Abstract
Androgens play a vital role in prostate cancer development, and their elimination and blockade are essential in the disease management. DHT is the key ligand for androgen receptor (AR) in the prostate. It is locally synthesized from testosterone. In the prostate, DHT is predominantly metabolized to α-diol and β-diol. Recent studies indicate that impaired DHT catabolism is associated with prostate cancer, signifying the necessity of a sensitive quantitative method for the determination of DHT and its metabolites.
In this work, an LC-MS/MS method for the simultaneous quantification of DHT and its metabolites was developed and validated. Steroid-free sera were prepared and used for the preparation of sera calibrators and quality controls (QCs). DHT and its metabolites along with their respective stable heavy isotope labeled analytes representing internal standards were first extracted with methyl tertiary-butyl ether (MTBE) and derivatized with picolinic acid (PA). The derivatized analytes were then extracted again with MTBE, dried under nitrogen and reconstituted in the mobile phase (80% methanol and 0.2% formic acid in water). Baseline chromatographic separation of the derivatized analytes was achieved isocratically on XTerra C18 column (2.1 × 100 mm) using the mobile phase at a flow rate of 0.25 mL/min. Quantitation was performed using multiple-reaction-monitoring mode with positive electrospray ionization. The method has calibration ranges from 0.0500 ng/mL to 50.0 ng/mL for DHT and its two metabolites with acceptable assay precision, accuracy, recovery, and matrix factor. It was applied to the determination of DHT and its metabolites in an animal study.
Keywords: Androgens, LC-MS/MS, Picolinic acid, Prostate cancer, Method validation
1. Introduction
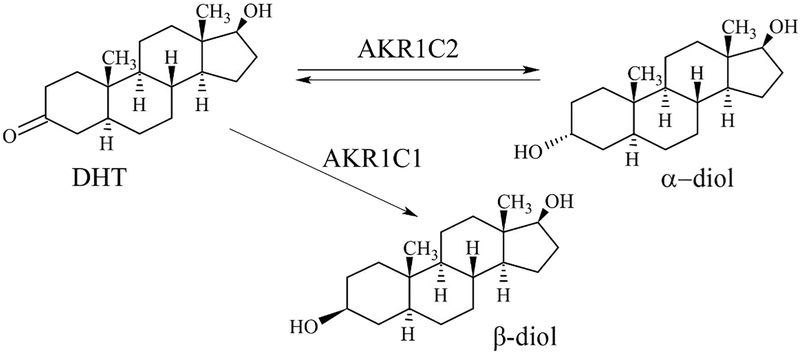
5α-dihydrotestosterone (DHT), also known as 5α-androstan-17β-ol-3-one, is an active androgenic hormone which is synthesized from testosterone by the enzyme 5α-reductase in the prostate gland, testes, hair follicles, and adrenal glands [1–3]. Modification of DHT at 17β-hydroxy or 3-keto position by the enzymes renders its inactivation. The enzymes involved in DHT metabolism are 3α-hydroxysteroid dehydrogenase (3α-HSD) and 3β-hydroxysteroid dehydrogenase (3β-HSD). 3α-HSD enzyme converts DHT to 5α-androstane-3α,17β-diol (α-diol) whereas 3β-HSD converts it to 5α-androstane-3β,17β-diol (β-diol) [4–7] as depicted in Fig. 1.
Fig. 1.
Metabolism of DHT depicting the formation of α-diol, and β-diol by the action of enzymes coded by the genes AKR1C1, and AKR1C2.
Prostate cancer is the third leading cause of cancer death for men in the United States [8]. There have been reports on the importance of DHT catabolism in the prostate and its role in regulating the intracellular pool of this androgen [9–14]. Studies suggest that there are selective losses of aldo-keto reductase family 1 member C1 (AKR1C1) and aldo-keto reductase family 1 member C2 (AKR1C2) genes which encode the enzymes (3α-HSD and 3β-HSD) in prostate cancer, indicating impaired DHT catabolism [15,16]. Studies have also shown that the changes in DHT metabolism are associated with the development of benign prostate hyperplasia [34], and the role of DHT meta-bolites in castration resistance in PC [12]. Therefore, simultaneous quantitative determination of DHT and its metabolites may contribute to the management of prostate cancer progression.
There are some quantitative techniques currently available for the determination of DHT including immunoassay, gas chromatography–mass spectrometry (GC–MS), and liquid chromatography-tandem mass spectrometry (LC-MS/MS). However, the commonly used immunoassay fails to exhibit accuracy and precision, especially at lower analyte concentrations, and the specificity of the assay is often compromised by non-specific adsorption of antibodies [17–20]. The GC–MS method usually employs chemical derivatization to form volatile derivatives of the analytes. Though the method is specific and sensitive, it is often associated with the thermal decomposition of the analyte derivatives and requires a large amount of serum (> 1 mL) [21–23]. Recently, several LC-MS/MS methods have been developed for the steroids, which use solid-phase extraction for sample extraction and gradient elution for separation of analytes from matrix interferences. Gradient elution often extends the run-time per sample since the column must be re-equilibrated before the subsequent analysis and solid-phase extraction is not cost-effective for routine analysis. Moreover, these LC-MS/MS methods use surrogate matrices (a mixture of aqueous and organic solutions) for the preparation of calibration standards and quality controls, which have significant differences in composition compared to the authentic matrices (e.g., serum, plasma, and tissue) [24–27]. There are a couple of LC-MS/MS methods reported for the quantitative determination of DHT and its metabolites using PA as derivatization reagent [35,36]. However, these methods used solid-phase extraction for the sample preparation, and gradient mode of elution with longer run time which significantly increases the time and cost incurred for sample analysis. Furthermore, none of the LC-MS methods developed so far for the quantitative determination of DHT metabolites are fully validated.
In this paper, we describe the development and validation of a specific and sensitive LC-MS/MS method for the simultaneous quantitation of DHT and its metabolites in mouse sera. This is the first fully validated quantitative method for α-diol and β-diol, which are indicators of hydroxysteroid dehydrogenase activities. This method uses a steroid-free sera matrix prepared by stripping the steroid with activated charcoal for the preparation of calibration standards and QCs, a liquid-liquid extraction (LLE) procedure for analyte extraction, and a reversed-phase C18 chromatographic column for separation. The lower limits of quantitation (LLOQ) are achieved by derivatization of analytes with picolinic acid. The separation of the derivatized analytes from the un-reacted derivatizing reagent and matrix interferences was achieved by isocratic elution. The method developed was applied for the quantitative determination of DHT, α-diol, and β-diol in mouse sera from an animal study focused on DHT catabolism in prostate cancer.
2. Experimental
2.1. Chemicals and materials
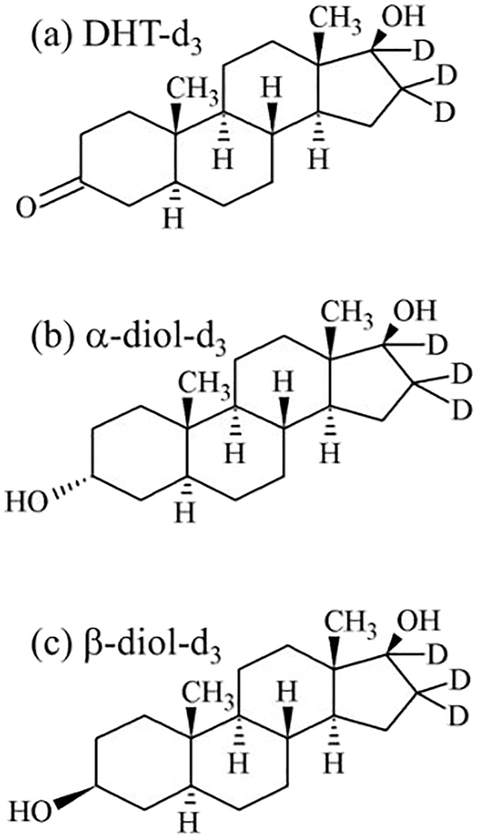
Certified reference materials of 5α-dihydrotestosterone (DHT, 1.00 mg/mL in methanol), 5α-dihydrotestosterone-16,16,17-d3 (DHT-d3, 100 μg/mL in methanol), 5α-androstane-3α,17β-diol (α-diol, 1.00 mg of lyophilized powder), 5α-androstan-3α,17β-diol-16,16,17-d3 (α-diol-d3, 1.00 mg of lyophilized powder), 5α-androstan-3β,17β-diol (β-diol, 1.00 mg of lyophilized powder), and 5α-androstan-3β,17β-diol- 16,16,17-d3 (β-diol-d3, 1.00 mg of lyophilized powder) were purchased from Cerilliant Corporation (Round Rock, Texas, USA) (Fig. 2). ACS-reagent-grade formic acid, activated charcoal, triethylamine (TEA), tetrahydrofuran (THF), 2-picolinic acid (PA), 4-(dimethylamino) pyridine (DMAP), 2-methyl-6-nitrobenzoic anhydride (MNBA), and 2-fluoro-1-methylpyridine (FMP) were purchased from Sigma-Aldrich (St. Louis, MO, USA). LC/MS-grade methanol, LC/MS-grade acetonitrile, HPLC-grade water, methyl-tertiary-butyl ether (MTBE), ethyl acetate, and hexane were purchased from Fisher Scientific (Pittsburgh, PA, USA). Six pooled blank mouse sera with specified lot numbers (IR12–061102, IR12–061101, IR12–9010, 10698, 10193, 10832) were purchased from Innovative Research (Novi, MI, USA).
Fig. 2.
