Abstract
As three decades ago, it was reported that adoptive T cell immunotherapy by infusion of autologous tumor infiltrating lymphocytes (TILs) mediated objective cancer regression in patients with metastatic melanoma. A new era of T cell immunotherapy arose since the improvement and clinical use of anti-CD19 chimeric antigen receptor T cells (CAR-T) for the treatment of refractory and relapsed B lymphocyte leukemia. However, several challenges and difficulties remain on the way to reach generic and effective T cell immunotherapy, including lacking a generic method for generating anti-leukemia-specific T cells from every patient. Here, we summarize the current methods of generating anti-leukemia-specific T cells, and the promising approaches in the future.
Keywords: Anti-leukemia T cell, T cell immunotherapy, TCR-T, CAR-T, T cell reprogramming
Abbreviations: TIL, infiltrating lymphocytes; CAR-T, chimeric antigen receptor T cells; CR, complete remission; CML, chronic myelogenous leukemia; TKI, tyrosine kinase inhibitor; APL, promyelocytic leukemia; allo-HSCT, allogeneic hematopoietic stem cell transplantation; GVL, graft-versus-leukemia; ACT, adoptive cellular immunotherapy; CTLs, cytotoxic T cells; DLI, donor lymphocyte infusion; HLA, human leukocyte antigen; IL-2, interleukin-2; WT1, Wilm's tumor antigen 1; FLT3-ITD, FLT3 internal tandem duplication; GVHD, graft-versus-host disease; TCR-T, TCR gene-modified T cell; TAA, tumor-associated antigen; scFv, single-chain variable fragment; Ig, immunoglobulin; B-ALL, cell acute lymphoblastic leukemia; HPCs, hematopoietic progenitor cells; hESC, human embryonic stem cell; iPSCs, induced pluripotent stem cells; iTs, induced functional T cells
1. Introduction
Leukemia is a group of blood cancers arising from hematopoietic stem/progenitor cells. Treatment of leukemia can be complex due to the variable types of leukemia and patient-related factors such as age. Stem cell transplantation is the major form of treatment, which most likely led to complete remission (CR). Moreover, targeted therapy and biological therapy are also commonly used in the clinic. The first targeted drug, anti-CD20 antibody, was approved for use in CD20 + B cell leukemia and lymphoma in 1997,1 and the first successful small molecule drug, the tyrosine kinase inhibitor (TKI) imatinib, which targets BCR-ABL, was introduced in clinical practice in 1998 to treat chronic myelogenous leukemia (CML).2 The benefit from TKI treatment is that greater than 90% of CML patients could live without leukemia burden. All-trans-retinoic acid (ATRA) treated patients with acute promyelocytic leukemia (APL) has a even higher remission rate.3 Increasing numbers of targeted drugs, such as BTK inhibitors, BCL-2 inhibitors, FLT3 inhibitors, and IDH inhibitors, have been used in clinical trials for treating different leukemia subtypes.4, 5, 6
Cancer-associated immune suppression can cause defectiveness of T cell-mediated anticancer immunity, which is an important factor for prognosis of leukemia.7 Reasonably, adoptive T cell immunotherapy may significantly improve the clinical outcome of leukemia. Evidence that tumor-infiltrating lymphocytes (TILs) could eliminate tumor cells was first described by Rosenberg.2 Subsequently, in the setting of allogeneic hematopoietic stem cell transplantation (allo-HSCT), donor NK and T cells were found to be capable of destroying both leukemic and leukemic stem cells, which is referred to as the graft-versus-leukemia (GVL) effect.8 In the same year, Kolb et al. reported that donor lymphocyte infusion (DLI) is effective for treating CML patients who suffer disease relapse after HSCT and other types of refractory and relapsed leukemia.9 Based on these discoveries, generation of anti-leukemia specific T cells has become a core interest for scientists in cell therapy field over the past several decades. Together with the success of second-generation CAR-T cells treating B cell leukemia, T cell immunotherapy, alone or in combination with other traditional leukemia treatments, has gradually become a powerful therapeutic strategy for B cell leukemia. While the challenges and difficulties remain in generating anti-leukemia-specific T cells, here we summarize and emphasize the current methods used for generation of anti-leukemia-specific T cells, and discuss promising methods that might be used in the future.
