebic drugs given for 10 days Comment: insufficient information on how blinding of participants, study personnel, and clinical outcome assessors was ensured |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | 10 to 12 days after start of treatment: There was no report of loss to follow‐up or dropouts, but there was no mention of how many were initially randomized. 21 cases that failed after administration of the primary drugs were randomized a second time to receive a different drug and were analysed twice under 2 different groups, but outcomes for these 21 were not reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | 40 days after start of treatment; however, no outcomes were reported. There was no report of loss to follow‐up or dropouts, but there was no mention of how many were initially randomized. 21 cases that failed after administration of the primary drugs were randomized a second time to receive a different drug and were analysed twice under 2 different groups, but the outcomes for these 21 were not reported |
| Selective reporting (reporting bias) | High risk | Published report did not include report of clinical outcomes |
| Other bias | High risk | Too many antiamoebic drugs were being compared (10 different drugs belonging to 6 different drug classes). Of the 346 enrolled, 346 were analysed initially, but 21 cases that failed after administration of the primary drugs were randomized a second time to receive a different drug and were analysed twice under 2 different groups Diagnosis of Intestinal amoebiasis was based only on stool microscopy demonstrating E histolytica, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Guevara 1980.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear; reported as "double‐blind", but blinding of participants, care providers, and outcome assessors not described Inclusion of all randomized participants: 92.5% (37/40) |
|
| Participants |
Numbers: 40 enrolled; 37/40 (92.5%) analysed; 2 in the quinfamide group and 1 in the teclozan group lost to follow‐up Inclusion criteria: adults with non‐dysenteric amoebiasis with trophozoites of E histolytica in recently emitted faecal material and/or in recto‐colonic mucosal exudate, recto‐colonic lesions suggestive of amoebiasis present or not, and not presenting clinical manifestations of acute amoebic recto‐colitis Exclusion criteria: those with clinical manifestations of acute amoebic recto‐colitis |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: Patients were hospitalized for 1 day, then were followed up as outpatients Date: 1980 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From the English translation: "The patients were randomly assigned to one of the treatment groups as they were incorporated into the study" Comment: no information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported to be a "double‐blind study", but it is unclear who was blinded. Different dosages of drugs were given (quinfamide 100 mg, 200 mg, or 300 mg 3 times in 1 day; teclozan 500 mg 3 times in 1 day), and the appearance of the drugs was not described Comment: It is not specifically mentioned who among participants, study personnel, and clinical outcome assessors was blinded |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not specifically mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 8 days after end of treatment: Not more than 2/30 from the quinfamide group and 1/10 from the teclozan group left ("abandoned") the study and were not included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 15 and 30 days after end of treatment: 2/30 from the quinfamide group and 1/10 from the teclozan group left ("abandoned") the study and were not included in the analysis |
| Selective reporting (reporting bias) | High risk | Final evaluation was based on parasitological outcomes, and it is unclear whether clinical outcomes were evaluated after treatment. Patients selected for enrolment included those with recto‐colonic lesions suggestive of amoebiasis, but results of rectosigmoidoscopy were not mentioned in the results. Results of laboratory monitoring for any abnormalities were not reported |
| Other bias | Unclear risk | Diagnosis of non‐dysenteric amoebiasis was based on demonstration of E histolytica in stools and rectal exudates taken by rectosigmoidoscopy, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Huggins 1982.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear; reported as "double‐blind", but blinding of participants, care providers, and outcome assessors not described Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 96 enrolled and analysed Inclusion criteria: adults with chronic intestinal amoebiasis and stool specimens positive for E histolytica by direct smear with Lugol's stain according to the Telemann‐Richter or Hoffman, Pons, and Janer methods Exclusion criteria: not stated |
|
| Interventions |
Not stated if Win 40.014 (quinfamide) and placebo tablets were identical in appearance |
|
| Outcomes |
|
|
| Notes |
Location: Clinical Hospital of the Federal University of Pernambuco, Brazil Date: 1982 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From the English translation: "The medication was administered according to a previously established routine, based on a randomised double‐blind study" Comment: no information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported to be a double‐blind study, but it is unclear who was blinded. The study drug, WIN 40.014, was given for 1 day at different frequencies: 100 mg as a single dose, every 12 hours, and every 8 hours. No information is provided on frequency of administration of placebo Comment: It is not specifically mentioned who among participants, study personnel, and clinical outcome assessors was blinded |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not specifically mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 7 days after end of treatment (day 7): No data were missing from all treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess whether there is risk for selective outcome reporting. Results of laboratory tests before or after treatment were not presented. Only 2 adverse effects were monitored ‐ nausea and headache; no mention of any other adverse effects monitored |
| Other bias | Unclear risk | Diagnosis of non‐dysenteric amoebic colitis was based on demonstration of E histolytica in stools, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Joshi 1975.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 60 enrolled and analysed Inclusion criteria: adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica Exclusion criteria: those who received antiamoebic treatment in the previous 1 month, pregnant women, dehydrated patients, and those with hepatic, renal, haematological, or ECG abnormalities |
|
| Interventions |
Treatment period was extended to 10 days in both groups when 5 days' treatment was inadequate to relieve symptoms or clear the stools of E histolytica |
|
| Outcomes |
|
|
| Notes |
Location: Ahmedabad, India Date: 1975 (date of publication only; actual study period not reported) Source of funding: not stated Tinidazole tablets (Fasigyn) were supplied by Pfizer Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "60 cases of symptomatic intestinal amoebiasis...were randomly allocated to treatment with tinidazole or metronidazole" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and regimens were used (tinidazole 600 mg twice daily and metronidazole 400 mg or 800 mg thrice daily), and treatment was extended to 10 days by the assessor when 5 days' treatment failed to relieve symptoms or clear E histolytica from the stools. Blinding of participants, study personnel, and clinical outcome assessors is not mentioned Comment: Blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 1 to 15 after end of treatment (days 5, 10, and 20 after start of treatment): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 20 to 25 days after end of treatment (day 30 after start of treatment): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | The published report mentions that "sigmoidoscopy was carried out wherever possible before and after treatment", but it is not mentioned in how many cases sigmoidoscopy was carried out. Results of sigmoidoscopy were not reported, although healing of ulcers was reported as one of the criteria for cure |
| Other bias | High risk | Diagnosis of intestinal amoebiasis was based only on stool microscopy demonstrating E histolytica, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR Duration of treatment was determined by persistence of clinical symptoms or E histolytica at end of treatment, and duration of treatment was variable in both groups, which was not considered in the analysis. Among those who showed clinical improvement and cleared E histolytica from the stools, 4 of 29 in the tinidazole group and 10 of 24 in the metronidazole group required 10 days' treatment. Participants were analysed together regardless of duration of treatment It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Kapadia 1968.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 100 enrolled and analysed Inclusion criteria: clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites and/or cysts of E histolytica Exclusion criteria: not stated |
|
| Interventions |
Not stated if chlorhydroxyquinoline and di‐diiodohydroxyquinoline were identical in appearance |
|
| Outcomes |
|
|
| Notes |
Location: Bombay, India Date: 1968 (date of publication only; actual study period not reported) Source of funding: not stated Supply of chlorhydroxyquinoline (Quixalin) from Sarabhai Chemicals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Two groups of randomly allocated 50 cases of amebiasis were treated by chlorohydroxyquinoline and di‐iodohydroxyquinoline...." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | All participants were treated with 2 tablets (250 mg each) of the drug thrice a day for 10 days, but blinding of participants, study personnel, and clinical outcome assessors was not mentioned |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of treatment (day 10): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Low risk | Published report includes pre‐specified outcomes |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based only on stool microscopy demonstrating E histolytica, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Karabay 1999.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 44 enrolled and analysed Inclusion criteria: acute amoebic dysentery and stool specimens positive for E histolytica cysts and/or trophozoites examined by 0.85% saline water, Lugol's solution, and trichrome stain Exclusion criteria: received treatment for diarrhoea in the last 10 days; those with pathogenic bacteria identified in stool culture |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: military hospital in Erzurum, Turkey Date: July 1998 to November 1998 Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were allocated at random into one or other treatment groups..." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and regimens were used (secnidazole 2 g single dose and metronidazole 750 mg thrice daily for 10 days), and blinding of participants, study personnel, and clinical outcome assessors was not mentioned Comment: Blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | Four and 11 days after end of treatment (day 14 and day 21): 1 participant in the metronidazole group missed day 14 follow‐up but came back for day 21 follow‐up. No losses to follow‐up or withdrawals from the secnidazole group. All participants randomized were included in the analysis, even the 3 participants on metronidazole who were non‐compliant with medications |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | High risk | Clinical outcomes were reported only as "average days of clearance of symptoms", but the number of participants analysed for clinical outcomes was not reported. Adverse effects were not reported or mentioned |
| Other bias | Unclear risk | Diagnosis of amoebic dysentery was based only on stool microscopy, and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Mansour‐Ghanaei 2003.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: double (participants, care providers, and outcome assessors – from personal communication with primary author) Inclusion of all randomized participants: 94.7% (54/57)) |
|
| Participants |
Numbers: 57 enrolled; 54 analysed; 3 non‐compliant participants (2 from the group without S boulardii and 1 from the group with S boulardii) were excluded from analysis Inclusion criteria: adults with amoebic dysentery presenting with mucous bloody diarrhoea, fever, and abdominal pain; stool specimens positive for haematophagous trophozoites of E histolytica (laboratory diagnostic method was not specified) Exclusion criteria: pregnant women; those on maintenance haemodialysis, steroids, or chemotherapy |
|
| Interventions |
S boulardii and placebo were identical in appearance |
|
| Outcomes |
|
|
| Notes |
Location: Shahid Beheshti Educational and Therapeutic Center in Shiraz, Iran Date: 21 March 1995 to 21 March 1996 Source of funding: not stated The study author was contacted and kindly provided data on method of blinding; however, no response was obtained regarding method of allocation concealment despite several follow‐up communications |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were then randomized to receive either metronidazole 750 mg and iodoquinol 650 mg thrice a day for 10 days or the same medications plus lyophilized Saccharomyces boulardii 250 mg orally thrice a day" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Reported to be double‐blind From correspondence with primary author: Placebo capsules were identical in appearance to S boulardii capsules Comment: Blinding of participants, study personnel, and clinical outcome assessors was adequate |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Reported to be double‐blind, and blinding of the microscopist examining the stools was probably done |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not determined |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 4 weeks after end of treatment: 2/29 from the metronidazole and iodoquinol group and 1/28 from the metronidazole and iodoquinol plus S boulardii group were excluded because of non‐compliance |
| Selective reporting (reporting bias) | Unclear risk | Published report includes pre‐specified outcomes. It is mentioned that participants reported no adverse reactions to S boulardii, but adverse effects in the group without Saccharomyces were not reported |
| Other bias | Unclear risk | Diagnosis of amoebic dysentery was based on both clinical presentation and presence of (haematophagous) amoeba trophozoites engulfing red blood cells in diarrhoeal stools. However, differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Mathur 1976.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 60 enrolled and 60 analysed Inclusion criteria: adults and adolescents with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica Exclusion criteria: received antiamoebic treatment in the previous 1 month; pregnant women; dehydrated patients; and those with hepatic, renal, hematological, or ECG abnormalities |
|
| Interventions |
Treatment period was extended to 10 days in both groups when 5 days' treatment was inadequate to relieve symptoms or clear the stools of E histolytica |
|
| Outcomes |
|
|
| Notes |
Location: India Date: 1976 (date of publication only; actual study period not reported) Source of funding: not stated Tinidazole tablets (Fasigyn) were supplied by Pfizer Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "60 cases of symptomatic intestinal amoebiasis were randomly allocated to treatment with tinidazole or metronidazole" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and regimens were used (tinidazole 600 mg twice daily and metronidazole 400 mg or 800 mg thrice daily), and treatment was extended to 10 days by the assessor when 5 days' treatment failed to relieve symptoms or clear E histolytica from the stools. Blinding of participants, study personnel, and clinical outcome assessors was not mentioned Comment: Blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 15 to 25 days after end of treatment (day 30): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | The report mentions that sigmoidoscopy was carried out wherever possible before and after therapy. It is not clear in how many cases sigmoidoscopy was done, even if healing of ulcers was 1 criterion for cure |
| Other bias | High risk | Diagnosis of intestinal amoebiasis was based only on stool microscopy demonstrating cysts or trophozoites of E histolytica, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined Duration of treatment was determined by persistent clinical symptoms or presence of E histolytica in the stools at end of treatment. Therefore, duration of treatment varied in both groups, which was not considered in the analysis. Four participants in each group required extension of the treatment period to 10 days |
Misra 1974.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear; reported as "single blind", but it is not stated who among participants, care providers, or outcome assessors was blinded Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 60 enrolled and analysed Inclusion criteria: adults and children with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica by direct smear or concentration method Exclusion criteria: antiamoebic treatment in the preceding 1 month before enrolment; pregnant women; severe anaemia |
|
| Interventions |
Treatment period was extended to 10 days in both groups when 5 days' treatment was inadequate to relieve symptoms or clear the stools of E histolytica |
|
| Outcomes |
|
|
| Notes |
Location: Medical College Hospital in Bhopal, India Date: 1974 (date of publication only; actual study period not reported) Source of funding: Pfizer Ltd for support and for supply of study drugs tinidazole (Fasigyn) and metronidazole (Flagyl) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Ten groups of 30 cases each were at random administered metronidazole and tinidazole" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported as "single‐blind", but it is unclear who was blinded. Different dosages and regimens were used (tinidazole 600 mg twice daily and metronidazole 400 mg thrice daily), and treatment was extended to 10 days when 5 days' treatment failed to relieve symptoms or clear E histolytica from the stools Blinding of the clinical outcome assessor was not specifically mentioned |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Reported as "single‐blind", but it is unclear if the microscopist examining the stools was blinded |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 10 to 15 days after end of treatment (day 20): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 20 to 25 days after end of treatment (day 30): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes |
| Other bias | High risk | Diagnosis of Intestinal amoebiasis was based only on stool microscopy (direct smear or concentration technique) demonstrating cysts or trophozoites of E histolytica, but differentiation from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined Duration of treatment was determined by persistence of clinical symptoms or E histolytica in the stools at end of treatment. Therefore, duration of treatment varied in both groups, which was not considered in the analysis. Treatment had to be extended to 10 days in 4 cases in the tinidazole group and in 5 cases in the metronidazole group, but these were not analysed separately |
Misra 1977.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 60 enrolled and analysed Inclusion criteria: adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica by direct smear or formol‐ether concentration technique, sigmoidoscopy for colonic ulcers, and parasitological examination of sigmoidoscopic scrapings Exclusion criteria: received antiamoebic treatment within the previous 4 weeks; pregnant women; dehydrated patients; evidence of hepatic, renal, haematological, or ECG abnormalities |
|
| Interventions |
Not stated whether tinidazole and metronidazole were identical in appearance |
|
| Outcomes |
|
|
| Notes |
Location: hospital in Bhopal, India Date: 1977 (date of publication only; actual study period not reported) Source of funding: not stated Unclear if Misra 1977 and Misra 1978 reported results for the same group of participants Several attempts were made to contact study authors, but no response was obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sixty patients with symptomatic intestinal amoebiasis were treated for 3 days with a single dose of 2 g of either tinidazole or metronidazole respectively by random order" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Both tinidazole and metronidazole were administered as 2 g single dose for 3 days, but blinding of the participants, study personnel, and clinical outcome assessors was not mentioned |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 20 to 25 days after end of treatment (day 30): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Criteria for cure included healing of ulcers seen on sigmoidoscopy, but these results were not mentioned |
| Other bias | Unclear risk | May be a duplicate of the Misra 1978 trial because of similar methods and numbers of enrolled participants Diagnosis of Intestinal amoebiasis was based on presence of E histolytica in the stools and in sigmoidoscopic scrapings using direct smear and concentration techniques and sigmoidoscopy for colonic ulcers. However, differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Misra 1978.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants: 98.3% (59/60) |
|
| Participants |
Numbers: 60 enrolled; 59 analysed, 1 randomized to tinidazole group excluded because it was discovered later that he had a history of ulcerative colitis Inclusion criteria: adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites and cysts of E histolytica by direct smear or formol‐ether concentration technique, sigmoidoscopy for colonic pathology Exclusion criteria: received antiamoebic treatment in the previous 4 weeks before enrolment |
|
| Interventions |
Not stated whether tinidazole and metronidazole were identical in appearance |
|
| Outcomes |
|
|
| Notes |
Location: hospital in Bhopal, India Date: 1978 (date of publication only; actual study period not reported) Source of funding: not stated Unclear if Misra 1977 and Misra 1978 reported results for the same group of participants Several attempts were made to contact the study author, but no response was obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "According to a predetermined random order, patients were assigned to wither tinidazole or metronidazole" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Blinding of participants, study personnel, and clinical outcome assessors was not mentioned |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 20 to 25 days after end of treatment (day 30): 1/30 in the tinidazole group was excluded from the analysis because of history of ulcerative colitis; no outcome data were missing in the metronidazole group |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes, including presence of colonic pathology on sigmoidoscopy |
| Other bias | Unclear risk | May be a duplicate publication of an earlier trial by the same author (Misra 1977) because of the identical number of enrolled participants and methods, although 1 participant in the tinidazole group was excluded from the analysis of the Misra 1978 trial Diagnosis of intestinal amoebiasis was based on presence of cysts or trophozoites of E histolytica in the stools using direct smear and concentration tests and sigmoidoscopy for colonic ulcers. However, differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Mohammed 1998.
| Methods |
Generation of allocation sequence: random numbers table Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 72.5% (50/69) |
|
| Participants |
Numbers: 69 enrolled; 50 analysed; 19 lost to follow‐up (11 in the praziquantel group, 8 in the metronidazole group); 3 in the praziquantel group had their treatment changed to metronidazole because of lack of response Inclusion criteria: adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for vegetative trophozoite forms (acute amoebic dysentery) or cysts of E histolytica; those who were cyst passers were treated with praziquantel alone and were not included in the review Exclusion criteria: not stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: outpatients in Iraq Date: 1993 to 1995 Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done according to a pre‐designed dispensing list (10 patients each) constructed from a table of random numbers..." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and regimens were used (praziquantel 40 mg/kg in a single dose and metronidazole 800 mg thrice daily), and blinding of participants, study personnel, or clinical outcome assessors was not mentioned Comment: Blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | High risk | One week after treatment: 11/37 missing from the praziquantel group and 8/32 missing from the metronidazole group. No reasons for missing data provided |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | High risk | The published report mentions that at the end of 28 days, "patients were assessed as per W.H.O. criterion." Frequency of loose stools per day and rate of disappearance of parasites in the stools were also reported but were not pre‐specified. Incomplete report of adverse effects (no report for metronidazole) |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based on stool microscopy demonstrating trophozoites or cysts of E histolytica, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Naoemar 1973.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: double (participants, care providers, and outcome assessors) Inclusion of all randomized participants: 100% at end of treatment and 1 month after end of treatment |
|
| Participants |
Numbers: 20 enrolled, 20 analysed Inclusion criteria: adults and children with bloody diarrhoea and stools positive for motile haematophagous trophozoites of E histolytica examined by eosin and iodine smears Exclusion criteria: anaemia or other diseases but exact conditions not stated |
|
| Interventions |
Both drugs given as follows: 2 to 6 years of age – 125 mg daily in 3 divided doses for 7 days; 7 to 12 years of age – 250 mg daily in 3 divided doses for 7 days; adults – 1500 mg daily in 3 divided doses for 5 days Ro 7‐0207 and metronidazole were identical in appearance (light yellow capsules) and were kept in numbered bottles |
|
| Outcomes |
|
|
| Notes |
Location: outpatient clinics in Jakarta, Indonesia Date: 1973 (date of publication only; actual study period not reported) Source of funding: Roche Far East Research Foundation for supply of drugs and support for the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All were given ambulatory treatment with either Ro7‐0207 or metronidazole according to a randomized numbering system" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Reported as "double‐blind", and drugs were given in identical physical forms (light yellow capsules) kept in bottles that were numbered Comment: Blinding of participants, study personnel, and clinical outcome assessors was adequate |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Reported as "double‐blind"; blinding of microscopist examining the stools probably was also done |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of treatment: No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | One month after end of treatment: Outcome for relapse was reported, and no withdrawals or losses to follow‐up were mentioned |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Unclear risk | Diagnosis of amoebic dysentery in children was based on presence of bloody stools with actively motile haematophagous E histolytica in the stools However, differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, and helminth parasites was determined Children and adults in the trial were given different dosages and duration of treatment (7 days in children, 5 days in adults) but were not analysed separately |
Nnochiri 1967.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: double (participants, care providers, and outcome assessors) Inclusion of all randomized participants: 100% at end of treatment; 96.7% (58/60) at 7 weeks after end of treatment |
|
| Participants |
Numbers: 60 with acute amoebic dysentery enrolled; 60 analysed at end of treatment, and 58 (96.8%) analysed 7 weeks after end of treatment Inclusion criteria: military personnel and their families with diagnosis of acute amoebic dysentery and stool specimens positive for E histolytica examined by saline and iodine‐stained smears Exclusion criteria: not stated |
|
| Interventions |
The 2 drug combinations with and without chloroquine were identical in appearance |
|
| Outcomes |
Not included in this review: results of stool examination at 3, 6, and 12 months after treatment; clearance of E histolytica from stools of 36 asymptomatic cyst carriers |
|
| Notes |
Location: Yaba Military Hospital in Lagos, Nigeria Date: August 1965 to July 1966 Source of funding: Messrs Boots Pure Drug Co Ltd, Nottingham, England |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sixty patients with acute amoebic dysentery were admitted...and placed in two groups on a randomized basis" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | From the report: "The two furamide combinations...were encapsulated and the capsules were made to look identical" Comment: Blinding of participants, study personnel, and clinical outcome assessors was done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Although it is not specifically mentioned, blinding of the microscopist examining the stools was probably done |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of treatment: No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 7 weeks after end of treatment: 1/34 from the diloxanide furoate‐tetracycline hydrochloride‐chloroquine phosphate group and 1/26 from the diloxanide furoate‐tetracycline hydrochloride group were missing from the analysis. Reasons for missing data were not reported Note: High attrition rates at 3, 6, and 12 months after end of treatment (10 soldiers treated for amoebic dysentery were transferred and were unable to report for 12‐month follow‐up). Results beyond 7 weeks were not included in the review because re‐infection could not be ruled out |
| Selective reporting (reporting bias) | Unclear risk | Published report included pre‐specified outcomes, although data on adverse effects were incomplete and the number of participants for whom adverse effects was ascertained was not specified for treatment groups |
| Other bias | Unclear risk | Diagnosis of amoebic dysentery was based only on stool microscopy, and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done Stool specimens from all acute dysenteric cases were cultured in appropriate culture media for enteric organisms, but it is not mentioned whether concomitant infection with other protozoa and helminth parasites was determined |
Padilla 2000.
