Abstract
Background
Prader-Willi syndrome (PWS) is a complex genetic neurodevelopmental disorder with endocrine disturbances, hyperphagia and often life-threatening obesity as key features. We investigated emotional-processing of food and eating behavior in PWS using startle response-modulation. Startle eyeblink response is an involuntary reflex activated by the autonomic nervous system in response to sudden or disturbing auditory/visual stimuli which may be modulated by the emotional valence of concurrently viewed visual stimuli.
Methodology
Differences in affective modulation of startle reflex were recorded in 13 individuals with PWS versus 8 healthy controls when viewing standard neutral, negative, positive and food-derived images. Electromyogram (EMG) of the orbicularis oculi muscle was measured in response to binaural white noise before and after consumption of a standard 500 Kcal meal. Participants reported their perceived emotional valence for each image, pre- and post-meal, using a 1-10 Likert rating scale.
Results
Subjective ratings of food images and urge to eat were significantly higher in PWS than controls and did not significantly decline post-meal. Acoustic startle responding was detected in PWS but was significantly lower than control participants under all conditions. Startle responses to food images in PWS were attenuated relative to other picture types with potentially abnormal emotional modulation of responses to non-food images which contrasted self-reported picture ratings. A stable positive emotional valence to food images was observed pre- and post-feeding with a sustained urge to consume food in PWS.
Conclusions
Emotional processing measured using startle modulation in response to non-food images was abnormal in PWS which may reflect unique physiological attributes such as hypotonia and abnormal skin conductivity due to increased fat mass. Alternatively, disruption of autonomic or sympathetic nervous system functioning reported in PWS may impact on hunger and/or food drive states. Our findings parallel attentional/processing attributes of affective stimuli reported in autism spectrum disorder and support the feasibility of eyeblink startle modulation to assess food motivation in PWS and provide preliminary data to optimize methodological parameters.
BACKGROUND
Prader-Willi syndrome (PWS) is a complex neurodevelopmental genetic disorder that affects multiple organ systems and characterized by central hypotonia, a poor suck with feeding problems and failure to thrive, hypogenitalism and hypogonadism during infancy with global developmental delay, behavioral problems and hyperphagia with marked obesity identified in early childhood. PWS is associated with a lack of paternally expressed genes on chromosome 15, typically from a paternally derived de novo deletion in the 15q11-q13 region in 70% of cases followed by maternal disomy 15 (both 15s from the mother) in about 25%.1–5 Of these symptoms, one behavioral characteristic that yields substantial health risk for PWS is the capacity for over-eating, weight gain and life-threatening obesity via hyperphagia. Obesity-related problems affect the US population at an increasing rate.6,7 Thus, understanding neural regulatory processes associated with over-eating and weight gain in PWS has implications for obesity in the general population.8
An inexpensive and broadly applicable method to assess physiological evidence of emotional processing in PWS is the study of emotional-modulation generated by the startle response. An acoustic stimulus (e.g., binaural 50 ms 95 sound pressure level (SPL) whitenoise via a headset) generally elicits a discernable and reliable motor response via EMG recording of the orbicularis oculi facial muscle at the inferior orbital region which can be modulated by visual stimulation of images with a recognized emotional valance. Viewing of positive (pleasant) images (e.g., puppies, smiling faces, etc) typically attenuate the amplitude of this response (e.g., resulting in a smaller EMG signal) while negative (unpleasant) images (e.g., crying faces, traumatic injuries, etc) typically potentiate responses (e.g., resulting in a larger EMG signal)9 as reflected in basic and clinical studies of emotional processing in humans in a variety of applications (e.g., psychiatric illness, addiction, appetite regulation).10 Startle is mediated by the autonomic nervous system and therefore an involuntary response. Life experiences can influence the startle response, resulting in sensitization or desensitization in response to certain stimuli.11,12
Hawk et al. [2004]13 demonstrated that visual processing of food stimuli (relative to viewing neutral stimuli such as viewing a cloth towel) can reduce startle amplitude of the EMG tracing in healthy human participants while craving food but not while they were not craving food suggesting the presence of a state-dependent positive emotional response. These results support the application of the startle-modification technique as a measure of the degree of hyperphagia in PWS. We predict that individuals with PWS will respond to food stimuli in a similar manner and that this technique will be useful in the assessment of food image processing in PWS and emotional processes associated with food cues. Furthermore, neuroimaging findings using functional MRI have identified aberrant responses to food stimuli following a meal relative to controls;8 thus, we examined startle-modulation at pre- and post-meal intervals at noontime to test patterns of startle plasticity in this paradigm.
