Abstract
Introduction
Rapid adoption of robotics has introduced a paradigm change in prostate cancer treatment, with more than 80% of prostatectomies performed robotically in 2015. For treatment of renal cell carcinoma (RCC), this change has not previously been reported. We evaluated trends in surgical management of RCC in Kaiser Permanente Southern California (KPSC) within the last 16 years, especially after adoption of robotics.
Methods
From January 1999 to September 2015, all KPSC members who underwent surgical treatment of suspected RCC were included retrospectively. Surgical approach, patient age, sex, clinicopathology, Charlson Comorbidity Index, and chronic kidney disease status were analyzed using robust Poisson multivariate regression.
Results
The study included 5237 patients. Partial nephrectomy was increasingly used during the study period, and its use surpassed radical nephrectomy in 2012. In a multivariate model, partial nephrectomy was associated with lower pathologic tumor stage (p < 0.001) and lower Charlson Comorbidity Index (p = 0.004) vs radical nephrectomy. Robot-assisted laparoscopic partial nephrectomy (RALPN) started in KPSC in March 2011, and its relative use among all RCC surgeries increased in the following 3 years by 125%, 45%, and 14%. Laparoscopic partial nephrectomy and laparoscopic radical nephrectomy were the most frequently used surgical approaches for localized RCC when RALPN started in 2011. However, RALPN surpassed laparoscopic partial nephrectomy and laparoscopic radical nephrectomy in 2012 and 2014, respectively.
Conclusion
During our study, partial nephrectomy became the most common surgery for treatment of localized RCC. Since 2014, RALPN has become the most common renal oncologic surgical modality in KPSC.
Keywords: cancer, implementation, kidney, kidney neoplasms laparoscopy, malignancy, minimally invasive, partial nephrectomy, practice patterns, RALPN, renal cell carcinoma, robot-assisted laparoscopic partial nephrectomy, robotic surgical procedures
INTRODUCTION
Renal cell carcinoma (RCC) is among the 10 most common cancers in both men and women (> 60,000 new cases per year, lifetime risk approximately 1.6%), with RCC comprising approximately 9 of 10 kidney cancers.1 Although radical nephrectomy was the standard of care in the past, the increased use of partial nephrectomy (PN) has correlated with the increased incidence of T1 renal masses owing to earlier detection.2,3 Current management options for patients with renal masses include surgery (radical nephrectomy or PN), ablative therapy (cryoablation and radiofrequency ablation), and active surveillance. The American Urological Association Practice Guidelines released in November 2009 recommended consideration of PN as a standard of treatment of small renal masses.4 The 2017 guidelines on localized RCC suggested that urologists should prioritize PN as the preferred surgical treatment of clinical T1a renal masses (< 4 cm) to minimize the risk of chronic kidney disease.5 Rates of active surveillance, as well as minimally invasive ablative techniques, have also increased, particularly in the elderly population.6 Extensive studies highlighting the use of each surgical modality in the 21st century have shown PN to have equivalent oncologic outcomes to radical nephrectomy, even for patients with larger T2 tumors, with the advantage of preserving nephrons and maximizing postoperative renal function.7,8 Furthermore, PN is being performed in patients with complex tumors, particularly at academic and high-volume centers.9
The emergence of robotics in urologic surgery has resulted in a paradigm shift in prostate cancer management, with more than 80% of all prostatectomies for prostate cancer being performed robotically in recent years.10 To our knowledge, this trend has not been described for RCC. Robot-assisted laparoscopic partial nephrectomy (RALPN) has been shown to have oncologic and functional outcomes similar to open partial nephrectomy (OPN), with improved perioperative outcomes and significantly fewer perioperative complications compared with a laparoscopic approach, even in those with complex renal tumors.11,12 We hereby include 16 years of trends in surgical management of RCC, with particular attention to the changes after introduction of robotics in our integrated health care system, Kaiser Permanente Southern California (KPSC).