| Methods |
Generation of allocation sequence: coin toss Allocation concealment: unclear Blinding: double‐blind (participants and outcome assessors for clinical and parasitological outcomes blinded; unclear whether care provider (main investigator) who administered the medications was blinded) Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 239 enrolled and analysed Inclusion criteria: children with clinical symptoms of non‐dysenteric amoebic colitis with at least 1 of 3 stool specimens positive for E histolytica cysts examined by direct smear using Faust concentration method Exclusion criteria: history of sensitivity to clioquinol or to metronidazole and its derivatives; children who had received antibacterial and/or antiparasitic drugs in the 15 days before their entry into the study; those with amoebic dysentery |
|
| Interventions |
|
|
| Outcomes |
Not included in this review: acceptability of taste |
|
| Notes |
Location: 2 urban federal elementary schools in Celaya, Guanajuato, Mexico (Urban Federal Elementary schools 'Carmen Serdan' and 'Juan Jesus de los Reyes') Date: 2000 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation (by tossing a coin) was performed progressively as patients were included in the study" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | From the report (blinding of participants and study personnel): "The medications were administered by the main investigator, and both patients and their parents were blinded to the antiamoebic drugs administered by removal of the labels from the bottles; however, the flavours and colours of these drugs are very different and this could have led to bias" From the report (blinding of clinical adverse events and acceptability): "A different investigator carried out a clinical evaluation on the fifth day, and she was also blinded to the patient" Comment: Blinding of participants, study personnel, and clinical outcome assessors was adequate |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | From the report: "The laboratory analyst was also blinded to the medication received by the children" Comment: Blinding of the microscopist examining the stools was done |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 7 days after end of treatment: No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | High risk | Parasitological efficacy was reported, but clinical evaluation included only specific adverse events with no mention of the number and proportion of participants who showed disappearance of or improvement in clinical symptoms after treatment |
| Other bias | Unclear risk | Diagnosis of amoebic dysentery was based only on stool microscopy with concentration techniques used, but differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is not mentioned whether concomitant infection with bacteria, other protozoa, or helminth parasites was determined |
Pamba 1990.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: single (only outcome assessors for parasitological response and rectosigmoidoscopy results were blinded; not stated whether assessors for clinical response were blinded) Inclusion of all randomized participants: 95.9% (400/417) at end of treatment for clinical cure only; for stool examination ‐ 100% (417/417) at end of treatment, 88.5% (369/417) 15 days after start of treatment, 67.6% (282/417) 30 days after start of treatment, and 51.3% (214/417) 60 days after start of treatment |
|
| Participants |
Numbers: 417 enrolled; 369/417 (88.5%) analysed 15 days after start of treatment, 282/417 (67.6%) analysed 30 days after start of treatment, and 214/417 (51.3%) analysed 60 days after start of treatment; recruitment to the etophamide plus aminosidine group was discontinued because of high incidence of diarrhoea; withdrawals not stated for the other groups Inclusion criteria: adults and children with clinical symptoms of intestinal amoebiasis with stool specimens positive for E histolytica by direct smear and a concentration method (not specified) Exclusion criteria: pregnant women; known allergy to the drugs; those with coexisting extraintestinal amoebiasis or other major diseases; treated with antiamoebic drugs in the 30 days before recruitment |
|
| Interventions |
|
|
| Outcomes |
Not included in this review: cumulative daily clearance of E histolytica from stools during treatment, at end of treatment, and on days 15, 30, and 60 after start of treatment; evolution of mild and severe amoebic ulcers seen on rectosigmoidoscopy; and anatomical cure (healing of previous ulceration) |
|
| Notes |
Location: 3 district hospitals of Kiambo, Machakos, and Kilifi in Kenya, Africa Date: 1990 (date of publication only; actual study period not reported) Source of funding: Farmitalia Carlo Erba |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to 6 different treatment groups" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Blinding of participants, study personnel, and clinical outcome assessors was not mentioned. Antiamoebic drugs (aminosidine, etophamide, nimorazole) were given in different dosages, were computed differently for adults and children, and were given singly and in combination. It was reported that "All drugs were administered under direct medical supervision", so the physician administering the drugs probably was not blinded and the clinical outcome assessor was not mentioned |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | From the report: "The persons in charge of stool examination and rectosigmoidoscopy were not informed of the drug being taken" Comment: Blinding of the microscopist examining the stools and the person doing the rectosigmoidoscopy was done |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of treatment: 17/115 in the combination group (all given etophamide‐aminosidine) were not analysed for clinical cure because of high incidence of diarrhoea; no data were missing for the monotherapy group. For parasitological outcomes, all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | High risk | From the report: "The percentage of patients reporting for recheck was 88.5% at 15 days, 67.6% at 30 days and 51.3% at 60 days", but the exact number of missing participants in each of the treatment groups was not given |
| Selective reporting (reporting bias) | High risk | Although clinical and parasitological outcomes defined in the methods were reported, the exact numbers of participants remaining in the study at specified time points were not reported. For parasitological cure, results were reported as cumulative daily clearance of amoebic forms from stools, which was not pre‐specified. Adverse effects or "drug tolerance" was incompletely reported |
| Other bias | High risk | Recruitment of participants in one group (etophamide plus aminosidine) was discontinued early owing to increased adverse effects (severe diarrhoea) Stool microscopy and rectosigmoidoscopy were used to diagnose intestinal amoebiasis and to differentiate invasive from non‐invasive forms, but differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done Other protozoal and bacterial infections (e.g. Campylobacter, Shigella, Balantidium) were mentioned by the trial author as causing ulcerative lesions in the distal gut indistinguishable from those caused by E histolytica, but this was not determined in the trial |
Panggabean 1980.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: reported as "double‐blind", but only care provider was blinded; blinding of participants and outcome assessors was not described Inclusion of all randomized participants: 62.5% (25/40) 1 week after treatment, 42.5% (17/40) 2 weeks after treatment, 27.5% (11/40) 3 weeks after treatment, and 15% (6/40) 4 weeks after treatment |
|
| Participants |
Numbers: 40 enrolled; 25/40 (62.5%) analysed 1 week after treatment, 17/40 (42.5%) analysed 2 weeks after treatment, 11/40 (27.5%) analysed 3 weeks after treatment, and 6/40 (15%) analysed 4 weeks after treatment Inclusion criteria: children with amoebic dysentery presenting with bloody stools and motile haematophagous trophozoites of E histolytica in stools examined by direct smear method with eosin 2% stain Exclusion criteria: not stated Concomitant intestinal infection: 35 participants included in the analysis had concomitant intestinal helminthic infection, and groups were comparable for numbers and types of concomitant intestinal helminthic infection (tinidazole group: Ascaris lumbricoides 10, Trichuris trichiura 26, Ancylostoma 2; ornidazole group: Ascaris lumbricoides 12, Trichuris trichiura 12, Ancylostoma 3) |
|
| Interventions |
Other interventions: Children with concomitant intestinal helminthic infection were given single‐dose pyrantel pamoate 10 mg/kg, and those with trichuriasis were given mebendazole 1 tablet twice daily for 3 consecutive days |
|
| Outcomes |
|
|
| Notes |
Location: outpatient clinic of the Sub‐department of Gastroenterology, Department of Child Health Medical School, General Hospital, Medan, Indonesia Date: January 1978 to June 1978 Source of funding: PT. Pfizer Indonesia and PT. Hoffmann‐La Roche |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "cases were randomly selected for either one of the groups" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported to be a double‐blind trial Quote: "The children were treated ambulatorily and the tablets were administered in the hospital daily under the supervision of the authors, without knowing which drug was being given" Comment: Participants and study personnel were blinded, but blinding of the clinical outcome assessor was not mentioned. It is unclear whether those administering the drugs are also the clinical outcome assessors |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of treatment (day 3): 4/20 missing from the tinidazole group (3 did not complete treatment, 1 did not return for follow‐up); 3/20 missing from the ornidazole group (1 did not return for follow‐up, reasons for 2 were not reported) |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | High risk | Four weeks after treatment: 15/20 missing from the tinidazole group (3 did not complete treatment, 14 did not return for follow‐up); 19/20 missing from the ornidazole group (17 did not return for follow‐up, reasons for 2 were not reported) |
| Selective reporting (reporting bias) | Unclear risk | Overall clinical and parasitological cure rates were reported until the end of the fourth week of follow‐up, but dropout rates for the 2 groups were high, and numbers for those who returned for follow‐up visits were decreasing |
| Other bias | Unclear risk | Trial enrolled only those children with bloody stools who showed motile trophozoites of E histolytica containing red blood cells in diarrhoeal stool. However, only stool microscopy was used to diagnose amoebic dysentery, and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, and helminth parasites was determined |
Pehrson 1983.
| Methods |
Generation of allocation sequence: unclear (unrecalled by primary author during personal communication) Allocation concealment: inadequate – no attempts to conceal treatment allocation (personal communication with primary author) Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 41 enrolled and analysed Inclusion criteria: adults and children with clinical symptoms of intestinal amoebiasis but no signs of invasion (e.g. no fever or acute dysentery) and stool specimens positive for trophozoites or cysts of E histolytica by direct smear or formol‐ether concentration technique by Ridley and Hawgood; had not received any antiamoebic drug during the previous year Exclusion criteria: acute dysenteric amoebiasis; liver abscess Concomitant intestinal infection: 17 participants had concomitant infection with other intestinal organisms (Giardia lamblia 9, Campylobacter jejuni 2, Hymenolepsis nana 1, Ascaris lumbricoides 1, Trichuris trichiura 1, Salmonella paratyphi A 1), but the distribution in the 2 groups was not specified |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: hospital in Stockholm, Sweden Date: 1983 (date of publication only; actual study period not reported) Source of funding: not reported The study author was contacted and kindly provided further data. Details on method of randomization could not be recalled by the trial author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In a predetermined, random order, the patients were allocated to two groups..." From correspondence with primary trial author: unrecalled method of randomization |
| Allocation concealment (selection bias) | High risk | From correspondence with primary trial author: no method used to conceal allocation sequence |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and frequencies were used (tinidazole 40 mg/kg in 1 daily dose for 5 days; combined tinidazole plus diloxanide furoate 20 mg/kg divided into 3 daily doses for 10 days), and blinding of participants and study personnel was not mentioned From correspondence with primary trial author: no method used to blind participants and study personnel |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Unclear if the microscopist examining the stools was blinded |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not determined |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | One month after end of treatment: No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Study report does not include results for clinical outcomes that would be expected to be reported for such a study |
| Other bias | Unclear risk | Diagnosis of non‐invasive amoebiasis was based only on presence of E histolytica on stool microscopy (direct microscopy and concentration technique), and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done Twelve participants had concomitant protozoal or helminth infection (9 with Giardia lamblia, 1 with Hymenolepsis nana, 1 with Ascaris lumbricoides, and 1 with Trichuris trichiura) and 5 had concomitant bacterial infection (2 with Shigella flexneri, 2 with Campylobacter jejuni, 1 with Salmonella paratyphi A). It is not specified whether these concomitant organisms were equally distributed in the two groups although the trial author reported that "the presence of parasites did not seem to affect the outcome of the treatment" |
Pehrson 1984.
| Methods |
Generation of allocation sequence: unclear (unrecalled by primary author during personal communication) Allocation concealment: inadequate – no attempts to conceal treatment allocation (personal communication with primary author) Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 30 enrolled and analysed Inclusion criteria: adults with clinical symptoms of intestinal amoebiasis but no signs of invasion (e.g. no fever or acute dysentery) and stool specimens positive for trophozoites or cysts of E histolytica examined by direct smear or formol‐ether concentration technique Exclusion criteria: not stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: Stockholm, Sweden Date: 1984 (date of publication only; actual study period not reported) Source of funding: not reported The study author was contacted and kindly provided further data. Details on method of randomization could not be recalled by the trial author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "Thirty consecutive, diagnosed cases of noninvasive amoebiasis...were randomly allocated in two groups" From correspondence with primary author: unrecalled method of randomization |
| Allocation concealment (selection bias) | High risk | From correspondence with primary trial author: No method was used to conceal allocation of treatment assignment |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | From correspondence with primary author: No method was used to blind participants and study personnel |
| Blinding (performance bias and detection bias) Parasitological outcomes | High risk | Stools were "examined by two very experienced laboratory technicians", but blinding of these lab technicians was not mentioned. Given that the study author confirmed that this was an open study, laboratory technicians probably were not blinded |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not determined |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | One month after end of treatment (day 30): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Study report does not include results for resolution of abdominal symptoms or results of specific adverse effects |
| Other bias | Unclear risk | Diagnosis of non‐invasive amoebiasis was based only on demonstration of cysts or trophozoites of E histolytica on stool microscopy (direct smear and concentration technique), but differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done Bacterial causes of diarrhoea ware excluded by cultures; sigmoidoscopy and colon X‐ray ware performed to rule out ulcerative colitis |
Prasad 1985.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: coded drug containers; code broken only at the end of the trial Blinding: double (participants, care providers, and outcome assessors) Inclusion of all randomized participants: 91.1% (164/180) |
|
| Participants |
Number: 180 patients with amoebiasis or giardiasis or both were enrolled; 164/180 (91.1%) were analysed, 90 with amoebiasis alone, 47 with giardiasis, and 27 with mixed infection with amoebiasis and giardiasis; 16/180 (8.9%) did not complete treatment and were dropped from the trial, but it is not stated whether those who dropped out had amoebiasis, giardiasis, or mixed infection Inclusion criteria: children with clinical symptoms of intestinal amoebiasis or giardiasis (diarrhoea, abdominal pain, dysentery, gastrocolic urgency, etc.) and whose stools were positive for amoebae or Giardia Exclusion criteria: not stated Concomitant intestinal infection: Ascaris lumbricoides present in 20%, Ancylostoma duodenale 9.9%, Enterobius vermicularis 1.8%, but distribution in the 2 groups not reported |
|
| Interventions |
|
|
| Outcomes |
Not included in this review: clinical and parasitological response in those with mixed amoebiasis and giardiasis infection; 12/63 from the metronidazole group and 15/101 from the fixed‐drug combination metronidazole plus furazolidone group had mixed amoebiasis and giardiasis and were not included in this review |
|
| Notes |
Location: paediatric outpatient department of S.N. Medical College, Agra, India Date: 1985 (date of publication only; actual study period not reported) Source of funding: not stated Attempts made to contact study authors were unsuccessful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "180 patients who entered the trial were randomly divided into two treatment groups" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Quote: "The codes of the two drugs were broken at the end of the trial..." Comment: Blinding of participants, study personnel, and clinical outcome assessors was not specifically mentioned but was implied |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Although not specifically mentioned, blinding of the microscopist examining the stools was probably done because it is mentioned that the "codes of the two drugs were broken at the end of the trial" |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | At end of treatment (day 7): 16 out of 180 participants enrolled did not complete treatment and were dropped from the trial, but the actual number and treatment groups to which these non‐compliant participants were randomized were not reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | High risk | Method for outcome evaluation was not pre‐specified. For those classified as "partial cure", it is unclear whether this pertains to clinical or parasitological outcome |
| Other bias | High risk | Diagnosis of amoebiasis was based on demonstration of cysts or trophozoites of E histolytica on stool microscopy, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR Participants with both amoebiasis and giardiasis were analysed separately, and only those with single infection with amoebiasis were included in this review Concomitant infection with other helminth parasites (Ascaris lumbricoides, Ancylostoma duodenale, Enterobius vermicularis) was determined, but distribution in the 2 groups was not reported Treatment duration was not uniform for all participants because duration of the treatment period ranged "from 5 to 10 days depending on the severity of disease and response to the therapy" |
Pudjiadi 1973.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: sequentially numbered coded drug containers supplied by Roche Far East Research Foundation, Hong Kong; sealed envelope containing the list of drugs opened only after the entire trial was finished Blinding: double (participants, care providers, and outcome assessors) Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 20 enrolled and analysed Inclusion criteria: children with bloody diarrhoea and stools positive for E histolytica examined by eosin and Lugol's solution Exclusion criteria: not stated Concomitant intestinal infection: Ascaris lumbricoides found in faeces of 6 participants, Trichuris trichiura found in faeces of 6 participants, but distribution in the 2 groups not specified |
|
| Interventions |
Both drugs were given as follows: up to 2 years of age – 62.5 mg, 2 to 6 years of age – 125 mg, and 6 to 12 years of age ‐ 250 mg daily, divided into 3 daily doses for 7 days |
|
| Outcomes |
|
|
| Notes |
Location: hospital at the Department of Child Health, Medical School University of Indonesia, Jakarta, Indonesia Date: 1973 (date of publication only; actual study period not reported) Source of funding: Roche Far East Research Foundation for supply of drugs and study grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The list stating which bottles contained Ro 7‐0207 or metronidazole was sent by Roche Far East Research Foundation, Hong Kong in a sealed envelope..." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "The list stating which bottles contained Ro 7‐0207 or metronidazole was sent by Roche Far East Research Foundation, Hong Kong in a sealed envelope and was only opened after the entire trial was finished" Comment: Allocation concealment was adequate |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Quote: "A double‐blind set containing ten bottles of Ro 7‐0207 125 mg capsules and 10 bottles of metronidazole capsules about 125 mg was supplied by the Roche Far East Research Foundation, Hong Kong. The bottles were numbered 191‐210 and contained either Ro 7‐0207 or metronidazole. The first admitted case was treated with capsules from bottle 191, the second with those from bottle 192, etc" Comment: Blinding of participants, study personnel, and clinical outcome assessors was adequate |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Although not specifically mentioned, blinding of the microscopist examining the stools was probably done |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of treatment (after 7 days of treatment): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based on stool microscopy demonstrating E histolytica, but differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done Concomitant infection with Ascaris lumbricoides and Trichuris trichiura was found in 6 cases each, but in which treatment group was not specified Concomitant infection with pathogenic bacteria and other protozoa was not determined |
Rossignol 2001.
| Methods |
Generation of allocation concealment: unclear Allocation concealment: unclear Blinding: double (participants, care providers, and outcome assessors) Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 91 enrolled but only 67 (74%) had Entamoeba histolytica (53 with single and 14 with mixed Giardia and Entamoeba infection); 67 analysed Inclusion criteria: adults and children with diarrhoea and stool specimens positive for cysts or trophozoites of E histolytica and/or E dispar alone or with concomitant Giardia intestinalis by direct smear, concentration technique, Ziehl‐Neelsen stain, and an immunofluorescent assay (MeriFluor Meridian Diagnostics) Exclusion criteria: pregnant women; using any drug with antiprotozoal activity within 2 weeks of enrolment; known to have or suspected or acquired immunodeficiency syndrome (AIDS) Concomitant intestinal infection: mixed Entamoeba histolytica and Giardia intestinalis infection in 6/36 (17%) participants in the nitazoxanide group and in 8/31 (26%) in the placebo group |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: outpatient clinic of the Department of Hepatology, Gastroenterology, and Infectious Diseases of the Benha University Hospital, governorate of Kalubia, Nile Delta, Egypt Date: 2001 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Reported as a double blind placebo‐controlled trial, where "each of the patients received 1 nitazoxanide 500mg yellow film‐coated tablets or a matching placebo tablet twice daily for 3 consecutive days" The trial author also reported that patients, personnel assessing clinical response, and laboratory personnel evaluating stool samples were blinded |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Laboratory personnel evaluating stool samples were blinded |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 5 days after end of treatment (day 7): 1/48 in the nitazoxanide group and 1/42 in the placebo group withdrew from the study before taking any study medication and were excluded from the analysis. Of those included in the study, 53 with E histolytica/E dispar alone (30 in the nitazoxanide group and 23 in the placebo group) were analysed for clinical cure, and 67 with E histolytica/E dispar and Giardia intestinalis (36 in the nitazoxanide group and 31 in the placebo group) were analysed for parasitological cure. No data were missing from both treatment groups |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based on stool microscopy demonstrating E histolytica, but differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done Stool culture was done to identify bacterial causes of diarrhoea, but other protozoa or helminth parasites were not identified |
Rossignol 2007.
