Abstract
Recent studies have shown that inconsistent results of safety and efficacy between sonothombolysis vs. non-sonothombolysis in acute ischemic stroke (AIS). We implemented a meta-analysis to explore the value of sonothrombolysis in AIS treatment. The MEDLINE, EMBASE, and Cochrane Library databases were searched for randomized controlled trials (RCTs) which had evaluated sonothrombolysis or ultrasound thrombolysis in AIS. One hundred five studies were retrieved and analyzed, among them, 7 RCTs were included in the current meta-analysis. In comparison with the non-sonothombolysis, sonothrombolysis significantly improved complete recanalization (RR 2.16, 95% CI 1.51 to 3.08, P < 0.001), complete or partial recanalization (RR 1.90, 95% CI 1.26 to 2.88, P = 0.002), there is also a tendency to improvement of ≥ 4 points in NIHSS score (RR 1.43, 95% CI 0.99 to 2.07, P = 0.057). However, sonothrombolysis and non-sonothrombolysis had insignificant differences in neurological recovery and adverse events. In subgroup analysis, we found that “With t-PA”, “NIHSS > 15”, “Treatment time ≤ 150min”, and “Age ≤ 65 years” are potential favorable factors for efficacy outcomes of sonothombolysis. Sonothrombolysis can significantly increase the rate of recanalization in patients with AIS compared with non-sonothrombolysis, but there is no significant effect on improving neurological functional recovery and avoiding complications.
Introduction
Stroke, along with cardiac disease and cancer are the most common reasons for permanent disability, which is the second leading cause of death worldwide among them [1, 2]. Ischemic stroke is characterized by the partial or complete loss of blood supply in part of the brain tissues and then causes dysfunction which occupies approximately 70–80% of all kinds of strokes [2, 3]. So far, when a sudden arterial occlusion occurs, followed by cerebral ischemic stroke, mechanical thrombolysis, and intravenous thrombolysis are the dominating methods to get recanalization, reduce the severity, and improve the outcomes [4]. In addition, sonothrombolysis is a novel method for the treatment of acute ischemic stroke (AIS). Sonothrombolysis by transcranial Doppler (TCD) or transcranial color-coded sonography (TCCS) with or without t-PA is a potential, promising, but debatable, the method for treating AIS patients [5]. Ultrasound can accelerate the blood flow near the thrombus, so we assumed that the application of TCD/TCCS could stir up the blood near the obstructed thrombus [6]. This way can also enhance the mixture of t-PA into a blood and increase the concentration of the t-PA near the occlusion consequently [7]. In addition, the pressure waves produced by TCD/TCCS may also augment the permeation of t-PA into the fibrin network and affect the binding of t-PA with fibrin directly. [8]
Microbubbles are air- or gas-filled microspheres [9] which were initially used to improve the echo-graphic quality of images by increasing the acoustic signal [10]. Application of TCD/TCCS has been shown to cause the vibration of microbubbles, resulting in the microbubbles sustaining absorption of energy until they explode and release the energy [11]. Hence, the ultrasound-induced blast of microbubbles may speed up the dissolution of thrombus. Microbubbles combined with TCD/TCCS were also applied to some other clinical trials, which might be able to get better clinical outcomes [12].
Recent years, there are several trials have consistently illustrated that sonthrombolysis can improve the outcomes of AIS [9, 13–17]. Eggers, J., et al. 2005 [14], Eggers, J., et al. 2008 [15] and Dwedar, A.Z., et al. 2014 [16] demonstrated that sonothrombolysis has positive effects on increasing the recanalization rate and improve the clinical outcomes of acute middle cerebral artery occlusion. CLOTBUST 2004 [17] and Molina, C.A., et al. 2006 [9] found that continuous TCD improves t-PA-induced arterial recanalization in patients with AIS, which did not significantly help patients to recover. TUCSON 2009 [13] concluded that ultrasound combined with microbubbles and t-PA showed higher recanalization rates and more favorable outcome rates compared with intravenous t-PA therapy alone. NOR-SASS 2017 [18] revealed that sonothrombolysis is safe, but there is no statistically significant clinical effect in unselected AIS patients.
Based on the above-mentioned clinical studies and trials, the efficacy and safety of sonothrombolysis for AIS are still unclear. There were been some similar systematic reviews that discuss sonothrombolysis in the treatment of AIS [5, 19]. However, our work represents an updated of the previous meta-analysis according to the publication of two novel studies in 2014 [16] and 2017 [18]. In addition, the current meta-analysis explores the impact of relevant factors on sonothrombolysis by more comprehensive subgroup analysis.
Materials and methods
Study protocol
This meta-analysis was written according to the preferred reporting items for systematic reviews and PRISMA statement (S1 File) [20]. At the start of this project, a drafted study protocol was made to consist with the Cochrane Collaboration format [21].
Eligibility criteria
Inclusion criteria were as follows: (a) Study type: RCT; (b) Language restriction: only English was available; (c) Participants: patients with AIS; (d) Intervention: sonothrombolysis; (e) Outcomes: Efficacy outcomes: excellent functional outcome (modified Rankin Scale (mRS) = 0–1) and good functional outcome (mRS = 0–2) based on mRS, early neurological improvement based on NHISS (NIHSS improve ≥ 4) and recanalization; Safety outcomes: intracranial hemorrhage, death (mRS = 6) and disability (mRS = 3–5). (f) Study years: We searched MEDLINE, EMBASE, and Cochrane Library to find related articles from January 2001 to May 2018. Exclusion criteria were as follows: (a) Study types: case reports, case reviews, retrospective studies, and cohort studies; (b) Control: positive control; (c) Conference abstracts without full text.
