Abstract
A kinship between cranial and pelvic visceral nerves of vertebrates has been accepted for a century. Accordingly, sacral preganglionic neurons are considered parasympathetic, as are their targets in the pelvic ganglia that prominently control rectal, bladder, and genital functions. Here, we uncover 15 phenotypic and ontogenetic features that distinguish pre- and postganglionic neurons of the cranial parasympathetic outflow from those of the thoracolumbar sympathetic outflow in mice. By every single one, the sacral outflow is indistinguishable from the thoracolumbar outflow. Thus, the parasympathetic nervous system receives input from cranial nerves exclusively and the sympathetic nervous system from spinal nerves, thoracic to sacral inclusively. This simplified, bipartite architecture offers a new framework to understand pelvic neurophysiology as well as development and evolution of the autonomic nervous system.
The allocation of the sacral autonomic outflow to the parasympathetic division of the visceral nervous system—as the second tier of a “cranio-sacral outflow”—has an ancient origin, yet a simple history: It is rooted in the work of Gaskell (1), was formalized by Langley (2), and has been universally accepted ever since [as in (3)]. The argument derived from several similarities of the sacral outflow with the cranial outflow: (i) anatomical—a target territory less diffuse than that of the thoracolumbar outflow, a separation from it by a gap at limb levels, and a lack of projections to the paravertebral sympathetic chain (1); (ii) physiological—an influence on some organs opposite to that of the thoracolumbar outflow (4); and (iii) pharmacological—an overall sensitivity to muscarinic antagonists (2). However, analysis of cellular phenotype was lacking. Here, we define differential genetic signatures and dependencies for parasympathetic and sympathetic neurons, both pre- and postganglionic. When we reexamine the sacral autonomic outflow of mice in this light, we find that it is better characterized as sympathetic than parasympathetic.
Cranial parasympathetic preganglionic neurons are born in the “pMNv” progenitor domain of the hindbrain (5) that expresses the homeogene Phox2b and produces, in addition, branchiomotor neurons (6). The postmitotic precursors migrate dorsally (7) to form nuclei (such as the dorsal motor nucleus of the vagus nerve) and project through dorsolateral exit points (7) in several branches of the cranial nerves to innervate parasympathetic and enteric ganglia. In contrast, thoracic and upper lumbar (hereafter “thoracic”) preganglionic neurons, which are sympathetic, are thought to have a common origin with somatic motoneurons (8, 9). By implication, they would be born in the pMN progenitor domain (just dorsal to p3)—thus from progenitors that express the basic helix-loop-helix (bHLH) transcription factor Olig2 (10). The sympathetic preganglionic precursors then segregate from somatic motoneurons to form the intermediolateral column in mammals (11), project in the ventral roots of spinal nerves together with axons of somatic motoneurons, and, via the white rami communicantes, synapse onto neurons of the paravertebral and prevertebral sympathetic ganglia.
We sought to compare the genetic makeup and dependencies of lower lumbar and sacral (hereafter “sacral”) preganglionic neurons with that of cranial (parasympathetic) and thoracic (sympathetic) ones. As representative of cranial preganglionic neurons, we focused on the dorsal motor nucleus of the vagus nerve, a cluster of neurons already well delineated at 13.5 days of embryonic development (E13.5), that expresses the vesicular acetylcholine transporter (VAChT) (Fig. 1B). Thoracic and sacral preganglionic neurons, which both form a mediolateral column in the spinal cord, did not express VAChT at this stage despite their eventual cholinergic nature. To localize them, we thus used their common marker nitric oxide synthase (NOS) (12) (Fig. 1, A and B), which was absent from the dorsal motor nucleus of the vagus nerve at E13.5 (Fig. 1B) or later (fig. S1). Thus, NOS expression characterizes thoracic and sacral, but not cranial, preganglionic neurons.
