Abstract
Preterm infants are at risk for acute kidney injury (AKI) for multiple reasons. Reports on the frequency and timeline of iatrogenic renal insults and potential consequences are limited. Our objectives are to estimate the prevalence and timing of exposure to nephrotoxic medications, and assess the association of these nephrotoxic medications with AKI in preterm infants.
We performed a retrospective chart review of infants <30 weeks postmenstrual age and/or <1500 g birth weight admitted to the neonatal intensive care units at Cincinnati Children's Hospital Medical Center and University of Cincinnati Medical Center from 2011 to 2014. We queried the electronic health record for exposures to nephrotoxic medications and/or radiologic contrast media and correlated to serum creatinine concentration proximate to the exposure. Using the Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria, we assessed the AKI rate associated with the exposures.
The cohort included 276 preterm infants. 233 (84%) received nephrotoxicity-associated medications. Antibiotics were the most common type (80%). AKI occurred in 9% of infants and was associated with exposure to a nephrotoxic medication. In a forward stepwise logistical regression, birth weight (OR: 0.995 (95% CI: 0.991-0.998), p=0.004) and number of exposures (OR: 1.83 (95% CI: 1.33-2.53), p=0.0002) were predictive of AKI.
Nephrotoxic medication exposure increased the odds of AKI in preterm and low birth weight infants. Future prospective surveillance through the electronic health record in addition to routine serum creatinine monitoring may reduce the rate of exposure and subsequent AKI.
Keywords: Acute kidney injury, Nephrotoxic, Very low birth weight infants, Amino glycosides, Premature infants
Introduction
Acute kidney injury (AKI) is associated with increased mortality and higher rates of chronic kidney disease in very low birth weight (VLBW, birth weight <1500 g) infants [1-4]. The etiology of AKI in premature and VLBW infants is multi factorial. Glomerulogenesis is incomplete prior to 34-36 weeks postmenstrual age and the existing nephrons are immature. Moreover, VLBW infants experience a high rate of extra-renal insults that are independently injurious to the kidneys such as sepsis and hemodynamic instability [5-7]. Further compounding the risk for AKI, many of the medications used in this population are potentially nephrotoxic [7,8].
Studies have indicated that nephrotoxic medication use is prevalent. Nephrotoxic antimicrobials, including vancomycin and aminoglycosides, are frequently used in the treatment of infections [7,9,10], and non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and indomethacin are used for the treatment of, or prophylaxis for intraventricular hemorrhage or patent ductus arteriosus closure [11,12]. In a cohort of non-critically ill children from birth to 18 years, 86% were exposed to 1 or more nephrotoxic medications from a list of 36 [13]. And in a small study that included VLBW infants, 87% were exposed to one or more nephrotoxic medications that encompassed antimicrobials, intravenous contrast and NSAIDS [10]. AKI was common in both studies [10,13].
The timing of nephrotoxic medication use and the temporal development of AKI in VLBW infants admitted to the neonatal intensive care unit (NICU) have not previously been described. The purpose of this study was to 1) Describe the frequency and timing of nephrotoxic medication use in VLBW infants, 2) Describe the temporal relationship between nephrotoxic medication use and the development of AKI, and 3) Determine if there were any factors associated with the development of AKI. We hypothesized that similar to previous studies, greater than 80% of all preterm infants will receive at least one nephrotoxic medication and that most of the exposures will occur in the first month of life. Additionally, we hypothesized that the rate of AKI will be associated with the frequency of nephrotoxic medication use.
Materials and Methods
Study population
We performed a retrospective chart review of all neonates <1500 g birth weight and/or <30 weeks postmenstrual age admitted between April 2011 and April 2014 to the NICU at the University of Cincinnati Medical Center (UCMC) or the NICU at Cincinnati Children's Hospital Medical Center (CCHMC) within 48 hours of birth. UCMC is a level 3 large birthing center in a metropolitan area. CCHMC is a level 4 major referral center with surgical capabilities. The study was approved by the Institutional Review Boards of both UCMC and CCHMC with a waiver of informed consent. Subjects were excluded if they were considered non-viable by the attending neonatologist at the time of birth, had congenital renal anomalies (e.g., congenital anomalies of the kidney and the urinary tract), or end stage kidney disease. These exclusion groups were identified by ICD-9 diagnosis codes.