METHODS
Consisting of 14 Medical Centers and hundreds of adjacent outpatient offices serving more than 4.2 million patients, KPSC has a racial and socioeconomic diversity reflective of the population of Southern California.
We performed an institutional review board-approved multicenter retrospective review of patients who underwent treatment of suspected RCC in our health care system from January 1999 to September 2015. The following treatment modalities were included in the final analysis: Open radical nephrectomy (ORN) and laparoscopic radical nephrectomy (LRN), OPN, laparoscopic partial nephrectomy (LPN), RALPN, and thermal ablation.
Four KPSC Medical Centers have a Da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA), and a core group of 22 robotic surgeons had experience with at least 100 robotic cases before receiving robotic privileges. This group of surgeons also serves as the privileging body for all approved robotic procedures for KPSC. In March 2011 RALPN was approved in KPSC. All patients age 18 years or older who underwent treatment of suspected localized RCC were included in our study. They were identified using the cancer registry as well as our electronic medical records. International Classification of Diseases, Ninth Revision and Current Procedural Terminology codes were used to extract patient clinicopathologic data as well as treatment modality. This included patient age, race, sex, Charlson Comorbidity Index (CCI), preoperative chronic kidney disease Stage 3 or later (defined as estimated glomerular filtration rate < 60 mL/min/1.73 m2, calculated using the modification of diet in renal disease equation13), hypertension, diabetes, tumor size as defined by pathologic T stage, and the year surgery was performed. Patients with locoregionally advanced disease, those who underwent multivisceral resection, as well as those who underwent renal surgery for nononcologic indications, were excluded from the analysis. Table 1 shows all exclusionary criteria.
Table 1.
Exclusionary criteria
| Criterion | No. excluded |
|---|---|
| Ureteral tumor | 150 |
| Nephroureterectomy | 181 |
| Splenectomy | 4 |
| Hepatic resection | 14 |
| Pancreatic resection | 21 |
| Bowel or colon resection | 92 |
| Thrombectomy | 96 |
| Donor nephrectomy | 1 |
| Acute or chronic pyelonephritis | 66 |
| Renal or perinephric abscess | 40 |
| Polycystic kidney disease | 49 |
| Renal laceration or disruption of renal parenchyma | 3 |
We plotted descriptive trends of PN, radical nephrectomy, and ablations as a percentage of total surgeries for the entire study period. Additionally, we plotted trends for RALPN vs other treatment modalities beginning in 2008, when more details about specific treatment modalities beyond simply PN or radical nephrectomy became available in our electronic medical databases. We investigated factors associated with the use of PN vs radical nephrectomy from 2009 (the year of release of the new American Urological Association guidelines on management of small renal masses) to 2015 using χ2 and analysis of variance statistical tests as well as multivariate analysis. A separate subanalysis using similar methods was performed looking at PN performed from 2011 to 2015, after RALPN was approved in our system, to examine factors associated with minimally invasive (RALPN or LPN) vs open approach. Of note, radical nephrectomy is not approved as a routine procedure on the robotic platform. Multivariate analyses used robust Poisson regression, which included quadratic time trends and included all covariates previously mentioned. All p values were based on 2-sided tests of significance, with statistical significance set at p < 0.05.