2. Resources and approaches for generating anti-leukemia-specific T cells
The direct resources of anti-leukemia T cells generating include patient autologous peripheral blood (PB), bone marrow (BM) and human leukocyte antigen (HLA) matched allogeneic individuals. While, there are several approaches for generating anti-leukemia-specific T cells, including ex vivo expansion, gene-modification as well as reprogramming. When using the method of reprogramming, the resource of T cell generation could be enlarged a great degree. Theoretically, all kinds of stem cells and somatic cells are reprogrammable. Here, we would like to introduce the methods had been tested either in clinical or in laboratory following the development of these techniques.
3. Autologous T cells from leukemia patients
Anti-tumor T cells were first identified from TILs in melanoma patients. After in vitro culture with interleukin-2 (IL-2), amplified autologous T cells from TILs were enriched and re-infused into patients, which consequently demonstrated an anti-melanoma effect.10 Similarly, anti-leukemia-specific cytotoxic T cells (CTLs) could be identified in peripheral blood and bone marrow from patients with leukemia. Candidate antigens for these cells should be unique or up-regulated by leukemic cells to reduce the risk of side effects on normal cells. Diverse autologous CTLs targeting different leukemia-associated antigens have been identified, including preferentially expressed antigens of Wilm's tumor antigen 1 (WT1) CTLs, preferentially expressed antigen of melanoma (PRAME) CTLs, the receptor for hyaluronic acid mediated motility (RHAMM/CD168) CTLs, and FLT3 internal tandem duplication (FLT3-ITD) CTLs from patients with leukemia.11, 12, 13, 14 Isolation and amplification of such leukemia antigen-specific T cells from leukemia patients is a straightforward approach for producing autologous CTLs against leukemia. However, due to the limited cell number and effect of leukemia microenvironment, ex vivo expanded leukemic-specific CTLs always show short lifespan and limited cytotoxic activity in vivo.15 Therefore, the use of allogeneic T cells to generate anti-leukemia T cell is an efficient and feasible approach.16
4. Allogeneic anti-leukemia T cells from donors
DLI could eliminate CML cells in CML relapse patients after allo-HSCT.17 Currently, DLI targeting multiple leukemia-associated antigens enhanced GVL effects for the treatment of leukemic relapse after allo-HSCT.18 However, graft-versus-host disease (GVHD) remains a major complication after DLI.19 Therefore, developing specific anti-leukemia T cells is important for improving the effects of allogeneic T cell treatment. The identification of T cells recognizing a specific leukemia antigen is an important step in developing autologous or allogeneic anti-leukemia T cells. Molecular and immunological techniques, such as GeneScan, Sanger sequencing, high-throughput TCR gene sequencing, tetramer analysis, and flow-cytometry combined with T cell function evaluation, allow for identification of leukemia-specific CTLs.20, 21, 22 In addition, co-administration of cytokines and antibodies further augment the potency of the DLI. In general, allogeneic anti-leukemia T cells could be induced after stimulation with leukemia antigen peptides derived from various leukemia-associated antigens such as WT-1, BCR-ABL, hTERT, PR-1, and NY-ESO-1.23, 24 For example, human leukocyte antigen A2 (HLA-A∗0201)-restricted, WT1-specific, donor-derived CD8+ T cells were induced by the WT1 peptide, which showed anti-leukemia activity in treating relapsed or high-risk leukemia patients after HSCT.
Additionally, the transferred T cells even maintained a long half-life.21 However, challenges remain in generating sufficient numbers of high-quality, antigen-specific T cells using autologous and allogeneic-derived antigen-specific T cells.25 Alternatively, engineered T cells may overcome the above limitations.
5. Redirected T cells
Screening and expansion of autologous or allogeneic T cells are laborious, time-consuming, and inefficient.26 Thus, engineered T cells have emerged as a new stage in precision cancer therapy. In this review, engineered T cells mainly mean TCR gene-modified T (TCR-T) cells and CAR-T cells. The idea is to enforce the expression of TCR or CAR genes on autologous or donor T cells so that they are supposed to specifically recognize leukemia antigens and enlarge their anti-leukemia cytotoxic signaling.25, 27 Except for mature T cells, HSCs are also can be endowed with those recognition and killing ‘weapons’. All of these methods have their unique advantages and disadvantages respectively, although the most successful method is CAR-T cell therapy right now. The progression of these three methods is summarized in the review.