| Methods |
Generation of allocation sequence: computer‐generated randomization Allocation concealment: adequate Blinding: double (participants, care providers, outcome assessors) Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 100 enrolled and 100 analysed; 2 participants in the placebo group lost to follow‐up and considered treatment failures Inclusion criteria: adults and children with diarrhoea; ≥ 1 enteric symptom; E histolytica/E dispar trophozoites identified in stool by microscopic examination using direct smear and concentration technique; stool positive for E histolytica by antigen‐based ELISA Exclusion criteria: other enteric pathogens identified by Ziehl‐Neelsen stain, immunofluorescent assay (MeriFluor Meridian Diagnostics), and stool culture; pregnant and lactating women; using any drug with antiprotozoal activity within 2 weeks of enrolment; and known or suspected to have acquired immunodeficiency syndrome (AIDS) or other immune deficiencies |
|
| Interventions |
|
|
| Outcomes |
Not included in this review: survival analysis of time from first dose to passage of last unformed stools (survival graph) |
|
| Notes |
Location: outpatient clinic of the Benha University Hospital, Benha, Egypt Date: 17 February 2004 to 2 October 2005 Source of funding: Romark Laboratories, L.C. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list was used |
| Allocation concealment (selection bias) | Low risk | Quote: "Upon enrolment, each patient was sequentially assigned a number corresponding to the number on his/her package of study medication" Comment: Allocation concealment was adequate |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Reported as a double‐blind placebo‐controlled trial, where "patients, principal investigators and their staffs, laboratory personnel and the study monitors were blinded" Trial reports that "packaging of study medications were prepared by the study sponsor" Comment: Blinding of participants, study personnel, and clinical outcome assessors was adequate |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Laboratory personnel evaluating stool samples were blinded |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 5 days after end of treatment (day 7): No data were missing from both treatment groups. Analysis was conducted for all participants randomised to the study and using a modified intention‐to‐treat population from which participants with no E histolytica cysts or trophozoites in their baseline stool sample and those with other identified enteric pathogens in their stool samples were excluded |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes were clinical response at day 7 and microbiological response between days 7 and 10. Survival analysis graph showing time from first dose to passage of last unformed stool was not pre‐specified |
| Other bias | Low risk | Study appears to be free of other sources of bias. Only those confirmed to be positive for E histolytica by the stool antigen ELISA test were included. Those with other identified enteric pathogens were excluded |
Rubidge 1970.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 39 enrolled and analysed Inclusion criteria: children with amoebic dysentery presenting with acute onset of diarrhoea with blood, mucus, and actively motile haematophagous trophozoites of E histolytica in stool specimens examined by direct smear and zinc sulphate flotation technique Exclusion criteria: not stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: hospital in Durban, South Africa Date: 1970 (date of publication only; actual study period not reported) Source of funding: not stated; metronidazole was supplied by Messrs May and Baker, Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "children were randomly allocated to one of the following two treatment schedules" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and regimens were used (metronidazole for 7 days; combination of dehydroemetine subcutaneous injection plus tetracycline for 7 days and diloxanide furoate for 10 days), and no blinding of participants, study personnel, and clinical outcome assessors was mentioned Comment: Blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 10 to 12 days after end of treatment (day 20 or 22): No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | After day 55: 1/19 in the combination dehydroemetine, tetracycline, and diloxanide furoate group was lost to follow‐up. No loss to follow‐up was mentioned in the metronidazole group |
| Selective reporting (reporting bias) | High risk | Outcomes and timing of determination of outcomes were not pre‐specified |
| Other bias | Unclear risk | Trial enrolled only children with amoebic dysentery, defined as acute bloody stools with motile haematophagous trophozoites of E histolytica in their stools However, only stool microscopy (using direct smear and zinc sulphate flotation technique) was used to demonstrate E histolytica in the stools, and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, and helminth parasites was determined |
Salles 1999.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 90.7% (275/303) included in evaluation for clinical efficacy; 99% (300/303) included in evaluation for parasitological efficacy |
|
| Participants |
Numbers: 303 enrolled; 275/303 (90.7%) included in evaluation for clinical efficacy; 300/303 (99%) included in evaluation for parasitological efficacy Inclusion criteria: children with clinical symptoms of intestinal amoebiasis with stool specimens positive for E histolytica by direct smear using the Faust and Katz method and no history of intolerance to imidazole drugs Exclusion criteria: history of vomiting in the past 48 hours; taken antiemetic drugs in the past 24 hours; treated with antiamoebic drugs in the past 15 days; symptoms of extraintestinal amoebiasis Concomitant intestinal infection: Groups were comparable for presence of other intestinal parasites (Ascaris lumbricoides, Tricuris trichiura, Giardia lamblia, Necator americanus, Ancylostoma, Hymenolepsis nana, Schistosoma, Enterobius vermicularis, Endolimax nana), except Strongyloides stercoralis, which was more frequent in the tinidazole group (3 participants) than in the secnidazole group (11 participants) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: 5 different centres in Brazil Date: 1999 (date of publication only; actual study period not reported) Source of funding: not stated One study author (Valfredo Costa) is connected with Rhodia Farma Ltd, the manufacturer of Secnidal (secnidazole) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly divided into 2 groups" Comment: insufficient information about the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Reported to be an open comparative multi‐centre study |
| Blinding (performance bias and detection bias) Parasitological outcomes | High risk | Reported to be an open comparative multi‐centre study |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Clinical and laboratory responses were determined on days 7 and 14 (5 or 12 days after end of treatment), but results were not reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | 19 days after end of treatment (day 21): Proportion remaining in the trial was 99.0% (300/303) for parasitological efficacy: 2/156 missing data from the secnidazole group and 1/147 missing data from the tinidazole group did not complete all 3 stool tests and were not included in the laboratory efficacy analysis. For clinical efficacy, proportion remaining was 90.7% (275/303): 18/156 missing data from the secnidazole group and 10/147 from the tinidazole group; reasons for missing data were not reported. Imbalance in quantity of missing data between the 2 groups and in the proportion of missing outcomes (18/156; 11.5%) compared with observed event risk (10/138; 7.2%) in the secnidazole group may induce clinically relevant bias in the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based on stool microscopy demonstrating E histolytica in the stools, but differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done Other parasites were identified in the 2 groups (Ascaris lumbricoides, Trichuris trichiura, Giardia lamblia, Strongyloides stercoralis) and were not statistically different, except Strongyloides stercoralis, which was more frequently found in the tinidazole group (P = 0.02). Concomitant infection with pathogenic bacteria or other protozoa was not determined |
Savas‐Erdeve 2009.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: adequate Blinding: open Inclusion of all randomized participants: 94.4% (85/90) |
|
| Participants |
Numbers: 90 enrolled; 85/90 (94.4%) analysed; 5 in the metronidazole plus Saccharomyces boulardii group excluded because of non‐compliance Inclusion criteria: children from 1 to 15 years of age who presented with E histolytica‐associated diarrhoea, defined as presence of compatible clinical presentations (acute diarrhoea, fever, and abdominal pain) and presence of E histolytica trophozoite engulfing red blood cells in diarrhoeal stool by light microscopy (fresh and trichrome staining) Exclusion criteria: children with severe intercurrent illnesses treated by any other antidiarrhoeal/antibiotics within 2 months, treated by probiotics within 1 week, severely malnourished, or with chronic disease/immune deficiency Concomitant intestinal infection: Stool cultures were obtained from all participants, and no positive stool cultures were reported for participants |
|
| Interventions |
|
|
| Outcomes |
Not included in this review: survival analysis graph of the number of stools per day during the 10‐day treatment period |
|
| Notes |
Location: outpatient in Turkey Date: January 2006 to April 2007 Source of funding: not stated The study author was contacted and kindly provided data on location (outpatient), type of amoebiasis (amoebic dysentery), randomization (randomly numbered by another person), allocation concealment (sequentially numbered sealed envelopes), and clinical outcomes (all improved by end of 10‐day treatment period) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 90 children were randomized into two groups" Comment: insufficient information about the sequence generation process even after correspondence |
| Allocation concealment (selection bias) | Low risk | From correspondence: "Envelopes were opaque and were prepared by a physician who was blind to the study. After preparation they were randomly numbered by another person" Comment: Allocation concealment was adequate |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Reported to be an "open prospective study" |
| Blinding (performance bias and detection bias) Parasitological outcomes | High risk | Reported to be an "open prospective study" |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | 14 days after end of treatment: 5/45 from the metronidazole plus S boulardii group were excluded because of non‐compliance with the study; none were missing from the metronidazole group Comment: Imbalance in quantity of missing data between the 2 groups and in the proportion of missing outcomes (5/45; 11%) compared with observed event risk (3/40; 7.5%) in the group receiving S boulardii may induce clinically relevant bias in intervention effect estimate |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes. Safety outcome were not pre‐specified, but study authors did not mention in the discussion that no side effects occurred among enrolled participants |
| Other bias | Unclear risk | Trial enrolled only children with clinical symptoms and presence of E histolytica engulfing red blood cells in diarrhoeal stools compatible with amoebic dysentery. However, trial author states as one limitation failure to do more specific diagnostic tests for amoebic dysentery such as stool antigen ELISA test or PCR to differentiate E histolytica from non‐pathogenic species Other causes of dysentery were ruled out by obtaining stool cultures on enrolment, but the presence of other protozoa or helminth parasites was not determined |
Shah 2016.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants: 88.5% (184/208); 8 patients did not agree to participate in the clinical trial, 6 patients dropped out owing to poor response, 4 patients were excluded owing to some serious side effects, and 4 were dropped because of allergic reaction |
|
| Participants |
Numbers: 184 patients complied with the criteria for inclusion ‐ 93 in the Herbal drug group and 91 in the metronidazole group Inclusion criteria: patients suffering from amoebiasis infection (confirmed by stool microscopy and antibody detection tests); no previous treatment against amoebiasis; living in Bahawalpur and Karachi division Exclusion criteria: concurrent physical illness, e.g. uncontrolled hypertension and diabetes mellitus; previous gastrointestinal surgery; any drug interaction or hypersensitivity; pregnant females; chronic diseases such as tuberculosis and cardiac myopathies; hospitalized for any serious disease |
|
| Interventions |
|
|
| Outcomes |
Not included in this review: improvement in intensity of symptoms |
|
| Notes |
Location: hospital, multi‐centre (Shifa‐ul‐mulk Memorial Hospital, Hamdard University Karachi, Hakeem, Pakistan)
Muhammad Said Shaheed Memorial Research Center, Bahawalpur and Bahawalpur Victoria Hospital, Bahawalpur Date: March 2010 to February 2012 Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to receive either herbal medicine or control allopathic treatment" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Described to be a "double blind, multicenter evaluation", but it is unclear who was blinded Comment: insufficient information on how blinding of participants, study personnel, and clinical outcome assessors was ensured |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Described to be a "double blind, multicenter evaluation", but it is unclear who was blinded Comment: insufficient information on how blinding of participants, study personnel, and clinical outcome assessors was ensured |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | At end of treatment: 184 patients who were included were analysed |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Unclear risk | Published report included pre‐specified outcomes. Adverse effects were incompletely reported. The treatment group to which the 4 participants who experienced serious side effects and the 4 who developed allergic reactions were assigned is not mentioned. It is reported that 57.4% of participants on metronidazole experienced mild side effects, including nausea and vomiting, but no further details were given. How many in the herbal group experienced adverse effects is not mentioned |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based on stool microscopy demonstrating E histolytica in the stools and antibody detection test, but differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done. At baseline, not all participants were positive on stool microscopy for amoebiasis Concomitant infection with pathogenic bacteria or other protozoa was not determined |
Siddiqui 2015.
| Methods |
Generation of allocation sequence: adequate Allocation concealment: adequate Blinding: unclear Inclusion of all randomized participants: 89.5% (153/171) |
|
| Participants |
Numbers: 171 enrolled; 153 analysed; 18/171 were not included in the analysis: 8/86 from the combination metronidazole + diloxanide furoate (7 refused to submit a second stool specimen; 1 left the city); 10/85 from the herbal group (8 refused to submit a second stool specimen; 2 changed physicians) Inclusion criteria: between the ages of 5 and 60 years with symptoms of amoebiasis (abdominal pain, blood in stool, or diarrhoea) and positive for E histolytica cyst or trophozoite by direct smear, Lugol's iodine smear, zinc sulphate floatation preparation, or formalin‐ether sedimentation method Exclusion criteria: congenital malformation, chronic diseases such as tuberculosis, or comorbid condition such as hypertension and diabetes; known hypersensitivity to study drugs; any other infection as shown by laboratory investigation |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: outpatient department of 2 centres in Pakistan (Shifa‐Ul‐Maluk Hospital, Gadap and Zahida Medical Centre, North Karachi) Date: October 2008 to December 2009 Source of funding: Hamdard University (Karachi, Pakistan) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Marked papers were prepared by a person who was not part of research team. Half (five) of each block of 10 were marked ‘Treatment Group 1' (TR1) and the rest marked as ‘Treatment Group 2' (TR2). Each eligible participant was invited to pick blindly, one sheet out of 10 available" Comment: adequate sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Marked papers were prepared by a person who was not part of research team" "These sheets were pulled out by the patient from a drawer at the time of informed consent, so allocation was concealed" Comment: adequate allocation concealment |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported to be a randomized double‐blind clinical trial Quote: "...physician and laboratory person were also blinded for the type of treatment" Comment: Although the physician was reported to be blinded, the formulations of the 2 study drugs, the regimen, and the duration were very different, and it is unclear how the physician and participants were blinded to the type of treatment received. It is not mentioned whether those administering the drugs were also the clinical outcome assessors. Attempts to contact the primary author for clarification were unsuccessful |
| Blinding (performance bias and detection bias) Parasitological outcomes | Low risk | Reported to be a randomized double‐blind clinical trial Quote: "...physician and laboratory person were also blinded for the type of treatment" Comment: Although the formulations of the 2 study drugs, the regimen, and the duration were very different, the laboratory person examining the stools probably was blinded |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | 5 days after end of treatment: 8/86 dropped out from the combination metronidazole + diloxanide furoate group (7 refused to submit a second stool specimen; 1 left the city); and 10/85 dropped out from the herbal group (8 refused to submit a second stool specimen; 2 changed physicians) Overall missing data are 10.5% (18/171). Except for 1 who left the city and 2 who remained symptomatic, 15 were symptom‐free but were not included in the analysis of clinical outcomes |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based on stool microscopy demonstrating E histolytica in the stools, but differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is unclear whether participants with other intestinal infections were not enrolled |
Singh 1977.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 93.3% (56/60) |
|
| Participants |
Numbers: 60 enrolled; 56 analysed; 3 participants in the tinidazole group and 1 in the metronidazole group did not comply with the regimen and were excluded from analysis Inclusion criteria: adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica by direct smear or formol‐ether concentration technique Exclusion criteria: received antiamoebic treatment in the previous 4 weeks before enrolment; pregnant women; dehydrated patients; evidence of hepatic, renal, haematological, or ECG abnormalities Concomitant intestinal infection: 12 had concomitant giardiasis, 6 in each group |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: medical outpatient department of the Government Medical College and Hospital, Patiala India Date: 1977 (date of publication only; actual study period not reported) Source of funding: not stated; tinidazole was supplied by Pfizer Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were allocated either to tinidazole or to metronidazole by random order" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different drugs and regimens were used (tInidazole 500 mg × 4 tabs and metronidazole 400 mg × 5 tabs once daily for 3 days), and blinding of participants, study personnel, and clinical outcome assessors was not mentioned Comment: The appearance of the drugs was not mentioned, and blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 28 days after end of treatment (day 30): 3/30 in the tinidazole group and 1/30 in the metronidazole group did not comply with the treatment regimen and were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Published report includes pre‐specified outcomes |
| Other bias | Unclear risk | Diagnosis of Intestinal amoebiasis was based only on demonstration of cysts or trophozoites of E histolytica on stool microscopy (direct smear or concentration technique), but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR Six participants each in the 2 treatment groups had concomitant giardiasis, although this probably did not introduce additional bias because of equal distribution between the 2 groups. It is not mentioned whether concomitant infection with pathogenic bacteria or helminth parasites was determined |
Sitepu 1982.
| Methods |
Generation of allocation sequence: random numbers table Allocation concealment: unclear Blinding: unclear; reported as "double‐blind", but the procedure for blinding participants, care providers, and outcome assessors was not described Inclusion of all randomized participants: 82% (41/50) included in analysis on third day or 2 days after treatment, 36% (18/50) 1 week after treatment |
|
| Participants |
Numbers: 50 enrolled; 41/50 (82%) analysed on the third day or 2 days after treatment, 18/50 (36%) analysed 1 week after treatment Losses to follow‐up: 9/51 (18%) were lost to follow‐up by the third day or 2 days after treatment ‐ 7 participants in the tinidazole group and 2 in the ornidazole group; 32/50 (64%) were lost to follow‐up 1 week after treatment ‐ 18 in the tinidazole group and 14 in the ornidazole group Inclusion criteria: children with amoebic dysentery presenting with bloody diarrhoea and motile haematophagous trophozoites of E histolytica in stools examined by direct smear method with eosin 1% stain Exclusion criteria: not stated Concomitant intestinal infection: trichuriasis (12 in tinidazole group and 15 in ornidazole group) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: outpatient clinic of the Pediatric Gastroenterology Subdivision, Department of Child Health, School of Medicine, University of North Sumatra/Dr Pirngadi Hospital, Medan, Indonesia Date: August 1978 to May 1979 Source of funding: PT. Pfizer Indonesia and PT. Hoffmann‐La Roche |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation to the tinidazole and ornidazole groups was done by random numbers" Comment: probably refers to table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported to be a double‐blind trial, but it is unclear who was blinded Comment: insufficient information on how blinding of participants, study personnel, and clinical outcome assessors was ensured |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Reported to be a double‐blind study, but blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | High risk | One day after treatment (day 2): 7/26 missing from the tinidazole group and 2/24 missing from the ornidazole group. Reason for non‐inclusion in the analysis was inability to return for at least 2 follow‐up visits. Imbalance in loss to follow‐up between the 2 groups may induce clinically relevant bias in the intervention effect estimate |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | High risk | One week after treatment: 18/26 missing from the tinidazole group and 14/24 missing from the ornidazole group. Reason for non‐inclusion in the analysis was inability to return for at least 2 follow‐up visits. The high number of losses to follow‐up in the 2 groups may induce clinically relevant bias in the intervention effect estimate |
| Selective reporting (reporting bias) | High risk | Only patients who returned for at least 2 follow‐up visits were included in the final evaluation. Outcomes for those who had only 1 evaluation were not reported. Adverse effects were not reported |
| Other bias | Unclear risk | Trial enrolled only children with bloody stools who showed motile trophozoites of E histolytica containing red blood cells in diarrhoeal stools. However, only stool microscopy was used to diagnose amoebic dysentery, and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is unclear how concomitant trichuriasis can affect evaluation of clinical response to antiamoebic drugs, but concomitant trichuriasis was found in similar numbers of children in the 2 groups (12 in the tinidazole group and 15 in the ornidazole group) |
Soedin 1985.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Number: 80 enrolled and analysed Inclusion criteria: children with clinical symptoms of acute intestinal amoebiasis with stool specimens positive for trophozoites or haematophagous forms of E histolytica Exclusion criteria: not stated |
|
| Interventions |
Co‐intervention: 2 cases in secnidazole group were given spasmolytics (unspecified) for stomach cramps |
|
| Outcomes |
|
|
| Notes |
Location: outpatient in the Padang Bulan Health Centre, Medan, Indonesia Date: September 1982 to September 1983 Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: Patients were "randomly allocated to one or the other of two treatment groups" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and regimens were used (secnidazole 2 g single dose; combination of tetracycline 750 mg given as 2 capsules thrice daily plus clioquinol 1 g given as 4 tablets once daily for 5 days). Blinding of participants, study personnel, and clinical outcome assessors was not mentioned Comment: Blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of treatment (day 5): No data were missing and no withdrawals or dropouts were reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 22 days after end of treatment (day 28): No data were missing and no withdrawals or dropouts were reported |
| Selective reporting (reporting bias) | High risk | Participants were asked to return to the clinic on days 1 to 7, 14, 21, and 28 for assessment of clinical and parasitological efficacy, but clinical cure was reported only until day 5, while parasitological failure was reported until day 28. Clinical outcomes on day 28 were not reported |
| Other bias | Unclear risk | Trial enrolled only children with bloody stools who showed trophozoites or haematophagous forms of E histolytica in the stools. However, only stool microscopy was used to diagnose amoebic dysentery, and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
Swami 1977.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants: 93.3% (56/60) |
|
| Participants |
Numbers: 60 enrolled; 56/60 (93.3%) analysed; 3/60 (5%) lost to follow‐up after day 4 (1 in tinidazole group, 2 in metronidazole group); 1 participant in the metronidazole group subsequently found to have amoebic liver abscess was excluded from the final analysis Inclusion criteria: adults with clinical symptoms of intestinal amoebiasis and stool specimens positive for trophozoites or cysts of E histolytica Exclusion criteria: received antiamoebic treatment in previous 4 weeks; pregnant women; patients with marked dehydration; concomitant serious illness (not specified) Type of amoebic colitis: tinidazole group: amoebic dysentery 20/29, non‐dysenteric amoebic colitis 9/29; metronidazole group: amoebic dysentery 22/27, non‐dysenteric amoebic colitis 5/27 |
|
| Interventions |
Treatment was extended if E histolytica persisted in the stool on the day following the last treatment period |
|
| Outcomes |
Not included in this review: number of participants who required extension of treatment beyond 3 days |
|
| Notes |
Geographic location: Visakhapatnam, India Date: 1977 (date of publication only; actual study period not reported) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients received either tinidazole or to metronidazole according to a randomization schedule" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Blinding of participants, study personnel, and clinical outcome assessors was not mentioned. Both tinidazole and metronidazole were administered in a single daily dose of 2 grams on 3 consecutive days. It is reported that "treatment period was extended if Entamoeba histolytica persisted in the stools following the last treatment day" Comment: Blinding of participants, study personnel, and clinical outcome assessors was unclear, and the appearance of the 2 drugs was not described |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 15 to 25 days after end of treatment (day 30): 1/30 missing data in the tinidazole group (owing to failure to return for follow‐up after day 4); 3/30 in the metronidazole group (2 did not return for follow‐up after day 4, 1 had concomitant amoebic liver abscess). Overall, 56/60 (93.3%) were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Published report included pre‐specified outcomes |
| Other bias | High risk | Diagnosis of intestinal amoebiasis was based only on stool microscopy demonstrating trophozoites or cysts of E histolytica, but differentiation of E histolytica from non‐pathogenic species was not done by more specific tests such as stool antigen ELISA or PCR It is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined Duration of treatment varied and was determined by persistence of E histolytica in the stools 1 day after treatment. Treatment was extended beyond the planned 3 days of treatment for 3 participants in the tinidazole group (4 days in 1 case and 5 days in 2 cases) and for 10 participants in the metronidazole group (5 days in 4 cases, 6 days in 4 cases, 8 days in 1 case). All cases were analysed together in the group, regardless of duration of treatment |
Toppare 1994.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: open Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 102 enrolled and analysed Inclusion criteria: children with gastrointestinal symptoms and stool specimens positive for haematophagous trophozoites of E histolytica Exclusion criteria: not stated Concomitant intestinal infection: All cases in both groups had negative stool cultures for pathogenic bacteria |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: Medical Center Hospital, Ankara, Turkey Date: 1994 (date of publication only; actual study period not reported) Source of funding: not stated Attempts to contact study authors were unsuccessful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sixty children were randomly allocated to receive secnidazole in a daily dose of 30 mg/kg for 3 days while the rest were given ornidazole in a dose of 15 mg/kg twice daily..." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Different dosages and regimens were used (secnidazole 30 mg/kg for 3 days; ornidazole 15 mg/kg twice daily for 10 days). Blinding of participants, study personnel, and clinical outcome assessors was not mentioned Comment: Blinding of participants, study personnel, and clinical outcome assessors probably was not done |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of the microscopist examining the stools was not mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | 10 days after end of treatment: No data were missing from both treatment groups; all randomized participants were included in the analysis |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Unclear risk | Not determined |
| Selective reporting (reporting bias) | High risk | Outcomes and analysis methods were not pre‐specified |
| Other bias | Unclear risk | Trial enrolled only children with gastrointestinal symptoms who were found to have haematophagous trophozoites of E histolytica in stool samples. However, only stool microscopy was used to diagnose amoebic dysentery, and differentiation of E histolytica from non‐pathogenic species by more specific tests such as stool antigen ELISA or PCR was not done Trial reported that all cases had negative stool cultures for pathogenic bacteria, but concomitant infection with other protozoa or helminth parasites was not determined |
Tripathi 1986.
