Abstract
Background:
Some studies have reported a potential association between usual source of health care and disability, but no one has explored the association with frailty, a state of early and potential reversible disability. We therefore aimed to explore the association between older persons' self-reported usual source of health care at baseline and the onset of frailty.
Methods:
Information regarding usual source of health care was captured through self-report and categorized as 1) private doctor's office, 2) public clinic, 3) Health Maintenance Organization (HMO), or 4) hospital clinic/emergency department (ED). Frailty was defined using the Study of Osteoporotic Fracture (SOF) index as the presence of at least two of the following criteria: (i) weight loss ≥5% between baseline and any subsequent follow-up visit; (ii) inability to do five chair stands; and (iii) low energy level according to the SOF definition. Multivariable Cox's regression analyses, calculating hazard ratios (HRs) with 95% confidence intervals (CIs), were undertaken.
Results:
Of the 4292 participants (mean age: 61.3), 58.7% were female. During the 8-year follow-up, 348 subjects (8.1% of the baseline population) developed frailty. Cox's regression analysis, adjusting for 14 potential confounders showed that, compared to those using a private doctor's office, people using a public clinic for their care had a significantly higher risk of developing frailty (HR = 1.56; 95%CI: 1.07–2.70), similar to those using HMO (HR = 1.48; 95%CI: 1.03–2.24) and those using a hospital/ED (HR = 1.76; 95%CI: 1.03–3.02). Conclusion: Participants receiving health care from sources other than private doctors are at increased risk of frailty, highlighting the need for screening for frailty in these health settings.
Keywords: Aged, Health care, Frailty
1. Introduction
As the population ages, the number of older people with frailty is expected to increase worldwide. Frail people are known to be at high risk for several deleterious outcomes, such as hospitalization, institutionalization and disability (Clegg et al., 2013), all conditions that contribute to increased costs for healthcare (McMillan and Hubbard, 2012).
Attention to frailty as a risk factor for deleterious outcomes highlights the importance of prevention, in order to attenuate the common progression from frailty to disability and then to death (Morley et al., 2013). Promoting prevention requires a conceptual framework tailored to maximize function in older age through consistent assessment and intervention strategies (Beard and Bloom, 2015). Such a coherent approach is lacking in most current health care settings (Beard and Bloom, 2015). Most older and complex patients are not cared for by geriatricians or in special geriatric units. Moreover, these patients are likely to receive care from multiple providers, challenging coordination of care. It has been suggested that Health Maintenance Organizations (HMOs) have the opportunity to better coordinate care, and in fact may have financial and other incentives to do so (Gaynor et al., 2004). However, some other studies have shown that HMOs have not met this promise (Ware Jr. et al., 1996; Burton et al., 2002; Porell and Miltiades, 2001).
Reviews of the literature have concluded that, for the general population, HMO enrollees and non-enrollees with indemnity insurance receive roughly comparable quality of care assessed by process and outcome measures (Hellinger, 1998; Miller and Luft, 1994). On the other hand, focusing only on older people, the Medical Outcomes Study found a greater decline in physical health for older patients in HMOs than in fee-for-service plans (Ware Jr. et al., 1996; Burton et al., 2002). Similarly, Porell et al. showed that the risk for functionally independent people becoming disabled within a year was lower in Fee-For-Service Medicare (FFS) individuals than among HMO enrollees (Porell and Miltiades, 2001).
While others study investigated the potential relationship between usual source of care and disability onset (Ware Jr. et al., 1996; Burton et al., 2002; Porell and Miltiades, 2001), none of these examined frailty, but this could be of importance since frailty is the first and still reversible step for the transition to disability (Clegg et al., 2013).
Given this background, the aim of the present study is to investigate the association between self-reported usual source of care among old people and the onset of frailty in a large cohort of North Americans at risk for or who have osteoarthritis, over a follow-up of 8 years. Based on previous studies, we hypothesized that persons using services other than FFS care would be at higher risk of developing frailty over time.