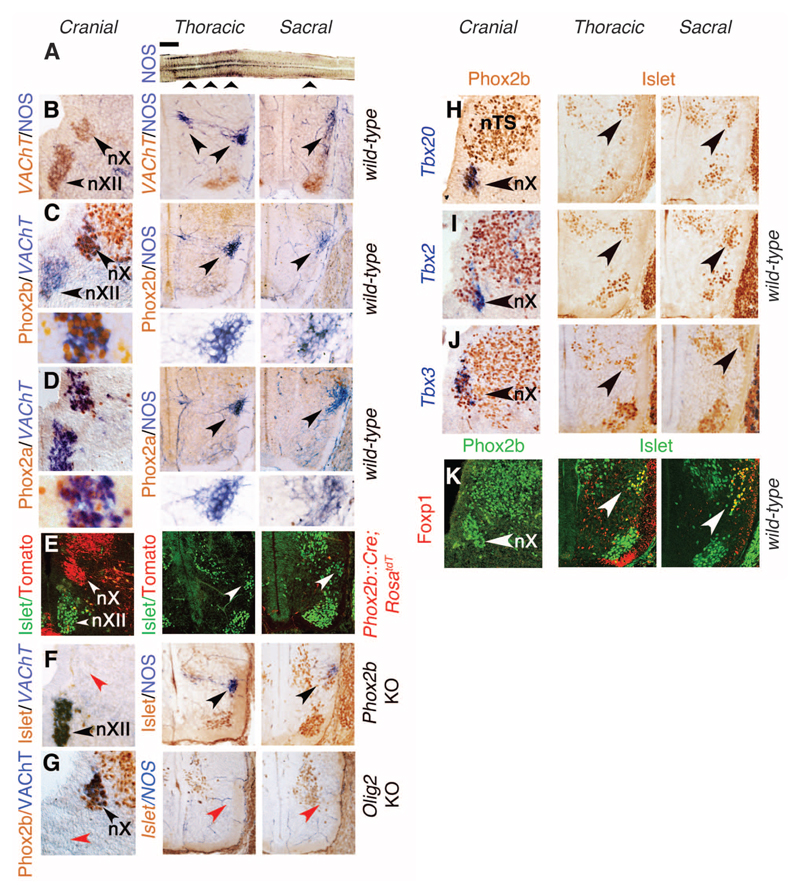
Fig. 1. Sacral preganglionic neurons develop like sympathetic, not parasympathetic, ones.
(A) Longitudinal thick section of the spinal cord reacted for a reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase activity indicative of NOS expression, revealing the thoracolumbar and sacral visceromotor columns (arrowheads) separated by a gap. (B to K) Transverse sections at E13.5 through the right half of the medulla (left column in both panels), thoracolumbar spinal cord (middle), and sacral spinal cord (right), stained with the indicated antibodies and probes, or for NOS expression, in the genetic backgrounds indicated on the right. (B) The dorsal motor nucleus of the vagus nerve (nX) expresses VAChT but not NOS, whereas the thoracic and sacral preganglionic neurons (arrowheads) express NOS but not yet VAChT. The ventrally located somatic motoneurons, including the hypoglossal nucleus (nXII) in the hindbrain, express VAChT. [(C) and (D)] Phox2b (C) and Phox2a (D) are expressed in nX but in neither thoracic nor sacral preganglionic neurons (arrowheads). Lower panels in (C) and (D): higher magnifications of the preganglionic neurons. (E) Neurons of nX but neither thoracic nor sacral preganglionic ones (labeled by an antibody to Islet1/2, white arrowheads) derive from Phox2b+ precursors, permanently labeled in a Phox2b::Cre;RosatdT background. (F) nX is missing in Phox2b knockouts (red arrowhead), but thoracic and sacral preganglionic neurons are spared (black arrowheads). (G) nX is spared in Olig2 knockouts (black arrowhead), but thoracic and sacral preganglionic neurons are missing (red arrowheads). nXII is also missing, as expected of a somatic motor nucleus (red arrowhead). [(H) to (J)] Tbx20, Tbx2, and Tbx3 are expressed in all or a subset of nX neurons (arrowheads in panels of the left column) but in no thoracic or sacral preganglionic neuron (arrowheads in panels of the middle and right columns). (K) Foxp1 is not expressed in the nX (arrowhead in left column) but is a marker of both thoracic and sacral preganglionic neurons (arrowheads in middle and right columns). nTS, nucleus of the solitary tract. Scale bars: 1 mm (A), 100 μm [(B) to (K)].
