Abstract
Purpose of review
The current review focuses on precise anesthesia for video-assisted thoracoscopic surgery (VATS) with the goal of enhanced recovery.
Recent findings
VATS has become an established and widely used minimally invasive approach with broad implementation on a variety of thoracic operations. In the current environment of enhanced recovery protocols and cost containment, minimally invasive VATS operations suggest adoption of individualized tailored, precise anesthesia. In addition to a perfect lung collapse for surgical interventions with adequate oxygenation during one lung ventilation, anesthesia goals include a rapid, complete recovery with adequate postoperative analgesia leading to early discharge and minimized costs related to postoperative inpatient services. The components and decisions related to precise anesthesia are reviewed and discussed including: letting patients remain awake versus general anesthesia, whether the patient should be intubated or not, operating with or without muscle relaxation, whether to use different separation devises, operating with different local and regional blocks and monitors.
Conclusion
The determining factors in designing a precise anesthesic for VATS operations involve consensus on patients’ tolerance of the associated side effects, the best practice or techniques for surgery and anesthesia, the required postoperative support, and the care team's experience.
Keywords: intercostal nerve block, thoracic anesthesia, vagal nerve block, video-assisted thoracoscopic surgery
INTRODUCTION
The major advance in video-assisted thoracoscopic surgery (VATS) procedures is related to the major pulmonary resections. With advances in endoscopic, robotic and endovascular techniques, VATS can be performed in a minimally invasive way in the management of most pulmonary [1–3], mediastinal, and pleural diseases. By avoiding a thoracotomy incision, VATS allows a shorter operating time and less postoperative morbidity [1,4▪]. Recently, uniportal VATS lobectomy combined with mediastinal lymphadenectomy has been reported to be a safe and feasible technique [5]. Substantial benefits of uniportal VATS in various operations [6▪,7,8▪,9] include less postoperative pain, blood loss, drainage time, and postoperative hospital stay [10,11]. Moreover, in selected patients, it was reported that tubeless VATS was feasible [12]. The application of these less invasive VATS techniques has prompted similar advancements in anesthetic management. A faster recovery with a best-fit postoperative analgesia and earlier return to normal activity have become new goals of anesthetic management above and beyond the conventional goals of satisfactory surgical fields and oxygenation during one lung ventilation (OLV). Therefore, added to an enhanced recovery after surgery protocol [13], precise anesthesia for VATS operations can play a major role in recovery.
Box 1.
no caption available
THE CONSIDERATIONS FOR PERFORMING PRECISE ANESTHESIA
The considerations of precise anesthesia include all the potential factors affecting recovery (age, smoking, male, cardiovascular disease, American Society of Anesthesiologists (ASA) status, preoperative lung function), surgical factors (operation fields, greater extent of resection) [14▪], and optimal postoperative analgesia with the goal of early respiratory rehabilitation, oral intake, and immobilization. Preoperative preparation, such as smoking cessation, rehabilitation, nutrition, and exercise, is indicated for aged patients and patients with chronic obstructive pulmonary disease (COPD). To perform precise anesthesia for VATS operations, an anesthetic combination has to be considered to avoid the disadvantages of some anesthetic practices as well as to provide satisfactory surgical fields and a postoperative analgesia that fits the recovery requirement.
The choices of general anesthesia and drugs applied to perform general anesthesia
General anesthetics, including intravenous anesthetics, inhalational agents, and narcotics, along with muscle relaxants and mechanical ventilation, have long been considered the standard approach for VATS operations. However, the concept of precise anesthesia can be summarized as the balance between the adverse effects of general anesthesia and the patients’ preexisting pulmonary and cardiac diseases. Reduced ventilation and depressed cardio vascular functions are concerns with general anesthesia in this patient population. The functional residual capacity (FRC) is reduced soon after anesthesia [15] and there is a cephalad shift of the diaphragm because of a loss of the diaphragmatic end-expiratory tone. Patients who were paralyzed with muscle relaxants exhibited a greater cephalad shift of the diaphragm. When the FRC falls below the closing capacity, lungs tend to collapse and airways tend to close. Consequently, hypoxemia develops easily, particularly in the elderly [16] and those patients with chronic obstructive pulmonary disease. As noted in previous reports, keeping patients awake to avoid those adverse effects was favorable in some particular operations [17,18]. However, most patients want to be sedated because of the discomfort from apneic sensations during surgical pneumothorax. With adequate monitoring, a precise sedation can be performed and adjusted [19].
