Abstract
Objective
To evaluate the predictive value of mean perfusion pressure (mPP) in the development of acute kidney injury (AKIN) after transcatheter aortic valve implantation (TAVI).
Methods
One hundred and forty seven consecutive patients with aortic stenosis (AS) were evaluated for this study and 133 of them were included. Mean arterial pressure (mAP) and central venous pressure (CVP) were used to calculate mPP before TAVI procedure (mPP = mAP-CVP). The occurrence of AKIN was evaluated with AKIN classification according to the Valve Academic Research Consortium-2 recommendations. The patients were divided into two groups according to the receiver operating characteristic (ROC) analysis of their mPP levels (high-risk group and low-risk group).
Results
The AKIN prevalence was 22.6% in this study population. Baseline serum creatinine level, glomerular filtration rate, amount of contrast medium, and the level of mPP were determined as predictive factors for the development of AKIN.
Conclusion
The occurrence of AKIN is associated with increased morbidity and mortality rates in patients with TAVI. In addition to the amount of contrast medium and basal kidney functions, our study showed that lower mPP was strongly associated with development of AKIN after TAVI.
Keywords: Transcatheter Aortic Valve Replacement, Kidney, Acute Kidney Injury, Perfusion
| Abbreviations, acronyms & symbols | ||||
|---|---|---|---|---|
| AKIN | = Acute kidney injury | GFR | = Glomerular filtration rate | |
| AR | = Aortic regurgitation | HR-G | = High-risk group | |
| AS | = Aortic stenosis | LR-G | = Low-risk group | |
| AUC | = Area under the curve | mAP | = Mean arterial pressure | |
| AVA | = Aortic valve area | mPP | = Mean perfusion pressure | |
| CAD | = Coronary artery disease | NRF | = Normal renal functions | |
| CHF | = Congestive heart failure | PCI | = Percutaneous coronary intervention | |
| CI | = Confidence interval | RBC | = Red blood cell | |
| CM | = Contrast mediums | ROC | = Receiver operating characteristic | |
| COPD | = Chronic obstructive pulmonary disease | SAVR | = Surgical aortic valve replacement | |
| CrCl | = Creatinine clearance | SBP | = Systolic blood pressure | |
| CVP | = Central venous pressure | STS | = Society of Thoracic Surgeons | |
| CT | = Computed tomography | TAVI | = Transcatheter aortic valve implantation | |
| DBP | = Diastolic blood pressure | VARC-2 | = Valve Academic Research Consortium-2 | |
| DM | = Diabetes mellitus | |||
INTRODUCTION
Aortic stenosis (AS) is one of the most common cardiac degenerative valvular diseases, with a prevalence of 3-5% in patients above 75 years of age[1]. Surgical aortic valve replacement (SAVR) is currently considered the gold standard treatment for severe symptomatic AS[2]. Transcatheter aortic valve implantation (TAVI) has emerged as an alternative to surgery for patients with severe symptomatic AS, particularly for those who were considered at intermediate to high risk for surgery[3,4]. Since its introduction in 2002, more than 200,000 patients have undergone TAVI globally.
Ageing, preexisting impaired kidney function, hemodynamic instability, congestive heart failure (CHF), diabetes mellitus (DM), anaemia, and the usage of great amount of contrast mediums (CM) are well-known risk factors for the development of acute kidney injury (AKIN) after TAVI[5,6].
Although these risk factors have been well known, the predictive value of mean perfusion pressure (mPP) in the development of AKIN has not been investigated yet. Therefore, in this study, we aimed to evaluate the predictive value of mPP in the development of AKIN after TAVI.
METHODS
In this study, 147 consecutive patients who had undergone TAVI procedure in our clinic between June 2013 and December 2015 were evaluated. One hundred and thirty three of them met the inclusion criteria and were included in this study. Patients who had invasive blood pressure monitorization, jugular venous catheter, and were hemodynamically stable were included in our study. Patients who had undergone renal replacement therapy, presented glomerular filtration rate (GFR) <30 ml/min/1.73 m2, had decompensated heart failure, received inotropic agents, had intra-aortic balloon pump, and received CM within the last 48 hours were excluded. Patients who had undergone additional procedures or received additional CM due to vascular complications, and had chronic pulmonary diseases or chronic liver diseases were also excluded from the study. Intravenous hydration therapy was started in patients with GFR <50 ml/min/1.73 m2 for 12 hours before the procedure and continued for 24-48 hours after TAVI. Patients' daily blood tests including creatinine were checked for three days before and three days after TAVI.