| Methods |
Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear; reported as "double‐blind", but procedure for blinding participants, care providers, and outcome assessors not described Inclusion of all randomized participants: 100% |
|
| Participants |
Numbers: 40 enrolled and analysed Inclusion criteria: adults with symptoms of intestinal amoebiasis and stool specimens positive for E histolytica by direct smear and formol‐ether concentration methods, sigmoidoscopy, colonic ulcer scrapings, and positive stool culture on NIH media Exclusion criteria: received amoebicidal drugs during previous 4 weeks; pregnant women; dehydrated patients; liver abscess and any evidence of hepatic, renal, haematological, and ECG abnormalities Concomitant intestinal infection: 4 in each group had concomitant Giardia lamblia in the stools |
|
| Interventions |
|
|
| Outcomes |
Not included in this review: frequency of loose stools/d from start of treatment |
|
| Notes |
Geographic location: hospital in Bhopal, India Date: 1986 (date of publication only; actual study period not reported) Source of funding: Ciba‐Geigy India Limited |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Forty hospitalized patients with intestinal amoebiasis...were administered either GO 10213 or metronidazole in dose of 150 mg and 400 mg thrice daily for 10 days at random" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Reported as "double‐blind", but it is unclear who was blinded. Different doses were used (GO 10213 150 mg and metronidazole 400 mg, both given thrice daily for 10 days), and the appearance of the drugs is not mentioned Comment: It is not specifically mentioned who among participants, study personnel, and clinical outcome assessors was blinded |
| Blinding (performance bias and detection bias) Parasitological outcomes | Unclear risk | Blinding of microscopist examining the stools was not specifically mentioned |
| Incomplete outcome data (attrition bias) For outcomes determined 1‐14 days after end of treatment | Low risk | At end of therapy (day 12): no dropouts |
| Incomplete outcome data (attrition bias) For outcomes determined 15‐60 days after end of treatment | Low risk | 18 days after end of treatment (day 28): 1/20 from the metronidazole group dropped out of the study because of increased severity of symptoms by the seventh day; no dropouts or withdrawals from the GO 10213 group |
| Selective reporting (reporting bias) | Unclear risk | The published report mentions that at the end of 28 days, "patients were assessed as per W.H.O. criterion". The frequency of loose stools per day and the rate of disappearance of parasites from the stools were also reported but were not pre‐specified |
| Other bias | Unclear risk | Diagnosis of intestinal amoebiasis was based only on stool microscopy (using direct smear and concentration techniques), sigmoidoscopy, and colonic ulcer scrapings demonstrating E histolytica, but differentiation from non‐pathogenic species was not specifically mentioned Four patients in each group had Giardia lamblia, but it is not mentioned whether concomitant infection with pathogenic bacteria, other protozoa, or helminth parasites was determined |
E histolytica: Entamoeba histolytica; ECG: electrocardiogram; ELISA: enzyme‐linked immunosorbent assay; NIH: National Institute of Health culture media; PCR: polymerase chain reaction; S boulardii: Saccharomyces boulardii; SGOT: aspartate aminotransferase; SGPT: alanine aminotransferase.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd‐Rabbo 1969 | Not an RCT |
| Abdallah 1969 | Not an RCT |
| Achar 1967 | Not an RCT |
| Ali Ata 1967 | Not an RCT |
| Alterio 1968 | Not an RCT |
| Amato Neto 1968 | Not an RCT |
| Apt 1976 | Not an RCT |
| Apt 1983 | Not an RCT. The English translation says that a sample of adult patients infected with E histolytica was divided into 2 groups but does not mention randomization |
| Arredondo 1993 | Ineligible study population: RCT that compared medical treatment with medical treatment plus liver puncture in patients with amoebic liver abscess |
| Atias 1972 | Not an RCT |
| Bakshi 1978 | Review of 17 RCTs conducted in India and comparing tinidazole with metronidazole over a 2‐year period |
| Banerjee 1976 | Not an RCT |
| Baranski 1966 | Not an RCT |
| Barroso 1969 | Not an RCT |
| Bassily 1987 | Not an RCT |
| Belkind 2004 | Ineligible study population: asymptomatic children positive for intestinal helminths or protozoa |
| Bezjak 1964 | Not an RCT |
| Bhatia 1998 | Ineligible study population: RCT comparing metronidazole with secnidazole in treating patients with amoebic liver abscess |
| Biagi 1966 | Wrong intervention: RCT comparing clefamide with placebo given not as treatment but as chemoprophylaxis for intestinal amoebiasis among asymptomatic carriers of E histolytica. Both the primary trial and the subsidiary trial by Biagi are probably duplicate publications of the same study because the 2 trials are similar in all aspects |
| Biagi 1978 | Not an RCT |
| Blanc 1965 | Not an RCT. Reports (1965 and 1966) by Blanc are probably duplicate publications of the same study because the 2 trials are similar in all aspects |
| Blessman 2002 | Ineligible study population: RCT comparing paromomycin with diloxanide furoate for treatment of asymptomatic carriers of E histolytica |
| Blessman 2003a | Wrong intervention and ineligible study population: RCT comparing metronidazole alone with ultrasound‐guided needle aspiration of the abscess in addition to metronidazole in patients with amoebic liver abscess |
| Botero 1967 | Not an RCT |
| Campos 1969 | Not an RCT |
| Capparelli 2016 | Not an RCT: a phase 1, open‐label study with 15 healthy adult participants to determine the pharmacokinetics of gold, given as auranofin, during and after 7 days of once‐daily oral dose administration |
| Cardoso Salles 1970 | Not an RCT: alternate allocation of patients with intestinal amoebiasis to receive 2 different doses of ethylchlordiphene |
| Cariry 1969 | Not an RCT |
| Chari 1970 | Not an RCT |
| Chaudhuri 1966 | Not an RCT |
| Cho 1972 | Not reported to be randomized but described as a double‐blind trial comparing Ro 7‐0207 vs metronidazole in treating participants with intestinal amoebiasis or E histolytica asymptomatic carriers; repeated attempts to gather more details from study authors were unsuccessful because the primary study author is deceased and the other study authors cannot be contacted |
| Cohen 1975 | Ineligible study population: RCT comparing chloroquine and metronidazole for treatment of amoebic liver abscess |
| da Cunha 1977 | Not an RCT |
| Datta 1974 | Ineligible study population: amoebic liver abscess |
| de Carvalho 1965 | Not an RCT |
| de la Rey 1989 | Wrong intervention and ineligible study population: RCT that randomized participants with amoebic liver abscess to either metronidazole alone or ultrasound‐guided aspiration of the abscess in addition to metronidazole |
| de Oliveira 1969 | Not an RCT |
| Delgado 1971 | Not an RCT |
| Devic 1974 | Not an RCT |
| Dhariwal 1963 | Not an RCT |
| Dinleyici 2009 | Quasi‐randomized clinical trial in which randomization was performed by alternating patient inclusion to 1 of 2 treatment groups: 1 group treated with metronidazole alone for 7 days, and the second group treated with metronidazole and lyophilized S boulardii, also given for 7 days |
| Donckaster 1957 | Not an RCT |
| dos Santos 1969 | Not an RCT |
| Doshi 1968 | Not an RCT |
| el Mofti 1965 | Not an RCT |
| Esquivel 1979 | Ineligible study population: RCT that compared metronidazole, emetine, or both for treating patients with amoebic liver abscess |
| Ey 1977 | Not an RCT |
| Felix 1966 | Not an RCT. Reports by Felix are probably duplicate publications of the same study because the 2 trials are similar in all aspects |
| Freeman 1990 | Wrong intervention and ineligible study population: compared efficacy of antiamoebic drug therapy plus needle aspiration vs antiamoebic drug therapy alone for patients with amoebic liver abscess |
| Gilman 1980 | Not an RCT: diagnostic validity study comparing conventional and immunofluorescent techniques for detection of E histolytica in rectal biopsy |
| Gorbea 1989 | Not an RCT |
| Hatchuel 1975 | Ineligible study population: double‐blind trial that compared tinidazole and metronidazole for treating patients with amoebic liver abscess |
| Hoekenga 1951 | Not an RCT |
| Holz 1965 | Not an RCT |
| Huggins 1965 | Not an RCT |
| Huggins 1969 | Not an RCT |
| Huggins 1974 | Not an RCT. Reports by Huggins are probably duplicate publications of the same study because the 2 trials are similar in all aspects |
| Huggins 1977 | Not an RCT |
| Huggins 1980 | Not an RCT |
| Huggins 1981 | Not an RCT |
| Irusen 1992 | Ineligible study population: amoebic liver abscess |
| Islam 1975 | Not an RCT |
| Islam 1978a | Ineligible study population: RCT that compared metronidazole and tinidazole for treating patients with amoebic liver abscess |
| Islam 1978b | Ineligible study population: amoebic liver abscess |
| Jain 1990 | Ineligible study population: open clinical trial that compared efficacy of various treatment regimens containing dehydroemetine and/or metronidazole for treating patients with hepatopulmonary amoebiasis |
| Jayawickrema 1975 | Ineligible study population: RCT that compared metronidazole with emetine and chloroquine for treatment of patients with hepatic amoebiasis |
| Kahbazi 2016 | Ineligible population: bacillary dysentery |
| Kaur 1972 | Not an RCT |
| Khalil 1987 | Not an RCT |
| Khokhani 1977 | Ineligible study population: RCT that compared metronidazole with emetine and chloroquine for treatment of patients with hepatic amoebiasis |
| Khokhani 1978 | Ineligible study population: RCT that compared metronidazole with tinidazole for treatment of patients with amoebic liver abscess |
| Konar 1963 | Not an RCT |
| Krishnaiah 2003 | Not an RCT: pharmacokinetic trial comparing 2 formulations of tinidazole given to healthy human volunteers |
| Kurt 2008 | Ineligible study population: RCT comparing metronidazole with single‐dose ornidazole for treatment of patients with dientamoebiasis |
| Laham 1951 | Not an RCT |
| Levy 1967 | Not an RCT |
| Martinez 1969 | Not an RCT |
| Masters 1979 | Not an RCT |
| Mathur 1974 | Not an RCT |
| McAuley 1992 | Not an RCT |
| McLeod 2014 | Not an RCT |
| Mendis 1984 | Ineligible study population: RCT that compared metronidazole with tinidazole for treatment of patients with hepatic amoebiasis |
| Misra 1976a | Not an RCT |
| Misra 1976b | Combination of an RCT involving 60 participants randomly assigned to either tinidazole or metronidazole and a non‐randomized trial involving 30 participants given tinidazole 600 mg twice daily for 5 to 10 days and another 20 patients given tinidazole at 2 g once daily for 3 days. No separate analysis was performed for randomized participants only. Several attempts to contact study authors were unsuccessful |
| Montovani 2009 | Not an RCT. Bioequivalence study comparing 2 oral formulations of secnidazole |
| Morales 1975 | Ineligible study population: RCT that compared intravenous metronidazole vs intramuscular emetine for treating patients with amoebic liver abscess |
| Murray 1980 | Wrong intervention: did not study effect of any antiamoebic drug for treating patients with amoebic colitis |
| Muzzafar 2006 | Ineligible study population: amoebic liver abscess |
| Nahrevanian 2008 | Ineligible study population and not an RCT: study to determine prevalence of Cryptosporidium in immunocompromised patients |
| Naik 1968 | Not an RCT |
| Nanavati 1965 | Not an RCT |
| O'Holohan 1972 | Not an RCT |
| Ohnishi 2014 | Not an RCT |
| Okeniyi 2007 | Ineligible study population: no mention of amoebic colitis |
| Olaeta 1996 | Not an RCT: quasi ‐randomized trial with alternate allocation of participants with intestinal amoebiasis to receive either quinfamide or etofamide |
| Omrani 1995 | Not an RCT |
| Orozco 1975 | Ineligible study population: amoebic liver abscess |
| Padilla 1995 | Ineligible study population: asymptomatic amoebic infection |
| Padilla 1998 | Unclear whether an RCT |
| Padilla 2002 | Wrong intervention and ineligible study population: RCT in which children whose stools became negative for E histolytica cysts and who were asymptomatic after 1 or 2 doses of quinfamide were randomized to 3 groups to determine whether administering quinfamide every 3 to 6 months resulted in reduced frequency of amoebic infection to below 27% |
| Pang 2014 | Not an RCT |
| Pimparkar 1966 | Not an RCT |
| Populaire 1980 | Not an RCT; pharmacokinetic study of secnidazole given to healthy human volunteers |
| Powell 1965a | Not an RCT |
| Powell 1965b | Ineligible study population: clinical trial of dehydroemetine, emetine, and chloroquine for treating patients with amoebic liver abscess |
| Powell 1965c | Ineligible study population: amoebic liver abscess |
| Powell 1965d | Not an RCT |
| Powell 1966a | Not an RCT |
| Powell 1966b | Not an RCT |
| Powell 1966c | Not an RCT |
| Powell 1967 | Ineligible study population: asymptomatic amoebic colitis |
| Powell 1968 | Report of 5 trials using metronidazole at different dosages and durations for treatment of patients with amoebic dysentery |
| Powell 1969a | Not an RCT |
| Powell 1969b | Review of several clinical trials using several amoebicides including niridazole, alone or in combination, for treatment of patients with amoebic dysentery or amoebic liver abscess |
| Powell 1969c | Guidelines on how to conduct drug trials in amoebiasis |
| Powell 1971a | Not an RCT |
| Powell 1971b | Letter relaying observations of study authors that no cases of liver abscess developed among patients with amoebic dysentery given chloroquine in addition to broad‐spectrum antibiotics or luminal amoebicides compared with those not given chloroquine |
| Powell 1972a | Report of clinical trials of new nitroimidazole derivatives for treating patients with amoebic liver abscess |
| Powell 1972b | Review on the evolution of drug therapy for amoebiasis that also presents the latest developments on niridazole, metronidazole, and other nitroimidazole drugs undergoing clinical trials at that time |
| Powell 1973 | Not an RCT |
| Prakash 1974 | Not an RCT: quasi‐randomized trial with alternate allocation of participants with intestinal amoebiasis to receive either tinidazole or metronidazole |
| Qureshi 1994 | Not an RCT |
| Qureshi 1997 | Not an RCT |
| Rodrigues 1968 | Not an RCT |
| Ruas 1973 | Ineligible study population: amoebic liver abscess |
| Ruchko 1978 | Not an RCT |
| Saha 1966 | Not an RCT |
| Saha 1970 | Not an RCT |
| Salem 1964 | Not an RCT |
| Salem 1967 | Not an RCT |
| Sandia 1977 | Not an RCT |
| Sangiuolo 1969 | Ineligible study population: patients had "acute gastroenteritis, food‐borne gastroenteritis, chronic enterocolitis, or ulcerative colitis". No mention of amoebic colitis or laboratory diagnosis of amoebic colitis among included patients |
| Sankale 1966 | Not an RCT |
| Sankale 1969 | Not an RCT |
| Sankale 1974 | Not an RCT |
| Satpathy 1988 | Ineligible study population: amoebic liver abscess |
| Schapiro 1967 | Not an RCT |
| Scragg 1968 | Ineligible study population: amoebic liver abscess |
| Scragg 1970 | Study population: amoebic liver abscess |
| Segal 1967 | Not an RCT |
| Sharif 2017 | Ineligible study population: bacillary dysentery |
| Sharma 1989 | Intervention and study populations: RCT that compared metronidazole alone vs needle aspiration of the abscess in addition to metronidazole in patients with amoebic liver abscess |
| Shrotriya 1985 | Not an RCT |
| Simjee 1985 | Ineligible study population: amoebic liver abscess |
| Simon 1967 | Not an RCT |
| Sinuhaji 1986 | Preliminary report of a trial on children with acute amoebic dysentery randomized to receive a single dose of metronidazole 50 mg/kg body weight/d or secnidazole 30 mg/kg body weight/d. Results were incomplete, and no final report of this trial was published. Attempts to contact study authors or the institution where the study was conducted were unsuccessful |
| Sladden 1964 | Not an RCT |
| Soh 1980 | Ineligible study population: amoebic liver abscess |
| Speich 2013 | Ineligible study population: asymptomatic school children |
| Spellberg 1969 | Study population: amoebic liver abscess |
| Spillman 1976 | Ineligible study population: RCT that compared metronidazole vs tinidazole for treating those with asymptomatic E histolytica infection and/or E hartmanni infection |
| Sutrisno 1978 | Not an RCT |
| Tandon 1997 | Wrong Intervention and ineligible study population: RCT that compared metronidazole alone vs needle aspiration of the abscess in addition to metronidazole in patients with amoebic liver abscess |
| Thompson 2015 | Not an RCT |
| Thoren 1990a | Ineligible study population: RCT that compared metronidazole, tinidazole, and diloxanide furoate for treating asymptomatic homosexual carriers of E histolytica |
| Thoren 1990b | Ineligible study population: asymptomatic E histolytica homosexual carriers |
| Tjaij 1969 | Not an RCT |
| Tjaij 1970 | Not an RCT |
| Vaidya 1983 | Not an RCT: pharmacokinetic study of Go.10213 that does not compare the drug vs placebo or another antiamoebic drug |
| Vakil 1967 | Not an RCT: alternate allocation of children and adults with amoebic dysentery, non‐dysenteric intestinal amoebiasis, or hepatic amoebiasis to receive intramuscular dehydroemetine or emetine |
| Vakil 1971 | Not an RCT |
| Vakil 1974 | Summary report of several clinical trials of various amoebicidal drugs conducted at 1 medical centre in Bombay, India, over the past 12 years |
| Valencia 1973 | Review on use of erythromycin stearate over the previous 3 years for 500 patients with intestinal amoebiasis, amoebic cysts, and other diseases of the colon |
| Vanijanonta 1985 | Ineligible study population: patients with amoebic liver abscess treated with low‐dose tinidazole and needle aspiration |
| Viswanathan 1968 | Not an RCT |
| Waddington 2018 | Protocol of an RCT but with wrong population: Study participants are Australian Aboriginal children aged greater than 3 months and less than 5 years with a primary diagnosis of acute gastroenteritis; no mention that those with intestinal amebiasis will be included |
| Wang 1971a | Not an RCT |
| Wang 1971b | Not an RCT: report of 2 cases of oxytetracycline‐resistant amoebic dysentery |
| Watson 1975 | Ineligible study population: amoebic infection of the eye |
| Welch 1978 | Not an RCT |
| Widjaya 1991 | Wrong Intervention and ineligible study population: RCT that compared various antiamoebic drug combinations vs percutaneous drainage in addition to combination drug therapy for treating patients with amoebic liver abscess |
| Wilmot 1962 | Not an RCT |
| Wolfe 1973 | Not an RCT |
| Wolfensberger 1968 | Not an RCT |
| Zuberi 1973 | Not an RCT |
E histolytica:Entamoeba histolytica; E hartmanii: Entamoeba hartmanii; RCT: randomized controlled trial; S boulardii: Saccharomyces boulardii.
Characteristics of ongoing studies [ordered by study ID]
NIAID 2016.
| Trial name or title | Phase IIa Randomized, Single‐blinded, Placebo‐controlled Clinical Trial of the Reprofiled Drug Auranofin for GI Protozoa |
| Methods | Randomized single‐blinded placebo‐controlled |
| Participants | 68 adults 18 to 65 years of age (34 per arm) with amebiasis identified by rapid EIA and positive antigen detection EIA of stool and with diarrhoea (defined as ≥ 3 loose stools) in the past 24 hours and assessed to be clinically stable and in otherwise good health Note: This study will also enrol 68 participants with stools positive by rapid EIA and positive antigen detection EIA for Giardia,but results will not be included in this review. Participants infected with both E histolyticaand Giardiawill be enrolled in the E histolyticastudy arm. Once the Entamoeba study arm is fully enrolled, any subsequent dual‐infected participants will be enrolled in the Giardiaarm |
| Interventions | • Auranofin 6 mg daily × 7 days • Placebo 6 mg daily × 7 days Note that auranofin is a gold‐containing chemical salt available as 3‐mg capsules |
| Outcomes | Primary outcome measure for E histolytica infection: • Proportion of participants with positive rapid EIA and positive antigen detection EIA for E histolytica and resolution of diarrhoea (< 3 loose stools/24 hours) by day 7 Secondary outcomes for E histolytica infection: • Proportion of participants with stools positive by rapid EIA and positive antigen detection EIA for E histolytica and trophozoites on smear at enrolment with parasitological response (no detection of trophozoites of E histolytica on microscopic exam by day 7 • Proportion of participants with stool positive rapid EIA and positive antigen detection EIA for E histolytica and trophozoites on smear at enrolment with parasitological response (no detection of trophozoites on microscopic exam or negative antigen detection) by days 3 and 5 • Rate of decrease of trophozoites/cyst load by qPCR in stools by days 3, 5, and 7 • Proportion of participants with negative stool antigen tests by days 3, 5, 7, and 14 • Proportion of participants with sustained cure (no detection of cysts or trophozoites by microscopic exam or negative antigen detection) at 14 and 28 days • Proportion of participants with relapse (same strain) or re‐infection (new strain) with positive stools at 14 and 28 days by genotyping initial versus subsequent strains |
| Starting date | 19 August 2016 |
| Contact information | Contact person: Sharon Reed; 18588222808; slreed@ucsd.edu Responsible party: National Institute of Allergy and Infectious Diseases (NIAID) |
| Notes | Location: International Center for Diarrheal Disease Research Bangladesh ‐ Parasitology, Dhaka, Bangladesh Sponsor: National Institute of Allergy and Infectious Diseases (NIAID) Estimated study completion date: 31 May 2019 |
Pfizer 2016.
| Trial name or title | Drug use investigation of paromomycin |
| Methods | Prospective cohort study |
| Participants | 200 participants 15 to 99 years old with intestinal amoebiasis |
| Interventions | Ameparomo (paromomycin) capsules 250 mg |
| Outcomes | Primary outcome: • Number of participants with adverse events (AEs) by seriousness and relationship to treatment [Time frame: maximum 10 days] Secondary outcome: • Number of participants with clinical response of cure [time frame: maximum 3 months] |
| Starting date | October 2015 |
| Contact information | Study director: Pfizer CT.gov Call Center |
| Notes | Location: not specified Sponsor: Pfizer Estmated completion date: February 2019 |
AE: adverse event; E histolytica: Entamoeba histolytica; EIA: enzyme immunoassay; qPCR: quantitative polymerase chain reaction.
Differences between protocol and review
Since many trials reported outcomes 28 days or one month after treatment, we decided to stratify outcomes from end of treatment to 14 days and 15 to 60 days after end of treatment, instead of reporting outcomes at end of treatment until seven days after treatment and eight to 21 days after end of treatment, as stated in the protocol (Gonzales 2006). We performed subgroup analysis, not mentioned in the protocol, based on clinical categories (amoebic dysentery, non‐dysenteric amoebic colitis, or not specified) and participant age (adults or children). Additional sources of heterogeneity explored included types of intestinal infection (Entamoeba histolytica infection alone or mixed intestinal infection), and criteria for determining outcomes (based on WHO 1969 criteria or other criteria). We were unable to undertake sensitivity analysis based on type of diagnostic test because only one included trial used stool antigen‐based ELISA to confirm E histolytica. However, we performed sensitivity analysis to determine the possible effect of pharmaceutical industry‐sponsored trials on trial quality.
