Abstract
Objective
To assess whether length of hospital stay is decreased among moderately preterm infants weaned from incubator to crib at a lower vs higher weight.
Study Design
This trial was conducted in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Infants with gestational ages 29–33 weeks, birthweight <1600 g, and in an incubator were randomly assigned to a weaning weight of 1600 or 1800 g. Within 60 to 100 g of weaning weight, the incubator temperature was decreased by 1.0°C to 1.5°C every 24 hours until 28.0°C. The infants were weaned to the crib following stable temperature at 36.5°C to 37.4°C for 8 to 12 hours. Clothing and bedcoverings were standardized. The primary outcome was length of hospital stay from birth to discharge; secondary outcomes included length of stay and growth velocity from weaning to discharge. Adverse events were monitored.
Results
Of 1565 infants screened, 885 were eligible, and 366 enrolled—187 to the 1600-g and 179 to the 1800-g group. Maternal and neonatal characteristics did not differ among weight groups. Length of hospital stay was a median of 43 days in the lower and 41 days in the higher weight group (P = .12). Growth velocity from completion of weaning to discharge was higher in the lower weight group, 13.7 g/kg/day vs 12.8 g/kg/day (P = .005). Groups did not differ in adverse events.
Conclusions
Among moderately preterm neonates, weaning from incubator to crib at a lower weight did not decrease length of stay, but was safe and was accompanied by higher weight gain after weaning.
Preterm infants are cared for in incubators to maintain their body temperature. Once clinically stable and approaching hospital discharge, they are weaned from the incubator to an open crib or cot. The timing of weaning from the incubator is important; weaning too early leads to cold stress and increased energy expenditure, whereas a delay in weaning may prolong hospital stay. Medically stable very preterm infants can be weaned from the incubator to the crib at a weight of <1800 g without adverse effects, as noted in a retrospective study, a prospective practice project and single-center clinical trials.1–4 A Cochrane database review concluded that very preterm infants can be safely weaned from the incubator to the crib at a body weight of 1600 g without adverse effects on temperature stability or weight gain.5–7 A multicenter trial reached the same conclusion.8
Guidelines for weaning from an incubator for moderately preterm infants (29 to 33 weeks gestational age) are not available and are adapted from those made for infants born at earlier gestations.9 Moderately preterm infants are a much larger group than very preterm infants, and a decrease in the length of hospital stay among moderately preterm infants would result in significant cost savings. A query conducted in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) revealed considerable variation in the practice of weaning moderately preterm infants from the incubator to the crib; 5 centers weaned only at 1800 g, 1 when infants reached 34 weeks postmenstrual age, and 2 centers had neither weight or gestational age criteria for weaning. There were variations regarding clinical status at weaning, infant clothing and bed coverings, and monitoring of body temperatures during the weaning process. This randomized, clinical trial was designed to test whether length of hospital stay would be decreased among moderately preterm infants weaned from an incubator to a crib at a lower vs a higher body weight (1600 g vs 1800 g).
Methods
The study (ClinicalTrials.gov: NCT02160002) was conducted at 17 clinical centers in the Eunice Kennedy Shriver NICHD NRN. The inclusion criteria were (a) moderately preterm infants (290/7–336/7 weeks gestational age) and <1600 g at birth, (b) weight <1540 g at screening, (c) age ≥48 hours, and (d) in an incubator. A room temperature of 22°C-25°C was encouraged per the American Academy of Pediatrics guidelines.10 Exclusion criteria were phototherapy for hyperbilirubinemia, respiratory support (>2 L/minute of oxygen therapy or positive pressure support), treatment for hypotension, multiple episodes of apnea (>5 episodes per hour),a major congenital anomaly, or designation for transfer to a referral hospital while in an incubator.
Treatment
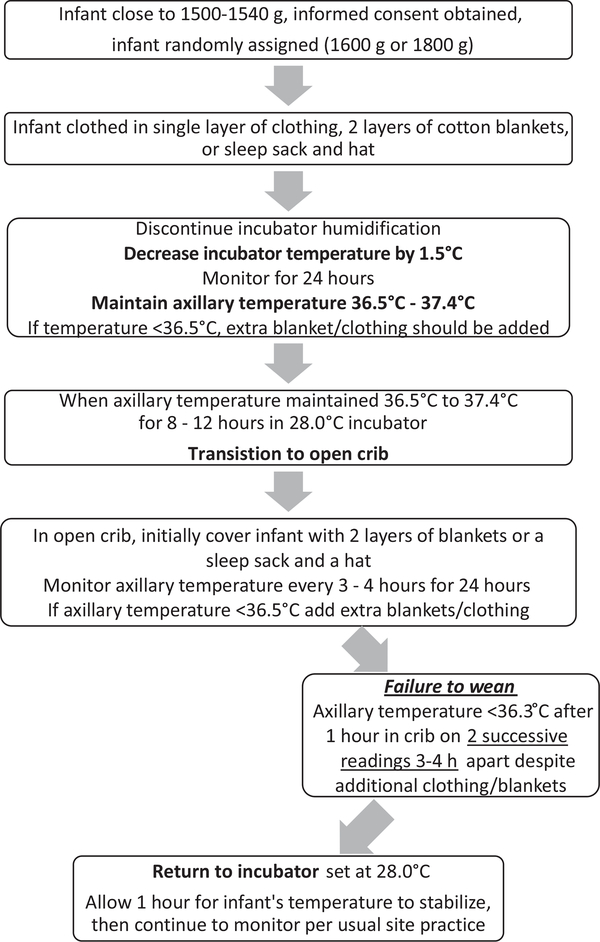
For each infant, the treatment group (weaning weight of 1600 g or 1800 g) was randomly assigned at the NRN data coordinating center using computer-generated random numbers. Assignments were stratified according to clinical center and gestational age (29–30 or 31–33 weeks of gestation). Infants in the study were placed on a standardized weaning protocol adapted from the Research Utilization Project Group2 (Figure 1; available at www.jpeds.com). The protocol was initiated at a weight that was 60–100 g below target weight in both groups for consistency across all centers. Infants were dressed in a single layer of clothing, cap, and booties and 2 layers of cotton blankets or a sleep sack. The incubator humidification was discontinued. The incubator temperature was decreased by 1.0°C to 1.5°C every 24 hours until 28.0°C and the infant’s axillary temperature was maintained at 36.5°C-37.4°C (97.7°F-99.3°F). The infant was transferred to a crib once the axillary temperature was stable for 8–12 hours in a 28.0°C incubator. In the crib, infants were covered with 2 layers of blankets or a sleep sack and a hat.