2. Methods
2.1. Data source and subjects
Data were obtained from the freely available (http://www.oai.ucsf.edu/) Osteoarthritis Initiative (OAI) database. Within the OAI, potential community-dwelling participants were recruited across four clinical sites in the United States of America (Baltimore, MD; Pittsburgh, PA; Pawtucket, RI; and Columbus, OH) between February 2004 and May 2006. In this database, people were included if they: (1) had knee OA with knee pain for a 30-day period in the past 12 months or (2) were at high risk of developing knee OA (Felson and Nevitt, 2004). The data were collected during the baseline and in screening evaluations and in subsequent evaluations over the 8 year period. All participants provided written informed consent.
The OAI study was given full ethics approval by the institutional review board of the OAI Coordinating Center, at the University of California in San Francisco.
2.2. Health care provider (exposure)
As part of the baseline data collection, trained interviewers asked each participant: “Where do you usually go for health care or advice about your health care”. The possible answers were: private doctor's office, public clinic, HMO, hospital outpatient clinic, emergency department (ED), or other. For the aims of this manuscript, data for ED and hospital clinics were merged.
2.3. Frailly (outcome)
In agreement with the Study of Osteoporotic Fracture (SOF) index (Ensrud et al., 2007; Veronese et al., 2017a) frailty was defined as the presence of ≥2 out of three of the following criteria:
weight loss ≥5% taking place between baseline and the follow-up examinations (at the baseline examination, a body mass index, BMI of < 20 kg/m2, a common cut-off for identifying underweight people the elderly (Veronese et al., 2015), was used, since no information regarding weight changes were recorded);
chair stand: the inability to rise from a chair five times without arm support (hereafter referred to as inability to carry out chair stands); and.
limited energy, based on the SF12 questionnaire response of either “a little of the time” or “none of the time” to the question “In the past 4 weeks, did you have a lot of energy?”
Assessment of the frailty outcome was made during the V01 (12 months), V03 (24 months), V05 (36 months), V06 (48 months), V08 (72 months) and V10 (96 months) evaluations compared to baseline.
2.4. Covariates
Multiple covariates were identified as potential confounding factors, including: body mass index (BMI); physical activity evaluated using the total score for the Physical Activity Scale for the Elderly scale (PASE) (Washburn et al., 1999); race; smoking history; educational attainment (college or higher vs. others); yearly income (< vs. ≥$50,000 or missing data); depressive symptoms assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) (Lewinsohn et al., 1997); and a validated general health measure of self-reported comorbidities assessed using the modified Charlson Comorbidity Index score (Katz et al., 1996); total energy intake per day (in kcal); medical insurance (categorized as yes vs. no); and transitions in health care during follow-up period assessed at the same time of frailty status.
2.5. Statistical analyses
Data on continuous variables were normally distributed according to the Kolmogorov-Smirnov test. Data were presented as means and standard deviation values (SD) for quantitative measures, and percentages for all categorical variables. p values were calculated using the Analysis of Variance (ANOVA) test with the Bonferroni's correction for continuous variables and the logistic regression analysis for categorical ones, taking people with private doctors as reference since this group was the largest.
To assess the relationship between health care provider and incident frailty, a Cox's regression analysis was conducted in which incident frailty was defined as the discrete “outcome,” time-to-event was the temporal factor, and the health care provider was the “exposure”. Deceased people were censored. The basic model was adjusted for age and sex. The fully adjusted model included also the following covariates: race (whites vs. others); body mass index (as continuous); education (degree vs. others); smoking habits (current and previous vs. others); yearly income (categorized as ≥ or < 50,000$ or missing data); Physical Activity Scale for Elderly score (as continuous); Charlson co-morbidity index; CES-D: Center for Epidemiologic Studies Depression Scale; total energy intake (as continuous); number of frailty indexes at baseline (one vs. none); medical insurance (yes vs. no); transitions in health care during follow-up period. Symptomatic knee OA (Veronese et al., 2017b) (i.e., the presence of painful knee and alterations suggestive for knee OA) was initially considered as covariate, but excluded since the prevalence was not difference across groups and was not associated with frailty at follow-up (p-value = 0.38 at the univariate analysis).