Mean Perfusion Pressure
Blood pressure of every patient was monitored using invasive monitorization during the 12 hours before TAVI procedure. Also during this period, mean arterial pressure (mAP) calculated by monitors was entered in patient files. Central venous pressure (CVP) was monitored with catheters implanted by anesthesiologists in all patients before valve implantation. The mPP was calculated using the formula MPP = mAP-CVP.
Acute Kidney Injury
The Valve Academic Research Consortium-2 (VARC-2) criteria were used to evaluate any complication occuring in TAVI patients[7]. VARC-2 recommends that the AKIN system should be used to diagnose AKIN. According to the AKIN system:
AKIN Stage 1: Increase in serum creatinine of 150-199% (1.5-1.99 × increase compared with baseline) or increase of ≥0.3 mg/dL (≥26.4 mmol/L) or urine output <0.5 mL/kg/h for >6 h but <12 h;
AKIN Stage 2: Increase in serum creatinine of 200-299% (2.0-2.99 × increase compared with baseline) or urine output <0.5 mL/kg/h for >12 h but <24 h;
AKIN Stage 3: Increase in serum creatinine of ≥300% (>3 × increase compared with baseline) or serum creatinine of ≥4.0 mg/dL (≥354 mmol/L) with an acute increase of at least 0.5 mg/dL (44 mmol/L) or urine output <0.3 ml/kg/h for >24 h or anuria for >12 h.
TAVI Procedure
Severe AS was diagnosed with echocardiographic methods. The situations of average aortic gradient > 40 mmHg, aortic valve area (AVA) <1 cm2, and valve area index (valve area/body surface area) <0.6 cm2 were considered to be severe AS[8]. The balloon-expandable Edwards Sapien XT valve (Edwards Lifesciences, Irvine, California, USA) was used for TAVI process. Vascular occlusion device (ProStar XL, Abbott Laboratories, North Chicago, Illinois, USA) was used in eligible patients in terms of femoral artery diameter and anatomy. The surgical cut-down method was applied in patients who were unsuitable for using iliac and femoral artery anatomy vascular closure device. Transoesophageal echocardiography and multislice computed tomography (CT) tests were done to determine the diameter of the aortic bioprosthesis implanted. In all patients, clopidogrel 75 mg and acetylsalicylic acid 100 mg were started before TAVI procedure. İohexol (Omnipaque, GE Healthcare), a nonionic low-osmolar monomeric CM, was used as the opaque material. The amount of CM was recorded during all TAVI procedure. Examinations such as CT and coronary angiography that required administration of CM, except TAVI procedures, were performed at least 72 hours before. Daily kidney function tests of all patients were monitored in our centre from admission to discharge. Creatinine levels were checked in the second week, and the first, third, sixth and twelfth month after TAVI procedure (COBAS Integra 400 plus, Roche Diagnostics).
The patients were prospectively followed during one year after TAVI. The informed consent form was obtained from each subject, and the study was conducted in accordance with the Helsinki Declaration. The study protocol was approved by the local ethics committee.
Study Groups
Based on the receiver operating characteristic (ROC) analysis, patients who had lower mPP values (<72 mmHg), which was determined as the threshold value for AKIN development, were included in the high-risk group (HR-G), and those who had higher mPP values (≥72 mmHg) were included in the low-risk group (LR-G). Additionally, characteristics of patients with normal renal functions (NRF) and those who developed AKIN were evaluated.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Chicago, Illinois, USA). The Kolmogorov-Smirnov test was used to assess the normality of distributions. Variables not normally distributed were expressed as medians (interquartile ranges). Normally distributed continuous variables were expressed as a mean ± standard deviation. The means for normally distributed continuous variables were compared by independent-samples t-tests. Skew-distributed continuous variables were compared using a Mann-Whitney U test. Pearsons x2 test and Fisher exact test were used to compare categorical variables. Univariate analyses were performed with the variables, such as age (years), left ventricular ejection fraction (%), mean aortic gradient (mmHg), Society of Thoracic Surgeons (STS) score (%), logistic EuroSCORE (%), mPP (mmHg), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), DM (%), hypertension (%), coronary artery disease (CAD, %), creatinine (mg/dl), GFR (ml/dk/1.73 m2), amount of CM (ml), pre-TAVI haemoglobin levels (g/dl), and red blood cell transfusion after TAVI (%). The backward stepwise multivariate regression analysis was performed with the variables of mPP, left ventricular ejection fraction, baseline creatinine, GFR, red blood cell (RBC) transfusion, amount of CM, SBP, and DBP; the P values of those was found to be P<0.10, by univariate analyses. A ROC curve analysis was performed to identify the optimal cut-off point of mPP to predict AKIN in patients with severe AS. The area under the curve (AUC) values were calculated as a measure of test accuracy. A two-sided P<0.05 was considered significant within a 95% confidence interval (CI). Kaplan-Meier survival plots were constructed from the index procedure and up to one year after that and compared using the log-rank test. P values <0.05 were accepted as statistically significant.