Search strategy and information sources
Three major databases: MEDLINE, EMBASE, and Cochrane Library were systematically searched by two authors independently (Z.C. and T.X.). The search strategy of the MEDLINE was to combine all the variables [(“ultrasound” AND “thrombolysis”) OR “sonothrombolysis”] AND “acute ischemic stroke.” Two independent investigators (Z.C. and T.X.) scanned the titles and abstract of all the studies to select applicable studies. The search strategy for EMBASE and the Cochrane Library is similar to what we used for searching MEDLINE. In addition, two investigators (Z.C. and T.X.) manually screened reference lists from RCTs and systematic reviews independently to ensure all relevant studies have been included in this study.
Study selection and data collection
All records from the systematic search in the electronic database and reference lists of RCTs and systematic reviews were evaluated by two authors (Z.C. and T.X.) independently following the eligibility criteria as mentioned above. After strict selection and evaluation, we collect the data from RCTs as follows: basic information on the included trials, inclusion, exclusion criteria for the participants, study design, and outcome assessments (Table 1).
Table 1. Baseline characteristics of the included studies and outcome events in the meta-analysis.
| Trials | Therapeutic centre | Publication | Inclusion Criteria | Exclusion Criteria | Study Design | Efficacy outcomes | Safety outcomes |
|---|---|---|---|---|---|---|---|
| Alexandrov 2004 | 5 centers in 3 countries | N Engl J Med |
|
|
2MHz TCD + tPA vs. tPAc |
|
|
| Eggers 2005 | 2 centers in 1 country | Neurology |
|
|
TCCS vs. none |
|
|
| Molina 2006 | 6 centers in 3 countries | Stroke |
|
|
2MHz TCD + tPA +MB vs. 2MHz TCD + tPA vs. tPA alone |
|
|
| Eggers 2008 | 4 centers in 1 country | Stroke |
|
|
1.8 MHz TCCS + tPA vs. tPA |
|
|
| Molina 2009 | 10 centers in 6 countries | Annals of Neurology |
|
|
2MHz TCD + tPA + uS vs. tPA |
|
|
| Dwedar 2014 | 1 center in 1 country | Neurology India |
|
|
2MHz TCD + aspirin vs. aspirin |
|
|
| Nacu 2017 | 5 centers in 1 country | Stroke |
|
|
2Mhz TCD + tPA +MB vs. tPA |
|
|
CLOTBUST: Combined Lysis Of Thrombus in Brain ischemia Using transcranial ultrasound and Systemic TPA; TPA: tissue plasminogen activator; TCD: transcranial Doppler; TIBI: Thrombolysis In Brain Ischemia; NIHSS: National Institute of Health Stroke Scale; mRS: modified Rankin scores; CR: complete recanalization; PR: partial recanalization; NR: no recanalization; NINDS: National Institute of Neurologic Disorders and Stroke; EICs: early ischemic changes; TCCS: transcranial color-coded sonography; MB: Microbubble; ICH: intracerebral hemorrhage; MCA: the middle cerebral artery; ACA: anterior cerebral artery; ICA: internal carotid artery; PCA: posterior cerebral artery; BI: barthel index; TUCSON: Transcranial Ultrasound in Clinical SONothrombolysis; uS: Microspheres; NOR-SASS: NORwegian Sonothrombolysis in Acute Stroke Study; MFV: mean flow velocity.
Risk of bias
The risk of bias plot in individual studies was created using the Review Manager 5.2 software. Uniform criteria of the Cochrane collaboration we applied to assess the risk of bias of RCTs, including selection bias, performance bias, detection bias, attrition bias, reporting bias, and some other potential biases.
Summary measures and synthesis of results
STATA (Version 12.0) was used for assessing the data. Dichotomous outcomes were analyzed as the risk ratio (relative risk [RR]; 95% confidence interval [CI]) and calculated using a random effect model. Statistical heterogeneity was estimated by the I2 statistic as follows: I2 < 30% means “low heterogeneity” I2 = 30 to 50% denotes “moderate heterogeneity,” and I2 > 50% represents “substantial heterogeneity.” Subgroup analyses were implemented to detect the application of microbubble and t-PA, the severity of Stroke, time from onset to treatment and patients’ ages. Sensitivity analysis was used to explore the stability of the consolidated results. Two-tailed test and a P value less than 0.05 was considered significant for all analyses.
Results
Six hundred thirty-eight titles and abstracts were identified through MEDLINE, EMBASE, and Cochrane Library in total (Fig 1). After removing the duplicates and irrelevant records, 105 articles were included, and 98 articles were excluded because of their types: 23 multiple reports on one RCT, 6 protocol studies, 6 post-hoc analysis, 4 meta-analysis, 5 comments, 32 reviews, and 20 nonrandomized clinical trials. Additionally, 2 conference abstracts without full-text Larrue, V., et al. 2007 [22] and Dinia, L., et al. 2016 [23] were also excluded from the current meta-analysis. Furthermore, the trial of Alexandrov et al. 2008 [24] was excluded due to lose the follow-up information of some patients at three-month (9 out of 15, or 60%) and the data of non-ultrasound control group was the same as CLOTBUST 2004 [17]. Ultimately, seven RCTs [9, 13–18] were eligible and contained 549 patients were included in qualitative synthesis (Fig 1). The main characteristics of those included studies are listed in Table 1.
