Abstract
Whether baseline metabolic tumor volume (TMTV) and total lesion glycolysis (TLG) measured by FDG-PET/CT affected prognosis of patients with lymphoma was controversial. We searched PubMed, EMBASE and Cochrane to identify studies assessing the effect of baseline TMTV and TLG on the survival of lymphoma patients. Pooled hazard ratios (HR) for overall survival (OS) and progression-free survival (PFS) were calculated, along with 95% confidence intervals (CI). Twenty-seven eligible studies including 2,729 patients were analysed. Patients with high baseline TMTV showed a worse prognosis with an HR of 3.05 (95% CI 2.55–3.64, p<0.00001) for PFS and an HR of 3.07 (95% CI 2.47–3.82, p<0.00001) for OS. Patients with high baseline TLG also showed a worse prognosis with an HR of 3.44 (95% CI 2.37–5.01, p<0.00001) for PFS and an HR of 3.08 (95% CI 1.84–5.16, p<0.00001) for OS. A high baseline TMTV was significantly associated with worse survival in DLBCL patients treated with R-CHOP (OS, pooled HR = 3.52; PFS, pooled HR = 2.93). A high baseline TLG was significantly associated with worse survival in DLBCL patients treated with R-CHOP (OS, pooled HR = 3.06; PFS, pooled HR = 2.93). The negative effect of high baseline TMTV on PFS was demonstrated in HL (pooled HR = 3.89). A high baseline TMTV was significantly associated with worse survival in ENKL patients (OS, pooled HR = 2.24; PFS, pooled HR = 3.25). A high baseline TLG was significantly associated with worse survival in ENKL patients (OS, pooled HR = 2.58; PFS, pooled HR = 2.99). High baseline TMTV or TLG predict significantly worse PFS and OS in patients with lymphoma. Future studies are warranted to explore whether TMTV or TLG could be integrated into various prognostic models for clinical decision making.
Introduction
Lymphoma continues to be the most common form of hematological malignancy worldwide [1,2]. Lymphoma is a heterogeneous group of biologically and clinically distinct neoplasms and have been historically divided into two distinct categories: non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) [3]. Although major progress has been made in the treatment of patients with lymphoma, many still fail to achieve a response or subsequently relapse [4]. These patients are not easily identified by existing pretreatment prognostic indexes such as the IPI (international prognostic index), IPS (international prognostic score for Hodgkin lymphoma [HL]), FLIPI (prognostic score for follicular lymphoma), MIPI (prognostic score for mantle cell lymphoma), and PIT (prognostic index for peripheral T cell lymphoma) or by conventional computed tomography (CT)–based response assessment [5–9]. Therefore, there is an urgent need for new prognostic and predictive markers which permit an accurate and early identify high-risk patient categories.
[18]Fluorine fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) has been recognized by the 2014 International Conference on Malignant Lymphoma imaging consensus guidelines as the standard imaging modality to evaluate glucose metabolism in fluorodeoxyglucose (FDG)-avid lymphoma tumors [10,11]. Its value for prognosis prediction at interim and end treatment has been recently investigated [12–14]. Many studies have also showed that quantitative volumetric parameters derived from baseline 18F-FDG PET such as total metabolic tumor volume (TMTV) or total lesion glycolysis (TLG) could predict outcome in diffuse large B-cell lymphoma [15–17], in follicular lymphoma [18], in peripheral T-cell lymphoma [19], in extranodal natural killer/T-cell lymphoma [20] and in Hodgkin lymphoma [21,22]. However, those studies evaluating the prognostic values of pre-therapy TMTV and TLG in in patients with various lymphoma subtypes showed inconclusive and contradictory results [23].
Therefore, the purpose of this meta-analysis was to evaluate the prognostic value of baseline TMTV or TLG by PET/CTin patients with lymphoma, in order to provide more evidence of their clinical value as prognostic biomarkers.
Materials and methods
Inclusion and exclusion criteria
A computerized search of PubMed, Embase and Cochrane was conducted to find relevant studies published prior to May 01, 2018. The following search terms were used: ("lymphoma"[MeSH Terms] OR lymphom*[All Fields] OR lymphoproliferative [All Fields] OR hodgkin*[All Fields] OR non-hodgkin* [All Fields]) AND ("Tomography, emission-computed"[MeSH Terms] OR ("positron emission tomograpy"[MeSH Terms]) OR (computed [All Fields] AND tomograph*[All Fields])) AND (prognos* OR predict* OR surviv* OR overall survival* OR recurrence* OR progress*). All searches were limited to human studies.
Eligible studies met the following criteria: (i) They were observational studies (retrospective or prospective) or clinical trials, (ii) the studies were limited to lymphoma, (iii) 18F-FDG PET was used as an initial imaging tool, (iv) patients had not undergone chemotherapy, immune-chemotherapy or radiotherapy before the 18F-FDG PET scan, (v) the volume of the lymphoma was measured, (vi) the survival data was reported. Studies were excluded if: (i) they were case reports, case series, review articles, editorials, letters or comments; (ii) the patient survival data was unavailable or insufficient to perform the meta-analysis, (iii) the data included was specifically for HIV-associated lymphoma, pediatric lymphoma, primary central nervous system lymphoma, primary testicular lymphoma, or primary mediastinal large B-cell lymphoma, or (iv) they included overlapping patients and data. Two reviewers (B.P. Guo and Q. Ke) independently selected the literature using a standardized protocol. Disagreements were resolved by discussion.
Quality assessment
The methodological quality of the primary manuscripts was independently evaluated by two reviewers (B.P. Guo and X.H. Tan) by means of the Newcastle-Ottawa-Scale (NOS) [24], which is used for the quality assessment of cohort and case-control studies. The NOS comprises three quality parameters: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). Studies with scores of six or more were considered to be of high quality. Any disparities between investigators were resolved by discussion. Study quality was not an exclusion criterion.
Data extraction
Data extraction was carried out by B.P Guo and independently confirmed by the other authors (X.H. Tan and H. Cen). The collected data included the following: Study characteristics: first author, year of publication, country, study design, imaging modalities, type of lymphoma, number of patients, treatment, tumor volume parameters (maximum threshold for PET volume auto-segmentation, and median MTV/TLG), MTV/TLG cut-off values, determination of MTV cut-off, median follow-up, and endpoints. Extracted data were entered onto a standardized Excel file (Microsoft Corporation). Discrepancies were resolved by discussion with coauthors.
We chose OS and PFS as endpoints for our meta-analysis. Overall survival is defined as the length of time from either the date of diagnosis or the date of recruitment in a study to the moment of death as a result of any cause. PFS is defined as the length of time from either the date of diagnosis or the date of recruitment in a study until lymphoma progression or death as a result of any cause.
Statistical analysis
The impact of MTV or TLG on survival was measured by estimating the effect size of the hazard ratios (HR). Pooled HRs of more than 1.00 indicated poor survival in the group with high MTV or TLG values when compared with the group with low values. For studies in which the HRs and CIs were not available, we used the method proposed by Parmar et al. [25] to derive estimates from survival curves. The point estimate of the HR was considered statistically significant at the p< 0.05 level if the 95% CI did not include the value 1.