The primary aim of our investigation was to examine the influence of food cues (at pre- and post-meal intervals) on the pattern of startle-modulation detected by the EMG tracing of the orbicularis oculi muscle in individuals with PWS and healthy adult controls in order to obtain preliminary information on responses found in the two subject groups. A secondary aim was to identify whether EMG readings in conjunction with other instruments as a measure of hyperphagia can be used in future intervention studies and ultimately reduce weight in individuals with PWS having the potential for direct application to obesity in the general population.
Methodology
Participants
Participants were referred from the local community or from Dr. Butler’s specialty genetics clinical practice. Informed consent was obtained from all study participants directly or from an authorized representative, typically a parent, with assent prior to enrollment with approval and oversight by the local Institutional Review Board. A total of 21 individuals were recruited for participation including 13 with PWS (mean age ± SD = 26.6 ± 11.0y, age range = 16-65y, 6 female, 7 male) and 8 healthy control adults (mean age ± SD = 31.6 ± 10.7y, age range = 16-65y, 5 female, 3 male). All participants were Caucasian. Eight of the participants with PWS had the 15q11-q13 type II deletion and five had maternal disomy 15. The average body mass index (BMI) for the PWS group was 32.3 ± 8.5 which is in the obese range. Gender, PWS genetic subtypes, growth parameters, medications and startle response data can be seen in Table 1. Participants were excluded on the basis of a positive history of schizophrenia-spectrum diagnoses, posttraumatic stress disorder, Tourette syndrome, Huntington disease, temporal lobe epilepsy, enuresis, obsessive compulsive disorder, seizures (past 12 months), head and/or brain injury (past 6 years), abnormal vision (PWS participants) or obesity status (controls). We also recruited participants as young as 16 years of age to assist with matching between the two subject groups. All participants had a history of normal hearing.
Table 1.
Descriptive Characteristics of Subjects with Prader-Willi Syndrome
| GENDER | PWS GENETIC SUBTYPE | AGE years |
HEIGHT cm |
WEIGHT kg |
BMI -kg/(m2) | PSYCHOTROPIC/BEHAVIORAL MEDICATIONS | STARTLE RESPONSE |
|---|---|---|---|---|---|---|---|
| F | Maternal Disomy 15 | 16 | 145 | 105.3 | 50.1 | Loratadine | Responder |
| M | Maternal Disomy 15 | 16 | 180 | 72.8 | 22.5 | Divalproex, Risperidone | Responder |
| F | 15q11-q13 Type II Deletion | 16 | 160 | 82.0 | 33.0 | NONE | Responder |
| M | 15q11-q13 Type II Deletion | 16 | 164 | 88.8 | 34.7 | NONE | Non Responder |
| F | Maternal Disomy 15 | 18 | 153 | 65.0 | 27.6 | NONE | Responder |
| M | 15q11-q13 Type II Deletion | 23 | 155 | 101.7 | 43.8 | NONE | Responder |
| M | 15q11-q13 Type II Deletion | 26 | 174 | 93.2 | 32.2 | Neurontin, Quetiapine fumarate | Responder |
| M | 15q11-q13 Type II Deletion | 27 | 172 | 66.0 | 23.5 | Divalproex, Fluoxetine, Loxapine | Responder |
| M | Maternal Disomy 15 | 28 | 169 | 84.6 | 29.5 | Cetirizine, Sertraline | Non Responder |
