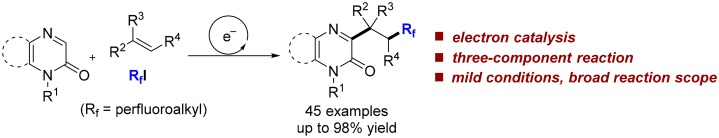
Abstract
A visible-light-initiated α-perfluoroalkyl-β-heteroarylation of various alkenes with perfluoroalkyl iodides and quinoxalin-2(1H)-ones is presented. This three-component radical cascade reaction allows an efficient synthesis of a range of perfluoroalkyl containing quinoxalin-2(1H)-one derivatives in moderate to excellent yields under mild conditions. Reactions proceed via acidic aminyl radicals that are readily deprotonated to give the corresponding radical anions able to sustain the radical chain as single electron transfer reducing reagents. Hence, the overall cascade classifies as an electron-catalyzed process.
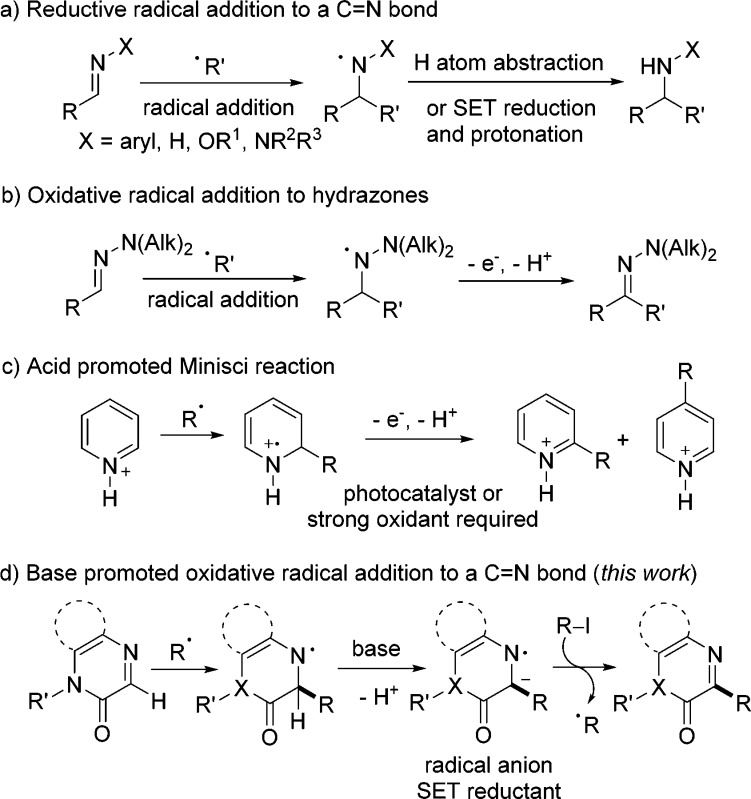
Radical addition reactions are important processes for C–C bond construction.1 Compared to the extensively explored intermolecular radical additions to C=C bonds, intermolecular radical addition to C=N bonds has received less attention.2,3 Along these lines, reductive radical addition to imines, oxime ethers, and hydrazones has been well explored (Scheme 1a).3 N-Radicals generated upon radical addition to such C=N bonds can either be reduced by a H atom transfer reagent or by a single-electron-transfer (SET) process followed by protonation to give amines, alkoxyamines, or hydrazines. Recently, radical α-C(sp2)–H functionalization in N,N-dialkylhydrazones has gained attention (Scheme 1b).4,5 In such cases, the adduct aminyl radical gets SET-oxidized, and deprotonation finally restores the C=N bond resulting in an overall oxidative C–H functionalization. The Minisci reaction, where the C=N double bond is part of a heteroarene, is a well-established and synthetically useful6 radical process comprising an addition step onto a C=N bond (Scheme 1c).7 Hence, alkyl radical addition to a protonated heteroarene, followed by SET-oxidation and deprotonation, provide an alkylated heteroarene generally with good ortho-selectivity. However, these reactions are usually conducted under strongly oxidizing conditions, and regioselectivity is often not complete. Considering these shortages, development of methods for mild oxidative and regioselective intermolecular radical addition to C=N bonds in particular in heteroarenes is still in demand and of importance.
Scheme 1. Different Strategies for Radical Addition to a C=N Bond.
The concept of “electron catalysis”8 has been recently implemented in various radical cascades including base-promoted homolytic aromatic substitutions (BHAS),9 radical substitution reactions (SRN1),10 radical Heck-type reactions,11 direct arene trifluoromethylations,12 and C-radical borylations,13 among other reactions,14 convincingly documenting the generality of that valuable concept.