Heterogeneity was assessed by means of Cochran Q and I2 statistics. I-square (I2) values of <30%, 30%-50%, 50%-75% and >75% were defined as low, moderate, substantial and considerable heterogeneity, respectively [26]. If heterogeneity existed between primary studies, a random effects model was used. Otherwise, a fixed effects model was used for the meta-analysis. Publication bias was assessed by visual inspection of the funnel plot and also by means of the Begg and Egger tests [27,28]. A p-value less than 0.05 indicates the existence of publication bias. All statistical analyses were performed by using RevMan 5.3 (Nordic Cochrane Centre).
Results
Literature search procedure
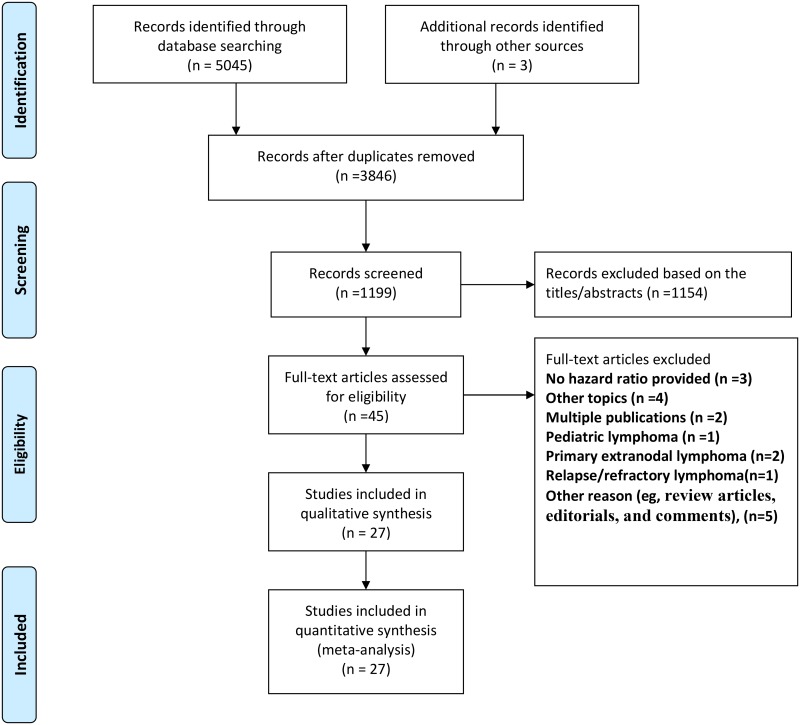
The PRISMA statement flowchart shows the process of literature screening and selection, as well as the reasons for exclusion (Fig 1). Our initial search yielded 5045 articles. After removing duplicates and screening the titles and abstracts, 45 articles were reviewed in more detail. Of the 45 full-text studies, 18 were excluded for the following reasons: 7 studies had incomplete or unavailable data and other topics; 5 reports were reviews and editorials; 1 study involved pediatric lymphoma; 3 studies included primary extranodal lymphoma and relapsed or refractory lymphoma; 2 studies were multiple publications. After reviewing the full texts, 27 studies were finally selected as potentially appropriate for inclusion in the meta-analysis [15–22,29–47].
Fig 1. Flow diagram of the systematic review and meta-analysis process.
Study characteristics
The 27 observational studies fulfilled the inclusion criteria, were published between 2012 and 2018 and are summarized in Table 1. Twenty-five studies were retrospective observational studies, and three studies were prospective multicenter trials. Seventeen studies included patients with diffuse large B cell lymphoma, three included patients with follicular lymphoma, one included patients with peripheral T-cell lymphoma, four studies included patients with extranodal natural killer/T cell lymphoma, and three studies included patients with Hodgkin lymphoma. The total sample size was 2729. Either TMTV or TLG was measured in 12 studies, and both were measured in 15 studies.
Table 1. Characteristics of studies included in the meta-analysis.
| Study | Year | Country | Study Design | NOS | Type of Lymphoma | Patients (No.) | Treatment regimen | Tumor Volume Parameters(MTV/TLG) | Cut-off values | Determination of MTV Cut off | Endpoints | Median follow-up | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Threshold (%) | Median MTV (cm3) | Median TLG | MTV (cm3) | TLG | |||||||||||
| Song et al.a | 2012 | Korea | R | 8 | DLBCL | 169 | R-CHOP | ≥2.5 | 198.1 | NR | 220 | NR | ROC analysis | PFS/OS | 36 months |
| Manohar et al. | 2012 | India | R | 6 | NHL§ | 51 | R-CHOP-like | Background- level † | 957 | 5356 | 416 | 3340 | ROC analysis | PFS/OS | 12 months |
| Kim et al. | 2013 | Korea | R | 8 | DLBCL | 140 | R-CHOP | Various‡ | NR | 415.5 | NR | 415.5 | N/A | PFS/OS | 28.5 months |
| Oh et al. | 2013 | Korea | R | 6 | DLBCL | 181 | R-CHOP | ≥2.5 | 156.89 | NR | 65.975 | NR | ROC analysis | PFS/OS | NR |
| Song et al. | 2013 | Korea | R | 7 | HL | 127 | ABVD | ≥2.5 | 142.6 | NR | 198 | NR | ROC analysis | PFS/OS | 45.8 months |
| Kim et al. | 2013 | Korea | R | 5 | ENKTL | 20 | CTx follow RT/ Only CTx | NR | 10.7 | 46.9 | 14.4 | 52.7 | ROC analysis | PFS/OS | 26.3 months |
| Esfahani et al. | 2013 | USA | R | 7 | DLBCL | 20 | R-CHOP | 50 | 379.16 | 704.77 | 379.16 | 704.77 | ROC analysis | PFS | mean 51.35 months |
| Sasanelli et al. | 2014 | France | R | 9 | DLBCL | 114 | R-CHOP/ R-ACVBP | 41 | 315 | 2974 | 550 | 4,576 | ROC analysis | PFS/OS | 39 months |
| Gallicchio et al. | 2014 | Italy | R | 5 | DLBCL | 52 | R-CHOP-like | 42 | 43 | 596.9 | 16.1 | 589.5 | ROC analysis | EFS/OS | 18 months |
| Kim et al. | 2014 | Korea | R | 5 | DLBCL | 96 | R-CHOP | ≥2.5 | 130.7 | NR | 130.7 | NR | ROC analysis | EFS/OS | 27.8 months |
| Adams et al. | 2015 | Netherland | R | 9 | DLBCL | 73 | R-CHOP | 40 | 272 | 2955.4 | 272 | 2955.4 | ROC analysis | PFS/OS | 2.7years |
| Schoder et al. | 2015 | USA | P | 6 | DLBCL | 65 | R-CHOP | Various‡ | 226 | NR | NR | NR | N/A | PFS/OS | 51 months |
| Kanoun et al. | 2015 | France | R | 8 | HL | 59 | CTx ± RT | Various‡ | 160 | NR | 313 | NR | ROC analysis | PFS/OS | 39 months |
| Mikhaeel et al. | 2016 | UK | R | 8 | DLBCL | 147 | R-CHOP | 41 | 595 | 4669.5 | 396 | 4541 | ROC analysis | PFS | 3.8 years |
| Zhou et al. | 2016 | China | R | 8 | DLBCL | 91 | R-CHOP | Background-level † | 50.7 | 497.3 | PFS: 70 OS: 78 | PFS: 827 OS: 726 | ROC analysis | PFS/OS | 30 months |
| Song et al.b | 2016 | Korea | R | 6 | DLBCL | 107 | R-CHOP | ≥2.5 | 526.8 | NR | 601.2 | NR | ROC analysis | PFS/OS | 40.8 months |
