Abstract
Nerve growth factor (NGF) is essential for the survival of sensory and sympathetic neurons during development. However, in the adult, NGF and its interaction with tropomyosin receptor kinase A receptor (TrkA) has been found to play a critical role in nociception and nervous system plasticity in pain conditions. Thus, various monoclonal antibody (mAb) therapies targeting this pathway have been investigated in the development of new pharmacotherapies for chronic pain. Although none of the mAbs against NGF are yet approved for use in humans, they look very promising for the effective control of pain. Recently, species-specific anti-NGF mAbs for the management of osteoarthritis (OA)-associated pain in dogs and cats has been developed, and early clinical trials have been conducted. Anti-NGF therapy looks to be both very effective and very promising as a novel therapy against chronic pain in dogs and cats. This review outlines the mechanism of action of NGF, the role of NGF in osteoarthritis, research in rodent OA models and the current status of the development of anti-NGF mAbs in humans. Furthermore, we describe and discuss the recent development of species-specific anti-NGF mAbs for the treatment of OA-associated pain in veterinary medicine.
Keywords: osteoarthritis, nerve growth factor, monoclonal antibody, pain, cytokines
Introduction
Osteoarthritis (OA) is a slowly progressive degenerative joint disease characterised by whole-joint structural changes including articular cartilage, synovium, subchondral bone and periarticular components, which can lead to pain and loss of joint function.1–4 It is considered to primarily affect the hip, stifle and elbow joints in dogs,5 although no comprehensive, prospective studies of the prevalence of canine OA throughout the skeleton have been performed. Although most commonly initiated early in life by developmental disease (eg, hip dysplasia), many other factors play a role in its development, including diet, genetics, environment, obesity and age.4 6 7 In cats, hip, stifle, hock and elbow joints are the most commonly affected synovial joints.3 Although much less is known about the aetiology of OA in cats, idiopathic OA is considered common, with congenital, traumatic, infectious, nutritional and immune-mediated causes having been documented, similar to other species.3 OA is a condition associated with clinical signs in a large percentage of the population, with an estimated minimum of 20 per cent to 30 per cent of dogs affected clinically and up to 40 per cent of all cats being affected clinically (90 per cent of all cats over 12 years of age).3 5 8 The disease is currently incurable with negative consequences related to pain, mobility impairment and decreased quality of life.9 Pain results in both local and distant deterioration of the musculoskeletal system as a result of decreased and altered mobility. The pathological processes of OA, such as joint capsule thickening and fibrosis, contribute to altered range of motion that compounds the musculoskeletal changes. Additionally, the ongoing nociceptive input into the CNS results in somatosensory system changes and central sensitisation10 11, which contributes to the perception of pain. The combined effects of pain, central sensitisation and activity impairment may have negative effects on the affective state, heightening anxiety, depression, sleep impairment12 and cognitive dysfunction as reported in humans.13 14
Currently, pharmacological treatment of pain centres around non-steroidal anti-inflammatory drugs (NSAIDs). These are used to relieve pain and promote functional improvement.2 Globally, several NSAIDs are approved for use in dogs, but only two NSAIDs are approved for use long-term in cats and only in certain countries. Despite their widespread use and obvious benefit in many cases, NSAIDs are not always sufficiently effective when used as monotherapy.15 Additionally, there are safety and tolerability concerns with their use in both dogs and cats.16–19 Beyond cyclooxygenase-inhibiting NSAIDs and the recently approved piprant NSAID, a prostaglandin receptor antagonist, grapiprant,20 treatment options for the control of pain are very limited. Furthermore, evidence for efficacy of so-called adjunctive analgesics is extremely limited15 21. Additionally, there are few proven non-drug therapies, and none that have been shown to provide rapid pain relief. Therefore, OA related-pain remains a challenging clinical entity to treat, and indeed, OA-associated pain is one of the most common reasons for euthanasia in dogs.22 23 Thus, there is an urgent need to develop effective treatments for OA-related pain in dogs and cats.
Advent of monoclonal antibodies
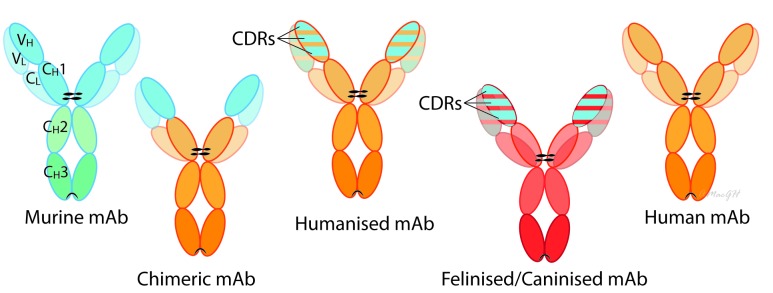
With greater understanding of pathogenesis of OA coupled with advances in biotechnology, specific immunomodulatory therapies called biological agents have been introduced for the treatment of joint diseases, specifically immune-mediated joint diseases. Biological agents are medical products that are isolated from a variety of natural sources (human, animal or microorganism). This is in contrast to most drugs that are chemically synthesised. Biological agents include a wide range of products such as vaccines, blood and blood components, gene therapy and recombinant therapeutic proteins. They also may be produced by biotechnology methods. In human medicine, biological products often offer the most effective means to treat a variety of medical illnesses and conditions. The monoclonal antibodies (mAbs) have proven to be extremely effective in a variety of diseases in humans. Monoclonal antibodies are produced from single B-lymphocyte clones in mice or through recombinant engineering. They are monovalent antibodies that specifically bind to target molecules including cytokines, receptors or cells.24 The binding results in blocking the activity of the target. They were first generated in mice in 1975 using a hybridoma technique25 involving immunising a certain species against a specific epitope on an antigen and obtaining B-lymphocytes from the animal’s lymphoid tissue. B-lymphocytes are then fused with an immortal myeloma cell, a type of B-cell tumour, and these hybridomas can be maintained in vitro and will continue to secrete antibodies with appropriate specificity. Administering purified non-human-derived antibodies to humans can cause immune reactions,26 and so antibody engineering technology has been developed to lower the risk of immune reactions.27 Engineering of antibodies by replacing mouse sequence-derived amino acids with human sequences has significantly reduced immunogenicity of this class of therapeutics.28 Over the last 25 years, chimeric, humanised and fully human mAbs have been developed for use in humans to decrease immunogenicity29 30 (figure 1). Chimeric mAbs are antibodies made by fusing the variable region from a murine-derived mAb, with the immunoglobulin (Ig) constant region from a human antibody.31 32 The resulting construct is approximately three quarters human.31 32 The next advance was the ‘humanization’ process.33 Initially, only the complementarity-determining regions (CDRs) (part of the variable domain from the original murine-derived mAb that binds to the specific antigen) are retained, resulting in a construct that is approximately 95 per cent human.31 32 Subsequently, fully human mAbs, which have no murine sequences, have been produced through transgenic mice and phage technologies.24 In general, the more humanised the construct is, the less immunogenic the mAb. However, the immunogenicity cannot be predicted based only on the amount of non-human sequence on the molecule. In fact, antidrug antibodies to even fully humanised mAbs have been reported due to factors such as aggregates and adjuvant-like contaminants, although these issues have largely been resolved by improvements in manufacturing and formulating practices.34 Moreover, other key factors that are relevant to the immunogenicity of a compound include route of administration (intravenous v subcutaneous), treatment paradigm (continuous v intermittent) and concurrent immunosuppressive therapy. In clinical practice, these factors have proven to be relevant considerations in the therapeutic use of mAbs.31 32 Immune responses to therapeutic mAbs are undesirable as they can neutralise the action of therapeutic mAbs, and hypersensitivity can result in morbidity and mortality.35 Importantly, some of the mAb immunogenicity appears to be idiotypic where patients who develop antibodies after treatment with 1 chimeric mAb might not be expected to demonstrate equal reactivity to another chimeric mAb.31 32
Figure 1.
Antibodies are large glycoproteins typically composed of two heavy chains and two light chains, each of which contain a variable domain (variable heavy (VH) or variable light (VL) and a constant domain (constant heavy (CH) or constant light (CL). The amino acid sequence of the variable domain varies greatly among antibodies and six ‘hyper-variable’ complementarity-determining region (CDR) loops within the variable domains give the antibody its specificity for binding to an antigen. In contrast, the constant domain is identical in all antibodies of the same isotype but differs in antibodies of different isotypes (IgA, IgD, IgE, IgG and IgM). The tail region of the constant domain (Fc region: CH2 and CH3) may direct immune effector functions by binding to cell receptors expressed on immune cells or initiating complementary-dependent cytotoxicity. Murine monoclonal antibodies (mAbs) are antibodies produced from individual cloned and immortalised mouse B cells. Most useful therapeutic antibodies have been constructed with the gamma immunoglobulin (IgG) isotype. Chimeric mAbs are antibodies made by fusing the genes encoding the variable region from a murine-derived mAb, with those from an immunoglobulin (Ig) constant region from a human antibody. Humanised mAbs retain only the CDRs (part of the variable domain from the original murine-derived mAb that binds to the specific antigen). Fully human mAbs have no murine sequences. Caninised and felinised mAbs are fully canine or feline specific. These can be made in several ways; for example, Nexvet have used a process of conversion based on alignment with immunoglobulin complementary DNA libraries (PETization).
There are multiple mechanisms by which mAbs produce their effect. These include blockade of ligand–receptor interaction or signalling pathways; altering cell populations (by engaging effector functions including the complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity and antibody dependent phagocytosis or apoptosis).29
The activation and sensitisation of peripheral nociceptors by inflammatory and hyperalgesic mediators such as cytokines is recognised as one of the main peripheral mechanisms responsible for joint pain.36 In parallel with an increased understanding of the role of the cytokines, chemokines and neurotrophins in joint pathology and pain,36 there has been growing interest the use of mAb therapy to target these molecules.29 Nerve growth factor (NGF) is one of the cytokines37 that has received significant attention as a key regulator involved in both inflammatory and neuropathic pain.38
This paper will review the role of NGF in the arthritic joint, efficacy of anti-NGF therapy based on murine OA models, the current status of the development of anti-NGF mAbs in humans and will also discuss the recent development of anti-NGF mAbs for the treatment of OA-associated pain in experimental and clinical studies in veterinary medicine.
