Abstract
Postpartum hemorrhage (PPH) remains a leading cause of maternal death worldwide, and it is important to understand the relative contributions of different risk factors. We assessed the incidence of these among cases of transvaginal delivery. Between June 2013 and July 2016, a prospective cohort study was conducted at a tertiary perinatal medical facility in Japan. Women were administered a questionnaire to ascertain risk factors for PPH, defined as a blood loss of 1,000 ml or more assessed using a calibrated under-buttocks drape and collection vessel at childbirth. We analyzed 1,068 transvaginal deliveries of singleton pregnancies. The incidence of PPH was 8.7%, and of severe PPH (1,500 ml blood loss or more) was 2.1%. Risk factors for postpartum hemorrhage among the deliveries were: fetal macrosomia (over 4000 g); pregnancy-induced hypertension; pregnancy generated by assisted reproductive technology; severe vaginal or perineal lacerations; and weight gain over 15 kg during pregnancy. Such high weight gain significantly increased the incidence of PPH compared with women showing less than 10 kg weight gain during pregnancy. Monitoring these identified risk factors could enable extra vigilance during labor, and preparedness for managing PPH in all women giving birth.
Background
Postpartum hemorrhage (PPH) is defined more than 500 ml of blood bleeding following vaginal delivery [1]. PPH is considered severe when blood loss exceeds 1,000 ml after a vaginal delivery, or results in signs or symptoms of circulating blood volume instability [1]. It is a major cause of maternal mortality especially in developing countries and is the cause of 25% of maternal deaths worldwide [2]. It is the most common maternal morbidity even in highly resourced countries and is increasing in incidence [3]. Sequelae of PPH include hypotension, anemia, and fatigue, which can make breastfeeding and maternal care of the newborn more difficult [4]. The Society of Obstetricians and Gynaecologists of Canada has published guidelines on the prevention and management of this complications [5]. They summarize the causes for PPH as related to abnormalities of one or more of four basic processes, namely the “four Ts”: tone, trauma, tissue, and thrombin. Atonic bleeding is major factor of PPH. Risk factors include antepartum and intrapartum conditions as including a history of PPH, multiple pregnancies, fetal macrosomia, primigravida, grand multiparity, older age, preterm births, genital tract injuries, non-use of oxytocin for PPH prophylaxis, labor induction, cesarean delivery and intra-uterine fetal deaths [1, 6–9]. However, 20% of patients who develop PPH have no risk factors, so providers must be prepared to treat it at every delivery [10]. There is little information on the magnitude and risk factors for PPH. Common causes include uterine atony, trauma including genital tract injuries, placental retentions and failure of the blood coagulation system. Uterine atony is responsible for the majority (75%) of cases of PPH [11]. To obstetrics providers, risk factor identification in the antenatal and intrapartum periods might enable timely interventions to prevent PPH. This study was undertaken to assess the incidence of, and risk factors for PPH among transvaginal deliveries at a tertiary perinatal medical facility in Japan.