| Cottereau et al. | 2016 | France | R | 8 | PTCL | 108 | CHOP-like/ ACVBP | 41 | 224 | 1155 | 230 | 1068 | ROC analysis | PFS/OS | 23 months |
| Meignan et al. | 2016 | France | R | 9 | FL 1-3a | 185 | R-CHOP/ R-CVP/R-FM | 41 | 297 | NR | 510 | NR | X-tile analysis | PFS/OS | 64 months |
| Chang et al. | 2017 | China | R | 6 | ENKTL | 52 | DDGP/SMILE | 40 | 11.2 | 46.4 | 16.1 | 44.7 | ROC analysis | PFS/OS | 19 months |
| Chang et al. | 2017 | Taiwan | R | 7 | DLBCL | 118 | R-CHOP | ≥2.5 | 550.4* | 3533.2* | 165.4 | 1204.9 | ROC analysis | PFS/OS | 28.7 months |
| Kesavan et al. | 2017 | Australia | P | 7 | FL | 68 | Iodine-131-rituximab | 41 | 510 | NR | 510 | NR | NR | TTNT/OS | 59 months |
| Song et al. | 2017 | Korea | R | 5 | ENKTL | 100 | CTx follow RT/CTx | ≥2.5 | 36.2 | NR | 94.2 | NR | ROC analysis | PFS/OS | NR |
| Cottereau et al. | 2018 | France | P | 9 | HL | 258 | ABVD | 41 | 67 | 332 | 147 | 495 | X-tile and ROC analysis | PFS/OS | 55 months |
| Ding et al. | 2018 | China | R | 7 | DLBCL | 72 | R-CHOP | 40 | 139.48 | 1413.77 | 67.71 | 1413.77 | ROC analysis | PFS/OS | 45 months |
| Toledano et al. | 2018 | France | R | 8 | DLBCL | 114 | R-CHOP/ R-CHOP-like | 41 | 275.8 | NR | 261.4 | 1325.80 | ROC analysis | PFS/OS | 40 months |
| Delfau-Larue et al. | 2018 | France | R | 8 | FL | 133 | R-CHOP/ R-CHOP-like/Chemo-free | 41 | 354 | NR | 510 | NR | X-tile and ROC analysis | PFS/OS | 48 months |
| Pak et al. | 2018 | Korea | R | 7 | ENKTL | 36 | NR | 40 | NR | NR | 7 | 45.8 | NR | RFS/OS | 20.6 months |
PET/CT, positron emission tomography/computed tomography; MTV, metabolic tumor volume; TLG, total lesion glycolysis; NOS, the Newcastle-Ottawa-Scale; DLBCL, diffuse large B cell lymphoma; HL, hodgkin lymphoma; NHL, non-hodgkin’s lymphoma; ENKTL, extranodal natural killer/T cell lymphoma; PTCL, peripheral T-cell lymphoma; FL, follicular Lymphoma; R, retrospective; P, prospective; NR, not reported; PFS, progression-free surviva; TTNT:time-to-next-treatment; IQR, interquartile range; ROC, receiver operator curve; N/A, not applicable; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; ABVD, doxorubicin,bleomycin, vinblastine, dacarbazine; CTx, chemotherapy; RT, radiotherapy; R-ACVBP: rituximab, doxorubicine, vindesine, cyclophosphamide, bleomycin, prednisolone; R-ICE, rituximab, etoposide, ifosfamide, carboplatin; R-CVP, rituximab, cyclophosphamide, vincristine and prednisolone; R-FM, rituximab, fludarabine and mitoxantrone; DDGP, dexamethasone, cisplatin, gemcitabline and pegaspargase; SMILE, dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide;
‡ tested various proposed thresholds, including 41%;
† MTV was measured by setting the tumor marginal threshold of liver SUVmean plus 3SDs. SUVmean in liver was calculated in a standard-sized ROI of 3cm in diameter;
§ Of the 51 patients, 39 (77%) had DLBCL, 8 had anaplastic large T-cell lymphoma and 4 had high-grade peripheral T-cell lymphoma.
a, In staged II and III patients without extranodal site involvement; b, In patients with bone marrow involvement of lymphoma.
*The mean values of total MTV and TLG were 550.4 ± 678.3 cm3 and 3533.2 ± 4394.1 cm3 respectively.
Three thresholding methods for the auto segmentation of PET volumes exist. A fixed SUV of 2.5 was used in 7 studies, the percentage of SUVmax (40%, 41%, 42% or 50%) was used in 18 studies, and in 2 studies the MTV was measured by setting the tumor margin threshold as the liver SUVmean plus 3SDs. In each study, patients were divided into 2 groups (high and low volume) based on the cut-off values. The MTV and TLG cut-off values were determined by means of receiver operating curve (ROC) and X-tile analyses. Receiver operating characteristics (ROCs) was used in 19 studies, receiver-operating characteristics and X-tile analysis in 3 studies, X-tile analysis in 1 study, and 4 studies did not provide cut-off information. The MTV cut-off values ranged between 10.7 and 595 cm3 and the TLG values ranged from 46.4 to 5356. The study quality assessed by means of the NOS was fair, with a median quality score of 8 (range 5–9).
Prognostic value of baseline metabolic tumor volume
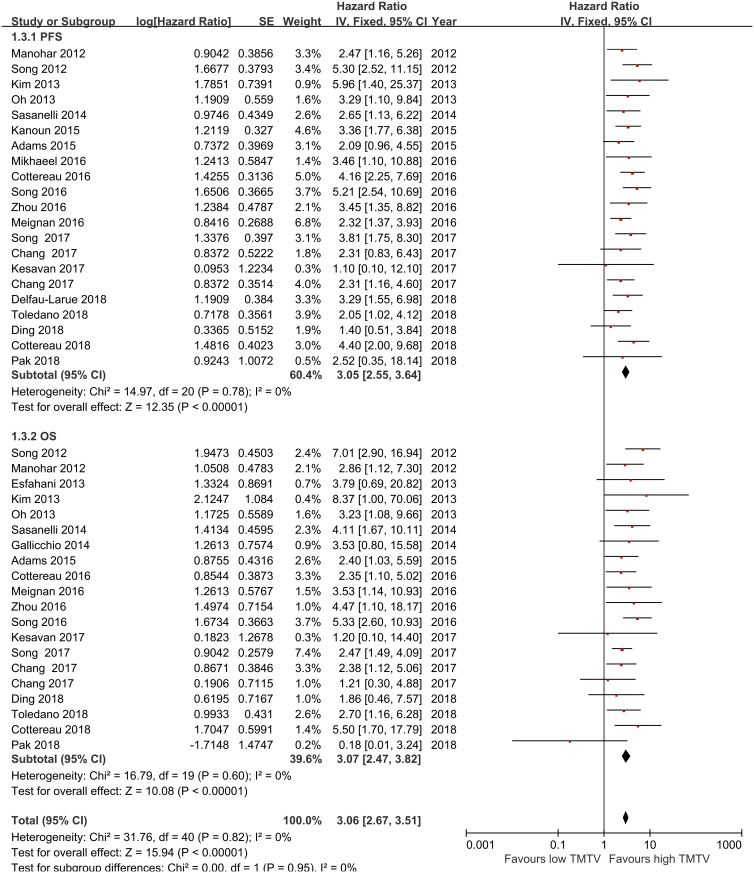
Twenty-one studies [15–19,21,22,29,30–35,37,39,43–47] on adult lymphoma were included for the analysis of the relationship between TMTV and PFS. The results of our meta-analysis showed that a high TMTV was associated with a shorter PFS than a low TMTV, with a pooled HR of 3.05 (95% CI, 2.55–3.64, p<0.00001). No significant heterogeneity was found across studies (I2 = 0.0%, P = 0.78). Twenty-one studies [15,17–20,22,29–35,38–40,43–47] reported data on TMTV and OS in adult lymphoma. The meta-analysis results demonstrated that a high TMTV was associated with shorter OS than a low TMTV, with a pooled HR of 3.07 (95%CI, 2.47–3.82, p<0.00001). No significant heterogeneity was found across studies (I2 = 0.0%, p = 0.60; Fig 2).