Multi-collinearity among covariates was assessed through variance inflation factor (VIF) (Miles, 2009), taking a cut-off of 2 as the criterion for exclusion. No covariates met this criterion and therefore none was excluded for this reason. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were calculated to estimate the strength of the associations between health care provider and incident frailty.
Several sensitivity analyses were conducted evaluating the interaction between health care provider and selected factors [i.e., age below or more/equal than 65 years, overweight/obese (≥25 kg/m2) vs. normal weight (18.5 kg/m2 < BMI ≥ 25 kg/m2), yearly income, gender, race, education, smoking habits, yearly income, number of frailty index at baseline categorized as one vs. none) in the association with frailty, but no one emerged as moderator of our findings (p > 0.10 for the interaction for all these parameters]. Finally, we used the propensity score that is a statistical matching technique that attempts to estimate the effect of a treatment by accounting for the covariates that predict receiving the treatment (Haukoos and Lewis, 2015). The propensity score, divided into quintiles, was estimated by using a logistic regression model regressing baseline health care provider on the above-mentioned covariates.
A p < 0.05 was deemed statistically significant. All analyses were performed using SPSS® software version 17.0 for Windows (SPSS Inc., Chicago, Illinois).
3. Results
3.1. Sample selection
The OAI dataset initially included a total of 4796 individuals. For 41 individuals, no information regarding their usual source of care was available, and 126 responded “others” when asked their health care provider. Twenty subjects were excluded because they were already frail at baseline, and 317 were excluded because no data regarding their frailty status were available during follow-up. Thus, 4292 participants were finally included.
3.2. Descriptive characteristics
The cohort consisted of 2519 females (58.7%) and 1773 males (41.3%), with a mean age of 61.3 years (±9.1 years; range: 45–79 years). A majority of this cohort (= 87.4%) reported a private doctor's clinic as their usual source of care.
Table 1 illustrates baseline characteristics by health care provider. Using participants reporting a private doctor's office as usual source of care as the reference (n = 3752), participants using a public clinic (n = 117) and those using hospital clinic/ED (n = 123) were significantly younger (p < 0.001 and p = 0.02, respectively), while no differences emerged in the comparison between HMO (n = 300) and those reporting a private doctor's office as usual care. There were no significant differences in terms of percentage of males across the 4 groups, except for a lower presence of males in those attending on a public clinic. People using a public clinic, HMO for their care and those using hospital/ED were less frequently whites, had a higher energy intake (except for HMO group), had lower education, and lower yearly income (Table 1). People using a public clinic or hospital/ED were less likely to have medical insurance than those using a private doctor.
Table 1.
Characteristics of the participants classified according to their baseline health care provider.
| Private doctors (n = 3752) |
Public clinic (n = 117) |
HMO (n = 300) |
Hospital clinic/ED (n = 123) |
||
|---|---|---|---|---|---|
| # or % (SD) | # or % (SD) | # or % (SD) | # or % (SD) | ||
| General characteristics | |||||
| Age (years) | 61.5 (9.1) | 57.8 (8.9)*** | 60.7 (9.1) | 59.4 (8.6)* | |
| Males (%) | 41.3 | 35.0*** | 42.0 | 47.2 | |
| PASE (points) | 162 (81) | 152 (84) | 158 (84) | 159 (88) | |
| White race (%) | 85.0 | 39.3*** | 63.3*** | 41.5*** | |
| Smoking (previous/current) (%) | 46.3 | 56.5 | 46.7 | 53.7 | |
| Daily energy intake (kcal) | 1393 (543) | 1655 (883)*** | 1454 (593) | 1583 (690)*** | |
| Graduate degree (%) | 31.9 | 13.7*** | 26.7*** | 17.2*** | |
| Yearly income (≥$50,000) (%) | 62.9 | 13.7*** | 52.7 | 26.8*** | |
| Medical insurance (%) | 89.8 | 57.1*** | 93.9 | 69.2*** | |
| Medical conditions | |||||
| BMI (kg/m2) | 28.5 (4.7) | 30.1 (5.5)*** | 29.4 (4.9)*** | 30.3 (5.2)*** | |
| CES-D (points) | 6.2 (6.6) | 12.4 (9.8)*** | 7.3 (6.7)* | 10.3 (9.1)*** | |
| Charlson co-morbidity index (points) | 0.4 (0.8) | 0.6 (1.0)* | 0.5 (1.1)* | 0.7 (1.0)** | |
| Frailty items | |||||
| Poor physical performance (%) | 10.0 | 29.1*** | 12.7 | 17.9*** | |
| Poor chair stands time (%) | 0.7 | 0.0 | 1.3 | 0.1 | |
| Weight loss (%) | 2.1 | 4.3 | 2.0 | 3.3 | |
Notes: The data are presented as means (with standard deviations) for continuous variables and number (with percentage).