RESULTS
Patients' General Characteristics
One hundred and thirty three patients (54.1% females; mean age of 78.1±7.5 years) were enrolled in this study. AKIN was recorded in 30 patients after TAVI (22.6%). Mean values of SBP, DBP, mAP, and mPP were lower in HR-G and AKIN groups. The mean value of CVP was higher in HR-G and AKIN groups. The average mean gradient of the aortic valve was 49.7±11.7 mmHg, the mean AVA was 0.65±0.11 cm2, and the mean value of left ventricular ejection fraction was 42 ±14.7%.
Procedural Data
A balloon-expandable Edwards SAPIEN XT valve was implanted via transfemoral access in all patients. The mean radiation time was 7.6±3.1 min. Mean CM and duration of procedure were 143.4±22.7 ml and 69.2±28.1 min, respectively. There weren't any significant difference between the two groups according to the numbers and durations of the rapid ventricular pacing.
Mean Perfusion Pressure and Acute Kidney İnjury
The values of mPP were significantly lower in AKIN patients (65.9±9.5 vs. 76.3±7.4 P<0.001). Age, gender, DM, hypertension, chronic obstructive pulmonary disease (COPD), CAD, moderate to high grade aortic regurgitation (AR) after TAVI, risk scores for TAVI, haemoglobin, cardiac systolic functions, mean aortic gradient, kidney function tests, amount of CM, previous coronary bypass surgery, and percutaneous coronary intervention (PCI) rates were similar between the two groups. SBP, DBP, mAP, and mPP levels were significantly lower in HR-G group, but CVP level was higher in HR-G group (Table 1).
Table 1.
Clinical, laboratory, echocardiographic, and angiographic characteristics of the study population
| HR-G (n=52) | LR-G (n=81) | P value | |
|---|---|---|---|
| Age (years) | 78.8±6.9 | 78.6±7.8 | 0.369 |
| Female gender, n (%) | 29 (55.8) | 43 (53.1) | 0.451 |
| STS score (%) | 13.3±4.3 | 14.6±6.7 | 0.472 |
| Logistic EuroSCORE (%) | 29.4 (15.9-38.2) | 24.7 (14.8-35.3) | 0.093 |
| AKIN, n (%) | 22 (42.3) | 8 (9.9) | <0.001 |
| Mortality, n (%) | 5 (9.6) | 3 (3.7) | 0.153 |
| Hemoglobin (g/dl) | 12.3±1.6 | 12.4±1.8 | 0.860 |
| RBC transfusion, n (%) | 15 (28.8) | 20 (24.7) | 0.785 |
| Left ventricle ejection fraction (%) | 41.9±10.4 | 42.6±11.1 | 0.726 |
| NT-pro BNP (pg/ml) | 4188±1388 | 3811±1258 | 0.640 |
| AVA (cm2) | 0.65±0.19 | 0.64±0.21 | 0.506 |
| Mean gradient (mmHg) | 49.1±12.9 | 50.2±10.6 | 0.590 |
| SBP (mmHg) | 120.1±9.8 | 132.7±7.3 | <0.001 |
| DBP (mmHg) | 57.4±8.1 | 69.6±9.4 | <0.001 |
| mAP (mmHg) | 78.1±9.7 | 90.5±8.3 | <0.001 |
| CVP (mmHg) | 13.2±2.9 | 10.9±2.5 | <0.001 |
| mPP (mmHg) | 64.1±7.1 | 79.7±5.3 | <0.001 |
| Creatinine (mg/dl) | 1.06±0.32 | 1.01±0.29 | 0.642 |
| eGFR (ml/dk/1.73 m2) | 60.15±14.6 | 62.69±16.7 | 0.348 |
| Diuretic, n (%) | 10 (19.2) | 13 (16.0) | 0.443 |
| RAAS blocker, n (%) | 24 (46.2) | 40 (49.4) | 0.214 |
| Beta-blocker, n (%) | 29 (55.7) | 50 (61.7) | 0.494 |
| Amount of contrast (ml) | 147 (115-245) | 140.5 (120-212) | 0.065 |
| AR after TAVI (≥ grade II) | 6 (11.5) | 10 (12.3) | 0.646 |
| Number of rapid pacing | 2.9±0.6 | 2.7±0.4 | 0.348 |
| Rapid pacing duration (second) | 41 (25-63) | 40 (21-55) | 0.642 |
| Previous CABG, n (%) | 10 (19.2) | 13 (16.0) | 0.443 |
| Previous PCI, n (%) | 9 (17.3) | 19 (23.8) | 0.254 |
| Diabetes mellitus, n (%) | 19 (36.5) | 27 (33.3) | 0.422 |
| Hypertension, n (%) | 21 (40.6) | 34 (41.9) | 0.908 |
| Hypercholesterolemia, n (%) | 20 (38.5) | 40 (49.4) | 0.145 |
| COPD, n (%) | 20 (38.5) | 26 (32,1) | 0.285 |
| CAD, n (%) | 25 (48.1) | 40 (49.4) | 0.512 |
| Intensive care unit (days) | 2 (1.3-3.2) | 1.5 (1.1-2.1) | 0.022 |
| Hospital duration (days) | 5 (3.5-6.2) | 4 (3.3-5.8) | 0.027 |
Values are number (%), mean ± standard deviation, or median [25th, 75th percentiles].