Radical anions (such as aryl radical anions and ketyl radical anions), able to sustain radical chains as SET-reductants, serve as intermediates in these processes. Along these lines, we assumed that the α-deprotonation of an aminyl radical, readily generated by addition of a C-radical to a C=N double bond, is a reasonable strategy for the generation of the corresponding radical anion. However, the problem in this approach lies in the low α-acidity of an aminyl radical. Facing that issue, we expected that a carbonyl group next to the C=N bond will increase the acidity of the α-C–H bond in the aminyl radical. Moreover, installing a carbonyl moiety will also influence the electrophilicity of the C=N radical acceptor. With this strategy in mind, we selected quinoxalin-2(1H)-ones15 as radical acceptors. Due to polar effects, electrophilic radicals should not react with quinoxalin-2(1H)-ones. This should allow running cascades, where the electrophilic radical is first captured by a nucleophilic C=C radical acceptor leading to a nucleophilic radical which is then trapped by the quinoxalin-2(1H)-one. In contrast to the Minisci reaction, neither protonation nor a stoichiometric external oxidant would be required. Herein, we disclose a simple and efficient α-perfluoroalkyl-β-heteroarylation of various mostly electron-rich alkenes with perfluoroalkyl iodides and quinoxalin-2(1H)-ones. This transformation should be of importance in particular to the field of medicinal chemistry since a structurally privileged heteroarene16 and the biologically important perfluoroalkyl moiety17 are added sequentially to an alkene.
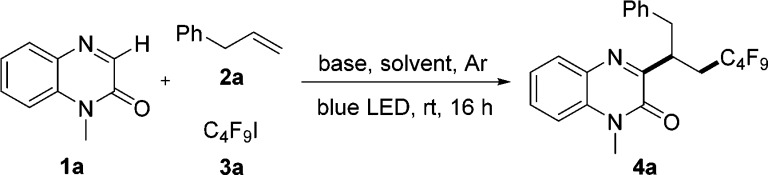
Inspired by our recent radical alkene 1,2-difunctionalizations,18,13b,14a we decided to utilize perfluoroalkyl iodides as C-radical precursors since they are readily SET-reduced. Initial screening was conducted with 1-methylquinoxalin-2(1H)-one (1a) as the electrophilic C-radical acceptor, allylbenzene (2a) as the nucleophilic acceptor, and perfluorobutyl iodide (3a) as the C-radical precursor in the presence of K3PO4 (2.0 equiv) as the base in dichloroethane (DCE) at room temperature. Initiation of the chain was conducted upon irradiation of the reaction mixture with a 10 W blue LED. Pleasingly, the targeted 3-alkylquinoxalin-2(1H)-one 4a was obtained in 10% yield (Table 1, entry 1). The yield was improved to 35% upon switching to DMF as the solvent (Table 1, entry 2). Other solvents such as CH3CN, 1,4-dixoane, and DMA provided worse results (Table 1, entries 3–5). When a weaker base such as K2HPO4 was used, 4a was not formed, indicating the basicity to be important. We therefore screened other bases and found DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) to be best suited, providing 4a in 49% yield (Table 1, entry 10). A higher yield (74%) was achieved upon increasing the concentration to 0.4 M (Table 1, entry 11), and 77% was obtained upon switching to NMP (N-methyl pyrrolidone) as the solvent (Table 1, entry 12). Varying the amount of DBU and allylbenzene revealed that the highest yield (88%) can be obtained when 3.0 equiv of DBU in combination with 2.5 equiv of 2a were used (Table 1, entry 14). Notably, the reaction did not work in the absence of base, and the yield was decreased to 24% without visible-light irradiation.
Table 1. Reaction Optimizationa,b.
| entry | base (equiv) | solvent | modifications | yield (%)b |
|---|---|---|---|---|
| 1 | K3PO4 (2.0) | DCE | 10 | |
| 2 | K3PO4 (2.0) | DMF | 35 | |
| 3 | K3PO4 (2.0) | CH3CN | 12 | |
| 4 | K3PO4 (2.0) | Dioxane | n.d. | |
| 5 | K3PO4 (2.0) | DMA | 19 | |
| 6 | K2HPO4 (2.0) | DMF | n.d. | |
| 7 | Cs2CO3 (2.0) | DMF | 44 | |
| 8 | Na3PO4 (2.0) | DMF | 6 | |
| 9 | Li3PO4 (2.0) | DMF | trace | |
| 10 | DBU (2.0) | DMF | 49 | |
| 11 | DBU (2.0) | DMF | 0.4 M concentration | 74 |
| 12 | DBU (2.0) | NMP | 0.4 M concentration | 77 |
| 13 | DBU (3.0) | NMP | 0.4 M concentration | 83 |
| 14 | DBU (3.0) | NMP | 0.4 M, 2a (2.5 equiv) | 88 (84)c |
| 15 | NMP | without base | n.d. | |
| 16 | DBU (3.0) | NMP | without light | 24 |
Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol, 2.0 equiv), 3a (0.4 mmol, 2.0 equiv), base, solvent (2.0 mL), 5 W blue LED, rt, Ar, 16 h.