p values were calculated using the Analysis of Variance (ANOVA) test with the Bonferroni’s correction for continuous variables and the logistic regression analysis for categorical ones, taking people with private doctors as reference.
Abbreviations: CES-D: Center for Epidemiologic Studies Depression Scale; PASE: Physical Activity Scale for the Elderly; BMI: body mass index; ED: emergency department; HMO: health maintenance organization.
p < 0.001.
p < 0.001.
p < 0.05.
People using a public clinic or HMO for their care and those using hospital/ED were more likely to be obese, reported significantly higher CES-D levels and poor physical performance (except for HMO) at baseline than those using a private doctor (Table 1), while no other significant differences emerged for the prevalence of frailty risk criteria.
3.3. Health care provider and incident frailty
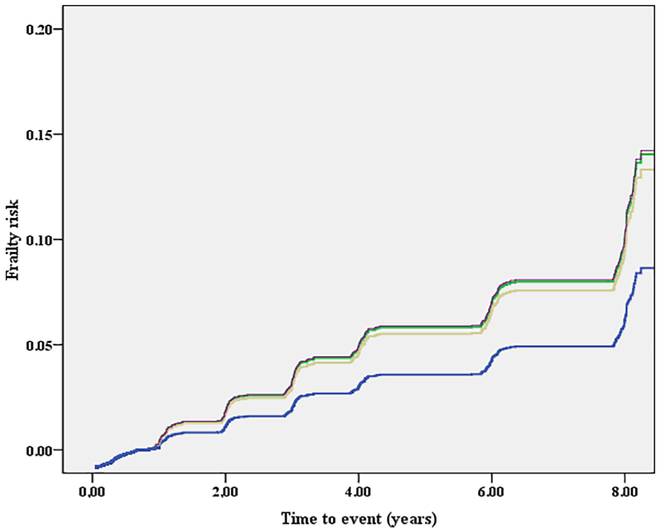
Over a mean follow-up of 8 years, 348 individuals (8.1% of the baseline population) became frail, for a global incidence of 12 (95%CI: 10–14) people per 1000 persons-years. It should be noted that of the 348 who became frail at some point during the follow-up period, 47 were no longer categorized as frail on subsequent assessments. The unadjusted incidence of frailty per 1000 persons per year was 10 for those using private doctors, 27 for those using public clinics, 17 for HMOs and 24 for those using a hospital clinic or ED (Fig. 1).
Fig. 1.
Association between different health settings and incident frailty, adjusted for potential confounders.
Legend: blue line identifies those using a private doctor's office; green those using a public clinic; yellow those using HMO; violet those attending to hospital/ED. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As shown in Table 2, the Cox's regression analysis, adjusting for 14 potential confounders, confirmed these findings. People using a public clinic for their care had a significantly higher risk of frailty of 56% (HR = 1.56; 95%CI: 1.07–2.70; p = 0.03); for those in HMOs, frailty rate was 48% higher (HR = 1.48; 95%CI: 1.03–2.24; p = 0.03), and those attending hospital/ED, there also was a significantly higher risk of 76% (HR = 1.76; 95%CI: 1.03–3.02; p = 0.04) (Table 2). Using a propensity-score divided in quintiles (between people using an HMO and those a private doctor), those in HMOs were at higher risk of frailty (HR = 1.52; 95%CI: 1.05–2.19; p = 0.03).