Methods
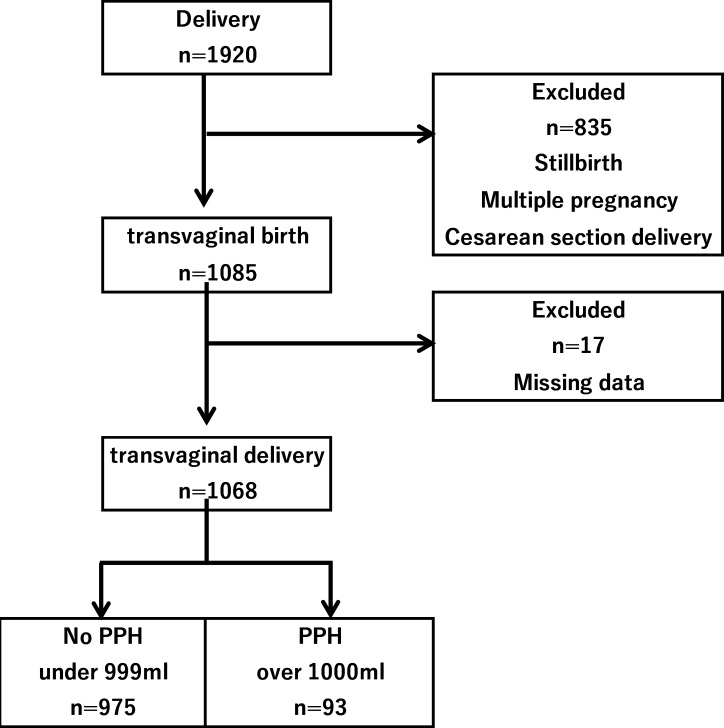
Our medical center is one of the tertiary perinatal medical facilities in Japan. Ethical approval was obtained from the ASO Iizuka Hospital, Iizuka, Japan. Informed written consent was obtained from all participants at enrolment. Between June 2013 and July 2016, a prospective cohort study was conducted. Women were administered a questionnaire to ascertain risk factors for PPH, defined as a blood loss of 1,000 ml or more at childbirth. PPH is defined as the blood loss of more than 500 ml within the first 24 hours following childbirth. In this study, we defined as the blood loss of more than 1,000 ml which influences results in signs or symptoms of circulating blood volume instability. Pregnant women were recruited at 22 weeks of gestation or greater. The study excluded women who underwent a caesarean-section delivery, or who had a stillbirth or a multiple pregnancy. Cases with data missing for the primary outcome of blood loss were excluded from the analysis (Fig 1). The health providers in the delivery rooms in these health facilities were trained in the data collection procedure and on the measurement of postpartum blood loss. During enrolment, interviewer-administered questionnaires were used to collect data on risk factors including maternal age, parity, maternal body weight and body mass index (BMI) before pregnancy, and weight gain during pregnancy. Gestational age at birth was calculated based on ultrasound scan estimations or on the mother’s recollection of her last normal menstrual period. The research team noted whether labor was induced or augmented with oxytocin, the mode of delivery (normal or assisted vaginal delivery), and any severe vaginal/perineal lacerations. The primary outcome was PPH defined as a blood loss of 1,000 ml or more after childbirth. If the blood loss is over 100ml, the vital sign deteriorated easily, so we defined more than 1000ml hemorrhage as PPH. The total blood loss collected in the calibrated conical receptacle was established by the attending midwife. The following variables were collected from medical record and questionnaire: maternal age; parity; the use of assisted reproductive technology (ART); maternal smoking habit; pregnancy-induced hypertension (PIH); maternal body weight and BMI before pregnancy; labor induction/augmentation by oxytocin; assisted vaginal delivery; severe vaginal/perineal lacerations; and neonatal birthweight. Multivariate Logistic regression analysis was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) and control for the potential confounders. Explanatory variables included in this model were those that statistically significant for outcome in the univariate analysis. However, we did not include gestational week and birth weight simultaneously in this model because the correlation between them was strong (Pearson correlation coefficient, r = 0.672, p<0.001). We did not include maternal age, maternal height, gestational age at delivery, weight gain during pregnancy, use of ART, PIH and severe vaginal/perineal lacerations were used for explanatory variables. Two-sided P values of < 0.05 were regarded as statistically significant. All analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC, USA). This study was approved by the institutional review board (IRB) of ASO Iizuka Hospital, Fukuoka, Japan.
Fig 1. Profiles of the study participants.