5.1. TCR-T cells
TCR-T cells are engineered by transducing autologous αβ or γδ T cells with a retroviral or lentiviral vector encoding TCR (an α chain noncovalently bound with a β chain) that recognizes peptides of interest and CD3ζ genes. When the engineered T cells recognize peptides bound to the major histocompatibility complex (MHC) on the surface of antigen-presenting or tumor cells, they become activated and start expanding.
The first TCR-T cell therapy was used in clinical trial for metastatic melanoma, whose TCR recognizing an HLA-A2–restricted peptide from a melanocytic differentiation antigen, melanoma antigen recognized by T cells 1 (MART-1).28 Afterward, to achieve the goal of sensitively recognizing malignant cells expressing low MART-1 antigen, higher-avidity TCR targeting the mutated MART-1 epitope was developed. However, despite an improved response rate, these higher-avidity TCR-T cells showed ‘on-target, off-tumor’ toxicity. The side-effect was induced by lower tumor-associated antigen (TAA) expression on normal tissue and cross-reactive epitopes present on normal cells occurred in more than half of the treated patients. Thus, killing tumor cells by TCR-targeting approaches brings safety concerns. Nonetheless, numerous studies have explored the potential of engineered TCRs both at the bench and in the clinic for treating hematological malignancies. NY-ESO-1 TCR-modified T cells demonstrated efficacies against MM.29 Engineered NY-ESO-1-TCR-T cells are now under evaluation in a late-stage clinical trial (NCT01343043, clinicaltrials.gov). WT-1 is also an interesting target for TCR transfer studies because it is persistently and highly expressed in AML, CML, and myelodysplastic syndrome (MDS). WT1-TCR-T cells successfully eliminated leukemia cells in xenograft mouse models and leukemia-bearing NOD/SCID mice.30, 31, 32 During the ASH (American Society of Hematology) meeting in 2014, Bar et al. reported that the infusion of escalating doses of donor-derived, virus-specific CD8+ T cells expressing high-affinity TCRs specific for the HLA A∗02:01-restricted WT1126-134 (RMFPNAPYL) epitope showed persist anti-leukemic activity in four of nine AML patients who belonged to high-risk AML and post-transplantation or who relapsed.33 To investigate safety and the kinetics of TCR-T cells, the first clinical trial using WT1-TCR-T cells (HLA-A∗24:02) in eight patients with refractory AML and high-risk MDS was performed. Four of five patients who had persistent WT1-TCR-T cells survived over 12 months. These results firstly demonstrate the potential of applying WT1-specific TCR-T cells in the clinic for leukemia immunotherapy.34
In general, engineered TCR-T cells are constructed using the recombinant TCR α and β chains to redirect the αβ T cells. However, major concerns include the mispairing of transferred TCRs with those endogenous TCRs and usage limitations by MHC restriction. To avoid mispairing, γδ T or NK cells are alternative TCR-T cell resources.35 More recently, the introduction of TCR αβ genes to HSC or reprogrammed T cells also showed feasibility in solving this problem.36, 37 While to overcome the MHC restriction barrier, CAR-T cells are a fantastic cell model against leukemia.