Table 2.
Association between baseline usual health care provider and incidence of frailty.
| Incidence per 1000 persons- year |
Basic adjusteda HR (95%CI) |
p value | Fully adjustedb HR (95%CI) |
p value | |
|---|---|---|---|---|---|
| Private doc- tors |
10 (9–12) | 1 [reference] | 1 [reference] | ||
| Public clinic |
27 (17–42) | 2.73 (1.69–4.41) |
< 0.001 | 1.56 (1.07–2.70) |
0.03 |
| HMO | 17 (12–23) | 1.65 (1.15–2.38) |
0.007 | 1.48 (1.03–2.24) |
0.03 |
| Hospital/ ED |
24 (15–37) | 2.72 (1.68–4.38) |
< 0.001 | 1.76 (1.03–3.02) |
0.04 |
Notes: All the data are presented as hazard ratios (HRs) with their 95% confidence intervals.
Abbreviations: CI: confidence intervals; HR: hazard ratio; ED: emergency department; HMO health maintenance organization.
Basic adjusted model included age and sex.
Fully adjusted model included as covariates: age (as continuous); sex; race (whites vs. others); body mass index (as continuous); education (degree vs. others); smoking habits (current and previous vs. others); yearly income (categorized as ≥ or < 50,000$ and missing data); Physical Activity Scale for Elderly score (as continuous); Charlson co-morbidity index; CES-D: Center for Epidemiologic Studies Depression Scale; total energy intake (as continuous); number of frailty indexes at baseline (one vs. none); medical insurance (yes vs. no); transitions in health care during follow-up period.
4. Discussion
In this longitudinal study, we found that during an 8-year follow-up period, compared to those using a private physician, individuals attending public clinic had a 56% higher risk of developing frailty, those attending HMO had a 48% higher risk, and people attending hospital clinic or ED as their usual source of care had 76% higher risk. These results were robust in the presence of several potential confounders at baseline and of transitions in self-reported health care during follow-up. While the data regarding public clinics and ED/hospital are hardly surprising, given expected concerns with continuity of care, the increased risk of frailty for HMO enrollees are more surprising. Previous studies comparing fee for service and HMO populations without regard to age, observe strong similarities in terms of processes of care (Miller and Luft, 1994; Sloss et al., 1987).
However, many authors have expressed concerns about care of older people with chronic diseases who are enrolled in HMOs. It is argued that HMOs' financial incentives may lead to reductions or delays in treatment, which may in turn exacerbate chronic illness and worsen functional status (Siu et al., 1986; Schlesinger, 1986; Hornbrook and Berki, 1985; Gillick, 1987). Indeed, HMO capitation payment systems do not distinguish between discretionary and necessary services in rewarding providers who contain costs by curtailing service use (Porell and Miltiades, 2001). Previously, in the 1990s, the Medical Outcomes Study reported a decline in physical health for older patients enrolled in HMOs compared to FFS (Ware Jr. et al., 1996). Similarly, Ware et al. observed that older HMO enrollees experience decline in physical health at rates almost double those for persons using FFS plans (Ware Jr. et al., 1996). Consistent with our findings, Porell et al. report that, among functionally independent people, the risk of becoming disabled in activities of daily living within a year were significantly lower among FFS individuals than HMO enrollees; while among older people who were already functionally impaired, neither HMO enrollment nor private supplementary insurance affected the risk of further functional decline (Porell and Miltiades, 2001). Shaughnessy et al. found a consistent pattern of superior outcomes for home health FFS patients, including reduction in the number of disabilities (Shaughnessy et al., 1994). In general, it appears that HMOs tend to approach some aspects of home health care with greater concerns for cost containment rather than focusing on rehabilitation, since fewer visits are provided, fewer personal care services are given, and there is a stronger orientation toward a medical approach (Shaughnessy et al., 1994).