Results
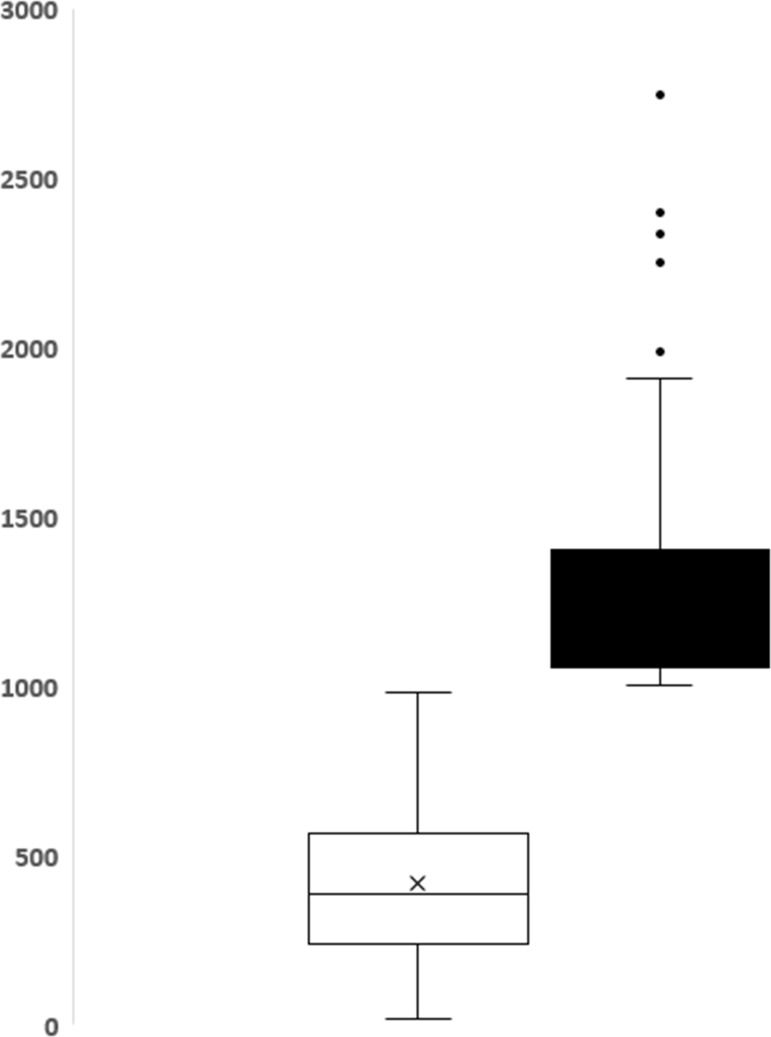
Fig 2 shows the median and interquartile range of blood loss. Table 1 shows the distribution of sociodemographic, antepartum and intrapartum factors of the study participants, and their association with PPH.
Fig 2. The median and interquartile range of blood loss.
Table 1. Characteristics of the study population (N = 1068).
| No PPH (n = 975) | PPH (n = 93) | Total (N = 1068) | minimum-maximum | |
|---|---|---|---|---|
| Maternal age at delivery | 30.4 ± 6.0 | 30.4 ± 6.0 | 30.4 ± 6.0 | (15–44) |
| Parity | 0.8 ± 1.1 | 0.9 ± 1.1 | 0.8 ± 1.1 | (0–6) |
| Pre-pregnant body weight (Kg) | 53.5 ± 10.9 | 54.1 ± 8.3 | 53.5 ± 10.7 | (32–105) |
| Pre-pregnant body mass index | 21.6 ± 4.1 | 21.7 ± 3.3 | 21.6 ± 4.1 | (14.6–41.2) |
| Weight gain during pregnancy (Kg) | 9.0 ± 5.4 | 10.8 ± 4.3 | 9.2 ± 5.3 | (-10.3–45.5) |
| Gestational week at delivery | 38.7 ± 2.2 | 39.3 ± 1.3 | 38.8 ± 2.1 | (23.1–42.3) |
| Blood loss (ml) | 425 ± 226 | 1,344 ± 386a | 505 ± 356 | (40–2745) |
| Neonatal birth weight (g) | 2,855 ± 511 | 3,237 ± 421a | 2,884 ± 515 | (580–4596) |
aSignificantly different from No PPH group.
The results are described by medians and interquartile ranges. Data collected among 1,068 women showed a mean blood loss of 505 ± 356 ml and ranged from 40 to 2,745 ml. Overall, 93 (8.7%) women had PPH (1,000 ml or more) and 22 (2.1%) had severe PPH (1,500 ml or more; Table 2).