Isolated yield based on 1a.
Reaction conducted at 1 mmol scale.
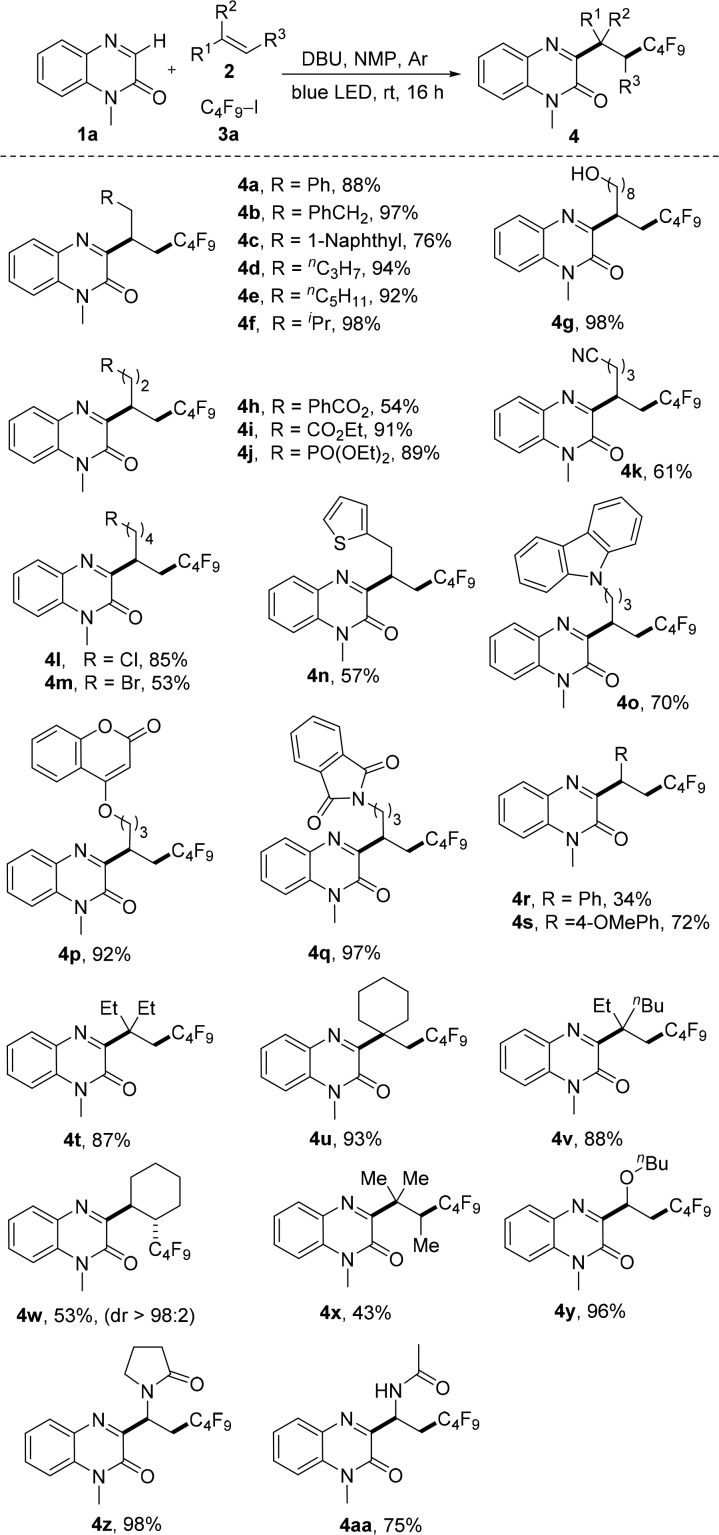
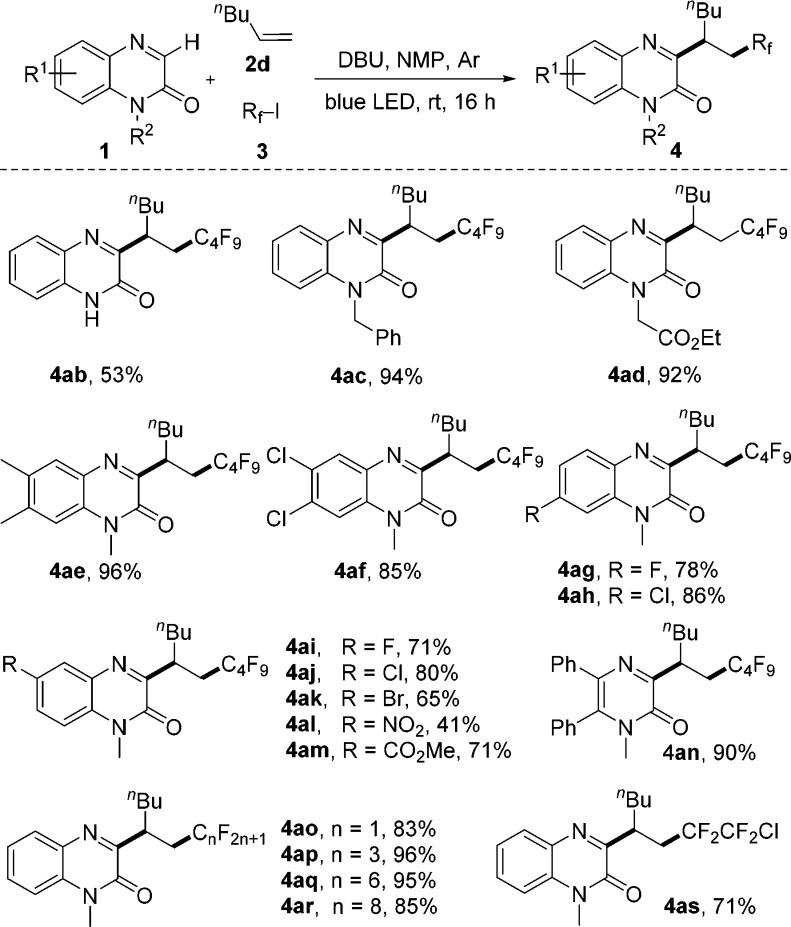
To document scope, various alkenes were investigated, keeping 3a as the radical precursor (Scheme 2). Reactions with nonfunctionalized alkenes proceeded smoothly to give 4a–f in high yields. Various functional groups including alcohol- (4g), ester- (4h, 4i), phosphonate- (4j), nitrile- (4k), and halo-substituents (4l, 4m) are tolerated (53–98%). Reactions with alkenes bearing heterocycles such as thiophene (4n), carbazole (4o), coumarin (4p), and isoindoline-1,3-dione (4q) proceeded smoothly. Styrene is an eligible acceptor (4r), and 1-methoxy-4-vinylbenzene also engages in this reaction (4s). Reactions with 2,2-disubstituted alkenes yielded the desired products 4t–4v in high yields (87–93%), showing that the process allows for construction of all carbon quaternary C-centers. The cascade also works with cyclohexene and 2-methylbut-2-ene (see 4w and 4x). Notably, reaction with cyclohexene occurred with complete trans-selectivity. The trans-configuration of 4w was assigned by NMR spectroscopy (see SI). An electron-rich enol ether (4y) and enamides (4z, 4aa) performed well, and the products were isolated in 75–98% yields.
Scheme 2. Difunctionalization of Various Alkenes,