Figure 1.
NICHD NRN weaning algorithm: initiate at 1500–1540 or 1700–1740 g.
Temperature was monitored in both groups every 3–4 hours for the first 24 hours after weaning to the crib. If a temperature of <36.5°C was recorded, extra clothing or blankets were added and temperature monitoring was continued. Infants who failed weaning to the crib were returned to the incubator with the temperature adjusted to maintain the infant’s temperature at 36.5°C-37.4°C. A second attempt at weaning was permitted within 72 hours. Infants who failed the second attempt were treated according to usual care at the center. If the infant’s temperature was >37.4°C during the study period, a layer of blanket or clothing was removed. A successful wean was defined as the ability to maintain axillary temperature of ≥36.3°C in the crib for the first 24 hours without return to the incubator.
Skin-to-skin care with parents was permitted throughout the study. To foster the cooperation of the nurses caring for the study participants, a nurse champion was identified at each center. A video of the weaning program was developed and reviewed by nursing staff. The family was contacted by telephone within 7–10 days of discharge. Information on any health care provider visit, infant’s weight, success with continuation of breastfeeding, and any hospital readmission was recorded.
Monitoring of Study
An independent Data Safety and Monitoring Committee appointed by the director of the NICHD monitored interim data and evaluated safety. The first evaluation was at accrual of 50% and the second at 75% of infants reaching discharge, transfer, or death. Safety was assessed by examining the number of infants with an axillary temperature of <36.3°C. Based on these 2 interim looks, Pocock stopping bounds were used to assess interim safety and O’Brien-Fleming bounds were used to assess interim efficacy.
Outcomes
The primary outcome was length of hospital stay from birth to discharge home. This outcome was selected because a decrease in the length of hospital stay has economic benefit and promotes earlier family attachment. Secondary outcomes included length of hospital stay between random assignment and discharge, postmenstrual age and growth measures at discharge, and growth velocity (weight in grams per kilograms per day)11,12 during weaning and from completion of weaning to 36 weeks postmenstrual age and to discharge. Adverse events monitored included moderate hypothermia (temperature 36.0°C-36.2°C), severe hypothermia (temperature <36.0°C), bradycardia, weight loss (>5% from initiation to 72 hours after completion of weaning), feeding intolerance, and any unexpected events. Any adverse event deemed as serious or unexpected by the clinical team was reported within 72 hours to the data coordinating center and the site institutional review board. The study protocol was approved by the institutional review board at each site. Written informed consent was obtained from a parent or guardian.
Statistical Analyses
There was limited published information to use for sample size calculations. The only clinical trial using a similar intervention with length of stay as the primary outcome was that of Zecca et al, conducted in Italy on 94 very preterm and moderately preterm infants with gestational ages of 27–35 weeks; infants were randomly assigned to weaning from the incubator at 1600 or 1800 g.7 The length of hospital stay was 23.5 days vs 33.0 days among infants weaned at lower and higher weight groups, respectively. For the current study, a more conservative effect size of 7 days was targeted; with a type 1 error of .05, power of 90%, assuming a standard deviation of 20 days for the primary outcome, a sample size of 366 infants was required. All data analyses were performed according to the intention-to-treat principle. All statistical analyses were conducted at the data coordinating center using SAS (version 9.3; SAS Institute, Cary, North Carolina) software.
Maternal and neonatal characteristics of infants in the 2 groups were compared using the χ2 or Fisher exact test for categorical variables and the nonparametric Wilcoxon test for continuous variables. The primary outcome was analyzed using nonparametric median regression, adjusting for study stratification factors of center and gestational age categories. Secondary outcomes were analyzed using robust Poisson regression for binary outcomes, with similar adjustment factors. All reported P values are 2 sided and not adjusted for multiple comparisons; P < .05 was considered statistically significant.
Results
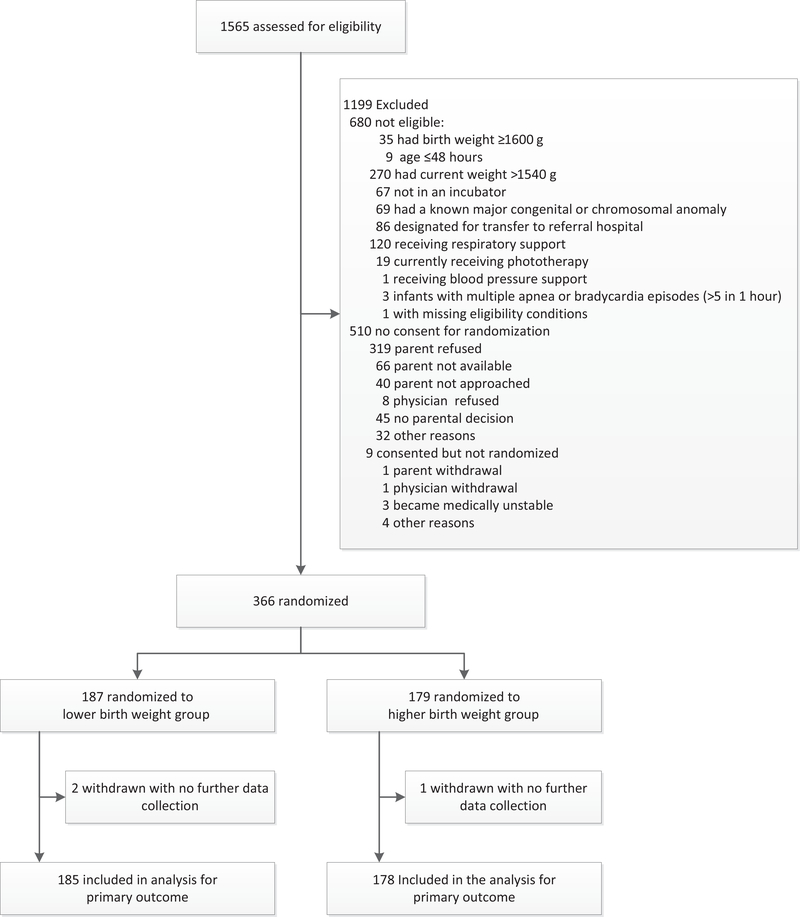
Enrollment started on February 15, 2015, and completed on April 29, 2016; of 1565 infants screened, 885 were eligible and 366 were enrolled; 187 were assigned to the lower and 179 to the higher weaning weight group (Figure 2). The parents of 2 infants in the lower weaning weight group and 1 in the higher weight group withdrew consent during study intervention; thus, 185 infants in the lower and 178 infants in the higher weaning weight group are included in the analysis of the primary outcome.