In contrast to cranial (parasympathetic) preganglionic neurons, thoracic (sympathetic) ones not only failed to express Phox2b or its paralogue Phox2a at E13.5 but also arose from Phox2b-negative progenitors and did not depend on Phox2b for their differentiation (Fig. 1, C to F, left and middle columns) but instead depended on Olig2 (Fig. 1G). Sacral preganglionic neurons shared all these features with thoracic ones (Fig. 1, C to G, middle and right columns). At E13.5, the T-box transcription factors Tbx20, Tbx2, and Tbx3 were expressed by cranial (parasympathetic) neurons but by neither thoracic (sympathetic) nor sacral preganglionic ones (Fig. 1, H to J, and fig. S2). The F-box transcription factor Foxp1, a determinant of thoracic preganglionic neurons (13), was expressed by sacral but not cranial preganglionic neurons (Fig. 1K). Differential expression of Phox2b, Tbx20, and FoxP1 between cranial and all spinal preganglionic neurons, thoracic and sacral, was still observed at E16.5 (fig. S3). In sum, the ontogeny and transcriptional signature of sacral preganglionic neurons was indistinguishable from that of thoracic ones and therefore sympathetic as well.
Thoracic and sacral preganglionic neurons share a settling site in the mediolateral region of the spinal cord and a ventral exit point for their axons, whereas cranial preganglionics have a less systematized topography and a dorsal axonal exit point. These similarities of thoracic with sacral, and differences of both with cranial, are at odds with the notion of craniosacral outflow since its first description (1).
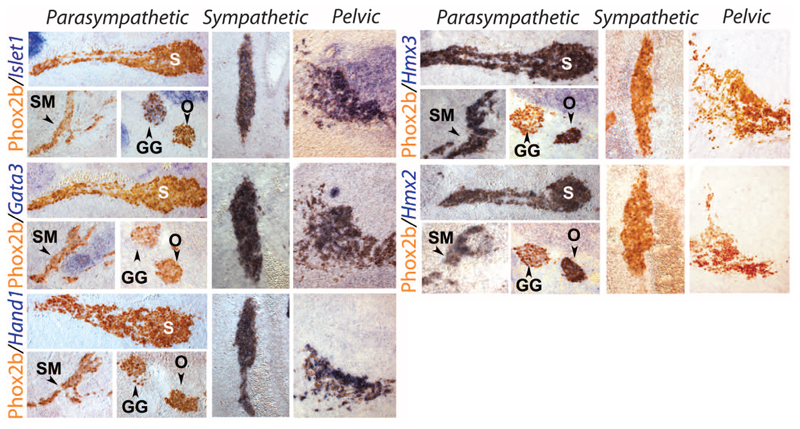
The targets of the sacral preganglionic neurons are in the pelvic plexus (figs. S4 and S5) and are considered, by definition, parasympathetic (14). Because a proportion of pelvic ganglionic neurons receive input from upper lumbar levels [half of them in rats (15)] and thus from sympathetic preganglionic neurons, the pelvic ganglion is considered mixed sympathetic and parasympathetic (16). This connectivity-based definition runs into a conundrum for cells that receive a dual lumbar/sacral input (17). The sympathetic identity of both thoracic and sacral preganglionic neurons that we unveil here makes the issue moot. Regardless, we looked for a cell-intrinsic criterion that would corroborate the sympathetic nature of all pelvic ganglionic cells in the form of genes differentially expressed in sympathetic versus parasympathetic ganglionic cells elsewhere in the autonomic nervous system. Neurotransmitter phenotypes do not map on the sympathetic/parasympathetic partition because cholinergic neurons in the pelvic ganglion comprise both “parasympathetic” and “sympathetic” ganglionic cells, as defined by connectivity (14), and bona fide sympathetic neurons of the paravertebral chain are cholinergic [reviewed in (18)]. However, we found that three transcription factors expressed and required in the sympathoadrenal lineage—Islet1 (19), Gata3 (20), and Hand1 (21)—were not expressed in parasympathetic ganglia such as the sphenopalatine, the submandibular, or the otic ganglia (Fig. 2 and fig. S6) [although Islet1 is expressed in ciliary ganglia (22) and Gata3 in cardiac ones (20), which thus diverge from the canonical parasympathetic molecular signature]. Conversely, we found that the two paralogous homeobox genes Hmx2 and Hmx3 are specific markers of all parasympathetic versus sympathetic ganglia and adrenal medulla (Fig. 2 and figs. S6 and S7). All cells of the pelvic ganglion were Islet1+, Gata3+, Hand1+, Hmx3–, and Hmx2– at E13.5 (Fig. 2) and at E16.5 (fig. S8), as were smaller scattered ganglia of the pelvic organs (fig. S8). Thus, all had a sympathetic transcriptional fingerprint. Similarly, the chicken ganglion of Remak, classically considered parasympathetic (23), displayed an Islet1+, Hand1+, Hmx3– signature, and thus is sympathetic (fig. S9).
Fig. 2. All pelvic ganglionic cells have a sympathetic, not parasympathetic, transcriptional signature.
Sagittal sections through parasympathetic ganglia (columns headed “Parasympathetic”), the lumbar paravertebral sympathetic chain (columns headed “Sympathetic”), and the pelvic ganglion (columns headed “Pelvic”) at E13.5, stained by inmmunohistochemistry for Phox2b, a determinant of all autonomic ganglia (31), and in situ hybridization for the indicated probes. GG, geniculate ganglion (a cranial sensory ganglion); O, otic ganglion; S, sphenopalatine ganglion; SM, submandibular ganglion (all parasympathetic ganglia).
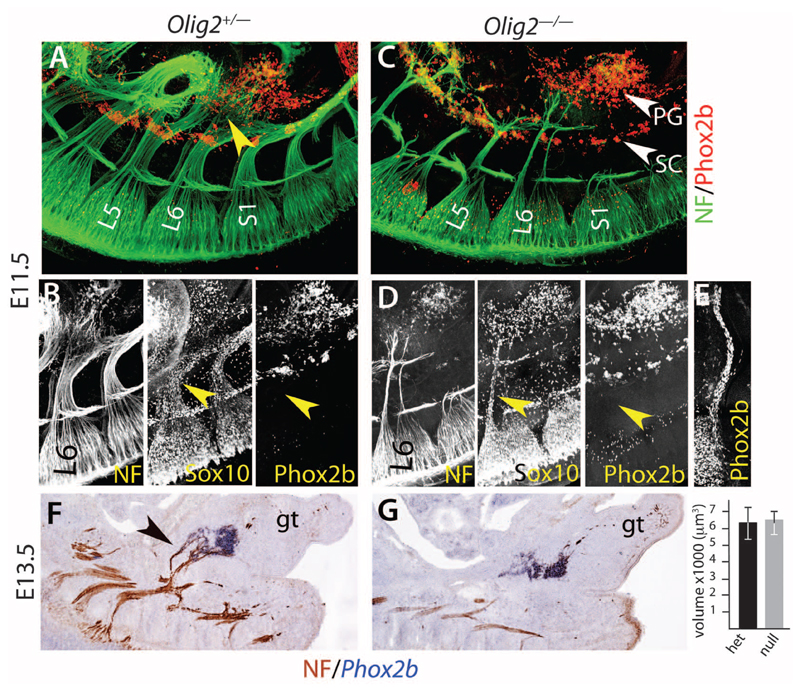
Finally, we tested the pelvic ganglion for the contrasted modes of development of sympathetic and parasympathetic ganglia. Parasympathetic ganglia, unlike sympathetic ones, arise through the migration of Sox10+/Phox2b+ Schwann cell precursors along their future preganglionic nerve toward the site of ganglion formation and do not form if these nerves are absent (24, 25). At E11.5, the lumbosacral plexus, which gives rise to the pelvic nerve, extended some fibers that reached the lateral and rostral edge of the pelvic ganglion anlagen, most of which was already situated well ahead of them (Fig. 3A and movie S1). These fibers were coated with Sox10+ cells, none of which, though, expressed Phox2b (Fig. 3B), in contrast to the cranial nerves that produce parasympathetic ganglia at the same stage (Fig. 3E). Deletion of all motor fibers in Olig2–/– embryos spared only two thin, presumably sensory, projections from the lumbosacral plexus (Fig. 3C), also devoid of Phox2b+ cells (Fig. 3D and fig. S10). Despite this massive atrophy, the pelvic ganglion appeared intact (Fig. 3C, fig. S10, and movie S2). This was verified quantitatively at E13.5 (Fig. 3, F and G). Thus, even though 50% of its cells are postganglionic to the pelvic nerve, the pelvic ganglion forms before and independently of it, as befits a sympathetic ganglion but contrary to parasympathetic ones.