RESULTS
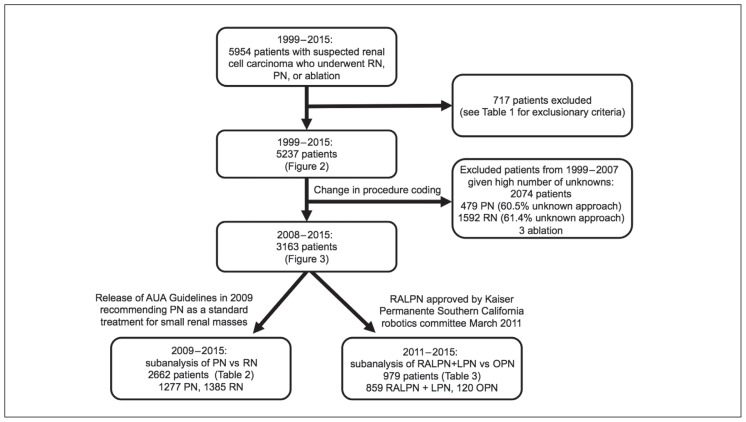
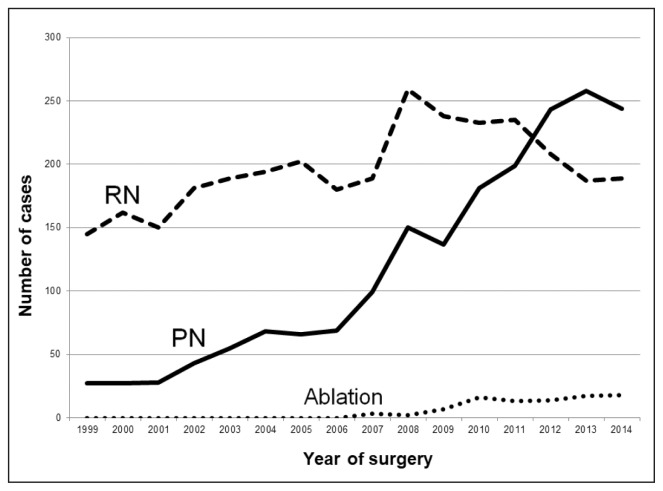
A total of 717 patients were excluded from our study because of potential confounding diagnoses or concomitant surgery (Table 1). The flowsheet in Figure 1 explains how the size of each cohort was determined. The total number of radical nephrectomy, PN, and ablative procedures performed in the remaining 5237 patients are depicted in Figure 2, which highlights the increased use of nephron-sparing surgery from 1999 through 2014. PN was increasingly performed during the study period; the percentage of all RCC patients undergoing PN increased yearly from 2009 to 2013. Given that data were not collected for the full calendar year of 2015, that last year of the study period was omitted from Figure 2. Because of medical and procedural coding changes in 2008, we were unable to accurately assess the surgical approach (laparoscopic vs open) for a large portion of PNs (60.5%, 290 of 479) and radical nephrectomies (61.4%, 979 of 1592) performed from 1999 to 2007. Thus, the graphic presentation of specific surgical approach focuses on 3163 patients treated from 2008 to 2015.
Figure 1.
Patient cohort sizes
AUA = American Urological Association; LPN = laparoscopic partial nephrectomy; OPN = open partial nephrectomy; PN = partial nephrectomy; RALPN = robot-assisted laparoscopic partial nephrectomy; RN = radical nephrectomy.
Figure 2.
Trends in treatment modality (1999–2014)
PN = partial nephrectomy; RN = radical nephrectomy.
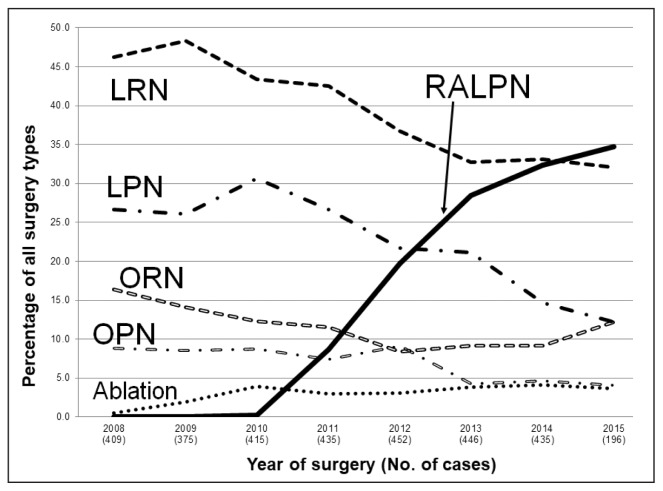
The percentage of each surgical approach as a total of all treatment modalities per year is shown in Figure 3. In March 2011, RALPN was approved by our robotics committee, and in the following 3 years its use increased per year by 125% (2012), 45% (2013), and 14% (2014). When RALPN was approved in 2011, LPN and LRN were the most frequently used surgical approaches for localized RCC. However, RALPN surpassed LPN and LRN in 2012 and 2014, respectively. Nephron-sparing surgery was increasingly performed throughout the study period, with PN accounting for 33.6% of surgeries in 2007 to 2009, compared with 48.6% of surgeries in 2010 to 2013 (p < 0.001).