Fig 1. The study search, selection, and inclusion process.
Outcomes analysis
All 7 RCTs [9, 13–18] include 549 patients are available for the analysis of efficacy and safety outcome.
1. Efficacy outcome
Sonothrombolysis group has an advantage over the non-sonothrombolysis group for patients in increasing the complete recanalization (RR 2.16, 95% CI 1.51 to 3.08, P < 0.001; Fig 2A), complete or partial recanalization (RR 1.90, 95% CI 1.26 to 2.88, P = 0.002; Fig 2B) and decreasing the no recanalization (RR 0.60, 95% CI 0.49 to 0.73, P < 0.001; Fig 2C). In addition, these was no significant difference between the excellent functional outcome (RR 1.39, 95% CI 0.86 to 2.25, P = 0.181; Fig 2D) and the good functional outcome (RR 1.23, 95% CI 0.88 to 1.73, P = 0.229; Fig 2E), which respectively were defined as the 3-month mRS = 0–1 or 0–2. However, compared with non-sonothrombolysis group, sonothrombolysis group also have a tendency, which can decrease NIHSS score more than 4 in AIS patients (RR 1.43, 95% CI 0.99 to 2.07, P = 0.057; Fig 2F). The heterogeneity test showed significant differences among studies (I2 = 60.6%, P = 0.038). (Fig 2E). To detect the source of the statistical heterogeneity, sensitivity analysis was performed. The sensitivity analysis showed that all of the consolidated results were stable (S1 Fig).
Fig 2. The pooled relative risk of the efficacy outcomes.
The diamond indicates the estimated relative risk (95% confidence interval) for all patients together. A, Complete recanlization. B, Complete or partial recanlization. C, No recanlization. D, Excellent function outcome. E, Good functional outcome. F, NIHSS improve ≥ 4.
2. Safety outcome
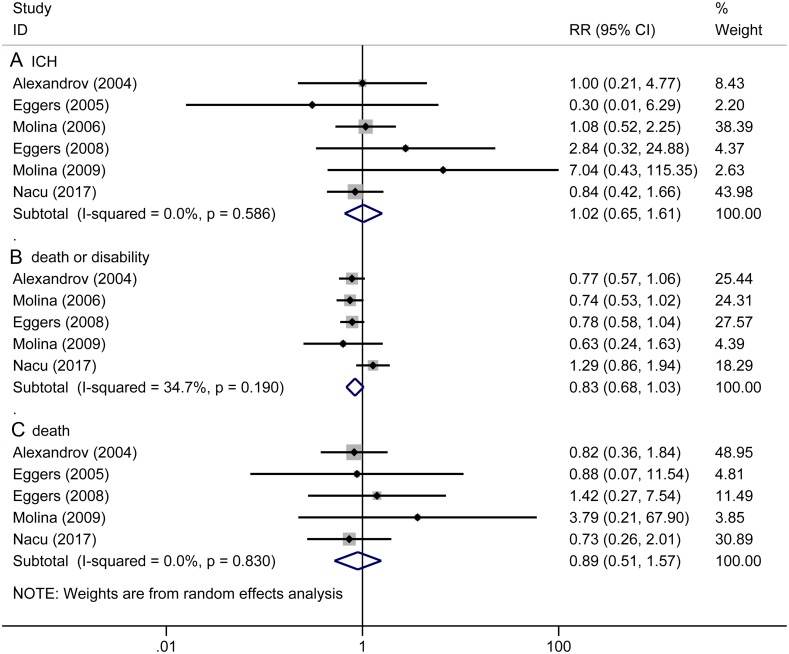
There exist no significant differences in preventing the adverse events, including: ICH (RR 1.02, 95% CI 0.65 to 1.61, P = 0.923, Fig 3A), death or disability (RR 0.83, 95% CI 0.68 to 1.03, P = 0.091, Fig 3B), death (RR 0.89, 95% CI 0.51 to 1.57, P = 0.698, Fig 3C) between sonothrombolysis group and control group.
Fig 3. The pooled relative risk of the safety outcomes.
The diamond indicates the estimated relative risk (95% confidence interval) for all patients together. A, ICH. B, Death or disability. C, Death.
Subgroup analysis
We performed subgroup analyses to assess the utility of microbubbles or t-PA combined with TCD/TCCS, the severity of stroke at the beginning, time of initial treatment and age of patients.
1. With/without microbubble
The subgroup without microbubble in sonothrombolysis group had a higher rate of excellent functional recovery (RR 1.74, 95% CI 1.00 to 3.01, P = 0.049; Table 2), good functional outcome (RR 1.43, 95% CI 1.03 to 1.99, P = 0.032; Table 2), NIHSS improvement ≥ 4 (RR 1.60, 95% CI 1.09 to 2.36, P = 0.017; Table 2) and simultaneously reduced the statistics of death or disability (RR 0.79, 95% CI 0.65 to 0.95, P = 0.011; Table 2) compared with non-sonothrombolysis group.