Reaction conditions: 1a (0.2 mmol), 2 (0.5 mmol, 2.5 equiv), 3a (0.4 mmol, 2.0 equiv), DBU (0.6 mmol), NMP (0.5 mL), 5 W blue LED, rt, Ar, 16 h.
Isolated yield based on 1a.
Next, the heteroarene and the C-radical precursor were varied in combination with 1-hexene as the radical acceptor (Scheme 3). A range of quinoxalin-2(1H)-ones and a pyrazin-2(1H)-one were subjected to this three-component reaction.
Scheme 3. Varying the Radical Acceptor and the Perfluoroalkyl Iodides,
Reaction conditions: 1 (0.2 mmol), 2d (0.5 mmol, 2.5 equiv), 3 (0.4 mmol, 2.0 equiv), DBU (0.6 mmol), NMP (0.5 mL), 10 W blue LED, rt, Ar, 16 h.
Isolated yield based on 1.
Substrates lacking the N-methyl group or with N-protecting groups such as the benzyl and the ethoxycarbonylmethyl group in place of the methyl moiety engaged in the cascade and 4ab–4ad were obtained in good yields. Quinoxalin-2(1H)-ones with electron-withdrawing or electron-donating substituents performed well, providing 4ae–4am in 41–96% yields. Various functional groups including fluoro-, chloro-, bromo-, nitro-, and ester-moieties were compatible (4ai–4am). A 5,6-disubstituted pyrazin-2(1H)-one was also an acceptor to afford 4an in 90% yield, indicating that a fused benzene ring at the heteroarene is not essential. Other perfluoroalkyl iodides gave the desired products 4ao–4ar in high yields.