Monoclonal antibodies targeting NGF
NGF and its pain pathway
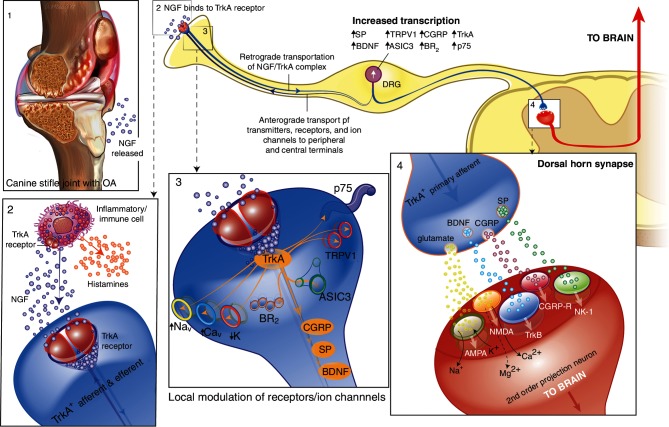
NGF was originally discovered as a critical factor for the development and maintenance of sensory and sympathetic neurons in the developing nervous system (reveiwed in ref 39). In the prenatal and early postnatal periods, NGF is required for survival of both sensory and sympathetic neurons.40 41 However, in adults, the main role of NGF in the periphery shifts from trophic support of sensory and sympathetic neurons to modulation of nociceptive neuronal activity.39 Preclinical and clinical research over the past several decades has clearly demonstrated the important role of NGF in nociceptor sensitisation in a wide variety of both acute and chronic pain states including postoperative and OA pain39 42–44 (figure 2). In this respect, NGF can be considered to be similar to prostaglandin E2 (PGE2), which also produces nociceptor sensitisation, and both play a role in the sensitisation of nerves following injury—the fundamental protective effect of pain. NGF is produced and released by peripheral tissues in response to noxious stimuli. It functions as a soluble signalling protein that mediates its activity via binding to two distinct cell surface receptors (NGFRs), the high-affinity NGF-specific tropomyosin receptor kinase A (TrkA) and the low affinity p75 neurotrophin receptor (p75NTR). When NGF binds to TrkA expressed on the peripheral terminals of sensory nerve fibres, the NGF/TrkA complex is internalised. The NGF/TrkA complex is retrogradely transported to the cell body of sensory neurons, located in the dorsal root ganglia (DRG). This modulates and/or increases the expression of a variety of cell surface receptors and ion channels involved in nociception including the transient receptor potential vanilloid 1, acid-sensing ion channels, bradykinin receptors, voltage-gated sodium channels, voltage-gated calcium channels and mechanotransducers. This results in an increase the excitability of primary afferent fibres (peripheral sensitisation) through phenotypic alterations. NGF/TrkA signalling also leads to transcriptional changes that result in the increased expression of pronociceptive neurotransmitters such as substance P, calcitonin gene-related peptide (CGRP) and brain-derived neurotrophic factor. Thus, NGF induces functional, as well as phenotypic, alterations in the primary afferent fibre. In the periphery, NGF also binds to TrkA located on mast cells and other immune cells and elicits the release of inflammatory mediators such as histamine, serotonin and NGF itself. Thus, NGF can trigger peripheral sensitisation and sensitises adjacent nociceptive neurons as a result of the release of these inflammatory mediators.39 42–44 In conditions where NGF is playing a pivotal role in the pronociceptive processes, an analgesic that blocks NGF/TrkA signalling may be useful.44 45
Figure 2.
Schematic diagram of the involvement of nerve growth factor (NGF) in nociception and nervous system plasticity. In osteoarthritis (box 1), NGF is produced and released by peripheral tissues (such as chondrocytes) and can bind to its receptor, TrkA located on primary afferent (sensory) fibres. In addition, NGF that is released in the periphery also binds to TrkA located on mast cells and other immune cells and subsequently elicits the release of inflammatory mediators such as histamine, serotonin (5HT) and NGF itself (box 2). When NGF binds to TrkA, on TrkA-positive primary afferent nerve fibres, the NGF/TrkA complex is internalised and retrogradely transported to the cell body of the sensory neurons that are located in the DRG. This modulates and/or increases the expression of a variety of cell surface receptors and ion channels involved in nociception including TRPV1, ASIC, BR2, Nav, Cav, K and putative mechanotransducers, which result in an increase the excitability of primary afferent fibres (peripheral sensitisation) (box 3). NGF/TrkA signalling also leads to transcriptional changes that result in the increased expression of pronociceptive neurotransmitters such as SP, CGRP and BDNF. When these peptidergic (TrkA-positive) primary afferent neurons are subsequently stimulated, release of these peptides, in addition to glutamate acting on AMPA receptors, and binding to their respective receptors (SP to NK-1, CGRP to CGRP-R, BDNF to TrkB) may cause strong depolarisation of the postsynaptic second order projection neuron (box 4). This will result in the removal of the magnesium (Mg2+) block of the NMDA receptor, facilitating cellular windup. This increases the probability of central sensitisation and facilitated transmission through the dorsal horn synapse and then, via third-order neurons, to the sensory cortex in the brain. Thus, NGF is involved in the processes of inflammation in the periphery, and also in the sensitisation of primary afferent neurons through alteration of their functional phenotype. 5-HT, 5-hydroxytryptamine; AMPA, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ASIC, acid-sensing ion channel; BDNF, brain-derived neurotrophic factor; BR2, bradykinin receptor 2; Cav, voltage-gated calcium channel; CGRP, calcitonin gene-related peptide; CGRP-R, calcitonin gene-related peptide receptor; DRG, dorsal root ganglia; K, delayed-rectifier potassium channel; Nav, voltage-gated sodium channel; NMDA; the glutamatergic N-methyl-D-aspartate; NGF, nerve growth factor; NK-1, neurokinin 1 receptor; p75, neurotrophin receptor; SP, substance P; trkA, tropomyosin receptor kinase A; trkB, tropomyosin receptor kinase B; TRPV1, transient receptor potential vanilloid 1.
It should be noted that several reports suggest that NGF and TrkA expression in the CNS may contribute to driving chronic pain.46 47 The present review is focused on peripherally restricted anti-NGF mAb therapies that do not readily cross the blood–brain barrier (BBB). However, previous reports using a small molecule pan-Trk inhibitor (which readily crosses the BBB and binds to TrkA, B and C with nanomolar affinity) showed very similar efficacy as peripherally restricted anti-NGF mAb therapies in attenuating both non-malignant and malignant skeletal pain.39 48 49 Thus, while peripherally restricted anti-NGF therapies presumably blocks NGF induced sensitisation and sprouting of nociceptors,39 it will be important to further define how NGF and TrkA in the CNS may also contribute to the induction and maintenance of chronic pain. Additionally, it will be important to define how anti-NGF therapies that are restricted to the periphery, such as mAbs, modulate centrally driven or maintained processes involved in chronic pain.
NGF and OA-associated pain and pathology
Evidence for a contribution of locally produced NGF to joint pathology, as well as pain in arthritic joints has emerged. NGF can induce joint pain via a direct sensitisation of nociceptors.50 Indeed, a single intra-articular (IA) injection of NGF into normal rat knees produced dose-dependent, long-lasting increases in pain behaviours (asymmetrical gait and allodynia at a site distal to the injected joint), joint swelling and synovial macrophage infiltration.51 Other work has shown that in arthritic joints, NGF is released by damaged cells including synovial cells and chondrocytes, and elevated NGF and/or its receptors are detected in synovium and articular cartilage in murine models of OA.52 53 Similarly, elevated NGF levels are found in synovial fluid, synovium, osteochondral junction and articular cartilage in human patients with arthritis.54–58 A recent work has shown elevated levels of NGF in synovial fluid in dogs with naturally occurring OA compared with healthy joints.38 Interestingly, experimental studies have demonstrated that augmented NGF gene expression and NGF immunoreactivity in the joint correlates with pain-related behavioural changes in surgically induced OA in mice.53 59 Administration of an anti-NGF/TrkA signalling molecule significantly decreased pain behaviours in a murine model of OA.59–63 The antinociceptive effect of anti-NGF antibody appeared to be equal to the highest tolerated dose of indomethacin, as assessed by a vocalization test in a complete Freud’s adjuvant (CFA) induced arthritic model in rats.60 In another study comparing the efficacy of different analgesics, Adams et al61 examined the ability of different analgesics (morphine, tramadol, NSAIDs and anti-NGF therapy) to attenuate gait impairment using a gait analysis system in the same arthritic model. They showed that intraperitoneal administration of anti-NGF mAb produced a profound improvement in gait parameters in a dose-dependent manner. Its effect was the same or greater than clinically efficacious doses of morphine and NSAIDs. Overall, basic science studies have shown a role of NGF in pain and pathology in OA and shown that anti-NGF therapies show robust analgesia that is equal to or greater than current analgesics.