Table 2. Prevalence of PPH.
| Blood loss (ml) | N = 1068 | % |
|---|---|---|
| less 499 | 648 | 60.7 |
| 500–1000 | 327 | 30.6 |
| 1000–1500 | 71 | 6.6 |
| over 1500 | 22 | 2.1 |
The use of ART, excessive weight gain (over 15 kg) during pregnancy, complicated PIH, severe vaginal/perineal lacerations and having a macrosomic baby were contributing factors for PPH (Table 3).
Table 3. Association between the risk factors and postpartum hemorrhage.
| Variable | No PPH n = 975 (%) | PPH n = 93 (%) | P value | |
|---|---|---|---|---|
| Age at birth | ||||
| under 19 | 51 (5.2) | 3 (3.2) | <0.05 | |
| 20–35 | 652 (66.9) | 62 (66.7) | Reference | |
| 35–40 | 228 (23.4) | 21 (22.6) | NS | |
| over 40 | 44 (4.5) | 7 (7.5) | <0.05 | |
| Pre-pregnant body mass index | ||||
| under 18.4 | 173 (17.7) | 18 (19.4) | NS | |
| 18.5–24.9 | 661 (67.8) | 59 (63.4) | Reference | |
| over 25.0 | 141 (14.5) | 16 (17.2) | NS | |
| Parity | ||||
| Primipara | 485 (49.7) | 44 (47.3) | Reference | |
| Multipara | 490 (50.3) | 49 (52.7) | NS | |
| Artificial reproductive technique | ||||
| No | 945 (96.9) | 84 (90.3) | Reference | |
| Yes | 30 (3.1) | 9 (9.7) | <0.01 | |
| Smoking habit | ||||
| No | 911 (93.4) | 86 (92.5) | Reference | |
| Yes | 64 (6.6) | 7 (7.5) | NS | |
| Weight gain during pregnancy (Kg) | ||||
| less 9.9 | 552 (56.6) | 36 (38.7) | Reference | |
| 10.0–14.9 | 337 (34.6) | 40 (43.0) | NS | |
| over 15.0 | 86 (8.8) | 17 (18.3) | <0.01 | |
| Gestational week at delivery (weeks) | ||||
| after 40/0 | 283 (29.0) | 31 (33.3) | NS | |
| 37/0-39/6 | 549 (56.3) | 56 (60.2) | Reference | |
| before 36/6 | 143 (14.7) | 6 (6.5) | <0.01 | |
| Pregnancy induced hypertension | ||||
| No | 913 (93.6) | 78 (83.9) | Reference | |
| Yes | 62 (6.4) | 15 (16.1) | <0.01 | |
| Labor induction/ augmentation by oxytocin | ||||
| No | 657 (67.4) | 60 (64.5) | Reference | |
| Yes | 318 (32.6) | 33 (35.5) | NS | |
| Assisted vaginal delivery | ||||
| No | 897 (92.0) | 84 (90.3) | Reference | |
| Yes | 78 (8.0) | 9 (9.7) | NS | |
| Severe vaginal/perineal laceration | ||||
| No | 834 (85.5) | 68 (73.1) | Reference | |
| Yes | 141 (14.5) | 25 (26.9) | <0.01 | |
| Neonatal birth weight (g) | ||||
| less 2499 | 209 (21.4) | 2 (2.2) | <0.01 | |
| 2500–3499 | 679 (69.6) | 67 (72.0) | Reference | |
| 3500–3999 | 77 (7.9) | 20 (21.5) | <0.05 | |
| over 4000 | 10 (1.0) | 4 (4.3) | <0.01 | |
Table 4, lists the factors associated with increased risk for PPH from the logistic regression analysis. These included the use of ART (OR 3.479; 95% CI 1.47–8.24); PIH (OR 3.159; 95% CI 1.65–6.06) and severe vaginal/perineal lacerations (OR 1.978; 95% CI 1.19–3.31).
Table 4. Adjusted odds ratio and corresponding 95% confidence intervals for delivery related outcomes.
| Odds ratio | 95% Confidence interval | |
|---|---|---|
| Artificial reproductive technique pregnancy | 3.48 | 1.47–8.24 |
| Pregnancy induced hypertension | 3.16 | 1.65–6.06 |
| Severe vaginal/perineal laceration | 1.98 | 1.12–3.31 |
All outcomes are adjusted for maternal age, maternal height, gestational age at delivery, weight gain during pregnancy, artificial reproductive technique pregnancy, pregnancy induced hypertension, severe vaginal/perineal laceration.
