Abstract
Inflammatory bowel disease (IBD), a collective term for Crohn disease and ulcerative colitis, is a polygenic disease thought to be triggered by environmental factors. A Western or westernized lifestyle may be a major driver of the growing incidence of IBD. IBD may represent dysregulated mucosal inflammation to gut microbiota. Despite many review articles on environmental factors in IBD, no consensus exists regarding which factor contributes most to trigger the onset of IBD. Identification and recognition of major environmental factors are prerequisite for effective disease treatment and prevention. Representative environmental factors such as smoking, breastfeeding, nonsteroidal anti-inflammatory drugs, antibiotic use in childhood, oral contraceptives, and appendectomy do not correlate with disease onset in most patients with IBD. In contrast, diet appears to be important in most cases of IBD. Diets rich in animal protein (risk factor) and deficient in dietary fiber (preventive factor) are characteristic of westernized diets in affluent societies. Recent research shows that westernized diets are associated with a reduced gut microbial diversity (dysbiosis), which may result in increased susceptibility to IBD and other common chronic diseases. Plant-based diets rich in dietary fiber are associated with increased microbial diversity. Recent reports on IBD therapy that replaced westernized diets with plant-based diets achieved far better outcomes than those previously reported in the literature. We believe that westernized diet-associated gut dysbiosis is the most ubiquitous environmental factor in IBD. Adoption of this concept may have the potential to provide a better quality of life for patients with IBD.
Keywords: Crohn disease, genetic factors triggered by environmental factors, inflammatory bowel disease, plant-based diets, polygenic disease, ulcerative colitis, western diet, westernized diet-associated gut dysbiosis
INTRODUCTION
The incidence of inflammatory bowel disease (IBD), a collective term for Crohn disease (CD) and ulcerative colitis (UC), has been increasing over time and expanding to different regions around the world, indicating that IBD is a global disease.1–6 Gut inflammation does not occur in the absence of gut microbiota.7 A dysregulated immune response to commensal bacteria may greatly contribute to IBD incidence and subsequent morbidity.
Like many other diseases, IBD is a multifactorial disease that occurs when genetic factors in a susceptible person are triggered by environmental factors. Recent genomewide association studies identified 200 susceptible loci involved in IBD.8 They are largely involved in 3 areas. The first area is related to innate immunity and autophagy (eg, NOD2, ATG161, IRGM, LRRK2). Namely, they recognize and clear microbial agents. The second is related to the adaptive immunity of interleukin-23 signaling and T-helper 17 cells (eg, IL23R, IL12B, STAT3, JAK2, TYK2) and interleukin-10 signaling (IL10). They regulate inflammatory response. The third is related to epithelial barrier function (eg, ECM1, CDH1, HNF4A, LAMB1, GNA12). They maintain the mucosal epithelial barrier against microbial invasion. The first appears to be implicated in CD only and the third in UC only. The second appears to be implicated in both CD and UC. These findings provide insight into the pathogenesis of IBD.8 It is obvious now that susceptible genes for IBD differ by ethnicity.2,8 In addition, it was recently demonstrated that host genetics influenced the composition of the gut microbiota. For example, healthy individuals with IBD-susceptible genes (NOD2, CARD9, ATG16L1, IRGM, and FUT2) exhibited a decreased abundance of Roseburia spp.9 Despite remarkable advances in genetics, the contribution of genetics to the onset of IBD is limited. Genetic-risk polymorphisms explain less than one-third of the heritability of the disease.8 Because the human genetic constitution has barely changed during its long history, the rapid increase in IBD incidence during the transition from a “developing” to “developed” nation can be explained not by genetic factors but by changes in environmental factors. It is recognized that a major driver of the growing incidence of IBD is westernization of lifestyle. However, the only lifestyle change recommended in current IBD guidelines is that patients with CD not smoke.3
Dysbiosis of the gut microbiota has been observed in IBD,1,2,4–6 and it is apparent now that gut microbiota is influenced by our diet. Thus, it seems critical to maintain gut symbiosis for the suppression of gut inflammation by consuming a suitable diet. With a suitable diet, substantial improvement in the prognosis can be expected. We believe that the lack of a suitable diet is the biggest issue faced in current IBD treatment. There are 2 steps to establishing a suitable diet for IBD. The first step is the recognition of the key factor (ie, westernized diet) among a variety of environmental factors in IBD, and the second step is investigation of a suitable diet for IBD. This commentary is the first step to try to recognize westernized diet as the key environmental factor in IBD.