Fig 2. Meta-analysis of the hazard ratios for PFS, and OS for high TMTV vs low TMTV.
Hazard ratios and 95% confidence intervals from individual studies are depicted as squares and horizontal lines, respectively. The pooled estimate is shown as a diamond shape, where the center represents the pooled HR and the horizontal borders represent the 95% CI. Hazard ratios are defined as high TMTV vs low TMTV, therefore a hazard ratio >1 represents a higher risk of death or progression associated with high TMTV. TMTV = total metabolic tumor volume, OS = overall survival, PFS = progression-free survival, CI = confidence interval.
Next, we examined the relationship between TMTV and the clinical outcome of different types of lymphomas. A meta-analysis of thirteen studies of DLBCL patients showed poorer PFS and OS in patients with high TMTV than in those with low TMTV, with pooled HRs for PFS and OS of 2.93 (95%CI, 2.29–3.73, p<0.001; heterogeneity: I2 = 0.0%, p = 0.481) and 3.52 (95%CI, 2.67–4.64, p<0.001; heterogeneity: I2 = 0.0%, p = 0.806), respectively. Three studies examined the prognostic significance of high TMTV in FL patients, and the pooled HRs for PFS and OS were 2.55 (95%CI, 1.65–3.92, p<0.001; heterogeneity: I2 = 0.0%, p = 0.622) and 2.89 (95%CI, 1.04–7.99, p<0.001; heterogeneity: I2 = 0.0%, p = 0.419), respectively. Four studies assessed the prognostic significance of high TMTV in ENKL patients, and the pooled HRs for PFS and OS were 3.25 (95%CI, 1.75–6.07, p<0.001; heterogeneity: I2 = 10.7%, p = 0.340) and 2.24 (95%CI, 1.23–4.08, p = 0.008; heterogeneity: I2 = 24%, p = 0.267), respectively. Two studies investigated the prognostic significance of high TMTV in HL patients, and the pooled HR for PFS was 3.89 (95%CI, 2.19–6.90, p<0.001; heterogeneity: I2 = 0.0%, p = 0.646) (Table 2). Notably, only one study provided relevant data on the correlation between TMTV and PTCL outcome and only one study provided data on the correlation between TMTV and OS in HL patients; therefore, the pooled analysis could not be performed.
Table 2. Pooled hazard ratios for PFS and OS according to TMTV.
| Study selection | N | PFS | N of cohorts | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Random-effects model | Heterogeneity | Random-effects model | Heterogeneity | |||||||
| Pooled HR (95% CI) | P value | I2 (%) | PH value | Pooled HR (95% CI) | P value | I2 (%) | PH value | |||
| Type of Lymphoma | ||||||||||
| DLBCL | 12 | 2.93(2.29–3.73) | <0.001 | 0 | 0.481 | 13 | 3.52(2.67–4.64) | <0.001 | 0 | 0.806 |
| FL | 3 | 2.55(1.65–3.92) | <0.001 | 0 | 0.622 | 2 | 2.89(1.04–7.99) | <0.001 | 0 | 0.419 |
| PTCL | 1 | 4.16(2.25–7.68) | <0.0001 | - | - | 1 | 2.35(1.10–5.04) | 0.028 | - | - |
| ENKTL | 4 | 3.25(1.75–6.07) | <0.001 | 10.7 | 0.34 | 4 | 2.24(1.23–4.08) | 0.008 | 24 | 0.267 |
| HL | 2 | 3.89(2.19–6.90) | <0.001 | 0 | 0.646 | 1 | 3.90(1.60–9.50) | 0.0032 | - | - |
| Study Design | ||||||||||
| Retrospective | 19 | 3.20(2.64–4.01) | <0.001 | 0 | 0.774 | 17 | 3.21(2.54–4.07) | <0.001 | 0 | 0.743 |
| Prospective | 3 | 2.81(1.72–4.59) | <0.001 | 11.2 | 0.342 | 3 | 1.91(0.51–7.15) | 0.337 | 23.1 | 0.273 |
| Sample size | ||||||||||
| ≥100 | 11 | 3.51(2.78–4.42) | <0.001 | 0 | 0.667 | 10 | 3.59(2.70–4.76) | <0.001 | 0 | 0.531 |
| <100 | 7 | 2.82(1.99–3.99) | <0.001 | 0 | 0.55 | 9 | 2.45(1.65–3.65) | <0.001 | 0 | 0.809 |
| Threshold | ||||||||||
| ≥2.5 | 5 | 3.93(2.76–5.60) | <0.001 | 0 | 0.553 | 5 | 3.65(2.38–5.61) | <0.001 | 28.9 | 0.229 |
| 41 | 6 | 2.56(1.83–3.58) | <0.001 | 0 | 0.554 | 5 | 3.56(2.22–5.70) | <0.001 | 0 | 0.772 |
| 40 | 3 | 2.19(1.21–3.98) | 0.01 | 0 | 0.98 | 3 | 1.80(0.84–3.83) | 0.129 | 5 | 0.349 |
| other | 4 | 3.20(2.02–5.05) | <0.001 | 0 | 0.753 | 4 | 3.69(1.89–7.22) | <0.001 | 0 | 0.817 |
N: number of studies; HR: hazard ratio; 95% CI: 95% confidence interval; PH: p values of Q test for heterogeneity test; OS: Overall survival; PFS: Progression free survival.
We also conducted subgroup analyses stratified by data collection method, sample size, and different threshold values. The subgroup analysis of retrospectively collected data showed pooled HRs for PFS and OS of 3.20 (95%CI, 2.64–4.01, p<0.001; heterogeneity: I2 = 0.0%, p = 0.774) and 3.21 (95%CI, 2.54–4.07, p<0.001; heterogeneity: I2 = 0.0%, p = 0.743), respectively. The prospectively collected data showed pooled HRs for OS and PFS of 2.81 (95%CI, 1.72–4.59, p<0.001; heterogeneity: I2 = 11.2%, p = 0.342) and 1.91 (95%CI, 0.51–7.15, p = 0.337; heterogeneity: I2 = 23.1%, p = 0.273), respectively. The subgroup analysis performed on the basis of sample size showed that the negative predictive value of high TMTV on PFS and OS was present both in samples with sizes ≥100 (PFS, HR: 3.51, 95%CI: 2.78–4.42, p<0.001; OS, HR: 3.59, 95%CI: 2.70–4.76, p<0.001) and <100 (PFS, HR: 2.82, 95%CI: 1.99–3.99, p< 0.001; OS, HR: 2.45, 95%CI:1.65–3.65, p<0.001). Lastly, the subgroup analyses by thresholds (≥ 2.5, 40%, 41%, and others) demonstrated that a high TMTV was associated with shorter PFS and OS than a low TMTV (Table 2).