Structures of internal standards. (a) DHT-d3; (b) α-diol-d3; (c) β-diol-d3.
2.2. Solutions
2.2.1. Stock and standard solutions
The certified solutions of DHT and DHT-d3 were used as stock solutions. The stock solutions of α-diol, β-diol, α-diol-d3, and β-diol-d3 were prepared at the concentration of 1.00 mg/mL each by dissolving the appropriate amount of each compound in a known volume of methanol. The working stock solutions of DHT, α-diol, β-diol, DHT-d3, α-diol-d3, and β-diol-d3 were prepared at the concentration of 10.0 μg/mL each by serial dilution of each stock solution with methanol. All the stocks and working stock solutions were kept in glass vials with caps and stored at −70 °C before use.
The solvent mixed standard working solutions containing DHT, α-diol, and β-diol at the concentrations of 1.00, 2.00, 3.00, 5.00, 10.0,20.0, 50.0, 100, 200, 800, 1.00 × 103 ng/mL were prepared by mixing appropriate amount of each working stock solution in a known volume of methanol. The solvent mixed internal standard working solution containing DHT-d3, α-diol-d3, and β-diol-d3 was prepared at a concentration of 80.0 ng/mL by mixing the appropriate amount of each working stock solution in a known volume of methanol.
2.2.2. Preparation of steroid-free sera
Eight grams of activated charcoal was added to 100 mL of pooled mouse sera in a 250 mL Pyrex Erlenmeyer flask and agitated overnight using Fisher Scientific Isotemp™ stirring hotplate with a magnetic stir bar in a cold room maintained at 4 °C. After agitation, the sera along with the activated charcoal was transferred to Fisher borosilicate glass tubes (16.0 × 100 mm) and was subjected to centrifugation by a Sorvall ST 40R centrifuge from Thermo Scientific (Waltham, MA, USA) at 2150 ×g at 4 °C for 30 min. Then the supernatant was transferred to another borosilicate glass tube and centrifuged again at 9000 ×g at 4 °C for 60 min. Finally, the supernatant from the second centrifugation was collected as steroid-free pooled mouse sera and stored in two sterile50.0 mL clear polypropylene (PP) conical bottom centrifuge tubes from Corning Inc. (Corning, NY, USA) and stored at −20 °C before use [28].
2.2.3. Sera calibrators and sera QCs
Sera calibrators at the concentrations of 0.0500, 0.100, 0.250, 0.500, 1.00, 5.00, 10.0, 50.0 ng/mL and QCs at the concentrations of0.150, 2.50, 40.0 ng/mL were prepared at room temperature by adding10.0 μL of their respective solvent mixed standard working solutions to 190 μL of steroid-free mouse sera in individual borosilicate glass tubes(13.0 × 100 mm). Independent solvent mixed standard working solutions were used for the preparation of all calibrators. The solvent mixed standard working solutions for calibrators and QCs were prepared from two independent certified stock solutions. The tubes were then vortexed for 5 s using MaxiMix II vortex mixer from Thermo Scientific and stored at −70 °C.
2.2.4. Preparation of derivatization reagent
The derivatization reagent solution was prepared by dissolving 10.0 mg of DMAP (0.082 mM), 20.0 mg of MNBA (0.058 mM), and 25.0 mg of PA (0.200 mM) in 1.00 mL of THF [29]. The chemicals were weighed using XS205 model analytical balance from Mettler Toledo (Columbus, Ohio, USA). The mixture solution was vortexed for 1 min for complete mixing. It was then left at room temperature for 10 min before use.
2.2.5. Mobile phase
The mobile phase was prepared by mixing 400 mL of methanol to 100 mL of water and 1.00 mL formic acid.
2.3. Sample preparation
2.3.1. Serum sample preparation
Two hundred microlitres aliquot of each mouse serum sample were mixed with 10.0 μL of solvent mixed internal standard working solution in borosilicate glass tube (13.0 × 100 mm). All sample tubes were vortexed for 5 s using Talboys Standard Analog Multi-Tube Vortex Mixer from Troemner Precision Weights and Laboratory Equipment (Thorofare, NJ, USA). All the sera calibrators, QCs, and mouse serum samples were allowed to stand at room temperature for 60 min after the addition of IS and before proceeding with further sample preparation. After 60 min, they were subjected to LLE by adding 2.00 mL of MTBE. Then the tubes were vortexed for 2 min, and the top layer of organic content was transferred to another borosilicate glass tube. The extraction was repeated once, and the organic layers were combined. The combined organic contents were dried in a TurboVap LV evaporator from Caliper Life Sciences (Hopkinton, MA, USA) under nitrogen gas pressure of 15 psi at 30 °C for 15 min.
2.3.2. Chemical derivatization of DHT and metabolites
To the dried extraction residue from the Section 2.3.1, 100 μL of the derivatization reagent and 100 μL of TEA were added. The tubes were vortexed for 5 s, and the reaction was allowed to proceed at room temperature for 30 min. After 30 min, 1.00 mL of 10% acetic acid aqueous solution (v/v) was added and vortexed for 5 s. This step was followed by LLE by adding 2.00 mL of MTBE and vortexed for 2 min, and the top layer of organic content was transferred to a borosilicate glass tube (12.0 × 75.0 mm). The extraction was repeated once, and the organic layers were combined. The tubes were dried in the evaporator at 30 °C under nitrogen gas pressure of 15 psi for 15 min. The residue was reconstituted in 100 μL of mobile phase for LC-MS/MS analysis.
2.3.3. Serum recovery study
Two hundred microlitres of the pooled mouse sera was aliquoted into five individual borosilicate glass tubes (13.0 × 100 mm) and 10.0 μL of solvent mixed internal standard working solution was added to each tube and vortexed for 5 s. They were allowed to stand for 0 min, 30 min, 60 min, 120 min, and 240 min on bench-top before subjecting to further sample preparation steps so that each tube corresponds to a time point. These samples processed at different time points were subjected to LC-MS/MS analysis.
2.4. LC-MS system
The LCMS system consisted of a LC-20AD HPLC unit with SIL-20AC autosampler from Shimadzu Scientific Instruments (Columbia, MD, USA) with XTerra MS C18 precolumn (2.10 mm × 10.0 mm, 3.50 μm) and XTerra MS C18 column (2.1 mm × 100 mm, 3.5 μm) from Waters Corporation (Milford, MA, USA); and the mass spectrometer used for this work is an AB Sciex API 3200 turbo-ion-spray triple quadrupole tandem mass spectrometer from AB Sciex LLC (Foster City, CA, USA). The LC-MS interface was controlled by AB Sciex Analyst (version 1.5.1) software. Analytical separation of the derivatized analytes was achieved on the column by isocratic elution with the mobile phase at a flow rate of 0.250 mL/min for 14 min. During each run, 40.0 μL of the reconstituted sample was injected into the system by the autosampler, which was maintained at 4 °C. Before initial sample analysis, the column was equilibrated with the mobile phase at the above flow rate for about 30 min.
The mass spectrometer was operated in the positive turbo-ion-spray ionization mode. The molecular ions and its fragmentation of the individual derivatized standards and the derivatized internal standards were determined by the direct infusion at individual concentrations of 500 ng/mL. The compound-dependent (i.e., declustering potential, entrance potential, collision energy, collision exit potential) and the source-dependent (i.e., curtain gas, collision assisted dissociation gas, ionization voltage, source temperature, sheath gas, desolvation gas) parameters were optimized by flow injection analysis of the derivatized analytes at individual concentrations of 500 ng/mL. The mass transitions used for multiple reaction monitoring (MRM) were m/z 396.3 > 255.3 for DHT, m/z 398.2 > 257.3 for α-diol, m/z 503.1 > 257.3 for β-diol, m/z 399.3 > 258.3 for DHT-d3, m/z 401.3 > 260.3 for α-diol-d3, and m/z 506.3 > 260.3 for β-diol-d3. The optimized instrument settings are as follows: declustering potential at 48 V, entrance potential at 5 V, collision energy at 28 psi, collision exit potential at 4 V, curtain gas at 20 psi, collision assisted dissociation gas at 3 psi, ionization voltage at 5500 V, source temperature at 500 °C, sheath gas at 60 psi, desolvation gas at 60 psi, and the quadrupoles were set at unit resolution. The switch valve on the mass spectrometer was programmed to divert the column eluate to waste for the first 3 min, then switch to the mass spectrometer for the next 10.5 min and finally return to waste at the end of each sample run.
2.5. Method validation
2.5.1. Assay selectivity and LLOQ
In order to examine the interfering compounds at the retention times and mass transitions of each standard and internal standard, six lots of steroid-free sera (190 μL of steroid-free sera from each lot) were spiked with standards (10.0 μL of 1.00 ng/mL solvent mixed standard working solution) and internal standards (10.0 μL of 80.0 ng/mL solvent mixed internal standard working solution). Another set of samples without analytes were prepared from the same six lots of steroid-free sera (190 μL of steroid-free sera from each lot) and was mixed with an analyte-free solvent (20.0 μL of methanol). Then, all the samples were subjected to extraction, PA derivatization, and final extraction before the LC-MS/MS analysis as described in Sections 2.3.1, and 2.3.2.
LLOQs were determined by analyzing five replicates of six calibrators at LLOQ concentrations of all the analytes on one of the validation days.