Table 2. Subgroup analysis of efficacy and safety outcomes.
| Efficacy outcomes | ||||||
| Excellent functional outcome | Good functional outcome | NIHSS improve ≥ 4 | ||||
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| 1.use microbubble | ||||||
| Yes | 1.155 (0.637, 2.095) | 0.635 | 1.154 (0.762, 1.747) | 0.499 | 1.267 (0.663, 2.419) | 0.474 |
| No | 1.738 (1.002, 3.014) | 0.049 | 1.432 (1.031, 1.988) | 0.032 | 1.600 (1.086, 2.356) | 0.017 |
| 2.use tPA | ||||||
| Yes | 1.349 (0.825, 2.207) | 0.233 | 1.231 (0.877, 1.728) | 0.229 | 1.284 (0.930, 1.771) | 0.129 |
| No | 4.444 (0.249, 79.42) | 0.311 | N/A | N/A | 4.661 (1.157, 18.78) | 0.030 |
| 3. Stroke severity at baseline | ||||||
| NIHSS ≤ 15 | 1.215 (0.681, 2.167) | 0.511 | 0.967 (0.701, 1.333) | 0.838 | 2.013 (0.556, 7.288) | 0.287 |
| NIHSS > 15 | 2.081 (0.584, 7.420) | 0.259 | 1.481 (1.081, 2.028) | 0.015 | 1.568 (1.074, 2.290) | 0.020 |
| 4.time to treatment (mins) | ||||||
| Time ≤ 150 | 1.720 (1.075, 2.754) | 0.024 | 1.387 (1.007, 1.909) | 0.045 | 1.566 (0.925, 2.650) | 0.095 |
| Time > 150 | 1.044 (0.494, 2.208) | 0.909 | 1.100 (0.631, 1.920) | 0.736 | 1.480 (0.814, 2.690) | 0.199 |
| 5.Age (years) | ||||||
| Age ≤ 65 | 6.185 (0.814, 47.01) | 0.078 | 4.737 (0.611, 36.72) | 0.137 | 2.767 (1.237, 6.187) | 0.013 |
| Age > 65 | 1.247 (0.809, 1.923) | 0.318 | 1.178 (0.859, 1.616) | 0.308 | 1.211 (0.869, 1.686) | 0.258 |
| Safety outcomes | ||||||
| ICH | Death or disability | Death | ||||
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| 1.use microbubble | ||||||
| Yes | 1.064 (0.587, 1.929) | 0.838 | 0.866 (0.517, 1.449) | 0.583 | 0.950 (0.282, 3.197) | 0.934 |
| No | 1.027 (0.512, 2.060) | 0.940 | 0.786 (0.653, 0.946) | 0.011 | 0.906 (0.450, 1.826) | 0.783 |
| 2.use tPA | ||||||
| Yes | 1.052 (0.665, 1.663) | 0.830 | 0.835 (0.678, 1.029) | 0.091 | 0.895 (0.501, 1.598) | 0.708 |
| No | 0.296 (0.014, 6.292) | 0.435 | N/A | N/A | 0.875 (0.066, 11.54) | 0.919 |
| 3. Stroke severity at baseline | ||||||
| NIHSS ≤ 15 | 1.025 (0.293, 3.585) | 0.969 | 1.030 (0.534, 1.986) | 0.930 | 0.872 (0.355, 2.144) | 0.765 |
| NIHSS > 15 | 1.158 (0.615, 2.183) | 0.650 | 0.766 (0.641, 0.916) | 0.003 | 0.909 (0.439, 1.882) | 0.797 |
| 4.time to treatment (mins) | ||||||
| Time ≤ 150 | 1.875 (0.591, 5.947) | 0.286 | 0.770 (0.625, 0.948) | 0.014 | 0.990 (0.489, 2.005) | 0.977 |
| Time > 150 | 0.916 (0.559, 1.499) | 0.726 | 0.965 (0.548, 1.698) | 0.901 | 0.744 (0.289, 1.919) | 0.541 |
| 5.Age (years) | ||||||
| Age ≤ 65 | 1.198 (0.139, 10.32) | 0.870 | 0.780 (0.583, 1.044) | 0.095 | 1.232 (0.303, 5.001) | 0.771 |
| Age > 65 | 1.004 (0.628, 1.605) | 0.987 | 0.859 (0.644, 1.145) | 0.299 | 0.840 (0.453, 1.559) | 0.580 |
RR: Relative Risk; CI: Confidence Interval; N/A: Not Applicable.
2. With/without t-PA
The subgroup without t-PA in sonothrombolysis group had a more effective NIHSS improvement (RR 4.66, 95% CI 1.16 to 18.78, P = 0.030; Table 2) compared with non-sonothrombolysis group. However, the subgroup with t-PA in sonothrombolysis group might be safer compared with non-sonothrombolysis group by showing a trend towards the attenuation of death or disability (RR 0.84, 95% CI 0.68 to 1.03, P = 0.091; Table 2).
3. NIHSS > 15 / NIHSS ≤ 15 at the beginning of stroke
The high stroke severity subgroup, patients’ NIHSS > 15 at the beginning of stroke in sonothrombolysis group was more likely to get good functional outcome (RR 1.48, 95% CI 1.08 to 2.03, P = 0.015; Table 2) and NIHSS improvement ≥ 4 (RR 1.57, 95% CI 1.07 to 2.29, P = 0.020; Table 2), also had less death or disability (RR 0.77, 95% CI 0.64 to 0.92, P = 0.003; Table 2) compared with non-sonothrombolysis group.