Prognostic value of baseline total lesion glycolysis
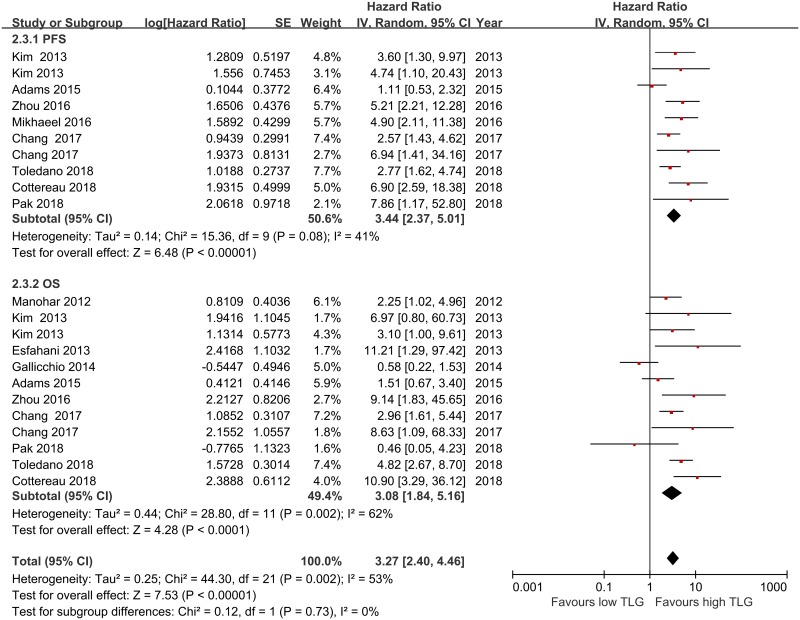
Ten studies [15–17,20,22,29,32,37,43,46] were included for the analysis of the relationship between TLG and PFS in adult lymphoma. The results of our meta-analysis showed that high TLG values were associated with shorter PFS than low TLG values, with a pooled HR of 3.44 (95%CI, 2.37–5.01, p<0.00001). There was moderate heterogeneity across the studies, but it did not reach statistical significance (I2 = 41.0%, p = 0.08). Twelve studies [15,17,20,22,29,32,34,37,38,40,43,46] reported data on TLG and OS in adult lymphoma. Because significant heterogeneity was found across the studies (I2 = 62.0%, P = 0.002), a pooled HR of 3.08 (95%CI, 1.84–5.16, p = 0.0001) was calculated on the basis of a random-effects model (Fig 3).
Fig 3. Meta-analysis of the hazard ratios for PFS and OS for high TLG vs low TLG.
Hazard ratios and 95% confidence intervals for death or progression associated with high vs low TLG. TLG = total lesion glycolysis, OS = overall survival, PFS = progression-free survival, CI = confidence interval.
Next, we examined the prognostic significance of TLG on different types of lymphomas. A meta-analysis of seven and eight studies involving DLBCL patients showed poorer PFS and OS in those with high TMTV values than in those with low TMTV values, with pooled HRs for OS and PFS of 3.06 (95%CI, 1.52–6.18, p = 0.002; heterogeneity: I2 = 67.3%, p = 0.003) and 2.93 (95%CI, 1.89–4.53, p<0.001; heterogeneity: I2 = 49.5%, p = 0.065), respectively. Three studies examined the prognostic significance of high TLG values in ENKL patients, and the pooled HRs for PFS and OS were 2.99 (95%CI, 1.83–4.89, p<0.001; heterogeneity: I2 = 0.0%, p = 0.503) and 2.58 (95%CI, 1.33–5.01, p = 0.005; heterogeneity: I2 = 19.0%, p = 0.291), respectively (Table 3). Only one study provided relevant data on the correlation between TLG and clinical outcome in PTCL patients and only one study provided data on the correlation between TLG and outcome in HL patients; therefore, the pooled analysis could not be performed.
Table 3. Pooled hazard ratios for PFS and OS according to TLG.
| Study selection | N | PFS | N | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Random-effects model | Heterogeneity | Random-effects model | Heterogeneity | |||||||
| Pooled HR (95% CI) | P value | I2 (%) | PH value | Pooled HR (95% CI) | P value | I2 (%) | PH value | |||
| Type of Lymphoma | ||||||||||
| DLBCL | 7 | 2.93(1.89–4.53) | <0.001 | 49.5 | 0.065 | 8 | 3.06(1.52–6.18) | 0.002 | 67.3 | 0.003 |
| PTCL | - | - | - | - | - | - | - | - | - | - |
| ENKTL | 3 | 2.99(1.83–4.89) | <0.001 | 0 | 0.503 | 3 | 2.58(1.33–5.01) | 0.005 | 19 | 0.291 |
| HL | 1 | 6.90(2.59–18.36) | 0.0001 | - | - | 1 | 10.9(3.29–36.23) | 0.0001 | - | - |
| Study Design | ||||||||||
| Retrospective | 10 | 2.97(2.03–4.35) | <0.001 | 35.7 | 0.122 | 11 | 2.28(1.40–3.71) | 0.001 | 50.1 | 0.029 |
| Prospective | 1 | 6.90(2.59–18.36) | 0.0001 | - | - | 1 | 10.9(3.29–36.23) | 0.0001 | - | - |
| Sample size | ||||||||||
| ≥100 | 5 | 4.60(2.83–7.47) | <0.001 | 0 | 0.59 | 4 | 3.54(1.56–8.06) | 0.003 | 62.6 | 0.045 |
| <100 | 6 | 2.73(1.53–4.86) | 0.001 | 48.7 | 0.083 | 9 | 2.07(1.21–3.55) | 0.008 | 54.9 | 0.023 |
| Threshold | ||||||||||
| ≥2.5 | 1 | 6.94(1.41–34.12) | 0.017 | - | - | 1 | 8.63(1.09–68.34) | 0.041 | - | - |
| 41 | 3 | 4.64(2.44–8.85) | <0.001 | 13.9 | 0.313 | 1 | 10.9(3.29–36.23) | 0.0001 | - | - |
| 40 | 3 | 2.16(0.94–4.96) | 0.07 | 60.6 | 0.079 | 3 | 1.90(0.91–3.98) | 0.086 | 43.7 | 0.169 |
| other | 3 | 4.52(2.48–8.21) | <0.001 | 0 | 0.86 | 5 | 3.53(1.98–6.28) | <0.001 | 3.1 | 0.389 |
N: number of studies; HR: hazard ratio; 95% CI: 95% confidence interval; PH: p values of Q test for heterogeneity test; OS: Overall survival; PFS: Progression free survival.