Controlled ventilation with muscle relaxants carries the risks of mechanical ventilation associated lung injuries [20] and residual muscle relaxation. Postoperative residual neuromuscular blockade not only delays the clinical recovery [21] but also increases the accidental awareness [22]. For rapid respiratory recovery and the necessity of respiratory rehabilitation, neuromuscular monitoring [21] or sugammadex [23] is strongly suggested to prevent residual muscle relaxation.
Some anesthesiologists believed that ideal candidates for awake or nonintubated VATS (NIVATS) are patients with multiple comorbidities [24]. However, the surgical needs should be taken into anesthetic consideration. For procedures not involving lung parenchyma, Katlic [25▪] has reported on more than 500 patients receiving VATS without intubation and muscle relaxants. In contrast, for anatomical resection requiring lobectomy or segmentectomy, a quiet smooth operation field with complete lung collapse is needed. Therefore, inhibition of the cough reflex is necessary for surgeons manipulating the airways and hilum during anatomical resection. In addition to intravenous narcotics, intrapulmonary or extrapulmonary vagal nerve blocks can suppress the cough reflex [26]. Thoracoscopic vagal nerve blocks can be performed precisely by surgeon under direct vision [27]. The injection sites of left and right thoracoscopic vagal blocks are different and illustrated in Fig. 1. The recovery from unilateral vagal nerve blocks can be monitored with cough performance and phonation without hoarseness.
FIGURE 1.
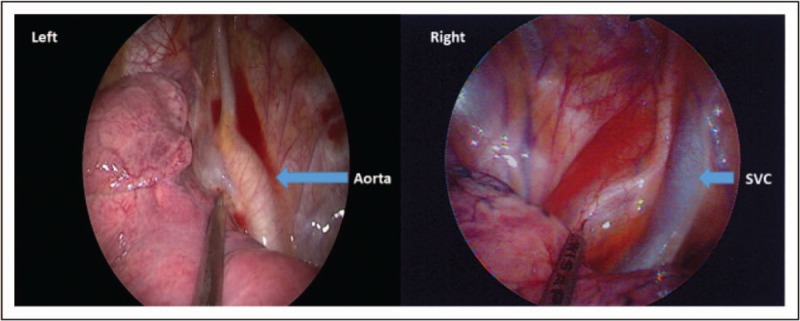
The injection site of left and right thoracoscopic vagal nerve blocks. SVC, superior vena cava.
The intraoperative opioid should also be chosen both based on duration of action and possible side effects. The continuous infusion of remifentanil set with target-controlled infusion (TCI) guarantees effective intraoperative analgesia and rapid extubation times with rapid recovery [28]. In recent years, postoperative narcotics requirement has been reduced with the popularity of regional blockades, even in pediatric patients [29], and there has been less analgesic requirement after minimized invasive surgeries.
The host's immune defense mechanisms and the effects on cancer progression are impacted by surgical stress, anesthesia, and perioperative analgesia [30▪]. Although inhalational agents were reported as preferred for better intraoperative microcirculation [31▪] and less postoperative complications [32▪▪], large-scale investigations have shown better survival rates for patients receiving total intravenous anesthesia (TIVA) [30▪]. As there is a trend of less invasiveness in thoracic operations and selecting regional blocks for postoperative analgesia, the impact of oncologic outcomes associated with operations and anesthesia will be limited in the future.