Patient demographics, proximate serum creatinine values (24 hours prior to, on the same day as, and 48 hours following anephrotoxic medication exposure) and outcome data were obtained by manual chart review from the electronic health record (EHR). The Medication Administration Records (MAR) were queried for administration and timing of nephrotoxic medications from a previously derived list Supplemental Table 1 [14]. We evaluated the prevalence of nephrotoxic medication use for all VLBW infants admitted to the UCMC and CCHMC NICUs during the study period. An exposure to a medication was defined as a continuous treatment course of that medication as determined by the NICU team. Doses separated by >24 hours from the preceding dose were considered separate exposures.
We assessed for the presence of AKI in patients who were exposed to at least one nephrotoxic medication from Supplementary Table 1. AKI was defined by the Kidney Disease Improving Global Outcome (KDIGO) serum creatinine criteria [15]. Baseline serum creatinine was defined as the trough prior to nephrotoxic medication exposure regardless of age of the neonate. If serum creatinine levels were not measured following the exposure, the exposure was assumed to not be associated with AKI. Therefore, all AKI events are temporally related to at least one potentially nephrotoxic medication. For the patients who had more than one episode of AKI, the serum creatinine must have returned to the previous trough or lower, and each episode was separated by at least 48 hours.
Data analysis
We assessed the prevalence of nephrotoxic medication exposure for all patients admitted to the NICU during the study period who were <1500 g birth weight and/or <30 weeks postmenstrual age (n = 276) (Figure 1). Next, patients were dichotomized based on a diagnosis of AKI related to nephrotoxic medication exposure. For these analyses, we included only those patients who were exposed to nephrotoxic medications (n=233) (Figure 1). Comparisons of continuous variables were performed using t-tests or Wilcoxon rank sum tests as appropriate. Categorical variables were compared using Chi-square or Fisher's exact tests as appropriate. A forward stepwise multivariable logistic regression was performed including all variables that were statistically significant in the univariate analyses (p=0.2). The data analysis was performed using SAS software 9.4, Cary, NC, USA.
Figure 1.

Study cohort.
Results and Discussion
Demographic data
Two hundred ninety-six premature VLBW infants were admitted to the NICU at the two study sites (UCMC and CCHMC) during the study period. Twenty infants were excluded because of non-viability (n=16) or for congenital renal anomalies (n=4). Therefore, 276 patients were included in the frequency of exposure analysis, (Figure 1). Demographic data is summarized in Table 1.
Table 1.
Demographic Data of All Patients.
| Variables | Total (n=276) |
|---|---|
| Gestational Age in Weeks,Mean (±SD) | 27.3 (±2.8) |
| Gender (Female)n (%) | 126 (46) |
| Birth Weight in Grams,Mean (±SD) | 991.4 (±336.9) |
| * Length of Stay in DaysMedian (IQR) | 59 (29-99) |
| Number of Exposures Median (IQR) | 1 (1-3) |
| Duration of Exposure in DaysMedian (IQR) | 3.5 (1-11) |
| Mortality n (%) | 38 (14) |
| Exposure to Nephrotoxic Medications, n (%) | 233 (84) |
Abbreviations: SD: standard deviation; IQR: interquartile range; n: number; %: percent
Length of stay was only evaluated in survivors (n = 238)
Frequency of nephrotoxic medication use
Two hundred and thirty-three patients (84%) had at least one exposure to a nephrotoxic medication during their stay in the NICU. The total number of exposures among the 233 patients was 666. The median number of nephrotoxic exposures per patient was 2 [IQR 1-4], and the median number of exposure days was 5 [IQR 2-15]. Antimicrobials accounted for 80% of all exposures (Figure 2). Gentamicin was the most common medication with 56% (n=155) of all VLBW infants receiving the medication at least once during their stay. The next most common medications exposures included tobramycin (27%), nafcillin (21%), ibuprofen (13%), intravenous contrast (12%) and indomethacin (11%). Exposure data are summarized in Table 2. The following medications were not encountered in our study cohort: Amikacin, Cefuroxime, Enalaprilat, Foscarnet, Gadobenate, Dimeglumine, Ganciclovir, Piperacillin (without Tazobactam), Ketorolac, Topiramate, Valcyclovir, and Valganciclovir
Figure 2.