2.5.2. Linearity of calibration curve
The calibration curve gives the concentration-response relationship. The calibrators for DHT, α-diol, and β-diol were prepared in steroid-free sera with a double blank (contains neither standards nor internal standards), a blank (contains only internal standards) and eight nonzero calibrators at the concentrations of 0.0500, 0.100, 0.250, 0.500,1.00, 5.00, 10.0, 50.0 ng/mL. The calibration curves for DHT or α-diol or β-diol were obtained by assigning the respective concentrations of DHT or α-diol or β-diol to the x-axis, and the peak area ratio of the respective PA derivatives of DHT or α-diol or β-diol to that of the corresponding IS to the y-axis. Subsequently, a 1/x2 weighting linear regression was performed for constructing the calibration curves.
2.5.3. Accuracy and precision
Accuracy was expressed as percent error (%RE) and precision as the coefficient of variation (CV). In this work, the intra-assay accuracy and precision were assessed by five replicate analyses of each QC sample in the same validation batch. The inter-assay accuracy and precision were evaluated by five parallel analyses of five identical QC samples at each of the three QC concentrations (0.150, 2.50, 40.0 ng/mL) over five validation batches.
2.5.4. Recovery and matrix factor (MF)
The absolute recovery of DHT, α-diol, or β-diol (or their respective IS) was determined by the mean peak area of DHT or α-diol or β-diol (or their respective IS) at a specific concentration in steroid-free sera matrix over the mean peak area of DHT, α-diol, or β-diol (or their respective IS) at the same concentration in the extracted steroid-free sera matrix multiplied by 100%. In other words, the recovery is calculated by the peak area of analyte (or respective IS) derived from spiking into the matrix, and spiking into the post-extraction matrix. The IS normalized recovery was determined by the absolute recovery of DHT, α-diol, or β-diol over that of the corresponding IS multiplied by 100%. For this study, DHT, α-diol, or β-diol QCs at three concentrations (0.150,2.50, 40.0 ng/mL) with a fixed concentration of their respective IS(4.00 ng/mL) were prepared in the steroid-free sera matrix and extracted steroid-free sera matrix.
The absolute MF of DHT, α-diol, or β-diol (or their respective IS) was determined by the mean peak area of DHT, α-diol, or β-diol (or their respective IS) at a specified concentration in the extracted steroid-free sera matrix over that of DHT, α-diol, or β-diol (or their respective IS) at the same concentration in the solvent matrix. In other words, MF is calculated by the peak area of analyte (or respective IS) derived from spiking into the post-extraction matrix, and the spiking into the solvent matrix. The IS normalized MF was determined by the absolute MF of DHT, α-diol, or β-diol over that of the corresponding IS. For this study, DHT, α-diol, or β-diol QCs at three concentrations (0.150, 2.50,40.0 ng/mL) with a fixed concentration of their respective IS (4.00 ng/mL) were prepared in six batches of steroid-free sera matrices and the reconstitution solution.
2.5.5. Stability studies
The stabilities of DHT, α-diol, and β-diol were investigated using the stock solution (i.e., 1.00 mg/mL); and low, and high QCs (0.150 and40.0 ng/mL) in steroid-free sera.
For stability study, the stock solution was placed on bench top at 23 °C for 6 and 24 h before dilution to 0.150 and 40.0 ng/mL. The QCs were placed on bench-top at 23 °C and in the autosampler at 4 °C (post-preparative) for 6 and 24 h. The QCs were also subjected to three freeze-and-thaw cycles where the samples were frozen at −20 °C for at least 24 h and then thawed at room temperature of 23 °C unassisted. For long-term storage study, the samples were stored at −70 °C for 40 days. For all the above stability studies, the solvent mixed internal standard working solution (80.0 ng/mL) was freshly prepared and added to each sample before sample extraction. The stabilities of DHT, α-diol, and β-diol were determined from five replicates by comparing the mean-peak-area ratios of the standards to their corresponding IS in the test samples to those of the freshly prepared samples, expressed as recovery percentages.
2.6. Method application
The LC-MS/MS method developed was applied to the measurement of DHT, α-diol, and β-diol concentrations in the animal study that was approved by the Case Western Reserve University Institutional Animal Care and Use Committee (IACUC). Blood was collected from 8-week old Ubc-Cre/HEXIM1fl/fl mice (created by mating floxed HEXIM1 mice with Ubc-Cre/ERT2 mice) via cardiac puncture using a 1.00 mL syringe and22.0 gauge needle from Becton, Dickinson and Company (Franklin Lakes, NJ, USA). The whole blood was transferred to a polypropylene microcentrifuge tube from VWR International (Radnor, PA, USA) and was allowed to clot by leaving it undisturbed at room temperature for 15–30 min. Then the clot was removed by centrifuging at 1000–2000 ×g at 4 °C for 5 min using an Eppendorf Microcentrifuge 5415C from Thermo Scientific (Waltham, MA, USA). Following centrifugation, the liquid component (serum) was immediately pipetted into a clean microcentrifuge tube and stored at −70 °C.
3. Results
3.1. Assay development
3.1.1. Chemical derivatization
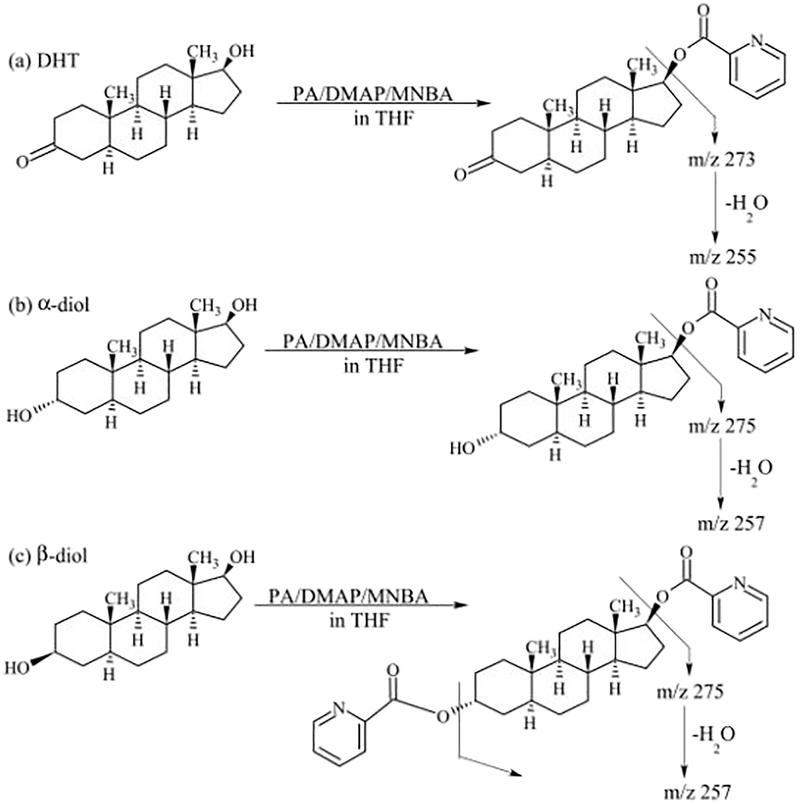
DHT and its metabolites were successfully derivatized using PA as the derivatization agent. Fig. 3 shows the typical reactions depicting the derivatization of DHT, α-diol, and β-diol. We choose the single hydroxyl group derivatized derivatives for α-diol, and double hydroxyl groups derivatized derivatives for β-diol for the subsequent study.
Fig. 3.
Derivatization of analytes with picolinic acid. (a) reaction with DHT; (b) reaction with α-diol; (c) reaction with β-diol.
3.1.2. Serum recovery study
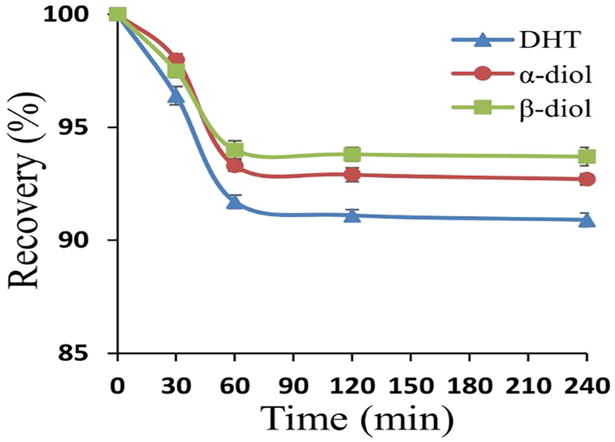
This study showed that the recoveries of stable isotope labeled analytes (i.e., DHT-d3, α-diol-d3, and β-diol-d3) decreased with time and reached a steady state at 60 min as shown in Fig. 4. This experimental outcome was applied to the consequent study.
Fig. 4.
Binding of serum protein to stable isotope labeled analytes, i.e., DHT-d3; α-diol-d3; β-diol-d3. Reaction condition is described in the Section 2.3.3. The concentration of stable isotope labeled analyte is 4.00 ng/mL.