4. Time of the initial treatment > 150min / ≤ 150min
The early treatment subgroup (≤ 150min) in sonothrombolysis group exhibited significantly differences in elevating excellent functional outcome (RR 1.72, 95% CI 1.08 to 2.75, P = 0.024; Table 2), good functional outcome (RR 1.39, 95% CI 1.01 to 1.91, P = 0.045; Table 2) and lowering death or disability (RR 0.77, 95% CI 0.63 to 0.95, P = 0.014; Table 2), compared with non- sonothrombolysis group.
5. Age > 65 years / ≤ 65 years
The patients ≤ 65 years subgroup in sonothrombolysis group were easier to get NIHSS improvement ≥ 4 (RR 2.77, 95% CI 1.24 to 6.19, P = 0.013; Table 2) and a tendency to get more excellent functional outcome (RR 6.19, 95% CI 0.81 to 47, P = 0.078; Table 2) and less death or disability (RR 0.78, 95% CI 0.58 to 1.04, P = 0.095; Table 2) compared with non- sonothrombolysis group.
The sensitivity analysis illustrated that all the consolidated statistics are stabilized.
Risk of bias in included studies
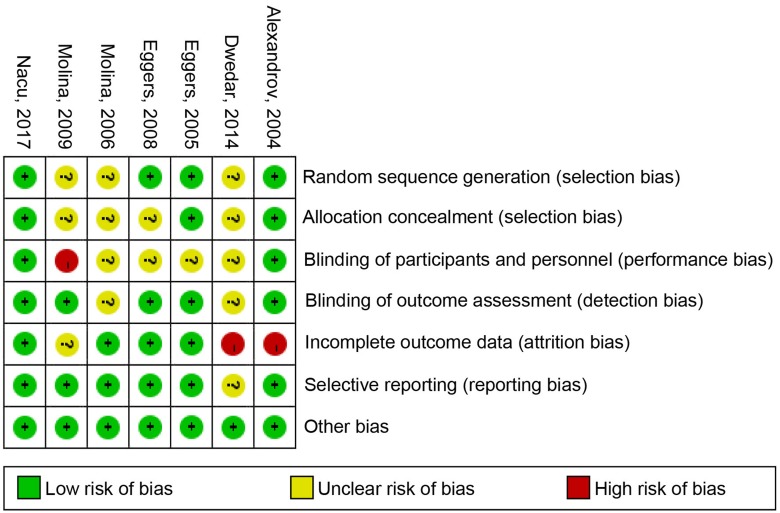
Details about the risk of bias of the included studies are shown in Fig 4. For the random sequence generation assessment, the risk of bias was unclear in 3 out of 7 studies. For the allocation concealment assessment, the risk of bias was unclear in 5 out of 7 studies. For the blinding of participants and personnel assessment, the risk of bias of four trial was unclear and 1 out of 7 trial had a high risk of bias. For the blinding of outcomes assessment, the risk of bias was unclear in 2 out of 7 studies. For incomplete outcome data, the risk of bias of 1 out of 7 trial was unclear and 2 out of 7 trials had a high risk of bias. For the selective reporting assessment, the risk of bias was unclear in 1 out of 7 study. There was no high risk, or unclear risk of bias was observed in other items.
Fig 4.
Risk of bias: A summary table for each risk of bias item for each study.
Publication bias
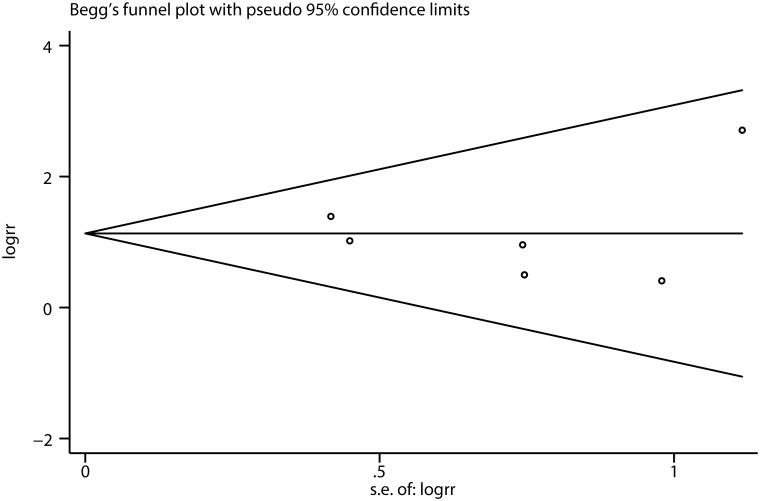
The funnel plot was applied to evaluate the publication bias, indicating that there is no evidence for publication bias (Fig 5). In addition, this evidence is confirmed by a formal statistical test (P = 0.967 of Egger’s test).
Fig 5. Begg funnel plot for publication bias test of each study.
Discussion
Based on the evidence of the current meta-analysis, sonothrombolysis for AIS might be debatable. When it comes to efficacy issues, we found that sonothrombolysis have a distinct benefit on the recanalization of occlusion, increasing the number of complete recanalization of occlusion, increasing complete or partial recanalization, and decreasing the frequency of the no recanalization than non-sonothrombolysis, which was in accordance with most other studies [5, 9, 14–17, 19, 25]. Although the specific mechanisms of sonothrombolysis were unclear [26], it has been hypothesized that TCD/TCCS could accelerate the movement of fluid around the thrombus so as to enhance the mixture of t-PA into the blood and consequently elevate the concentration of this drug near the occlusion [6, 7]. In addition, the pressure waves produced by TCD/TCCS may also augment the permeation of t-PA into the fibrin network and affect the binding of t-PA with fibrin directly [8]. Moreover, some studies believe that sustainable ultrasound exposure may result in vessel vasodilatation probably owing to the elevation of the activity of nitric oxide synthase, which leads to temporal vasodilation which improved reperfusion of local cerebral tissue [27, 28].