Differences between review and review update
MLMG, LFD, and EGM authored the protocol and the previous published review version (Gonzales 2006; Gonzales 2009). For this review update, EGM stepped down from the review author team, and JSA joined as a review author. We updated epidemiological data on amoebiasis and amoebic colitis. We re‐classified nitazoxanide, initially classified as a luminal amoebicide in the earlier version of this review, as a tissue amoebicide in Table 3 since more recent studies reported effectiveness of this drug against invasive trophozoites. We added four specific objectives to Gonzales 2009 to provide a more focused direction for the review.
We created a study flow diagram based on the PRISMA template (Figure 1). We prepared a 'Risk of bias' table for each included trial, including the four new trials added to this review update. We summarized continuous data (duration of clinical symptoms) that were measured in the included studies in a new table (Table 5). We assessed the certainty of the evidence for two important outcomes (tinidazole compared with metronidazole as treatment for amoebic colitis, and combination therapy compared with metronidazole alone as treatment for amoebic colitis) using the GRADE approach (GRADE 2004), and we presented this information in ‘Summary of fIndings' tables (Table 1; Table 2).
Contributions of authors
MLMG conceived and designed the review, co‐ordinated its development, and prepared initial drafts of the Background and Methods, selected studies, extracted data, synthesized data in RevMan 5, and prepared the initial draft of the Results, Discussion, and ‘Summary of findings' tables. LFD advised MLMG about design and co‐ordination of the review and, together with MLMG, selected studies and extracted data, assessed risk of bias, and contributed to the Discussion and Authors' conclusions. JSA evaluated full‐text articles, extracted data from the included trials, resolved differences between the other two review authors regarding assessment of papers, and contributed to the Discussion and Authors' conclusions.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Project number 300342‐104
Declarations of interest
MLMG has no known conflicts of interest. LFD was an invited lecturer on a talk sponsored by Wyeth Nutrition. She has no other conflicts of interest to declare. JSA has no known conflicts of interest.
Unchanged
References
References to studies included in this review
Asrani 1995 {published data only}
- Asrani CH, Damle SS, Ghotge VV, Gokhale AS, Jalgaonkar M, Pai Kakode PR, et al. Efficacy and safety of metronidazole versus a combination of metronidazole and diiodohydroxyquinoline for the treatment of patients with intestinal amebiasis: a Primary Care Physician Research Group Study. Current Therapeutic Research 1995;56(7):678‐83. [Google Scholar]
Awal 1979 {published data only}
- Awal ARMA, Ali S. Tinidazole in the treatment of symptomatic intestinal amoebiasis. Current Therapeutic Research 1979;26(6):962‐6. [Google Scholar]
Batra 1972 {published data only}
- Batra SK, Ajmani NK, Chuttani HK. Evaluation of 1‐methyl‐2‐(4'‐fluorophenyl)‐5‐nitroimidazole. Journal of Tropical Medicine and Hygiene 1972;75(2):40‐1. [PubMed] [Google Scholar]
Botero 1974 {published data only}
- Botero D. Double blind study with a new nitroimidazole derivative, Ro 7‐0207, versus metronidazole in symptomatic intestinal amebiasis. American Journal of Tropical Medicine and Hygiene 1974;23(5):1000‐1. [DOI] [PubMed] [Google Scholar]
Botero 1977 {published data only}
- Botero D, Perez A. Treatment of intestinal amoebiasis and vaginal trichomoniasis with panidazole and its comparison with metronidazole. Transactions of the Royal Society of Tropical Medicine and Hygiene 1977;71(6):508‐11. [DOI] [PubMed] [Google Scholar]
Chunge 1989 {published data only}
- Chunge CN, Estambale BB, Pamba HO, Chitayi PM, Munanga PN, Kang'ethe S. Comparison of four nitroimidazole compounds for treatment of symptomatic amoebiasis in Kenya. East African Medical Journal 1989;66(11):724‐7. [PubMed] [Google Scholar]
Davila 2002 {published data only}
- Davila‐Gutierrez C, Vasquez C, Trujillo‐Hernandez B, Huerta M. Nitazoxanide compared with quinfamide and mebendazole in the treatment of helminthic infections and intestinal protozoa in children. American Journal of Tropical Medicine and Hygiene 2002;66(3):251‐4. [DOI] [PubMed] [Google Scholar]
Donckaster 1964 {published data only}
- Donckaster R, Atias A, Faiguenbaum J, Jarpa A, Sapunar J, Cuello E. Chronic intestinal amebiasis. Therapeutic trial of antibiotics, chemotherapeutics and placebos [Amibiasis intestinal cronica. Ensayo terapeutico con antibioticos, quimioterapicos y placebo]. Boletin Chileno de Parasitologia 1964;19:46‐54. [PubMed] [Google Scholar]
Guevara 1980 {published data only}
- Guevara L. Evaluation of the tolerance and efficiency of quinfamide, a new intraluminal amebicide, in man (one day treatment). Double blind study. Revista de Gastroenterologia de Mexico 1980;45(2):93‐7. [PubMed] [Google Scholar]
Huggins 1982 {published data only}
- Huggins D. Double‐blind clinical trial with WIN 40.014 in the treatment of intestinal chronic amebiasis [Ensaio clinico duplo‐cego com o WIN 40.014 no tratamento da amebiase intestinal cronica]. Folha Medica 1982;85 Suppl 1:869‐70. [Google Scholar]
Joshi 1975 {published data only}
- Joshi HD, Shah BM. A comparative study of tinidazole and metronidazole in treatment of amoebiasis. The Indian Practitioner 1975;28:295‐302. [Google Scholar]
Kapadia 1968 {published data only}
- Kapadia RM, Pathak HY, Apte SP. Chlorhydroxyquinoline and di‐iodohydroxyquinoline in amoebiasis: a comparative study. Journal of the Indian Medical Association 1968;51(3):125‐7. [PubMed] [Google Scholar]
Karabay 1999 {published data only}
- Karabay O, Godekmerden A. Comparison of therapeutic efficacies of the single dose secnidazole versus 10‐day metronidazole in acute amebiasis [Akut intestinal amebiyaz tedavisinde tek doz seknidazol ile 10 gunluk metronidazolun etkinliklerinin karsilastirilmasi]. Klimik Dergisi 1999;12(2):82‐4. [Google Scholar]
Mansour‐Ghanaei 2003 {published data only}
- Mansour‐Ghanaei F, Dehbashi N, Yazdanparast K, Shafaghi A. Efficacy of Saccharomyces boulardii with antibiotics in acute amoebiasis. World Journal of Gastroenterology 2003;9(8):1832‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathur 1976 {published data only}
- Mathur SN, Itigi A, Rao PD, Krishnaveni, Rai V. Evaluation of tinidazole in treatment of amoebiasis. Indian Medical Gazette 1976;15:361‐4. [Google Scholar]
Misra 1974 {published data only}
- Misra NP, Laiq SM. Comparative trial of tinidazole and metronidazole in intestinal amebiasis. Current Therapeutic Research 1974;16(12):1255‐63. [PubMed] [Google Scholar]
Misra 1977 {published data only}
- Misra NP, Gupta RC. A comparison of a short course of single daily dosage therapy of tinidazole with metronidazole in intestinal amoebiasis. Journal of International Medical Research 1977;5(6):434‐7. [DOI] [PubMed] [Google Scholar]
Misra 1978 {published data only}
- Misra NP. A comparative study of tinidazole and metronidazole as a single daily dose for three days in symptomatic intestinal amoebiasis. Drugs 1978;15 Suppl 1:19‐22. [DOI] [PubMed] [Google Scholar]
Mohammed 1998 {published data only}
- Mohammed KA, Strak SK, Jawad AM, Al‐Sadoon IO, Mahdi NK. Effectiveness of praziquantel in treatment of intestinal amoebiasis and giardiasis. Eastern Mediterranean Health Journal 1998;4(1):161‐3. [Google Scholar]
Naoemar 1973 {published data only}
- Naoemar SA, Rukmono B. Clinical trial of Ro 7‐0207, a nitroimidazole derivative, in amoebic dysentery. Southeast Asian Journal of Tropical Medicine and Public Health 1973;4(3):417‐20. [PubMed] [Google Scholar]
Nnochiri 1967 {published data only}
- Nnochiri E. Oral chemotherapy in amoebic dysentery ‐ potentiating effect of chloroquine on the action of diloxanide furoate. Journal of Tropical Medicine and Hygiene 1967;70(9):224‐8. [PubMed] [Google Scholar]
Padilla 2000 {published data only}
- Padilla N, Diaz R, Munoz M. Efficacy and safety of quinfamide versus secnidazole in the management of amoebic non‐dysenteric colitis in children. Clinical Drug Investigation 2000;20(2):89‐93. [DOI] [PubMed] [Google Scholar]
Pamba 1990 {published data only}
- Pamba HO, Estambale BB, Chunge CN, Donno L. Comparative study of aminosidine, etophamide and nimorazole, alone or in combination, in the treatment of intestinal amoebiasis in Kenya. European Journal of Clinical Pharmacology 1990;39(4):353‐7. [DOI] [PubMed] [Google Scholar]
Panggabean 1980 {published data only}
- Panggabean A, Sutjipto A, Aldy D, Sutanto AH, Siregar H. Tinidazole versus ornidazole in amebic dysentery in children (a double blind trial). Paediatrica Indonesiana 1980;20(11‐12):229‐35. [PubMed] [Google Scholar]
Pehrson 1983 {published data only}
- Pehrson P, Bengtsson E. Treatment of non‐invasive amoebiasis. A comparison between tinidazole alone and in combination with diloxanide furoate. Transactions of the Royal Society of Tropical Medicine and Hygiene 1983;77(6):845‐6. [DOI] [PubMed] [Google Scholar]
Pehrson 1984 {published data only}
- Pehrson P, Bengtsson E. Treatment of non‐invasive amoebiasis ‐ a comparison between tinidazole and metronidazole. Annals of Tropical Medicine and Parasitology 1984;78(5):505‐8. [DOI] [PubMed] [Google Scholar]
Prasad 1985 {published data only}
- Prasad R, Jagota SC, Mathur PP, Taneja V. Drug trial of ‘Dependal' ‐ M suspension against metronidazole suspension in amoebiasis and giardiasis. Indian Medical Gazette 1985;119(7):219‐23. [Google Scholar]
Pudjiadi 1973 {published data only}
- Pudjiadi SH, Sunoto, Suharjono, Kadri N. A new oral amoebicid (RO 7‐0207) in the treatment of intestinal amoebiasis. Paediatrica Indonesiana 1973;13(4):113‐9. [PubMed] [Google Scholar]
Rossignol 2001 {published data only}
- Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double‐blind, placebo‐controlled study of nitazoxanide. Journal of Infectious Diseases 2001;184(3):381‐4. [DOI] [PubMed] [Google Scholar]
Rossignol 2007 {published data only}
- Rossignol JF, Kabil SM, Gohary Y, Younis AM. Nitazoxanide in the treatment of amoebiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(10):1025‐31. [DOI] [PubMed] [Google Scholar]
Rubidge 1970 {published data only}
- Rubidge CJ, Scragg JN, Powell SJ. Treatment of children with acute amoebic dysentery. Comparative trial of metronidazole against a combination of dehydroemetine, tetracycline, and diloxanide furoate. Archives of Disease in Childhood 1970;45(240):196‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salles 1999 {published data only}
- Salles JMC, Bechara C, Tavares AM, Martins M, Sobrinho JG, Dietrich‐Neto F, et al. Comparative study of the efficacy and tolerability of secnidazole suspension (single dose) and tinidazole suspension (two days dosage) in the treatment of amebiasis in children. Brazilian Journal of Infectious Diseases 1999;3(2):80‐8. [PubMed] [Google Scholar]
Savas‐Erdeve 2009 {published and unpublished data}
- Savas‐Erdeve S, Gokay S, Dallar Y. Efficacy and safety of Saccharomyces boulardii in amebiasis‐associated diarrhea in children. Turkish Journal of Pediatrics 2009;51(3):220‐4. [PubMed] [Google Scholar]
Shah 2016 {published data only}
- Shah SMA, Usmanghani K, Akhtar N, Akram M, Asif HM, Hasan MM. Clinical study on the efficacy of Amoebex (coded herbal drug) compared with metronidazole for the treatment of amoebic dysentery. Pakistan Journal of Pharmaceutical Sciences 2016;29(6):2005‐14. [PubMed] [Google Scholar]
Siddiqui 2015 {published data only}
- Siddiqui MI, Usmanghani K. Comparison of how well allopathic and herbal medicine work for the treatment of Entamoeba histolytica. Journal of Medicinal Plants Research 2015;9(9):301‐9. [Google Scholar]
Singh 1977 {published data only}
- Singh G, Kumar S. Short course of single daily dosage treatment with tinidazole and metronidazole in intestinal amoebiasis: a comparative study. Current Medical Research and Opinion 1977;5(2):157‐60. [DOI] [PubMed] [Google Scholar]
Sitepu 1982 {published data only}
- Sitepu N, Lubis CP, Sutanto AH, Siregar H. Minute treatment with tinidazole and ornidazole in children with amebic dysentery. Paediatrica Indonesiana 1982;22(7‐8):132‐5. [PubMed] [Google Scholar]
Soedin 1985 {published data only}
- Soedin K, Alfien Syukran OK, Fadillah A, Sidabutar P. Comparison between the efficacy of a single dose of secnidazole with a 5‐day course of tetracycline and clioquinol in the treatment of acute intestinal amoebiasis. Pharmatherapeutica 1985;4(4):251‐4. [PubMed] [Google Scholar]
Swami 1977 {published data only}
- Swami B, Lavakusulu D, Devi CS. Tinidazole and metronidazole in the treatment of intestinal amoebiasis. Current Medical Research and Opinion 1977;5(2):152‐6. [DOI] [PubMed] [Google Scholar]
Toppare 1994 {published data only}
- Toppare MF, Kitapci F, Senses DA, Yalcinkaya F, Safa Kaya I, Dilmen U. Ornidazole and secnidazole in the treatment of symptomatic intestinal amoebiasis in childhood. Tropical Doctor 1994;24(4):183‐4. [DOI] [PubMed] [Google Scholar]
Tripathi 1986 {published data only}
- Tripathi BM, Misra NP, Tiwari A. A double‐blind trial of GO 10213 and metronidazole in intestinal amoebiasis. Current Therapeutic Research 1986;39(2):178‐82. [Google Scholar]
References to studies excluded from this review
Abdallah 1969 {published data only}
- Abdallah A, Gad‐el‐Mawla N, el‐Kordy MI. Erythromycin stearate in intestinal amoebiasis. Journal of the Egyptian Medical Association 1969;52(2):168‐73. [PubMed] [Google Scholar]
Abd‐Rabbo 1969 {published data only}
- Abd‐Rabbo H, Montasir M. A trial of oral dehydroemetine compounds in the treatment of amoebiasis. Journal of Tropical Medicine and Hygiene 1969;72(3):64‐7. [PubMed] [Google Scholar]
Achar 1967 {published data only}
- Achar ST, Rama Row GV. Clinical trials with dehydroemetine and paromomycin in acute amoebic dysentery in children. Journal of the Indian Medical Association 1967;49(8):370‐2. [PubMed] [Google Scholar]
Ali Ata 1967 {published data only}
- Ali Ata AH, el‐Raziky ES. Trials of BT 436 in amoebiasis. Zeitschrift für Tropenmedizin und Parasitologie 1967;18(3):321‐6. [PubMed] [Google Scholar]
Alterio 1968 {published data only}
- Alterio DL. Clinico‐parastilogological evaluation of an anti‐ameba therapeutic activity of a new non‐absorbable erythromycin stearate preparation [Avaliacao clinico‐parasitologica da atividade terapeutica antiamebiana de uma nova preparacao de estearato de eritromicina nao absorvivel]. Hospital (Rio de Janeiro, Brazil) 1968;73(4):1207‐13. [PubMed] [Google Scholar]
Amato Neto 1968 {published data only}
- Amato Neto V, Wanderley RA. Use of an erythromycin stearate preparation of regulated intestinal release in the treatment of intestinal amebiasis [Observacoes preliminares sobre o emprego de preparacao de estearato de eritromicina, de liberacao intestinal regulada, no tratamento da amebiase intestinal]. Hospital (Rio de Janeiro, Brazil) 1968;73(2):583‐9. [PubMed] [Google Scholar]
Apt 1976 {published data only}
- Apt W, Perez C, Gabor M, Doren G. Treatment of chronic amebiasis with nitrimidazole [Tratamiento de la amibiasis intestinal cronica con nimorazol]. Revista Medica de Chile 1976;104(11):791‐3. [PubMed] [Google Scholar]
Apt 1983 {published data only}
- Apt W, Perez C, Miranda C, Gabor M, Doren G. Treatment of intestinal amebiasis and giardiasis with ornidazole [Tratamiento de la amebiasis intestinal y giardiasis con ornidazole]. Revista Medica de Chile 1983;111(11):1130‐3. [PubMed] [Google Scholar]
Arredondo 1993 {published data only}
- Arredondo‐Cortes E, Gonzalez‐Gonzales JA, Bosques‐Padilla F, Elizondo Riojos G, Barragan‐Villareal. A randomized controlled trial of medical treatment vs medical treatment plus puncture in amoebic liver abscess [abstract]. Gut 1993;34(3):S43. [Google Scholar]
Atias 1972 {published data only}
- Atias A, Sapunar J, Parodi V, Perez C, Gabor M, Stagno S, et al. Treatment of chronic intestinal amebiasis with teclozan [Tratamiento de la amibiasis intestinal cronica con teclozan]. Boletin Chileno de Parasitologia 1972;27:119‐21. [PubMed] [Google Scholar]
Bakshi 1978 {published data only}
- Bakshi JS, Ghiara JM, Nanivadekar AS. How does tinidazole compare with metronidazole? A summary report of Indian trials in amoebiasis and giardiasis. Drugs 1978;15 Suppl 1:133‐42. [DOI] [PubMed] [Google Scholar]
Banerjee 1976 {published data only}
- Banerjee RN, Singh J, Basu AK. Metronidazole in the treatment of amoebiasis. The Indian Practitioner 1976;29:208‐16. [Google Scholar]
Baranski 1966 {published data only}
- Baranski MC. Treatment of chronic intestinal amebiasis with the compound of diiodohydroxyquinoline, tetracycline and chloroquine [Tratamento da amebiase intestinal cronica pela associacao de diiohidroxiquinoleina, tetraciclina e cloroquina]. Revista Brasileira de Medicina 1966;23(11):774‐8. [PubMed] [Google Scholar]
Barroso 1969 {published data only}
- Barroso E, Ruiloba J. Treatment of intestinal amebiasis with eticlordifene [Tratamento de la amebiasis intestinal con eticlordifene]. Revista de Investigacion Clinica 1969;21(2):195‐203. [PubMed] [Google Scholar]
Bassily 1987 {published data only}
- Bassily S, Farid Z, El‐Masry NA, Mikhail EM. Treatment of intestinal E. histolytica and G. lamblia with metronidazole, tinidazole and ornidazole: a comparative study. Journal of Tropical Medicine and Hygiene 1987;90(1):9‐12. [PubMed] [Google Scholar]
Belkind 2004 {published data only}
- Belkind‐Valdovinos U, Belkind‐Gerson J, Sanchez‐Francia D, Espinoza‐Ruiz MM, Lazcano‐Ponce E. Evaluation of nitazoxanide in single dose and for three days in intestinal parasitism [Evaluacion de la nitazoxanida en dosis unica y por tres dias en parasitosis intestinal]. Salud Publica de Mexico 2004;46(3):333‐40. [DOI] [PubMed] [Google Scholar]
Bezjak 1964 {published data only}
- Bezjak B, Breitenfeld V. Mexaform in the treatment of amoebiasis [Mexaform in der amobiasis‐therapie]. Munchener Medizinische Wochenschrift 1964;106(42):1946‐6. [PubMed] [Google Scholar]
Bhatia 1998 {published data only}
- Bhatia S, Karnad DR, Oak JL. Randomized double‐blind trial of metronidazole versus secnidazole in amebic liver abscess. Indian Journal of Gastroenterology 1998;17(2):53‐4. [PubMed] [Google Scholar]
Biagi 1966 {published data only}
- Biagi FF, Lopez RM, Gonzalez C, Gutierrez M. Chemoprophylaxis of amebiasis using clefamide in an open community [Quimoprofilaxis de la amibiasis con clefamida en una comunidad abierta]. Gaceta Medica de Mexico Sao Paolo 1966;96(2):183‐90. [PubMed] [Google Scholar]
- Biagi FF, Lopez RM, Gonzalez C, Gutierrez M. Chemoprophylaxis of amebiasis using clefamide in an open community [Quimoprofilaxis de la amibiasis con clefamida en una comunidad abierta]. Revista do Instituto de Medicina Tropical de São Paulo 1966;8(5):235‐40. [PubMed] [Google Scholar]
Biagi 1978 {published data only}
- Biagi F, Munoz J, Gonzalez C. Treatment of amoebiasis with drugs acting on intestinal lumen and tissue [Tratamiento de la amibiasis con medicamentos de accion luminal y tisular]. Prensa Medica Mexicana 1978;43(1‐2):59‐60. [PubMed] [Google Scholar]
Blanc 1965 {published data only}
- Blanc F, Denjean B, Felix H, Nosny Y, Pene P, Renaud R. Trial treatment of amoebiasis by oral administration of 2‐dehydroemetine [Essai de traitement de l'amibiase par l'administration orale de la 2‐dehydroemetine]. Academie Nationale de Medecine La Presse Medicale 1965;149(16‐17):360‐5. [PubMed] [Google Scholar]
- Blanc F, Denjean B, Felix H, Nosny Y, Pene P, Reynaud R, Sankale M. Treatment of amoebiasis with oral 2‐dehydroemetine [Le traitement de l'amibiase par la 2‐dehydroemetine orale]. La Presse Medicale 1966;74(2):51‐4. [PubMed] [Google Scholar]
Blessman 2002 {published data only}
- Blessman J, Tannich E. Treatment of asymptomatic intestinal Entamoeba histolytica infection. New England Journal of Medicine 2002;347(17):1384. [DOI] [PubMed] [Google Scholar]
Blessman 2003a {published data only}
- Blessman J, Binh HD, Hung DM, Tannich E, Burchard G. Treatment of amoebic liver abscess with metronidazole alone or in combination with ultrasound‐guided needle aspiration: a comparative, prospective and randomized study. Tropical Medicine and International Health 2003;8(11):1030‐4. [DOI] [PubMed] [Google Scholar]
Botero 1967 {published data only}
- Botero D. Treatment of intestinal amoebiasis with diloxanide furoate, tetracycline and chloroquine. Transactions of the Royal Society of Tropical Medicine and Hygiene 1967;61(6):769‐73. [DOI] [PubMed] [Google Scholar]
Campos 1969 {published data only}
- Campos R. Treatment of intestinal amebiasis using erythromycin stearate under controlled release [Tratamento de amebiase intestinal pelo estearato de eritromicina de liberacao regulada]. Revista Brasileira de Medicina 1969;26(2):113‐4. [PubMed] [Google Scholar]
Capparelli 2016 {published data only}
- Capparelli EV, Bricker‐Ford R, Rogers MJ, McKerrow JH, Reed SL. Phase I clinical trial results of auranofin, a novel antiparasitic agent. Antimicrobial Agents and Chemotherapy 2016;61(1):pii: e01947‐16. [DOI: 10.1128/AAC.01947-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardoso Salles 1970 {published data only}
- Cardoso Salles JM, Gundim Leitao E. Treatment of intestinal amebiasis using ethyl chlordiphene. Comparative study [Tratamento da amebiase intestinal com eticlordifene. Estudo comparativo]. Hospital (Rio de Janeiro, Brazil) 1970;77(6):2073‐80. [PubMed] [Google Scholar]
Cariry 1969 {published data only}