3.1.3. MS detection
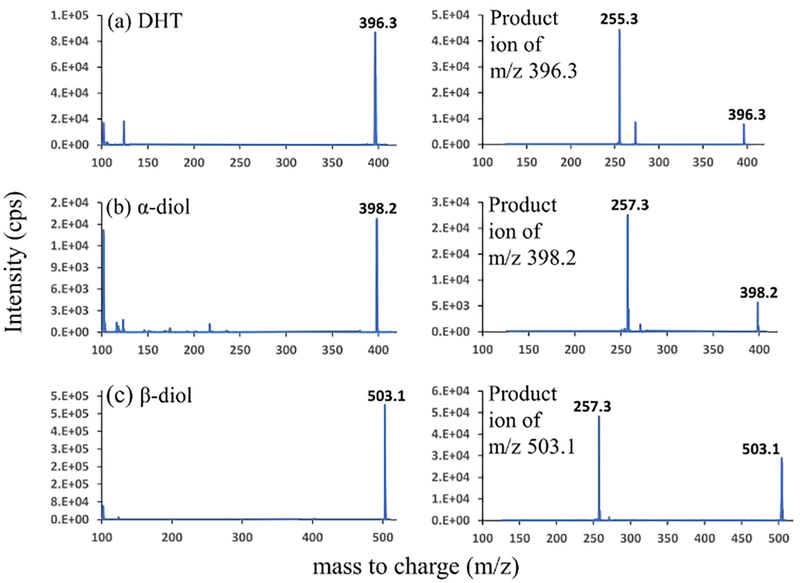
Fig. 5 shows the mass spectra of the PA derivatized DHT, α-diol, and β-diol. The precursor ions were m/z 396.3, m/z 398.2, m/z 503.1 for DHT, α-diol, and β-diol respectively. The fragmentation of the respective precursor ions produced m/z 255.3 for DHT, m/z 257.3 for α-diol, and β-diol as major product ions. Based on this experimental outcome, the mass transitions used for multiple reaction monitoring (MRM) were m/z 396.3 > 255.3 for DHT, m/z 398.2 > 257.3 for α-diol, m/z 503.1 > 257.3 for β-diol.
Fig. 5.
Positive-ESI-mass spectra and product ion spectra of picolinyl derivatives of (a) DHT; (b) α-diol; (c) β-diol at an individual concentration of 500 ng/mL. The condition is described in Section 2.3.
3.1.4. LC separation
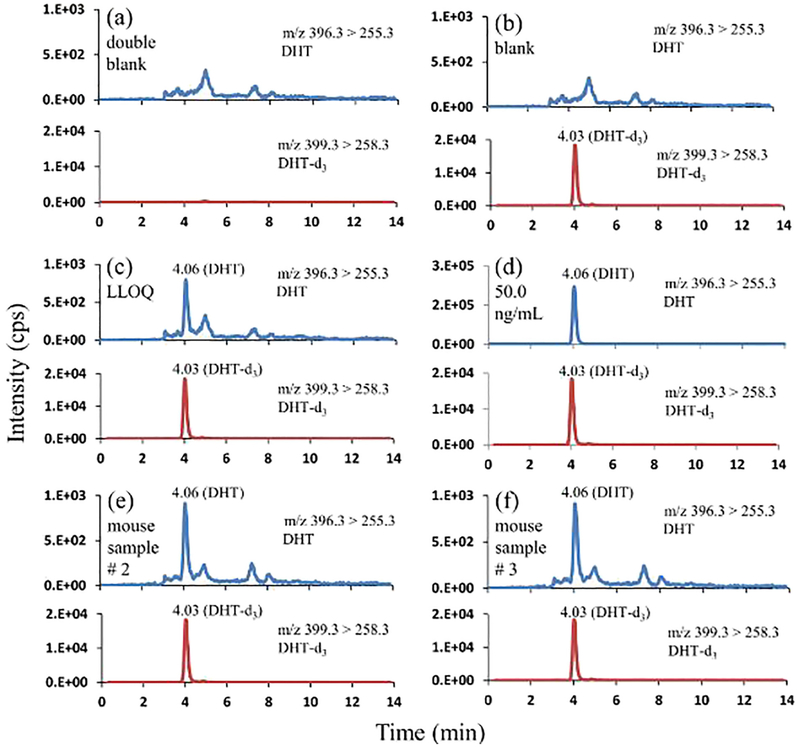
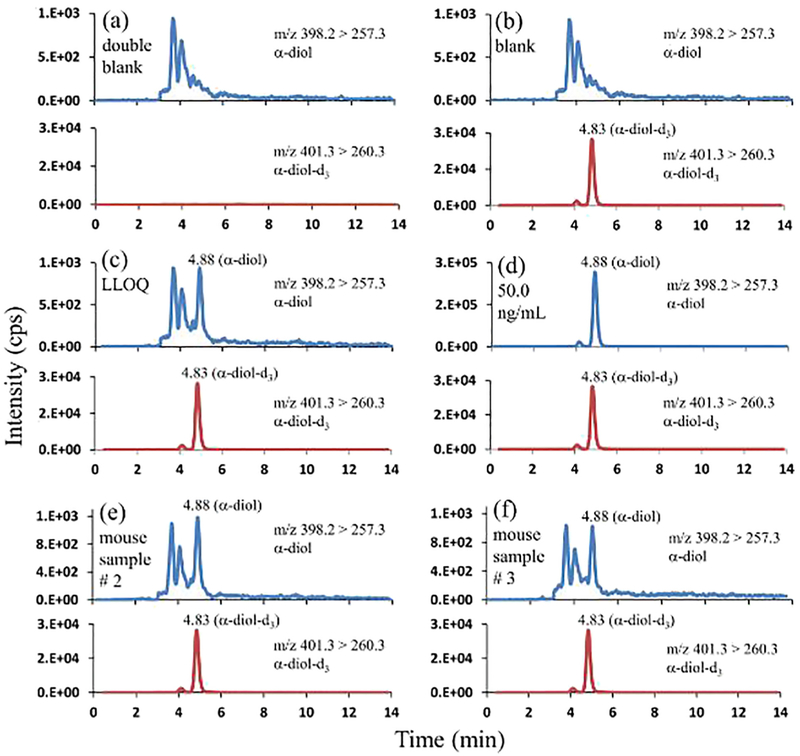
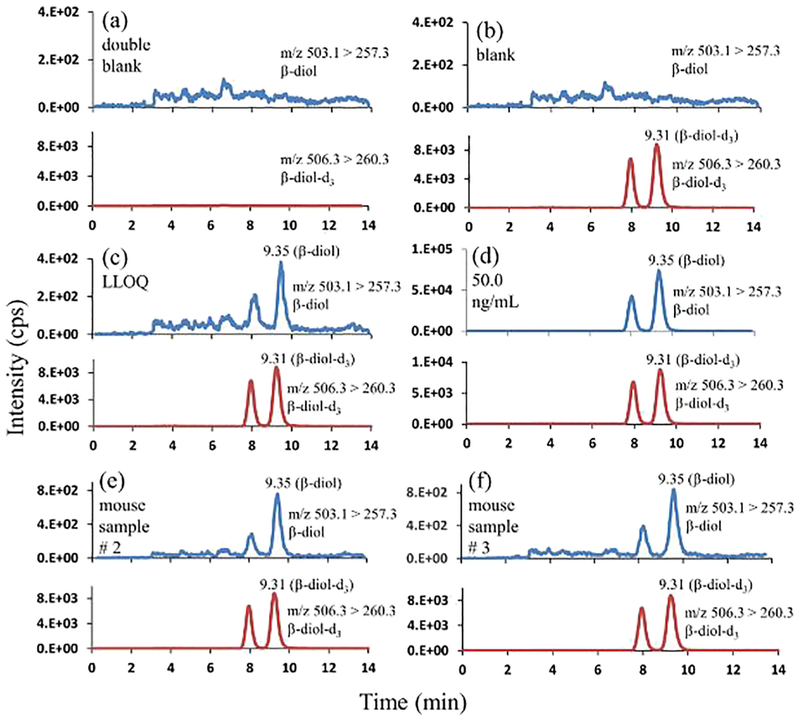
Baseline separation between the analytes of interest was achieved in isocratic mode of elution using 80% methanol and 0.2% formic acid in water as the mobile phase. Figs. 6, 7, and 8 represent the chromatographic separation of the DHT, α-diol, and β-diol with the retention times of 4.06 min, 4.88 min, and 9.35 min respectively.
Fig. 6.
Representative mass chromatograms of DHT and DHT-d3 (a) in double blank pooled sera (with neither DHT nor DHT-d3); (b) in blank pooled sera (with DHT-d3 only, 4.00 ng/mL); (c) in pooled sera at LLOQ (0.0500 ng/mL; DHT-d3, 4.00 ng/mL); (d) in pooled sera at 50.0 ng/mL DHT, and 4.00 ng/mL DHT-d3; (e) in mouse serum sample # 2 at 0.0700 ng/mL DHT, and 4.00 ng/mL DHT-d3; (f) in mouse serum sample # 3 at 0.0600 ng/mL DHT, and 4.00 ng/mL DHT-d3.
Fig. 7.
Representative mass chromatograms of α-diol, and α-diol-d3 (a) in double blank pooled sera (with neither α-diol nor α-diol-d3); (b) in blank pooled sera (with α-diol-d3 only, 4.00 ng/mL); (c) in pooled sera at LLOQ (0.0500 ng/mL; α-diol-d3, 4.00 ng/mL); (d) in pooled sera at 50.0 ng/mL α-diol, and 4.00 ng/mL α-diol-d3; (e) in mouse serum sample # 2 at 0.130 ng/mL α-diol, and 4.00 ng/mL α-diol-d3; (f) in mouse serum sample # 3 at 0.0900 ng/mL α-diol, and 4.00 ng/mL α-diol-d3.
Fig. 8.
Representative mass chromatograms of β-diol, and β-diol-d3 (a) in double blank pooled sera (with neither β-diol nor β-diol-d3); (b) in blank pooled sera (with β-diol-d3 only, 4.00 ng/mL); (c) in pooled sera at LLOQ (0.0500 ng/mL; β-diol-d3, 4.00 ng/mL); (d) in pooled sera at 50.0 ng/mL β-diol, and 4.00 ng/mL β-diol-d3; (e) in mouse serum sample # 2 at 0.100 ng/mL β-diol, and 4.00 ng/mL β-diol-d3; (f) in mouse serum sample # 3 at 0.110 ng/mL β-diol, and 4.00 ng/mL β-diol-d3.