Peripheral and central neuronal plasticity are key mechanisms in the development and maintenance of chronic pain64 and evidence points to NGF as being a major determinant of plasticity in both the peripheral and CNS. Small doses of exogenous NGF delivered to intact DRG or into the intrathecal space in rats triggered a persistent hyperalgesia, indicative of peripheral and central sensitisation, respectively.65 66 A recent study reported the effect of anti-NGF therapy on peripheral and CNS plasticity and associated hyperalgesia.53 Behavioural tests showed significantly reduced OA-associated pain and hyperalgesia in mice injected with anti-NGF mAb compared with a saline injected control group, suggesting that anti-NGF therapy is a potentially effective analgesic treatment for both the peripheral and the central plasticity components of chronic pain. It is worth noting that reducing sensitisation by NGF signalling blockade is not anticipated to block normal, protective, nociceptive signalling unlike traditional analgesics such as opiates.67
One potential mechanism behind OA-associated pain is ectopic sprouting of sensory and sympathetic nerve fibres. With the progression of OA in humans, nerve sprouting along new blood vessels can be detected, both into structures that are normally not innervated (eg, non-calcified cartilage, the osteochondral junction and meniscus), as well as normally innervated structures (eg, synovium).53 56 57 68 69 NGF is considered to be one of the main factors inducing nerve sprouting and neuromas in response to tissue and/or nerve injury, and NGF may promote angiogenesis itself.39 56 Studies of tissue from human patients with OA and rheumatoid arthritis have showed that the NGF immunoreactivity in pathological joint tissue was significantly higher than normal joint tissues. These studies showed NGF immunoreactivity was associated with increased sensory nerve innervation and sprouting (CGRP positive neurons) in the synovium and at the osteochondral junction.56 57 Interestingly, data suggest NGF activity may be correlated with symptoms. Investigators compared tissue from human patients with similar macroscopic chondropathy, half of whom were symptomatic and half of whom were asymptomatic knee chondropathy patients.53 57 In these reports, the NGF immunoreactivity was significantly greater in the synovium from patients with symptomatic OA compared those with asymptomatic OA.53 57 Supporting this association between NGF immunoreactivity and symptoms, Kc et al53 reported that sensory nerve (protein gene product 9.5 positive neurons) fibre innervation was markedly increased in the synovium from patients with symptomatic OA, and this change was colocalised with augmented NGF immunoreactivity. This may suggest that upregulation of sensory neurons and associated NGF/TrkA signalling are better correlated with OA symptoms than are cartilage lesions. In an experimental study in mice, sensory (CGRP and neurofilament 200 positive neurons) and sympathetic nerve fibres (tyrosine hydroxylase positive neurons) in synovium and periosteum were significantly increased following the injection of CFA.70 In the same mouse model, administration of anti-NGF mAb significantly reduced the sprouting of these sensory and sympathetic nerve fibres in synovium in the OA joint and attenuated joint pain.71 There appears to be good evidence that NGF may be responsible for pathological nerve sprouting in arthritis, and that this, and the presence of NGF, correlates well with clinical signs of pain.
Although NGF is known to induce angiogenesis that could contribute to inflammation, the effects of anti-NGF therapy on experimentally induced synovitis are mixed. One study reported anti-NGF therapy significantly decreased synovitis and cellular infiltration,62 while other studies have concluded that anti-NGF therapy did not alter synovitis and cellular infiltration.63 71 72 In clinical orthopaedics in humans and veterinary species, there is ongoing debate as to whether OA pain treatments should have anti-inflammatory effects to be optimally effective.
As cartilage damage progresses in rodent models of OA, gene expression and immunoreactivity of NGF in articular cartilage are increased.53 73 Similarly, in human patients, immunoreactivity of NGF and its receptors in articular cartilage and chondrocytes are elevated with severity of cartilage damage.53 58 Recently, an in vitro study suggested that NGF signalling is a contributing factor in articular cartilage degeneration in OA. In this study, human cartilage tissue explants were harvested from early OA joints and cultured in serum-free medium with or without NGF for 14 days. NGF treatment resulted in extracellular matrix catabolism indicated by increased sulfated glycosaminoglycan release and matrix metalloproteinase (MMP) levels and activity.58 Additionally, treatment with NGF neutralising antibody inhibited increased MMP levels and enhanced the level of tissue inhibitor of matrix metalloprotease-1 in OA cartilage explants. However, the severity of cartilage damage in rats administered anti-NGF mAb was the same as those given saline control in a sodium monoiodoacetate (MIA) model of OA.63 72 Overall, at present, it is unclear if anti-NGF therapy has any potential protective effect on articular cartilage.
Thus, given the role of NGF in nociception and contribution to OA disease progression, various ways of preventing activation of NGF/TrkA have been developed, including capturing free NGF, preventing NGF binding to TrkA or inhibition of TrkA function.39 42–44 Methods for capturing free NGF and inhibition of TrkA function have been advanced into clinical trials in humans.
Potential beneficial effects of NGF
This review focuses on the treatment of pain via inhibition of NGF. However, there are potential beneficial effects of NGF. NGF is essential for the development and phenotypic maintenance of neurons in the peripheral nervous system (PNS) and for the functional integrity of cholinergic neurons in the CNS.74 In the PNS, in addition to the role in the regulation of neurotransmitters and neuropeptides synthesis, NGF has the protective action on the survival of degenerating peripheral nerve cells.39 Deprivation of NGF can lead to damage of neurons, while exogenous NGF administration can promote peripheral nerve growth and re-establish functional activity.74 Thus, NGF attracted clinicians for the potential clinical application to neurodegenerative diseases. However, the phase III clinical trials did not show positive results after administration of human recombinant NGF. Additionally, undesired adverse events (AEs), such as the peripheral pain, were observed.75 Clinical trials were halted.74 A specific role of NGF has been proposed for the cholinergic neuron population of the CNS.76 NGF contributes to the maintenance of cell morphology and physiology of cholinergic neurons, which are highly dependent on NGF during both development and adulthood.77 Studies demonstrated that NGF was able to promote survival of basal forebrain cholinergic neurons, known to degenerate in age-related disorders (such as in Alzheimer’s disease (AD)).78 This leads to the hypothesis that intracerebral administration of NGF might reduce or prevent brain neuronal degeneration of patients with AD. However, clinical studies were stopped due to only mild neurological improvements and AEs, including systemic pain.79 In more recent years, other strategies have been applied for the delivery of NGF into the damaged brain neurons and to bypass safely the BBB (eg, nose-to-brain).74 NGF may affect a variety of CNS neurons, other than cholinergic origin, including the visual system.80 NGF and TrkA are expressed by many tissues in the eye.81 Topically applied NGF eye drops can reach the retina and the optic nerve,82 and there is interest in topically applied NGF for neurodegenerative diseases, such as glaucoma and neurotrophic keratitis.83 Moreover, eye NGF topical administration enhanced tear release in humans and bulldogs suffering of dry eye.74 Of note in these discussions is the fact that anti-NGF mAbs are confined to the periphery because of the BBB.
Recent data have suggested that NGF is a pleiotropic factor and its actions extend beyond the nervous system.76 NGF is produced and used by several cell types including structural (eg, epithelial cells and endotherial cells), accessory (eg, glial cells and astrocytes) and immune cells (eg, lymphocytes and mast cells).74 During the last two decades, evidence has been accumulated supporting the hypothesis that NGF possesses potential therapeutic properties on tissue healing (cutaneous and corneal ulcers), cardiomyopathy, and myocardial ischaemia.74
Development of anti-NGF therapy for humans
Although none of the mAbs against NGF are yet approved for use in humans, anti-NGF mAbs are in development as treatments for several pain conditions. Currently, three drugs that capture free NGF have been developed: tanezumab (humanised mAb; Pfizer, in collaboration with Eli Lilly), fulranumab (fully human mAb; Amgen) and fasinumab (fully human mAb; Regeneron Pharmaceuticals, in collaboration with Sanofi). In studies performed thus far, they have been administered intravenously or subcutaneously every four weeks to eight weeks and have demonstrated dose-dependent efficacy in human patients with moderate to severe pain associated with symptomatic knee or hip OA.84–86 In a study in human patients with knee or hip OA, tanezumab reduced OA pain and improved function more than that observed with NSAIDs or opiates.87 The most common AEs observed across the clinical trials performed so far were peripheral oedema, arthralgia, extremity pain and neurosensory symptoms (primarily paraesthesia, hypoesthesia and hyperaesthesia).43 However, overall, the AE rate has been small (1 per cent to 10 per cent in most studies), and anti-NGF mAb therapy has been generally well tolerated by the human patients.43 88–91 Abnormal sensory symptoms tended to occur within a short time after the first dose and were generally transient but tended to develop more frequently with higher doses of anti-NGF mAbs.43 84 Overall, symptoms were generally considered mild to moderate in severity and did not generally result in early exit from the study.43 Furthermore, most symptoms were transient and resolved without permanent sequelae within one month.43 Thus, based on work in rodent models and human clinical trials, anti-NGF mAb therapy looks promising for the effective control of OA pain. However, the development programmes for anti-NGF mAbs were temporary put on clinical hold by the Food and Drug Administration (FDA) due to an increased incidence of serious joint-related AEs from 2010 to 2012. The incidence of serious joint-related AEs were initially postulated to be osteonecrosis and/or rapidly progressive OA (RPOA) in the hip, knee and shoulder joints, which lead to early than expected joint replacement. These AEs were reported in 83 patients who had either received tanezumab monotherapy or tanezumab and NSAIDs. The incidence rate was 9 and 23.9 per 1000 patient-years (the sum of events divided by the duration of administration of tanezumab), respectively. These AEs occurred during phase II and III trials in the tanezumab development programme where the mean duration of treatment was 199 days.92 The incidence of joint destruction was higher in the patients with longer exposure of anti-NGF mAbs, larger doses of anti-NGF mAbs and concurrent use of NSAIDs.42 92 However, serious joint-related AEs were observed in some patients following a single treatment of anti-NGF mAb.91 Several cases occurred in multiple joints and also in non-index joints.92 Following extensive adjudication of these AEs,92 less than 1 per cent of the AEs were deemed to be due to osteonecrosis and the majority classified as RPOA. Characteristics of RPOA are rapid clinical deterioration (increase in pain) and radiographic progression of joint degeneration.93 The cause of these cases of RPOA is not currently understood and experimental studies found no evidence of a direct adverse effect on bone healing or joint health (bone, cartilage, joint vasculature or joint innervation) in animals (rodents) treated with anti-NGF mAbs at large multiples of the clinical exposure.94 95 In human medicine, the most prominent concern around anti-NGF revolves around RPOA. Although theories have been proposed, the cause of anti-NGF related RPOA remains unclear.96 Overloading, resulting from increased activity and weight-bearing due to good analgesia (analgesic arthropathy), immune reactions and neuropathic arthropathy (nerve damage resulting in loss of ability to feel the joint and decreases in joint stability) have been suggested as potential factors leading to RPOA following anti-NGF therapy.91 97 98 The increased incidence of RPOA associated with concurrent NSAID use is not understood, but it is possible that NSAIDs contribute to RPOA through prostaglandin-dependent and prostaglandin-independent mechanisms, including increasing the risk of microvascular thrombotic events in bone and inhibiting the repair of subchondral microfractures.91 However, this is speculative at the moment. To the authors’ best knowledge, the influence of coadministration of anti-NGF therapy and NSAIDs on joint health has not yet been evaluated in basic studies, and the reason for the increased incidence of RPOA associated with concurrent use of anti-NGF mAb and NSAIDs has not been elucidated. It appears the recommendation will be to avoid use of NSAIDs concurrently with anti-NGF therapy (see below). Another concern with using anti-NGF mAb was whether this therapy could cause loss of sensory or sympathetic nerve fibres in the adult as NGF itself is known to be required for the normal development of sensory and sympathetic nerve fibres in developing animals and humans. Experiments in both mice and monkeys showed that anti-NGF therapy did not induce loss of sensory or sympathetic nerve fibres in the skin or bone nor any sign of injury or degeneration in the cell bodies of sensory neurons in the DRG.94 99 100 Interestingly a partial FDA clinical hold was placed on all anti-NGF programmes from 2012 to 2015 due to anatomical changes in the cell bodies of postganglionic neurons, which was based on an observed reduction in size and neuronal count of primates receiving very high doses of anti-NGF therapy. However, subsequent detailed toxicology studies in monkeys did not demonstrate any reduction in sympathetic function or neuronal cell counts.89 101 While it did appear that primates exposed to high and prolonged doses of anti-NGF did have a reduced size of postganglionic sympathetic cell bodies, this change in cell body size returned to normal upon cessation of anti-NGF administration.100 In light of these data, a risk minimisation plan has been incorporated subsequent anti-NGF human studies by excluding patients with ongoing disorders of the sympathetic nervous system.100