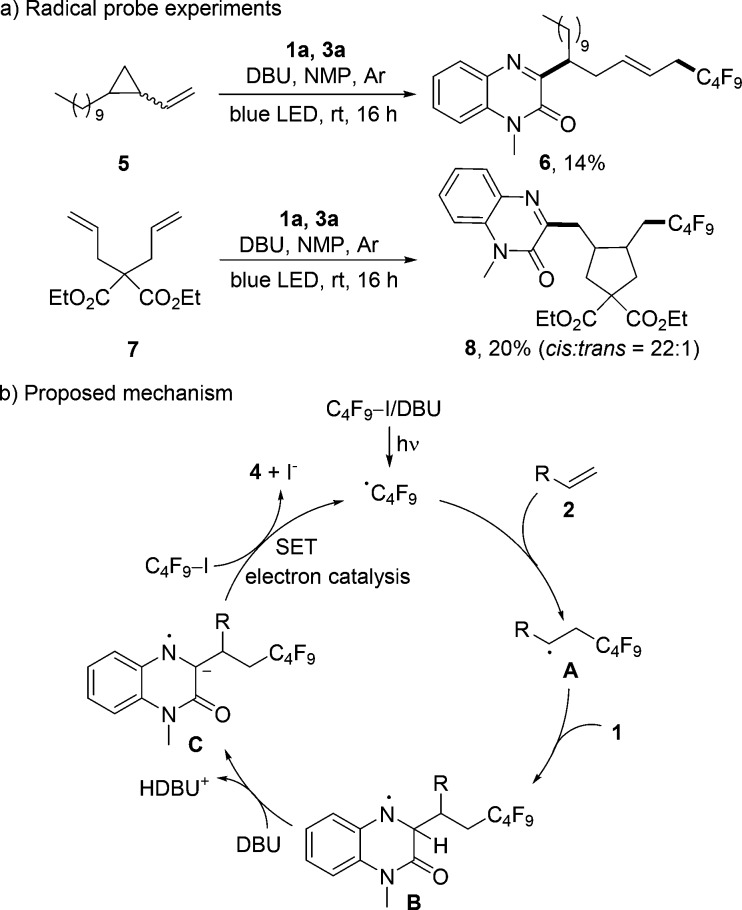
Regarding mechanism, radical probe experiments were carried out (Scheme 4a). Reaction of 1-decyl-2-vinylcyclopropane (5) with 1a and 3a provided exclusively the ring-opened product 6, and the reaction of diethyl 2,2-diallylmalonate 7 with 1a and 3a gave the cyclized product 8. The cis-selectivity was assigned based on the Beckwith–Houk model for the radical 5-exo-cyclization.19 We assume that initiation proceeds by a light-mediated C–I bond homolysis of a Rf–I/DBU complex20 that absorbs in the visible region (see SI) to generate the corresponding Rf-radical. This electrophilic radical then chemoselectively adds to 2 to afford A. The addition of the nucleophilic radical A then occurs selectively at the electron-deficient C=N bond of 1 to deliver B. The acidic aminyl radical B will be deprotonated by DBU to give the radical anion C, which is a strong reducing reagent able to reduce Rf–I via single-electron-transfer to eventually give 4 along with the Rf-radical that sustains the chain.8
Scheme 4. Radical Clock Experiments and Proposed Mechanism.
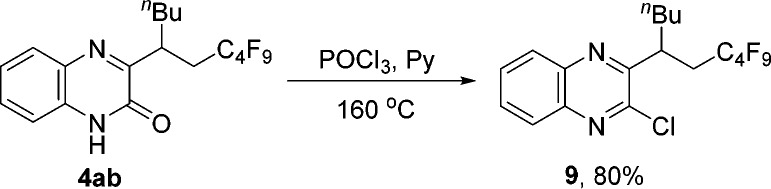
Finally, to show the value of the method, quinoxalin-2(1H)-one 4ab was reacted with POCl3 to give the synthetically valuable chlorinated quinoxaline 9 in 80% yield (Scheme 5). The C–Cl bond in compounds of type 9 is known to engage in different coupling reactions to access diverse multifunctionalized quinoxaline derivatives.15a
Scheme 5. Follow-up Chemistry.
In summary, we have developed an α-perfluoroalkyl-β-heteroarylation of alkenes. The cascade comprises two highly chemoselective radical addition steps that are controlled by polar effects. The acidic aminyl radical intermediate is readily deprotonated by DBU to generate a radical anion acting as a SET-reducing reagent. The reaction belongs to an electron-catalyzed process. Notably, electron-catalyzed three component cascades are not well explored to date,13a and transition-metal-free radical alkene alkylation with concomitant β-heteroarylation is not well established.21 Our method provides an efficient approach to an interesting compound class containing a structurally privileged heteroarene moiety, and cascades proceed with good to excellent yields.
Acknowledgments
This work was supported by the Alexander von Humboldt Foundation (postdoctoral fellowship to D.Z.) and the European Research Council (ERC Advanced Grant agreement No. 692640).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.8b03849.