We also conducted subgroup analyses stratified by data collection method, sample size, and different threshold values. The subgroup analysis of retrospectively collected data showed pooled HRs for OS and PFS of 2.28 (95%CI, 1.40–3.71, p = 0.001; heterogeneity: I2 = 50.1%, p = 0.029) and 2.97 (95%CI, 2.03–4.35, p<0.001; heterogeneity: I2 = 35.7%, p = 0.122), respectively. Only one study involved prospectively collected data, so the pooled analysis could not be performed. The subgroup analysis conducted by sample size showed that the negative predictive value of high TMTV on PFS and OS was present both in samples with sizes ≥100 (PFS, HR: 4.60, 95%CI: 2.83–7.47, p<0.001; OS, HR: 3.54, 95%CI: 1.56–8.06, p<0.001) and <100 (PFS, HR: 2.73, 95%CI: 1.53–4.86, p = 0.001; OS, HR: 2.07, 95%CI:1.21–3.55, p = 0.008). A meta-analysis of three studies with a 40% threshold showed there was a trend for a correlation between high TLG values and lymphoma prognosis, with pooled HRs for OS and PFS of 1.90 (95%CI, 0.91–3.98, p = 0.086; heterogeneity: I2 = 43.7%, p = 0.169) and 2.16 (95%CI, 0.94–4.96, p = 0.07; heterogeneity: I2 = 60.6%, p = 0.079), respectively. The meta-analysis of studies using other thresholds (including the liver SUVmean plus 3SDs, 50%) showed a negative correlation between high TLG values and lymphoma prognosis, with pooled HRs for OS and PFS of 3.53 (95%CI, 1.98–6.28, p<0.001; heterogeneity: I2 = 3.1%, p = 0.389) and 4.52 (95%CI, 2.48–8.21, p<0.001; heterogeneity: I2 = 0.0%, p = 0.860), respectively. The subgroup analysis selecting a 41% threshold showed a negative predictive value of high TLG on PFS, with a pooled HR for PFS of 4.64 (95%CI, 2.44–8.85, p<0.001; heterogeneity: I2 = 13.9%, p = 0.313) (Table 3). Only one study used a threshold ≥2.5, so the pooled analysis could not be performed.
Publication bias
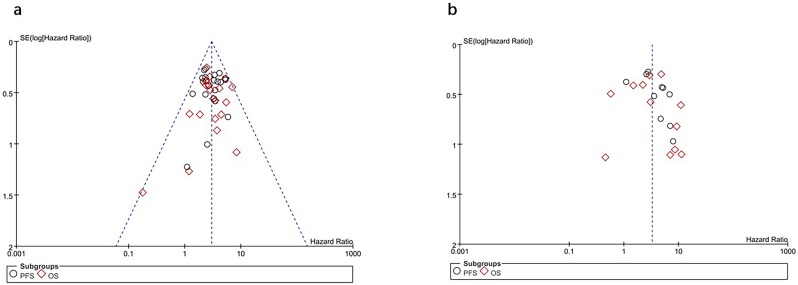
Inspection of the funnel plot and formal statistical tests (TMTV: Egger test, p = 0.931; Begg test, p = 0.867; TLG: Egger test, p = 0.200; Begg test, p = 0.236; Fig 4) showed no evidence of publication bias in the meta-analysis of the prognostic significance of baseline metabolic tumor volume and total lesion glycolysis in adult lymphoma.
Fig 4. Assessment of publication bias using Funnel plot analysis.
(a) Funnel plot of hazard ratio for overall survival and progression-free survival for high TMTV (horizontal axis) and the standard error (SE) for the hazard ratio (vertical axis). (b) Funnel plot of hazard ratio for overall survival and progression-free survival for high TLG (horizontal axis) and the standard error (SE) for the hazard ratio (vertical axis). Each study is represented by one circle. The vertical line represents the pooled effect estimate.
Discussion
Main findings
This meta-analysis comprehensively and systematically reviewed the current available literature and found that: (1) A high baseline TMTV significantly predicted poor OS and shorter PFS in adult lymphoma patients (p<0.00001 and p<0.00001, respectively); (2) A high baseline TMTV was significantly associated with reduced survival in DLBCL patients treated with R-CHOP and predicted poor OS and PFS for different types of lymphomas, such as FL, ENKL and HL. The evidence supporting this association was consistent in most subgroup analyses (retrospective data collection, ethnicity, sample size, and different thresholds). The analysis of prospectively collected data and studies using a 40% threshold suggested a trend towards poor OS; however, these results were not statistically significant; (3) A high baseline TLG significantly predicted poor OS and shorter PFS in adult lymphoma patients (p<0.00001 and p = 0.005, respectively); (4) A high baseline TLG was significantly associated with reduced survival in DLBCL patients treated with R-CHOP and predicted poor OS and PFS in different types of lymphomas, such as FL, ENKL and HL. The evidence of this association was consistent in most subgroup analyses (data collection method, ethnicity, sample size, and different thresholds).
Metabolic tumor volumes can be segmented by using various methods, such as a fixed SUV threshold, a percentage (based on the percentage of maximum uptake in the lesion), a threshold adjusted to the tumor-to-background ratio, or a gradient [23]. Reproducibility is the key for reliable volumetric tumor segmentation. Different TMTV measurement methods have been used in various types of lymphoma, each with specific advantages and disadvantages. A method based on the 41% SUVmax threshold is recommended by the European Association of Nuclear Medicine (EANM) for TMTV measurement of solid tumors. It has been developed in patients with HL and DLBCL, showing good reproducibility [48]. Different thresholding methods were used for PET volume auto-segmentation in the studies included herein; however, a threshold of 41% or 40% of the SUVmax was widely used. In our study, we conducted subgroup analyses stratified by different thresholds (≥2.5, 41%, and others). The results demonstrated that high TMTV or TLG values were associated with shorter PFS and OS. Subgroup stratification based on a threshold of 40% of the SUVmax showed that a high TMTV was a negative predictor of PFS; however, it did not significantly predict poor PFS and OS in the case of TLG.
Baseline MTV by PET/CT, is a promising prognostic indicator in patients with lymphoma, which is better than using size-defined bulk [16, 33]. TLG, which is the MTV multiplied by the mean SUV in the volume, is also prognostic [37], but appears no better than MTV in prediction of survival in lymphoma [16].
Several retrospective studies have shown that metabolic tumor volume (MTV) is a strong predictor of prognosis irrespective of the method [19,21,49]. However, cut-offs used to divide patients into high and low risk groups by MTV are highly dependent on the patient population and the method used. A fixed 41% SUVmax relative thresholding method has been applied successfully in different subtypes of lymphoma, but probably overestimated the volume of lesions with low SUVmax, particularly for smaller VOIs [19,21,48]. The 2.5 method could include the volume of nontumor regions located between small distant nodes with high uptake [50]. The 2.5 method probably overestimated MTV in approximately 12% of patients who had low FDG uptake in the liver or liver involvement by lymphoma [49]. Furthermore, the negative and positive predictive values of the 41% method have been shown to be superior to other methods, which results in excellent outcome prediction in other subtypes of lymphoma [21]. Generally, current evidence showed that metabolic tumor volume values were significantly influenced by the choice of the method used for determination of volume. However, no significant differences were found in term of prognosis [21]. In clinical practice, a consensus on the most accurate method or an optimal cut-off to define the MTV for specific lymphoma subtypes will be required, which will require validation in multicenter prospective trials.