After reviewing the data, the FDA advisory committee concluded that these serious joint AEs were probably related to the anti-NGF treatment. However, clinical trials for the development of anti-NGF mAbs in human have been restarted in 2015 with the adoption of a risk mitigation strategy, which included monitoring patient overuse of the skeletal using accelerometers, dosing restrictions and a recommendation against concomitant NSAID use in patients with OA. The decision by the FDA to allow the continued development of the drug class was made due to the potential significant benefit of anti-NGF therapy for a multitude of pain conditions and the absence of any direct link between the administration of anti-NGF mAbs and joint destruction. Indeed, in 2017, tanezumab received FDA fast track designation, recognising the significant potential benefit from this therapeutic. A recent search of the National Institutes of Health web site (ClinicalTrials.gov) revealed that tanezumab is currently in phase III studies in the patients with OA of the hip and knee and chronic lower back pain with the bone cancer pain trials being conducted outside the USA.90
Glenmark Pharmaceuticals Ltd. has developed a TrkA antagonist (GBR 900) with the target indication being the treatment of chronic pain. This antibody has completed phase I enabling preclinical development programme102 and a phase I trial in normal volunteers (ClinicalTrials.gov identifier: NCT02235727). In the Good laboratory practice (GLP) toxicity studies, no dose-limiting toxicities were detected with GBR 900, even at high doses. This potentially differentiates GBR 900 from anti-NGF antibodies that do show preclinical toxicity. Reportedly, preclinical head-to-head comparisons with anti-NGF antibodies in animal models of inflammatory pain demonstrated that GBR 900’s efficacy profile compares favourably with that of anti-NGF antibodies.102 Another TrkA inhibitor (GZ389988) has been developed by Sanofi, formulated for IA administration to control OA pain. In the rat MIA model, a single IA injection of GZ389988 resulted in more normal weight-bearing for four weeks without any significant histopathological changes in joint tissues compared with placebo.103 Interestingly, IA injection into the contralateral joint had no effect on the ipsilateral limb joint pain, suggesting that IA injection does not result in substantial systemic exposure, which may limit the risk of AEs. A proof-of-concept study to assess the efficacy, tolerability and safety of GZ389988 in human patients with painful OA of the knee has recently completed, but results are not yet available (ClinicalTrials.gov identifier: NCT02845271).
Development of anti-NGF mAbs for the dog and cat
Canine and feline NGF are closely homogenous to NGF in other species, such as human and mice. However, as mAbs from one species often can induce an immune response when used without modification in another species, for therapeutic purposes, antibodies need to be species specific to reduce the risk of immunoreactions to the antibody. It is likely that several companies have developed or are developing technology to create species-specific antibodies for the veterinary market (as evidenced by the recent approval of Zoetis’ interleukin (IL)-31 mAB (https://www.zoetisus.com/products/dogs/cytopoint/)), but in the pain arena, the only publicly available information at the time of writing this review was for the company Nexvet, which was recently purchased by Zoetis (http://news.zoetis.com/press-release/investors/zoetis-acquire-nexvet-biopharma-innovator-monoclonal-antibody-therapies-comp). Nexvet developed technology to create anti-NGF mAbs specifically for canine and feline use. In this approach, complementary DNA libraries are used to compare the natural variations in the sequences of the heavy and light chain of the mAb between donor species (human or rodent) and target species. This comparison enables the determination of the minimal number of changes at each position in the amino acid sequences that are required to convert the donor mAb variable region heavy and light chain sequences into mAb sequences containing only amino acids identified within target species. At sites where amino acid changes are necessary, the most similar amino acid in the matrix of the target species is substituted.
This results in 100 per cent species-specific mAb sequences that carry a lower risk of rejection due to immunoreaction, while preserving high affinity and potent bioactivity. Nexvet successfully converted the rat anti-NGF mAb (αD11) into caninised and felinised anti-NGF mAbs with the goal of managing pain states, including OA.
Development of anti-NGF therapy for canine OA
Early work with Nexvet’s fully caninised anti-NGF mAb (ranevetmab) indicated a favourable PK profile (mean tissue distribution phase half-life of approximately 12 hours and a mean plasma half-life time of nine days) and no evidence of an acute neutralising immunogenic response in dogs.104 In the kaolin injection pain model in dogs, efficacy was seen (reduced lameness).104 Currently, there are two published clinical trials that evaluate the efficacy of single intravenous injection of ranevetmab (0.2 mg/kg).105 106 Both studies required a two-week withdrawal period of NSAIDs prior to the study starting, and NSAIDs were not permitted to be used throughout the study period, in order to best assess efficacy of the anti-NGF therapy. In a randomised and double-blind study where all dogs received ranevetmab, the safety and clinical effect was examined using an owner completed questionnaire—the Canine Brief Pain Inventory (CBPI) score.105 Nine dogs with OA received a single injection of ranevetmab during the 10 weeks of study period (either at the start, two or four weeks into the study), with owners blinded to the time of injection. They were evaluated every two weeks for six weeks following injection. At other evaluation times, the dogs received sterile saline, and the owners were unaware of the evaluation time at which the mAb had been administered. This study showed that significantly lower CBPI scores were seen compared with baseline scores until four weeks after treatment, and although values were not statistically significant, CBPI scores at six weeks after administration were still lower than baseline scores. In another randomised, double-blind, placebo-controlled pilot study,106 26 dogs suffering from OA pain were allocated to placebo or treatment group based on predominant site of problem (fore or hind limb impairment) and CBPI score. The dogs were assessed every two weeks for four weeks using objective accelerometry (which measures activity and movement) and subjective, owner-completed clinical metrology instruments (CMIs, CBPI, Client-Specific Outcome Measures (CSOM) and Liverpool Osteoarthritis in Dogs) for the evaluation of efficacy. The dogs that received ranevetmab had significant improvement in all three CMIs compared with baseline scores throughout the study period and significantly greater activity compared with placebo group during the daytime period (09.00–17.00). Additionally, the distribution of success/failure rates for the CBPI and CSOM was very similar to previous studies using carprofen and grapiprant.20 107 In both clinical studies, no AEs associated with treatment were reported. Finally, although the data have not been published yet, a pivotal, multicentre, placebo-controlled, randomised, double blind study was conducted at 12 sites (http://ir.nexvet.com/phoenix.zhtml?c=253841&p=irol-newsArticle&ID=2176442: this was announced on the website, but it is no longer active). Two hundred and sixty-two dogs with OA were enrolled in this study and allocated to treatment or placebo on a 2:1 ratio. They received ranevetmab subcutaneously once per month for three months. The efficacy and safety of the drug were evaluated using CMIs (CBPI and CSOM) over 84 days. Subcutaneous administration of ranevetmab appeared to result in a statistically significant improvement on the assessed level of pain as measured using CBPI improvement success/fail criteria as well as using CSOM improvement success/fail criteria. Furthermore, subjective veterinarian assessment of limb function and joint pain were also numerically superior to placebo for each composite variable at most evaluation points and included a statistically significant overall treatment effect for lameness, particularly relevant given the large placebo effect seen in veterinarian lameness assessments.108
Basic science studies have evaluated the IA route of administration, but as yet, there are no reports of studies looking at IA injection in the dog. Although there are no other publications on anti-NGF mAbs and dogs, a recent search revealed that Zoetis LLC109 and Abbott Laboratories110 as well as Nexvet Biopharma111–114hold patents for anti-NGF mAb in dogs.
Development of anti-NGF therapy for feline OA
Currently, to our knowledge, Abbott Laboratories110 and Nexvet Biopharma115 hold a patent for anti-NGF mAbs in cats. However, the only published data in cats are for the Nexvet feline anti-NGF mAb.
Initial pharmacokinetic and efficacy evaluation of frunevetmab, Nexvet’s fully felinised anti-NGF mAb, were performed by investigators using eight cats administered four different doses (from 2.0 mg/kg to 28 mg/kg) subcutaneously with plasma concentrations measured over a 42-day period following injection.116 Frunevetmab had a peak plasma concentration of approximately three days and a mean plasma half-life time of nine days. It was well tolerated at dosages up to 28 mg/kg. In placebo-controlled, unblinded work, using the kaolin injection pain model of cats showed that a single dose of 2 mg/kg significantly decreased subjective lameness scores compared with placebo treatment.116 No AEs were seen. A single clinical trial has been published.117 This was a blinded, placebo-controlled pilot study conducted in 34 cats with OA-associated pain to assess the efficacy of a single dose of frunevetmab (0.4 mg/kg or 0.8 mg/kg, subcutaneously) over a nine-week period. A two-week wash-out period from NSAIDs was required prior to the study, and cats were not permitted to be on NSAIDs during the study period. Outcome measures were objectively measured using collar-mounted accelerometers and CMIs.117 Objective accelerometry revealed a significant increase in activity compared with placebo treatment from two weeks to six weeks after injection. Furthermore, the mean activity increase over placebo over first three weeks after treatment (12.9 per cent) was greater than the increase over placebo produced by daily administration of 0.035 mg/kg of meloxicam (5.97 per cent) over a three-week period in an earlier study.118 Subjective owner assessments showed a significant effect of treatment over the first three weeks after administration, the first time in the published literature that owners have been able to correctly distinguish between treatment and placebo in cats with OA-associated pain under a parallel design, placebo-controlled study. This is particularly noteworthy given the very high caregiver placebo effect seen in feline chronic pain studies.119 The anti-NGF mAb was well tolerated, with a single injection of frunevetmab producing positive treatment effects with a duration of up to six weeks. Although the data have not been published yet, multicentre, placebo-controlled, randomised, double-blind pilot field study that enrolled 126 cats with OA was conducted over three months to examine the efficacy of intravenous and subcutaneous monthly administration of frunevetmab (http://ir.nexvet.com/phoenix.zhtml?c=253841&p=irol-newsArticle&ID=2176442: this was announced on the website, but it is no longer active). It appeared that both routes of administration were effective and, when they were combined for analysis, frunevetmab demonstrated statistically significant efficacy over placebo at multiple time points using CMIs (CSOM and Feline Musculoskeletal Pain Index). No significant adverse safety signals were observed in this study. In December 2016, Nexvet announced initiation of pivotal efficacy study of frunevetmab in cats: a placebo-controlled, randomised, double blind study with a target enrolment of 250 cats with OA at approximately 20 clinical sites around the USA. Enrolled cats will be randomly assigned to receive frunevetmab or placebo at a 2:1 ratio. Each cat will receive three doses, with each dose given 28 days apart. There are no updates on this at the time of publication.