Several review articles on environmental factors in IBD have been published since 2016.1–6 Abegunde et al1 listed environmental factors under the headings lifestyle, pharmacologic agents, surgery, and so on. The European Crohn’s and Colitis Organisation Environmental Factors Working Group3 provided 22 evidence-based guidelines for current clinical practice. van der Sloot et al4 quantified environmental factors and listed them by exposures during life stages (childhood exposures, adulthood exposures, and lifelong exposures). Bernstein5 focused on factors relating to the gut microbiota. Kaplan and Ng2 proposed cutting the global incidence of IBD by 50% by 2032, which is exactly 1 century after the first description of CD in the literature in 1932. However, no statement was found in these reviews regarding the specific environmental factor or factors that may contribute the most to trigger IBD onset. This is most likely because of the current lack of firm evidence identifying the greatest environmental factors. Without recognition of key environmental factors, clinicians will continue conventional medical care which is inadequate and nonoptimal. Therefore, identification of the environmental factor most responsible for IBD is critical for treatment and prevention.
We previously reported in 2011 that diet-associated gut microbiota is the greatest environmental factor in IBD.10 We have been treating patients with IBD on the basis of this concept.11–14 The more we practice, the more we are convinced of the utility of diet-based therapy in IBD. In addition, there is growing evidence in the medical literature concerning the sequence of diet, gut microbiota, and health, which further supports our view.15 In this commentary, we describe why we think the greatest environmental risk factor in IBD is westernized diet-associated gut microbial dysbiosis.
RECOGNITION OF INFLAMMATORY BOWEL DISEASE AS A LIFESTYLE DISEASE
The etiology of IBD has been described as unknown. The term idiopathic is often added: Idiopathic IBD. As already described here, we believe it is clear that westernized lifestyle is a major driver of the growing incidence of IBD. Other common chronic diseases are also lifestyle related (eg, obesity, diabetes, coronary artery disease, stroke), and IBD is probably no exception. If the cause of the disease is unknown, we cannot do anything more than conventional treatment. If the disease is related to lifestyle, lifestyle modification may prevent initiation of the disease process or possibly improve the disease course.
CURRENT PROBLEMS IN INFLAMMATORY BOWEL DISEASE
No recommendation for lifestyle modification is stated in the guidelines except for nonsmoking for patients with CD.3 It is noteworthy that smoking is firmly appreciated as a risk and exacerbating factor in CD. Recent study in Eastern Asian countries, however, did not reproduce the results found in Western countries.2 The most common question asked by patients with IBD is what should I eat? Clinicians cannot adequately answer this question.
The lack of identification of the key environmental factor is the biggest problem in current IBD practice. If it is identified, modification of the implicated lifestyle may improve disease outcomes.
MOST UBIQUITOUS ENVIRONMENTAL FACTOR IN INFLAMMATORY BOWEL DISEASE
Common environmental factors in IBD found across the recent reviews1–6 are listed in Table 1. Among the factors in Table 1, 4 criteria may be helpful in identifying the most important environmental factor. First, the factor should be a protective factor or a risk factor in both UC and CD. Second, the factor should be a protective factor or risk factor in all geographic areas. Third, most patients with IBD should be exposed to the factor. Finally, the factor should influence gut microbiota considering that gut microbial dysbiosis is consistently observed in IBD.1–3,5,6
Table 1.