5.2. CAR-T
CAR-T cells overcome some of the limitations of engineered TCR-T by using a single-chain variable fragment (scFv) derived from immunoglobulin (Ig) variable domains that can specifically recognize antigens combined with costimulatory domains from receptors such as CD28, OX40, and CD137 (also known as 4-1BB). T cells armed with CARs possess strong anti-tumor cytotoxicity based on their direct targeting of tumor antigens without the necessity of recognizing MHC binding domains, which are commonly down-regulated on tumor cells.38, 39 Anti-CD19 CAR-T cell therapy was the first great success based on CAR-T approach, which was approved by the U.S. Food and Drug Administration (FDA) for the treatment of refractory pre-B cell acute lymphoblastic leukemia (B-ALL) and diffuse large B cell lymphoma.38 In addition to CD19, CARs targeting different tumor cell-surface antigens, such as CD33, CD123, CD138, and BCMA, have been developed for clinical trials in treating AML and MM.38, 40, 41
5.3. HSC-TCR/CAR-T
As mentioned above, although TCR-T cells show the efficacy of anti-leukemia in vivo, the introduction of exogenous TCR genes into terminally differentiated T cells also brings disadvantages. First, the activation and expansion process also activate the existing endogenous TCRs, and the exogenous TCR chains can mispair with the endogenous TCR chains. These unwanted chimeric TCRs might cause unknown antigen specificity or result in autoimmune effects, or reducing the efficacy of leukemia-specific TCRs, although that these lethal autoimmune disorders only detected in mouse models but not yet verifying in human beings. Second, to generate adequate dose of T cells for infusion, TCR and CAR gene-redirected T cells always experience multiple-rounds of ex vivo divisions. Consequently, the expanded T cells demonstrate a terminally differentiated T cell phenotype (Loss of CD62L and/or CCR7) and reduced regenerative potential. The reduced persistence and activity of these cells in vivo might partially address the leukemia relapse after T cell infusion. An alternative approach to generate redirected T cells avoiding these weaknesses is the introduction of leukemia-specific TCR or CAR genes into autologous or exogenous HSCs.36, 42 An additional advantage of CAR-engineered HSCs is that not only long-term persistent CAR-specific T cells can be generated but also CAR-specific NK and myeloid cells can be produced, providing broader and quicker anti-tumor activity. Several pre-clinical studies using CAR-HSC-derived TCR-T cells, by approaches including in vitro murine OP9-DL1/4 co-culture system,43, 44 CAR-HSC transplantation of NOD/SCID/IL2Rg−/− (NSG) immune-deficient mice or a NSG humanized mouse model simultaneously transplanted with human thymus and fetal liver tissues (BLT mice),42, 45, 46, 47 have demonstrated their therapeutic efficacies comparable to those TCR-T cells from other resources. In addition, De Oliveira et al. demonstrated that anti-CD19 CAR-engineered HSC-derived T cells carrying a suicide gene gave rise to a persistent, multi-cell lineage, HLA-independent immunotherapy against B-lineage malignancies in xenograft animal model,48 however, it may further need to confirm the feasibility of those CAR-HSC derived T cells which development in a human thymic like micro-environment in the humanized BTL mouse models.49 Recently, cord blood hematopoietic progenitor cells (HPCs) transduced with a CAR/TCR with or without CD28 co-stimulatory domains could differentiate ex vivo into mature T cells when co-cultured with an OP9-DL1 stromal feeder layer, implying their potent as a new CAR-T/TCR-T resource in clinical settings where autologous HSC transplants are performed to add a GVL effect.36, 48, 50 However, this type of engineered HSC might lead to neoplasia caused by insertional mutagenesis in the engineered HSCs. Further, the speed of T cell generation by engineered HSCs is slow and the efficacy of T cells is seriously dependent on the status of individual patients’ thymus environment. Taken together, TCR- or CAR-modified HSCs show potential advantages and disadvantages. Overcoming these drawbacks requires technique innovation.