Several methods for autosegmentation of PET volumes exist (e g, threshold-based, gradient-based, statistical, and texture-based methods) [51]. All methods have strengths and limitations. Reproducibility is the key for tumor segmentation in routine practice [23]. There is no universally accepted reproducible and practical method for tumor segmentation. Recently, Yu et al. reported a new semi-automatic approach that applies first an anatomical multi-atlas segmentation on the CT images to remove the organs having hyper uptake value on PET images. Using a CRFs (Conditional Random Fields) model, the rate of good detection of lymphoma is 100% in 11 patients [52]. Meanwhile, this new semi-automatic approach has the best dice index for the real lymphoma regions. However, this new methodology will require prospective validation in sufficiently large patient cohorts.
Among the included studies, there were mainly two different approaches to define the optimal TMTV cut-off value as a predictor of survival: X-tile analysis, receiver operating curve (ROC) analysis, or both. X-tile analysis is the primary approach for reliable cut-point determination. This method creates separate training and validation data sets, improving the robustness of the analysis [53]. In the studies included in this meta-analysis, ROC was widely used. This method defines the optimal cut-off point as the value whose sensitivity and specificity are closest to the value of the area under the ROC curve, and for which the absolute value of the difference between the sensitivity and specificity values is minimal. This method is recommended for finding the true cut-off point [54]. Meignan et al. [18] used another restricted cubic spline to define the optimal TMTV cut-off point. Splines are used to model the relationship between TMTV as a continuous variable and survival time, but their contribution to optimal cut-off point definition is minimal. Subgroup analyses based on different MTV cut-off values demonstrated that patients with a high TMTV had shorter PFS and OS than those with a low TMTV.
A major strength of this meta-analysis is that it complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [55]. In addition, we extracted the maximum information from the included studies by a thorough qualitative review and quantitative meta-analysis.
Our study also has some limitations. Firstly, nearly all of the included studies were retrospective, which may result in confounding and detection bias. Secondly, patients with different types of lymphoma were treated with different therapeutic regimes. Thirdly, PET scans were performed using scanners of different generations, which may potentially affect the calculation of the SUV and therefore, of TMTV and TLG as well. Similarly, the FDG uptake times were difficult to standardize. Based on all of the above, the clinical heterogeneity of the included studies could be an issue. Finally, our meta-analysis was based on data from published trials, and we did not obtain individual patient data.
Conclusions
Our meta-analysis suggests that high baseline metabolic tumor volumes or total lesion glycolysis measured by FDG-PET/CT predict significantly worse overall survival and progression-free survival in patients with lymphoma. Therefore, TMTV and TLG may serve as new prognostic biomarkers. In view of our findings, future clinical trials with patients with different types of lymphoma are warranted to determine whether these novel findings can be integrated into various prognostic models, with the goal of achieving better risk stratification and treatment selection.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Linet MS, Brown LM, Mbulaiteye SM, Check D, Ostroumova E, Landgren A, et al. International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0–19 years. Int J Cancer. 2016;138(8):1862–74. 10.1002/ijc.29924 [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th eds Lyon, France: International Agency for Research on Cancer press; 2017. [Google Scholar]
- 4.Cheson BD. International staging and response criteria for lymphomas In: O’Brien S, Vose JM, Kantarjian HM. Management of Hematological Malignancies. New York, NY: Cambridge University Press; 2010. pp.277–58. [Google Scholar]
- 5.Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Ann Oncol. 2014;25(11):2124–33. 10.1093/annonc/mdu109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moccia AA, Donaldson J, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. International Prognostic Score in advanced-stage Hodgkin’s lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30(27):3383–8. 10.1200/JCO.2011.41.0910 [DOI] [PubMed] [Google Scholar]
- 7.Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–65. 10.1182/blood-2003-12-4434 [DOI] [PubMed] [Google Scholar]
- 8.Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–65. 10.1182/blood-2007-06-095331 [DOI] [PubMed] [Google Scholar]
- 9.Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103(7):2474–9. 10.1182/blood-2003-09-3080 [DOI] [PubMed] [Google Scholar]
- 10.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–58. 10.1200/JCO.2013.53.5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams HJ, Nievelstein RA, Kwee TC. Prognostic value of complete remission status at end-of-treatment FDG-PET in R-CHOP-treated diffuse large B-cell lymphoma: systematic review and meta-analysis. Br J Haematol. 2015;170(2):185–91. 10.1111/bjh.13420 [DOI] [PubMed] [Google Scholar]
- 13.El-Galaly TC, Pedersen MB, Hutchings M, Mylam KJ, Madsen J, Gang AO, et al. Utility of interim and end-of-treatment PET/CT in peripheral T-cell lymphomas: A review of 124 patients. Am J Hematol. 2015;90(11):975–80. 10.1002/ajh.24128 [DOI] [PubMed] [Google Scholar]
- 14.Adams HJ, Nievelstein RA, Kwee TC. Prognostic value of interim FDG-PET in Hodgkin lymphoma: systematic review and meta-analysis. Br J Haematol. 2015;170(3):356–66. 10.1111/bjh.13441 [DOI] [PubMed] [Google Scholar]
- 15.Chang CC, Cho SF, Chuang YW, Lin CY, Chang SM, Hsu WL, et al. Prognostic significance of total metabolic tumor volume on (18)F-fluorodeoxyglucose positron emission tomography/ computed tomography in patients with diffuse large B-cell lymphoma receiving rituximab-containing chemotherapy. Oncotarget. 2017;8(59):99587–600. 10.18632/oncotarget.20447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikhaeel NG, Smith D, Dunn JT, Phillips M, Moller H, Fields PA, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43(7):1209–19. 10.1007/s00259-016-3315-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams HJ, de Klerk JM, Fijnheer R, Heggelman BG, Dubois SV, Nievelstein RA, et al. Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole-body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol. 2015;94(6):532–9. 10.1111/ejh.12467 [DOI] [PubMed] [Google Scholar]
- 18.Meignan M, Cottereau AS, Versari A, et al. Baseline Metabolic Tumor Volume Predicts Outcome in High-Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. J Clin Oncol. 2016;34(30):3618–26. 10.1200/JCO.2016.66.9440 [DOI] [PubMed] [Google Scholar]
- 19.Cottereau AS, Becker S, Broussais F, Casasnovas O, Kanoun S, Roques M, et al. Prognostic value of baseline total metabolic tumor volume (TMTV0) measured on FDG-PET/CT in patients with peripheral T-cell lymphoma (PTCL). Ann Oncol. 2016;27(4):719–24. 10.1093/annonc/mdw011 [DOI] [PubMed] [Google Scholar]
- 20.Kim CY, Hong CM, Kim DH, Son SH, Jeong SY, Lee SW, et al. Prognostic value of whole-body metabolic tumour volume and total lesion glycolysis measured on (1)(8)F-FDG PET/CT in patients with extranodal NK/T-cell lymphoma. Eur J Nucl Med Mol Imaging. 2013;40(9):1321–9. 10.1007/s00259-013-2443-6 [DOI] [PubMed] [Google Scholar]