In our study, people using a public clinic or an HMO had a significantly lower yearly income than those using private physicians. Ware et al. associated poverty with worse outcomes among HMOs patients (Ware Jr. et al., 1986). In their study, the low income group assigned to the HMO reported significantly more bed-days per year, more serious symptoms, and also a greater risk of death than those assigned free FFS care (Ware Jr. et al., 1986). Analogously, in another study of over 2000 patients during a 4-year follow-up period, older and poor chronically ill patients had worse physical health outcomes in HMOs than in FFS systems (Ware Jr. et al., 1996). However, it should be noted that in our research, we adjusted our analyses for income and the findings remain robust. Finally, we found that people using a public clinic or HMO for their care and those using hospital/ED were more likely to be non-white. Lower socioeconomic status and lower rates of health insurance coverage for those using hospital clinics or EDs among African-Americans and Hispanics could influence our findings. Moreover, it has been observed that people in these ethnic groups who are enrolled in managed care plans are at a higher risk than are whites in these plans (Cunningham and Kohn, 2000). In this context, other international studies reported that low socioeconomic status can be a strong risk factor for frailty. For example, in 11,390 community-dwelling European older people, 61.8% of the variation of poverty risk on frailty level was explained by direct and indirect effects. In particular, psychosocial factors such as perceived control and social isolation are among the strongest contributors of frailty status (Stolz et al., 2017). On the other hand, there are potential positive aspects of HMOs for addressing racial and ethnic disparities, as enrollees are more likely to have a usual source of care, a higher probability of a recent physician visit and lower use of the ER, regardless of race (Hargraves et al., 2001).
Finally, people using a HMO were more likely to be obese and more likely to be depressed than those having a private doctor. These two conditions are important independent risk factors for frailty. The association between BMI and frailty has been shown to be U-shaped (Hubbard et al., 2010; Porter Starr et al., 2014), with obesity at mid-life associated with increased risk of frailty and disability during advanced ages (Stenholm et al., 2014). The relationship between frailty and depression is important, too. Depression is a reversible risk factor for frailty, as noted t in our systematic review and meta-analysis which showed that depressed people had four-fold increased risk of becoming frail, even after accounting for potential confounders (Soysal et al., 2017). The increased prevalence of obesity and depression among people using an HMO, may partially explain the observed higher risk of developing frailty among HMO enrollees, than among those having a private doctor. Since physical exercise seems to have important antidepressant effect (Schuch et al., 2016a; Schuch et al., 2016b), we strongly recommend to encourage this kind of intervention in people attending on HMO. Several practice implications are suggested by these findings. For example, because exercise is a strategy for achieving healthy weight and also because exercise is associated decreases in symptoms of depression, HMO's might consider implementing age and health appropriate exercise interventions.
The findings of our study should be interpreted within its limitations. First, the participants enrolled in the OAI are at high risk of knee OA or they have OA, and therefore may have increased risk for incident frailty. Consequently, the application of our findings to the general population is limited. Second, due to the limited number of cases, we were not able to separate those who use EDs as their usual source of care from those who use hospital-based clinics. Thus, further research is needed to determine how the care provided in each of these settings might be altered to reduce the risk of frailty. Third, the identification of incident frailty is hampered by the fact that the definitions of frailty at baseline and at follow-up were somewhat different because weight was not recorded at baseline. Fourth, the comorbidities were self-reported, potentially introducing bias into our findings. Fifth, we were able to categorize the presence of medical insurance only as yes vs. no, while it is likely that this confounder can affect in different ways the onset of frailty. Sixth, the population who go to a hospital ED is very different from those whose usual source of care is a private doctor's office or a public clinic or an HMO, potentially introducing another source of bias, though we made every effort to address this by adjusting for potential confounders. Finally, this project was conducted solely with a population receiving care in the United States. Because of substantial differences in health care systems across countries, it is difficult to apply the findings more broadly. However, to the degree that certain aspects of a health system (i.e., capitated managed populations fee for service care) are used for care, the findings may be informative. Certainly, observations regarding patient specific risk factors (e.g., depression, obesity, poverty) are applicable to patients in other countries.