| F | 15q11-q13 Type II Deletion | 29 | 150 | 74.1 | 32.9 | Aripiprazole, Benzonatate, Benzotropine, 70Sertraline | Responder |
| F | 15q11-q13 Type II Deletion | 37 | 146 | 56.0 | 28.7 | NONE | Non Responder |
| F | Maternal Disomy 15 | 40 | 143 | 86.3 | 42.2 | Cetirizine, Divalproex, Fluoxetine | Non Responder |
| M | 15q11-q13 Type II Deletion | 54 | 156 | 85.7 | 34.8 | Benzonatate, Cetirizine, Fluoxetine | Responder |
Study Procedures
Control subjects were asked to report their most recent height and weight at baseline and a brief hearing test was performed to detect any obvious hearing deficits that might impact acoustic perception and response prior to study testing. The skin surface over the orbicularis oculi and mastoid muscles were cleaned to prepare for EMG recording and reduce impedance using disposable alcohol wipes (Becton-Dickinson alcohol swabs, 70% isopropyl alcohol) and allowed to dry for approximately three minutes prior to EMG electrode placement (Figure 1). Two BIOPAC systems EL503 electrodes (Goleta, CA) were situated over the orbicularis oculi, with the first electrode placement under alignment with the pupil and the second electrode placed approximately 10 mm lateral to the first electrode at the lateral canthus of the eye (approximately at a 45-degree angle) for the recording of eyeblink activity. A third BIOPAC systems EL503 electrode was situated on the skin surface over the mastoid, serving as a ground electrode. Muscle contractions generated by the orbicularis oculi were recorded by a BIOPAC systems bioamplifier (model EMG 100c) passing 10-500 Hz signals, sampling at a rate of 2000/second and amplified by a factor of 5000.
Figure 1.
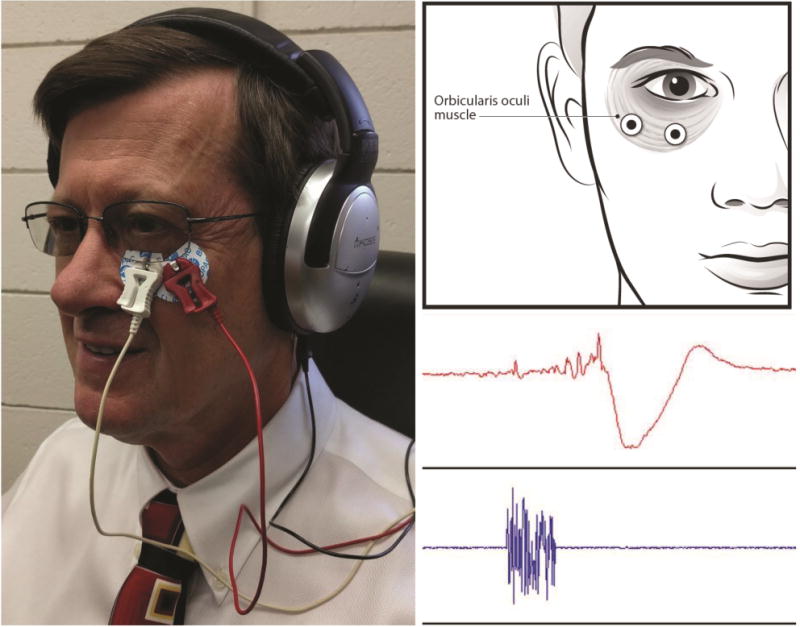
Placement of EMG recording electrodes over the lower orbital portion of the orbicularis oculi muscle is shown in the diagram (upper right). The complete startle eyeblink response montage is shown on the left with the isolated ground electrode placed on the mastoid process and head phones. The lower right electromyographic recording shows the characteristic acoustic stimuli (lower-blue) and muscle blink response (upper-red).
Startle-responses to acoustic stimulation (50 ms 95 SPL whitenoise) were assessed at two lead-intervals (2500 ms and 6000 ms) to measure emotional responses (e.g., early and late, respectively). Startle responses were assessed during viewing of images (e.g., food, puppies, etc.) and between images (e.g., washout periods; no images) on a standard desktop computer monitor screen. All participants were presented with hard copies of images following the trial and were asked to rate how they felt about viewing the pictures (images) on a 1-10 Likert scale (1 = extremely negative, 10 = extremely positive) as well as their desire to eat when shown food pictures (1 = none, 10 very strong). After the first assessment, participants were debriefed and asked to consume a uniform standardized 500 Kcal meal prepared by the local dietary services and provided to the investigative team on the morning of the study visit and refrigerated until use. Following the consumption of the standardized meal, the participants were asked to repeat the procedures, picture viewing and rating-task sessions within 2 hours of eating.