Figure 3.
Trends in treatment modality before and after introduction of robotic approach (2008–2015)
LPN = laparoscopic partial nephrectomy; LRN = laparoscopic radical nephrectomy; OPN = open partial nephrectomy; ORN = open radical nephrectomy; RALPN = robot-assisted laparoscopic partial nephrectomy.
We performed a subanalysis comparing factors associated with the use of PN and radical nephrectomy from 2009 to 2015. Neither race (p = 0.369) nor sex (p = 0.105) was associated with nephron-sparing surgery in univariate or multivariate analyses. Patients undergoing radical nephrectomy were more likely to have preoperative chronic kidney disease Stage 3 or later (22.5% radical nephrectomy vs 17.6% PN, p = 0.003) in univariate analysis, but significance was removed in the multivariate analysis once other covariates were included in the model. Higher pathologic T stage was associated with the use of radical nephrectomy (p < 0.001) in the univariate and multivariate analyses. Patients with pathologic T3 tumors were 69% less likely to have received PN (relative risk [RR] = 0.31, 95% confidence interval [CI] = 0.25–0.38, p < 0.001] compared with those with T1 (< 7-cm) tumors. Patients with T2 tumors were 86% less likely to have undergone PN (RR = 0.14, 95% CI = 0.10–0.20, p < 0.001) than those with T1 tumors. Recipients of PN had a slightly younger median age at diagnosis compared with radical nephrectomy recipients (60 vs 63 years old, p < 0.001) in univariate analysis, and this remained significant in the multivariate analysis. Hypertension was associated with the use of radical nephrectomy, although this lost significance in the multivariate analysis. In univariate and multivariate models, a higher CCI score (p = 0.004) was associated with the use of radical nephrectomy (Table 2).
Table 2.
Partial nephrectomy (PN) vs radical nephrectomy (RN) (2009–2015)
| Patient data | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| PN (n = 1277), no. (%) | RN (n = 1385), no. (%) | p valuea | RR (95% CI) | p valueb | |
| Median age, years | 60 | 63 | < 0.001 | 0.99 (0.99-0.99) | < 0.001 |
| Hypertension | 808 (63.3) | 951 (68.7) | 0.003 | 1.06 (0.97–1.15) | 0.221 |
| Diabetes | 356 (27.9) | 434 (31.3) | 0.051 | 0.98 (0.89–1.08) | 0.665 |
| Chronic kidney disease (CKD) stage | |||||
| Preoperative CKD 1/2 | 971 (82.4) | 1012 (77.5) | 0.003 | 0.96 (0.85–1.08) | 0.528 |
| Preoperative CKD 3+ | 208 (17.6) | 293 (22.5) | |||
| Cancer stage | |||||
| T1 | 1153 (91.6) | 720 (54.0) | < 0.001 | — | — |
| T2 | 26 (2.1) | 269 (20.2) | 0.14 (0.10–0.20) | < 0.001 | |
| T3 | 80 (6.3) | 344 (25.8) | 0.31 (0.25–0.38) | < 0.001 | |
| Race/ethnicityc | |||||
| White | 630 (49.8) | 697 (50.6) | 0.369 | — | 0.066 |
| Hispanic | 405 (32.0) | 413 (30.0) | 0.96 (0.88–1.04) | ||
| Black | 139 (11.0) | 176 (12.8) | 0.84 (0.74–0.96) | ||
| Asian | 91 (7.2) | 90 (6.5) | 0.98 (0.85–1.14) | ||
| Sex | |||||
| Men | 778 (60.9) | 886 (64.0) | 0.105 | — | — |
| Women | 499 (39.1) | 499 (36.0) | 1.03 (0.96–1.11) | 0.421 | |
| Charlson Comorbidity Index (CCI) | |||||