The choice of different local and regional blocks
Local and regional anesthesia has a fundamental role in maximizing all potential advantages of minimally invasive techniques like VATS. Thoracic epidural analgesia (TEA) remains to be the intraoperative and postoperative gold standard for thoracotomy. However, although epidural anesthesia was reported not to disturb respiratory or parenchymal functions [33], TEA leads to the development of bilateral sympathetic nerve block with reduced cardiac output (CO) [34] and a high risk of hypotension. Recently, regional blocks with less incidence of hypotension such as intercostal nerve blockades [35] and paravertebral blocks [36] are chosen more often for VATS operations with combination of sedation. These nerve blocks remain a good component of postoperative multimodal analgesia especially for minimal-invasive VATS operations. As a result, the determining factors in selecting the regional technique of choice include the consensus on best practice or technique, the tolerance of the associated side effects, and the anesthesiologist's experience.
The choice of tracheal intubation and lung separation instruments
Satisfactory surgical fields with complete lung collapse is mandatory for VATS operations. Although spontaneous negative breathing facilitates lung collapse in NIVATS, the spectrum of indication contains procedure-dependent and patient-dependent factors. While choosing intubated VATS, OLV via lung separation and isolation plays a key role in airway management. Techniques on inserting lung isolation instruments such as double-lumen tubes (DLTs) or bronchial blockers [37–39] and knowledge in tracheobronchial anatomy by fibroptic bronchoscopy (FOB) is routinely requested for anesthesiologists [40]. Recently, a new VivaSight DL (EF-View Ltd, Misgav, Israel) with a camera embedded in the tube's right side has been reported to intubate to the correct position without FOB [41] minimizing insertion injuries and continually monitoring the position of the lung separation device. In addition, there are also reports on how to select the best-fit DLTs to minimize DLT-associated injuries, especially for small-sized Asian people [42]. With recently developed easily inserted rigid-angle bronchial blockers, the role of FOB has also changed to be effective on directing bronchial blockers to the target side but not ensuring satisfaction of surgical fields [43].
NIVATS has been transformed from awake VATS to monitored VATS [19] and can be performed under general anesthesia with supraglottic airway devices [44▪]. There are more and more NIVATS performed for different VATS operations all over the world [17–19] in minor operations [44▪] as well as in major operations [45], even for geriatric patients [46]. The key role of anesthesiologists on NIVATS is to provide a well controlled anesthesia combined with optimal regional blocks and optimal sedation. Optimal anesthetic depth is essential to maintain oxygenation, CO2 elimination, and a smooth respiratory movement for safe surgery. Bispectral index (BIS) monitoring allows advantages to fit the fluctuation in anesthetic requirement with regional anesthesia and surgical manipulation. It also reduces the recovery time after waking, mainly by reducing the administration of general anesthetics as well as the risk of adverse events [47].
The choices of anesthetic management during one lung ventilation
To achieve adequate oxygenation during OLV, the main issues are to maintain adequate CO and to reduce the ventilation/perfusion mismatch. In addition to avoiding the cardiovascular depression of general anesthesia, fluid therapy needs to be administered critically to avoid overload. The application of minimally invasive monitors based on ventilation-induced pulse pressure variations has its limitations in protective ventilation strategies [48] and low tidal volume ventilation during OLV [49]. In addition, ‘volume responsiveness’ should not be equated to volume deficiency. So, minimal discontinuation of oral hydration and early feeding should be encouraged [50]. In addition to maintaining CO during anesthesia, there are ways to reduce the ventilation/perfusion mismatch during OLV such as supplying oxygen to the nondependent lung, boosting the oxygen content in the shunt, and increasing oxygen reserve [51,52]. Respiratory management of ventilation during VATS, including during OLV, needs to be optimized to prevent not only intraoperative hypoxemia, but also postoperative acute lung injury.