- Cariry NA, Silva MA. Treatment of intestinal amoebiasis with teclozan (Falmonox). Comparative study of therapeutic schemes [Tratamento da amebiase intestinal com teclozan (Falmonox). Estudo comparativo de esquemas terapeuticos]. Hospital (Rio de Janeiro, Brazil) 1969;76(3):1033‐7. [PubMed] [Google Scholar]
Chari 1970 {published data only}
- Chari MV, Gadiyar BN. A new drug (MK‐910) in the therapy of intestinal and hepatic amebiasis. First results of clinical trial. American Journal of Tropical Medicine and Hygiene 1970;19(6):926‐8. [DOI] [PubMed] [Google Scholar]
Chaudhuri 1966 {published data only}
- Chaudhuri RN, Saha TK. Combined therapy of amoebic dysentery. Bulletin of the Calcutta School of Tropical Medicine 1966;14(1):22. [PubMed] [Google Scholar]
Cho 1972 {published data only}
- Cho KM, Cha HY, Soh CT. Clinical trials of R‐0207 against Entamoeba histolytica infections (double blind trials versus metronidazole). Yonsei Reports on Tropical Medicine 1972;3:123‐33. [Google Scholar]
Cohen 1975 {published data only}
- Cohen HG, Reynolds TB. Comparison of metronidazole and chloroquine for the treatment of amoebic liver abscess. A controlled trial. Gastroenterology 1975;69(1):35‐41. [PubMed] [Google Scholar]
da Cunha 1977 {published data only}
- Cunha AS, SIlva EF, Mello SM. Clinical trial with the imidazol compound R.P. 14539 in intestinal amebiasis [Avaliacao terapeutico do composto imidazolico R.P. 14539 na amebiase intestinal]. Revista do Instituto de Medicina Tropical de São Paulo 1977;19(5):342‐8. [PubMed] [Google Scholar]
Datta 1974 {published data only}
- Datta DV, Singh SA, Chhuttani PN. Treatment of amoebic liver abscess with emetine hydrochloride, niridazole, and metronidazole. A controlled clinical trial. American Journal of Tropical Medicine and Hygiene 1974;23(4):586‐9. [DOI] [PubMed] [Google Scholar]
de Carvalho 1965 {published data only}
- Carvalho HT, Coura LC, Silva JR. Treatment of intestinal amebiasis ‐ preliminary results of a trial with a new drug, Bayer 2456 (amebicide) [Tratamento da amebiase intestinal ‐ resultados preliminares de ensaio com um novo medicamento, o Bayer 2456 (amoebacide)]. Revista Brasileira de Medicina 1965;22(9):562‐6. [PubMed] [Google Scholar]
de la Rey 1989 {published data only}
- Rey Nel J, Simjee AE, Patel A. Indications for aspiration of amoebic liver abscess. South African Medical Journal 1989;75(8):373‐6. [PubMed] [Google Scholar]
Delgado 1971 {published data only}
- Delgado y Garnia R, Chavez‐Esgueda JM. Etofamide in the treatment of children with acute intestinal amebiasis [Etofamida en el tratamiento de ninos con amibiasis intestinal aguda]. Prensa Medica Mexicana 1971;36(7‐8):358‐61. [PubMed] [Google Scholar]
de Oliveira 1969 {published data only}
- Oliveira CA. Therapeutic experience in the use of erythromycin stearate in chronic intestinal amebiasis [Experiencia terapeutica com estearato de eritromicina de liberacao regulada, na amebiase intestinal cronica]. Hospital (Rio de Janeiro, Brazil) 1969;76(1):175‐8. [PubMed] [Google Scholar]
Devic 1974 {published data only}
- Devic J, Dosen H. Our initial experiences in the treatment of the intestinal amebiasis with 2‐dehydroemetine. Medicinski Pregled 1974;27(1‐2):79‐83. [PubMed] [Google Scholar]
Dhariwal 1963 {published data only}
- Dhariwal RK, Verma NP, Nioguy C, Pal SK, Singh SS, Chatterjee AK, et al. Clinical trial with entamide furoate in acute amebic dysentery. Indian Journal of Medical Science 1963;17:825‐6. [PubMed] [Google Scholar]
Dinleyici 2009 {published data only}
- Dinleyici EC, Eren M, Yargic ZA, Dogan N, Vandenplas Y. Clinical efficacy of Saccharomyces boulardii and metronidazole compared to metronidazole alone in children with acute bloody diarrhea caused by amebiasis: a prospective, randomized, open label study. American Journal of Tropical Medicine and Hygiene 2009;80(6):953‐5. [PubMed] [Google Scholar]
Donckaster 1957 {published data only}
- Donckaster R, Sapunar J, Donoso A. Treatment of chronic intestinal amebiasis with tetracycline and chloroquine with bismuth glycoarsanilate and parasitological control by the combined telemann and polvinyl alcohol method [Ensayo terapeutico de la amibiasis intestinal cronica con tetraciclina y chloroquina glicolilarsanilato de bismuto y control parasitologico con los metodos de telemann y alcohol polivinilico combinados]. Boletin Chileno de Parasitologia 1957;12(2):24‐9. [PubMed] [Google Scholar]
Doshi 1968 {published data only}
- Doshi JC, Doshi MJ, Vaidya AB, Mehta JM, Sheth UK. Niridazole in amebic dysentery and hepatic amebiasis. American Journal of Tropical Medicine and Hygiene 1968;17(5):702‐8. [DOI] [PubMed] [Google Scholar]
dos Santos 1969 {published data only}
- dos Santos Moraise ML. Clinico‐pathological results using erythromycin stearate in the treatment of intestinal amebiasis [Resultados clinico‐parasitologicos obtidos com o uso do estearato de eritromicina de liberacao regulada no tratamento da amebiase intestinal]. Hospital (Rio de Janeiro, Brazil) 1969;75(4):1367‐74. [PubMed] [Google Scholar]
el Mofti 1965 {published data only}
- Mofti A, Ayadi A. Trial of a new amoebicidal agent (chloro‐hydroxyquinoline). Journal of the Egyptian Medical Association 1965;48(2):142‐6. [PubMed] [Google Scholar]
Esquivel 1979 {published data only}
- Esquivel Lopez A, Gonzales Espinola G, Garcia Garduno JR, Guarner Dalias V. Various considerations in the treatment of amoebic liver abscess [Algunas consideraciones en el tratamiento del absceso hepatico amibiano]. Revista De Gastroenterologia De Mexico 1979;44(2):51‐6. [PubMed] [Google Scholar]
Ey 1977 {published data only}
- Ey JL. Treatment of amoebiasis with metronidazole and entamide furoate. Ethiopian Medical Journal 1977;15:101‐5. [PubMed] [Google Scholar]
Felix 1966 {published data only}
- Felix H, Freyria J, Maillard A. 2‐dehydro‐emetine administered per os in the treatment of amoebiasis. Therapeutical tests [La 2‐dehydro‐emetine administree par voie orale dans le traitement de l'amibiase. Essais therapeutiques]. Le Journal de Medecine de Lyon 1966;47(104):1211‐6. [PubMed] [Google Scholar]
- Felix H, Freyria J, Maillard A. Recent developments. 2‐dehydro‐emetine administered orally in the treatment of amoebiasis. Therapeutic trials (50 personal cases) [La 2‐dehydro‐emetine administree par voie orale dans le traitement de l'amibiase. Essais therapeutiques (a propos de 50 cas personnels)]. Archives Francaises des Maladies de l'Appareil Digestif 1966;55(10):909‐14. [PubMed] [Google Scholar]
Freeman 1990 {published data only}
- Freeman O, Akamaguna A, Jarikre LN. Amoebic liver abscess: the effect of aspiration on the resolution or healing time. Annals of Tropical Medicine and Parasitology 1990;84(3):281‐7. [DOI] [PubMed] [Google Scholar]
Gilman 1980 {published data only}
- Gilman R, Islam M, Paschi S, Goleburn J, Ahmad F. Comparison of conventional and immunofluorescent techniques for the detection of Entamoeba histolytica in rectal biopsies. Gastroenterology 1980;78(3):435‐9. [PubMed] [Google Scholar]
Gorbea 1989 {published data only}
- Gorbea Robles MC, Eternod JG, Velasquez FG, Cupido JD. Comparative study in intestinal amebiasis and giardiasis in infants and pre‐school: efficacy and tolerance of secnidazole vs metronidazole and etofamide [Estudio comparativo en amebiasis y giardiasis intestinal del lactante y pre‐escolar: eficacia y tolerancia del secnidazole vs metronidazol y etofamida]. Investigacion Medicina Inst 1989;16:79‐82. [Google Scholar]
Hatchuel 1975 {published data only}
- Hatchuel W. Tinidazole for the treatment of amoebic liver abscess. South African Medical Journal 1975;49(45):1879‐81. [PubMed] [Google Scholar]
Hoekenga 1951 {published data only}
- Hoekenga MT. A comparison of aureomycin and carbarsone in the treatment of intestinal amebiasis. American Journal of Tropical Medicine and Hygiene 1951;31(4):423‐5. [DOI] [PubMed] [Google Scholar]
Holz 1965 {published data only}
- Holz J. Comparison of natural and synthetical emetine as amebicide in children. Paediatrica Indonesiana 1965;5(3):793‐801. [PubMed] [Google Scholar]
Huggins 1965 {published data only}
- Huggins D. Treatment of amebiasis. Results obtained with diloxanide furoate [Tratamento da amebiase. Resultados obtidos com o furoato de diloxanida]. Revista do Instituto de Medicina Tropical de São Paulo 1965;7(2):110‐1. [PubMed] [Google Scholar]
Huggins 1969 {published data only}
- Huggins D. Clinical trial of a new salt: ethyl chordiphene, in the treatment of chronic intestinal amebiasis [Ensaio clinico com um novo sal: etil clordifene, no tratamento da amebiase intestinal cronica]. Anais da Escola Nacional de Saude Publica e de Medicina Tropical 1969;3(1):93‐5. [PubMed] [Google Scholar]
Huggins 1974 {published data only}
- Huggins D. Chemoprophylaxis of amebiasis: clinical trial with a new drug etophamide, in an open community [Quimoprofilaxia da amebiase, ensaio clinico com uma nova substancia‐etofamida, em uma comunidade aberta]. Anais do Instituto de Higiene e Medicina Tropical 1974;2(1‐4):545‐51. [PubMed] [Google Scholar]
- Huggins D. Clinical and chemoprophilatical trial with a new drug used against amebiasis in an open community [Ensaio clinico e quimoprofilatico com etofamida, uma substancia amebicida numa comunidade aberta]. Revista Brasiliera de Clinica e Terapeutica 1974;3(7):279‐84. [Google Scholar]
Huggins 1977 {published data only}
- Huggins D. Clinical test with teclozan in the treatment of amebic dysentery with a dose of 24 hours [Ensaio clinico com teclozan no tratamento da colite amebiana nao desinterica na dose de 24 horas]. Anais do Instituto de Higiene e Medicina Tropical 1977‐1978;5(1‐4):329‐31. [PubMed] [Google Scholar]
Huggins 1980 {published data only}
- Huggins D. Clinical trial of etophamide in the treatment of chronic intestinal amebiasis. G.E.N. 1980;34(1):51‐4. [PubMed] [Google Scholar]
Huggins 1981 {published data only}
- Huggins D. Clinical trials with RO 7‐0207, ornidazole, in the treatment of chronic intestinal amebiasis [Ensaio clinico com Ro 7‐0207, ornidazol, no tratamento da amebiase intestinal cronica]. Folha Medica 1981;82(4):445‐6. [Google Scholar]
Irusen 1992 {published data only}
- Irusen EM, Jackson TF, Simjee AE. Asymptomatic intestinal colonization by pathogenic Entamoeba histolytica in amebic liver abscess: prevalence, response to therapy, and pathogenic potential. Clinical Infectious Diseases 1992;14(4):889‐93. [DOI] [PubMed] [Google Scholar]
Islam 1975 {published data only}
- Islam N, Hasan M. Tinidazole in the treatment of intestinal amoebiasis. Current Therapeutic Research 1975;17(2):161‐5. [PubMed] [Google Scholar]
Islam 1978a {published data only}
- Islam N, Hasan K. Tinidazole and metronidazole in hepatic amoebiasis. Drugs 1978;15 Suppl 1:26‐9. [DOI] [PubMed] [Google Scholar]
Islam 1978b {published data only}
- Islam N, Hasan M. Tinidazole and metronidazole in hepatic amoebiasis. Journal of Tropical Medicine and Hygiene 1978;81(1):20‐2. [PubMed] [Google Scholar]
Jain 1990 {published data only}
- Jain NK, Madan A, Sharma TN, Sharma DK, Mandhana RG. Hepatopulmonary amoebiasis. Efficacy of various treatment regimens containing dehydroemetine and/or metronidazole. Journal of the Association of Physicians of India 1990;38(4):269‐71. [PubMed] [Google Scholar]
Jayawickrema 1975 {published data only}
- Jayawickrema US, Lionel ND. Comparison of metronidazole with emetine and chloroquine in the treatment of hepatic amoebiasis ‐ a controlled double blind study. Ceylon Medical Journal 1975;20(2):99‐102. [PubMed] [Google Scholar]
Kahbazi 2016 {published data only}
- Kahbazi M, Ebrahimi M, Zarinfar N, Arjomandzadegan M, Fereydouni T, Karimi F, Najmi AR. Efficacy of synbiotics for treatment of bacillary dysentery in children: a double‐blind, randomized, placebo‐controlled study. Advances in Medicine 2016;2016(3194010):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 1972 {published data only}
- Kaur J, Mathur TN. Comparative drug trials in symptomatic and asymptomatic non‐dysenteric amoebic colitis. Indian Journal of Medical Research 1972;60(10):1547‐53. [PubMed] [Google Scholar]
Khalil 1987 {published data only}
- Khalil HM, Fawzy AF, Sarwat MA. Trials of furazol and some other drugs in intestinal amoebiasis. Journal of the Egyptian Society of Parasitology 1987;17(2):417‐25. [PubMed] [Google Scholar]
Khokhani 1977 {published data only}
- Khokhani RC, Garud AD, Deodhar KP, Sureka SB, Kulkarni M, Damle VB. Comparative study of tinidazole and metronidazole in amoebic liver abscess. Current Medical Research and Opinion 1977;5(2):161‐3. [DOI] [PubMed] [Google Scholar]
Khokhani 1978 {published data only}
- Khokhani RC, Garud AD, Deodhar KP, Sureka SB, Kulkarni M, Damle VB. Treatment of amoebic liver abscess with tinidazole and metronidazole. Drugs 1978;15 Suppl 1:23‐5. [DOI] [PubMed] [Google Scholar]
Konar 1963 {published data only}
- Konar NR, Mandal AK. Clinical trial of dehydroemetine in amoebiasis. Journal of the Indian Medical Association 1963;41(11):529‐34. [PubMed] [Google Scholar]
Krishnaiah 2003 {published data only}
- Krishnaiah YSR, Muzib YI, Bhaskar P, Satyanarayana V, Latha K. Pharmacokinetic evaluation of guar gum‐based colon‐targeted drug delivery systems of tinidazole in healthy human volunteers. Drug Delivery 2003;10(4):263‐8. [DOI] [PubMed] [Google Scholar]
Kurt 2008 {published data only}
- Kurt O, Girginkardesler N, Balcioglu IC, Ozbilgin A, Ok UZ. A comparison of metronidazole and single‐dose ornidazole for the treatment of dientamoebiasis. Clinical Microbiology and Infections 2008;14:601‐4. [DOI] [PubMed] [Google Scholar]
Laham 1951 {published data only}
- Laham E. Clinical trial of bismuth glycolyl arsenilate in intestinal amebiasis [Essai clinique du glycolyl arsenilate de bismuth dans l'amibiase intestinale]. Revue Medicale du Moyen‐Orient 1951;8(1):96‐9. [PubMed] [Google Scholar]
Levy 1967 {published data only}
- Levy A, Martinez AA, Castro ML. Sustained release erythromycin stearate in the therapy of intestinal amebiasis [Estearatao de eritromicina de liberacao regulada no tratamento da amebiase intestinal]. Revista Brasileira de Medicina 1967;24(6):413‐5. [PubMed] [Google Scholar]
Martinez 1969 {published data only}
- Martinez AA, Levy A. Efficiency of the combination erythromycin‐hexocylium in the treatment of dysentery syndromes [Eficacia da associacao eritromicina‐hexociclio no tratamento de sindromes disentericas]. Revista Brasileira de Medicina 1969;26(12):759‐62. [PubMed] [Google Scholar]
Masters 1979 {published data only}
- Masters DK, Hopkins AD. Therapeutic trials of four amoebicide regimens in rural Zaire. Journal of Tropical Medicine and Hygiene 1979;82(5):99‐101. [PubMed] [Google Scholar]
Mathur 1974 {published data only}
- Mathur TN, Kaur J. Non‐dysenteric amoebic colitis treated with two grammes of metronidazole given as a single dose for two days. Indian Journal of Medical Research 1974;62(8):1208‐11. [PubMed] [Google Scholar]
McAuley 1992 {published data only}
- McAuley JB, Juranek DD. Paromomycin in the treatment of mild‐to‐moderate intestinal amebiasis. Clinical Infectious Diseases 1992;15(3):551‐2. [DOI] [PubMed] [Google Scholar]
McLeod 2014 {published data only}
- McLeod C, Morris PS, Snelling TL, Carapetis JR, Bowen AC. Nitazoxanide for the treatment of infectious diarrhoea in the Northern Territory, Australia 2007‐2012. Rural and Remote Health 2014;14(2):2759. [PubMed] [Google Scholar]
Mendis 1984 {published data only}
- Mendis S, Dharmasena BD, Jayatissa SK. Comparison of tinidazole with metronidazole in the treatment of hepatic amoebiasis: a controlled double blind study. Ceylon Medical Journal 1984;29(2):97‐100. [PubMed] [Google Scholar]
Misra 1976a {published data only}
- Misra NP, Laiq SM. Tinidazole in intestinal amoebiasis. Antiseptic 1976;73(7):371‐3. [PubMed] [Google Scholar]
Misra 1976b {published data only}
- Misra NP, Laiq SM. Tinidazole in intestinal amoebiasis. Journal of the Association of Physicians of India 1976;24(4):231‐5. [PubMed] [Google Scholar]
Montovani 2009 {published data only}
- Montovani PAB, Pinto AMP, Santos MBD, Vieira DL, Prado AWD, Manfio JL. Bioavailability of two oral formulas of secnidazole in healthy volunteers. Brazilian Journal of Pharmaceutical Sciences 2009;45(4):687‐92. [Google Scholar]
Morales 1975 {published data only}
- Morales‐Mareles P, Suarez‐Sanchez F, Boom RA. Random double blind comparison of intravenous metronidazole and intramuscular emetine in acute amebic liver abscess [Tratamiento doble ciego al azar con metronidazole i.v. o emetina i.m. en el absceso hepatico amibiano agudo]. Prensa Medica Mexicana 1975;40(3‐4):124‐6. [Google Scholar]
Murray 1980 {published data only}
- Murray MJ, Murray A, Murray CJ. The salutary effect of milk on amoebiasis and its reversal by iron. British Medical Journal 1980;280(6228):1351‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muzzafar 2006 {published data only}
- Muzaffar J, Madan K, Sharma MP, Kar P. Randomized, single‐blind, placebo‐controlled multicenter trial to compare the efficacy and safety of metronidazole and satranidazole in patients with amebic liver abscess. Digestive Disease Science 2006;51(12):2270‐3. [DOI] [PubMed] [Google Scholar]
Nahrevanian 2008 {published data only}
- Nahrevanian H, Assmar M. Cryptosporidiosis in immunocompromised patients in the Islamic Republic of Iran. Journal of Microbiology Immunology and Infection 2008;41:74‐7. [PubMed] [Google Scholar]
Naik 1968 {published data only}
- Naik BK, Saboo RM. Quixalin. A drug trial. Journal of the Association of Physicians of India 1968;16(5):313‐6. [PubMed] [Google Scholar]
Nanavati 1965 {published data only}
- Nanavati MB, Damany SJ, Joshi HD. Clinical trial of dehydroemetine (Ro 1‐9334) in amoebiasis. Indian Practitioner 1965;18:259‐63. [PubMed] [Google Scholar]
O'Holohan 1972 {published data only}
- O'Holohan DR, Hugoe‐Matthews JH. Single‐dose and short course regimens of metronidazole in the treatment of amoebiasis in Malaysia. Annals of Tropical Medicine and Parasitology 1972;66(2):181‐6. [DOI] [PubMed] [Google Scholar]
Ohnishi 2014 {published data only}
- Ohnishi K, Sakamoto N, Kobayashi K‐I, Iwabuchi S, Nakamura‐Uchiyama F, Ajisawa A, et al. Subjective adverse reactions to metronidazole in patients with amebiasis. Parasitology International 2014;63(5):698‐700. [DOI] [PubMed] [Google Scholar]
Okeniyi 2007 {published data only}
- Okeniyi JA, Ogunlesi TA, Oyelami OA, Adeyemi LA. Effectiveness of dried Carica papaya seeds against human intestinal parasitosis: a pilot study. Journal of Medicine and Food 2007;10(1):194‐6. [DOI] [PubMed] [Google Scholar]
Olaeta 1996 {published data only}
- Olaeta Elizalde R, Perez Huacuja R, Najera Ruano A. Comparison of quinfamide versus etofamide in the Mexican population with intestinal amebiasis [Comparacion quinfamida vs etofamida en poblacion Mexicana con amibiasis intestinal]. Acta Gastroenterologica Latinoamericana 1996;26(5):277‐80. [PubMed] [Google Scholar]
Omrani 1995 {published data only}
- Omrani G, Rastegar‐Lari A, Khamse M. Effect of single dose of secnidazole in treatment of intestinal amoebiasis. Journal of the Pakistan Medical Association 1995;45(6):159. [PubMed] [Google Scholar]
Orozco 1975 {published data only}
- Orozco Garcia O, Perez Nava J, Gonzalez Diaz R. Clinical evaluation of nimorazole in amebic liver abscess (author's transl)]. Prensa Medica Mexicana 1975;40(11‐12):378‐82. [PubMed] [Google Scholar]
Padilla 1995 {published data only}
- Padilla N, Figueroa R, Rivera MR, Guerrero S. Comparative study in quinfamide and etofamide in the treatment of asymptomatic amoebic infection [Estudio comparativo entre quinfamida y etofamida en el tratamiento de la infección amibiana asintomática]. Revista Mexicana de Pediatría 1995;62(1):5‐7. [Google Scholar]
Padilla 1998 {published data only}
- Padilla‐Raygoza N, Alarcon‐Ginori A, Figueroa‐Ferrari RC, Munoz‐Rodriguez M. Comparison of the effect of quinfamide and nitazoxanide in the treatment of nondysenteric intestinal amebiasis in children [Comparacion del efecto de la quinfamida y de la nitazoxanida en el tratamento de la amibiasis intestinal no disenterica en ninos]. Revista Mexicana de Pediatria 1998;65(5):196‐9. [Google Scholar]
Padilla 2002 {published data only}
- Padilla N, Diaz R, Alarcon A, Barreda R. Antiamoebic chemoprophylaxis using quinfamide in children: a comparative study. Scientific World Journal 2002;2:1070‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pang 2014 {published data only}
- Pang X, Zhang Y, Gao R, Zhong K, Zhong D, Chen X. Effects of rifampin and ketoconazole on pharmacokinetics of morinidazole in healthy Chinese subjects. Antimicrobial Agents and Chemotherapy 2014;58(10):5987‐5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pimparkar 1966 {published data only}
- Pimparkar BD, Raghavan P, Satoskar RS. A clinical trial of 1‐dichloroacetyl‐4‐methylpiperidine (R.D. 7098), a new anti‐amoebic drug. Transactions of the Royal Society of Tropical Medicine and Hygiene 1966;60(2):237‐40. [DOI] [PubMed] [Google Scholar]
Populaire 1980 {published data only}
- Populaire P, Decouvelaere B, Renard A, Pasquier P. Study of seric concentrations and urinary excretion of secnidazole after oral administration in man. Comparison with tinidazole [Etude des taux seriques et de l'elimination urinaire du secnidazole apres administration orale chez l'homme comparaison avec le tinidazole]. Pathologie et Biologie 1980;28(9):621‐4. [PubMed] [Google Scholar]
Powell 1965a {published data only}
- Powell SJ, MacLeod I, Wilmot AJ, Elsdon‐Dew R. The treatment of acute amoebic dysentery: trials of dehydroemetine, of dehydroemetine‐bismuth‐iodide, and of dehydroemetine and dehydroemetine‐bismuth‐iodide in combination. Annals of Tropical Medicine and Parasitology 1965;59:205‐7. [DOI] [PubMed] [Google Scholar]