3.2. Assay validation
3.2.1. Selectivity and LLOQ
The developed method was examined for selectivity and LLOQ. As illustrated in the representative chromatograms of the double blank steroid-free sera (Figs. 6a, 7a, and 8a) recorded at the mass transitions of the individual standards and internal standards of DHT, α-diol, and β-diol, there were no detectable interferences at their respective retention times. The representative mass chromatograms of the single blanks (Figs. 6b, 7b, and 8b) showed that there were no interferences from the IS at the respective retention times of standards.
The LLOQs of this method for DHT, α-diol, and β-diol were same at the concentration of 0.0500 ng/mL. The accuracy and precision of measured LLOQs from six lots were ≤ ± 4% and ≤4% for DHT, ≤ ± 4% and ≤4% for α-diol, and ≤ ± 4% and ≤4% for β-diol respectively (Table 1).
Table 1.
Accuracy and precision of DHT, α-diol, and β-diol at LLOQ in six individual lots of mouse sera (n = 5).
| Sera | [XN]a (ng/mL) | DHT | α-diol | β-diol | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| b ± SDc(ng/mL) | CVd (%) | REe (%) | ± SD (ng/mL) | CV(%) | RE (%) | ± SD (ng/mL) | CV(%) | RE (%) | ||
| Lot 1 | 0.0500 | 0.051 ± 0.002 | 4 | 2 | 0.051 ± 0.001 | 2 | 2 | 0.051 ± 0.002 | 4 | 2 |
| Lot 2 | 0.0500 | 0.051 ± 0.001 | 2 | 2 | 0.050 ± 0.002 | 4 | 0 | 0.051 ± 0.002 | 4 | 2 |
| Lot 3 | 0.0500 | 0.049 ± 0.002 | 4 | −2 | 0.050 ± 0.002 | 4 | 0 | 0.051 ± 0.002 | 4 | 2 |
| Lot 4 | 0.0500 | 0.051 ± 0.002 | 4 | 2 | 0.052 ± 0.002 | 4 | 4 | 0.049 ± 0.001 | 2 | −2 |
| Lot 5 | 0.0500 | 0.052 ± 0.002 | 4 | 4 | 0.051 ± 0.002 | 4 | 2 | 0.049 ± 0.002 | 4 | −2 |
| Lot 6 | 0.0500 | 0.052 ± 0.002 | 4 | 4 | 0.049 ± 0.002 | 4 | −2 | 0.053 ± 0.003 | 6 | 6 |
| Mean of 6 lots | 0.0500 | 0.051 ± 0.001 | 1 | 2 | 0.051 ± 0.001 | 2 | 1 | 0.051 ± 0.001 | 2 | 1 |
[XN] = nominal concentration of analyte sera calibrator.
= mean value of measured analyte concentration.
SD = standard deviation of replicate measurements.
CV = SD/ × 100%.
RE = ( − [XN])/[XN] × 100%.
3.2.2. Linearity of calibration curve
The calibration equations for DHT, α-diol and β-diol derived from five individual calibration curves from three validation batches using 1/x2 weighting linear regression were y = 0.354 (± 0.005)x + 0.006 (± 0.001) with a correlation coefficient of 0.999 for DHT, y = 0.286 (± 0.008)x + 0.003 (± 0.001) with a correlation coefficient of 0.999 for α-diol, y = 0.339 (± 0.009)x + 0.008 (± 0.002) with a correlation coefficient of 1.00 for β-diol. The accuracy and precision of individual calibrators were ≤ ± 6% and ≤ 8% for DHT, ≤ ± 5% and ≤9% for α-diol, and ≤ ± 7% and ≤9% for β-diol respectively in steroid-free sera (Table 2).
Table 2.
Accuracy and precision of sera DHT, α-diol, and β-diol calibrators over five validation batches.
| [XN] (ng/ml) | DHT | α-diol | β-diol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ± SD (ng/ml) | CV (%) | RE (%) | ± SD (ng/ml) | CV (%) | RE (%) | ± SD (ng/ml) | CV (%) | RE (%) | |
| 0.0500 | 0.051 ± 0.003 | 6 | 2 | 0.052 ± 0.002 | 4 | 4 | 0.051 ± 0.002 | 4 | 2 |
| 0.100 | 0.099 ± 0.007 | 7 | −1 | 0.105 ± 0.008 | 8 | 5 | 0.095 ± 0.005 | 5 | −5 |
| 0.250 | 0.23 ± 0.02 | 9 | −8 | 0.26 ± 0.02 | 8 | 4 | 0.26 ± 0.02 | 8 | 4 |
| 0.500 | 0.53 ± 0.03 | 6 | 6 | 0.52 ± 0.02 | 4 | 4 | 0.51 ± 0.04 | 8 | 2 |
| 1.00 | 0.96 ± 0.06 | 6 | −4 | 1.05 ± 0.06 | 6 | 5 | 0.96 ± 0.07 | 7 | −4 |
| 5.00 | 5.0 ± 0.1 | 2 | 0 | 5.3 ± 0.3 | 6 | 6 | 5.2 ± 0.3 | 6 | 4 |
| 10.0 | 9.9 ± 0.1 | 1 | −1 | 10.4 ± 0.3 | 3 | 4 | 9.6 ± 0.4 | 4 | −4 |
| 50.0 | 48 ± 1 | 2 | −4 | 52 ± 3 | 6 | 4 | 54 ± 1 | 2 | 8 |
3.2.3. Intra- and inter-day accuracy and precision
The intraday accuracy and precision were ≤ ± 9% and ≤4% for DHT, ≤ ± 6% and ≤4% for α-diol, and ≤ ± 8% and ≤4% for β-diol respectively, whereas the interday accuracy and precision were ≤ ± 8% and ≤5% for DHT, ≤ ± 4% and ≤4% for α-diol, and ≤ ± 4% and ≤5% for β-diol respectively (Table 3).
Table 3.
Intra- and inter-assay accuracy and precision of DHT, α-diol, and β-diol in pooled mouse sera (n = 5).
| Intra-Assaya | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DHT | α-diol | β-diol | |||||||
| [XN] (ng/ml) |
± SD (ng/ml) |
CV (%) |
RE (%) |
± SD (ng/ml) |
CV (%) |
RE (%) |
± SD (ng/ml) |
CV (%) |
RE (%) |
| 0.150 | 0.14 ± 0.01 | 7 | −7 | 0.15 ± 0.01 | 7 | 0 | 0.15 ± 0.01 | 7 | 0 |
| 2.50 | 2.30 ± 0.02 | 1 | −8 | 2.43 ± 0.03 | 1 | −3 | 2.51 ± 0.07 | 3 | 0 |
| 40.0 | 37 ± 1 | 3 | −8 | 38 ± 2 | 5 | −5 | 39 ± 1 | 3 | −3 |
| Inter-Assayb | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DHT | α-diol | β-diol | |||||||
| [XN] (ng/ml) |
± SD (ng/ml) |
CV (%) |
RE (%) |
± SD (ng/ml) |
CV (%) |
RE (%) |
± SD (ng/ml) |
CV (%) |
RE (%) |
| 0.150 | 0.14 ± 0.01 | 7 | −7 | 0.15 ± 0.01 | 7 | 0 | 0.16 ± 0.01 | 6 | 7 |
| 2.50 | 2.4 ± 0.1 | 4 | −4 | 2.5 ± 0.1 | 4 | 0 | 2.47 ± 0.05 | 2 | −1 |
| 40.0 | 36.7 ± 0.4 | 1 | −8 | 38.6 ± 0.5 | 1 | −4 | 39.8 ± 0.7 | 2 | −1 |
Measured by five replicate measurements of each QC sample within a validation batch.
Measured by five parallel measurements of five identical QC samples at each concentration over five validation batches.
3.2.4. Recovery and MF
The recoveries of DHT, α-diol, and β-diol at three different QC concentrations in steroid-free sera were summarized in Table 4. The absolute recoveries of DHT, α-diol, and β-diol were in the ranges of 89–91%, 102–108%, and 89–95% respectively, whereas the IS normalized recoveries of DHT, α-diol, and β-diol were in the ranges of 98–103%, 102–103%, and 99–100% respectively.
Table 4.
Recovery of DHT, α-diol, and β-diol in pooled mouse sera (n = 5).
| [XN] (ng/ml) | DHT | α-diol | β-diol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Recoveryxa- ± SD (%) |
RecoveryIS − b ± SD (%) |
IS Normalized Recoveryc - ± SD (%) |
Recoveryx - ± SD (%) |
RecoveryIS -
± SD (%) |
IS Normalized Recovery -± SD (%) |
Recoveryx - ± SD (%) |
RecoveryIS - ± SD (%) |
IS Normalized Recover-y ± SD (%) |
|
| 0.150 | 90 ± 4 | 92 ± 6 | 98 ± 4 | 106 ± 3 | 104 ± 5 | 102 ± 3 | 89 ± 6 | 90 ± 3 | 99 ± 3 |
| 2.50 | 89 ± 3 | 86 ± 5 | 103 ± 3 | 108 ± 5 | 105 ± 3 | 103 ± 8 | 92 ± 5 | 93 ± 2 | 99 ± 6 |
| 40.0 | 91 ± 5 | 90 ± 3 | 101 ± 5 | 102 ± 6 | 100 ± 2 | 102 ± 2 | 95 ± 3 | 95 ± 4 | 100 ± 4 |
RecoveryX = (mean peak area value of spiked analyte in steroid-free sera matrix)/(mean peak area value of spiked analyte in extracted steroid-free sera matrix) × 100%.