- 21.Kanoun S, Tal I, Berriolo-Riedinger A, Rossi C, Riedinger JM, Vrigneaud JM, et al. Influence of Software Tool and Methodological Aspects of Total Metabolic Tumor Volume Calculation on Baseline [18F]FDG PET to Predict Survival in Hodgkin Lymphoma. PLoS One. 2015;10(10):e0140830 10.1371/journal.pone.0140830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottereau AS, Versari A, Loft A, Casasnovas O, Bellei M, Ricci R, et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood. 2018;131(13):1456–63. 10.1182/blood-2017-07-795476 [DOI] [PubMed] [Google Scholar]
- 23.Kostakoglu L, Chauvie S. Metabolic Tumor Volume Metrics in Lymphoma. Semin Nucl Med. 2018;48(1):50–66. 10.1053/j.semnuclmed.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 25.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence--;inconsistency. J Clin Epidemiol. 2011;64(12):1294–302. 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pak K, Kim BS, Kim K, Kim IJ, Jun S, Jeong YJ, et al. Prognostic significance of standardized uptake value on F18-FDG PET/CT in patients with extranodal nasal type NK/T cell lymphoma: A multicenter, retrospective analysis. Am J Otolaryngol. 2018;39(1):1–5. 10.1016/j.amjoto.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 30.Song MK, Chung JS, Yhim HY, Lim SN, Kim SJ, Han YH, et al. Tumor necrosis and complete resection has significant impacts on survival in patients with limited-stage upper aerodigestive tract NK/T cell lymphoma. Oncotarget. 2017;8(45):79337–46. 10.18632/oncotarget.18107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesavan M, Boucek J, MacDonald W, McQuillan A, Turner JH. Imaging of Early Response to Predict Prognosis in the First-Line Management of Follicular Non-Hodgkin Lymphoma with Iodine-131-Rituximab Radioimmunotherapy. Diagnostics (Basel). 2017;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang Y, Fu X, Sun Z, Xie X, Wang R, Li Z, et al. Utility of baseline, interim and end-of-treatment (18)F-FDG PET/CT in extranodal natural killer/T-cell lymphoma patients treated with L-asparaginase/pegaspargase. Sci Rep. 2017;7:41057 10.1038/srep41057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MK, Chung JS, Shin HJ, Lee SM, Lee SE, Lee HS, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91(5):697–703. 10.1007/s00277-011-1357-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manohar K, Mittal BR, Bhattacharya A, Malhotra P, Varma S. Prognostic value of quantitative parameters derived on initial staging 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with high-grade non-Hodgkin’s lymphoma. Nucl Med Commun. 2012;33(9):974–81. 10.1097/MNM.0b013e32835673ec [DOI] [PubMed] [Google Scholar]
- 35.Oh MY, Chung JS, Song MK, Shin HJ, Lee HS, Lee SM, et al. Prognostic value of Waldeyer’s ring involvement of diffuse large B-cell lymphoma treated with R-CHOP. Int J Hematol. 2013;97(3):397–402. 10.1007/s12185-013-1282-3 [DOI] [PubMed] [Google Scholar]
- 36.Song MK, Chung JS, Lee JJ, Jeong SY, Lee SM, Hong JS, et al. Metabolic tumor volume by positron emission tomography/computed tomography as a clinical parameter to determine therapeutic modality for early stage Hodgkin’s lymphoma. Cancer Sci. 2013;104(12):1656–61. 10.1111/cas.12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TM, Paeng JC, Chun IK, Keam B, Jeon YK, Lee SH, et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the International Prognostic Index for patients with diffuse large B cell lymphoma. Cancer. 2013;119(6):1195–202. 10.1002/cncr.27855 [DOI] [PubMed] [Google Scholar]
- 38.Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with (18)F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging. 2013;3(3):272–81. [PMC free article] [PubMed] [Google Scholar]
- 39.Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(11):2017–22. 10.1007/s00259-014-2822-7 [DOI] [PubMed] [Google Scholar]
- 40.Gallicchio R, Mansueto G, Simeon V, Nardelli A, Guariglia R, Capacchione D, et al. F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;92(5):382–9. 10.1111/ejh.12268 [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Hong J, Kim SG, Hwang KH, Kim M, Ahn HK, et al. Prognostic Value of Metabolic Tumor Volume Estimated by (18) F-FDG Positron Emission Tomography/Computed Tomography in Patients with Diffuse Large B-Cell Lymphoma of Stage II or III Disease. Nucl Med Mol Imaging. 2014;48(3):187–95. 10.1007/s13139-014-0280-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoder H, Zelenetz AD, Hamlin P, Gavane S, Horwitz S, Matasar M, et al. Prospective Study of 3’-Deoxy-3’-18F-Fluorothymidine PET for Early Interim Response Assessment in Advanced-Stage B-Cell Lymphoma. J Nucl Med. 2016;57(5):728–34. 10.2967/jnumed.115.166769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou M, Chen Y, Huang H, Zhou X, Liu J, Huang G. Prognostic value of total lesion glycolysis of baseline 18F-fluorodeoxyglucose positron emission tomography/computed tomography in diffuse large B-cell lymphoma. Oncotarget. 2016;7(50):83544–53. 10.18632/oncotarget.13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song MK, Yang DH, Lee GW, Lim SN, Shin S, Pak KJ, et al. High total metabolic tumor volume in PET/CT predicts worse prognosis in diffuse large B cell lymphoma patients with bone marrow involvement in rituximab era. Leuk Res. 2016;42:1–6. 10.1016/j.leukres.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 45.Ding CY, Guo Z, Sun J, Yang WP, Li TR. Prognostic value of pretreatment (18)F-FDG PET-CT for patients with advanced diffuse large B-cell lymphoma. Zhonghua Zhong Liu Za Zhi. 2018;40(7):528–33. 10.3760/cma.j.issn.0253-3766.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 46.Toledano MN, Desbordes P, Banjar A, Gardin I, Vera P, Ruminy P, et al. Combination of baseline FDG PET/CT total metabolic tumour volume and gene expression profile have a robust predictive value in patients with diffuse large B-cell lymphoma.Eur J Nucl Med Mol Imaging. 2018;45(5):680–88. 10.1007/s00259-017-3907-x [DOI] [PubMed] [Google Scholar]
- 47.Delfau-Larue MH, van der Gucht A, Dupuis J, Jais JP, Nel I, Beldi-Ferchiou A, et al. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: distinct prognostic value in follicular lymphoma. Blood Adv. 2018;2(7):807–16. 10.1182/bloodadvances.2017015164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meignan M, Sasanelli M, Casasnovas RO, Luminari S, Fioroni F, Coriani C, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging. 2014;41(6):1113–22. 10.1007/s00259-014-2705-y [DOI] [PubMed] [Google Scholar]
- 49.Ilyas H, Mikhaeel NG, Dunn JT, Rahman F, Møller H, Smith D, et al. Defining the optimal method for measuring baseline metabolic tumour volume in diffuse large B cell lymphoma. Eur J Nucl Med Mol Imaging. 2018;45(7):1142–54. 10.1007/s00259-018-3953-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soret M, Bacharach SL, Buvat I: Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48(6):932–45. 10.2967/jnumed.106.035774 [DOI] [PubMed] [Google Scholar]
- 51.Schöder H, Moskowitz C. Metabolic Tumor Volume in Lymphoma: Hype or Hope? J Clin Oncol. 2016;34(30):3591–94. 10.1200/JCO.2016.69.3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu Y, Decazes P, Lapuyade-Lahorgue J, Gardin I, Vera P, Ruan S. Semi-automatic lymphoma detection and segmentation using fully conditional random fields. Comput Med Imaging Graph. 2018;70:1–7. 10.1016/j.compmedimag.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 53.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9. 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 54.Unal I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput Math Methods Med. 2017;2017:3762651 10.1155/2017/3762651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.