Figure 2.
Flow of moderately preterm infants through a trial of incubator weaning at a lower weight vs a higher weight.
Maternal and Neonatal Characteristics
Maternal and neonatal characteristics did not differ between the 2 groups. Among infants in the 1600-g weaning weight group, the axillary temperatures and postmenstrual ages were lower than infants in the 1800-g weaning weight group (P < .05 and P< .01, respectively).The incubator temperature was higher in the lower compared with the higher weaning weight group (P < .01; Table I). The proportion of infants who were small for gestational age was high in both groups because only moderately preterm infants with a birthweight of <1600 g were included in this trial.
Table I.
Maternal and neonatal characteristics*
| Lower weight group (n = 187) | Higher weight group (n = 179) | |
|---|---|---|
| Maternal characteristics | ||
| Maternal age, y | 28.8 ± 5.8 | 28.9 ± 6.1 |
| Gravida | 2(1–4) | 2(1–4) |
| Para | 2(1–3) | 2(1–3) |
| Married | 74 (34) | 79 (44) |
| Education: high school degree | 106 (57) | 104 (59) |
| Race | ||
| Black | 76 (41) | 72 (40) |
| White | 99 (53) | 97 (54) |
| Multiple gestation | 60 (32) | 47 (26) |
| At least 1 prenatal visit | 180 (97) | 174 (97) |
| Maternal diabetes requiring insulin or oral medications | 20 (11) | 10(6) |
| Maternal hypertension | 84 (45) | 87 (49) |
| Hypertension before pregnancy | 35 (43) | 27 (32) |
| Antepartum hemorrhage | 19(10) | 15(8) |
| Clinical and histologic chorioamnionitis | 7(4) | 12(8) |
| Rupture of membrane ≥18 h | 30 (37) | 30 (38) |
| Complete course of antenatal steroids | 128(81) | 127 (83) |
| Antibiotics during this admission | 127 (70) | 119(68) |
| Magnesium sulfate before delivery | 128 (70) | 136 (77) |
| Cesarean delivery | 136 (73) | 122 (68) |
| Neonatal characteristics: preintervention | ||
| Transferred from birth hospital | 14(7) | 11 (6) |
| Male | 93 (50) | 92 (51) |
| Gestational age, wk | 31.2 ± 1.3 | 31.1 ± 1.2 |
| Small for gestational age | 82 (44) | 61 (34) |
| Apgar score at 1 min | 7 (5–8) | 7 (5–8) |
| Apgar score at 5 min | 8 (7–9) | 8 (7–9) |
| Delivery room resuscitation: intubation | 25 (13) | 25 (14) |
| Birth weight, g | 1306± 204 | 1335±179 |
| Birth length, cm | 39.0 ± 2.8 | 39.4 ± 2.0 |
| Birth head circumference, cm | 27.4 ± 1.4 | 27.6 ± 1.4 |
| Age at randomization, d | 14(9–20) | 12.5(9–18) |
| Neonatal characteristics: at intervention | ||
| Weight at start of weaning in incubator, g | 1527 ± 30 | 1717 ± 57 |
| Infant axillary temp at start of weaning, °C | 36.8 ± 0.5 | 36.9 ± 0.3† |
| Incubator temp at start of weaning, °C | 29.6 ± 1.9 | 28.7 ± 1.8‡ |
| Ambient (room) temp at start of weaning, °C | 23.7 ± 1.6 | 23.6 ± 1.1 |
| At start of weaning clothed in single layer, with 2 layers blankets or sleep sack and cap | 157 (89) | 143 (88) |
| Postmenstrual age at start of weaning, wk | 33.8 ± 1.3 | 34.3 ± 1.2‡ |
Values are mean ±SD, n (%), or median (IQR).
Percentages are based on the number of mothers or infants for whom data were available.
Because of rounding, not all percentages sum to 100.
p<.05
p<.01
Primary and Secondary Outcomes
The primary outcome of length of hospital stay from birth to discharge was a median of 43 days (IQR, 32–55 days) in the lower and 41 days (IQR, 33–52 days) in the higher weaning weight group (Table II). The adjusted median difference in length of hospital stay was not significantly different between the 2 groups (P = .12). The secondary outcome, length of stay from random assignment to discharge, was also not significantly different between groups. Fewer infants in the lower weaning weight group had a successful wean from incubator to crib on the first attempt at weaning. Infants in both groups continued to gain weight during the 72 hours after weaning from the incubator to the crib.
Table II.