Powell 1965b {published data only}
- Powell SJ, Wilmot AJ, Macleod IN, Elsdon‐Dew R. A comparative trial of dehydroemetine, emetine hydrochloride and chloroquine in the treatment of amoebic liver abscess. Annals of Tropical Medicine and Parasitology 1965;59(4):496‐9. [DOI] [PubMed] [Google Scholar]
Powell 1965c {published data only}
- Powell SJ, Wilmot AJ, Macleod IN, Elsdon‐Dew R. Dehydroemetine in the treatment of amoebic liver abscess. Annals of Tropical Medicine and Parasitology 1965;59(2):208‐9. [DOI] [PubMed] [Google Scholar]
Powell 1965d {published data only}
- Powell SJ, MacLeod I, Wilmot AJ, Elsdon‐Dew R. Further drug trials in acute amoebic dysentery: demethylchlortetracycline, methacycline, ampicillin and chlorhydroxyquinoline. Transactions of the Royal Society of Tropical Medicine and Hygiene 1965;59(6):709‐11. [Google Scholar]
Powell 1966a {published data only}
- Powell SJ, MacLeod I, Wilmot AJ, Elsdon‐Dew R. Ambilhar in amoebic dysentery and amoebic liver abscess. Lancet 1966;i:20‐2. [DOI] [PubMed] [Google Scholar]
Powell 1966b {published data only}
- Powell SJ, MacLeod I, Wilmot AJ, Elsdon‐Dew R. Late release oral dehydroemetine in acute amoebic dysentery. Journal of Tropical Medicine and Hygiene 1966;69:153‐4. [Google Scholar]
Powell 1966c {published data only}
- Powell SJ, Wilmot AJ, MacLeod I, Elsdon‐Dew R. The effect of a nitro‐thiazole derivative, Ciba 32,644‐Ba, in amebic dysentery and amebic liver abscess. American Journal of Tropical Medicine and Hygiene 1966;15(3):300‐2. [DOI] [PubMed] [Google Scholar]
Powell 1967 {published data only}
- Powell SJ, Wilmot AJ, Macleod IN, Elsdon‐Dew R. A comparative trial of dehydroemetine and emetine hydrochloride in identical dosage in amoebic liver abscess. Annals of Tropical Medicine and Parasitology 1967;61(1):26‐8. [DOI] [PubMed] [Google Scholar]
Powell 1968 {published data only}
- Powell SJ. Metronidazole in the treatment of amoebic dysentery. Indian Practitioner 1968;21:696‐700. [Google Scholar]
Powell 1969a {published data only}
- Powell SJ, Wilmot AJ, Elsdon‐Dew R. Single and low dosage regimens of metronidazole in amoebic dysentery and amoebic liver abscess. Annals of Tropical Medicine and Parasitology 1969;63(2):139‐42. [DOI] [PubMed] [Google Scholar]
Powell 1969b {published data only}
- Powell SJ, Wilmot AJ, Elsdon‐Dew R. The use of niridazole alone and in combination with other amoebicides in amoebic dysentery and amoebic liver abscess. Annals of the New York Academy of Science 1969;160(2):749‐54. [DOI] [PubMed] [Google Scholar]
Powell 1969c {published data only}
- Powell SJ. Drug trials in amoebiasis. Bulletin of the World Health Organization 1969;40(6):956‐8. [PMC free article] [PubMed] [Google Scholar]
Powell 1971a {published data only}
- Powell SJ, Elsdon‐Dew R. Evaluation of metronidazole and MK910 in invasive amebiasis. American Journal of Tropical Medicine and Hygiene 1971;20(6):839‐41. [DOI] [PubMed] [Google Scholar]
Powell 1971b {published data only}
- Powell SJ, Elsdon‐Dew R. Chloroquine in amoebic dysentery. Transactions of the Royal Society of Tropical Medicine and Hygiene 1971;65(4):540. [DOI] [PubMed] [Google Scholar]
Powell 1972a {published data only}
- Powell SJ, Elsdon‐Dew R. Some new nitroimidazole derivatives. Clinical trials in amebic liver abscess. American Journal of Tropical Medicine and Hygiene 1972;21(5):518‐20. [DOI] [PubMed] [Google Scholar]
Powell 1972b {published data only}
- Powell SJ. Latest developments in the treatment of amebiasis. Advances in Pharmacology and Chemotherapy 1972;10:91‐103. [DOI] [PubMed] [Google Scholar]
Powell 1973 {published data only}
- Powell SJ, Rubidge CJ, Elsdon‐Dew R. Clinical trials of benzoyl metronidazole suspension in amoebic dysentery and amoebic liver abscess. South African Medical Journal 1973;47(12):507‐8. [PubMed] [Google Scholar]
Prakash 1974 {published data only}
- Prakash C, Bansal BC, Bansal MR. A comparative study of tinidazole and metronidazole in symptomatic intestinal amoebiasis. Journal of the Association of Physicians of India 1974;22(7):527‐9. [PubMed] [Google Scholar]
Qureshi 1994 {published data only}
- Qureshi H, Baqai B, Zuberi SJ, Ahmed W, Qureshi SA. Efficacy of secnidazole in the treatment of intestinal amoebiasis. Journal of the Pakistan Medical Association 1994;44(4):93‐4. [PubMed] [Google Scholar]
Qureshi 1997 {published data only}
- Qureshi H, Ali A, Baqai R, Ahmed A. Efficacy of a combined diloxanide furoate‐metronidazole preparation in the treatment of amoebiasis and giardiasis. Journal of International Medical Research 1997;25(3):167‐70. [DOI] [PubMed] [Google Scholar]
Rodrigues 1968 {published data only}
- Rodrigues LD, Jafferian PA, Villela MdeP, Mello EB. Comparative study on 3 amebicides: teclozine, clefamide and a combination of clefamide and iodo‐chloro‐oxyquinolines and streptomycin [Estudo comparativo de tres amebicidas: o teclozine, a clefamida e a associacao de clefamida com iodo‐cloro‐oxiquinoleina e estreptomicina]. Hospital (Rio de Janeiro, Brazil) 1968;74(5):1563‐73. [PubMed] [Google Scholar]
Ruas 1973 {published data only}
- Ruas A, Correia MH, Valle JC do, Ribeiro JA. RO 7‐0207 in amoebic liver abscess. Comparative study of the effects of RO 7‐0207 and metronidazole. Central African Journal of Medicine 1973;19(6):128‐32. [PubMed] [Google Scholar]
Ruchko 1978 {published data only}
- Ruchko AF. Clinical and treatment characteristics of amebiasis in children in a hot climate. Pediatriia, Akusherstvo i Ginekologiia 1978;Sept‐Oct(5):28‐9. [PubMed] [Google Scholar]
Saha 1966 {published data only}
- Saha TK, Chaudhuri RN. Clinical trial of furoxone in amoebic dysentery. Bulletin of the Calcutta School of Tropical Medicine 1966;14(1):22. [PubMed] [Google Scholar]
Saha 1970 {published data only}
- Saha TK, Mandal JN. Niridazole in amoebic dysentery. Journal of the Indian Medical Association 1970;55(4):127‐9. [PubMed] [Google Scholar]
Salem 1964 {published data only}
- Salem HH, Rabbo HA. Clinical trials with dehydroemetine dihydrochloride in the treatment of acute amoebiasis. Journal of Tropical Medicine and Hygiene 1964;67:137‐41. [PubMed] [Google Scholar]
Salem 1967 {published data only}
- Salem SN. Clinical trial of oral dehydroemetine in intestinal amoebiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1967;61(6):774‐5. [DOI] [PubMed] [Google Scholar]
Sandia 1977 {published data only}
- Sandia OG, Dobbins Filho J, Leite IC, Harris PB. Comparative therapeutic trial of ornidazole and metronidazole in chronic amebiasis [Ensaio terapeutico comparativo entre ornidazole e metronidazol em amebiase cronica]. Revista do Instituto de Medicina Tropical de São Paulo 1977;19(1):52‐6. [PubMed] [Google Scholar]
Sangiuolo 1969 {published data only}
- Sangiuolo F. Antimicrobial and antispastic effect of a combination of canulase and iodochloroxyquinoline (Septo‐canulase) in various acute and chronic enteropathies [Sull'azione antimicrobica ed antispastica di una associazione di canulase con iodoclorossichinolina (Septo‐canulase) in alcune enteropatie acute e croniche]. Rassegna Internazionale di Clinica e Terapia 1969;49(19):1231‐50. [PubMed] [Google Scholar]
Sankale 1966 {published data only}
- Sankale M, Moulanier M. Treatment of amebiasis with oral dehydroemetine [Le traitement de l'amibiase par la dehydroemetine orale]. Therapie 1966;21(3):733‐41. [PubMed] [Google Scholar]
Sankale 1969 {published data only}
- Sankale M, Satge P, Lariviere M, Moulanier M, Bourgeade A, Debroise C, et al. Efficacy of niridazole in amoebic dysentery. Annals of the New York Academy of Science 1969;160(2):755‐63. [DOI] [PubMed] [Google Scholar]
Sankale 1974 {published data only}
- Sankale M, Coly D, Niang I. Treatment of amoebiasis with a drinkable suspension of metronidazole [Traitement de l'amibiase par une suspension buvable de metronidazole]. Therapie 1974;29:411‐5. [PubMed] [Google Scholar]
Satpathy 1988 {published data only}
- Satpathy BK, Acharya SK, Satpathy S. Comparative study of intravenous metronidazole and intramuscular dehydroemetine in amoebic liver abscess. Journal of the Indian Medical Association 1988;86(2):38‐40. [PubMed] [Google Scholar]
Schapiro 1967 {published data only}
- Schapiro MM. Intestinal amebiasis. A preliminary clinical trial of furamide T/c. American Journal of Tropical Medicine and Hygiene 1967;16(6):704‐7. [PubMed] [Google Scholar]
Scragg 1968 {published data only}
- Scragg JN, Powell SJ. Emetine hydrochloride and dehydroemetine combined with chloroquine in the treatment of children with amoebic liver abscess. Archives of Disease in Childhood 1968;43(227):121‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scragg 1970 {published data only}
- Scragg JN, Powell SJ. Metronidazole and niridazole combined with dehydroemetine in treatment of children with amoebic liver abscess. Archives of Disease in Childhood 1970;45(240):193‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Segal 1967 {published data only}
- Segal J. Clinico‐paracytological and therapeutic study with a new preparation of erythromycin stearate of controlled release (A‐16535) [Estudo clinico‐parasitologico e terapeutico com uma nova preparacao de estearato de eritromicina de liberacao regulada (A‐16535)]. Revista Brasileira de Medicina 1967;24(8):626‐32. [PubMed] [Google Scholar]
Sharif 2017 {published data only}
- Sharif A, Kashani HH, Nasri E, Soleimani Z, Sharif MR. The role of probiotics in the treatment of dysentery: a randomized double‐blind clinical trial. Probiotics and Antimicrobial Proteins 2017;21:1‐6 [Epub ahead of print]. [DOI: 10.1007/s12602-017-9271-0] [DOI] [PubMed] [Google Scholar]
Sharma 1989 {published data only}
- Sharma MP, Rai RR, Acharya SK, Ray JC, Tandon BN. Needle aspiration of amoebic liver abscess. BMJ 1989;299(6711):1308‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrotriya 1985 {published data only}
- Shrotriya V, Dabral SB, Maheshwari BB, Gupta SC, Maheshwari BB. A controlled trial of Diarex and tinidazole in chronic intestinal amoebiasis. Medicine and Surgery 1985;25(1):8‐9, 16. [Google Scholar]
Simjee 1985 {published data only}
- Simjee AE, Gathiram V, Jackson TF, Khan BF. A comparative trial of metronidazole v. tinidazole in the treatment of amoebic liver abscess. South African Medical Journal. Suid‐Afrikaanse Tydskrif Vir Geneeskunde 1985;68(13):923‐4. [PubMed] [Google Scholar]
Simon 1967 {published data only}
- Simon M, Shookhoff HB, Terner H, Weingarten B, Parker JG. Paromomycin in the treatment of intestinal amebiasis; a short course of therapy. American Journal of Gastroenterology 1967;48(6):504‐11. [PubMed] [Google Scholar]
Sinuhaji 1986 {published data only}
- Sinuhaji AB, Lubis CP, Daulay HRM, Lubis IT, Jufri A, Sutanto AH. A double‐blind trial between metronidazole and secnidazole in acute amebic dysentery in children (Preliminary Report). Paediatrica Indonesiana 1986;26:9‐14. [PubMed] [Google Scholar]
Sladden 1964 {published data only}
- Sladden DL, Taylor E, Livingstone DJ. A clinical trial of a new compound (dehydroemetine bismuth iodide, Ro 4,3076) in amoebic dysentery. Central African Journal of Medicine 1964;10(11):412‐3. [PubMed] [Google Scholar]
Soh 1980 {published data only}
- Soh CT, Cho MJ, Choi HJ, Hur JD. Double blind test of ornidazole versus tinidazole against amoebic liver abscess. Yonsei Reports on Tropical Medicine 1980;11(1):43‐50. [Google Scholar]
Speich 2013 {published data only}
- Speich B, Marti H, Ame SM, Ali SM, Bogoch II, Utzinger J, Albonico M, et al. Prevalence of intestinal protozoa infection among school‐aged children on Pemba Island, Tanzania, and effect of single‐dose albendazole, nitazoxanide and albendazole‐nitazoxanide. Parasites & Vectors 2013;6(3):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spellberg 1969 {published data only}
- Spellberg MA. Treatment of amoebic liver abscess. American Journal of Gastroenterology 1969;51(4):298‐302. [PubMed] [Google Scholar]
Spillman 1976 {published data only}
- Spillman R, Ayala SC, Sanchez CE. Double blind test of metronidazole and tinidazole in the treatment of asymptomatic Entamoeba histolytica and Entamoeba hartmanni carriers. American Journal of Tropical Medicine and Hygiene 1976;25(4):549‐51. [DOI] [PubMed] [Google Scholar]
Sutrisno 1978 {published data only}
- Sutrisno D, Ismail D, Sebodo T, Ismangun, Noerhajati S. Nitrimidazine (Naxogin) in the treatment of children with intestinal amoebiasis. Paediatrica Indonesiana 1978;18(7‐8):217‐23. [PubMed] [Google Scholar]
Tandon 1997 {published data only}
- Tandon A, Jain AK, Dixit VK, Agarwal AK, Gupta JP. Needle aspiration in large amoebic liver abscess. Tropical Gastroenterology: Official Journal of the Digestive Diseases Foundation 1997;18(1):19‐21. [PubMed] [Google Scholar]
Thompson 2015 {published data only}
- Thompson CN, Phan MVT, Minh Hoang N, Minh P, Vinh NT, Thuy CT, et al. A prospective multi‐center observational study of children hospitalized with diarrhea in Ho Chi Minh City, Vietnam. American Journal of Tropical Medicine and Hygiene 2015;92(5):1045‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thoren 1990a {published data only}
- Thoren K, Hakansson C, Bergstrom T, Johnaisson G, Norkrans G. Treatment of asymptomatic amebiasis in homosexual men. Clinical trials with metronidazole, tinidazole, and diloxanide furoate. Sexually Transmitted Diseases 1990;17(2):72‐4. [DOI] [PubMed] [Google Scholar]
Thoren 1990b {published data only}
- Thoren K, Hakansson C, Bergstrom T, Johnaisson G, Norkrans G. Treatment of asymptomatic amoebiasis in homosexual men: clinical trials with metronidazole, tinidazole and diloxanide furoate [abstract]. Genitourinary Medicine 1990;66(5):411. [DOI] [PubMed] [Google Scholar]
Tjaij 1969 {published data only}
- Tjaij JK, Raid N, Irawati T, Siregar D, Kwo IT, Tan BE. Mexaform and entobex therapy in amebic dysentery. Paediatrica Indonesiana 1969;9(5):210‐4. [PubMed] [Google Scholar]
Tjaij 1970 {published data only}
- Tjaij JK, Raid N, Sutanto AH. Clinical trials of oral dehydro‐emetine tablets (Ro 1‐9334/10) in amebic dysentery in children. Paediatrica Indonesiana 1970;10(4):139‐48. [PubMed] [Google Scholar]
Vaidya 1983 {published data only}
- Vaidya AB, Ray DK, Mankodi NA, Paul T, Sheth UK. Phase I tolerability and antiamoebic activity studies with 1‐methylsulphonyl‐3‐(1‐methyl‐5‐nitro‐2‐imidazolyl)‐2‐imidazolidinone (Go 10213): a new antiprotozoal agent. British Journal of Clinical Pharmacology 1983;16(5):517‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vakil 1967 {published data only}
- Vakil BJ, Shah SC, Moses JM. The comparative value of dehydroemetine and emetine in amebiasis. Journal of the Association of Physicians of India 1967;15(5):223‐8. [PubMed] [Google Scholar]
Vakil 1971 {published data only}
- Vakil BJ, Dalal AJ, Mehta AJ, Mody NC. Clinical evaluation of oral dehydroemetine in amoebiasis. Journal of the Association of Physicians of India 1971;19(5):403‐8. [PubMed] [Google Scholar]
Vakil 1974 {published data only}
- Vakil BJ, Dalal NJ. Comparative evaluation of amoebicidal drugs. Progress in Drug Research 1974;18:353‐64. [DOI] [PubMed] [Google Scholar]
Valencia 1973 {published data only}
- Valencia‐Parparcen J. Erythromycin in the treatment of intestinal amebiasis and other processes of the digestive tract [La eritromicina en el tratamiento de la amibiasis intestinal y otros procesos del tubo digestivo]. G.E.N. 1973;28(1):29‐37. [PubMed] [Google Scholar]
Vanijanonta 1985 {published data only}
- Vanijanonta S, Bunnag D, Looareesuwan S, Harinasuta T. Low dose tinidazole in the treatment of amoebic liver abscess. Southeast Asia Journal of Tropical Medicine and Public Health 1985;16(2):253‐6. [PubMed] [Google Scholar]
Viswanathan 1968 {published data only}
- Viswanathan M, Krishnaswami CV. Therapeutic trials with oral dehydroemetine in intestinal amoebiasis. Journal of the Indian Medical Association 1968;51(8):381‐3. [PubMed] [Google Scholar]
Waddington 2018 {published data only}
- Waddington CS, McLeod C, Morris P, Bowen A, Naunton M, Carapetis J, et al. The NICE‐GUT trial protocol: a randomised, placebo controlled trial of oral nitazoxanide for the empiric treatment of acute gastroenteritis among Australian Aboriginal children. BMJ Open 2018;8:e019632. [DOI: 10.1136/bmjopen-2017-019632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1971a {published data only}
- Wang LT, Sung JL. Trials of metronidazole in amebic dysentery and amebic liver abscess. Taiwan Yi Xue Hue Za Zhi 1971;70(7):405‐8. [PubMed] [Google Scholar]
Wang 1971b {published data only}
- Wang LT, Yang SP. Studies on oxytetracycline resistant amebic dysentery. Taiwan Yi Xue Hue Za Zhi 1971;70(3):131‐4. [PubMed] [Google Scholar]
Watson 1975 {published data only}
- Watson PG. Amoebic infection of the eye. Transactions of the Ophthalmological Societies of the United Kingdom 1975;95(2):204‐6. [PubMed] [Google Scholar]
Welch 1978 {published data only}
- Welch JS, Rowsell BJ, Freeman C. Treatment of intestinal amoebiasis and giardiasis. Efficacy of metronidazole and tinidazole compared. Medical Journal of Australia 1978;1(9):469‐71. [PubMed] [Google Scholar]
Widjaya 1991 {published data only}
- Widjaya P, Bilic A, Babic Z, Ljubicic N, Bakula B, Pilas V. Amoebic liver abscess: ultrasonographic characteristics and results of different therapeutic approaches. Acta Medica Iugoslavica 1991;45(1):15‐21. [PubMed] [Google Scholar]
Wilmot 1962 {published data only}
- Wilmot AJ, Powell SJ, MacLeod I, Elsdon‐Dew R. Paromomycin in acute amoebic dysentery. Annals of Tropical Medicine and Parasitology 1962;56:383‐6. [DOI] [PubMed] [Google Scholar]
Wolfe 1973 {published data only}
- Wolfe MS. Nondysenteric intestinal amoebiasis. JAMA 1973;224(12):1601‐4. [PubMed] [Google Scholar]
Wolfensberger 1968 {published data only}
- Wolfensberger HR. Amoebiasis: clinical trials of dehydroemetine late‐release tablets (Ro 1‐9334/20) compared with parenteral dehydroemetine and niridazole. Transactions of the Royal Society of Tropical Medicine and Hygiene 1968;62(6):831‐7. [DOI] [PubMed] [Google Scholar]
Zuberi 1973 {published data only}
- Zuberi SJ, Ibrahim M. Tinidazole in amoebiasis. Practitioner 1973;211:93‐5. [PubMed] [Google Scholar]
References to ongoing studies
NIAID 2016 {published data only}
- National Institute of Allergy and Infectious Diseases (NIAID). Phase IIa Randomized, Single‐blinded, Placebo‐controlled Clinical Trial of the Reprofiled Drug Auranofin for GI Protozoa. clinicaltrials.gov Last Update Posted: September 15, 2017. [ClinicalTrials.gov identifier: NCT02736968]
Pfizer 2016 {published data only}
- Drug Use Investigation of Paromomycin. clinicaltrials.gov Last update posted: March 6, 2018. [ClinicalTrials.gov identifier NCT02680665 ]
Additional references
AAP 2015
- American Academy of Pediatrics. Amebiasis. In: Kimberlin DW, Brady MT, Jackson MA, Long SS editor(s). Red Book: 2015 Report of the Committee on Infectious Diseases. 30th Edition. Elk Grove Village, IL: American Academy of Pediatrics, 2012:228‐30. [Google Scholar]
Abd‐Alla 2002
- Abd‐Alla MD, Ravdin JI. Diagnosis of amoebic colitis by antigen capture ELISA in patients presenting with acute diarrhoea in Cairo, Egypt. Tropical Medicine and International Health 2002;7(4):365‐70. [DOI] [PubMed] [Google Scholar]
Adagu 2002
- Adagu IS, Nolder D, Warhurst DC, Rossignol JF. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica, and Trichomonas vaginalis. Journal of Antimicrobial Chemotherapy 2002;49:103‐11. [DOI] [PubMed] [Google Scholar]
Allen 2010
- Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD003048.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bansal 2006
- Bansal D, Sehgal R, Chawla Y, Malla N, Mahajan RC. Multidrug resistance in amoebiasis patients. Indian Journal of Medical Research 2006;124(2):189‐94. [PubMed] [Google Scholar]
Barwick 2002
- Barwick RS, Uzicanin A, Lareau S, Malakmadze N, Imnadze P, Iosava M, et al. Outbreak of amebiasis in Tbilisi, Republic of Georgia, 1998. American Journal of Tropical Medicine and Hygiene 2002;67(6):623‐31. [DOI] [PubMed] [Google Scholar]
Bercu 2007
- Bercu TE, Petri WA, Behm JW. Amebic colitis: new insights into pathogenesis and treatment. Current Gastroenterology Reports 2007;9(5):429‐33. [DOI] [PubMed] [Google Scholar]
Blessman 2003
- Blessman J, Ali IKM, Ton Nu PA, Dinh BT, Ngo Viet TQ, Van AL, et al. Longitudinal study of intestinal Entamoeba histolytica infections in asymptomatic adult carriers. Journal of Clinical Microbiology 2003;41(10):4745‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braga 1996
- Braga LL, Lima AAM, Sears CL, Newman RD, Wuhib T, Paiva CA, et al. Seroepidemiology of Entamoeba histolytica in a slum in Northeastern Brazil. American Journal of Tropical Medicine and Hygiene 1996;55(6):693‐7. [DOI] [PubMed] [Google Scholar]
Braga 1998
- Braga LL, Mendonca Y, Paiva CA, Sales A, Cavalcante ALM, Mann BJ. Seropositivity for and intestinal colonization with Entamoeba histolytica and Entamoeba dispar in individuals in Northeastern Brazil. Journal of Clinical Microbiology 1998;36(10):3044‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. British Medical Journal 2005;331(7521):897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Catalla‐Lopez 2014
- Catala‑Lepez F, Tobias A, Cameron C, Moher D, Hutton B. Network meta‑analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatology International 2014;34(11):1489‐96. [DOI] [PubMed] [Google Scholar]
CDC 2010
- CDC. Laboratory diagnosis of amebiasis: Entamoeba histolytica and Entamoeba dispar. CDC, Atlanta, GA, 2010. http://www.cdc.gov/dpdx/resources/pdf/benchAids/entamoeba_benchaid.pdf (accessed 12 April 2015).