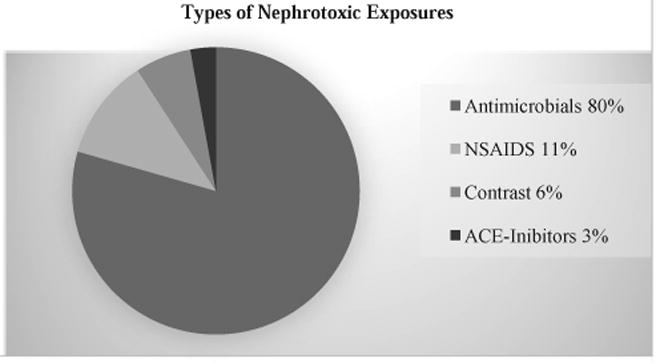
Exposure Types.
Table 2.
Frequency of Nephrotoxic Medications.
| Number and % of Exposures | Frequency in All Patients (n=276) | Exposure Frequency in Patients with AKI (n = 21) | |
|---|---|---|---|
| Acyclovir | 6 (1%) | 6 (2%) | 3 (14%) |
| Amphotericin B | 2 (0.3%) | 4 (1%) | 1 (5%) |
| Captopril | 8 (1%) | 7 (3%) | 0 (0%) |
| Cefotaxime | 8 (1%) | 7 (3%) | 3 (14%) |
| Ceftazidime | 1 (0.2%) | 1 (0.4%) | 1 (5%) |
| Enalapril | 11 (2%) | 8 (3%) | 0 (0%) |
| Gentamicin | 233 (35%) | 155 (56%) | 19 (91%) |
| Ibuprofen | 46 (7%) | 35 (13%) | 4 (19%) |
| Indomethacin | 30 (5%) | 29 (11%) | 8 (38%) |
| Iohexol/ Iodixane | 42 (6%) | 33 (12%) | 1 (5%) |
| Nafcillin | 91 (14%) | 58 (21%) | 13 (62%) |
| Piperacillin/ Tazobactam | 36 (6%) | 30 (11%) | 5 (24%) |
| Ticarcillin/ Lavulonic acid | 1 (0.2%) | 1 (0.4%) | 1 (5%) |
| Tobramycin | 120 (18%) | 75 (27%) | 13 (62%) |
| Vancomycin | 31 (5%) | 27 (10%) | 6 (29%) |
Acute kidney injury
Twenty-seven discrete episodes of AKI occurred in 21 (9%) different infants. Five infants had 2 separate episodes of AKI and 1 infant had 3 episodes of AKI. Six infants had KIDGO stage 1 AKI; 9 infants had stage 2 AKI; 6 infants had stage 3 AKI.
AKI was associated with a lower gestational age (p<0.0001), having a lower birth weight (p<0.0001), increased total number of nephrotoxic medication exposures (p<0.0001), and increased number of days of nephrotoxic medication exposures (p=0.05). Survivors with AKI had longer length of stay compared to those without AKI (median: 120, IQR: 64-156 days) compared to those without AKI (median 69, IQR: 36-106 days) (p=0.03). A greater proportion of patients with AKI (n = 10, 48%) died compared to those without AKI (n = 28, 11%) (p< 0.0001).Mortality was high in all three stages of AKI (stage 1 = 50%, stage 2 = 33% and stage 3 = 67%). Comparison of demographic and outcome data for patients with and without AKI are summarized in Table 3. AKI occurred early in the NICU course: 41% during the first week of life, 19% during the second week of life and 15% during the third week of life (Figure 3).
Table 3.