| CCI 0 | 229 (17.9) | 132 (9.5) | < 0.001 | — | 0.004 |
| CCI 1 | 0.96 (0.82–1.13) | ||||
| CCI 2 | 760 (59.5) | 780 (56.3) | 0.85 (0.75–0.96) | ||
| CCI 3 | 0.89 (0.77–1.01) | ||||
| CCI 4 | 0.87 (0.74–1.02) | ||||
| CCI ≥ 5 | 288 (22.6) | 473 (34.2) | 0.75 (0.64–0.88) | ||
| Year of surgery | |||||
| 2009 | 130 (10.2) | 238 (17.2) | < 0.001 | Adjusted for year of surgery; results not shown | |
| 2010 | 165 (12.9) | 234 (16.9) | |||
| 2011 | 186 (14.6) | 236 (17.0) | |||
| 2012 | 229 (17.9) | 209 (15.1) | |||
| 2013 | 241 (18.9) | 188 (13.6) | |||
| 2014 | 226 (17.7) | 191 (13.8) | |||
χ2 tests for independence were used for categorical variables; Wilcoxon rank sum test were used for continuous variables. Boldface values are statistically significant.
Based on likelihood ratio statistic. Boldface values are statistically significant.
Numbers for race/ethnicity do not sum to 100 because there were insufficient numbers to include those.
CI = confidence interval; RR = relative risk.
In our analysis comparing use of minimally invasive PN (LPN or RALPN) with OPN from 2011 to 2015, after approval of RALPN, we found those undergoing surgery in later years were more likely to receive a minimally invasive approach (p = 0.001). In the univariate analysis, higher T stage (p = 0.04) and CCI score (p = 0.001), as well as hypertension (p = 0.031), were associated with an open approach, but these associations did not remain in the multivariate analysis. There were similar findings when we excluded LPN and compared only RALPN vs OPN during this same period. Certain races were more likely to receive a minimally invasive approach on univariate analysis. This was shown in the multivariate analysis, as Asians (RR = 1.1, 95% CI = 1.05–1.21, p = 0.001) and Hispanics (RR = 1.1, 95% CI = 1.01–1.13, p = 0.028) were more likely to undergo minimally invasive PN than were whites (Table 3).
Table 3.
Minimally invasive partial nephrectomy vs open partial nephrectomy (OPN) (2009–2015)
| Patient data | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| RALPN + LPN (n = 859 +n = 1277), no. (%) | OPN (n = 120), no. (%) | p valuea | RR (95% CI) | p valueb | |
| Median age, years | 60 | 61 | 0.463 | 1.00 (1.00-1.00) | 0.323 |
| Hypertension | 536 (62.4) | 87 (72.5) | 0.031 | 0.97 (0.91–1.03) | 0.363 |
| Diabetes | 245 (28.5) | 38 (31.7) | 0.477 | 1.02 (0.95–1.09) | 0.576 |
| Chronic kidney disease (CKD) stage | |||||
| Preoperative CKD 1/2 | 633 (82.3) | 86 (76.1) | 0.112 | 0.99 (0.91–1.08) | 0.881 |
| Preoperative CKD 3+ | 136 (17.7) | 27 (23.9) | |||
| Cancer stage | |||||
| T1 | 783 (91.8) | 101 (85.6) | 0.040 | — | — |
| T2 | 21 (2.5) | 3 (2.5) | 0.99 (0.83–1.17) | 0.873 | |
| T3 | 49 (5.7) | 14 (11.9) | 0.88 (0.76–1.03) | 0.101 | |
| Race/ethnicityc | |||||
| White | 399 (47.1) | 73 (60.8) | 0.019 | — | 0.010 |
| Hispanic | 288 (34.0) | 34 (28.3) | 1.07 (1.01–1.13) | ||
| Black | 96 (11.3) | 10 (8.3) | 1.08 (1.10–1.60) | ||
| Asian | 65 (7.3) | 3 (2.5) | 1.13 (1.05–1.21) | ||
| Sex | |||||