Fig. 3. The pelvic ganglion forms independently of its nerve, like sympathetic and unlike parasympathetic ones.
(A and C). Whole-mount immunofluorescence with the indicated antibodies on E11.5 embryos either heterozygous (A) or homozygous (C) for an Olig2 null mutation. The nascent pelvic nerves [yellow arrowhead in (A)] seem to derive mostly from the L6 nerve at that stage. The Olig2 null mutation (C) spares two thin sensory pelvic projections. The pelvic ganglion (PG) lies ahead of most fibers in both heterozygous and mutant background. (B and D). View of the L6 nerve, covered with Sox10+ cells but no Phox2b+ cells (yellow arrowheads), unlike cranial nerves that give rise to parasympathetic ganglia at the same stage [Jacobson’s nerve in (E)]. (F and G) In situ hybridization for Phox2b and immunohistochemistry for neurofilament (NF) on heterozygous and homozygous Olig2 knockouts at E13.5, when parasympathetic ganglia have formed elsewhere in the body. Graph: the pelvic ganglion has the same volume whether its preganglionic nerve is present [black arrowhead in (F)] or not (6369 μm3 ± 1066 versus 6441 μm3 ± 919, P = 0.96, n = 5 embryos). gt, genital tubercle; L5 and L6, 5th and 6th lumbar roots; S1, 1st sacral root; SC, sympathetic chain.
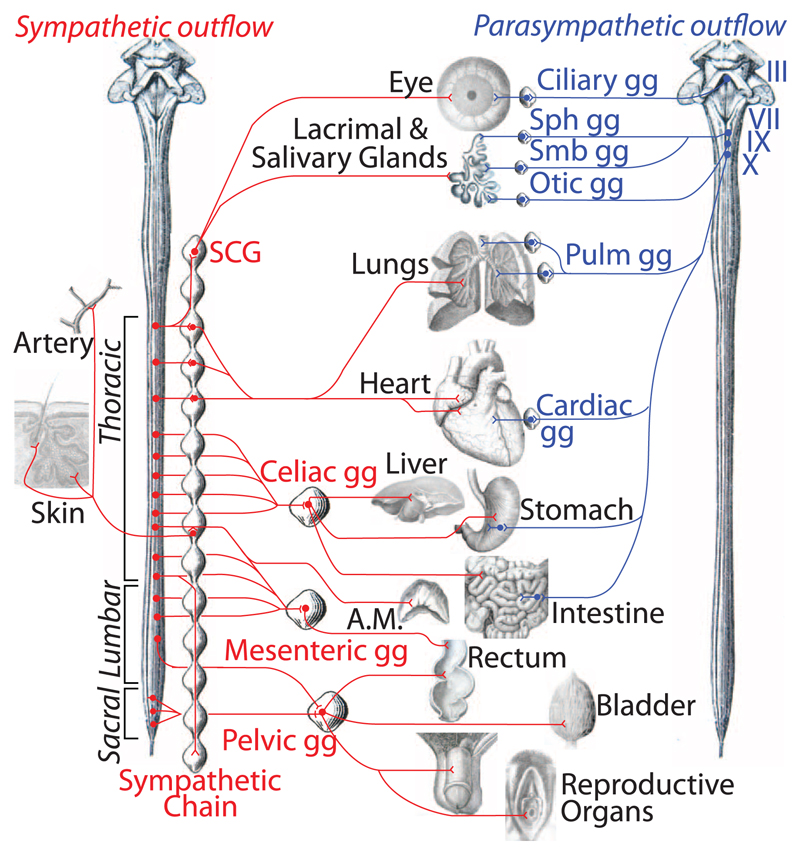
Thus, the sacral visceral nervous system is the caudal outpost of the sympathetic outflow (Fig. 4 and fig. S11), the autonomic nervous system being divided in a cranial and a spinal autonomic system, in line with certain evolutionary speculations (26). This new understanding of the anatomy accounts for many data that were at odds with the previous one. For example, although schematics generally represent the sacral pathway to the rectum as disynaptic—i.e., vagal-like—[e.g., (3)], it is in fact predominantly (27) if not exclusively (28) trisynaptic—i.e., sympathetic-like (29). Despite the dogma of lumbosacral antagonism on the bladder detrusor muscle, the lumbar inhibition is experimentally absent (4) or of dubious functional relevance (30). The synergy of the lumbar and sacral pathway for vasodilatation in external sexual organs [reviewed in (29)] shows a continuity of action—rather than antagonism, as the old model suggested—across the gap between the thoracolumbar and sacral outflows.