Generally, anti-NGF mAb exposure is limited to peripheral tissues, because mAbs do not cross the BBB,120 although recent work has been performed developing techniques to allow mAbs to cross the BBB.121 However, anti-NGF mAbs can pass through the placental blood barrier and also be excreted in milk,122 and their use should be avoided in pregnant or lactating animals. Anti-NGF mAbs from the maternal circulation cause fetal abnormalities in rodents, and in pregnant non-human primates, they caused increased rates of stillbirth, and increased postpartum infant mortality and morbidity, decreased infant growth, sensory and sympathetic nerve system changes and decreased infant primary antibody responses.123
Current limitations of our knowledge of anti-NGF therapy for canine and feline OA
Overall, in both dogs and cats, significant improvement has been seen in both subjective and objective (activity) measures following administration of anti-NGF mAbs, suggesting a positive analgesic effect of the same magnitude or greater than that expected with NSAIDs. However, the current assessment of the response to anti-NGF mAbs has not included gait analysis, which was the mainstay of assessment of NSAIDs until recently. Gait analysis is most suited to dogs with asymmetrical gait, and the dogs recruited to the studies performed thus far had multiple joint OA, better reflecting the majority of dogs with OA.106 This is why gait analysis was not used, and an alternative objective measure, activity monitoring, was used. However, it would be interesting to know how anti-NGF therapy compares with NSAIDs using gait analysis in dogs, as well as in cats.124–126 Although not yet performed, Quantitative Sensory Testing10 11 127 could be used to more completely assess sensory function changes, as the most common AEs in human use is abnormal peripheral sensation.43
While no anti-NGF therapy-related AEs in dogs and cats have been seen, the safety of long-term exposure over years, possibly starting early in life, needs to be determined. Currently, the longest follow-up time after anti-NGF mAb therapy in dogs and cats is three months, compared with the mean trial duration of 199 days in humans. Additionally, the safety of concomitant use of NSAIDs with anti-NGF mAb therapy has not been elucidated. This will be important information because in addition to higher dose and longer exposure to anti-NGF mAbs, concurrent use of NSAIDs was a risk factor for the development of RPOA in the human trials.42 92 Interestingly, RPOA is not a recognised or described phenomenon in dogs or cats, anecdotally or otherwise. Additionally, the normal rates of progression of radiographic OA in veterinary species has not been defined, leaving no ‘benchmark’ against which to evaluate potential cases of RPOA. Such a benchmark will be important in order to distinguish clinicians’ focus on the progression of OA in cases that receive an anti-NGF mAb from true RPOA. Currently, there are no data to inform whether NSAIDs can be used with anti-NGF mAbs, nor what sort of withdrawal period (if any) is needed for NSAIDs prior to animals receiving anti-NGF mAbs.
It is likely that if approved, anti-NGF therapy will be used in joint diseases of differing aetiopathogenesis, and there is much to learn about the role of NGF and the effects of anti-NGF in relation to the differing pathologies, such as immune-mediated joint disease versus non-inflammatory OA.
One final consideration is that if the pain relief is indeed greater than seen with NSAIDs, it may be prudent to control the increase in activity seen after administration of the anti-NGF mAb in order to prevent musculoskeletal damage due to overuse of a poorly conditioned body. This is speculative but something that should be considered as clinical use begins.
Potential use of anti-NGF in other pain conditions
Endogenous levels of NGF are increased in a wide range of painful disorders such as inflammatory arthritis, degenerative intervertebral disc disease, prostatitis and cancer. Administration of anti-NGF mAb has been shown to provide effective analgesia in a number of animal models of human disease including inflammatory arthritis, fracture pain, joint surgery, cancer pain and pancreatic pain.71 94 128–130 Interestingly, in nearly all of conditions, anti-NGF on average reduced pain by 30 per cent to 50 per cent and this anti-NGF reduction of pain does not appear to dissipate with time of treatment.
Cancer pain
An estimated 30 per cent to 50 per cent of human patients with cancer experience moderate to severe cancer-related pain, and in advanced or metastatic cancer, 75 per cent to 95 per cent of human patients report life-altering cancer induced pain.131 Although pain arises from numerous causes, bone metastasis is the most common cause of cancer pain. This occurs in 60 per cent to 84 per cent of patients.132 133 However, therapeutics such as opioids, which are commonly used in these patients, are not fully effective in many patients and often have significant side effects. In addition, the use of opioids is contributing to the opioid epidemic.134 Thus, in humans, a significant unmet need remains for development of novel agents for cancer pain treatments. Preclinical and clinical research over the past several decades have suggested that anti-NGF mAb therapy might have an impact on the management of cancer pain.90 135 136 Almost all sensory innervation of bone is provided by nociceptors that express CGRP and TrkA, which are susceptible to the blockade by anti-NGF therapy.128 Additionally, cancers express TrkA and p75NTR and are stimulated by NGF, which is thought to contribute to cancer progression.137
Rodent models suggest significant therapeutic potential in cancer pain in humans, and the same is likely true of companion animals. To our knowledge, no efficacy studies with anti-NGF mAb have been performed. However, a study showed that the majority of canine osteosarcoma primary tumours and pulmonary metastases expressed TrkA protein, and its signalling protected against apoptosis since blockade of NGF/TrkA signalling induced apoptosis of canine osteosarcoma cell lines.138 Thus, anti-NGF mAb may provide significant relief of bone cancer pain90 and slow the progression rate of tumour growth.137
Other orthopaedic pain conditions (fracture and joint surgery)
Several rodent studies using hind limb fracture models (femur and tibia) in mice demonstrate the analgesic potential of anti-NGF therapy. In these models in mice, anti-NGF or anti-TrkA mAbs were administered intraperitoneally following establishment of the fracture, and pain was assessed using observational pain behaviour, ground reaction forces, mechanical allodynia and recording of locomotor activity. The treatment with both anti-NGF and anti-TrkA mAbs significantly reduced pain-related behaviour.70 94 95 139 Furthermore, the efficacy of anti-NGF mAb therapy in reducing pain-related behaviours was comparable with or greater than that achieved by subcutaneous administration of therapeutic dose of morphine.70 In these studies, three to four weeks after fracture, bone healing was assessed by various techniques such as mechanical testing, histomorphometric analysis, microcomputed tomography and radiographical analysis. They concluded that blockade of NGF/TrkA signalling does not influence fracture healing when compared with vehicle control group.
In a mouse model of knee joint surgery pain (drilling and coring the trochlear groove of the femur), pre-emptive intraperitoneal administration of anti-NGF mAb significantly attenuated reduction of spontaneous activity and frequency of vertical rearing following surgery compared with vehicle treated mice.130
Such work suggests potential for the use of anti-NGF mAbs for perioperative orthopaedic pain, and such therapy would be welcome in veterinary medicine where proven analgesic efficacy that can be provided following discharge from the clinic is very limited. However, much work is needed to define any potential side effects related to healing or reinnervation of surgical sites.
Visceral pain
Tanezumab has been studied in two proof-of-concept trials involving chronic visceral pain in humans.140 Patients with interstitial cystitis were administered a single dose of intravenous tanezumab, a human anti-NGF mAb, 200 µg/kg (n=34) or placebo (n=30), and assessed over 16 weeks using questionnaires.140 Tanezumab provided a statistically significant reduction in pain scores compared with placebo over the first six weeks and reduced urgency frequency. In a study evaluating patients with moderate-to-severe chronic prostatitis/chronic pelvic pain syndrome, 30 patients received a single intravenous dose of tanezumab (20 mg) and 32 received a placebo.141 They were followed over 16 weeks after injection. Although average pain score and urgency episode frequency trended downwards at a six-week follow-up, neither outcome achieved significance. Anti-NGF therapy may have potential in visceral pain syndromes; however, visceral pain syndromes are not yet well defined in companion animals, except for interstitial cystitis in cats.
The main symptom of patients with pancreatitis is severe abdominal pain. NGF was found to be upregulated and notably expressed in the pancreas in both human patients with chronic pancreatitis and a rat model of acute and chronic pancreatitis.142 143 Furthermore, in the study of patients with chronic pancreatitis, the expression levels of NGF and TrkA mRNA in pancreas were strongly related with pain intensity and frequency.142 Although, to the best of our knowledge, no clinical study has been conducted, several studies in acute or chronic pancreatitis models in rats showed robust reduction of pain following an administration of blockade of NGF/TrkA signalling.129 144 145 Anti-NGF therapy may be useful for the pain management of acute or chronic pancreatitis in companion animals.
Conclusion
Overall, the rationale for using anti-NGF therapy in several pain conditions is strong. Current evidence indicates that anti-NGF mAb therapy has positive analgesic effects and is well tolerated for up to three months in dogs and cats suffering from OA-associated pain. Additionally, the efficacy of single injection appears to last at least four to six weeks and the magnitude of effect appears the same as, or greater than, that expected with NSAIDs. Thus, anti-NGF mAb therapy could be an alternative to the pharmacological pain management options currently available. Of particular relevance at the moment is the fact that anti-NGF therapy is a non-opioid analgesic. Further studies are needed to better understand the level of analgesic effect and the duration, and also what potential AEs and immunogenicity may occur.