AKIN=acute kidney injury; AR=aortic regurgitation; AVA=aortic valve area; BNP=b-type natriuretic peptide; CABG=coronary artery bypass graft; CAD=coronary artery disease; COPD=chronic obstructive pulmonary disease; CVP=central venous pressure; DBP=diastolic blood pressure; eGFR=estimated glomerular filtration rate; HR-G=High-risk group; LR-G=Low-risk group; mAP=mean arterial pressure; mPP=mean perfusion pressure; NT=N-terminal; PCI=percutaneous coronary intervention; RAAS=renin-angiotensin-aldosterone system; RBC=red blood cell; SBP=systolic blood pressure; STS=Society of Thoracic Surgeons; TAVI=transcatheter aortic valve implantation
AKI and Risk Factors
There was no significant difference between AKIN and NRF groups in terms of CAD, DM, hypertension, and COPD. The AKIN group had longer hospitalisation duration (5.5 vs. 4 days, P=0.014). After TAVI procedure, 2 (2.2%) patients needed permanent pacemaker implantation due to atrioventricular conduction block. Transfusion rates were higher in the AKIN group (13 vs. 22, P=0.032). Patients in AKIN and NRF groups did not show a significant difference in terms of diuretic, renin-angiotensin-aldosterone blocker, and beta-blocker therapy (Table 1). The amount of CM was significantly higher in the AKIN group (148 vs. 138 ml, P=0.028). Eight patients died during the study period. Total mortality was higher in the AKIN group than in the NRF group (16.6% vs. 2.9%, P=0.015; Table 2).
Table 2.
Baseline characteristics in patients with or without AKIN (NRF group).
| AKIN group (n=30) | NRF (n=103) | P value | |
|---|---|---|---|
| Age (years) | 78.2±7.3 | 77.6±8.1 | 0.699 |
| Female gender, n (%) | 14 (46.7) | 58 (56.3) | 0.234 |
| STS score (%) | 14.4±5.3 | 13.2±5.7 | 0.154 |
| Logistic EuroSCORE (%) | 27.5 (14.7-36.1) | 26.5 (15.0-37.1) | 0.742 |
| Mortality, n (%) | 5 (16.6) | 3 (2.9) | 0.015 |
| Hemoglobin (g/dl) | 11.1±1.7 | 11.3±1.8 | 0.716 |
| RBC transfusion, n (%) | 13 (43.3) | 22 (21.4) | 0.032 |
| Left ventricle ejection fraction, % | 41.9±10.4 | 42.6±11.1 | 0.245 |
| NT-pro BNP (pg/ml) | 4315±1492 | 3656±1168 | 0.524 |
| AVA (cm2) | 0.61±0.22 | 0.68±0.28 | 0.605 |