Environmental factors in inflammatory bowel disease (IBD)
| Environmental factor | Role in IBD | Exposure to most patients with IBD | Relevance to gut microbiota | ||
|---|---|---|---|---|---|
| UC | CD | Mode of role | |||
| Lifestyle | |||||
| Smoking | P | R | Divergent in IBD | No | Yes |
| Diet | |||||
| Animal protein | R | R | Identical in IBD | Yes | Yes |
| Dietary fiber | N | P | Neither identical nor divergent | Yes | Yes |
| Tea or coffee | P | P | Identical in IBD | Yes | Unknown |
| Low levels of vitamin D | R | R | Identical in IBD | No | Unknown |
| Breastfeedinga | P | P | Identical in IBD | No | Yes |
| Pharmacologic agents | |||||
| NSAID | R | R | Identical in IBD | No | Unknown |
| Antibiotics in childhood | Divergent among ethnic groups | No | Yes | ||
| Oral contraceptives | R | R | Identical in IBD | No | Unknown |
| Dipeptidyl peptidase-4 inhibitors | R | N | No | Unknown | |
| Vaccination | N | N | Yes | Unknown | |
| Other factors | |||||
| Appendectomy | P | R | Divergent in IBD | No | Unknown |
| Air pollution | R | R | Identical in IBD | No | Unknown |
CD = Crohn disease; N = neither protective nor risk factor; NSAID = nonsteroidal anti-inflammatory drug; P = protective factor; R = risk factor; UC = ulcerative colitis.
Being breastfed as a baby is a protection against the development of IBD. Duration of breastfeeding is not specified.
The epidemiology of IBD is similar in both CD and UC. This means that the role of the most ubiquitous environmental factor in IBD should not be divergent between UC and CD but should be common to both UC and CD. Smoking and appendectomy have a divergent role in IBD; each is a protective factor for UC but a risk factor for CD (Table 1). Therefore, these are not the key factor. It is noteworthy that some environmental factors identified in Western countries are not the same in Asia.2 For example, antibiotic use in childhood is a risk factor for IBD in Western countries, but it is found to be a protective factor in recent Asian studies. Similar to the role of the environmental factors in UC and CD, the role of the key environmental factor is not divergent but must be identical across geographic areas. Therefore, antibiotic use in childhood is not the key factor.
According to the hygiene hypothesis, an improvement in sanitation with limited exposure to microbes results in impaired immune response, causing immune-mediated diseases including IBD. Hygiene factors studied include sanitary facilities (toilet, water supply), family size and birth order, pets, and farm animals. There is insufficient evidence to support or refute the hygiene hypothesis.1–3 A positive association between urban air pollution and IBD has been described. However, it is difficult to interpret whether IBD is a direct consequence of pollution.3 Although stress and anxiety are shown to be related to IBD relapse, they have not been proved to be risk factors for the development of IBD.3
The most ubiquitous environmental factor for IBD should be present in most patients with IBD. Low levels of vitamin D, breastfeeding, nonsteroidal anti-inflammatory drugs, oral contraceptive use, dipeptidyl peptidase-4 inhibitors, air pollution, and Helicobacter pylori infection—all on the list (Table 1)—do not apply to the majority of patients with IBD. The remaining factor in the list in Table 1 is diet. All patients with IBD are exposed to food. Although dietary fiber is protective for CD, its protective effect is absent in UC.1,3,5 Animal protein is a risk factor for IBD.5,11 Diet influences gut microbiota. Namely, diet meets all 4 conditions for the key environmental factor. Increased consumption of animal protein and decreased consumption of dietary fiber are characteristic of dietary westernization.11 Wealth inevitably induces dietary westernization, which explains the high incidence of IBD in wealthy nations. It can be concluded that a westernized diet is the critical environmental factor in IBD.10,11
RATIONALE FOR HOW WESTERNIZED DIET CAUSES INFLAMMATORY BOWEL DISEASE
Research on the gut microbiota has advanced our understanding about the key role of the gut microbiota in health and disease.15 The diseases extend beyond the confines of the gut (IBD) to various chronic diseases: Obesity, diabetes, coronary artery disease, stroke, rheumatoid arthritis, cancer, psychiatric diseases, and others. Gut microbiota is beginning to be recognized as an endocrine organ.16 The gut microbiota affects the immune system, host metabolism, cardiovascular system, enteric nervous system, brain and behavior, and stress/hypothalamic-pituitary-adrenal axis. Microbial diversity plays an important role in gut and systemic homeostasis. Reduced microbial diversity (dysbiosis) is commonly observed in a variety of chronic diseases, including IBD.17 In patients with IBD, bacterial levels are known to fluctuate up and down compared with healthy controls. Numbers of Fusobacterium, adherent-invasive Escherichia coli and Enterobacter organisms are increased, and Firmicutes and Bacteroidetes phyla, Faecalibacterium prausnitzii, Roseburia hominis, Bifidobacterium, and Prevotella are decreased.2,6 We tend to recognize things outside the body as environmental factors. However, the gut microbiota inside the body has been identified as an environmental factor for obesity. The presence of gut microbiota is a prerequisite for gut inflammation.7 Furthermore, it is also known that the gut microbiota is formed by our diet.