Acknowledgments
We are grateful to Alice MacGregor Harvey for the medical illustrations and to David Gearing for comments on figures.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ME was supported by 2A42AR055042-02. BDXL received research funding from Nexvet for work in cats and dogs. BDXL is a paid consultant of Zoetis Animal Health. PWM was supported by grants from National Cancer Institute, National Institute of Neurological Disorders and Stroke, and the Veterans Administration and has received support from Abbott, Abbvie, Advanced Targeting Systems, Amgen, Array, Johnson & Johnson, Lilly, Medtronic, Merck, Pfizer, Plexxikon, Rinat, Roche and Sanofi-Aventis.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. The Lancet 2005;365:965–73. 10.1016/S0140-6736(05)71086-2 [DOI] [PubMed] [Google Scholar]
- 2.Sanderson RO, Beata C, Flipo RM, et al. . Systematic review of the management of canine osteoarthritis. Vet Rec 2009;164:418–24. 10.1136/vr.164.14.418 [DOI] [PubMed] [Google Scholar]
- 3.Lascelles BD. Feline degenerative joint disease. Vet Surg 2010;39:2–13. 10.1111/j.1532-950X.2009.00597.x [DOI] [PubMed] [Google Scholar]
- 4.Innes JF. Arthritis Tobias KM, Johnston SA. Veterinary surgery: small animal. Philadelphia: Saunders, 2012:1078–112. [Google Scholar]
- 5.Johnson J, Austin C, Breur G. Incidence of canine appendicular musculoskeletal disorders in 16 Veterinary Teaching Hospitals from 1980 through 1989. Veterinary and Comparative Orthopaedics and Traumatology 1994;07:56–69. 10.1055/s-0038-1633097 [DOI] [Google Scholar]
- 6.van Hagen MA, Ducro BJ, van den Broek J, et al. . Incidence, risk factors, and heritability estimates of hind limb lameness caused by hip dysplasia in a birth cohort of boxers. Am J Vet Res 2005;66:307–12. 10.2460/ajvr.2005.66.307 [DOI] [PubMed] [Google Scholar]
- 7.Smith GK, Lawler DF, Biery DN, et al. . Chronology of hip dysplasia development in a cohort of 48 Labrador retrievers followed for life. Vet Surg 2012;41:20–33. 10.1111/j.1532-950X.2011.00935.x [DOI] [PubMed] [Google Scholar]
- 8.Lascelles BDX, Dong Y-H, Marcellin-Little DJ, et al. . Relationship of orthopedic examination, goniometric measurements, and radiographic signs of degenerative joint disease in cats. BMC Vet Res 2012;8:10 10.1186/1746-6148-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DC, Boston RC, Coyne JC, et al. . Ability of the Canine Brief Pain Inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc 2008;233:1278–83. 10.2460/javma.233.8.1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillot M, Moreau M, Heit M, et al. . Characterization of osteoarthritis in cats and meloxicam efficacy using objective chronic pain evaluation tools. Vet J 2013;196:360–7. 10.1016/j.tvjl.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 11.Knazovicky D, Helgeson ES, Case B, et al. . Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain 2016;157:1325–32. 10.1097/j.pain.0000000000000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knazovicky D, Tomas A, Motsinger-Reif A, et al. . Initial evaluation of nighttime restlessness in a naturally occurring canine model of osteoarthritis pain. PeerJ 2015;3:e772 10.7717/peerj.772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S-W, Wang W-T, Chou L-C, et al. . Osteoarthritis increases the risk of dementia: a nationwide cohort study in Taiwan. Sci Rep 2015;5:10145 10.1038/srep10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Kudesia P, Shi Q, et al. . Anxiety and depression in patients with osteoarthritis: impact and management challenges. Open Access Rheumatol 2016;8:103–13. 10.2147/OARRR.S93516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lascelles BDX, Gaynor JS, Smith ES, et al. . Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. J Vet Intern Med 2008;22:53–9. 10.1111/j.1939-1676.2007.0014.x [DOI] [PubMed] [Google Scholar]
- 16.Lascelles BDX, Court MH, Hardie EM, et al. . Nonsteroidal anti-inflammatory drugs in cats: a review. Vet Anaesth Analg 2007;34:228–50. 10.1111/j.1467-2995.2006.00322.x [DOI] [PubMed] [Google Scholar]
- 17.Rychel JK. Diagnosis and treatment of osteoarthritis. Top Companion Anim Med 2010;25:20–5. 10.1053/j.tcam.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Marino CL, Lascelles BDX, Vaden SL, et al. . Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014;16:465–72. 10.1177/1098612X13511446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belshaw Z, Asher L, Dean RS. The attitudes of owners and veterinary professionals in the United Kingdom to the risk of adverse events associated with using non-steroidal anti-inflammatory drugs (NSAIDs) to treat dogs with osteoarthritis. Prev Vet Med 2016;131:121–6. 10.1016/j.prevetmed.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rausch-Derra L, Huebner M, Wofford J, et al. . A prospective, randomized, masked, placebo-controlled multisite clinical study of grapiprant, an EP4 Prostaglandin Receptor Antagonist (PRA), in dogs with osteoarthritis. J Vet Intern Med 2016;30:756–63. 10.1111/jvim.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.KuKanich B. Outpatient oral analgesics in dogs and cats beyond nonsteroidal antiinflammatory drugs. Vet Clin North Am Small Anim Pract 2013;43:1109–25. 10.1016/j.cvsm.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 22.Moore GE, Burkman KD, Carter MN, et al. . Causes of death or reasons for euthanasia in military working dogs: 927 cases (1993-1996). J Am Vet Med Assoc 2001;219:209–14. 10.2460/javma.2001.219.209 [DOI] [PubMed] [Google Scholar]
- 23.Lawler DF, Evans RH, Larson BT, et al. . Influence of lifetime food restriction on causes, time, and predictors of death in dogs. J Am Vet Med Assoc 2005;226:225–31. 10.2460/javma.2005.226.225 [DOI] [PubMed] [Google Scholar]
- 24.Liu JKH. The history of monoclonal antibody development – progress, remaining challenges and future innovations. Ann Med Surg 2014;3:113–6. 10.1016/j.amsu.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975;256:495–7. 10.1038/256495a0 [DOI] [PubMed] [Google Scholar]
- 26.Sgro C. Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: bibliographic review. Toxicology 1995;105:23–9. 10.1016/0300-483X(95)03123-W [DOI] [PubMed] [Google Scholar]
- 27.Tsurushita N, Hinton PR, Kumar S. Design of humanized antibodies: from anti-Tac to Zenapax. Methods 2005;36:69–83. 10.1016/j.ymeth.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 28.Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods 2005;36:3–10. 10.1016/j.ymeth.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 29.Khan AH, Sadroddiny E. Licensed monoclonal antibodies and associated challenges. Hum Antibodies 2015;23:63–72. 10.3233/HAB-150286 [DOI] [PubMed] [Google Scholar]
- 30.Zheng S, Hunter DJ, Xu J, et al. . Monoclonal antibodies for the treatment of osteoarthritis. Expert Opin Biol Ther 2016;16:1529–40. 10.1080/14712598.2016.1229774 [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Ballow M. Monoclonal antibodies and fusion proteins and their complications: targeting B cells in autoimmune diseases. J Allergy Clin Immunol 2010;125:814–20. 10.1016/j.jaci.2010.02.025 [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Chinen J, Kavanaugh A. Immunomodulator therapy: monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J Allergy Clin Immunol 2010;125:S314–S323. 10.1016/j.jaci.2009.08.018 [DOI] [PubMed] [Google Scholar]
- 33.Jones PT, Dear PH, Foote J, et al. . Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986;321:522–5. 10.1038/321522a0 [DOI] [PubMed] [Google Scholar]
- 34.Harding FA, Stickler MM, Razo J, et al. . The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2010;2:256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. . Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis 2007;66:921–6. 10.1136/ard.2006.065615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014;2014:1–19. 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonini S, Rasi G, Bracci-Laudiero ML, et al. . Nerve growth factor: neurotrophin or cytokine? Int Arch Allergy Immunol 2003;131:80–4. 10.1159/000070922 [DOI] [PubMed] [Google Scholar]
- 38.Isola M, Ferrari V, Miolo A, et al. . Nerve growth factor concentrations in the synovial fluid from healthy dogs and dogs with secondary osteoarthritis. Veterinary and Comparative Orthopaedics and Traumatology 2011;24:279–84. 10.3415/VCOT-10-04-0051 [DOI] [PubMed] [Google Scholar]
- 39.Mantyh PW, Koltzenburg M, Mendell LM, et al. . Antagonism of nerve growth factor-trka signaling and the relief of pain. Anesthesiology 2011;115:189–204. 10.1097/ALN.0b013e31821b1ac5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley C, Spencer SD, Nishimura MC, et al. . Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 1994;76:1001–11. 10.1016/0092-8674(94)90378-6 [DOI] [PubMed] [Google Scholar]
- 41.Smeyne RJ, Klein R, Schnapp A, et al. . Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 1994;368:246–9. 10.1038/368246a0 [DOI] [PubMed] [Google Scholar]
- 42.McKelvey L, Shorten GD, O’Keeffe GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J Neurochem 2013;124:276–89. 10.1111/jnc.12093 [DOI] [PubMed] [Google Scholar]
- 43.Bannwarth B, Kostine M. Targeting nerve growth factor (ngf) for pain management: what does the future hold for ngf antagonists? Drugs 2014;74:619–26. 10.1007/s40265-014-0208-6 [DOI] [PubMed] [Google Scholar]
- 44.Chang DS, Hsu E, Hottinger DG, et al. . Anti-nerve growth factor in pain management: current evidence. Journal of pain research 2016;9:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirose M, Kuroda Y, Murata E. NGF/TrkA signaling as a therapeutic target for pain. Pain Practice 2016;16:175–82. 10.1111/papr.12342 [DOI] [PubMed] [Google Scholar]
- 46.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of ngf-induced hyperalgesia. Eur J Neurosci 1994;6:1903–12. 10.1111/j.1460-9568.1994.tb00581.x [DOI] [PubMed] [Google Scholar]
- 47.Michael GJ, Kaya E, Averill S, et al. . TrkA immunoreactive neurones in the rat spinal cord. J Comp Neurol 1997;385:441–55. [DOI] [PubMed] [Google Scholar]
- 48.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, et al. . Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer Pain. Mol Pain 2010;6:1744-8069-6-87 10.1186/1744-8069-6-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghilard JR, Freeman KT, Jimenez-Andrade JM, et al. . Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non-malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone 2010b;8:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001;24:677–736. 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashraf S, Mapp PI, Burston J, et al. . Augmented pain behavioural responses to intra-articular injection of nerve growth factor in two animal models of osteoarthritis. Ann Rheum Dis 2014;73:1710–8. 10.1136/annrheumdis-2013-203416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Z, Nagata K, Iijima T. Immunohistochemical study of NGF and its receptors in the synovial membrane of the ankle joint of adjuvant-induced arthritic rats. Histochem Cell Biol 2000;114:453–9. [DOI] [PubMed] [Google Scholar]