| Mean gradient (mmHg) | 51.5±13.8 | 49.2±10.8 | 0.355 |
| SBP (mmHg) | 121.9±10.6 | 129.5±9.7 | <0.001 |
| DBP (mmHg) | 57.8±8.1 | 66.9±7.7 | <0.001 |
| mAP (mmHg) | 79.5±7.8 | 87.5±6.9 | <0.001 |
| CVP (mmHg) | 13.6±3.2 | 11.2±2.6 | <0.001 |
| mPP (mmHg) | 65.9±9.5 | 76.3±7.4 | <0.001 |
| Creatinine (mg/dl) | 1.17±0.36 | 0.99±0.26 | 0.003 |
| eGFR (ml/dk/1.73 m2) | 53.4±15.0 | 64.1±15.4 | 0.001 |
| Diuretic, n (%) | 8 (26.7) | 15 (14.6) | 0.548 |
| RAAS blocker, n (%) | 16 (53.3) | 48 (46.6) | 0.214 |
| Beta-blocker, n (%) | 18 (60.0) | 51 (49.5) | 0.406 |
| Amount of contrast (ml) | 148 (114-258) | 138 (120-225) | 0.028 |
| AR after TAVI (≥ grade II), n (%) | 5 (16.7) | 11 (10.7) | 0.646 |
| Number of rapid ventricular pacing, n | 3±0.8 | 2.7±0.4 | 0.442 |
| Rapid pacing duration (second) | 43 (26-67) | 40.5 (22-53) | 0.721 |
| Previous CABG, n (%) | 7 (23.3) | 16 (15.5) | 0.143 |
| Previous PCI, n (%) | 8 (26.7) | 20 (19.4) | 0.254 |
| Diabetes mellitus, n (%) | 10 (33.3) | 36 (35.0) | 0.527 |
| Hypertension, n (%) | 11 (36.7) | 44 (42.7) | 0.174 |
| Hypercholesterolemia, n (%) | 11 (36.7) | 49 (47.6) | 0.199 |
| COPD, n (%) | 12 (40.0) | 34 (33.0) | 0.309 |
| CAD, n (%) | 14 (46.7) | 51 (49.5) | 0.424 |
| Intensive care unit (days) | 2 (1.3-3.4) | 1.5 (1.1-1.8) | 0.001 |
| Hospital duration (days) | 5.5 (3.4-6.8) | 4 (3.1-5.2) | 0.014 |
Values are number (%), mean ± standard deviation, or median [25th, 75th percentiles].
AKIN=acute kidney injury; AR=aortic regurgitation; AVA=aortic valve area; BNP=b-type natriuretic peptide; CABG=coronary artery bypass graft; CAD=coronary artery disease; COPD=chronic obstructive pulmonary disease; CVP=central venous pressure; DBP=diastolic blood pressure; eGFR=estimated glomerular filtration rate; HR-G=High-risk group; LR-G=Low-risk group; mAP=mean arterial pressure; mPP=mean perfusion pressure; NT=N-terminal; PCI=percutaneous coronary intervention; RAAS=renin-angiotensin-aldosterone system; RBC=red blood cell; SBP=systolic blood pressure; STS=Society of Thoracic Surgeons; TAVI=transcatheter aortic valve implantation
AKIN Predictors and Survival