RecoveryIS = (mean peak area value of spiked IS in steroid-free sera matrix)/(mean peak area value of spiked IS in extracted steroid-free sera matrix) × 100%.
IS Normalized Recovery = (RecoveryX)/(RecoveryIS) × 100%.
The absolute MFs of DHT, α-diol, and β-diol were in the ranges of 0.95–1.04, 0.93–1.04, and 0.95–1.06 respectively, whereas the IS normalized MFs of DHT, α-diol, and β-diol were in the ranges of 0.97–1.04, 0.97–1.05, and 0.98–1.06. These results are summarized in Table 5.
Table 5.
Matrix factors of DHT, α-diol, and β-diol in six individual lots of mouse sera (n = 5).
| Sera | [XN] (ng/ml) | DHT | α-diol | β-diol | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MFxa ± SD | MFISb ± SD | IS normalized MFc ± SD | MFX ± SD | MFIS ± SD | IS normalized MF ± SD | MFX ± SD | MFIS ± SD | IS normalized MF ± SD | ||
| Lot 1 | 0.150 | 0.99 ± 0.02 | 0.96 ± 0.01 | 1.03 ± 0.02 | 0.99 ± 0.06 | 1.00 ± 0.01 | 0.99 ± 0.05 | 1.00 ± 0.02 | 0.98 ± 0.01 | 1.02 ± 0.03 |
| 2.50 | 0.98 ± 0.01 | 0.97 ± 0.02 | 1.01 ± 0.03 | 0.93 ± 0.05 | 0.95 ± 0.01 | 0.98 ± 0.05 | 0.99 ± 0.02 | 0.97 ± 0.01 | 1.02 ± 0.03 | |
| 40.0 | 1.02 ± 0.01 | 0.99 ± 0.04 | 1.03 ± 0.05 | 0.99 ± 0.03 | 0.98 ± 0.02 | 1.01 ± 0.05 | 1.01 ± 0.03 | 0.98 ± 0.04 | 1.03 ± 0.04 | |
| Lot 2 | 0.150 | 1.03 ± 0.02 | 1.03 ± 0.03 | 1.00 ± 0.02 | 1.01 ± 0.04 | 0.99 ± 0.01 | 1.02 ± 0.04 | 0.97 ± 0.03 | 0.97 ± 0.02 | 1.00 ± 0.02 |
| 2.50 | 1.01 ± 0.03 | 1.01 ± 0.01 | 1.00 ± 0.03 | 0.99 ± 0.03 | 0.96 ± 0.04 | 1.03 ± 0.04 | 0.95 ± 0.05 | 0.96 ± 0.02 | 0.99 ± 0.03 | |
| 40.0 | 0.99 ± 0.02 | 0.98 ± 0.03 | 1.01 ± 0.03 | 0.98 ± 0.04 | 1.00 ± 0.02 | 0.98 ± 0.06 | 0.98 ± 0.02 | 0.97 ± 0.03 | 1.01 ± 0.03 | |
| Lot 3 | 0.150 | 0.97 ± 0.04 | 0.98 ± 0.02 | 0.99 ± 0.06 | 0.96 ± 0.03 | 0.99 ± 0.03 | 0.97 ± 0.06 | 0.99 ± 0.05 | 0.98 ± 0.01 | 1.01 ± 0.05 |
| 2.50 | 1.04 ± 0.01 | 0.99 ± 0.06 | 1.05 ± 0.01 | 1.04 ± 0.02 | 1.01 ± 0.03 | 1.03 ± 0.03 | 1.03 ± 0.02 | 1.01 ± 0.05 | 1.02 ± 0.02 | |
| 40.0 | 0.95 ± 0.03 | 0.94 ± 0.02 | 1.01 ± 0.02 | 0.98 ± 0.04 | 0.99 ± 0.02 | 0.99 ± 0.04 | 1.06 ± 0.01 | 1.00 ± 0.03 | 1.06 ± 0.04 | |
| Lot 4 | 0.150 | 0.97 ± 0.04 | 0.99 ± 0.01 | 0.98 ± 0.05 | 0.96 ± 0.03 | 0.98 ± 0.02 | 0.98 ± 0.05 | 1.02 ± 0.03 | 1.04 ± 0.02 | 0.98 ± 0.03 |
| 2.50 | 0.99 ± 0.02 | 0.98 ± 0.03 | 1.01 ± 0.05 | 1.02 ± 0.01 | 1.01 ± 0.04 | 1.01 ± 0.05 | 1.04 ± 0.02 | 1.02 ± 0.03 | 1.02 ± 0.02 | |
| 40.0 | 1.02 ± 0.04 | 1.01 ± 0.02 | 1.01 ± 0.02 | 1.04 ± 0.03 | 1.02 ± 0.01 | 1.02 ± 0.03 | 0.99 ± 0.03 | 1.01 ± 0.04 | 0.98 ± 0.03 | |
| Lot 5 | 0.150 | 0.95 ± 0.03 | 0.98 ± 0.02 | 0.97 ± 0.05 | 0.97 ± 0.02 | 0.99 ± 0.04 | 0.98 ± 0.04 | 0.97 ± 0.04 | 0.98 ± 0.03 | 0.99 ± 0.03 |
| 2.50 | 0.98 ± 0.04 | 0.99 ± 0.02 | 0.99 ± 0.04 | 0.99 ± 0.04 | 1.01 ± 0.03 | 0.98 ± 0.04 | 1.05 ± 0.04 | 1.03 ± 0.03 | 1.02 ± 0.03 | |
| 40.0 | 1.02 ± 0.03 | 1.01 ± 0.04 | 1.01 ± 0.04 | 1.05 ± 0.01 | 1.03 ± 0.04 | 1.02 ± 0.05 | 1.00 ± 0.03 | 0.99 ± 0.04 | 1.01 ± 0.03 | |
| Lot 6 | 0.150 | 1.01 ± 0.02 | 0.99 ± 0.03 | 1.02 ± 0.05 | 0.99 ± 0.06 | 0.94 ± 0.03 | 1.05 ± 0.02 | 0.99 ± 0.05 | 1.00 ± 0.03 | 0.99 ± 0.05 |
| 2.50 | 0.99 ± 0.02 | 0.95 ± 0.03 | 1.04 ± 0.03 | 0.98 ± 0.03 | 0.97 ± 0.01 | 1.01 ± 0.04 | 0.97 ± 0.04 | 0.98 ± 0.02 | 0.99 ± 0.06 | |
| 40.0 | 0.98 ± 0.04 | 1.00 ± 0.04 | 0.98 ± 0.04 | 1.03 ± 0.05 | 0.99 ± 0.02 | 1.04 ± 0.05 | 0.99 ± 0.05 | 0.98 ± 0.03 | 1.01 ± 0.03 | |
MFX = (mean peak area value of spiked analyte in extracted steroid-free sera matrix)/(mean peak area value of spiked analyte in the mobile phase).
MFIS = (mean peak area of spiked IS in the extracted steroid-free sera matrix)/(mean peak area of spiked IS in the mobile phase).
IS normalized MF = MFX/MFIS.
3.2.5. Stability studies
The stabilities of DHT, α-diol, and β-diol were determined and summarized in Table 6. The stock solutions and sera QCs were stable on bench top at room temperature of 23 °C for at least 24 h before sample preparation with recoveries of 99–102% and 91–94% respectively for DHT; 98–100% and 91–94% respectively for α-diol; 99–101% and 91–94% respectively for β-diol. The QC samples were also stable post-preparative for at least 24 h in autosampler maintained at 4 °C with recoveries of 96–99% for DHT, 99–102% for α-diol and 97–101% for β-diol. The recoveries of sera QCs after three freeze-thaw cycles were 98–102% for DHT, 95–104% for α-diol and 97–104% for β-diol, whereas the long-term storage of 40 days at −70 °C had recoveries of 93–97% for DHT, 94–98% for α-diol and 92–95% for β-diol.
Table 6.
Stabilities of DHT, α-diol, and β-diol under various test conditions (n = 5).
| Condition | Temperature | Sample | Recovery ± SD (%) | |||||
|---|---|---|---|---|---|---|---|---|
| DHT | α-diol | β-diol | ||||||
| 6h | 24 h | 6h | 24 h | 6h | 24 h | |||
| Bench-top | 23 °C | Stock solutiona | 102 ± 1 | 100 ± 1 | 100 ± 2 | 99 ± 3 | 101 ± 2 | 100 ± 1 |
| Stock solutionb | 99 ± 2 | 101 ± 1 | 100 ± 3 | 98 ± 4 | 99 ± 3 | 99 ± 2 | ||
| Bench-top | 23 °C | Low QCc | 94 ± 1 | 92 ± 3 | 93 ± 2 | 94 ± 4 | 91 ± 6 | 92 ± 3 |
| High QCc | 92 ± 2 | 91 ± 4 | 91 ± 4 | 92 ± 2 | 94 ± 5 | 93 ± 5 | ||
| Auto sampler | 4°C | Low QC | 97 ± 3 | 99 ± 5 | 101 ± 2 | 99 ± 3 | 97 ± 4 | 99 ± 3 |
| HighQC | 96 ± 5 | 98 ± 4 | 102 ± 4 | 100 ± 3 | 101 ± 1 | 98 ± 4 | ||
| 3 Freeze-thaw cycles | −20°C to 23 °C | Low QC | 100 ± 3 | 98 ± 3 | 99 ± 2 | 104 ± 2 | 97 ± 5 | 99 ± 3 |
| HighQC | 101 ± 5 | 102 ± 2 | 95 ± 4 | 100 ± 4 | 101 ± 4 | 104 ± 2 | ||
| Long-term. (40 days) | −70°C | Low QC | 93 ± 3 | 94 ± 5 | 95 ± 6 | |||
| HighQC | 97 ± 5 | 98 ± 6 | 92 ± 5 | |||||
The concentration of the stock solution was 1.00 mg/mL, which was measured by serial dilution to 0.150 ng/mL in the mobile phase.