Image Presentation
The study used images from the International Affective Picture System database with standardized, normative emotional valence and arousal.12 Image presentation was similar to event-related fMRI or EEG/ERP designs as reported by Holsen et al. [2006].8 The total number of picture + startle trials per acquisition are described below:
| Picture Type | Startle Probe Position |
|---|---|
| 15 positive picture trials | (5 “early” 2500 ms; 5 “late” 6000 ms) |
| 15 neutral picture trials | (5 “early” 2500 ms; 5 “late” 6000 ms) |
| 15 negative picture trials | (5 “early” 2500 ms; 5 “late” 6000 ms) |
| 15 black screen (baseline) trials | (5 probes at 4250 ms) |
Total time to complete the trial sequence was 35 to 40 minutes (10-15 minutes preparation, ~20 minutes for picture viewing sequence).
Electromyography Scoring Parameters
Startle response amplitudes were scored using a latency window of 20-100 ms post-startle response-eliciting stimulus onset and startle responses were defined as the largest time-locked EMG event present during this interval. Startle response onsets were quantified using the raw EMG signal, whereas startle response amplitudes were quantified using the integrated EMG signal. Startle plasticity was calculated as a percent change score to provide an index of difference in EMG amplitude on pre-pulse inhibition (PPI) trials relative to control (startle-alone) trials to adjust for relative differences in individuals’ baseline startle amplitudes as recommended by Blumenthal et al. [2004]14 using the following formula: (average test startle trial EMG – average startle-alone trial EMG/average startle-alone trial EMG) × 100). This formula served as a standard measure to calculate the primary dependent variable (e.g., percent-change startle response plasticity) across study phases and participants as inter-subject startle response magnitudes can vary significantly.14 EMG impedance was standardized by application methods across all study participants.
Statistics
The primary omnibus statistic employed a factorial between-groups design comparing EMG startle responses across the image categories and time of testing (4×2×2×2 – Image type: Positive, Negative, Neutral, Food; Probe lead interval: 2500 ms, 6000 ms; Meal time/Phase: pre-meal, post-meal; Group: Prader-Willi syndrome participants, controls). Post hoc t-tests were employed to further investigate trends in the dataset. A 4×2×2 repeated measures analysis of variance model was employed for picture ratings, with picture-type and phase corresponding to within-subject factors and group corresponding to a between-subject factor. Repeated measures ANOVA were followed by post-hoc t-tests with assumed unequal variance as per Levene’s test for equality of variances.15
Results
The primary a priori hypothesis to be tested in our study held that individuals with PWS would demonstrate a disproportionately large positive emotional response to food stimuli (in both pre- and post-meal conditions) as indexed by suppressed acoustic startle eyeblink responses elicited during visual processing of food images. Exploratory post-hoc analyses were used to characterize overall emotional processing relative to neutral, negative, and positive cues in this syndrome population relative to controls who are expected to have “normal” emotional responses(negative>neutral> positive)9 detailed by startle response findings. Of the 21 participants initially recruited for investigation, 13 individuals responded to acoustic stimulation with sufficient EMG facial muscle amplitude recordings for analysis. Eight subjects [four with PWS (two male, two female) and four controls (three male, one female)] were not considered for further analysis due to insufficient startle response or non-responder status. One additional participant from the PWS group and one control subject were excluded due to abnormally high startle responses which consistently registered between two and three standard deviations above the mean.