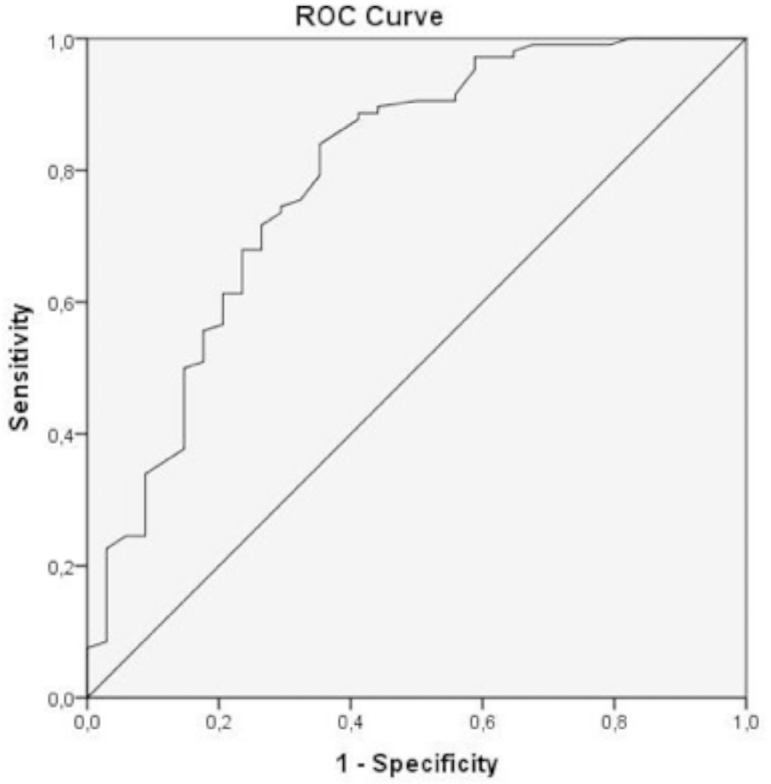
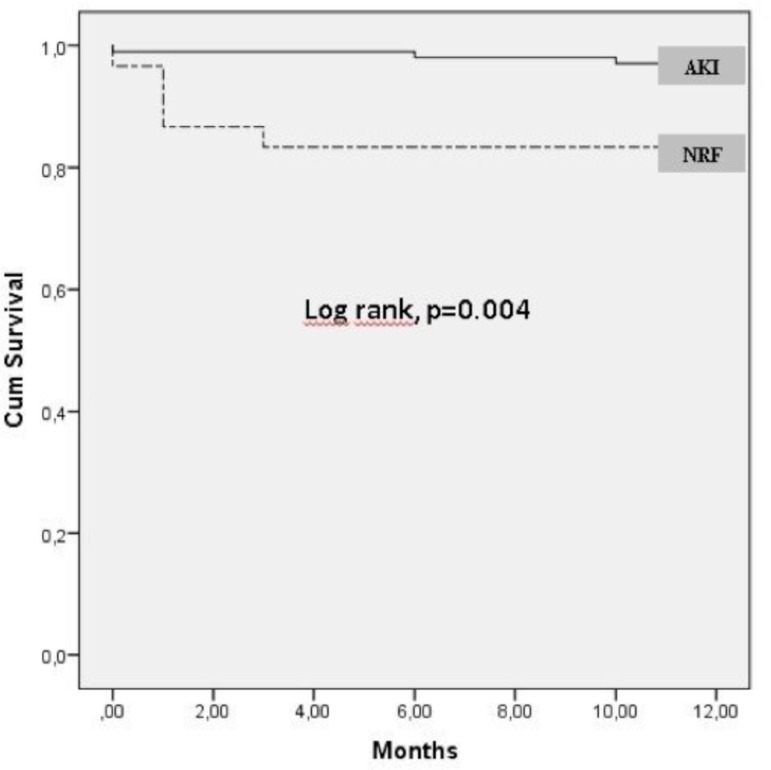
In univariate analysis, mPP, left ventricle ejection fraction, baseline creatinine, GFR, red blood cell transfusion, amount of CM, SBP, and DBP were found to be significantly associated with AKIN (P<0.01 for all parameters). Thus, the multivariate regression analysis was performed with these variables; baseline creatinine, baseline GFR, amount of CM, and mPP were found to be significant predictors of AKIN (Table 3). The ROC analysis of the significant variables in multivariate regression analysis revealed that the cut-off value of mPP was 72 mmHg to predict the development of AKIN (AUC, 0.813; 95% CI, 0.721-0.905; sensitivity, 72%; specificity, 84%; Figure 1). Mortality rates were significantly higher in AKIN patients. Kaplan-Meier survival curves for patients with and without AKIN (NRF group) showed a significantly lower survival rate up to 1 year in the overall AKIN group (16.6% vs. 0.03% log-rank, P=0.02; Figure 2).
Table 3.
Results of multivariate regression analysis for predictors of post-TAVI AKIN.
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| mPP (mmHg) | 5.1 | 2.7-8.5 | 0.013 |
| Amount of contrast (ml) | 7.0 | 3.2-11.1 | 0.008 |
| RBC transfusion, n | 0.7 | 0.3-1.4 | 0.385 |
| Baseline creatinine (mg/dl) | 2.1 | 1.1-3.3 | 0.044 |
| Baseline GFR (ml/min/1.73 m2) | 2.6 | 1.4-4.1 | 0.032 |
| SBP (mmHg) | 0.5 | 0.2-1.1 | 0.782 |
| DBP (mmHg) | 0.6 | 0.4-1.1 | 0.582 |
| Left ventricle ejection fraction (%) | 1.3 | 0.7-2.0 | 0.248 |
AKIN=acute kidney injury; CI=confidence interval; DBP=diastolic blood pressure; GFR=glomerular filtration rate; mPP=mean perfusion pressure; RBC=red blood cell; SBP=systolic blood pressure; TAVI=transcatheter aortic valve implantation
Fig. 1.
ROC curve of mean perfusion pressure. The mean perfusion pressure value which can predict the acute kidney injury development was determined as 72 mmHg in ROC analysis [AUC: 0.813 (95% CI; 0.721-0.905); sensitivity, 72%; specificity, 84%]. AUC = area under the curve; CI = confidence interval; ROC = receiver operating characteristics.