Demographic Data of Preterm Neonates with and without AKI.
| Variables | Infants Exposed (n=233) | AKI (n=21) | Non-AKI (n=212) | p value |
|---|---|---|---|---|
| Gestational Age inWeeks, Mean (± SD) | 26.6 (± 2.2) | 25 (± 1.7) | 26.8 (± 2.1) | <0.0001 |
| Gender (Female) n (%) | 105 (45%) | 11 (52%) | 94 (44%) | 0.5 |
| Birth Weight inGrams, Mean (± SD) | 942 (± 327) | 696.9 (± 187.7) | 966.6 (± 328.3) | <0.0001 |
| * Length of Stay in Days, Median (IQR) | 71 (37-108) | 120 (64-156) | 69 (36-106) | 0.03 |
| Number of Exposures, Median (IQR) | 2 (1-4) | 5 (3-9) | 2 (1-3) | <0.0001 |
| Duration of Exposure in Days, Median (IQR) | 5 (2-15) | 12 (4-29) | 5 (2-13) | 0.05 |
| Mortality, n (%) | 38 (16) | 10 (48) | 28 (13) | 0.0004 |
Abbreviations: SD: standard deviation; n: number; %: percent; IQR: interquartile range
The number of survivors exposed to nephrotoxic medications was 195, of which 11 had AKI and 184 did not have AKI.
Figure 3.
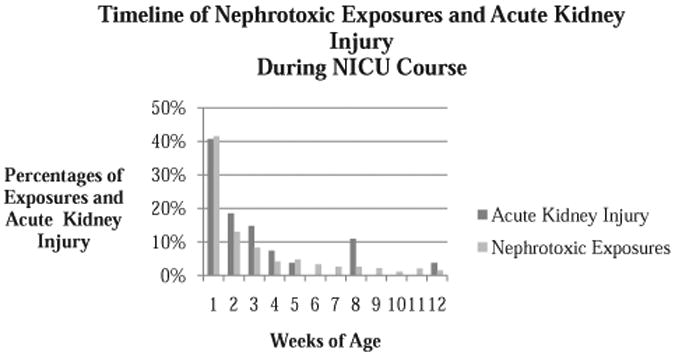
Timeline of Nephrotoxic Exposures and Acute Kidney Injury during NICU Course by weeks of age.
Nephrotoxic medication exposures and AKI
Patients with AKI were exposed to a median of 5 (IQR: 3-9) nephrotoxic medications compared to those without AKI, who were only exposed to 2 (IQR: 1-3) (p< 0.0001). Patients with AKI were exposed to nephrotoxic medications for a median of 12 days (IQR: 4-29) compared to a median of 5 days (IQR: 2-13) in those without AKI (p = 0.05), (Table 3).
Similar to the timing of AKI, 41% of all nephrotoxic exposures happened in week 1 of age, 13% in week 2, and 8% in week 3. The percentage of exposures continued to decrease over time with very few occurring beyond 3 months of life, 11% (Figure 3).
In a multivariable logistic regression analysis, lower birth weight (OR 0.995, 95%: CI 0.991-0.998) (p=0.004) and a greater number of nephrotoxic medication exposures (OR 1.83, 95%: CI 1.33-2.53) (p=0.0002) remained significant predictors of AKI in patients who were exposed to at least 1 nephrotoxic medication during their NICU stay. The odds of AKI in VLBW infants decreased for each 5-gram increase in birth weight.
Discussion
We performed an assessment of nephrotoxic medication exposure among VLBW infants admitted to 2 large NICUs. We found that VLBW infants are frequently exposed to nephrotoxic medications in the NICU, with antimicrobials being the predominant type. More than half of the exposures occurred within the first two weeks of age, and two-thirds of them in the first month of life. In fact, over three-fourths of the nephrotoxic medication exposures occurred during the first 40 days of life, a period that coincides with a critical and active stage of postnatal glomerulogenesis [5]. Gentamicin, which has been shown to inhibit glomerulogenesis in an animal model [17], accounted for 50% of exposures. We also evaluated the AKI rate temporally associated with the nephrotoxic medication exposures and its risk factors. Our regression analysis demonstrated that higher birth weight was protective for developing AKI, with the odds of AKI in VLBW infants decreasing for each 5-gram increase in birth weight. This suggests that infants with intrauterine growth restriction may be at higher risk for developing nephrotoxic medication associated AKI beyond the risk posed by prematurity. Furthermore, the odds of AKI increased by 1.83 times for each additional nephrotoxic medication added to the treatment regimen.