Picture Type Ratings
Self-reported ratings of image valence were generally consistent with presupposed expectations of the control and experimental PWS subjects. Repeated measures ANOVA modeling identified a main effect of picture type (F=52.6, df=3, p<0.001), a main effect of phase (pre- to post-meal) (F=7.5, df=1, p=0.01) as well as a picture type by phase interaction (F=3.7, df=3, p=0.03), but overall no main effect of group, group by phase, nor picture type by group interaction was found (Figure 2). Significant group differences were found in key subsets of the data, specifically food rating and “urge to eat.” An independent samples t-test, assuming unequal variances showed that the food ratings for individuals with PWS [mean= 8.8(1.7)] were significantly higher than controls [mean= 6.5(1.3)] before eating (t=3.4, df=18, p=0.003) and remained significantly higher in the post-meal phase [mean=7.1 (3.1) PWS, mean=5.2 (0.8) controls, t=2.1, df=14, p=0.05, see Table 2 and Figure 2]. Self-reported ratings of “urge to eat” were also significantly higher in PWS than controls before (mean=8.3 (2.6) PWS, mean=5.8 (1.9) control, t=2.5, df=18, p=0.02) and after (mean=7.2(3.2) PWS, mean=2.9(1.3) control, t=4.3, df=17, p=0.001) the meal (see Table 2 and Figure 2). The change in the self-reported “urge to eat” from pre- to post-meal was greater among the control group compared to the PWS group. Globally, excluding group comparisons, there was a significant change in the urge to eat among all participants from pre- to post-meal (t=3.5, df=20, p=0.002).
Figure 2.
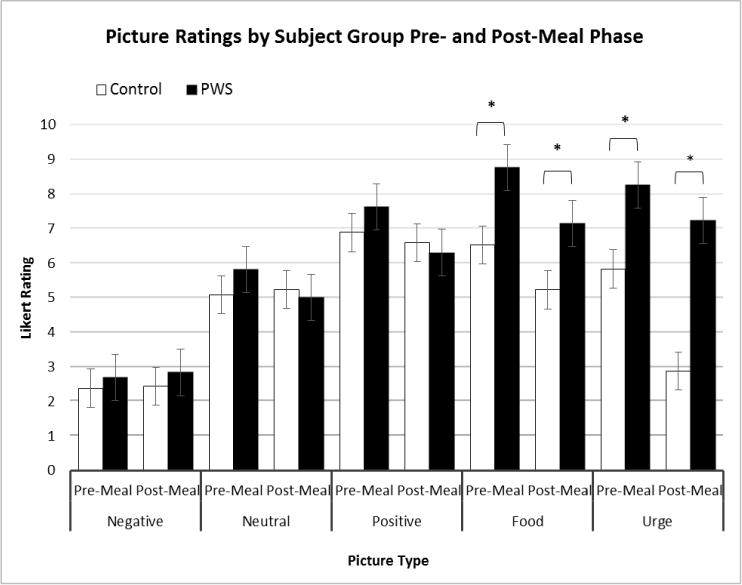
Subjective self-reported picture rating subdivided by subject group (PWS and control), phase (pre- and post-meal intervals), and picture type (positive, neutral, and negative valence, food images, and urge to eat). Group differences were compared using two-tailed independent samples t-tests.* p<0.05.
Table 2.
Picture Ratings for Prader-Willi Syndrome and Control Groups
| Picture Type | Phase | Control Mean (SD) | PWS Mean (SD) | t (2-tailed) | df | p value |
|---|---|---|---|---|---|---|
| Negative | Pre-Meal | 2.39 (0.87) | 2.70 (1.81) | 0.5 | 18.3 | 0.61 |
| Post-Meal | 2.44 (0.66) | 2.84 (2.50) | 0.6 | 14.6 | 0.59 | |
| Neutral | Pre-Meal | 5.08 (0.15) | 5.82 (2.47) | 1.1 | 12.2 | 0.30 |
| Post-Meal | 5.24 (0.49) | 5.02 (2.27) | −0.4 | 13.8 | 0.73 | |
| Positive | Pre-Meal | 6.89 (0.96) | 7.64 (1.72) | 1.3 | 18.9 | 0.22 |
| Post-Meal | 6.60 (0.61) | 6.31 (2.27) | −0.4 | 14.6 | 0.67 | |
| Food | Pre-Meal | 6.53 (1.27) | 8.77 (1.71) | 3.4 | 18.1 | 0.003* |
| Post-Meal | 5.23 (0.80) | 7.15 (3.10) | 2.1 | 14.5 | 0.05* | |
| Urge | Pre-Meal | 5.84 (1.89) | 8.27 (2.57) | 2.5 | 18.2 | 0.02* |
| Post-Meal | 2.89 (1.31) | 7.24 (3.23) | 4.3 | 17.2 | 0.001* |
Summary of self-reported responses based upon a Likert (1-10) rating scale. Mean (SD) ratings for picture type (positive, neutral, and negative valence, and food images) and urge to eat are presented by phase (pre- and post-meal) and group (PWS and control).