| Men | 525 (61.1) | 68 (56.7) | 0.350 | — | — |
| Women | 334 (38.9) | 52 (43.3) | 0.96 (0.91–1.01) | 0.117 | |
| Charlson Comorbidity Index (CCI) | |||||
| CCI 0 | 170 (19.8) | 13 (10.8) | 0.043 | — | 0.091 |
| CCI 1 | 1.02 (0.94–1.12) | ||||
| CCI 2 | 503 (58.6) | 69 (57.5) | 0.98 (0.90–1.06) | ||
| CCI 3 | 0.97 (0.89–1.06) | ||||
| CCI 4 | 0.93 (0.83–1.03) | ||||
| CCI ≥ 5 | 186 (21.7) | 38 (31.7) | 0.89 (0.80–0.99) | ||
| Year of surgery | |||||
| 2011 | 154 (17.9) | 32 (26.7) | 0.001 | Adjusted for year of surgery; results not shown | |
| 2012 | 187 (21.8) | 41 (34.2) | |||
| 2013 | 221 (25.7) | 19 (15.8) | |||
| 2014 | 205 (23.9) | 20 (16.7) | |||
χ2 tests for independence were used for categorical variables; Wilcoxon rank sum test, for continuous variables. Boldface values are statistically significant.
Based on likelihood ratio statistic. Boldface values are statistically significant.
Numbers for race/ethnicity do not sum to 100 because there were insufficient numbers to include those.
CI = confidence interval; LPN = laparoscopic partial nephrectomy; RALPN = robot-assisted laparoscopic partial nephrectomy; RR = relative risk.
DISCUSSION
During the past decade, PN has become the standard treatment for most patients with T1 renal masses.4,5 In our study of patients with suspected localized RCC, nephron-sparing surgery has overtaken rates of radical nephrectomy beginning in 2012 as depicted in Figure 2. The adoption of the robotic platform has resulted in a paradigm shift in the surgical management of RCC at KPSC, with RALPN becoming the most common type of oncologic renal surgery performed in 2014. Our study serves to highlight trends in the surgical approach to RCC within an integrated health care system.
A growing body of evidence suggests that the percentage of renal parenchyma preserved during surgery is one of the most important modifiable factors predicting long-term renal functional outcomes after surgical excision of the kidney.14,15 The 2017 European Association of Urology Guidelines on RCC consider PN to be the treatment of choice for T1b (4 cm – 7 cm) RCC given the procedure’s similar oncologic outcomes and improved renal function, metabolic, and cardiovascular outcomes compared with radical nephrectomy.16 Recent studies have highlighted the increased use of PN, even in patients with T2 tumors.8 A recent population-based study of 1836 patients from Australia has shown an increased incidence of PN for treatment of T1 RCC from 2009 to 2013.17 This was consistent with our finding of steadily increasing use of PN in our study of 5237 patients from 1999 to 2014 (Figure 2). We also demonstrated that patients undergoing PN in recent years had a younger median age at diagnosis than did those undergoing radical nephrectomy (Table 2). This difference may be attributable to the fact that younger patients are generally healthier and therefore more capable of tolerating the increased morbidity associated with PN. Additionally, there is likely more of an attempt to preserve nephrons in the younger patient.