Cedillo‐Rivera 2002
- Cedillo‐Rivera R, Chavez B, Gonzales‐Robles A, Tapia A, Yepez‐Mulia L. In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis, and Trichomonas vaginalis trophozoites. Journal of Eukaryotic Microbiology 2002;49(3):201‐8. [DOI] [PubMed] [Google Scholar]
Chen 2007
- Chen Y, Zhang Y, Yang B, Qi T, Lu H, Cheng X, Tachibana H. Seroprevalence of Entamoeba histolytica infection in HIV infected patients in China. American Journal of Tropical Medicine and Hygiene 2007;77:825–8. [PubMed] [Google Scholar]
Choudhuri 2012
- Choudhuri G, Rangan M. Amebic infection in humans. Indian Journal of Gastroenterology 2012;31(4):153–62. [DOI] [PubMed] [Google Scholar]
Dans 2006
- Dans L, Martinez E. Amoebic dysentery. Clinical Evidence 2006;15:1007‐13. [PubMed] [Google Scholar]
Fotedar 2007
- Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Laboratory diagnostic techniques for Entamoeba species. Clinical Microbiology Reviews 2007;20:511‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2005
- Fox LM, Saravolatz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clinical Infectious Diseases 2005;40:1173–80. [DOI] [PubMed] [Google Scholar]
Gathiram 1987
- Gathiram V, Jackson TFHG. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of Entamoeba histolytica. South African Medical Journal 1987;72:669‐72. [PubMed] [Google Scholar]
Gatti 2002
- Gatti S, Swierczynski G, Robinson F, Anselmi M, Corrales J, Moreira J, et al. Amebic infections due to the Entamoeba histolytica‐Entamoeba dispar complex: a study of the incidence in a remote rural area of Ecuador. American Journal of Tropical Medicine and Hygiene 2002;67(1):123‐7. [DOI] [PubMed] [Google Scholar]
Goldenberg 2013
- Goldenberg JZ, Ma SSY, Saxton JD, Martzen MR, Vandvik PO, Thorlund K, et al. Probiotics for the prevention of Clostridium difficile‐associated diarrhea in adults and children. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD006095.pub3] [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(1490):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunther 2011
- Gunther J, Shafir S, Bristow B, Sorvillo F. Short report: amebiasis‐related mortality among United States residents, 1990–2007. American Journal of Tropical Medicine and Hygiene 2011;85(6):1038–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haque 1997
- Haque R, Faruque ASG, Hahn P, Lyerly DM, Petri WA Jr. Entamoeba histolytica and Entamoeba dispar infection in children in Bangladesh. Journal of Infectious Diseases 1997;175(3):734‐6. [DOI] [PubMed] [Google Scholar]
Haque 1998
- Haque R, Ali IKM, Akther S, Petri WA Jr. Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. Journal of Clinical Microbiology 1998;36(2):449‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haque 1999
- Haque R, Ali IM, Petri WA Jr. Prevalence and immune response to Entamoeba histolytica infection in preschool children in Bangladesh. American Journal of Tropical Medicine and Hygiene 1999;60(6):1031‐4. [DOI] [PubMed] [Google Scholar]
Haque 2001
- Haque R, Ali IM, Sack RB, Farr BM, Ramakrishnan G, Petri WA Jr. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. Journal of Infectious Diseases 2001;183(12):1787‐93. [DOI] [PubMed] [Google Scholar]
Haque 2002
- Haque R, Duggal P, Ali IM, Hossain MB, Mondal D, Sack RB, et al. Innate and acquired resistance to amebiasis in Bangladeshi children. Journal of Infectious Diseases 2002;186(4):547‐52. [DOI] [PubMed] [Google Scholar]
Haque 2003
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA Jr. Amebiasis. New England Journal of Medicine 2003;348(16):1565‐73. [DOI] [PubMed] [Google Scholar]
Hayat 2016
- Hayat F, Azam A, Shin D. Recent progress on the discovery of antiamoebic agents. Bioorganic & Medicinal Chemistry Letters 2016;26(21):5149–59. [DOI] [PubMed] [Google Scholar]
Herbinger 2011
- Herbinger KH, Fleischmann E, Weber C, Perona P, Löscher T, Bretzel G. Epidemiological, clinical, and diagnostic data on intestinal infections with Entamoeba histolytica and Entamoeba dispar among returning travelers. Infection 2011;39(6):527‐35. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hung 2008
- Hung CC, Ji DD, Sun HY, Lee YT, Hsu SY, Chang SY, et al. Increased risk for Entamoeba histolytica infection and invasive amebiasis in HIV seropositive men who have sex with men in Taiwan. PLoS Neglected Tropical Diseases 2008;2(2):e175.doi:10.1371/journal.pntd.0000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2010
- James R, Barratt J, Marriott D, Harkness J, Stark D. Short report: seroprevalence of Entamoeba histolytica infection among men who have sex with men in Sydney, Australia. American Journal of Tropical Medicine and Hygiene 2010;83(4):914–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kappagoda 2011
- Kappagoda S, Singh U, Blackburn BG. Antiparasitic therapy. Mayo Clinic Proceedings 2011;86(6):561‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamp 1999
- Lamp KC, Freeman CD, Klutman NE, Lacy MK. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clinical Pharmacokinetics 1999;36(5):353‐73. [DOI] [PubMed] [Google Scholar]
Lee 2015
- Lee KC, Lu CC, Hu WS, Lin SE, Chen HH. Colonoscopic diagnosis of amebiasis: a case series and systematic review. International Journal of Colorectal Disease 2015;30(1):31–41. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Li 1996
- Li E, Stanley SL Jr. Parasitic diseases of the liver and intestines. Gastroenterology Clinics 1996;25(3):471‐92. [DOI] [PubMed] [Google Scholar]
Looke 1987
- Looke DFM. Metronidazole and tinidazole compared. Australian Prescriber 1987;10(2):35‐7. [Google Scholar]
Mackey‐Lawrence 2011
- Mackey‐Lawrence N, Petri Jr WA. Amoebic dysentery. BMJ Clinical Evidence 2011;2011:0918. [PMC free article] [PubMed] [Google Scholar]
Marie 2013
- Marie C, Petri WA Jr. Amoebic dysentery. BMJ Clinical Evidence 2013;2013:0918. [PMC free article] [PubMed] [Google Scholar]
Mondal 2006
- Mondal D, Petri WA, Sack RB, Kirkpatrick BD, Haque R. Entamoeba histolytica‐associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006;100(11):1032‐8. [DOI] [PubMed] [Google Scholar]
Monro 1974
- Monro AM. Blood levels of chemotherapeutic drugs and the pharmacokinetics of tinidazole and metronidazole. Current Medical Research and Opinion 1974;2(3):130‐6. [DOI] [PubMed] [Google Scholar]
Morfl 2012
- Morfl L, Singh U. Entamoeba histolytica: a snapshot of current research and methods for genetic analysis. Current Opinion in Microbiology 2012;15(4):469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagata 2012
- Nagata N, Shimbo T, Akiyama J, Nakashima R, Nishimura S, Yada T, et al. Risk factors for intestinal invasive amebiasis in Japan, 2003–2009. Emerging Infectious Diseases 2012;18(5):717‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagpal 2012
- Nagpal I, Raj I, Subbarao N, Gourinath S. Virtual screening, identification and in vitro testing of novel inhibitors of O‐acetyl‐L‐serine sulfhydrylase of Entamoeba histolytica. PLoS One 2012;7(2):e30305. [DOI: 10.1371/journal.pone.0030305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nesbitt 2004
- Nesbitt RA, Mosha FW, Katki HA, Ashraf M, Assenga C, Lee CM. Amebiasis and comparison of microscopy to ELISA technique in detection of Entamoeba histolytica and Entamoeba dispar. Journal of the National Medical Association 2004;96(5):671‐7. [PMC free article] [PubMed] [Google Scholar]
Ochoa 2005
- Ochoa TJ, White AC Jr. Nitazoxanide for treatment of intestinal parasites in children. Pediatric Infectious Disease Journal 2005;24(7):641‐2. [DOI] [PubMed] [Google Scholar]
Okamoto 2005
- Okamoto M, Kawabe T, Ohata K, Togo G, Hada T, Katamoto T, et al. Amebic colitis in asymptomatic subjects with positive fecal occult blood test results: clinical features different from symptomatic cases. American Journal of Tropical Medicine and Hygiene 2005;73(5):934‐5. [PubMed] [Google Scholar]
Parashar 2005
- Parashar A, Arya R. Nitazoxanide. Indian Pediatrics 2005;42:1161‐5. [PubMed] [Google Scholar]
Park 2007
- Park WB, Choe PG, Jo JH, Kim SH, Bang JH, Kim HB, et al. Amebic liver abscess in HIV‐infected patients, Republic of Korea. Emerging Infectious Diseases 2007;13:516–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petri 2003
- Petri WA. Therapy of intestinal protozoa. Trends in Parasitology 2003;19(11):523‐6. [DOI] [PubMed] [Google Scholar]
Petri 2009
- Petri WA Jr, Mondal D, Peterson KM, Duggal P, Haque R. Association of malnutrition with amebiasis. Nutrition Reviews 2009;67(Suppl 2):S207‐15. [DOI] [PubMed] [Google Scholar]
Petri 2010
- Petri WA Jr, Haque R. Entamoeba species, including amoebiasis. In: Mandell GL, Bennett JE, Dolin R editor(s). Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th Edition. Philadelphia, PA: Churchill Livingstone Elsevier, 2010:3411‐25. [Google Scholar]
Ralston 2011
- Ralston KS, Petri WA Jr. Tissue destruction and invasion by Entamoeba histolytica. Trends in Parasitology 2011;27(6):254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramos 2005
- Ramos F, Moran P, Gonzalez E, Garcia G, Ramiro M, Gomez A, et al. High prevalence rate of Entamoeba histolytica asymptomatic infection in a rural Mexican community. American Journal of Tropical Medicine and Hygiene 2005;73(1):87‐91. [PubMed] [Google Scholar]
Ravdin 2005
- Ravdin JI. Entamoeba histolytica (Amebiasis). In: Mandell GL, Bennett JE, Dolin R editor(s). Principles and Practice of Infectious Diseases. 6th Edition. Philadelphia: Churchill Livingstone, 2005:3097‐3111. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera 1998
- Rivera WL, Tachibana H, Kanbara H. Field study on the distribution of Entamoeba histolytica and Entamoeba dispar in the Northern Philippines as detected by the polymerase chain reaction. American Journal of Tropical Medicine and Hygiene 1998;59(6):916‐21. [DOI] [PubMed] [Google Scholar]
Rivera 2006
- Rivera WL, Santos SR, Kanbara H. Prevalence and genetic diversity of Entamoeba histolytica in an institution for the mentally retarded in the Philippines. Parasitology Research 2006;98:106‐10. [DOI] [PubMed] [Google Scholar]
Salit 2009
- Salit IE, Khairnar K, Gough K, Pillai DR. A possible cluster of sexually transmitted Entamoeba histolytica: genetic analysis of a highly virulent strain. Clinical Infectious Diseases 2009;49:346–53. [DOI] [PubMed] [Google Scholar]
Samarawickrema 1997
- Samarawickrema NA, Brown DM, Upcroft JA, Thammapalerd N, Upcroft P. Involvement of superoxide dismutase and pyruvate: ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. Journal of Antimicrobial Chemotherapy 1997;40(6):833‐40. [DOI] [PubMed] [Google Scholar]
Samie 2006
- Samie A, Obi LC, Bessong PO, Stroup S, Houpt E, Guerrant RL. Prevalence and species distribution of E. histolytica and E. dispar in the Venda Region, Limpopo, South Africa. American Journal of Tropical Medicine and Hygiene 2006;75(3):565‐71. [PubMed] [Google Scholar]
Shirley 2016
- Shirley D‐A, Moonah S. Fulminant amebic colitis after corticosteroid therapy: a systematic review. PLoS Neglected Tropical Diseases 2016;10(7):e0004879. [DOI: 10.1371/journal.pntd.0004879] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shirley 2018
- Shirley DT, Farr L, Watanabe K, Moonah S. A review of the global burden, new diagnostics, and current therapeutics for amebiasis. Open Forum Infectious Diseases 2018;5(7):ofy161. [DOI: 10.1093/ofid/ofy161] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanley 2003
- Stanley SL Jr. Amoebiasis. Lancet 2003;361(9362):1025‐34. [DOI] [PubMed] [Google Scholar]
Stark 2008
- Stark D, Hal SJ, Matthews G, Harkness J, Marriott D. Invasive amebiasis in men who have sex with men, Australia. Emerging Infectious Diseases 2008;14:1141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talamas‐Lara 2014
- Talamás‐Lara D, Chávez‐Munguía B, González‐Robles A, Talamás‐Rohana P, Salazar‐Villatoro L, Durán‐Díaz Á, et al. Erythrophagocytosis in Entamoeba histolytica and Entamoeba dispar: a comparative study. BioMed Research International 2014;2014:626259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanyuksel 2003
- Tanyuksel M, Petri WA Jr. Laboratory diagnosis of amebiasis. Clinical Microbiology Reviews 2003;16(4):713‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanyuksel 2005
- Tanyuksel M, Yilmaz H, Ulukanligil M, Araz E, Cicek M, Koru O, et al. Comparison of two methods (microscopy and enzyme‐linked immunosorbent assay) for the diagnosis of amebiasis. Experimental Parasitology 2005;110(3):322‐6. [DOI] [PubMed] [Google Scholar]
Tarleton 2006
- Tarleton JL, Haque R, Mondal D, Shu J, Farr BM, Petri WA Jr. Cognitive effects of diarrhea, malnutrition, and Entamoeba histolytica infection on school age children in Dhaka, Bangladesh. American Journal of Tropical Medicine and Hygiene 2006;74(3):475‐81. [PubMed] [Google Scholar]
The Medical Letter 2013
- The Medical Letter. Drugs for parasitic infections. 3rd edition. 2013. https://secure.medicalletter.org/article‐share?a=143a&p=tg&title=Drugs%20for%20Parasitic%20Infections&cannotaccesstitle=1 (accessed 1 September 2017).
Tracy 2001
- Tracy JW, Webster LT Jr. Drugs used in the chemotherapy of protozoal infections: amebiasis, giardiasis, trichomoniasis, leishmaniasis, and other protozoal infections. In: Hardman JG, Limbird LE, Goodman Gilman A editor(s). Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th Edition. New York: McGraw Hill Medical Publishing Division, 2001:1097‐1120. [Google Scholar]
Tsai 2006
- Tsai JJ, Sun HY, Ke LY, Tsai KS, Chang SY, Hsieh SM, et al. Higher seroprevalence of Entamoeba histolytica infection is associated with human immunodeficiency virus Type 1 infection in Taiwan. American Journal of Tropical Medicine and Hygiene 2006;74:1016–9. [PubMed] [Google Scholar]
Verkere 2012
- Verkerke HP, Petri WA, Marie CS. The dynamic interdependence of amebiasis, innate immunity, and undernutrition. Seminars in Immunopathology 2012;34(6):771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1986
- Walsh JA. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Reviews of Infectious Disease 1986;8(2):228‐38. [DOI] [PubMed] [Google Scholar]
Wassman 1999
- Wassman C, Hellberg A, Tannich E, Bruchhaus I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron‐containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. Journal of Biological Chemistry 1999;274(37):26051‐6. [DOI] [PubMed] [Google Scholar]
Watanabe 2011
- Watanabe K, Gatanaga H, Cadiz AE, Tanuma J, Nozaki T, Oka S. Amebiasis in HIV‐1‐infected Japanese men: clinical features and response to therapy. PLoS Neglected Tropical Diseases 2011;5(9):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1969
- WHO Expert Committee on Amoebiasis. Amoebiasis. Report of a WHO Expert Committee. World Health Organization Technical Report Series 1969;421:1‐52. [PubMed] [Google Scholar]
WHO 1985
- World Health Organization. Amoebiasis and its control. A WHO Meeting. Bulletin of the World Health Organization 1985;63(3):417‐26. [PMC free article] [PubMed] [Google Scholar]
WHO 1994
- World Health Organization. Diarrhoeal Disease Control Programme. The Management of Bloody Diarrhoea in Young Children [WHO/CDD/94.49]. Geneva: World Health Organization, 1994. [Google Scholar]
WHO 1995
- World Health Organization. WHO Model Prescribing Information: Drugs Used in Parasitic Diseases. 2nd Edition. Geneva: World Health Organization, 1995. [Google Scholar]
WHO 1997
- World Health Organization/Pan American Health Organization/UNESCO. Amoebiasis. Weekly Epidemiological Record 1997;72:97‐9. [Google Scholar]
WHO 2005
- World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers [WHO/FCH/CAH/05.1]. 4th Edition. Geneva: World Health Organization, 2005. [Google Scholar]
Wilson 2012
- Wilson IW, Weedall GD, Hall N. Host–parasite interactions in Entamoeba histolytica and Entamoeba dispar: what have we learned from their genomes?. Parasite Immunology 2012;34:90‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ximenez 2011
- Ximénez C, Morán P, Rojas L, Valadez A, Gómez A, Ramiro M, et al. Novelties on amoebiasis: a neglected tropical disease. Journal of Global Infectious Diseases 2011;3(2):166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2013
- Zhou F, Li M, Li X, Yang Y, Gao C, Jin Q, Gao L. Seroprevalence of Entamoeba histolytica infection among Chinese men who have sex with men. PLoS Neglected Tropical Diseases 2013;7(5):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gonzales 2006
- Gonzales MLM, Dans LF, Martinez EG. Antiamoebic drugs for treating amoebic colitis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD006085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzales 2009
- Gonzales MLM, Dans LF, Martinez EG. Antiamoebic drugs for treating amoebic colitis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006085.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