Significant group differences compared using two-tailed independent samples t-tests.
Startle Response Trends
PWS participants were able to elicit measurable acoustic startle reflex as assessed through eyeblink response of the orbicularis oculi muscle to binaural white noise. PWS individuals exhibited lower overall baseline startle response for all experimental conditions relative to the control group shown in Figure 3. A repeated measures ANOVA model of the average EMG startle response by condition revealed a main effect of subject group (F=5.6, df=1, p=0.04). A main effect of picture type (F=5.2, df=3, p=0.03) was found indicating differences in image valence and a main effect of meal phase (F=12.1, df=1, p=0.006) with both subject groups showing suppressed responding from pre- to post-meal phase - a characteristic of habituation to the acoustic stimulus and imagery. A picture type by group interaction (F=7.5, df=3, p=0.01) and a picture type by phase interaction (F=3.9, df=3, p=0.05) was observed as well as a trend in the group by phase interaction term (F= 3.5, df=3, p=0.09). Both the PWS and the control groups exhibited attenuated acoustic startle response when exposed to food relative to neutral and negative images. Whereas the control group adhered to the classic startle response paradigm of potentiated startle in response to negative images and attenuated startle in response to positive images, the PWS group exhibited potentiation in startle in response to both positive and negative images. Lead interval between exposure to the affective image and onset of the acoustic stimulus (2500 ms and 6000 ms) did not appear to have a significant effect on responding (p=0.11), therefore the two lead interval groups were merged.
Figure 3.
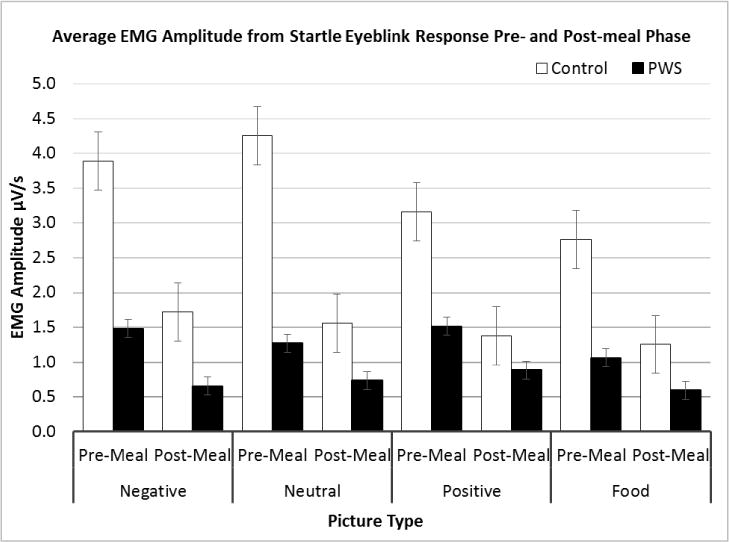
Average EMG amplitude from startle response subdivided according to subject group (PWS and control), phase (pre- and post-meal interval), and picture type (positive, neutral, and negative valence, food images, and urge to eat). A repeated measures ANOVA found a significant main effect of group (F=5.6, df=1, p=0.04), a main effect of picture type (F=5.2, df=3, p=0.03), a main effect of meal phase (F=12.1, df=1, p=0.006), a picture type by group interaction (F=7.5, df=3, p=0.01), as well as a picture type by phase interaction (F=3.9, df=3, p=0.05).
Discussion
Our study provided an exploratory characterization of emotional processing and modulation of acoustic startle eyeblink responses to visual images in PWS. Acoustic startle eyeblink responses to positive, negative, neutral and food-related stimuli were assessed and correlated with self-reported ratings of their emotional valence. Self-reported ratings of image valence were generally consistent with presupposed expectations of the control and PWS subjects with positive valence rated high and images with a negative valence consistently rated lower regardless of pre- or post-meal phase. As anticipated, PWS participants reported higher favorability ratings of food images relative to their control counterparts during both pre- and post-meal phases of the experiment and higher than corresponding “positive” valence images. Further, PWS post-meal food ratings remained high where control individuals were significantly attenuated; consistent with the knowledge base regarding food related activity in this rare obesity-related condition.