Robotic technology and its applications to urologic surgery continue to advance, and its feasibility in advanced tumors, including those necessitating inferior vena cava tumor thrombectomy, is under investigation.18 Although randomized controlled trial data have yet to prove the oncologic efficacy of RALPN, initial oncologic outcomes after RALPN appear to be comparable to OPN, with improved perioperative outcomes. A recent study of 110 patients who underwent RALPN, with a median tumor size of 2.6 cm and follow-up of 62 months, demonstrated 5-year overall survival and recurrence-free survival of 91.% and 97.8%, respectively.19 Perioperative outcomes for our patient population are described in a previous study by Banapour et al,12 who compared 862 patients who underwent RALPN, LPN, or OPN from 2007 to 2014 at KPSC. The authors found that, after matching for tumor complexity, minimally invasive approach was associated with less intraoperative blood loss, shorter length of stay, and less change in estimated glomerular filtration rate compared with the open approach.12 A review of 19 cohort studies comparing RALPN and OPN in a combined 3551 patients identified lower rates of major and minor postoperative complications, lower transfusion rates, and shorter length of hospital stay in patients who underwent RALPN.20 RALPN has become increasingly utilized in our health care system since its approval and is now the most common renal oncologic surgery performed. The application of RALPN is not limited to small tumors. Results of a collaborative study of 298 patients with T2 tumors who underwent RALPN showed a 5% rate of Clavien-Dindo Grade 3 or higher postoperative complications, with acceptable renal functional and oncologic outcomes; these results suggest that RALPN can be used to treat larger tumors with appropriate patient selection.21
In our population of patients who underwent PN from 2011 to 2015, there was no association between T2 or T3 tumors and an open vs minimally invasive approach. A high CCI score was found on multivariate analyses to be significantly associated with the use of radical nephrectomy rather than nephron-sparing surgery, as well as open rather than minimally invasive approach in the PN cohort. We hypothesize that patients with more comorbidities are less capable of tolerating the increased surgical and anesthetic risks associated with nephron-sparing surgery. Interestingly, we also found an association of Asian and Hispanic race/ethnicity with the use of minimally invasive technology. However, it is important to note that this is an observational study and that there may be variables associated with geographic and ethnic variation with respect to access to a Da Vinci System. In our integrated health care system, all patients have equal access to robotic surgery.
Another limitation is that this is a retrospective study populated by data from administrative codes. Some of the surgical modality cohorts may be underpowered to identify their associations with clinicopathologic data. This could be potentially revisited in the future after accumulating more years of data to compare these surgeries. Furthermore, our study is limited by the change in procedural coding practices and the large percentage of radical nephrectomy and PN procedures performed from 1999 to 2007 of unknown approach (open vs laparoscopic). Nonetheless, this study provides insight into the current trends in renal oncologic surgery in a large population-based study within a health care system.
CONCLUSION
From 1999 to 2015, the treatment of localized RCC has changed dramatically; use of PN has increased and surpassed radical nephrectomy in 2012. In our health care system, after adoption of the robotic platform in 2011, RALPN has rapidly become the most common surgical modality (since 2014). This information may aid in the understanding of contemporary treatment options for individual patients with localized RCC. On a population level, it may shed light on the effects of clinical guidelines and introduction of a new technology on the treatment of localized RCC.
Acknowledgment
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.CEUR-WS. CEUR Workshop Proceedings [Internet] Aachen, Germany: RWTH Aachen University; 2018. [cited 2018 Aug 10]. Available from: http://ceur-ws.org/ [Google Scholar]