CONCLUSION
As VATS operations become more popular and less invasive on various surgical procedures, precise anesthesia should be performed to improve short-term and long-term outcomes. By considering the patients and the surgical factors with clear recovery goals, anesthesiologists can perform a tailored anesthetic using a combination of general anesthetics and regional blocks with optimal airway management for different patients with less invasive monitors.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for nonsmall cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013; 16:244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puri V, Meyers BF. Video-assisted thoracoscopic surgery lobectomy for lung cancer. Surg Oncol Clin 2013; 22:27–38. [DOI] [PubMed] [Google Scholar]
- 3.Yang CF, Sun Z, Speicher PJ, et al. Use and outcomes of minimally invasive lobectomy for stage I non-small cell lung cancer in the national cancer data base. Ann Thorac Surg 2016; 101:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Onaitis MW, Furnary AP, Kosinski AS, et al. Prediction of long-term survival after lung cancer surgery for elderly patients in the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg 2018; 105:309–316. [DOI] [PubMed] [Google Scholar]; Procedure choice are potentially modifiable predictors of long-term survival after lung cancer resection.
- 5.Cai Y, Han Y, Zhang N, et al. Modular uniportal video-assisted thoracoscopic lobectomy and lymphadenectomy: a novel pattern of endoscopic lung cancer resection. J Laparoendosc Adv Surg Tech A 2017; 27:1230–1235. [DOI] [PubMed] [Google Scholar]
- 6▪.Ng CSH, He JX, Rocco G. Innovations and technologies in thoracic surgery. Eur J Cardiothorac Surg 2017; 52:203–205. [DOI] [PubMed] [Google Scholar]; Anesthetic management may be modified by new technology in thoracic surgery.
- 7.Mineo TC, Sellitri F, Fabbi E, Ambrogi V. Uniportal nonintubated lung metastasectomy. J Vis Surg 2017; 3:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪.Masmoudi H, Etienne H, Sylvestre R, et al. Three hundred fifty-one patients with pneumothorax undergoing uniportal (single port) video-assisted thoracic surgery. Ann Thorac Surg 2017; 104:254–260. [DOI] [PubMed] [Google Scholar]; Uniportal VATS is as effective as multiportal VATS or thoracotomy on treating pneumothorax.
- 9.Dmitrii S, Pavel K. Uniportal video-assisted thoracic surgery esophagectomy. Thorac Surg Clin 2017; 27:407–415. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Ma H, Chen S. Enhanced recovery after surgery using uniportal video-assisted thoracic surgery for lung cancer: a preliminary study. Thorac Cancer 2018; 9:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abouarab AA, Rahouma M, Kamel M, et al. Single versus multi-incisional video-assisted thoracic surgery: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 2018; 28:174–185. [DOI] [PubMed] [Google Scholar]
- 12.Yang S-M, Wang M-L, Hung M-H, et al. Tubeless uniportal thoracoscopic wedge resection for peripheral lung nodules. Ann Thorac Surg 2017; 103:462–468. [DOI] [PubMed] [Google Scholar]
- 13.Martin LW, Sarosiek BM, Harrison MA, et al. Implementing a thoracic enhanced recovery program: lessons learned in the first year. Ann Thorac Surg 2018; 105:1597–1604. [DOI] [PubMed] [Google Scholar]
- 14▪.Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons lung cancer resection risk model: higher quality data and superior outcomes. Ann Thorac Surg 2016; 102:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]; An update of lung cancer resection risk model was started.