Primary and secondary outcomes
| Outcomes | Lower weight group (n = 185) | Higher weight group (n = 178) | P value* | |
|---|---|---|---|---|
| Primary outcome | Median differences (95% CI) | |||
| Length of hospital stay from birth to discharge, median (IQR), d | 43 (32–55) | 41 (33–52) | 2.00 (−0.50 to 4.50) | .12 |
| Secondary outcomes | ||||
| Postmenstrual age at discharge, median (IQR), wk | 37.1 (36.3–38.9) | 37.0 (36.0–38.3) | 0.29 (−0.07 to 0.64) | .11 |
| Length of hospital stay from randomization to discharge, median (IQR), d | 28 (19–39) | 27.5 (20–37) | 0.09 (−1.93 to 2.12)* | .93 |
| Adjusted risk ratio (95% CI) | ||||
| First attempt at weaning, n (%)† | 178 (96) | 167 (94) | 1.03 (0.98–1.08) | .23 |
| Infant transition to open crib after first attempt, n (%) | 166 (93) | 162 (97) | 0.96 (0.91–1.00) | .06 |
| Successful wean to open crib after first attempt, n (%)‡ | 151 (92) | 157 (98) | 0.93 (0.89–0.98) | .004 |
| Second attempt at weaning, n (%)*,§ | 15 (65) | 3(50) | ||
| Successful wean to open crib after second attempt, n (%)* | 12 (80) | 2(67) | ||
| Return to incubator within 72 h of successful wean, n (%)¶ | 12(8) | 8(5) | 1.55 (0.69–3.76) | .33 |
| Median/mean differences (95% CI) | ||||
| Length of stay until discharge criteria met, median (IQR), d** | 38.0 (25.0–50.0) | 36.5 (27.0–47.0) | 1.30 (−1.08 to 3.67) | .28 |
| Weight at status: discharge/transfer, mean (SD), g†† | 2336 (648) | 2354 (522) | 4.84 (−107.5 to 117.2) | .93 |
| Length at status, mean (SD), cm‡‡ | 44.6 (3.2) | 44.9 (2.9) | −0.15 (−0.76 to 0.45) | .62 |
| Head circumference at status, mean (SD), cm§§ | 31.9 (2.2) | 32.0 (2.0) | −0.04 (−0.46 to 0.37) | .83 |
| Growth velocity from start of weaning to 36 wk PMA, median (IQR), g/kg/d¶¶ | 16.4 (14.1–18.2) | 15.9 (14.4–17.9) | 0.64 (−0.19to1.47) | .13 |
| Growth velocity after weaning to crib to discharge, median (IQR), g/kg/d*** | 13.7 (12.1–15.7) | 12.8 (10.2–15.1) | 0.92 (0.28 to 1.56) | .005 |
| Adjusted risk ratio (95% CI) | ||||
| Mortality, n (%) | 0(0) | 0(0) | ||
| Transferred to another facility, n (%)††† | 11 (6) | 9(5) | 1.18 (0.50–2.79) | .70 |
| Readmissions after first hospitalization, n (%)‡‡‡ | 6(3) | 2(1) | 2.99 (0.61–14.60) | .18 |
For continuous outcomes parameter estimates (95% CI) and for categorical outcomes risk ratios (95% CI) are presented. For outcomes for which the median (IQR) is reported, median regression was performed. For outcomes for which mean (SD) is reported, linear regression was performed. For categorical outcomes, Poisson regression analysis was performed. Site and gestational age were adjusted in all models unless specified otherwise. All adjusted risk ratios/parameter estimates compare low weight group vs high weight group. For outcomes with small sample size adjusted risk ratio/parameter estimates and P values are not reported.
Seven in the lower weight group and 11 in the higher weight group were weaned by bedside nurses without following study protocol and do not have any weaning data.
There was 1 missing in lower weight group and 2 missing in the higher weight group.
There were 3 missing second attempt data in lower weight group and 2 missing second attempt data in higher weight group.
There were 2 missing in lower weight group and 1 missing in higher weight group. Model not adjusted for site because of convergence issues.
Data were missing for 20 in each group.
There was 1 missing in the higher weight group.
There were 11 missing in lower weight group and 9 missing in the higher weight group.
There were 3 missing in lower weight group and 5 missing in the higher weight group.
There were 91 infants (40 lower weight and 51 higher weight group) who were discharged home and 15 (9 lower weight and 6 higher weight group) transferred to another hospital at 36 weeks postmenstrual age (PMA). There were 257 (136 lower weight and 121 higher weight group) who remained in the hospital at 36 weeks PMA. There were 26 infants (10 lower weight and 16 higher weight group) who were still in the incubator at 36 weeks PMA. There were 20 (7 lower weight and 13 higher weight group) infants who were missing the date of start of weaning.
There were 6 missing in lower weight group and 2 in higher weight group.
Model is not adjusted for site because of convergence issues.
There were 11 missing data in the lower weight group and 10 in the higher weight group. Model is not adjusted for site because of convergence issues.
Length of stay until discharge criteria were met and weight, length, and head circumference did not differ between groups at discharge or transfer to another hospital. Growth velocity (median) after weaning to the crib until hospital discharge was significantly higher among infants in the lower compared with the higher weaning weight group (13.7 g/kg/day vs 12.8 g/kg/day, respectively; P = .005, Table II). The median postnatal age at first oral feed was 16 and 18 days, respectively, in the lower and higher weaning weight groups; age at attaining full feeds was 33 days in both groups.