When it comes to safety issues, sonothrombolysis or non-sonothrombolysis might not influence the occurrence of ICH, disability or death at 3 months in patients with AIS. The results indicated that sonothrombolysis did not increase the adverse events compared with non-sonothrombolysis. In consideration of the sonothrombolysis hypothetical mechanisms, sonothrombolysis may improve the recanalization in short-term, but 3 months’ outcomes were impacted by more factors such as age, hyperlipidemia, and recurrence of stroke [6, 7, 27–30]. These factors may lead to the bias of the efficacy and safety results of 3 months’ outcomes, so that we may miss the long-term curative effect of sonothrombolysis.
The subgroup analysis on the basis of microbubbles demonstrated that sonothrombolysis without microbubbles was more effective and safer than non- sonothrombolysis thrombolysis through the subgroup data of excellent functional outcome (P = 0.049), good functional outcome (P = 0.032), NIHSS improvement ≥ 4 (P = 0.017) and death or disability (P = 0.011). However, in the sonothrombolysis with microbubbles subgroup, there were no significant differences between sonothrombolysis and non-sonothrombolysis in above mentioned outcome. In theory, the sonothrombolysis plus microbubble therapy should achieve better efficacy than non-sonothrombolysis. However, we got the opposite conclusion according to the sonothrombolysis plus microbubble subgroup anylsis. These results were different from many other articles and trials [9, 13, 19, 31, 32] It is possible that only two RCTs nvolve microbubbles, so that a small sample size is difficult to make a difference. In addition, microbubbles were supposed to work by absorbing energy, releasing energy, explosion [12, 33] and they might not only accelerate the dissolution of clots [34, 35] but also can cause the vascular endothelial damage directly [36]. Therefore, the value of sonothrombolysis plus microbubbles requires more randomized controlled trials to confirm.
Another subgroup analysis, compared with non-sonothrombolysis without t-PA subgroup, sonothrombolysis without t-PA subgroup showed more patients whose NIHSS decrease ≥ 4 (P = 0.030). However, when compared sonothrombolysis with t-PA subgroup with non-sonothrombolysis with t-PA subgroup, there is no significant difference in efficacy outcomes. Taken together, we concluded sonothrombolysis is a valuable method to treat AIS and t-PA also play an important role in thrombolysis. Therefore, t-PA is a favorable factor in sonothrombolysis for AIS.
From the rest of subgroup analysis, we found that sonothrombolysis might be more effective and safer for patients whose NIHSS scores at the beginning of the stroke were higher than 15 or the time of initial treatment was less than 150 min or ages were younger than 65 years, compared with non-sonothrombolysis group.
On the basis of our knowledge, most of previous systematic reviews and meta-analysis brought in several non-randomized types of research [19, 25, 34, 37, 38]. Combining all the results of no RCTs was heterogeneous so that these systematic reviews were flawed. Different from above-mentioned systematic reviews, all patients in the present meta-analysis were intervened by sonothrombolysis or non-sonothrombolysis and were randomized, which was the best way to divide risk factors equally over the two groups. This is the second meta-analysis about the sonothrombolysis all evidenced from RCTs (randomized clinical trials). In the first article Ricci et al. 2012 [5] included 5 RCTs, and our meta-analysis included 2 relatively novel RCTs, Dwedar et al. 2014 [16] and NOR-SASS 2017 [18], which was not used in preceding systematic reviews and meta-analysis. These two novel RCTs have a large sample size. Besides, we did further subgroup analysis including 5 relevant factors, which provide more comprehensive comparisons between sonothrombolysis and non-sonothrombolysis. With the data from new RCTs [16, 18], we might come to more definitive conclusions in order to guide clinical treatment. The following limitations of our meta-analysis should be noticed. Firstly, this meta-analysis was performed on base on the limited statistics. We only pooled 7 published RCTs15, 19-24 totally 549 patients were included (sonothrombolysis group, n = 302; control group, n = 247) to analysis the efficacy and safety of sonothromblolysis for AIS. Secondly, the included RCTs showed heterogeneity in the data of complete or partial recanalization (I2 = 42.2%), excellent functional outcome (I2 = 44.1%), good functional outcome (I2 = 60.6%) and death or disability (I2 = 34.7%). Although the sensitivity analysis demonstrated that all the consolidated statistics were stabilized, these disadvantages of the included studies could not be ignored. Thirdly, there was the possibility of selection bias in our meta-analysis, because we excluded some RCTs only with abstract reported in some meetings like Larrue et al. 2007 [22] and Dinia et al. 2016 [23]. Fourthly, our meta-analysis statistics could not represent all kinds of sonothrombolysis treatment, since we restricted it to TCD/TCCS-induced high-frequency ultrasound treatment (1.8–2 MHz).
In addition to the above limitations, sonothrombolysis itself has limitations in the treatment of AIS. Only patients with occlusion of the proximal middle cerebral artery can benefit significantly by sonothrombolysis. Therefore, the population suitable for treatment is limited. Furthermore, mechanical thrombectomy is currently recommended for the treatment of a large artery occlusion in patients with AIS. Sonothrombolysis is a relatively time consuming and operator-dependent procedure and presents many limitations, mainly technical, according to the presence of a temporal bone window to be performed. However, sonothrombolysis remains a treatment modality when patients admitted to stroke centers without endovascular competence until secondary transport to the intervention center.