- 15.Warner DO, Warner MA, Ritman EL. Human chest-wall function while awake and during halothane anesthesia 0.1. Quiet breathing. Anesthesiology 1995; 82:6–19. [DOI] [PubMed] [Google Scholar]
- 16.Gunnarsson L, Tokics L, Gustavsson H, Hedenstierna G. Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. Br J Anaesth 1991; 66:423–432. [DOI] [PubMed] [Google Scholar]
- 17.Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012; 143:47–54. [DOI] [PubMed] [Google Scholar]
- 18.Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013; 95:445–452. [DOI] [PubMed] [Google Scholar]
- 19.Mineo TC, Tacconi F. From ‘awake’ to ‘monitored anesthesia care’ thoracic surgery: a 15 year evolution. Thorac Cancer 2014; 5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsevier, Landsberg JW. e1-e13, chapter 19. Clinical practice manual for pulmonary and critical care medicine 1st edition2018. [Google Scholar]
- 21.Murphy GS, Szokol JW, Avram MJ, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg 2013; 117:133–141. [DOI] [PubMed] [Google Scholar]
- 22.Pandit JJ, Andrade J, Bogod DG, et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. Br J Anaesth 2014; 113:549–559. [DOI] [PubMed] [Google Scholar]
- 23.Paton F, Paulden M, Chambers D, et al. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. Br J Anaesth 2010; 105:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tacconi F, Pompeo E. Nonintubated video-assisted thoracic surgery: where does evidence stand? J Thorac Dis 2016; 8:S364–S375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪.Katlic MR. Five hundred seventy-six cases of video-assisted thoracic surgery using local anesthesia and sedation: lessons learned. J Am Coll Surg 2018; 226:58–63. [DOI] [PubMed] [Google Scholar]; VATS surgery using local anesthesia and sedation is safe and effective for pleural disease and some lung diseases.
- 26.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 2006; 152:223–242. [DOI] [PubMed] [Google Scholar]
- 27.Hung M-H, Hsu H-H, Chan K-C, et al. Nonintubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014; 46:620–625. [DOI] [PubMed] [Google Scholar]
- 28.Deng X, Zhu T. Clinical comparison of propofol-remifentanil TCI with sevoflurane induction/maintenance anesthesia in laparoscopic cholecystectomy. Pak J Med Sci 2014; 30:1017–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semmelmann A, Kaltofen H, Loop T. Anesthesia of thoracic surgery in children. Paediatr Anaesth 2018; 28:326–331. [DOI] [PubMed] [Google Scholar]
- 30▪.Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology 2016; 124:69–79. [DOI] [PubMed] [Google Scholar]; IV anesthesia was associated with a higher survival rate after lung resection.
- 31▪.Cho YJ, Bae J, Kim TK, et al. Microcirculation measured by vascular occlusion test during desflurane–remifentanil anesthesia is superior to that in propofol–remifentanil anesthesia in patients undergoing thoracic surgery: subgroup analysis of a prospective randomized study. J Clin Monit Comput 2017; 31:989–997. [DOI] [PubMed] [Google Scholar]; Desflurane-remifentanil anesthesia presented a better microcirculation than propofol-remifentanil anesthesia in VATS operations.
- 32▪▪.de la Gala F, Pineiro P, Reyes A, et al. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. Br J Anaesth 2017; 119:655–663. [DOI] [PubMed] [Google Scholar]; Sevoflurane, compared to propofol, reduces the frequency of postoperative pulmonary complications and systemic inflammation in lung resection surgery.