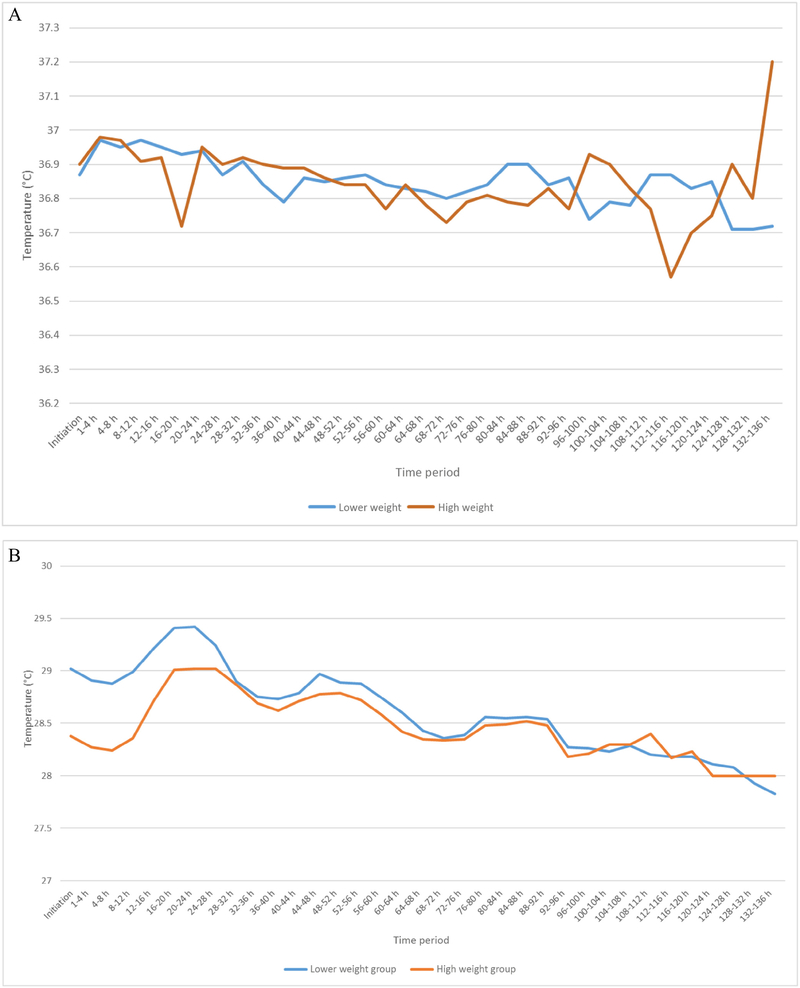
There were no deaths in either group and no difference in the number of infants transferred to another hospital or the number of infants who were rehospitalized after discharge (Table II). The neonatal hospital course did not differ between the 2 groups (Table III). The infants’ axillary temperatures and incubators’ temperatures during the weaning phase are noted in Figure 3, A and B (available at www.jpeds.com). Eighteen infants had missing temperature data owing to protocol deviations by bedside nurses. There were 36 adverse events among the study participants (Table IV). Of the 13 infants with moderate cold stress, 10 were in the lower weaning weight group. Five infants had severe cold stress, 4 of whom were in the lower weaning weight group. Six infants with bradycardia and 6 infants with feeding intolerance were distributed equally between the 2 groups. One infant in the lower weaning weight group developed a pneumoperitoneum that was serious and life threatening, but not considered to be related to the study. Nineteen percent of participants had a protocol deviation (Table V; available at www.jpeds.com); the frequency was not different between the 2 groups.
Table III.
Hospital course
| Outcomes | Lower weight group (n = 185) | Higher weight group (n = 178) | Adjusted risk ratio (95% CI)* | P value* |
|---|---|---|---|---|
| Oxygen at 28 days, n (%)§,† | 24 (13) | 16(9) | 1.53 (0.85–2.72) | .15 |
| Patent ductus arteriosus, n (%)¶,† | 14(9) | 12(7) | 1.16 (0.56–2.40) | .69 |
| Grade III or IV intracranial hemorrhage, among those with head ultrasound, n (%)**,† | 7/151 (5) | 1/155 (0.7) | 7.19(0.91–57.17) | .06 |
| Late onset sepsis, n (%)† | 5(3) | 1 (0.6) | 5.00 (0.60–41.94) | .14 |
| Proven necrotizing enterocolitis, n (%)††,† | 4 (2) | 6 (3) | 0.67 (0.20–2.31) | .52 |
| Retinopathy of prematurity among infants screened, n (%)† | 17/123 (14) | 13/114 (11) | 1.22 (0.62,2.39) | .57 |
| If retinopathy, highest stage, n (%) | ||||
| I | 14(82) | 8(61) | ||
| II | 2(12) | 4(31) | ||
| III | 1 (6) | 1 (8) | ||
| Still in hospital at 36 weeks, n (%) | 136 (73) | 121 (68) | 1.09 (0.96–1.23) | .20 |
| Still in hospital at 36 weeks postmenstrual age‡ due to | ||||
| Respiratory morbidity only | 1 (1) | 3 (2) | 0.28 (0.03–2.82) | .28 |
| Apnea/bradycardia only | 21 (15) | 21 (17) | 0.85 (0.40–1.79) | .66 |
| Inadequate oral feeds only | 44 (32) | 37 (31) | Reference | Reference |
| Other reason | 10(7) | 7 (6) | 1.19(0.41–3.45) | .74 |
| Multiple reasons | 60 (44) | 53 (44) | 0.96 (0.54–1.71) | .88 |
Adjusted P values and risk ratios calculated from Poisson regression analysis. Site and gestational age was adjusted for in the models. All risk ratios compare the low weight group vs high weight group.
Not adjusted for sites because of model convergence issues.
Generalized logistic regression used.
There were 6 missing data in lower weight group and 3 in the higher weight group.
One infant missing in higher weight group.
One infant missing data in each group.
One infant in the lower weight group developed pneumoperitoneum and necrotizing enterocolitis during study period.
Figure 3.
A, Infant axillary temperature during weaning from incubator to crib. B, Incubator temperature during weaning from incubator to the crib.
Table IV.