Conclusion
In conclusion, this meta-analysis demonstrated that sonothrombolysis treatment for AIS by TCD/TCCSc had significant efficacy on promoting recanalization and the upward tendency of the number of NIHSS improvement ≥ 4. However, it is not significantly different from other efficacy and safety outcomes. Some factors such as with t-PA, NIHSS > 15, treatment ≤ 150 min and Age ≤ 65 years may be potential advantages of sonothombolysis. These evidences can provide confident insights for further research on sonothombolysis in patients with AIS.
Supporting information
Fig 2E sensitivity analysis was performed to detect the source of statistical heterogeneity. which showed that all of the consolidated results were stable.
(TIF)
(DOC)
Acknowledgments
We thank all colleagues who provided us with the information we needed for this study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No.81571115).
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–54. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–23. 10.1016/S0140-6736(08)60694-7 . [DOI] [PubMed] [Google Scholar]
- 3.Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. . [DOI] [PubMed] [Google Scholar]
- 4.McManus JJ. Oxygen consumption of the beetle, Dermestes maculatus. Comparative biochemistry and physiology A, Comparative physiology. 1974;49(1A):169–73. . [DOI] [PubMed] [Google Scholar]
- 5.Ricci S, Dinia L, Del Sette M, Anzola P, Mazzoli T, Cenciarelli S, et al. Sonothrombolysis for acute ischaemic stroke. The Cochrane database of systematic reviews. 2012;10:CD008348 10.1002/14651858.CD008348.pub3 . [DOI] [PubMed] [Google Scholar]
- 6.Tsivgoulis G, Alexandrov AV. Ultrasound-enhanced thrombolysis in acute ischemic stroke: potential, failures, and safety. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2007;4(3):420–7. 10.1016/j.nurt.2007.05.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skoloudik D, Bar M., Skoda O., Vaclavik D., Hradilek R., Herzig R., & Kanovsky P. Efficacy of sonothrombotripsy versus sonothrombolysis in recanalization of intracranial arteries. European Journal of Neurology. 2006;13:1. CN-00603701.17112344 [Google Scholar]
- 8.Alexandrov AV, Demchuk A. M., Burgin W. S., Robinson D. J., & Grotta J. C. Ultrasound-Enhanced Thrombolysis for Acute Ischemic Stroke: Phase I. Findings of the CLOTBUST Trial. Journal of neuroimaging. 2004;14(2):5. Epub 2004. CN-00488040. [PubMed] [Google Scholar]
- 9.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37(2):425–9. 10.1161/01.STR.0000199064.94588.39 . [DOI] [PubMed] [Google Scholar]
- 10.Perren F, Loulidi J, Poglia D, Landis T, Sztajzel R. Microbubble potentiated transcranial duplex ultrasound enhances IV thrombolysis in acute stroke. Journal of thrombosis and thrombolysis. 2008;25(2):219–23. 10.1007/s11239-007-0044-6 . [DOI] [PubMed] [Google Scholar]
- 11.Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Maisterra O, Delgado P, et al. Do bubble characteristics affect recanalization in stroke patients treated with microbubble-enhanced sonothrombolysis? Ultrasound in medicine & biology. 2008;34(10):1573–7. 10.1016/j.ultrasmedbio.2008.02.011 . [DOI] [PubMed] [Google Scholar]
- 12.Auboire L, Sennoga CA, Hyvelin JM, Ossant F, Escoffre JM, Tranquart F, et al. Microbubbles combined with ultrasound therapy in ischemic stroke: A systematic review of in-vivo preclinical studies. PloS one. 2018;13(2):e0191788 10.1371/journal.pone.0191788 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina CA, Barreto AD, Tsivgoulis G, Sierzenski P, Malkoff MD, Rubiera M, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Annals of neurology. 2009;66(1):28–38. 10.1002/ana.21723 . [DOI] [PubMed] [Google Scholar]
- 14.Eggers J, Seidel G, Koch B, Konig IR. Sonothrombolysis in acute ischemic stroke for patients ineligible for rt-PA. Neurology. 2005;64(6):1052–4. 10.1212/01.WNL.0000154599.45969.D6 . [DOI] [PubMed] [Google Scholar]
- 15.Eggers J, Konig IR, Koch B, Handler G, Seidel G. Sonothrombolysis with transcranial color-coded sonography and recombinant tissue-type plasminogen activator in acute middle cerebral artery main stem occlusion: results from a randomized study. Stroke. 2008;39(5):1470–5. 10.1161/STROKEAHA.107.503870 . [DOI] [PubMed] [Google Scholar]
- 16.Dwedar AZ, Ashour S, Haroun M, El Nasser AA, Moustafa RR, Ibrahim MH, et al. Sonothrombolysis in acute middle cerebral artery stroke. Neurology India. 2014;62(1):62–5. 10.4103/0028-3886.128308 . [DOI] [PubMed] [Google Scholar]
- 17.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. The New England journal of medicine. 2004;351(21):2170–8. 10.1056/NEJMoa041175 . [DOI] [PubMed] [Google Scholar]
- 18.Nacu A, Kvistad CE, Naess H, Oygarden H, Logallo N, Assmus J, et al. NOR-SASS (Norwegian Sonothrombolysis in Acute Stroke Study): Randomized Controlled Contrast-Enhanced Sonothrombolysis in an Unselected Acute Ischemic Stroke Population. Stroke. 2017;48(2):335–41. 10.1161/STROKEAHA.116.014644 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saqqur M, Tsivgoulis G, Nicoli F, Skoloudik D, Sharma VK, Larrue V, et al. The role of sonolysis and sonothrombolysis in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and case-control studies. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2014;24(3):209–20. 10.1111/jon.12026 . [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535 10.1136/bmj.b2535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700 10.1136/bmj.b2700 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larrue V, Viguier A., Arnaud C., Cognard C., Petit R., Rigal M., et al. Trancranial ultrasound combined with intravenous microbubbles and tissue plasminogen activator for acute ischemic stroke: a randomized controlled study. Stroke. 2007;38 (2):1. Epub 2007, February. 001.17138953 [Google Scholar]
- 23.Dinia L, Prats-Sánchez L, Carrera D, Delgado-Mederos R, Marín-Bueno R, Camps-Renom P, et al. Sonothrombolysis potentiated by microbubbles in acute ischemic stroke: a prospective randomized pilot study. The 2nd European Stroke Organisation Conference 2016: European Stroke Organisation; 2016. p. 37.