5.4. Reprogramed T cells
The ACT usually use T cells that directly infiltrated the tumor microenvironment after being transferred with TCRs or CARs; however, T cells from cancer patients occasionally demonstrate functional exhaustion, senescence, and reduced response to activation.7, 51, 52, 53 To overcome the disadvantages faced in CAR-T and TCR-T cell production, an alternative approach has been attempted in the T cell regenerative field i.e., generating tumor-targeting T cells from human embryonic stem cell (hESC) or induced pluripotent stem cells (iPSCs).52, 54, 55 In contrast to T cells generated from mouse ESCs, the induction of T cells from hESCs demonstrated limited success due to the failure of mimicking human thymus microenvironment in vivo. Generation of T cells from patient somatic cell-reprogrammed iPSCs demonstrated great potential in recent years. Certain types of somatic cells, such as B and CD8+ antigen-specific T cells, could be reprogrammed into iPSC cells by transduction with Yamanaka, and further differentiated into T cells.56, 57, 58 iPSCs reprogrammed from T cells are called T-iPSCs. In 2013, two groups demonstrated that terminally differentiated antigen-specific CD8+ T cells (MART-1 and HIV-1-epitope) could be rejuvenated through reprogramming. These rejuvenated T cells can recognize their cognate peptides while possessing prolonged telomeres, a memory T cell phenotype, and an anti-specific antigen immune response.56, 59
Interestingly, induced functional T cells (iT) can be generated via the expression of the HSC-specific transcription factor Hoxb5 in mouse pro-pre-B cells in vivo.60 This trans-differentiation approach implicates a de novo method producing engineered CAR-T/TCR-T in vivo if the role of HOXB5 is conserved in human cell trans-differentiation.61
5.5. Summary and future directions
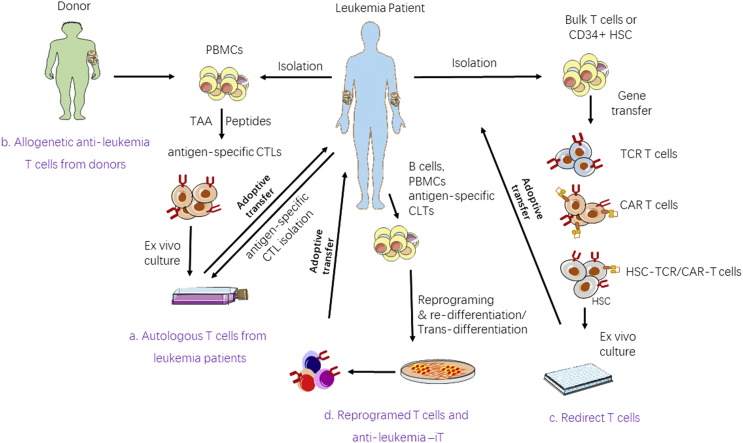
T cell therapy plays a vital role in the treatment of leukemia, particularly for refractory and relapsed patients after HSCT. A large number of clinical trials have demonstrated that adoptive transfer of CAR-T cells induces remarkable responses and durable remission in patients with relapsed/refractory B cell leukemia and lymphoma. Similar strategies are even extended to the treatment of AML and MM. However, cytokine release syndrome (CRS), neurotoxicity, CAR-T cell exhaustion, and unpredictable pharmacology toxicity due to heterogeneity of individual products remain in CAR-T cell therapy. Universal CAR-T cells derived from healthy donors, knocked out endogenous TCR gene and exhaustion genes which could prevent the generation of GVHD in MHC-mismatched recipients and functioning in long-term would be an ideal CAT-T cell product which is the so called off the shelf CAR-T cells.62, 63, 64 A great challenge is disease relapse after CAR-T cell therapy, the production of the ‘off shelf therapy T cells’ which targeted different leukemia antigens may meet the salvage therapy needs of T cells when detection of leukemia relapse with a changed leukemia cell clone. An advantage of TCR-T cells is that they are capable of recognizing intracellular antigens and covering the shortage of CAR-T cells that can only recognize surface antigens.38 With the development of non-viral human genome-targeting T cell techniques such as CRISPR-Cas9, the utilization of TCR-T cells for leukemia therapy may rapidly grow.65 Moreover, reprogrammed T cells may be a source for producing anti-leukemia-iTs (Fig. 1).61
Fig. 1.
Schematic diagram of approaches for generating anti-leukemia T cells for adoptive T cell immunotherapy. (a) PBMCs from patients are either stimulated with appropriate specificity against leukemia antigens, or antigen-specific CTLs isolated directly from patients. After initial stimulation, leukemia-specific T cells are selected and expanded ex vivo and then re-infused into the same patients. (b) Generation of antigen-specific CTLs from healthy donors targeting patient leukemia cells. (c) Flowchart of redirected T-cell-based adoptive immunotherapeutic. Bulk T cells/CD34+ HSCs from patients/donors can be redirected with TCRs/CARs and then cultured in a suitable environment ex vivo to generate antigen-specific T cells for the ACT. (d) B cells or antigen-specific T cells can be reprogramed into iPSCs and re-differentiated or directly trans-differentiated into rejuvenated antigen-specific T cells ex vivo for ACT. PBMCs: peripheral blood mononuclear cells; CTLs: cytotoxic T cells; TCR: T-cell receptor; CAR: chimeric antigen receptor; HSC: hematopoietic stem cells; T-iPSC: T cell-induced pluripotent stem cell.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Nos. 91642111 and 81770152), the Guangdong Provincial Basic Research Program (No. 2015B020227003), the Guangdong Provincial Applied Science and Technology Research & Development Program (No. 2016B020237006) and the Guangzhou Science and Technology Project (Nos. 201510010211, 201807010004, and 201803040017).