5. Conclusions
In this study, participants who report private FFS physicians as their usual source of care are at lower risk of developing frailty, even after considering the influence of several potentially important confounders. Research has identified many shortcomings in the care of old people, including lack of comprehensive assessment, of early detection of predisability status, and of coordination and continuity of care. Our work suggests that there are lost opportunities for identifying those at high risk for developing frailty and providing effective interventions, particularly in organizations such as HMOs with capitated populations. Identifying specialized structures and programs for management and prevention of disability in old people should be considered. Future longitudinal studies, with fewer differences in baseline characteristics and in the general population, are needed to better understand whether people attending on HMOs are at increased risk of frailty and which interventions are more efficacious in preventing frailty in this specific setting.
Acknowledgements
Funding sources: The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. NS and JRH were supported by the United States National Institute for Diabetes, Digestive and Kidney Diseases (grant no. R44DK103377).
Sponsor's role: the sponsors had no role in the design, methods, subject recruitment, data collection, analysis or preparation of this paper.
Footnotes
Conflict of interest: Prof. RB serves on non-profit Board of Directors for American Federation for Aging Research and Neighborhood Health Plan of Rhode Island.
References
- Beard JR, Bloom DE, 2015. Towards a comprehensive public health response to population ageing. Lancet 385 (9968), 658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton LC, Weiner JP, Stevens GD, Kasper J, 2002. Health outcomes and Medicaid costs for frail older individuals: a case study of a MCO versus fee-for-service care. J. Am. Geriatr. Soc. 50 (2), 382–388. [DOI] [PubMed] [Google Scholar]
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K, 2013. Frailty in elderly people. Lancet 381 (9868), 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham PJ, Kohn L, 2000. Health plan switching: choice or circumstance? Health Aff. 19 (3), 158–164. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Taylor BC, et al. , 2007. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J. Gerontol. A Biol. Sci. Med. Sci. 62 (7), 744–751. [DOI] [PubMed] [Google Scholar]
- Felson DT, Nevitt MC, 2004. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum. Dis. Clin. North Am. 30 (4), 783–797. [DOI] [PubMed] [Google Scholar]
- Gaynor Martin, Rebitzer James B., Taylor Lowell J., 2004. Physician incentives in health maintenance organizations. J. Polit. Econ. 112 (4), 915–931. [Google Scholar]
- Gillick MR, 1987. The impact of health maintenance organizations on geriatric care. Ann. Intern. Med. 106 (1), 139–143. [DOI] [PubMed] [Google Scholar]
- Hargraves JL, Cunningham PJ, Hughes RG, 2001. Racial and ethnic differences in access to medical care in managed care plans. Health Serv. Res. 36 (5), 853–868. [PMC free article] [PubMed] [Google Scholar]
- Haukoos JS, Lewis RJ, 2015. The propensity score. JAMA 314 (15), 1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellinger FJ, 1998. The effect of managed care on quality: a review of recent evidence. Arch. Intern. Med. 158 (8), 833–841. [DOI] [PubMed] [Google Scholar]
- Hornbrook MC, Berki SE, 1985. Practice mode and payment method. Effects on use, costs, quality, and access. Med. Care 23 (5), 484–511. [DOI] [PubMed] [Google Scholar]
- Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K, 2010. Frailty, body mass index, and abdominal obesity in older people. J. Gerontol. A Biol. Sci. Med. Sci. 65 (4), 377–381. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW, 1996. Can comorbidity be measured by questionnaire rather than medical record review? Med. Care 34 (1), 73–84. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB, 1997. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging 12 (2), 277–287. [DOI] [PubMed] [Google Scholar]
