Adjuvants promote the primary T cell response as well as the conversion of T cells from naive to memory. In this issue of Immunity, Sawa et al. (2009) show that a number of adjuvants that act via Toll-like receptors (TLRs) can induce production of hepatocyte IL-7. The study mainly focuses on lipopolysaccharide (LPS) but also shows that the liver produces IL-7 in response to other TLR stimuli, including CpG, poly(I-C), imiquimod, zymosan, and Complete Freund’s adjuvant (CFA).
IL-7 is required for survival of most T cell subsets, and its expression has been proposed to be important for regulating T cell numbers. Production of IL-7 by stromal cells has previously been thought to be constitutive, and therefore consumption of IL-7 by T cells via their IL-7 receptors has been proposed to play an important role in T cell homeostasis (Mazzucchelli and Durum, 2007). Although this constitutive IL-7 production model remains tenable in lymphoid organs, Sawa et al. now show that hepatocytes can produce immunologically stimulatory amounts of IL-7 in response to adjuvants that act via TLRs (Figure 1). IL-7 has therefore become, in a sense, one of the acute-phase reactants. The hepatocyte response is distinct from spleen and lymph node stromal cells, which did not upregulate IL-7 in response to TLR ligands.
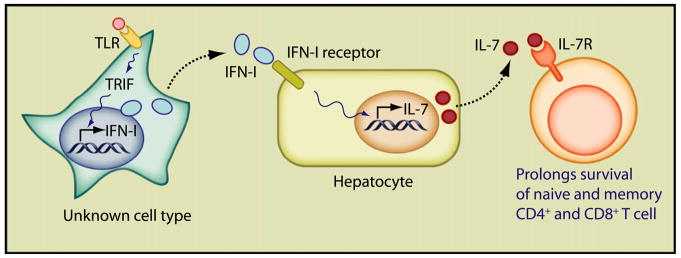
Figure 1. Triggering of Several Different TLRs in an Unknown Cell Type Induces Type I IFN Expression via the TRIF Signaling Pathway.
Type I IFN acts on hepatocytes, inducing IL-7 expression and release. IL-7 then prolongs the survival of naive and memory T cells.
Proof that the liver was the IL-7 source primarily relied on clever application of hydrodynamic DNA injection, which is selectively taken up by hepatocytes. Sawa et al. used this method to introduce interfering RNA for IL-7, which in turn blocked a number of TLR-mediated effects on T cells, demonstrating that liver was the major IL-7 source. TLR effects mediated by hepatocyte IL-7 included enhanced CD4+ and CD8+ T cell survival (Figure 1), augmented CTL activity, and experimental autoimmune encephalomyelitis (EAE) via promotion of a Th17 response. In the absence of TLR ligands, the liver did not appear to be a major contributor of IL-7 to T cell survival; under normal conditions, IL-7 is thought to be provided by stromal cells in lymphoid organs, although this remains to be rigorously proven.
The hepatocyte response was shown to be indirect (Figure 1). An intermediate, undefined cell responded to TLR ligands and, via the Toll/interleukin-1R homologous-domain-containing adaptor protein inducing interferon-beta (TRIF) signaling pathway, induced synthesis of type I interferon, which in turn acted on hepatocytes eliciting IL-7 production. IL-7 induction had been reported to be driven in vitro by IFN-γ (Ariizumi et al., 1995; Oshima et al., 2004) and tumor necrosis factor (TNF-α) (Weitzmann et al., 2000) and in vivo by keratinocyte growth factor (KGF) (Min et al., 2002) or IL-6 (Sawa et al., 2006). Although IFN-γ induces IL-7 production from thymic cell lines, from our experience it does not induce it in the liver, and therefore the effect is apparently restricted to type I IFNs, i.e., the innate rather than the adaptive immune response.
It was not determined where T cells encountered this hepatic IL-7 elicited by adjuvants. The IL-7 effects included increased T cell numbers in spleen and lymph node, as well as liver. It is possible that T cells circulate through liver and encounter IL-7 near the hepatocyte, in paracrine fashion. It seems less likely that hepatic IL-7 acted at a distance from the liver: the serum amounts reported in the study, although elevated, appear too low to have a biological effect. In addition, it has not been determined where T cells encounter IL-7 under normal circumstances, and this is an important issue that remains to be addressed.