Adverse events among all participants
| Adverse events categories | Infants (n) | Duration mean (SD), d | Relatedness to study* | Intensity† and seriousness | Expectedness |
|---|---|---|---|---|---|
| Moderate cold stress (36.2°C-36.0°C) | 13 | 2.7 (3.3) | Possibly = 7 | Mild = 8 | Expected = 8 |
| Probably = 2 Definitely = 4 | Moderate = 5 | Unexpected = 5 | |||
| All not serious | |||||
| Severe cold stress (<36.0°C) | 5 | 1 (0) | Unlikely = 1 Possibly = 1 | Moderate = 1 | Expected = 4 |
| Probably = 2 Definitely = 1 | Severe = 4. | Unexpected = 1 | |||
| Of 5, other medical condition = 2, not serious = 3 | |||||
| Bradycardia | 6 | 8.6 (15.3) | None = 2 | Mild = 3 | Expected = 5 |
| Unlikely = 2 possibly = 2 | Moderate = 3, all not serious | Unexpected = 1 | |||
| Feeding intolerance | 6 | 3.6 (3.2) | None = 2 | Mild = 3 | Expected = 3 |
| Unlikely = 1 Possibly = 3 | Moderate = 2 | Unexpected = 3 | |||
| Severe = 1, other medical condition = 1 and 5 not serious | |||||
| Desaturations | 1 | 3.0 (—) | Possibly | Moderate, not serious | Unexpected |
| Necrotizing enterocolitis stage Ila | 1 | None | Moderate, with other medical condition | Unexpected | |
| Respiratory distress | 1 | Unlikely | Moderate, not serious | Expected | |
| Tachycardia with desaturations | 1 | 17.0 (—) | None | Mild | Expected |
| Increasing heart failure | 1 | 26.0 (—) | None | Moderate | Unexpected |
Relatedness to study: none, unlikely, possibly, probably, definitely.
Intensity: mild, moderate, severe, life threatening.
Table V.
Protocol deviations
| Protocol deviation category | Total PD events | Number of infants with each PD | Events in low weight group | Events in high weight group |
|---|---|---|---|---|
| Transferred to crib before reaching weight category | 10 | 10 | 3 | 7 |
| Transferred to crib before 8 h of stable temperature in incubator | 11 | 11 | 4 | 7 |
| Incubator at <28°C at beginning of weaning process | 2 | 2 | 1 | 1 |
| Incubator weaned at >1.5°C over a 24-h period during weaning process | 3 | 3 | 1 | 2 |
| Infants had elevated temperature in incubator or too hot and therefore algorithm not followed | 11 | 10 | 6 | 5 |
| Infant not transferred to crib after >12 h of stable temperature | 12 | 12 | 9 | 3 |
| Returned to crib after transfer for incorrect indication | 7 | 5 | 6 | 1 |
| Other reason for not following protocol | 5 | 5 | 2 | 3 |
| Temperature of <36.5° C, but no additional layers added as per protocol | 6 | 4 | 4 | 2 |
| Transferred out of NRN | 4 | 4 | 2 | 2 |
| Total | 71 | 38 | 33 |
Discussion
Our study evaluated whether weaning moderately preterm infants from an incubator to a crib at a lower weight (1600 g) compared with a higher weight (1800 g) would result in a shorter length of hospital stay. The trial demonstrated no difference in the length of stay between infants weaned at lower vs higher weights. There was no difference in the rate of returning infants to the incubator after successful weaning in the 2 groups. The growth velocity of infants after weaning to the crib until hospital discharge was higher among infants in the lower weight than in the higher weight group.
Weaning of very low birth weight infants from the incubator to the crib using a research-based protocol was first reported in 1994; 270 infants with a birth weight of 1118 ± 270 g and a gestational age of 29 ± 3 weeks were weaned at a weight of 1598 ± 136 g (mean ± SD).2 Hospital practices regarding weight at weaning from the incubator to the crib continue to be highly variable. In a data registry of 2098 infants from 579 hospitals in the US, only 8.3% of infants 22–30 weeks or less were weaned from the incubator to the crib at weight <1600 g.1 The first randomized clinical trial of weaning <1500-g birthweight infants from the incubator to the crib at either 1700 or 1800 g weight found no difference in length of hospital stay between the 2 groups.3 Prospective studies of infants with birth weights of <1600 g from New Zealand and Italy have demonstrated the feasibility and safety of early weaning from incubators to cribs.6,13 Families have more access to infants cared for in cribs with the ability to promote skin-to-skin care and breastfeeding, as noted in a recent Family Integrated Care model.14
Two clinical trials have examined weight at weaning from incubator to crib among preterm infants in the last decade. Investigators in Italy studied 94 infants with a birthweight of 750–1600 g who were weaned from the incubator at 1600 or 1800 g when they were medically stable. The primary outcome, length of hospitalization, was shorter in the 1600-g group compared with the 1800-g group (23.5 days vs 33.0 days; P = .002).7 The current clinical trial focuses on moderately preterm infants, with a larger sample size, and fewer exclusion criteria. The median lengths of hospital stay in the current trial were not significantly different in the 2 groups. Investigators in Australia studied 182 infants with birth weights from 526 to 1680 g who were weaned from the incubator at 1600 or 1800 g.8 Primary outcome measures were temperature stability and average daily weight gain for 14 days after transfer to the crib. Infants in the 1600-g group had fewer temperatures of <36.4°C (17% vs 30%; P = .03) and higher average daily weight gain (17 g/kg/day vs 14 g/kg/day; P < .001) compared with those in the 1800-g group. In the current trial, no differences in the frequency of moderate or severe cold stress between infants in the lower and higher weaning weight group were noted.