Overall startle responses were attenuated in PWS relative to control participants under all experimental conditions. Participants with PWS showed suppression of acoustic startle response when exposed to food images which is characteristic of modulation by images with positive emotional valance. However, emotional modulation of PWS startle responses to non-food images appeared abnormal, yielding potentiated responses to both positive and negative stimuli in contrast to self-reported picture ratings which showed the expected response with regard to the emotional valance of positive relative to negative images. The observed abnormalities in emotional modulation of startle responses in PWS may reflect our small sample size or heterogeneity within the PWS population related to underlying genetic subtype or level of cognitive function of the sample although the PWS subjects had comparable levels of cognition. However, these findings could also reflect abnormal emotional processing in individuals with PWS.
A recent investigation by Whittington and Holland [2011]16 investigated the ability of individuals with PWS to discriminate facial expressions to discern emotional states of others in a study of social cognition. That study demonstrated that those with PWS were only able to correctly identify roughly half (~55%) of the emotional stimuli. The present results conceptually build upon these findings demonstrating abnormal emotional processing using startle modulation. In line with this view, Key and Dykens [2008]17 demonstrated that individuals with PWS (depending on genetic subtype) appear to have differential motivation for food vs non-food stimuli per their analysis of event-related potentials (ERPs). Collectively, these results suggest that individuals with PWS demonstrate differential responding to emotional, motivational, and behavioral responding to external stimuli at various points of information-processing.
Emotional modulation of startle responses is regulated by the neurologic activity of the amygdala which may be blunted in individuals with intellectual disability18,19 and could be a contributing factor in the levels and profile of baseline startle responses observed in our participants with PWS. There is a paucity of data regarding the impact of cognitive function on startle response profiles of individuals with intellectual disabilities including PWS. A similar potentiation of both negative and positive imagery, relative to neutral images in startle response despite correct assessment of emotional valence has been reported previously in individuals diagnosed with autism spectrum disorder (ASD),20 although our PWS participants were not diagnosed with autism. However, our findings may reflect similarities in attentional attributes or underlying emotional processing of affective stimuli in PWS and ASD.
The strength of ocular muscle contractions as well as skin conductivity may have been impacted by reduced muscle tone, a characteristic of PWS, along with increased fat mass reducing sensitivity of EMG recordings and/or applicability of the startle response model in the PWS population. In addition, autonomic dysfunction with diminished parasympathetic nervous system activity has been reported in PWS21 including impaired postprandial nervous system response to meals22 that may play a role in emotional processing and food drive in PWS. Impaired growth, innervation and function of the sympathetic nervous system have also been reported in animal models of PWS.23 The startle response methodology in PWS may require adjustments such as increased acoustic stimulus intensity in order to maximize eyeblink contractions. Replicative studies are needed to fully characterize the impact of the level of cognitive function, attention, autonomic dysfunction and related co-variates such as sex and genetic subtype on startle responses in PWS and whether data interpretations can equate to fMRI observations in this rare obesity-related genetic disorder. Our findings support the feasibility of eyeblink startle modulation to assess food motivation in PWS and provide preliminary data to optimize methodological parameters.
CONCLUSION
Subjective ratings of food images and urge to eat were significantly higher in PWS than controls and did not significantly decline post-meal but startle responses to food images in PWS were attenuated relative to other picture types. This may imply that emotional processing measured using startle modulation in response to non-food images was abnormal in PWS and may reflect unique physiological attributes such as hypotonia and abnormal skin conductivity due to increased fat mass or altered disruption of autonomic or sympathetic nervous system functioning previously reported in PWS thereby impacting hunger and/or food drive states. Our findings parallel attentional/processing attributes of affective stimuli reported in autism spectrum disorder.
Acknowledgments
We thank the study participants and acknowledge financial support from the Prader-Willi Syndrome Association (USA), The Headley Family Scholarship, Friends of Kyleigh Ellington and the National Institute of Child Health and Human Development (NICHD) grant HD02528.