Footnotes
Peer review under responsibility of Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences.
Contributor Information
Zhenyi Jin, Email: jinzhenyijnu@163.com.
Ling Xu, Email: lingxu114@163.com.
Yangqiu Li, Email: yangqiuli@hotmail.com.
References
- 1.McLaughlin P., Grillo-Lopez A.J., Link B.K. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol: Offic J Am Soc Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S.A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 3.Burnett A.K., Russell N.H., Hills R.K. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:1295–1305. doi: 10.1016/S1470-2045(15)00193-X. [DOI] [PubMed] [Google Scholar]
- 4.Kriegsmann K., Kriegsmann M., Witzens-Harig M. Acalabrutinib, a second-generation bruton's tyrosine kinase inhibitor. Recent results cancer res Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2018;212:285–294. doi: 10.1007/978-3-319-91439-8_14. [DOI] [PubMed] [Google Scholar]
- 5.Perl A.E. The role of targeted therapy in the management of patients with AML. Blood Adv. 2017;1:2281–2294. doi: 10.1182/bloodadvances.2017009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Poeta G., Postorino M., Pupo L. Venetoclax: bcl-2 inhibition for the treatment of chronic lymphocytic leukemia. Drugs Today. 2016;52:249–260. doi: 10.1358/dot.2016.52.4.2470954. [DOI] [PubMed] [Google Scholar]
- 7.Kasakovski D., Xu L., Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol. 2018;11:91. doi: 10.1186/s13045-018-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz M.M., Gale R.P., Sondel P.M. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 9.Kolb H.J., Mittermuller J., Clemm C. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 10.Rosenberg S.A., Packard B.S., Aebersold P.M. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 11.Driessche A.V., Gao L., Stauss H.J. Antigen-specific cellular immunotherapy of leukemia. Leukemia. 2005;19:1863. doi: 10.1038/sj.leu.2403930. [DOI] [PubMed] [Google Scholar]
- 12.Graf C., Heidel F., Tenzer S. A neoepitope generated by an FLT3 internal tandem duplication (FLT3-ITD) is recognized by leukemia-reactive autologous CD8+ T cells. Blood. 2007;109:2985–2988. doi: 10.1182/blood-2006-07-032839. [DOI] [PubMed] [Google Scholar]
- 13.Greiner J., Li L., Ringhoffer M. Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood. 2005;106:938–945. doi: 10.1182/blood-2004-12-4787. [DOI] [PubMed] [Google Scholar]
- 14.Rezvani K., Yong A.S.M., Tawab A. Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia. Blood. 2009;113:2245. doi: 10.1182/blood-2008-03-144071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg S.A., Restifo N.P., Yang J.C., Morgan R.A., Dudley M.E. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Canc. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber G., Gerdemann U., Caruana I. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia. 2013;27:1538–1547. doi: 10.1038/leu.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolb H.J., Schattenberg A., Goldman J.M. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041. [PubMed] [Google Scholar]
- 18.Anguille S., Van Tendeloo V.F., Berneman Z.N. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. 2012;26:2186–2196. doi: 10.1038/leu.2012.145. [DOI] [PubMed] [Google Scholar]
- 19.Levine J.E., Barrett A.J., Zhang M.J. Donor leukocyte infusions to treat hematologic malignancy relapse following allo-SCT in a pediatric population. Bone Marrow Transplant. 2008;42:201–205. doi: 10.1038/bmt.2008.135. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt M., Li L., Giannopoulos K. Chronic myeloid leukemia cells express tumor-associated antigens eliciting specific CD8+ T-cell responses and are lacking costimulatory molecules. Exp Hematol. 2006;34:1709–1719. doi: 10.1016/j.exphem.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Chapuis A.G., Ragnarsson G.B., Nguyen H.N. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5(174) doi: 10.1126/scitranslmed.3004916. 174ra127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Z., Luo Q., Lu S. Oligoclonal expansion of TCR Vdelta T cells may be a potential immune biomarker for clinical outcome of acute myeloid leukemia. J Hematol Oncol. 2016;9:126. doi: 10.1186/s13045-016-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vonka V. Immunotherapy of chronic myeloid leukemia: present state and future prospects. Immunotherapy. 2010;2:227. doi: 10.2217/imt.10.2. [DOI] [PubMed] [Google Scholar]