Several studies have investigated the role of nephrotoxic medication exposure and the development of AKI in the pediatric population. In one retrospective study that included non-critically ill children aged 1 day to 18 years, greater than 80% of the study population was exposed to at least one nephrotoxic medication, and 34% developed AKI [13]. Similar to our finding, the risk of AKI increased with exposure to more nephrotoxic medications [13]. A small retrospective study of VLBW infants evaluated the role of nephrotoxic medications (predominantly antimicrobials, NSAIDS, and contrast medium). Exposure to one or more nephrotoxic medications occurred in 87% of patients. In this study, infants with AKI received more nephrotoxic medications per day [10]. Similarly, 84% of our study population was exposed to at least one nephrotoxic medication and we demonstrated an independent risk for AKI with increasing nephrotoxic medication exposures.
Increased awareness of nephrotoxic medications associated AKI from these retrospective studies has resulted in important quality improvement initiatives with the main objective to decrease the rate of nephrotoxic medication exposures and AKI. Goldstein et al. conducted a prospective quality improvement project implementing a systematic electronic health record (EHR) screening and detection support system in non-critically ill hospitalized children. In this initiative, an “exposure” was defined as receipt of an intravenous aminoglycoside for greater than 48 hours, or 3 simultaneous nephrotoxic medications derived from a previous study [13]. The authors found that 3% of patients admitted to the hospital were exposed to nephrotoxic medications, and AKI occurred in 25% of unique exposed patients [14]. With implementation of routine serum creatinine monitoring for exposed patients, the authors observed a 42% decrease in the AKI intensity rate, which is a normalized duration (per 100 days) of AKI per susceptible days. In a follow-up study, the authors reported a further reduction (38%) in overall medication exposure rate (38%) and in the AKI incidence (from 2.96 to 1.06 per 1000 patient days) with an estimation of 633 exposures and 398 AKI episodes avoided [18]. To our knowledge, there are currently no similar screening and detection support processes in NICUs for identifying at risk patients. Our study highlights that the odds of AKI increases with each additional nephrotoxic medication exposure, and this study could lay the foundation for designing and implementing a similar EHR alert system in the high-risk and vulnerable low birth weight infants admitted to the NICU.
This study has several strengths and limitations that warrant discussion. The major strengths are: the sample encompasses a large cohort of patients from 2 NICUs, and we directly assessed the temporal relationship between measures of serum creatinine and nephrotoxic medication exposure in defining the AKI event. There are, however, several important limitations. First, the study is a retrospective chart review, and thus we can only establish associations and not causality. Second, the rate of AKI is likely an underestimation of the true incidence among exposed patients. This is likely due to a combination of factors including the retrospective nature of the study, the assessment of only the 48 hours following the exposure, and the lack of standardized surveillance of serum creatinine. Third, therapeutic drug monitoring for assessment of drug clearance and thus renal function was not included in the analysis. This may have provided insight into the renal function in some patients in whom a serum creatinine measurement was not performed. Fourth, we did not evaluate the reason for initiation of the nephrotoxic medication, co-morbidities, or the clinical state that may have contributed to the development of AKI. Finally, the reliance on serum creatinine for defining AKI has significant limitations. For example, levels measured in the first few days of life may reflect maternal creatinine. Moreover, the current KDIGO definition for AKI may not be appropriate for VLBW infants.
Conclusions
VLBW infants are at high risk for nephrotoxic medication associated AKI, with higher birth weight being protective. An increased number of nephrotoxic medications prescribed increases the odds of AKI. Medication type and serum creatinine monitoring should be considered in this vulnerable population to prevent AKI.