- 53.Kc R, Li X, Kroin JS, et al. . PKCδ null mutations in a mouse model of osteoarthritis alter osteoarthritic pain independently of joint pathology by augmenting NGF/TrkA-induced axonal outgrowth. Ann Rheum Dis 2016;75:2133–41. 10.1136/annrheumdis-2015-208444 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Aloe L, Tuveri MA, Carcassi U, et al. . Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis & Rheumatism 1992;35:351–5. 10.1002/art.1780350315 [DOI] [PubMed] [Google Scholar]
- 55.Seidel MF, Herguijuela M, Forkert R, et al. . Nerve growth factor in rheumatic diseases. Semin Arthritis Rheum 2010;40:109–26. 10.1016/j.semarthrit.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 56.Walsh DA, McWilliams DF, Turley MJ, et al. . Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 2010;49:1852–61. 10.1093/rheumatology/keq188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoppiello LA, Mapp PI, Wilson D, et al. . Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol 2014;66:3018–27. 10.1002/art.38778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang Y, Hu C, Yu S, et al. . Cartilage stem/progenitor cells are activated in osteoarthritis via interleukin-1β/nerve growth factor signaling. Arthritis Res Ther 2015;17:327 10.1186/s13075-015-0840-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNamee KE, Burleigh A, Gompels LL, et al. . Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain 2010;149:386–92. 10.1016/j.pain.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 60.Shelton DL, Zeller J, Ho WH, et al. . Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain 2005;116:8–16. 10.1016/j.pain.2005.03.039 [DOI] [PubMed] [Google Scholar]
- 61.Adams BL, Guo W, Gors RT, et al. . Pharmacological interrogation of a rodent forced ambulation model: leveraging gait impairment as a measure of pain behavior pre-clinically. Osteoarthritis Cartilage 2016;24:1928–39. 10.1016/j.joca.2016.05.022 [DOI] [PubMed] [Google Scholar]
- 62.Nwosu LN, Mapp PI, Chapman V, et al. . Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheum Dis 2016;75:1246–54. 10.1136/annrheumdis-2014-207203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu L, Nwosu LN, Burston JJ, et al. . The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthritis Cartilage 2016;24:1587–95. 10.1016/j.joca.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arendt-Nielsen L, Fernández-de-las-Peñas C, Graven-Nielsen T. Basic aspects of musculoskeletal pain: from acute to chronic pain. Journal of Manual & Manipulative Therapy 2011;19:186–93. 10.1179/106698111X13129729551903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou XF, Deng YS, Xian CJ, et al. . Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J Neurosci 2000;12:100–5. 10.1046/j.1460-9568.2000.00884.x [DOI] [PubMed] [Google Scholar]
- 66.Pezet S, Onténiente B, Jullien J, et al. . Differential regulation of NGF receptors in primary sensory neurons by adjuvant-induced arthritis in the rat. Pain 2001;90(1-2):113–25. 10.1016/S0304-3959(00)00393-6 [DOI] [PubMed] [Google Scholar]
- 67.Cheppudira BP, Trevino AV, Petz LN, et al. . Anti-nerve growth factor antibody attenuates chronic morphine treatment-induced tolerance in the rat. BMC Anesthesiol 2015;16:73 10.1186/s12871-016-0242-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashraf S, Wibberley H, Mapp PI, et al. . Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis 2011;70:523–9. 10.1136/ard.2010.137844 [DOI] [PubMed] [Google Scholar]
- 69.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol 2012;8:390–8. 10.1038/nrrheum.2012.80 [DOI] [PubMed] [Google Scholar]
- 70.Jimenez-Andrade JM, Martin CD, Koewler NJ, et al. . Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain 2007;133:183–96. 10.1016/j.pain.2007.06.016 [DOI] [PubMed] [Google Scholar]
- 71.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, et al. . Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis & Rheumatism 2012;64:2223–32. 10.1002/art.34385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishikawa G, Koya Y, Tanaka H, et al. . Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthritis Cartilage 2015;23:925–32. 10.1016/j.joca.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 73.Im HJ, Kim JS, Li X, et al. . Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum 2010;62:2995–3005. 10.1002/art.27608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aloe L, Rocco M, Balzamino B, et al. . Nerve growth factor: a focus on neuroscience and therapy. Current Neuropharmacol 2015;13:294–303. 10.2174/1570159X13666150403231920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? International Review of Neurobiology 2002;50:393–413. [DOI] [PubMed] [Google Scholar]
- 76.Bracci-Laudiero L, De Stefano ME. NGF in early embryogenesis, differentiation, and pathology in the nervous and immune systems. Curr Top Behav Neurosci 2016;29:125–52. 10.1007/7854_2015_420 [DOI] [PubMed] [Google Scholar]
- 77.Higgins GA, Koh S, Chen KS, et al. . NGF induction of NGF receptor gene expression and cholinergic neuronal hypertrophy within the basal forebrain of the adult rat. Neuron 1989;3:247–56. 10.1016/0896-6273(89)90038-X [DOI] [PubMed] [Google Scholar]
- 78.Fischer W, Wictorin K, Björklund A, et al. . Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 1987;329:65–8. 10.1038/329065a0 [DOI] [PubMed] [Google Scholar]
- 79.Tuszynski MH, Thal L, Pay M, et al. . A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med 2005;11:551–5. 10.1038/nm1239 [DOI] [PubMed] [Google Scholar]
- 80.Vantini G, Schiavo N, Di Martino A, et al. . Evidence for a physiological role of nerve growth factor in the central nervous system of neonatal rats. Neuron 1989;3:267–73. 10.1016/0896-6273(89)90251-1 [DOI] [PubMed] [Google Scholar]
- 81.Micera A, Lambiase A, Aloe L, et al. . Nerve growth factor involvement in the visual system: implications in allergic and neurodegenerative diseases. Cytokine Growth Factor Rev 2004;15:411–7. 10.1016/j.cytogfr.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 82.Ferrari MP, Mantelli F, Sacchetti M, et al. . Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs 2014;28:275–83. 10.1007/s40259-013-0079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H, Wang R, Thrimawithana T, et al. . The nerve growth factor signaling and its potential as therapeutic target for glaucoma. Biomed Res Int 2014;2014:1–10. 10.1155/2014/759473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lane NE, Schnitzer TJ, Birbara CA, et al. . Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med Overseas Ed 2010;363:1521–31. 10.1056/NEJMoa0901510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanga P, Katz N, Polverejan E, et al. . Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain 2013;154:1910–9. 10.1016/j.pain.2013.05.051 [DOI] [PubMed] [Google Scholar]
- 86.Tiseo PJ, Kivitz AJ, Ervin JE, et al. . Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain 2014;155:1245–52. 10.1016/j.pain.2014.03.018 [DOI] [PubMed] [Google Scholar]
- 87.Schnitzer TJ, Ekman EF, Spierings EL, et al. . Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis 2015;74:1202–11. 10.1136/annrheumdis-2013-204905 [DOI] [PubMed] [Google Scholar]
- 88.Kivitz AJ, Gimbel JS, Bramson C, et al. . Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013;154:1009–21. 10.1016/j.pain.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 89.Brown MT, Herrmann DN, Goldstein M, et al. . Nerve safety of tanezumab, a nerve growth factor inhibitor for pain treatment. J Neurol Sci 2014;345:139–47. 10.1016/j.jns.2014.07.028 [DOI] [PubMed] [Google Scholar]
- 90.Sopata M, Katz N, Carey W, et al. . Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 2015;156:1703–13. 10.1097/j.pain.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 91.Hochberg MC, Tive LA, Abramson SB, et al. . When is osteonecrosis not osteonecrosis?: adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol 2016;68:382–91. 10.1002/art.39492 [DOI] [PubMed] [Google Scholar]
- 92.Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015;23(Suppl 1):S18–S21. 10.1016/j.joca.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 93.Wenham CY, Grainger AJ, Conaghan PG. The role of imaging modalities in the diagnosis, differential diagnosis and clinical assessment of peripheral joint osteoarthritis. Osteoarthritis Cartilage 2014;22:1692–702. 10.1016/j.joca.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 94.Koewler NJ, Freeman KT, Buus RJ, et al. . Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. Journal of Bone and Mineral Research 2007;22:1732–42. 10.1359/jbmr.070711 [DOI] [PubMed] [Google Scholar]
- 95.Rapp AE, Kroner J, Baur S, et al. . Analgesia via blockade of NGF/TrkA signaling does not influence fracture healing in mice. Journal of Orthopaedic Research 2015;33:1235–41. 10.1002/jor.22892 [DOI] [PubMed] [Google Scholar]
- 96.Flemming DJ, Gustas-French CN. Rapidly progressive osteoarthritis: a review of the clinical and radiologic presentation. Curr Rheumatol Rep 2017;19:42 10.1007/s11926-017-0665-5 [DOI] [PubMed] [Google Scholar]
- 97.Fukui K, Kaneuji A, Fukushima M, et al. . Inversion of the acetabular labrum triggers rapidly destructive osteoarthritis of the hip: representative case report and proposed etiology. J Arthroplasty 2014;29:2468–72. 10.1016/j.arth.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 98.LaBranche TP, Bendele AM, Omura BC, et al. . Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann Rheum Dis 2017;76:295–302. 10.1136/annrheumdis-2015-208913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown M, Koltzenburg M, Nguyen H, et al. . Tanezumab does not cause sympathetic nervous system dysfunction in clinical osteoarthritis studies (P3.303). Neurology 2015;84. [Google Scholar]
- 100.Belanger P, Butler P, Butt M, et al. . From the cover: evaluation of the effects of tanezumab, a monoclonal antibody against nerve growth factor, on the sympathetic nervous system in adult cynomolgus monkeys (macaca fascicularis): a stereologic, histomorphologic, and cardiofunctional assessment. Toxicological Sciences 2017;158:319–33. 10.1093/toxsci/kfx089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bannwarth B, Kostine M. Nerve growth factor antagonists: is the future of monoclonal antibodies becoming clearer? Drugs 2017;77:1377–87. 10.1007/s40265-017-0781-6 [DOI] [PubMed] [Google Scholar]
- 102.Lange J, 2014. Glenmark’s novel monoclonal antibody GBR 900 for treatment of chronic pain entering human trials. http://www.prnewswire.com/news-releases/glenmarks-novel-monoclonal-antibody-gbr-900-for-treatment-of-chronic-pain-entering-human-trials-257130311.html (accessed 29 Apr 2017).