- 24.Bornhäuser M., Thiede C., Platzbecker U. Prophylactic transfer of BCR-ABL-, PR1-, and WT1-reactive donor T cells after T cell-depleted allogeneic hematopoietic cell transplantation in patients with chronic myeloid leukemia. Blood. 2011;117:7174–7184. doi: 10.1182/blood-2010-09-308569. [DOI] [PubMed] [Google Scholar]
- 25.Rouce R.H., Sharma S., Huynh M., Heslop H.E. Recent advances in T-cell immunotherapy for haematological malignancies. Br J Haematol. 2017;176:688–704. doi: 10.1111/bjh.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riddell S.R. Hematology American Society of Hematology Education Program; 2007. Engineering antitumor immunity by T-cell adoptive immunotherapy; pp. 250–256. [DOI] [PubMed] [Google Scholar]
- 27.Vonderheide R.H., June C.H. Engineering T cells for cancer: our synthetic future. Immunol Rev. 2014;257:7–13. doi: 10.1111/imr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan R.A., Dudley M.E., Wunderlich J.R. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapoport A.P., Stadtmauer E.A., Binder-Scholl G.K. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue S.A., Gao L., Hart D. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 2005;106:3062–3067. doi: 10.1182/blood-2005-01-0146. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara H., Ochi T., Ochi F. Antileukemia multifunctionality of CD4(+) T cells genetically engineered by HLA class I-restricted and WT1-specific T-cell receptor gene transfer. Leukemia. 2015;29:2393–2401. doi: 10.1038/leu.2015.155. [DOI] [PubMed] [Google Scholar]
- 32.Xue S.A., Gao L., Thomas S. Development of a Wilms' tumor antigen-specific T-cell receptor for clinical trials: engineered patient's T cells can eliminate autologous leukemia blasts in NOD/SCID mice. Haematologica. 2010;95:126–134. doi: 10.3324/haematol.2009.006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar M., Chapuis A.G., Schmitt T.M. Transferred donor-derived virus specific CD8+T cells that have been transduced to express a WT1-specific T cell receptor can persist and provide anti-leukemic activity in AML patients post-transplant. Blood. 2014;124(21) 3939-3939. [Google Scholar]
- 34.Tawara I., Kageyama S., Miyahara Y. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood. 2017;130:1985–1994. doi: 10.1182/blood-2017-06-791202. [DOI] [PubMed] [Google Scholar]
- 35.van der Veken L.T., Hagedoorn R.S., van Loenen M.M., Willemze R., Falkenburg J.H., Heemskerk M.H. Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res. 2006;66:3331–3337. doi: 10.1158/0008-5472.CAN-05-4190. [DOI] [PubMed] [Google Scholar]
- 36.Gschweng E., De Oliveira S., Kohn D.B. Hematopoietic stem cells for cancer immunotherapy. Immunol Rev. 2014;257:237–249. doi: 10.1111/imr.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serwold T., Hochedlinger K., Swindle J., Hedgpeth J., Jaenisch R., Weissman I.L. T-cell receptor-driven lymphomagenesis in mice derived from a reprogrammed T cell. Proc Natl Acad Sci U S A. 2010;107:18939–18943. doi: 10.1073/pnas.1013230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.June C.H., O'Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 39.Kingwell K. CAR T therapies drive into new terrain. Nat Rev Drug Discov. 2017;16:301–304. doi: 10.1038/nrd.2017.84. [DOI] [PubMed] [Google Scholar]
- 40.Bu D.X., Singh R., Choi E.E. Pre-clinical validation of B cell maturation antigen (BCMA) as a target for T cell immunotherapy of multiple myeloma. Oncotarget. 2018;9:25764–25780. doi: 10.18632/oncotarget.25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maus M.V., Fraietta J.A., Levine B.L., Kalos M., Zhao Y., June C.H. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starck L., Popp K., Pircher H., Uckert W. Immunotherapy with TCR-redirected T cells: comparison of TCR-transduced and TCR-engineered hematopoietic stem cell-derived T cells. J Immunol. 2014;192:206–213. doi: 10.4049/jimmunol.1202591. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y., Parkhurst M.R., Zheng Z. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67:2425–2429. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Lent A.U., Nagasawa M., van Loenen M.M. Functional human antigen-specific T cells produced in vitro using retroviral T cell receptor transfer into hematopoietic progenitors. J Immunol. 2007;179:4959–4968. doi: 10.4049/jimmunol.179.8.4959. [DOI] [PubMed] [Google Scholar]