- 2.Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969 Mar;101(3):297–301. doi: 10.1016/s0022-5347(17)62331-0. [DOI] [PubMed] [Google Scholar]
- 3.Štimac G, Reljić A, Pezelj I, et al. The evolution of partial nephrectomy for kidney tumors—are we abandoning the basic principles of Robson’s radical nephrectomy? Acta Clin Croat. 2014 Dec;53(4):455–61. [PubMed] [Google Scholar]
- 4.Campbell SC, Novick AC, Belldegrun A, et al. Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical stage T1 renal mass. J Urol. 2009 Oct;182(4):1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017 Sep;198(3):520–9. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 6.Tan HJ, Filson CP, Litwin MS. Contemporary, age-based trends in the incidence and management of patients with early-stage kidney cancer. Urol Oncol. 2015 Jan;33(1):21.e19–26. doi: 10.1016/j.urolonc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Pierorazio PM, Johnson MH, Patel HD, et al. Management of renal masses and localized renal cancer: Systematic review and meta-analysis. J Urol. 2016 Oct;196(4):989–99. doi: 10.1016/j.juro.2016.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J. Predicting renal functional outcomes after surgery for renal cortical tumours: A multifactorial analysis. BJU Int. 2010 Aug;106(4):489–92. doi: 10.1111/j.1464-410x.2009.09147.x. [DOI] [PubMed] [Google Scholar]
- 9.Maurice MJ, Zhu H, Kim SP, Abouassaly R. Increased use of partial nephrectomy to treat high-risk disease. BJU Int. 2016 Jun;117(6B):E75–86. doi: 10.1111/bju.13262. [DOI] [PubMed] [Google Scholar]
- 10.Oberlin DT, Flum AS, Lai JD, Meeks JJ. The effect of minimally invasive prostatectomy on practice patterns of American urologists. Urol Oncol. 2016 Jun;34(6):255.e1–5. doi: 10.1016/j.urolonc.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Shao J, Ma X, Du Q, Gong H, Zhang X. Robotic and open partial nephrectomy for complex renal tumors: A matched-pair comparison with a long-term follow-up. World J Urol. 2017 Jan;35(1):73–80. doi: 10.1007/s00345-016-1849-8. [DOI] [PubMed] [Google Scholar]
- 12.Banapour P, Abdelsayed GA, Bider-Canfield Z, Elliott PA, Kilday PS, Chien GW. Nephrometry score matched robotic vs laparoscopic vs open partial nephrectomy. J Robot Surg. 2018 Dec;12(4):679–85. doi: 10.1007/s11701-018-0801-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Levey A, Coresh J, Balk E, et al. National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003 Jul;139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Simmons MN, Fergany AF, Campbell SC. Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J Urol. 2011 Aug;186(2):405–10. doi: 10.1016/j.juro.2011.03.154. [DOI] [PubMed] [Google Scholar]
- 15.Jabaji R, Palazzi KL, Mehrazin R, et al. Determinants of renal functional decline after open partial nephrectomy: A comparison of warm, cold, and non-ischemic modalities. Can J Urol. 2014 Feb;21(1):7126–33. [PubMed] [Google Scholar]
- 16.Ljungberg B, Albiges L, Bensalah K, et al. EAU Guidelines on Renal Cell Carcinoma: 2017 Update [Internet] Arnheim: The Netherlands; 2017. [cited 2018 Oct 15]. Available from: http://uroweb.org/guideline/renal-cell-carcinoma/ [Google Scholar]
- 17.White V, Marco DJT, Bolton D, et al. Trends in the surgical management of stage 1 renal cell carcinoma: Findings from a population-based study. BJU Int. 2017 Nov;120( Suppl 3):6–14. doi: 10.1111/bju.13889. [DOI] [PubMed] [Google Scholar]
- 18.Maurice MJ, Ramirez D, Kaouk JH. Advances in robotic-assisted treatments for renal cell carcinoma. Curr Opin Urol. 2016 Sep;26(5):417–23. doi: 10.1097/mou.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 19.Andrade HS, Zargar H, Caputo PA, et al. Five-year oncologic outcomes after transperitoneal robotic partial nephrectomy for renal cell carcinoma. Eur Urol. 2016 Jun;69(6):1149–54. doi: 10.1016/j.eururo.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Xia L, Wang X, Xu T, Guzzo TJ. Systematic review and meta-analysis of comparative studies reporting perioperative outcomes of robot-assisted partial nephrectomy versus open partial nephrectomy. J Endourol. 2017 Sep;31(9):893–909. doi: 10.1089/end.2016.0351. [DOI] [PubMed] [Google Scholar]
- 21.Bertolo R, Autorino R, Simone G, et al. Outcomes of robot-assisted partial nephrectomy for clinical T2 renal tumors: A multicenter analysis (ROSULA Collaborative Group) Eur Urol. 2018 Aug;74(2):226–32. doi: 10.1016/j.eururo.2018.05.004. [DOI] [PubMed] [Google Scholar]