Recently, research is unraveling the relationship between diet and microbial diversity.15,17 We have coevolved with gut microbiota to exist in a symbiotic relationship. Westernized diets tend to cause gut dysbiosis (reduced microbial diversity), resulting in poor production of microbial metabolites such as short-chain fatty acids, which have diverse effects in maintaining homeostasis. In addition, these diets promote expansion and activity of colonic mucus-degrading bacteria, resulting in barrier dysfunction.18 In contrast, a plant-based diet rich in dietary fiber increases microbial diversity and produces beneficial microbial metabolites.15,17 These observations indicate that westernized diets increase susceptibility to not only IBD19 but also other chronic diseases. The precise mechanisms underlying how diet induces microbial dysbiosis and results in onset of individual chronic diseases are not yet fully understood.
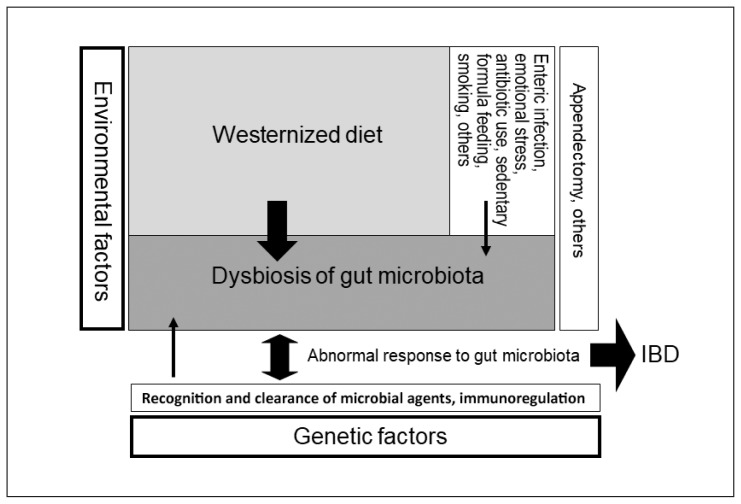
There have been many schemas of the pathogenesis of IBD together with environmental factors. Most of them depict factors in parallel without indicating the degree of contribution to the pathogenesis. The schema for clinicians should be simple but provide clear information. Our schematic pathogenesis is presented in Figure 1. This schema is a modification of the original.10
Figure 1.
Schematic pathogenesis of inflammatory bowel disease (IBD).a
a IBD occurs in genetically susceptible persons when triggered by environmental factors. Width of arrows reflects the degree of the contributing role in the pathogenesis of IBD. The greatest environmental factor is gut dysbiosis (imbalance of gut microbiota), which is formed by a westernized diet, namely, westernized diet-associated gut dysbiosis. This schema is a modification of the original. Reprinted with permission.10
CLINICAL OUTCOME OF MANIPULATING WESTERNIZED DIET
Interventional studies to modify risk factors to reduce relapse or to improve patient outcomes are worthwhile. By replacing a westernized diet with a plant-based diet in treatment, we have achieved and published far better outcomes than those reported in both the active stage and quiescent stage in CD11,12 and in UC.13 We now treat mild cases of UC with a plant-based diet first, not with medication.13,14
It is clear that treatment based on the etiopathogenesis of the disease is optimal. Identification of the most ubiquitous environmental factor can lead to modification of the environmental factor. Our westernized diet is one of the major factors in current common chronic diseases. Plant-based diets are listed as variations of US Department of Agriculture healthy eating patterns and are recommended to the public to prevent common chronic diseases.20,21 The spread of a healthy diet may halt the further increase in IBD incidence or decrease the incidence. We know, however, that such spread is quite slow without a supportive public campaign such as the campaign against smoking.