- 45.Greenblatt M.B., Vrbanac V., Tivey T., Tsang K., Tager A.M., Aliprantis A.O. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PloS One. 2012;7(9) doi: 10.1371/journal.pone.0044664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vatakis D.N., Koya R.C., Nixon C.C. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2011;108:E1408–E1416. doi: 10.1073/pnas.1115050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitchen S.G., Bennett M., Galic Z. Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice. PloS One. 2009;4(12) doi: 10.1371/journal.pone.0008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larson S.M., Truscott L.C., Chiou T.T. Pre-clinical development of gene modification of haematopoietic stem cells with chimeric antigen receptors for cancer immunotherapy. Hum Vaccines Immunother. 2017;13:1094–1104. doi: 10.1080/21645515.2016.1268745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lan P., Tonomura N., Shimizu A., Wang S., Yang Y.G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 50.Van Caeneghem Y., De Munter S., Tieppo P. Antigen receptor-redirected T cells derived from hematopoietic precursor cells lack expression of the endogenous TCR/CD3 receptor and exhibit specific antitumor capacities. OncoImmunology. 2017;6(3) doi: 10.1080/2162402X.2017.1283460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crespo J., Sun H., Welling T.H., Tian Z., Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crompton J.G., Clever D., Vizcardo R., Rao M., Restifo N.P. Reprogramming antitumor immunity. Trends Immunol. 2014;35:178–185. doi: 10.1016/j.it.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pawelec G. Immunosenescence and cancer. Biogerontology. 2017;18:717–721. doi: 10.1007/s10522-017-9682-z. [DOI] [PubMed] [Google Scholar]
- 54.Ando M., Nakauchi H. Off-the-shelf' immunotherapy with iPSC-derived rejuvenated cytotoxic T lymphocytes. Exp Hematol. 2017;47:2–12. doi: 10.1016/j.exphem.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Karagiannis P., Iriguchi S., Kaneko S. Reprogramming away from the exhausted T cell state. Semin Immunol. 2016;28:35–44. doi: 10.1016/j.smim.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Vizcardo R., Masuda K., Yamada D. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell stem cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Themeli M., Kloss C.C., Ciriello G. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31:928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loh Y.H., Hartung O., Li H. Reprogramming of T cells from human peripheral blood. Cell stem cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishimura T., Kaneko S., Kawana-Tachikawa A. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell stem cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Zhang M., Dong Y., Hu F. Transcription factor Hoxb5 reprograms B cells into functional T lymphocytes. Nat Immunol. 2018;19:279–290. doi: 10.1038/s41590-018-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu L., Jin Z., Li Y. CD8+ iT cell, a budding star for cancer immunotherapy. Cell Biol Toxicol. 2018;34(4):1–3. doi: 10.1007/s10565-018-9442-0. [DOI] [PubMed] [Google Scholar]
- 62.Tasian S.K. Acute myeloid leukemia chimeric antigen receptor T-cell immunotherapy: how far up the road have we traveled? Therapeutic Adv Hematol. 2018;9:135–148. doi: 10.1177/2040620718774268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCreedy B.J., Senyukov V.V., Nguyen K.T. Off the shelf T cell therapies for hematologic malignancies. Best Pract Res Clin Haematol. 2018;31:166–175. doi: 10.1016/j.beha.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Ghorashian S., Amrolia P., Veys P. Open access? Widening access to chimeric antigen receptor (CAR) therapy for ALL. Exp Hematol. 2018;66:5–16. doi: 10.1016/j.exphem.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Roth T.L., Puig-Saus C., Yu R. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018;559:405–409. doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]