The growth velocity of moderately preterm infants from weaning to the crib to hospital discharge was greater in the 1600-g compared with the 1800-g weaning weight group in this study. However, growth measures at discharge/transfer (weight, height, and head circumference) and postmenstrual age at discharge were similar in both groups. Small for gestational age infants may wean at a lower weight than appropriate for gestational age infants; in this study the frequencies of small for gestational age infants were not significantly different in the lower weaning and higher weaning weight groups. Other studies of preterm infants have also noted accelerated weight gain after transfer to the crib.1,13 Measurements of resting energy expenditure using indirect calorimetry while in the incubator and after weaning to the crib demonstrate an increase in the metabolic rate of preterm infants after weaning from the incubator.15–17 Skinfold thickness, which measures deposition of subcutaneous fat, is also increased after weaning from the incubator to the cooler environment of the crib in preterm infants.18
The strengths of this study are its focus on moderately preterm infants and the relatively large number of infants enrolled from multiple centers across the country. We have demonstrated the feasibility and safety of weaning moderately preterm infants from the incubator to the crib at 1600 g body weight compared with 1800 g, without significantly increasing the length of hospital stay. The limitations of this study are that the intervention was unmasked, and the rate of parental refusal was relatively high (36%). Eighteen infants had missing temperature profiles owing to protocol deviations. Secondary outcome data at 36 weeks postmenstrual age could not be collected among 106 infants because they had been discharged or transferred to another hospital (Table II).After this trial was initiated, we reported that 38% of moderately preterm infants are discharged before 36 weeks postmenstrual age.19 In addition, 26 moderately preterm infants enrolled in the study were still in incubators at 36 weeks postmenstrual age (Table II); the lack of weaning data on these infants accounted for the missing data for some secondary outcomes. Other limitations include a lack of data on duration of skin-to-skin contact, breastfeeding, or nutritional intake of infants during the study.
Among moderately preterm neonates, weaning from the incubator to the crib at a lower weight of 1600 g compared with a higher weight of 1800 g did not decrease the length of hospital stay. Weaning at the lower weight was safe and was accompanied by greater weight gain after weaning.
Acknowledgments
We thank our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Funding and Disclosures
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s Trial on Incubator Weaning of Moderately Preterm Infants (NCT02160002) through cooperative agreements. Although NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, A.D. (Data Coordinating Center Principal Investigator) and S.S. (Data Coordinating Center Statistician) had full access to all of the data in the study, and with the NRN Center Principal Investigators, take responsibility for the integrity of the data and accuracy of the data analysis. The authors declare no conflicts of interest.
Glossary
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
Appendix
The following investigators are additional members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development NRN and participated in this study:
NRN Steering Committee Chair: Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904)—Martin Keszler, MD; Angelita M. Hensman, MS, RNC-NIC, BSN; Elisa Vieira, RN, BSN.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80)—Anna Marie Hibbs, MD; Bonnie S. Siner, RN.
Children’s Mercy Hospital (U10 HD68284)—William E. Truog, MD; Eugenia K. Pallotto, MD, MSCE; Howard W. Kilbride, MD; Cheri Gauldin, RN, MSN, CCRC; Anne Holmes, RN, MSN, MBA-HCM, CCRC; Kathy Johnson RN, CCRC.
Cincinnati Children’s Hospital Medical Center, University of Cincinnati Medical Center, and Good Samaritan Hospital (U10 HD27853, UL1 TR77)—Kurt Schibler, MD; Suhas G. Kallapur, MD; Cathy Grisby, BSN, CCRC; Barbara Alexander, RN; Estelle E. Fischer, MHSA, MBA; Lenora Jackson, CRC; Kristin Kirker, CRC; Jennifer Jennings, RN, BSN; Sandra Wuertz, RN, BSN, CLC; Greg Muthig, BA.
Duke University School of Medicine, University Hospital, University of North Carolina, WakeMed Health and Hospitals, and Duke Regional Hospital (U10 HD40492,UL1 TR1117, UL1 TR1111)—C. Michael Cotten, MD, MHS; Ronald N. Goldberg, MD; Theresa Roach, RN; Joanne Finkle, RN, JD; Kimberley A. Fisher, PhD, FNP-BC, IBCLC; Matthew M. Laughon, MD, MPH; Carl L. Bose, MD; Janice Bernhardt, MS, RN; Cindy Clark, RN; Stephen D. Kicklighter, MD; Ginger Rhodes-Ryan, ARNP, MSN, NNP-BC.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, UL1 TR454)—Ellen C. Hale, BS, RN, CCRC; Yvonne Loggins, RN; Diane I. Bottcher, RN, MSN.
Eunice Kennedy Shriver National Institute of Child Health and Human Development—Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children at Indiana University Health, and Eskenazi Health (U10 HD27856, UL1 TR6)—Heidi Harmon MD, MS. Dianne E. Herron, RN, CCRC; Shirley I. Wright-Coltart, RN, CCRP.
Nationwide Children’s Hospital, The Research Institute at Nationwide Children’s Hospital, The Ohio State University Wexner Medical Center, The Ohio State College of Medicine, Center for Perinatal Research (U10 HD68278)—Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Julie Gutentag, RN, BSN; Courtney Park, RN, BSN; Julie C. Shadd, BSN, RD; Margaret Sullivan, BA; Jennifer L. Grothause, BA, RN, BSN; Melanie Stein, RRT, BBS; Erna Clark, BA; Rox Ann Sullivan, RN, BSN.
RTI International (U10 HD36790)—Dennis Wallace, PhD; Kristin M. Zaterka-Baxter, RN, BSN, CCRP; Margaret Crawford, BS, CCRP; Jeanette O’Donnell Auman, BS.
Stanford University and Lucile Packard Children’s Hospital (U10 HD27880,UL1 TR93)—David K. Stevenson, MD; Lou Ann Herfert, MSN, NNP; M. Bethany Ball, BS, CCRC; Gabrielle T. Goodlin, BAS: Melinda S. Proud, RCP; R. Jordan Williams, BA.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216)—Namasivayam Ambalavanan, MD; Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN.
University of California—Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270)—Teresa Chanlaw, MPH; Rachel Geller, RN, BSN.
University of Iowa and Mercy Medical Center (U10 HD53109, UL1 TR442)—Dan L. Ellsbury, MD; Jane E. Brumbaugh, MD; Karen J. Johnson, RN BSN; Donia B. Campbell, RNC-NIC; Jacky R. Walker, RN.