Fig. 4. Revised anatomy of the autonomic nervous system.
The efferent path of the autonomic nervous system is made up of a spinal sympathetic outflow (left, in red) and a cranial parasympathetic outflow (right, in blue). Sympathetic targets in the skin other than arteries are piloerector muscles and sweat glands. III, oculomotor nerve; VII, facial nerve; IX, glosso-pharyngeal nerve; X, vagus nerve; A.M., adrenal medulla; gg, ganglion; Pulm, pulmonary; SCG, superior cervical ganglion; Sph, sphenopalatine; Smb, submandibular. For a larger version, see fig. S11.
The sympathetic identity of all sacral and pelvic autonomic neurons, which our data unveil, provides a new framework for discoveries on pelvic neuroanatomy and physiology.
Supplementary Material
Acknowledgments
We thank the Imaging Facility of Institut de Biologie de l'École Normale Supérieure (IBENS), which is supported by grants from Fédération pour la Recherche sur le Cerveau, Région Ile-de-France DIM NeRF 2009 and 2011 and France-BioImaging. We thank A. Shihavuddin and A. Genovesio for help with image analysis, the animal facility of IBENS, C. Goridis for helpful comments on the manuscript, and all the members of the Brunet laboratory for discussions. This study was supported by the Centre National de la Recherche Scientifique, the Ecole Normale Supérieure, Institut National de la Santé et de la Recherche Médicale, Agence Nationale de la Recherche (ANR) award ANR-12-BSV4-0007-01 (to J.-F.B), Fondation pour la Recherche Médicale (FRM) award DEq. 2000326472 (to J.-F.B.), the Investissements d’Avenir program of the French government implemented by the ANR (referenced ANR-10-LABX-54 MEMO LIFE and ANR-11-IDEX-0001-02 Paris Sciences et Lettres Research University). I.E.-M. was supported by the French Ministry of Higher Education and Research and the FRM award FDT20160435297. Work in W.D.R.’s laboratory is supported by the European Research Council (grant agreement 293544) and Wellcome (100269/Z/12/Z). The supplementary materials contain additional data.