- 33.Lundh R, Hedenstierna G. Ventilation-perfusion relationships during anaesthesia and abdominal surgery. Acta Anaesthesiol Scand 1983; 27:167–173. [DOI] [PubMed] [Google Scholar]
- 34.Miro M, Sanfilippo F, Perez F, et al. Influence of the thoracic epidural anesthesia on the left ventricular function: an echocardiographic study. Minerva Anestesiol 2017; 83:695–704. [DOI] [PubMed] [Google Scholar]
- 35.Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: a retrospective cohort study of 238 cases. Medicine (Baltimore) 2015; 94:e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Fang S, Wang Q, et al. Patient-controlled paravertebral block for video-assisted thoracic surgery: a randomized trial. Ann Thorac Surg 2018; 106:888–894. [DOI] [PubMed] [Google Scholar]
- 37.Clayton-Smith A, Bennett K, Alston RP, et al. A comparison of the efficacy and adverse effects of double-lumen endobronchial tubes and bronchial blockers in thoracic surgery: a systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 2015; 29:955–966. [DOI] [PubMed] [Google Scholar]
- 38.Seo JH, Kwon TK, Jeon Y, et al. Comparison of techniques for double-lumen endobronchial intubation: 90° or 180° rotation during advancement through the glottis. Br J Anaesth 2013; 111:812–817. [DOI] [PubMed] [Google Scholar]
- 39.Mourisse J, Liesveld J, Verhagen A, et al. Efficiency, efficacy, and safety of EZ-blocker compared with left-sided double-lumen tube for one-lung ventilation. Anesthesiology 2013; 118:550–561. [DOI] [PubMed] [Google Scholar]
- 40.Loop T, Spaeth J. Airway management in thoracic anesthesia with double-lumen tube. Anasthesiol Intensivmed Notfallmed Schmerzther 2018; 53:174–185. [DOI] [PubMed] [Google Scholar]
- 41.Levy-Faber D, Malyanker Y, Nir RR, et al. Comparison of VivaSight double-lumen tube with a conventional double-lumen tube in adult patients undergoing video-assisted thoracoscopic surgery. Anaesthesia 2015; 70:1259–1263. [DOI] [PubMed] [Google Scholar]
- 42.Shiqing L, Wenxu Q, Jin Z, Youjing D. The combination of diameters of cricoid ring and left main bronchus for selecting the ‘Best Fit’ double-lumen tube. J Cardiothorac Vasc Anesth 2018; 32:869–876. [DOI] [PubMed] [Google Scholar]
- 43.Wang ML, Wang YP, Hung MH, et al. Is fibre-optic bronchoscopy necessary to confirm the position of rigid-angled endobronchial blockers before thoracic surgery? A randomized controlled trial. Eur J Cardiothorac Surg 2018; 53:241–246. [DOI] [PubMed] [Google Scholar]
- 44▪.Irons JF, Miles LF, Joshi KR, et al. Intubated versus nonintubated general anesthesia for video-assisted thoracoscopic surgery – a case–control study. J Cardiothorac Vasc Anesth 2017; 31:411–417. [DOI] [PubMed] [Google Scholar]; Non-intubated general anesthesia bares a trend toward shorter recovery and shorter hospitalization days.
- 45.Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011; 254:1038–1043. [DOI] [PubMed] [Google Scholar]
- 46.Wu C-Y, Chen J-S, Lin Y-S, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013; 95:405–411. [DOI] [PubMed] [Google Scholar]
- 47.Oliveira CR, Bernardo WM, Nunes VM. Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Braz J Anesthesiol 2017; 67:72–84. [DOI] [PubMed] [Google Scholar]
- 48.Umari M, Falini S, Segat M, et al. Anesthesia and fast-track in video-assisted thoracic surgery (VATS): from evidence to practice. J Thorac Dis 2018; 10:S542–S554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raphael J, Regali LA, Thiele RH. Hemodynamic monitoring in thoracic surgical patients. Curr Opin Anaesthesiol 2017; 30:7–16. [DOI] [PubMed] [Google Scholar]
- 50.Senturk M, Orhan Sungur M, Sungur Z. Fluid management in thoracic anesthesia. Minerva Anestesiol 2017; 83:652–659. [DOI] [PubMed] [Google Scholar]
- 51.Wang ML, Hung MH, Chen JS, et al. Nasal high-flow oxygen therapy improves arterial oxygenation during one-lung ventilation in nonintubated thoracoscopic surgery. Eur J Cardiothorac Surg 2018; 53:1001–1006. [DOI] [PubMed] [Google Scholar]
- 52.Russell WJ. Hypoxaemia associated with one-lung anaesthesia: an alternative approach. Br J Anaesth 2011; 107:818.Author's reply 818. [DOI] [PubMed] [Google Scholar]