Discussion
The overall incidence of PPH was 8.7% and that of severe PPH was 2.1%. The risk factors for PPH were the use of ART, PIH, severe vaginal/perineal lacerations and having a macrosomic baby. The incidence of PPH in this study was higher than that reported previously. Sosa et al. reported that 10.8% of woman lost more than 500 ml and 1.9% lost greater than 1,000 ml [12]. Calvert et al. reported that the prevalence of PPH (blood loss >500 ml) ranged from 7.2% in Oceania to 25.7% in Africa [13]. The prevalence of severe PPH (blood loss >1,000 ml) was highest in Africa at 5.1% and lowest in Asia at 1.9%. This high incidence of PPH in our study may have been influenced by the characteristics of the study population. Our hospital is a single tertiary perinatal medical facility in Japan that is reported to have higher rates of PPH. We excluded cesarean deliveries and multiple pregnancies in this study as the former associated with an increased risk of PPH [14,15]. Multiple pregnancies are also associated with an increased risk of PPH [15,16]. Singleton transvaginal deliveries were analyzed in this study to identify the risk factor clearly. The risk for PPH was highest for women using ART. This is consistent with previous studies [17,18]. Thus, Zhu et al. reported that placental adherence occurred more frequently in a group of women after using ART.17 Placental adherence reflects abnormal development, and is an independent risk factor for PPH. However, there was no placental adhesion or uterine inversion in our study. Another possibility is the presence of uterine myomas or uterine anomalies, but we did not investigate maternal uterine factors. Complicated PIH was the second major risk factor in our study. Hematological abnormalities can develop in some women with PIH and the levels of plasma clotting factors may decrease. Other possible and not registered confounders were the use of magnesium sulfate, increased blood loss via vasodilatation, a tocolytic effect predisposing to uterine atony, prolonged bleeding time through inhibition of platelet activity and red cell deformities [19]. Lacerations and hematomas resulting from birth trauma can cause significant blood loss that can be lessened by hemostasis and timely repair. Sutures for hemostasis should be placed if direct pressure does not stop the bleeding. Episiotomy increases blood loss as well as the risk of anal sphincter tears and should be avoided unless urgent delivery is necessary and the perineum is felt to be a limiting factor in achieving delivery [20]. Additionally, it was clear from our study that excessive weight gain during pregnancy was associated with an increased risk of PPH. Excessive weight gain can lead to a macrosomic baby, which will overdistend the uterus and uterine atony. There is no data, if macrosomia inducted earlier to prevent the PPH.
This study had limitations. First, our data set included only Japanese women, and it is unclear whether the results can be extrapolated to women of other ethnic groups. Second, we are in a tertiary obstetrics center. This might have introduced selective bias in the patient background characteristics. Third, this study did not include maternal uterine factors. Fourth, we did not investigate the duration of labor and HELLP syndrome associated with PIH.
In conclusion, the risk factors for PPH in our setting were the use of ART, PIH, severe vaginal/perineal lacerations and macrosomic neonates. Extra vigilance during the antenatal and peripartum periods is needed to identify women at risk and enable early intervention to prevent PPH. It is important to remember that we need to prepare for PPH in all women giving birth, as some develop it without any known risk factors.
Supporting information
(XLSX)
Acknowledgments
We thank all our medical staff, midwives and nurses for their devotion to the infants involved in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sterweil P, Nygren P, Chan BK, Helfand M. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol 2006; 108:1039–1047. [DOI] [PubMed] [Google Scholar]
- 2.Trends in maternal mortality: 1990 to 2015. (Accessed Dec 1, 2016, at http://www.who.int/reproductivehealth/publications/monitoring/maternal-mortality-2015/en) 2015.