References and Notes
- 1.Gaskell WH. J Physiol. 1886;7:1–80.9. doi: 10.1113/jphysiol.1886.sp000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langley JN. The Autonomic Nervous System: Part I. W. Heffer; Cambridge: 1921. [Google Scholar]
- 3.Kandel E, Schwartz J, Jessell T, Siegelbaum S, Hudspeth AJ. Principles of Neural Science. Fifth Edition. McGraw-Hill Professional; 2012. [Google Scholar]
- 4.Langley JN, Anderson HK. J Physiol. 1895;19:71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briscoe J, et al. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 6.Pattyn A, Hirsch M, Goridis C, Brunet JF. Development. 2000;127:1349–1358. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- 7.Guthrie S. Nat Rev Neurosci. 2007;8:859–871. doi: 10.1038/nrn2254. [DOI] [PubMed] [Google Scholar]
- 8.Prasad A, Hollyday M. J Comp Neurol. 1991;307:237–258. doi: 10.1002/cne.903070207. [DOI] [PubMed] [Google Scholar]
- 9.Markham JA, Vaughn JE. J Neurobiol. 1991;22:811–822. doi: 10.1002/neu.480220803. [DOI] [PubMed] [Google Scholar]
- 10.Alaynick WA, Jessell TM, Pfaff SL. Cell. 2011;146:178–178.e1. doi: 10.1016/j.cell.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelps PE, Barber RP, Vaughn JE. J Comp Neurol. 1993;330:1–14. doi: 10.1002/cne.903300102. [DOI] [PubMed] [Google Scholar]
- 12.Anderson CR. Neurosci Lett. 1992;139:280–284. doi: 10.1016/0304-3940(92)90571-n. [DOI] [PubMed] [Google Scholar]
- 13.Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Keast JR. Int Rev Cytol. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- 15.Keast JR. Neuroscience. 1995;66:655–662. doi: 10.1016/0306-4522(94)00595-v. [DOI] [PubMed] [Google Scholar]
- 16.Kuntz A, Moseley RL. J Comp Neurol. 1936;64:63–75. [Google Scholar]
- 17.De Groat WC, Booth AM, Krier J. In: Integrative Functions of the Autonomic Nervous System. Brooks CM, Koizumi K, Sato A, editors. University of Tokyo Press; Tokyo: 1979. pp. 234–245. [Google Scholar]
- 18.Ernsberger U, Rohrer H. Cell Tissue Res. 1999;297:339–361. doi: 10.1007/s004410051363. [DOI] [PubMed] [Google Scholar]
- 19.Huber K, et al. Dev Biol. 2013;380:286–298. doi: 10.1016/j.ydbio.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsarovina K, et al. Development. 2004;131:4775–4786. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- 21.Doxakis E, Howard L, Rohrer H, Davies AM. EMBO Rep. 2008;9:1041–1047. doi: 10.1038/embor.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber L, Ferdin M, Holzmann J, Stubbusch J, Rohrer H. Dev Biol. 2012;363:219–233. doi: 10.1016/j.ydbio.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Yntema CL, Hammond WS. J Exp Zool. 1955;129:375–413. [Google Scholar]
- 24.Dyachuk V, et al. Science. 2014;345:82–87. doi: 10.1126/science.1253281. [DOI] [PubMed] [Google Scholar]
- 25.Espinosa-Medina I, et al. Science. 2014;345:87–90. doi: 10.1126/science.1253286. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson S. In: Autonomic Nerve Function in the Vertebrates, Zoophysiology. Farner DS, editor. Vol. 13. Springer-Verlag; New York: 1983. chap. 2. [Google Scholar]
- 27.Olsson C, et al. J Comp Neurol. 2006;496:787–801. doi: 10.1002/cne.20965. [DOI] [PubMed] [Google Scholar]
- 28.Fukai K, Fukuda H. J Physiol. 1985;362:69–78. doi: 10.1113/jphysiol.1985.sp015663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jänig W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostatis. Cambridge Univ. Press; Cambridge, UK: 2006. [Google Scholar]
- 30.de Groat WC, Saum WR. J Physiol. 1972;220:297–314. doi: 10.1113/jphysiol.1972.sp009708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.