We do not know how long it will take until the key environmental factor in IBD is firmly determined. Until then, we must analyze the available data for the second-best measure. As discussed earlier, the data point to westernized diet-associated gut microbial dysbiosis as the most ubiquitous environmental factor in IBD. Wide appreciation of this environmental factor by gastroenterologists will likely improve our therapeutic strategies and provide far better outcomes in IBD treatment.
CONCLUSION
We believe that westernized diet-associated gut microbial dysbiosis is the most ubiquitous environmental factor in IBD. Appreciation of this concept may help provide a better quality of life for patients with IBD.
Acknowledgment
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Abegunde AT, Muhammad BH, Bhatti O, Ali T. Environmental risk factors for inflammatory bowel diseases: Evidence based literature review. World J Gastroenterol. 2016 Jul 21;22(27):6296–317. doi: 10.3748/wjg.v22.i27.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017 Feb;152(2):313–21.e2. doi: 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Maaser C, Langholz E, Gordon H, et al. European Crohn’s and Colitis Organisation topical review on environmental factors in IBD. J Crohns Colitis. 2017 Aug 1;11(8):905–20. doi: 10.1093/ecco-jcc/jjw223. [DOI] [PubMed] [Google Scholar]
- 4.van der Sloot KWJ, Amini M, Peters V, Dijkstra G, Alizadeh BZ. Inflammatory bowel diseases: Review of known environmental protective and risk factors involved. Inflamm Bowel Dis. 2017 Sep;23(9):1499–509. doi: 10.1097/mib.0000000000001217. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein CN. Review article: Changes in the epidemiology of inflammatory bowel disease―clues for aetiology. Aliment Pharmacol Ther. 2017 Nov;46(10):911–9. doi: 10.1111/apt.14338. [DOI] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: A review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018 Jan;15(1):39–49. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 7.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994 Dec 1;180(6):2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015 Sep;47(9):979–86. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imhann F, Vich Vila A, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018 Jan;67(1):108–19. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba M, Tsuda H, Abe T, Sugawara T, Morikawa Y. Missing environmental factor in inflammatory bowel disease: Diet-associated gut microflora. Inflamm Bowel Dis. 2011 Aug;17(8):E82–3. doi: 10.1002/ibd.21745. [DOI] [PubMed] [Google Scholar]
- 11.Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010 May 28;16(20):2484–95. doi: 10.3748/wjg.v16.i20.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba M, Tsuji T, Nakane K, et al. Induction with infliximab and a plant-based diet as first-line (IPF) therapy for Crohn disease: A single-group trial. Perm J. 2017;21:17–009. doi: 10.7812/TPP/17-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiba M, Nakane K, Tsuji T, et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: A single-group trial. Perm J. 2018;22:17–167. doi: 10.7812/TPP/17-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiba M, Sugawara T, Komatsu M, Tozawa H. Onset of ulcerative colitis in the second trimester after emesis gravidarum: Treatment with plant-based diet. Inflamm Bowel Dis. 2018 Apr 23;24(5):e8–9. doi: 10.1093/ibd/izy121. [DOI] [PubMed] [Google Scholar]
- 15.Singh RK, Chang HW, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017 Apr 8;15(1):73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: The neglected endocrine organ. Mol Endocrinol. 2014 Aug;28(8):1221–38. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014 Nov 4;20(5):779–86. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016 Nov 17;167(5):1339–53.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolan KT, Chang EB. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol Nutr Food Res. 2017 Jan;61(1) doi: 10.1002/mnfr.2016001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.2015 Dietary Guidelines Advisory Committee. Dietary guidelines for Americans 2015–2020. 8th ed. Washington, DC: US Department of Health and Human Services; 2015. p. 35. [Google Scholar]
- 21.Grant JD. Time for change: Benefits of a plant-based diet. Can Fam Physician. 2017 Oct;63(10):744–6. [PMC free article] [PubMed] [Google Scholar]