References
- 1.Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990;35:319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91(2):398–402. [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MG, Lee PDK, Whitman BY. Management of Prader-Willi Syndrome. 3rd. New York: Springer; 2006. [Google Scholar]
- 4.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 5.Butler MG. Single gene and syndromic causes of obesity: Illustrative examples. Prog Mol Biol Transl Sci. 2016;140:1–45. doi: 10.1016/bs.pmbts.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The continuing epidemic of obesity in the United States. JAMA. 2000;284:1650–1651. doi: 10.1001/jama.284.13.1650. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity. 2006;14(6):1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrana SR, Spense EL, Lang PJ. The startle probe response: A new measure of emotion? J Abnorm Psychol. 1988;97(4):487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- 10.Filion DL, Dawson ME, Schell AM. The psychological significance of human eyeblink modification: a review. Biol Psychol. 1998;47(1):1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- 11.Miller MW, Patrick CJ, Levenston GK. Affective imagery and the startle response: probing mechanisms of modulation during pleasant scenes, personal experiences, and discrete negative emotions. Psychophysiology. 2002;39(4):519–529. doi: 10.1017/s0048577202394095. [DOI] [PubMed] [Google Scholar]
- 12.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- 13.Hawk LW, Baschnagel JS, Ashare RL, Epstein LH. Craving and startle modification during in vivo exposure to food cues. Appetite. 2004;43(3):285–294. doi: 10.1016/j.appet.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Blumenthal TD, Elden A, Flaten MA. A comparison of several methods used to quantify prepulse inhibition of eyeblink responding. Psychophysiology. 2004;41(2):326–332. doi: 10.1111/j.1469-8986.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- 15.Levene H. Robust tests for equality of variances. In: Ingram Olkin, Harold Hotelling, et al., editors. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Stanford University Press; 1960. pp. 278–292. [Google Scholar]
- 16.Whittington J, Holland T. Recognition of emotion in facial expression by people with Prader-Willi syndrome. J Intellect Disabil Res. 2011;55(1):75–84. doi: 10.1111/j.1365-2788.2010.01348.x. [DOI] [PubMed] [Google Scholar]
- 17.Key AP, Dykens EM. ‘Hungry Eyes’: visual processing of food images in adults with Prader-Willi syndrome. J Intellect Disabil Res. 2008;52(Pt 6):536–46. doi: 10.1111/j.1365-2788.2008.01062.x. 2008. [DOI] [PubMed] [Google Scholar]
- 18.Ballinger EC, Cordeiro L, Chavez AD, Hagerman RJ, Hessl D. Emotion potentiated startle in fragile X syndrome. J Autism Dev Disord. 2014;44(10):2536–2546. doi: 10.1007/s10803-014-2125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borji R, Zghal F, Zarrouk N, Sahli S, Rebai H. Individuals with intellectual disability have lower voluntary muscle activation level. Res Dev Disabil. 2014;35(12):3574–3581. doi: 10.1016/j.ridd.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 20.Wilbarger JL, McIntosh DN, Winkielman P. Startle modulation in autism: positive affective stimuli enhance startle response. Neuropsychologia. 2009;47(5):1323–1331. doi: 10.1016/j.neuropsychologia.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 21.DiMario FJ, Jr, Dunham B, Burleson JA, Moskovitz J, Cassidy SB. An evaluation of autonomic nervous system function in patients with Prader-Willi syndrome. Pediatrics. 1994;93(1):76–81. [PubMed] [Google Scholar]
- 22.Purtell L, Jenkins A, Viardot A, Herzog H, Sainsbury A, Smith A, Loughnan G, Steinbeck K, Campbell LV, Sze L. Postprandial cardiac autonomic function in Prader-Willi syndrome. Clin Endocrinol (Oxf) 2013;79(1):128–33. doi: 10.1111/cen.12084. [DOI] [PubMed] [Google Scholar]
- 23.Tennese AA, Gee CB, Wevrick R. Loss of the Prader-Willi syndrome protein necdin causes defective migration, axonal outgrowth, and survival of embryonic sympathetic neurons. Dev Dyn. 2008;237(7):1935–43. doi: 10.1002/dvdy.21615. [DOI] [PubMed] [Google Scholar]