The increase in T cells was not accompanied by increased BrdU incorporation, suggesting that IL-7 did not increase T cell proliferation, which in their study was already quite vigorous in some T cell subsets. Thus, T cells proliferate and die under normal conditions, and the TLR-hepatic-IL-7 effect is to protect from cell death. In contrast, pharmacological doses of IL-7 can induce substantial cell division. This difference probably reflects the concentration of IL-7; low IL-7 amounts, as in the adjuvant effect, promote survival but not cell division, whereas high doses induce both survival and cell cycling.
The hepaticIL-7increase did not enhance primary T cell responses to antigen. This is consistent with most previous studies, which show little effect of IL-7 on primary responses, in part because of the loss of IL-7 receptor expression on activated T cells. However, hepatic IL-7 was shown to prolong the CTL and IFN-γ responses promoted by TLR ligands, perhaps because IL-7 receptor reappears on memory T cells.
The reported liver response to LPS was transient. Il7 mRNA message peaked at 3 hr and was back to baseline by 6 hr; the numbers of T cells peaked on day 3 and returned to normal levels at day 6. Despite the transience of LPS-induced IL-7, a long-lasting effect was observed in the EAE model, in which hepatic IL-7 exacerbated disease weeks later. There could be several explanations for an enduring effect of IL-7 in EAE. One possibility is that CFA and pertussis toxin are used in EAE, rather than LPS, and perhaps they elicit a more durable hepatic IL-7 expression. Another intriguing possibility is that there is something peculiarly autoreactive about the T cells that expand in the presence of IL-7. Perhaps the cells that liver IL-7 promotes are slightly more self reactive. It has often been noted that lymphopenia promotes autoimmunity, and in lymphopenia, IL-7 would be more abundant. Fortunately, patients treated with IL-7 have not, as yet, shown autoimmune sequelae, and clinicians are very cognizant of this possible risk. However, previous studies have connected IL-7 with EAE (Bebo et al., 2000) and multiple sclerosis (Traggiai et al., 2001).
The IL-7 protein cannot be visualized by immunohistochemistry in tissues because protein expression is too low. For this reason, there remain many fundamental questions, including those about the sites in which T cells encounter IL-7, as well as regulation of protein amounts. We do not know whether mRNA expression is the only mechanism controlling the protein amounts. There could be constraints on translation as there are for IL-15. We also do not know what becomes of IL-7 protein after it is produced in tissues. Although a small protein such as IL-7 would have a very short half-life in serum, it is possible that IL-7 in tissues associates with extra-cellular matrix and could be available for weeks—this is another potential explanation of how a transient increase in IL-7 transcripts could generate a sustained supply of protein. Another unrelated but vexing question is why IL-7 antibodies have so little effect in vivo, whereas dramatic effects are shown by transfer of T cells into Il7−/− recipients. Fresh approaches are needed to probe this essential protein.
Onto the playing field of adjuvanticity and autoimmunity, the study by Sawa et al. introduces important new participants: IFNs, hepatocytes, and IL-7. It is the game plan that remains elusive.
References
- Ariizumi K, Meng Y, Bergstresser PR, Takashima A. J Immunol. 1995;154:6031–6039. [PubMed] [Google Scholar]
- Bebo BF, Jr, Schuster JC, Adlard K, Vandenbark AA, Offner H. Cytokine. 2000;12:324–331. doi: 10.1006/cyto.1999.0564. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli R, Durum SK. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, Lacey DL, Blazar BR, Weinberg KI. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- Oshima S, Nakamura T, Namiki S, Okada E, Tsuchiya K, Okamoto R, Yamazaki M, Yokota T, Aida M, Yamaguchi Y, et al. Mol Cell Biol. 2004;24:6298–6310. doi: 10.1128/MCB.24.14.6298-6310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S, Kamimura D, Jin GH, Morikawa H, Kamon H, Nishihara M, Ishihara K, Murakami M, Hirano T. J Exp Med. 2006;203:1459–1470. doi: 10.1084/jem.20052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T, Kamimura D, Hirano T, Murakami M. Immunity . 2009;30:447–457. doi: 10.1016/j.immuni.2009.01.007. this issue. [DOI] [PubMed] [Google Scholar]
- Traggiai E, Biagioli T, Rosati E, Ballerini C, Mazzanti B, Ben NA, Massacesi L, Vergelli M. J Neuroimmunol. 2001;121:111–119. doi: 10.1016/s0165-5728(01)00433-7. [DOI] [PubMed] [Google Scholar]
- Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Blood. 2000;96:1873–1878. [PubMed] [Google Scholar]