- 24.Alexandrov AV, Mikulik R, Ribo M, Sharma VK, Lao AY, Tsivgoulis G, et al. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke. 2008;39(5):1464–9. 10.1161/STROKEAHA.107.505727 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsivgoulis G, Eggers J, Ribo M, Perren F, Saqqur M, Rubiera M, et al. Safety and efficacy of ultrasound-enhanced thrombolysis: a comprehensive review and meta-analysis of randomized and nonrandomized studies. Stroke. 2010;41(2):280–7. 10.1161/STROKEAHA.109.563304 . [DOI] [PubMed] [Google Scholar]
- 26.Rubiera M, Alexandrov AV. Sonothrombolysis in the management of acute ischemic stroke. American journal of cardiovascular drugs: drugs, devices, and other interventions. 2010;10(1):5–10. 10.2165/11316850-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 27.Daffertshofer M, Hennerici M. Ultrasound in the treatment of ischaemic stroke. The Lancet Neurology. 2003;2(5):283–90. . [DOI] [PubMed] [Google Scholar]
- 28.Eggers J, Koch B, Meyer K, Konig I, Seidel G. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Annals of neurology. 2003;53(6):797–800. 10.1002/ana.10590 . [DOI] [PubMed] [Google Scholar]
- 29.Leung T, Leung H, Soo YO, Mok VC, Wong KS. The prognosis of acute symptomatic seizures after ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2017;88(1):86–94. 10.1136/jnnp-2015-311849 . [DOI] [PubMed] [Google Scholar]
- 30.Quinn TJ, Singh S, Lees KR, Bath PM, Myint PK, Collaborators V. Validating and comparing stroke prognosis scales. Neurology. 2017;89(10):997–1002. 10.1212/WNL.0000000000004332 . [DOI] [PubMed] [Google Scholar]
- 31.Tsivgoulis G, Culp WC, Alexandrov AV. Ultrasound enhanced thrombolysis in acute arterial ischemia. Ultrasonics. 2008;48(4):303–11. 10.1016/j.ultras.2007.11.008 . [DOI] [PubMed] [Google Scholar]
- 32.Meairs S, Culp W. Microbubbles for thrombolysis of acute ischemic stroke. Cerebrovascular diseases. 2009;27 Suppl 2:55–65. 10.1159/000203127 . [DOI] [PubMed] [Google Scholar]
- 33.Schleicher N, Tomkins AJ, Kampschulte M, Hyvelin JM, Botteron C, Juenemann M, et al. Sonothrombolysis with BR38 Microbubbles Improves Microvascular Patency in a Rat Model of Stroke. PloS one. 2016;11(4):e0152898 10.1371/journal.pone.0152898 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Saint Victor M, Crake C, Coussios CC, Stride E. Properties, characteristics and applications of microbubbles for sonothrombolysis. Expert opinion on drug delivery. 2014;11(2):187–209. 10.1517/17425247.2014.868434 . [DOI] [PubMed] [Google Scholar]
- 35.Molina CA, Alexandrov AV. Transcranial ultrasound in acute stroke: from diagnosis to therapy. Cerebrovascular diseases. 2007;24 Suppl 1:1–6. 10.1159/000107373 . [DOI] [PubMed] [Google Scholar]
- 36.Wood SC, Antony S, Brown RP, Chen J, Gordon EA, Hitchins VM, et al. Effects of ultrasound and ultrasound contrast agent on vascular tissue. Cardiovascular ultrasound. 2012;10:29 10.1186/1476-7120-10-29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balucani C, Alexandrov AV. Ultrasound- and microspheres-enhanced thrombolysis for stroke treatment: state of the art. Current cardiology reports. 2010;12(1):34–41. 10.1007/s11886-009-0082-0 . [DOI] [PubMed] [Google Scholar]
- 38.Amaral-Silva A, Pineiro S, Molina CA. Sonothrombolysis for the treatment of acute stroke: current concepts and future directions. Expert review of neurotherapeutics. 2011;11(2):265–73. 10.1586/ern.11.3 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig 2E sensitivity analysis was performed to detect the source of statistical heterogeneity. which showed that all of the consolidated results were stable.
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.