- 103.Flannery CR, Moran N, Blasioli D, et al. . Efficacy of a novel, locally delivered TrkA inhibitor in preclinical models of OA and joint pain. Osteoarthritis Cartilage 2015;23(suppl 2):A45–A46. 10.1016/j.joca.2015.02.100 [DOI] [Google Scholar]
- 104.Gearing DP, Virtue ER, Gearing RP, et al. . A fully caninised anti-NGF monoclonal antibody for pain relief in dogs. BMC Vet Res 2013;9:226 10.1186/1746-6148-9-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Webster RP, Anderson GI, Gearing DP. Canine brief pain inventory scores for dogs with osteoarthritis before and after administration of a monoclonal antibody against nerve growth factor. Am J Vet Res 2014;75:532–5. 10.2460/ajvr.75.6.532 [DOI] [PubMed] [Google Scholar]
- 106.Lascelles BDX, Knazovicky D, Case B, et al. . A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC Vet Res 2015;11:101 10.1186/s12917-015-0413-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brown DC, Bell M, Rhodes L. Power of treatment success definitions when the canine brief pain inventory is used to evaluate carprofen treatment for the control of pain and inflammation in dogs with osteoarthritis. Am J Vet Res 2013;74:1467–73. 10.2460/ajvr.74.12.1467 [DOI] [PubMed] [Google Scholar]
- 108.Conzemius MG, Evans RB. Caregiver placebo effect for dogs with lameness from osteoarthritis. J Am Vet Med Assoc 2012;241:1314–9. 10.2460/javma.241.10.1314 [DOI] [PubMed] [Google Scholar]
- 109.Bergeron LM, Bainbridge G, Dunham SA, et al. , 2013. Caninized anti-ngf antibodies and methods thereof. http://www.google.com/patents/WO2013184871A1?cl=en (accessed 29 May 2017).
- 110.Lacey SE, Barbon JA, Chhaya M, et al. , 2012. Anti-ngf antibodies and their use. http://www.google.com/patents/WO2012024650A2?cl=en (accessed 29 May 2017).
- 111.Gearing D, 2012. Anti-nerve growth factor antibodies and methods of preparing and using the same. https://www.google.com/patents/WO2012153121A1?cl=en (accessed 29 May 2017).
- 112.Gearing D, 2014. Anti-nerve growth factor antibodies and methods of preparing and using the same. https://www.google.com/patents/EP2705054A1?cl=en (accessed 29 May 2017).
- 113.Gearing D. Anti-nerve growth factor antibodies and methods of preparing and using the same. 2015. https://www.google.com/patents/US20150017154 (Accessed May 29, 2017).
- 114.Gearing D, 2016b. Anti-nerve growth factor antibodies for treating itching. https://www.google.com/patents/WO2016035052A1?cl=en (accessed 29 May 2017).
- 115.Gearing D, 2016a. Anti-nerve growth factor antibodies and methods of preparing and using the same. https://www.google.ch/patents/US9328164 (accessed 29 May 2017).
- 116.Gearing DP, Huebner M, Virtue ER, et al. . In vitro and in vivo characterization of a fully felinized therapeutic anti-nerve growth factor monoclonal antibody for the treatment of pain in cats. J Vet Intern Med 2016;30:1129–37. 10.1111/jvim.13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gruen ME, Thomson AE, Griffith EH, et al. . A feline-specific anti-nerve growth factor antibody improves mobility in cats with degenerative joint disease-associated pain: a pilot proof of concept study. J Vet Intern Med 2016;30:1138–48. 10.1111/jvim.13972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gruen ME, Griffith EH, Thomson AE, et al. . Criterion validation testing of clinical metrology instruments for measuring degenerative joint disease associated mobility impairment in cats. PLoS One 2015;10:e0131839 10.1371/journal.pone.0131839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gruen ME, Dorman DC, Lascelles BDX. Caregiver placebo effect in analgesic clinical trials for cats with naturally occurring degenerative joint disease-associated pain. Vet Rec 2017;180:473 10.1136/vr.104168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pearson J, Johnson EM, Brandeis L. Effects of antibodies to nerve growth factor on intrauterine development of derivatives of cranial neural crest and placode in the guinea pig. Dev Biol 1983;96:32–6. 10.1016/0012-1606(83)90308-1 [DOI] [PubMed] [Google Scholar]
- 121.Yu YJ, Zhang Y, Kenrick M, et al. . Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med 2011;3:ra44 10.1126/scitranslmed.3002230 [DOI] [PubMed] [Google Scholar]
- 122.Gorin PD, Johnson EM. Experimental autoimmune model of nerve growth factor deprivation: effects on developing peripheral sympathetic and sensory neurons. Proceedings of the National Academy of Sciences 1979;76:5382–6. 10.1073/pnas.76.10.5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bowman CJ, Evans M, Cummings T, et al. . Developmental toxicity assessment of tanezumab, an anti-nerve growth factor monoclonal antibody, in cynomolgus monkeys (Macaca fascicularis). Reprod Toxicol 2015;53:105–18. 10.1016/j.reprotox.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 124.Aragon CL, Hofmeister EH, Budsberg SC. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc 2007;230:514–21. 10.2460/javma.230.4.514 [DOI] [PubMed] [Google Scholar]
- 125.Schnabl E, Bockstahler B. Systematic review of ground reaction force measurements in cats. Vet J 2015;206:83–90. 10.1016/j.tvjl.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 126.Gagnon A, Brown D, Moreau M, et al. . Therapeutic response analysis in dogs with naturally occurring osteoarthritis. Vet Anaesth Analg 2017;44:1373–81. 10.1016/j.vaa.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 127.Addison ES, Clements DN. Repeatability of quantitative sensory testing in healthy cats in a clinical setting with comparison to cats with osteoarthritis. J Feline Med Surg 2017;19:1274–82. 10.1177/1098612X17690653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mantyh WG, Jimenez-Andrade JM, Stake JI, et al. . Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010;171:588–98. 10.1016/j.neuroscience.2010.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu Y, Colak T, Shenoy M, et al. . Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis. Gastroenterology 2011;141:370–7. 10.1053/j.gastro.2011.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Majuta LA, Guedon J-MG, Mitchell SAT, et al. . Anti–nerve growth factor therapy increases spontaneous day/night activity in mice with orthopedic surgery–induced pain. Pain 2017;158:605–17. 10.1097/j.pain.0000000000000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Portenoy RK, Lesage P. Management of cancer pain. The Lancet 1999;353:1695–700. 10.1016/S0140-6736(99)01310-0 [DOI] [PubMed] [Google Scholar]
- 132.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997;69:1–18. 10.1016/S0304-3959(96)03267-8 [DOI] [PubMed] [Google Scholar]
- 133.Langford DJ, Paul SM, Tripathy D, et al. . Trajectories of pain and analgesics in oncology outpatients with metastatic bone pain during participation in a psychoeducational intervention study to improve pain management. The Journal of Pain 2011;12:652–66. 10.1016/j.jpain.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 2013;154(Suppl 1):S54–S62. 10.1016/j.pain.2013.07.044 [DOI] [PubMed] [Google Scholar]
- 135.Sevcik MA, Ghilardi JR, Peters CM, et al. . Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005;115:128–41. 10.1016/j.pain.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 136.Miyagi M, Ishikawa T, Kamoda H, et al. . The efficacy of nerve growth factor antibody in a mouse model of neuropathic cancer pain. Exp Anim 2016;65:337–43. 10.1538/expanim.16-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Demir IE, Tieftrunk E, Schorn S, et al. . Nerve growth factor & TrkA as novel therapeutic targets in cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2016;1866:37–50. 10.1016/j.bbcan.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 138.Shor S, Fadl-Alla BA, Pondenis HC, et al. . Expression of nociceptive ligands in canine osteosarcoma. J Vet Intern Med 2015;29:268–75. 10.1111/jvim.12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sabsovich I, Wei T, Guo TZ, et al. . Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain 2008;138:47–60. 10.1016/j.pain.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Evans RJ, Moldwin RM, Cossons N, et al. . Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol 2011;185:1716–21. 10.1016/j.juro.2010.12.088 [DOI] [PubMed] [Google Scholar]
- 141.Nickel JC, Atkinson G, Krieger JN, et al. . Preliminary assessment of safety and efficacy in proof-of-concept, randomized clinical trial of tanezumab for chronic prostatitis/chronic pelvic pain syndrome. Urology 2012;80:1105–10. 10.1016/j.urology.2012.07.035 [DOI] [PubMed] [Google Scholar]
- 142.Friess H, Zhu Z-W, di Mola FF, et al. . Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg 1999;230:615–24. 10.1097/00000658-199911000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Toma H, Winston J, Micci MA, et al. . Nerve growth factor expression is up-regulated in the rat model of L-arginine-induced acute pancreatitis. Gastroenterology 2000;119:1373–81. 10.1053/gast.2000.19264 [DOI] [PubMed] [Google Scholar]
- 144.Winston JH, Toma H, Shenoy M, et al. . Acute pancreatitis results in referred mechanical hypersensitivity and neuropeptide up-regulation that can be suppressed by the protein kinase inhibitor k252a. J Pain 2003;4:329–37. 10.1016/S1526-5900(03)00636-9 [DOI] [PubMed] [Google Scholar]
- 145.Zhu Y, Mehta K, Li C, et al. . Systemic administration of anti-NGF increases A-type potassium currents and decreases pancreatic nociceptor excitability in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2012;302:G176–G181. 10.1152/ajpgi.00053.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]