The concentration of the stock solution was 1.00 mg/mL, which was measured by serial dilution to 40.0 ng/mL in the mobile phase.
The concentrations of sera low and high QCs were 0.150 ng/mL and 40.0 ng/mL, respectively.
3.3. Assay application
The validated LC-MS/MS method was applied to the measurement of DHT, α-diol, and β-diol in mouse serum samples. The mouse serum samples were collected according to the procedure described in Section 2.6. These samples were prepared according to the procedure outlined in Section 2.3. Our preliminary results showed that the concentration ranges of the 8-week old Ubc-Cre/HEXIM1fl/fl mice were below LLOQ −0.0700 ng/mL for DHT, 0.0600–0.130 ng/mL for α-diol, and below LLOQ-0.110 ng/mL for β-diol (Table 7).
Table 7.
Measured concentrations of DHT, α-diol, and β-diol in mouse serum samples.
| Mouse serum sample | [DHT] (ng/mL) | [α-diol] (ng/mL) | [β-diol] (ng/mL) |
|---|---|---|---|
| # 1 | 0.0500 | 0.0700 | < LLOQ |
| # 2 | 0.0700 | 0.130 | 0.100 |
| # 3 | 0.0600 | 0.0900 | 0.110 |
| # 4 | 0.0600 | 0.100 | 0.0600 |
| # 5 | < LLOQ | 0.0700 | < LLOQ |
| # 6 | < LLOQ | 0.0600 | < LLOQ |
| # 7 | 0.0600 | 0.0900 | 0.0500 |
4. Discussion
4.1. Assay development
4.1.1. Chemical derivatization
The chemical derivatization of the analytes was used to achieve lower limits of detection. The derivatization agents examined for this work were 2-fluoro-1-methylpyridine (FMP), and picolinic acid (PA) and the best result was obtained using PA. The derivatization method is based on the reaction of PA with the hydroxyl groups of the analytes forming their respective picolinyl derivatives. Since the spatial orientations of the two hydroxyl groups in α-diol (axial at 3α, equatorial at 17β), and β-diol (equatorial at 3α, 17β) are different, our results show that this resulted in the differential reactivity of PA towards the hydroxyl groups. We found that α-diol formed single hydroxyl group derivatized derivative as the major product, and double hydroxyl groups derivatized derivative as a minor product. Whereas, β-diol formed only double hydroxyl groups derivatized derivative.
4.1.2. Serum recovery study
This recovery study was conducted to estimate the times needed to achieve steady state recoveries of the internal standards in sera. This study suggested that the shortest wait time after the addition of internal standards is 60 min before proceeding with LLE.
4.1.3. MS detectionIn
this method the chemical derivatization was used to enhance MS ionization efficiency. By introducing the easily ionizable groups, the picolinyl derivatives provided an ESI response about 10-fold higher compared to the non-derivatives.
4.1.4. LC separation
To achieve separation initially, we used an acetonitrile/water mobile phase system which worked reasonably well in gradient elution mode but could not separate DHT and α-diol in isocratic elution mode. Since the isocratic elution mode is robust regarding retention time re-producibility and does not require column re-equilibration time for the sequential injection, we preferred this mode and were able to achieve baseline separation of the analytes using methanol/water mobile phase system. Therefore, we used 80% methanol and 0.2% formic acid in water as the mobile phase for subsequent study. Stereoisomers are not easily separated on a C 18 column, but we were able to achieve the separation of α-diol, and β-diol on a C 18 column due to their large molecular structure, and differential derivatization with PA. During the method development, we extended the elution time to a much longer period and did not see any long-retained peaks in the respective MRM channels. To increase the longevity of the column, we have been practicing the column wash procedure recommended by the manufacturer after daily usage.
4.2. Assay validation
4.2.1. Selectivity and LLOQ
Selectivity is the ability of an analytical method to differentiate and quantify the analyte in the presence of other components in the sample. This test must demonstrate the lack of significant interference in the chromatographic regions of the analytes and internal standards. The results depicted that there were no significant interferences which can impact the method developed.
The LLOQ of the method for an analyte was defined as the concentration of standard calibrator at the lowest concentration of the analyte in a calibration plot, which had both accuracy and precision of ≤ ± 20% by five replicate measurements [30]. The LLOQs of this method for DHT, α-diol, and β-diol met the acceptance criteria.
4.2.2. Linearity of calibration curve
The calibration curves for DHT, α-diol, and β-diol were constructed in steroid-free sera with a double blank (contains neither standards nor internal standards), a blank (contains only internal standards) and eight non-zero calibrators (0.0500, 0.100, 0.250, 0.500, 1.00, 5.00, 10.0, 50.0 ng/mL), with the concentration of each IS at 4.00 ng/mL. The conditions for constructing a calibration curve are ≤ ± 20% deviation of the LLOQ from nominal concentration and ≤ ± 15% deviation of standards other than LLOQ from nominal concentration. The simplest mathematical linear regression that adequately described the concentration-response relationship was used for constructing the calibration curves. The regression and the weighting was consistently used for all the analytes throughout the study.
4.2.3. Intra- and inter-day accuracy and precision
The accuracy (%RE) of an analytical method describes the closeness of the mean test results obtained by the method to the true value (concentration) of the analyte. The precision (CV) of an analytical method describes the closeness of individual measures of an analyte when the procedure is repeatedly applied to multiple aliquots of a single homogenous volume of the biological matrix. According to FDA criteria for bioanalytical method validation, %RE and CV of the validation batches are ≤ ± 15% [30]. The method developed met the acceptance criteria for intra- and inter-day accuracy and precision.
4.2.4. Recovery and MF
The recovery of an analyte in an assay is the detector response obtained from an amount of the analyte added to and extracted from the biological matrix, compared to the detector response obtained for the true concentration the pure authentic standard. Recovery gives an estimate of the extraction efficiency of an analytical method within the limits of variability. Protein precipitation using acetonitrile/methanol (v/v, 3:1) was used, but it failed to remove the interfering compounds. LLE was tried using various solvents ethyl acetate (EA), hexane, MTBE, EA/MTBE (v/v, 1:1) and EA/hexane (v/v, 3:2). Double LLE using MTBE produced the desired results. Our experiments showed that the double LLE increased the recovery of analytes from 69 to 74% to 89–108%. The results depicted that the LLE using MTBE worked well for this study, and the stable isotope labeled analytes as internal standards were able to normalize the recovery effects.
MF is determined to estimate the suppression or enhancement of ionization by co-eluting compounds, which is common in ESI source. This is calculated to estimate the effect of co-eluting compounds with the standard and IS on the performance of the assay. Ideally, it is desirable to have the IS normalized MF close to 1 for the effect to be minimal on the performance of assay. The results showed that the use of stable isotope labeled analytes as internal standards were able to correct the matrix effects.
4.2.5. Stability studies
Stability studies were performed to determine if the analytes of interest are stable in matrices (solvent, and sera) throughout the sample preparation and LC-MS analysis. The solvent solutions and sera QCs stability was tested on bench top at room temperature for a maximum of 24 h before sample preparation which cover the duration of time taken to extract the samples. The QC stability is assessed post-preparative in autosampler for a maximum of 24 h to ensure that the analytes are stable post extraction in LC-MS analysis conditions. The real samples undergo stress during freeze-thaw process and to estimate the stability of the analytes during this stressed condition, freeze-thaw stability is performed. Freeze-thaw cycles are performed at an interval of at least 12 h between the cycles at anticipated sample storage temperature. Long-term stability assessment is performed to cover the longest time from sample collection to analysis, and in our case we performed it for 40 days. These studies indicated that the analytes were stable and there was no significant deviation in quantitation of DHT, α-diol, and β-diol under the developed experimental conditions.
4.3. Assay application
Studies from the Montano laboratory indicated that HEXIM1 protein interacts directly with the androgen receptor (AR) and prevents androgen-independent prostate cancer cell proliferation [31], and also suggests that HEXIM1 play a role in the metabolism of AR agonist, DHT. To provide in vivo support for the role of HEXIM1 in the inhibition of prostate tumorigenesis, the Montano laboratory created HEXIM1 knockout (Ubc-Cre/HEXIM1fl/fl) mouse model. Preliminary studies were conducted using sera from these mice to test our methodology for quantifying DHT, α-diol, and β-diol. While levels of α-diol and β-diol in mouse sera were not previously reported, the concentration range we observed for DHT included values reported by others [32,33].
Analysis of alterations in the steroid metabolic pathway are essential due to hormone dependency of prostate cancer and underscore these metabolic pathways as critical targets of therapy. The simultaneous quantification of DHT and its metabolites from the serum of HEXIM1 knockout mice should help us to determine the role of HEXIM1 in androgen metabolism. The enzymatic pathways present in mouse and human prostate share some similarities and some differences, and the enzymes regulated by HEXIM1 are similar in the mouse and human prostate [37]. The method developed describes the simultaneous quantitation of DHT and its metabolites and is expected to contribute to the advancement of prostate cancer research.