Fig. 2.
Kaplan-Meier curves for overall survival in AKIN and non-AKIN (NRF) groups. AKIN = acute kidney injury; NRF = normal renal functions.
DISCUSSION
In our study, we found out that baseline creatinine, GFR, amount of CM, and lower mPP values were significantly associated with the development of AKIN in patients with TAVI.
Development of AKIN is strongly associated with increased major adverse cardiac events after TAVI procedure[9-11]. While 40% of the patients suffer from AKIN after SAVR, prevalence of AKIN ranges from 3.4% to 57% after TAVI procedure[10,12,13]. In accordance with previous studies, in our study 33 (21.2%) patients developed AKIN after TAVI. Excessive CM usage, hypotension, rapid ventricular pacing, balloon aortoplasty, valve implantation, and embolisation of aortic plaque are considered intra-operative risk factors for AKIN after TAVI[12,14].
As it is well known, CM reduce oxygen availability to the renal medulla and cause renal ischemia[15]. Several studies demonstrated that CM/GFR and CM/creatinine clearance (CrCl) rates in invasive procedures are predictors of renal failure[16-18]. It can be useful to calculate basal GFR and CrCl before TAVI, to be used to calculate upper limits for CM. The mAP might be decreased below 50 mmHg during rapid ventricular pacing. These hypotensive stages can contribute to the development of AKIN. Therefore, number and duration of rapid ventricular pacing used for our patients during TAVI were recorded in our study. Although basal kidney function tests are important for impairment of renal function, other factors, such as mPP, amount of CM, and rapid ventricular pacing, should be kept in mind to predict renal impairment.
In addition to many factors affecting the development of AKIN, hemodynamic parameters of patients before an invasive procedure are also important indicators of potential renal complications. Renal hypoperfusion is the most important cause of AKIN after SAVR and TAVI[11,19]. Renal perfusion pressure is the most important predictor of renal blood flow. A normal renal perfusion pressure should be between 60 and 100 mmHg[20]. However, there isn't any non-invasive method that can directly measure it. Renal perfusion pressure can be estimated using mPP, mAP, and CVP levels. Especially, mPP calculated with mAP and CBP is shown to be an important indicator for the continuation of NRF[21]. A strong association has been demonstrated between mPP levels and GFR in various diseases[22]. Based on the study results, mPP values obtained with invasive monitorization in advanced AS patients were found to be a significant factor in predicting the development of AKIN. The predictive value of AKIN development was calculated as 72 mmHg using ROC analysis (Figure 1). The percentage of AKIN was much higher in the HR-G group (42.3% vs. 9.9%, P<0.001). The calculation of mPP is based on the difference between mAP and CVP. Since mAP values of the HR-G group were lower and CVP values were higher, mPP values in this group were low (Table 1). The value of mPP is more closely associated with renal perfusion pressure, compared to mAP and CVP[21,22].
Limitations of the Study
The main limitation of this study is the small number of patients included. Additional information can be obtained in longer follow-up periods. In our study, TAVI using balloon expandable prosthesis was performed, and it would be useful to conduct similar studies with a self-expandable prosthesis.
CONCLUSION
These findings led to the conclusion that among the patients with similar renal functions, who received a similar amount of CM, those with lower mPP are at higher risk for AKIN development. A model was created with regression analysis to identify the factors affecting the development of AKIN in our study.
The mortality rate in patients who developed AKIN after TAVI ranges from 7.8% to 29%[6,23]. In our study, the one-year mortality rate was 16.7% in patients who developed AKIN and 2.9% in patients who did not. The presence of AKIN increased the mortality in a one-year period approximately by 5.5 times. Therefore, preventing the development of AKIN should be an important goal to minimise TAVI complications.
In our study, the amount of CM, basal kidney function tests, and lower mPP levels (mPP <72 mmHg) were strongly associated with the development of AKIN after TAVI. Further studies are needed to evaluate the association between mPP and AKIN in patients treated with TAVI.
| Authors’ roles & responsibilities | |
|---|---|
| IG | Analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| LC | Analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| BS | Conception and design of the work; acquisition of data; analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| MZ | Conception and design of the work; acquisition of data; analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| MBA | Conception and design of the work; acquisition of data; analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| HK | Conception and design of the work; acquisition of data; analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| ZC | Conception and design of the work; acquisition of data; analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| BY | Conception and design of the work; acquisition of data; analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| SU | Conception and design of the work; acquisition of data; analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
| HD | Conception and design of the work; acquisition of data; analysis and interpretation of data; drafting the paper; revising the work; approval of the final version |
Footnotes
This study was carried out at Near East University Hospital, Near East Boulevard, Nicosia, Cyprus.