Experimental procedures and product characterization (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For reviews and book chapters:; a Curran D. P.; Porter N. A.; Giese B.. Stereochemistry of Radical Reactions: Concepts, Guidelines and Synthetic Applications; Wiley-VCH Verlag: Weinheim, Germany, 1996. [Google Scholar]; b Radicals in Organic Synthesis; Sibi M. P.; Renaud P., Eds.; Wiley-VCH: New York, 2001. [Google Scholar]; c Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu C., Studer A., Eds.; John Wiley and Sons: Chichester, U.K., 2012. [Google Scholar]; d Bar G.; Parsons A. F. Chem. Soc. Rev. 2003, 32, 251. 10.1039/b111414j. [DOI] [PubMed] [Google Scholar]
- For reviews of radical additions to C = N bonds:; a Miyabe H. Synlett 2012, 23, 1709. 10.1055/s-0031-1290378. [DOI] [Google Scholar]; b Friestad G. K. Tetrahedron 2001, 57, 5461. 10.1016/S0040-4020(01)00384-2. [DOI] [Google Scholar]; c Miyabe H.; Ueda M.; Naito T. Synlett 2004, 1140. 10.1055/s-2004-822889. [DOI] [Google Scholar]; d Friestad G. K. Eur. J. Org. Chem. 2005, 2005, 3157. 10.1002/ejoc.200500232. [DOI] [Google Scholar]; e Friestad G. K.; Mathies A. K. Tetrahedron 2007, 63, 2541. 10.1016/j.tet.2006.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Yamada K.; Tomioka K. Chem. Rev. 2008, 108, 2874. 10.1021/cr078370u. [DOI] [PubMed] [Google Scholar]
- Selected examples:; a Friestad G. K.; Shen Y.; Ruggles E. Angew. Chem., Int. Ed. 2003, 42, 5061.(Angew. Chem. 2003, 115, 5215) 10.1002/anie.200352104. [DOI] [PubMed] [Google Scholar]; b Rono L. J.; Yayla H. G.; Wang D. Y.; Armstrong M. F.; Knowles R. R. J. Am. Chem. Soc. 2013, 135, 17735. 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]; c Vo C.-V. T.; Luescher M. U.; Bode J. W. Nat. Chem. 2014, 6, 310. 10.1038/nchem.1878. [DOI] [PubMed] [Google Scholar]; d Slater K.; Friestad G. K. J. Org. Chem. 2015, 80, 6432. 10.1021/acs.joc.5b00863. [DOI] [PubMed] [Google Scholar]
- For rewiews of C(sp2)–H functionalization of hydrazones:; a Xu P.; Li W.; Xie J.; Zhu C. Acc. Chem. Res. 2018, 51, 484. 10.1021/acs.accounts.7b00565. [DOI] [PubMed] [Google Scholar]; b Xu X.; Zhang J.; Xia H.; Wu J. Org. Biomol. Chem. 2018, 16, 1227. 10.1039/C8OB00056E. [DOI] [PubMed] [Google Scholar]; c Guo R.; Chen J. RSC Adv. 2018, 8, 17110. 10.1039/C8RA02533A. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Prieto A.; Bouyssi D.; Monteiro N. Eur. J. Org. Chem. 2018, 2018, 2378. 10.1002/ejoc.201701600. [DOI] [Google Scholar]
- For selected examples of C(sp2)–H functionalization of hydrazones:; a Xu P.; Wang G.; Zhu Y.; Li W.; Cheng Y.; Li S.; Zhu C. Angew. Chem., Int. Ed. 2016, 55, 2939.(Angew. Chem.2016, 128, 2992) 10.1002/anie.201508698. [DOI] [PubMed] [Google Scholar]; b Xie J.; Zhang T.; Chen F.; Mehrkens N.; Rominger F.; Rudolph M.; Hashmi A. S. K. Angew. Chem., Int. Ed. 2016, 55, 2934.(Angew. Chem.2016, 128, 2987) 10.1002/anie.201508622. [DOI] [PubMed] [Google Scholar]; c Janhsen B.; Studer A. J. Org. Chem. 2017, 82, 11703. 10.1021/acs.joc.7b00934. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhang M.; Duan Y.; Li W.; Xu P.; Cheng J.; Yu S.; Zhu C. Org. Lett. 2016, 18, 5356. 10.1021/acs.orglett.6b02711. [DOI] [PubMed] [Google Scholar]
- Duncton M. A. J. MedChemComm 2011, 2, 1135. 10.1039/c1md00134e. [DOI] [Google Scholar]