University of New Mexico Health Sciences Center (U10 HD53089, UL1 TR41)—Kristi Watterberg MD; Conra Backstrom Lacy, RN; Sandy Sundquist Beauman, MSN, RNCNIC; Carol Hartenberger, MPH, RN, CCRC.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (U10 HD68244)—Haresh Kirpalani, MB, MSc; Eric C. Eichenwald, MD; Sara B. DeMauro, MD, MSCE; Noah Cook, MD; Aasma S. Chaudhary, BS, RRT; Soraya Abbasi, MD; Toni Mancini, RN, BSN, CCRC; Dara Cucinotta.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (U10 HD68263, UL1 TR42)—Satyan Lakshminrusimha, MD; Ronnie Guillet, MD, PhD; Ann Marie Scorsone, MS; Julianne Hunn, BS; Rosemary Jensen; Holly I.M. Wadkins, MA; Stephanie Guilford, BS; Ashley Williams, MSEd.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689)—Myra Wyckoff, MD; Luc P. Brion, MD; Diana M. Vasil, RNC-NIC; Lijun Chen, PhD, RN; Lizette E. Torres, RN.
Wayne State University, University of Michigan, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385)—Athina Pappas, MD; Bogdan Panaitescu, MD, Shelley Handel, AD; Diane F. White, RT; Mary Christensen, RT; Stephanie A. Wiggins, MS.
Footnotes
List of additional members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network is available at www.jpeds.com (Appendix).
Trial Registration ClinicalTrials.gov NCT02160002.
Portions of this study were presented at the Pediatric Academic Societies annual meeting, May 5–8, 2018, Toronto, Ontario.
References
- 1.Schneiderman R, Kirkby S, Turenne W, Greenspan J. Incubator weaning in preterm infants and associated practice variation. J Perinatol 2009;29:5704. [DOI] [PubMed] [Google Scholar]
- 2.Medoff-Cooper B Transition of the preterm infant to an open crib. J Obstet Gynecol Neonatal Nurs 1994;23:329–35. [DOI] [PubMed] [Google Scholar]
- 3.Sutter TW, Phan D, Pierchala CE, Rishel W. Weaning of premature infants from the incubator to an open crib. J Perinatol 1988;8:193–8. [PubMed] [Google Scholar]
- 4.Gibson E, Medoff-Cooper B, Nuamah IF, Gerdes J, Kirkby S, Greenspan J. Accelerated discharge of low birth weight infants from neonatal intensive care: a randomized, controlled trial. The Early Discharge Study Group. J Perinatol 1998;18(6 Pt 2 Su):S17–23. [PubMed] [Google Scholar]
- 5.New K, Flenady V, Davies MW. Transfer of preterm infants for incubator to open cot at lower versus higher body weight. Cochrane Database Syst Rev 2011;(9):CD004214 Review. doi: 10.1002/14651858.CD004214.pub4. [DOI] [PubMed] [Google Scholar]
- 6.West CR, Williams M, Weston PJ. Feasibility and safety of early transfer of premature infants from incubators to cots: a pilot study. J Paediatr Child Health 2005;41:659–62. [DOI] [PubMed] [Google Scholar]
- 7.Zecca E, Corsello M, Priolo F, Tiberi E, Barone G, Romagnoli C. Early weaning from incubator and early discharge of preterm infants: randomized clinical trial. Pediatrics 2010;126:e651–6. [DOI] [PubMed] [Google Scholar]
- 8.New K, Flint A, Bogossian F, East C, Davies MW. Transferring preterm infants from incubators to open cots at 1600 g: a multicentre randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2012;97:F88–92. [DOI] [PubMed] [Google Scholar]
- 9.Whyte RK. Neonatal management and safe discharge of late and moderate preterm infants. Semin Fetal Neonatal Med 2012;17:153–8. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics Committee on Fetus and Newborn. Hospital discharge of the high-risk neonate. Pediatrics 2008;122:111926. [PubMed] [Google Scholar]
- 11.Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics 2005;116:1466–73. [DOI] [PubMed] [Google Scholar]
- 12.Patel AL, Engstrom JL, Meier PP, Jegier BJ, Kimura RE. Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J Perinatol 2009;29:618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone G, Corsello M, Papacci P, Priolo F, Romagnoli C, Zecca E. Feasibility of transferring intensive cared preterm infants from incubator to open crib at 1600 grams. Ital J Pediatr 2014;40:41. doi: 10.1186/1824-7288-40-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien K, Robson K, Bracht M, Cruz M, Lui K, Alvaro R, et al. Effectiveness of Family Integrated Care in neonatal intensive care units on infant and parent outcomes: a multicenter, multinational, cluster-randomised controlled trial. Lancet Child Adolesc Health 2018;2:245–54. [DOI] [PubMed] [Google Scholar]
- 15.Dollberg S, Mimouni FB, Weintraub V. Energy expenditure in infants weaned from a convective incubator. Am J Perinatol 2004;21:253–6. [DOI] [PubMed] [Google Scholar]
- 16. Lei TH, Lien R, Hsu JF, Chiang MC, Fu RH. Effect of body weight on temperature control and energy expenditure in preterm infants. Pediatr Neonatol 2010;51:178–81. [DOI] [PubMed] [Google Scholar]
- 17.Berger I, Marom R, Mimouni F, Kopelovich R, Dollberg S. Weight at weaning of preterm infants from incubator to bassinet: a randomized clinical trial. Am J Perinatol 2014;31:535–40. [DOI] [PubMed] [Google Scholar]
- 18.Heimler R, Sumners JE, Grausz JP, Kien CL, Glaspey JC. Thermal environment change in growing premature infants: effect on general somatic growth and subcutaneous fat accumulation. Pediatrics 1981;68:82–6. [PubMed] [Google Scholar]
- 19.Walsh MC, Bell EF, Kandefer S, Saha S, Carlo WA, D’angio CT et al. Neonatal outcomes of moderately preterm infants compared to extremely preterm infants. Pediatr Res 2017;82:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]