No financial support.
No conflict of interest.
REFERENCES
- 1.Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromso study. Heart. 2013;99(6):396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 2.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 3.Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J. 1987;8(5):471–483. doi: 10.1093/oxfordjournals.eurheartj.a062307. [DOI] [PubMed] [Google Scholar]
- 4.Cribier A. Development of transcatheter aortic valve implantation (TAVI): a 20-year odyssey. Arch Cardiovasc Dis. 2012;105(3):146–152. doi: 10.1016/j.acvd.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95(1):13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 6.Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochellière R, Doyle D, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31(7):865–874. doi: 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33(19):2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 8.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. ESC Committee for Practice Guidelines (CPG) Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) European Association for Cardio-Thoracic Surgery (EACTS): Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2012;42(4):S1–44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 9.Elhmidi Y, Bleiziffer S, Deutsch MA, Krane M, Mazzitelli D, Lange R, et al. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis. 2014;107(2):133–139. doi: 10.1016/j.acvd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Najjar M, Salna M, George I. Acute kidney injury after aortic valve replacement: incidence, risk factors and outcomes. Expert Rev Cardiovasc Ther. 2015;13(3):301–316. doi: 10.1586/14779072.2015.1002467. [DOI] [PubMed] [Google Scholar]
- 11.Genereux P, Kodali SK, Green P, Paradis JM, Daneault B, Rene G, et al. Incidence and effect of acute kidney injury after transcatheter aortic valve replacement using the new valve academic research consortium criteria. Am J Cardiol. 2013;111(1):100–105. doi: 10.1016/j.amjcard.2012.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thongprayoon C, Cheungpasitporn W, Srivali N, Ungprasert P, Kittanamongkolchai W, Greason KL, et al. Acute kidney injury after transcatheter aortic valve replacement: a systematic review and meta-analysis. Am J Nephrol. 2015;41(4-5):372–382. doi: 10.1159/000431337. [DOI] [PubMed] [Google Scholar]
- 13.Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 14.Scherner M, Wahlers T. Acute kidney injury after transcatheter aortic valve implantation. J Thorac Dis. 2015;7(9):1527–1535. doi: 10.3978/j.issn.2072-1439.2015.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudnick MR, Kesselheim A, Goldfarb S. Contrast-induced nephropathy: how it develops, how to prevent it. Cleve Clin J Med. 2006;73(1):75–80. doi: 10.3949/ccjm.73.1.75. 83-7. [DOI] [PubMed] [Google Scholar]
- 16.Wang XC, Fu XH, Wang YB, Jia XW, Wu WL, Gu XS, et al. Prediction of contrast-induced nephropathy in diabetics undergoing elective percutaneous coronary intervention: role of the ratio of contrast medium volume to estimated glomerular filtration rate. Chin Med J. 2011;124(6):892–896. [PubMed] [Google Scholar]
- 17.Gul I, Zungur M, Tastan A, Okur FF, Damar E, Uyar S, et al. The importance of contrast volume/glomerular filtration rate ratio in contrast-induced nephropathy patients after transcatheter aortic valve implantation. Cardiorenal Med. 2015;5(1):31–39. doi: 10.1159/000369943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbieri L, Verdoia M, Marino P, Suryapranata H, De Luca G, Novara Atherosclerosis Study Group Contrast volume to creatinine clearance ratio for the prediction of contrast-induced nephropathy in patients undergoing coronary angiography or percutaneous intervention. Eur J Prev Cardiol. 2016;23(9):931–937. doi: 10.1177/2047487315614493. [DOI] [PubMed] [Google Scholar]
- 19.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 20.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panwar R, Lanyon N, Davies AR, Bailey M, Pilcher D, Bellomo R. Mean perfusion pressure deficit during the initial management of shock: an observational cohort study. J Crit Care. 2013;28(5):816–824. doi: 10.1016/j.jcrc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Ostermann M, Hall A, Crichton S. Low mean perfusion pressure is a risk factor for progression of acute kidney injury in critically ill patients: a retrospective analysis. BMC Nephrol. 2017;18(1):151–151. doi: 10.1186/s12882-017-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuis RJ, Rodés-Cabau J, Sinning JM, van Garsse L, Kefer J, Bosmans J, et al. Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2012;5(5):680–688. doi: 10.1161/CIRCINTERVENTIONS.112.971291. [DOI] [PubMed] [Google Scholar]