5. Conclusion
A quantitative and selective LC-MS/MS method has been developed for the simultaneous determination of DHT, α-diol, and β-diol in mouse sera. The method used LLE for sample preparation to extract the analytes, reverse phase chromatography for analytes separation and tandem mass spectrometry for quantitation. This method was validated according to US FDA guidance for industry on bioanalytical method validation and was successfully applied to the measurement of DHT, α-diol, and β-diol in mouse serum samples. This method should have broad application to measure DHT and its metabolites in transgenic/knockout mouse models for preclinical studies.
Acknowledgements
This research was supported by Translational Research and Pharmacology Core Facility of Case Comprehensive Cancer Center, Case Western Reserve University (NIH P30 CA43703).
References
- [1].Amory JK, Anawalt BD, Matsumoto AM, Page ST, Bremner WJ, Wang C, Swerdloff RS, Clark RV, The effect of 5α-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men, J. Urol 179 (2008) 2333–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Newman T, DHT (dihydrotestosterone): what is DHT’s role in male pattern baldness? Medical News Today (2015), http://www.medicalnewstoday.com/articles/68082.php, Accessed date: 2 December 2017.
- [3].Qoubaitary A, Swerdloff RS, Wang C, Advances in male hormone substitution therapy, Expert. Opin. Pharmacother 6 (2005) 1493–1506. [DOI] [PubMed] [Google Scholar]
- [4].Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM, Human type 3 3alpha-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells, Endocrinology 144 (2003) 2922–2932. [DOI] [PubMed] [Google Scholar]
- [5].Hsing AW, Hormones and prostate cancer: what’s next? Epidemiol. Rev 23 (2001) 42–58. [DOI] [PubMed] [Google Scholar]
- [6].Span PN, Sweep CG, Benraad TJ, Smals AG, 3 Alpha-hydroxysteroid oxidoreductase activities in dihydrotestosterone degradation and back-formation in rat prostate and epididymis, J. Steroid Biochem. Mol. Biol 58 (1996) 319–324. [DOI] [PubMed] [Google Scholar]
- [7].Biswas MG, Russell DW, Expression cloning and characterization of oxidative 17beta- and 3alpha-hydroxysteroid dehydrogenases from rat and human prostate,J. Biol. Chem 272 (1997) 15959–15966. [DOI] [PubMed] [Google Scholar]
- [8].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2017, CA Cancer J. Clin 67 (2017) 7–30. [DOI] [PubMed] [Google Scholar]
- [9].Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M,Palackal N, Ratnam K, Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones, Biochem. J 351 (2000) 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Penning TM, Molecular endocrinology of hydroxysteroid dehydrogenases, Endocr. Rev 18 (1997) 281–305. [DOI] [PubMed] [Google Scholar]
- [11].Dufort I, Labrie F, Luu-The V, Human types 1 and 3 3 alpha-hydroxysteroid dehydrogenases: differential lability and tissue distribution, J. Clin. Endocrinol. Metab 86 (2001) 841–846. [DOI] [PubMed] [Google Scholar]
- [12].Ando T, Nishiyama T, Takizawa I, Ishizaki F, Miyashiro Y, Takeda K, Hara N,Tomita Y, Dihydrotestosterone synthesis pathways from inactive androgen 5alpha-androstane-3beta, 17beta-diol in prostate cancer cells: inhibition of intratumoural 3beta-hydroxysteroid dehydrogenase activities by abiraterone, Sci. Rep 6 (2016) 32198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM, Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action, J. Biol. Chem 279 (2004) 10784–10795. [DOI] [PubMed] [Google Scholar]
- [14].Zeng CM, Chang LL, Ying MD, Cao J, He QJ, Zhu H, Yang B, Aldo-keto reductase AKR1C1-AKR1C4: functions, regulation, and intervention for anti-cancer therapy, Front. Pharmacol 8 (2017) 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rizner TL, Penning TM, Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism, Steroids 79 (2014) 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ji Q, Chang L, Stanczyk FZ, Ookhtens M, Sherrod A, Stolz A, Impaired dihydrotestosterone catabolism in human prostate cancer: critical role of AKR1C2 as a pre-receptor regulator of androgen receptor signaling, Cancer Res. 67 (2007) 1361–1369. [DOI] [PubMed] [Google Scholar]
- [17].Dorgan JF, Fears TR, McMahon RP, Aronson Friedman L, Patterson BH, Greenhut SF, Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry, Steroids 67 (2002) 151–158. [DOI] [PubMed] [Google Scholar]
- [18].Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS, Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry, J. Clin. Endocrinol. Metab 89 (2004) 534–543. [DOI] [PubMed] [Google Scholar]
- [19].Warner MH, Kane JW, Atkin SL, Kilpatrick ES, Dehydroepiandrosterone sulphate interferes with the Abbott Architect direct immunoassay for testosterone, Ann. Clin. Biochem 43 (2006) 196–199. [DOI] [PubMed] [Google Scholar]
- [20].Tate J, Ward G, Interferences in immunoassay, The Clinical Biochemist Reviews 25 (2004) 105–120. [PMC free article] [PubMed] [Google Scholar]
- [21].Shackleton CH, Mass spectrometry: application to steroid and peptide research, Endocr. Rev 6 (1985) 441–486. [DOI] [PubMed] [Google Scholar]
- [22].Salerno R, Moneti G, Forti G, Magini A, Natali A, Saltutti C, Di Cello V,Costantini A, Serio M, Simultaneous determination of testosterone, dihydrotestosterone and 5 alpha-androstan-3 alpha,−17 beta-diol by isotopic dilution mass spectrometry in plasma and prostatic tissue of patients affected by benign prostatic hyperplasia. Effects of 3-month treatment with a GnRH analog, J. Androl 9 (1988) 234–240. [DOI] [PubMed] [Google Scholar]
- [23].Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH, Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS), J. Steroid Biochem. Mol. Biol 121 (2010) 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Licea-Perez H, Wang S, Szapacs ME, Yang E, Development of a highly sensitive and selective UPLC/MS/MS method for the simultaneous determination of testosterone and 5alpha-dihydrotestosterone in human serum to support testosterone replacement therapy for hypogonadism, Steroids 73 (2008) 601–610. [DOI] [PubMed] [Google Scholar]
- [25].Wang C, Shiraishi S, Leung A, Baravarian S, Hull L, Goh V, Lee PW,Swerdloff RS, Validation of a testosterone and dihydrotestosterone liquid chromatography tandem mass spectrometry assay: interference and comparison with established methods, Steroids 73 (2008) 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang D, Zhang M, Rapid quantitation of testosterone hydroxyl metabolites by ultra-performance liquid chromatography and mass spectrometry, J. Chromatogr. B Anal. Technol. Biomed. Life Sci 855 (2007) 290–294. [DOI] [PubMed] [Google Scholar]
- [27].Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, Goh VH,Ridgway EC, Wierman ME, Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry, Steroids 76 (2011) 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Oakey RE, Green B, Leake RE (Eds.), Steroid Hormones: A Practical Approach, IRL Press, Oxford, 1987, p. 278 (£17 ISBN 0–947946-53–5, Biochem. Educ. 16 (1988) 53–53). [Google Scholar]
- [29].Yamashita K, Miyashiro Y, Maekubo H, Okuyama M, Honma S, Takahashi M,Numazawa M, Development of highly sensitive quantification method for testosterone and dihydrotestosterone in human serum and prostate tissue by liquid chromatography-electrospray ionization tandem mass spectrometry, Steroids 74 (2009) 920–926. [DOI] [PubMed] [Google Scholar]
- [30].US Food and Drug Administration, Guidance for Industry: Bioanalytical Method Validation, https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf, (2001), Accessed date: 2 December 2017.
- [31].Yeh I, Song K, Wittmann BM, Bai X, Danielpour D, Montano MM, HEXIM1 plays a critical role in the inhibition of the androgen receptor by antiandrogens, Biochem. J 462 (2014) 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McNamara DT Hardwood U Simanainen KA Walters M Jimenez DJ Handelsman, Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry, J. Steroid Biochem. Mol. Biol 121 (2010) 611–618. [DOI] [PubMed] [Google Scholar]
- [33].Harwood DT, Handelsman DJ, Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization, Clin. Chim. Acta Int. J. Clin. Chem 409 (2009) 78–84. [DOI] [PubMed] [Google Scholar]
- [34].Isaacs JT, Coffey DS, Changes in dihydrotestosterone metabolism associated with the development of canine benign prostatic hyperplasia, Endocrinology 108 (1981) 445–453. [DOI] [PubMed] [Google Scholar]
- [35].Zang T, Tamae D, Mesaros C, Wang Q, Huang M, Blair IA, Penning TM, Simultaneous quantitation of nine hydroxy-androgens and their conjugates in human serum by stable isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry, J. Steroid Biochem. Mol. Biol 165 (2017) 342–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Arai S, Miyashiro Y, Shibata Y, Tomaru Y, Kobayashi M, Honma S, Suzuki K, Effect of castration monotherapy on the levels of adrenal androgens in cancerous prostatic tissues, Steroids 76 (2011) 301–308. [DOI] [PubMed] [Google Scholar]
- [37].McNamara KM, Handelsman DJ, Simanainen U, The mouse as a model to investigate sex steroid metabolism in the normal and pathological prostate, J. Steroid Biochem. Mol. Biol 131 (2012) 107–121, 10.1016/j.jsbmb.2011.10.009. [DOI] [PubMed] [Google Scholar]