- 3.Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC pregnancy and childbirth 2009; 9:55 10.1186/1471-2393-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson JF, Heal LJ, Roberts CL, Ellwood DA. Women’s breastfeeding experiences following a significant primary postpartum haemorrhage: A multicenter cohort study. Int Breastfeed J 2010; 5:5 10.1186/1746-4358-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies GA, Tessier JL, Woodman MC, Lipson A, Hahn PM. Maternal hemodynamics after oxytocin bolus compared with infusion in the third stage of labor: a randomized controlled trial. Obstet Gynecol 2005; 105:294–299. 10.1097/01.AOG.0000148264.20909.bb [DOI] [PubMed] [Google Scholar]
- 6.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG 2008;115:1265–1272. 10.1111/j.1471-0528.2008.01859.x [DOI] [PubMed] [Google Scholar]
- 7.Oberg AS, Hernandez-Diaz S, Palmsten K, Almqvist C, Bateman BT. Patterns of recurrence of postpartum hemorrhage in a large population-based cohort. Am J Obstet Gynecol 2014; 210:229.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bais JM, Eskes M, Pel M, Bonsel GJ, Bleker OP. Postpartum haemorrhage in nulliparous women: incidence and risk factors in low and high risk women. A Dutch population-based cohort study on standard (> or = 500 ml) and severe (> or = 1000 ml) postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol 2004; 115:166–172. 10.1016/j.ejogrb.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 9.Ford JB, Patterson JA, Seeho SK, Roberts CL. Trends and outcomes of postpartum haemorrhage, 2003–2011. BMC Pregnancy Childbirth 2015; 15:334 10.1186/s12884-015-0788-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magann EF, Evans S, Hutchinson M, Collins R, Howard BC, Morrison JC. Postpartum hemorrhage after cesarean delivery: an analysis of risk factors. South Med J 2015; 98:681–685. [DOI] [PubMed] [Google Scholar]
- 11.Lutomski JE, Byrne BM, Devane D, Greene RA. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG 2012; 119:306–314. 10.1111/j.1471-0528.2011.03198.x [DOI] [PubMed] [Google Scholar]
- 12.Sosa CG, Althabe F, Belizan JM, Buekens P. Use of oxytocin during early stages of labor and its effect on active management of third stage of labor. Am J Obstet Gynecol 2011; 204:238.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvert C, Thomas SL, Ronsmans C, Wagner KS, Adler AJ, Filippi V. Identifying regional variation in the prevalence of postpartum haemorrhage: a systematic review and meta-analysis. PLoS One 2012; 7:e41114 10.1371/journal.pone.0041114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheldon WR, Blum J, Vogel JP, Souza JP, Gülmezoglu AM, Winikoff B; WHO Multicountry Survey on Maternal and Newborn Health Research Network. Postpartum haemorrhage management, risks, and maternal outcomes: findings from the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG 2014; 121(Suppl 1):5–13. [DOI] [PubMed] [Google Scholar]
- 15.Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol 2013; 209:449 e1–7 [DOI] [PubMed] [Google Scholar]
- 16.Sosa CG, Althabe F, Belizan JM, Buekens P. Risk factors for postpartum hemorrhage in vaginal deliveries in a Latin-American population. Obstet Gynecol 2009; 113:1313–1319. 10.1097/AOG.0b013e3181a66b05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, et al. Maternal and Live-birth Outcomes of Pregnancies following Assisted Reproductive Technology: A Retrospective Cohort Study. Sci Rep 2016;6:35141 10.1038/srep35141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elenis E, Svanberg AS, Lampic C, Skalkidou A, Åkerud H, Sydsjö G. Adverse obstetric outcomes in pregnancies resulting from oocyte donation: a retrospective cohort case study in Sweden. BMC Pregnancy Childbirth. 2015;8;15:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Schmidt auf Altenstadt JF, Hukkelhoven CW, van Roosmalen J, Bloemenkamp KW. Pre-eclampsia increases the risk of postpartum haemorrhage: A nationwide cohort study in the Netherlands. PLoS One. 2015; 8;12:e81959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shmueli A, Gabbay Benziv R, Hiersch L, Ashwal E, Aviram R, Yogev Y, et al. Episiotomy—risk factors and outcomes. J Matern Fetal Neonatal Med 2016; 29:1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.