Supplementary Material
Supplemental Table 1: Nephrotoxic Medications (Adapted from the list compiled for older infants and children).
Acknowledgments
The authors report a statement of financial support. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number UL1TR000077. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AKI
Acute Kidney Injury
- VLBW
Very Low Birth Weight
- NSAIDs
Non-steroidal Anti-inflammatory Medications
- NICU
Neonatal Intensive Care Unit
- UCMC
University of Cincinnati Medical Center
- CCHMC
Cincinnati Children's Hospital Medical Center
- KDIGO
Kidney Disease Improving Global Outcomes
- GFR
Glomerular Filtration Rate
Footnotes
Conflict of Interest: The authors have no other financial or ethical conflicts of interest to disclose.
References
- 1.Carmody JB, Charlton JR. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics. 2013;131:1168–1179. doi: 10.1542/peds.2013-0009. [DOI] [PubMed] [Google Scholar]
- 2.Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. 2009;24:991–997. doi: 10.1007/s00467-009-1133-x. [DOI] [PubMed] [Google Scholar]
- 3.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 4.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011;69:354–358. doi: 10.1203/PDR.0b013e31820b95ca. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez MM, Gomez A, Abitbol C, Chandar J, Montané B, Zilleruelo G. Comparative renal histomorphometry: a case study of oligonephropathy of prematurity. Pediatr Nephrol. 2005;20:945–949. doi: 10.1007/s00467-004-1800-x. [DOI] [PubMed] [Google Scholar]
- 6.Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005;45:96–101. doi: 10.1053/j.ajkd.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Cataldi L, Leone R, Moretti U, De Mitri B, Fanos V, Ruggeri L, et al. Potential risk factors for the development of acute renal failure in preterm newborn infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2005;90:F514–519. doi: 10.1136/adc.2004.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuzzolin L, Fanos V, Pinna B, di Marzio M, Perin M, Tramontozzi P, et al. Postnatal renal function in preterm newborns: a role of diseases, drugs and therapeutic interventions. Pediatr Nephrol. 2006;21:931–938. doi: 10.1007/s00467-006-0118-2. [DOI] [PubMed] [Google Scholar]
- 9.Tugay S, Bircan Z, Cağlayan C, Arisoy AE, Gökalp AS. Acute effects of gentamicin on glomerular and tubular functions in preterm neonates. Pediatr Nephrol. 2006;21:1389–1392. doi: 10.1007/s00467-006-0131-5. [DOI] [PubMed] [Google Scholar]
- 10.Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med. 2014;27:1485–1490. doi: 10.3109/14767058.2013.860522. [DOI] [PubMed] [Google Scholar]
- 11.Fanos V, Antonucci R, Zaffanello M. Ibuprofen and acute kidney injury in the newborn. Turk J Pediatr. 2010;52:231–238. [PubMed] [Google Scholar]
- 12.Kömhoff M, Wang JL, Cheng HF, Langenbach R, McKanna JA, Harris RC, et al. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int. 2000;57:414–422. doi: 10.1046/j.1523-1755.2000.00861.x. [DOI] [PubMed] [Google Scholar]
- 13.Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011;6:856–863. doi: 10.2215/CJN.08110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013;132:e756–767. doi: 10.1542/peds.2013-0794. [DOI] [PubMed] [Google Scholar]
- 15.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 16.Akinola O, Noronha C, Oremosu A, Kusemiju O, Okanlawon OA. The effect of the cyclooxygenase blockers, ibuprofen on the development of glomeruli in Sprague-Dawley rats. Niger Postgrad Med J. 2003;10:46–50. [PubMed] [Google Scholar]
- 17.Gilbert T, Gaonach S, Moreau E, Merlet-Benichou C. Defect of nephrogenesis induced by gentamicin in rat metanephric organ culture. Lab Invest. 1994;70:656–666. [PubMed] [Google Scholar]
- 18.Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90:212–221. doi: 10.1016/j.kint.2016.03.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Nephrotoxic Medications (Adapted from the list compiled for older infants and children).