- McMillan GJ, Hubbard RE, 2012. Frailty in older inpatients: what physicians need to know. QJM 105 (11), 1059–1065. [DOI] [PubMed] [Google Scholar]
- Miles J, 2009. Tolerance and variance inflation factor. In: Wiley StatsRef: Statistics Reference Online. [Google Scholar]
- Miller RH, Luft HS, 1994. Managed care plan performance since 1980. A literature analysis. JAMA 271 (19), 1512–1519. [PubMed] [Google Scholar]
- Morley JE, Vellas B, van Kan GA, et al. , 2013. Frailty consensus: a call to action. J. Am. Med. Dir. Assoc. 14 (6), 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porell FW, Miltiades HB, 2001. Disability outcomes of older Medicare HMO enrollees and fee-for-service Medicare beneficiaries. J. Am. Geriatr. Soc. 49 (5), 615–631. [DOI] [PubMed] [Google Scholar]
- Porter Starr KN, McDonald SR, Bales CW, 2014. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J. Am. Med. Dir. Assoc. 15 (4), 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M, 1986. On the limits of expanding health care reform: chronic care in prepaid settings. Milbank Q. 64 (2), 189–215. [PubMed] [Google Scholar]
- Schuch FB, Deslandes AC, Stubbs B, Gosmann NP, Silva CT, Fleck MP, 2016a. Neurobiological effects of exercise on major depressive disorder: a systematic review. Neurosci. Biobehav. Rev. 61, 1–11. [DOI] [PubMed] [Google Scholar]
- Schuch FB, Vancampfort D, Rosenbaum S, et al. , 2016b. Exercise for depression in older adults: a meta-analysis of randomized controlled trials adjusting for publication bias. Rev. Bras. Psiquiatr. 38 (3), 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy PW, Schlenker RE, Hittle DF, 1994. Home health care outcomes under capitated and fee-for-service payment. Health Care Financ. Rev. 16 (1), 187–222. [PMC free article] [PubMed] [Google Scholar]
- Siu AL, Brook RH, Rubenstein LZ, 1986. Medicare capitation and quality of care for the frail elderly. Health Care Financ. Rev. 57–63 (Spec No). [PMC free article] [PubMed] [Google Scholar]
- Sloss EM, Keeler EB, Brook RH, Operskalski BH, Goldberg GA, Newhouse JP, 1987. Effect of a health maintenance organization on physiologic health. Results from a randomized trial. Ann. Intern. Med. 106 (1), 130–138. [DOI] [PubMed] [Google Scholar]
- Soysal P, Veronese N, Thompson T, et al. , 2017. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res. Rev. 36, 78–87. [DOI] [PubMed] [Google Scholar]
- Stenholm S, Strandberg TE, Pitkala K, Sainio P, Heliovaara M, Koskinen S, 2014. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland Follow-up Survey. J. Gerontol. A Biol. Sci. Med. Sci. 69 (1), 73–78. [DOI] [PubMed] [Google Scholar]
- Stolz E, Mayerl H, Waxenegger A, Freidl W, 2017. Explaining the impact of poverty on old-age frailty in Europe: material, psychosocial and behavioural factors. Eur. J. Pub. Health 27 (6), 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese N, Cereda E, Solmi M, et al. , 2015. Inverse relationship between body mass index and mortality in older nursing home residents: a meta-analysis of 19,538 elderly subjects. Obes. Rev. 16 (11), 1001–1015. [DOI] [PubMed] [Google Scholar]
- Veronese N, Stubbs B, Noale M, et al. , 2017a. Polypharmacy is associated with higher frailty risk in older people: an 8-year longitudinal cohort study. J. Am. Med. Dir. Assoc. 18 (7), 624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese N, Shivappa N, Stubbs B, et al. , 2017b. The relationship between the dietary inflammatory index and prevalence of radiographic symptomatic osteoarthritis: data from the Osteoarthritis Initiative. Eur. J. Nutr 10.1007/s00394-017-1589-6. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr., Brook RH, Rogers WH, et al. , 1986. Comparison of health outcomes at a health maintenance organisation with those of fee-for-service care. Lancet 1 (8488), 1017–1022. [DOI] [PubMed] [Google Scholar]
- Ware JE Jr., Bayliss MS, Rogers WH, Kosinski M, Tarlov AR, 1996. Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. Results from the Medical Outcomes Study. JAMA 276 (13), 1039–1047. [PubMed] [Google Scholar]
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA, 1999. The physical activity scale for the elderly (PASE): evidence for validity. J. Clin. Epidemiol. 52 (7), 643–651. [DOI] [PubMed] [Google Scholar]