- a Minisci F.; Bernardi R.; Bertini F.; Galli R.; Perchinunno M. Tetrahedron 1971, 27, 3575. 10.1016/S0040-4020(01)97768-3. [DOI] [Google Scholar]; b Minisci F.; Fontana F.; Vismara E. J. Heterocycl. Chem. 1990, 27, 79. 10.1002/jhet.5570270107. [DOI] [Google Scholar]; c Seiple B.; Su S.; Rodriguez R. A.; Gianatassio R.; Fujiwara Y.; Sobel A. L.; Baran P. S. J. Am. Chem. Soc. 2010, 132, 13194. 10.1021/ja1066459. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Molander G. A.; Colombel V.; Braz V. A. Org. Lett. 2011, 13, 1852. 10.1021/ol2003572. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Cheng W.-M.; Shang R.; Fu Y. ACS Catal. 2017, 7, 907. 10.1021/acscatal.6b03215. [DOI] [Google Scholar]; f Cheng W.-M.; Shang R.; Fu M.-C.; Fu Y. Chem. - Eur. J. 2017, 23, 2537. 10.1002/chem.201605640. [DOI] [PubMed] [Google Scholar]
- a Studer A.; Curran D. P. Nat. Chem. 2014, 6, 765. 10.1038/nchem.2031. [DOI] [PubMed] [Google Scholar]; b Studer A.; Curran D. P. Angew. Chem., Int. Ed. 2016, 55, 58.(Angew. Chem.2016, 128, 58) 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- a Studer A.; Curran D. P. Angew. Chem., Int. Ed. 2011, 50, 5018. 10.1002/anie.201101597. [DOI] [PubMed] [Google Scholar]; b Yanagisawa S.; Ueda K.; Taniguchi T.; Itami K. Org. Lett. 2008, 10, 4673. 10.1021/ol8019764. [DOI] [PubMed] [Google Scholar]; c Liu W.; Cao H.; Zhang H.; Zhang H.; Chung K. H.; He C.; Wang H.; Kwong F. Y.; Lei A. J. Am. Chem. Soc. 2010, 132, 16737. 10.1021/ja103050x. [DOI] [PubMed] [Google Scholar]; d Sun C.-L.; Li H.; Yu D.-G.; Yu M.; Zhou X.; Lu X.-Y.; Huang K.; Zheng S.-F.; Li B.-J.; Shi Z.-J. Nat. Chem. 2010, 2, 1044. 10.1038/nchem.862. [DOI] [PubMed] [Google Scholar]
- For reviews:; a Rossi R. A.; Pierini A. B.; Peñéñory A. B. Chem. Rev. 2003, 103, 71. 10.1021/cr960134o. [DOI] [PubMed] [Google Scholar]; b Bunnett J. F. Acc. Chem. Res. 1978, 11, 413. 10.1021/ar50131a003. [DOI] [Google Scholar]; Selected recent examples:; c Kiriyama K.; Okura K.; Tamakuni F.; Shirakawa E. Chem. - Eur. J. 2018, 24, 4519. 10.1002/chem.201800011. [DOI] [PubMed] [Google Scholar]
- a Shirakawa E.; Zhang X.; Hayashi T. Angew. Chem., Int. Ed. 2011, 50, 4671. 10.1002/anie.201008220. [DOI] [PubMed] [Google Scholar]; b Sun C.-L.; Gu Y.-F.; Wang B.; Shi Z.-J. Chem. - Eur. J. 2011, 17, 10844. 10.1002/chem.201101562. [DOI] [PubMed] [Google Scholar]; c Rueping M.; Leiendecker M.; Das A.; Poisson T.; Bui L. Chem. Commun. 2011, 47, 10629. 10.1039/c1cc14297f. [DOI] [PubMed] [Google Scholar]
- a Zhang B.; Mück-Lichtenfeld C.; Daniliuc C. G.; Studer A. Angew. Chem., Int. Ed. 2013, 52, 10792.(Angew. Chem.2013, 125, 10992) 10.1002/anie.201306082. [DOI] [PubMed] [Google Scholar]; b Wang Q.; Dong X.; Xiao T.; Zhou L. Org. Lett. 2013, 15, 4846. 10.1021/ol4022589. [DOI] [PubMed] [Google Scholar]
- a Cheng Y.; Mück-Lichtenfeld C.; Studer A. J. Am. Chem. Soc. 2018, 140, 6221. 10.1021/jacs.8b03333. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wu J.; He L.; Noble A.; Aggarwal V. K. J. Am. Chem. Soc. 2018, 140, 10700. 10.1021/jacs.8b07103. [DOI] [PubMed] [Google Scholar]
- Selected examples:; a Dewanji A.; Mück-Lichtenfeld C.; Studer A. Angew. Chem., Int. Ed. 2016, 55, 6749.(Angew. Chem.2016, 128, 6861) 10.1002/anie.201601930. [DOI] [PubMed] [Google Scholar]; b Kischkewitz; Okamoto M. K.; Mück-Lichtenfeld C.; Studer A. Science 2017, 355, 936. 10.1126/science.aal3803. [DOI] [PubMed] [Google Scholar]
- For selected examples of C–H functionalization of quinoxalin-2(1H)-ones:; a Liu S.; Huang Y.; Qing F.-L.; Xu X.-H. Org. Lett. 2018, 20, 5497. 10.1021/acs.orglett.8b02451. [DOI] [PubMed] [Google Scholar]; b Gao M.; Li Y.; Xie L.; Chauvin R.; Cui X. Chem. Commun. 2016, 52, 2846. 10.1039/C5CC08049E. [DOI] [PubMed] [Google Scholar]; c Yin K.; Zhang R. Org. Lett. 2017, 19, 1530. 10.1021/acs.orglett.7b00310. [DOI] [PubMed] [Google Scholar]; d Yang L.; Gao P.; Duan X.-H.; Gu Y.-R.; Guo L.-N. Org. Lett. 2018, 20, 1034. 10.1021/acs.orglett.7b03984. [DOI] [PubMed] [Google Scholar]; e Yuan J.; Liu S.; Qu L. Adv. Synth. Catal. 2017, 359, 4197. 10.1002/adsc.201701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinoxalin-2(1H)-ones widely exist in bioactive compounds:; a TenBrink R. E.; Im W. B.; Sethy V. H.; Tang A. H.; Carter D. B. J. Med. Chem. 1994, 37, 758. 10.1021/jm00032a008. [DOI] [PubMed] [Google Scholar]; b Monge A.; Martinez-Crespo F. J.; Cerai A. L.; Palop J. A.; Narro S.; Senador V.; Marin A.; Sainz Y.; Gonzalez M.; Hamilton E.; Barker A. J. J. Med. Chem. 1995, 38, 4488. 10.1021/jm00022a014. [DOI] [PubMed] [Google Scholar]; c Shi L.-L.; Zhou H.; Wu J.-F.; Li X. Mini-Rev. Org. Chem. 2014, 12, 96. 10.2174/1570193X11666141029004418. [DOI] [Google Scholar]; d Wagle S.; Adhikari A. V.; Kumari N. S. Indian J. Chem. 2008, 47, 439. [Google Scholar]
- a Hird M. Chem. Soc. Rev. 2007, 36, 2070. 10.1039/b610738a. [DOI] [PubMed] [Google Scholar]; b Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Chem. Soc. Rev. 2008, 37, 320. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; c Wang X.; Ye Y.; Zhang S.; Feng J.; Xu Y.; Zhang Y.; Wang J. J. Am. Chem. Soc. 2011, 133, 16410. 10.1021/ja207775a. [DOI] [PubMed] [Google Scholar]; d Müller K.; Faeh C.; Diederich F. Science 2007, 317, 1881. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- a Hartmann M.; Li Y.; Studer A. J. Am. Chem. Soc. 2012, 134, 16516. 10.1021/ja307638u. [DOI] [PubMed] [Google Scholar]; b Li Y.; Studer A. Angew. Chem., Int. Ed. 2012, 51, 8221.(Angew. Chem.2012, 124, 8345) 10.1002/anie.201202623. [DOI] [PubMed] [Google Scholar]; c Li Y.; Hartmann M.; Daniliuc C. G.; Studer A. Chem. Commun. 2015, 51, 5706. 10.1039/C5CC00591D. [DOI] [PubMed] [Google Scholar]; d Hartmann M.; Li Y.; Mück-Lichtenfeld C.; Studer A. Chem. - Eur. J. 2016, 22, 3485. 10.1002/chem.201504852. [DOI] [PubMed] [Google Scholar]; e Hartmann M.; Li Y.; Studer A. Org. Biomol. Chem. 2016, 14, 206. 10.1039/C5OB02210J. [DOI] [PubMed] [Google Scholar]; f Tang X.; Studer A. Chem. Sci. 2017, 8, 6888. 10.1039/C7SC02175E. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Tang X.; Studer A. Angew. Chem., Int. Ed. 2018, 57, 814.(Angew. Chem.2018, 130, 822) 10.1002/anie.201710397. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Gerleve C.; Kischkewitz M.; Studer A. Angew. Chem., Int. Ed. 2018, 57, 2441.(Angew. Chem.2018, 130, 2466) 10.1002/anie.201711390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Beckwith A. L. J.; Schiesser C. H. Tetrahedron 1985, 4, 3925. 10.1016/S0040-4020(01)97174-1. [DOI] [Google Scholar]; b Spellmeyer D. C.; Houk K. N. J. Org. Chem. 1987, 52, 959. 10.1021/jo00382a001. [DOI] [PubMed] [Google Scholar]
- Postigo A. Eur. J. Org. Chem. 2018, 2018, 6391. 10.1002/ejoc.201801079. [DOI] [Google Scholar]
- β-Heteroarylation of alkenes via cascade Minisci reaction:; a McCallum T.; Barriault L. Chem. Sci. 2016, 7, 4754. 10.1039/C6SC00807K. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liu Z.; Liu Z.-Q. Org. Lett. 2017, 19, 5649.β-(Hetero)arylation of alkenes via intramolecular (hetero)aryl migration: 10.1021/acs.orglett.7b02788. [DOI] [PubMed] [Google Scholar]; c Wu Z.; Wang D.; Liu Y.; Huan L.; Zhu C. J. Am. Chem. Soc. 2017, 139, 1388. 10.1021/jacs.6b11234. [DOI] [PubMed] [Google Scholar]; d Li L.; Gu Q.-S.; Wang N.; Song P.; Li Z.-L.; Li X.-H.; Wang F.-L.; Liu X.-Y. Chem. Commun. 2017, 53, 4038. 10.1039/C6CC09215B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.