Abstract
Oxidative phosphorylation not only generates cellular energy via ATP synthesis, but also controls the intracellular oxygen level to minimize oxygen toxicity resulting from reactive oxygen species (ROS). These species include superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH). While the rate of mitochondrial respiration determines the intracellular oxygen concentration, the relationship between oxygen concentration and ROS generation is not fully understood. We hypothesized that mitochondrial respiration controls intracellular oxygen concentration which in turn regulates ROS generation. To test this hypothesis, we used two prostate cancer cell lines; PC-3 cells, which have low mitochondrial genome (mtDNA) content and low mitochondrial respiratory activity, and LNCaP cells, which have high mtDNA content and high mitochondrial respiratory activity. PC-3 cells exhibited high mitochondrial oxygen concentration and generated more O2− as well as •OH when compared to LNCaP cells which showed low mitochondrial oxygen concentration and reduced levels of O2− and •OH. Exogenous hypoxic conditions (0.2% O2) reduced mitochondrial oxygen concentration and the levels of ROS, whereas exogenous hyperoxic conditions (40% O2) increased mitochondrial oxygen concentration and increased the levels of ROS. These results support the hypothesis that mitochondrial respiration regulates the intracellular oxygen concentration and in turn the generation of ROS.
Keywords: Mitochondria, Intracellular oxygen, Reactive oxygen species, Prostate cancer
Introduction
As atmospheric oxygen level increased, some organisms evolved to survive from oxygen toxicity by acquiring defense mechanisms. One mechanism is the use of detoxifying enzymes, such as catalases and superoxide dismutases [1,2]. Additionally, a recent study reported that Desulfovibrio gigas, a strict anaerobe, contains a metabolic pathway using rubredoxin:oxygen oxidoreductase (ROO) which enables the bacteria to survive transient contact with oxygen [3]. ROO reduces oxygen to water directly in order to lower oxygen toxicity [3]. Oxygen toxicity is associated with the production of reactive oxygen species (ROS) such as superoxide and hydroxyl radical [4,5]. ROS have important roles in normal cell signaling and homeostasis; however, ROS are also known to be cytotoxic and implicated in many human diseases, including cancer [6]. The cytotoxicity of ROS is due to their reactivity with key cellular biomolecules including enzymes, membrane lipids, and nucleic acids. Superoxide (O2−) is generated when an oxygen molecule is reduced with a free electron. Studies in reperfusion systems have shown that reduction of mitochondrial respiratory chain by NADH increases superoxide generation via the accumulation of free electrons [7]. Previous studies from our laboratory showed that mitochondrial respiration dictated intracellular oxygen concentration and downstream events, such as membrane localization and activation of Ras/MAPK signaling pathway via regulating the mevalonate pathway in prostate cancer system [8–10]. However, the relationship between intracellular oxygen concentration/consumption and ROS generation are poorly understood. In this study we used validated probes [11,12] to measure mitochondrial oxygen concentration and ROS to test the hypothesis that intracellular oxygen concentration, determined by mitochondrial respiration, regulates ROS generation.
Materials and Methods
Materials
MitoSOX [3,8-phenanthridinediamine, 5-(6′-triphenylphosphoniumhexyl)-5,6 dihydro-6-phenyl] and HPF [ 3’-(p-hydroxyphenyl) fluorescein] were purchased from Molecular Probes (Invitrogen, Carlsbad, CA). Mitochondrial-specific BTP (mitoBTP) [acetylacetonatobis [2-(2’-benzothienyl)pyridinato-kN,kC3’]iridium(III)] was kindly provided by Dr. Zhang. Rotenone and manganese (III) tetrakis (1-methyl-4-pyridyl) (MnTMPyP) were obtained from Sigma Chemical Co. (St. Louis, MO).
Cell culture and transfection
The PC-3 prostate cancer cell line was purchased from American Type Culture Collection and the LNCaP prostate cancer cell line was purchased from UROCOR (Oklahoma City, OK). Cell lines were cultured in RPMI media plus 5% fetal calf serum (FCS, Life Technologies) and maintained at 37°C under atmospheric oxygen conditions with 5% CO2 unless noted otherwise.
Live cell confocal microscopy
Cells were maintained in a stage-mounted atmospheric box (Pathology Devices) at 37°C, 5% CO2, and 75% humidity during the course of the experiments. All microscopic samples were analyzed on an Olympus Fluoview FV1000 laser confocal microscope. Images from all microscopy experiments were processed using the FV10-ASW 3.1 Viewer (Olympus).
Measurement of mitochondrial oxygen concentration
For detection of mitochondrial oxygen concentration, mitoBTP (515 nm excitation/620 nm emission) was utilized in the indicated experiments at a concentration of 500 nM in RPMI medium. BTP is an iridium complex that exhibits phosphorescence in low-oxygen conditions [11]. This phosphorescence is readily quenched by molecular oxygen in a cell. mitoBTP, described in detail by Murase et al. [12], shows the specific localization to the mitochondria and the extent of quenching is dependent upon mitochondrial oxygen concentration. Samples were incubated in the presence of mitoBTP for 1 h before imaging. In all experiments, cells were plated and grown overnight at a cell density of 105 cells in glass-bottom 35 mm dishes (Mattek). For hypoxic experiments, cells were incubated in the atmosphere box for 6 h at 0.2% O2/5% CO2 following addition of mitoBTP. For hyperoxic experiments, cells were incubated in the atmosphere box for 6 h at 40% O2/5% CO2 following addition of BTP. For normoxic conditions cells were incubated in the presence of BTP in a normal cell culture incubator for 1 h. Phosphorescent values of each cell were computed by measuring phosphorescence of a cell divided by an area of a whole cell using FV10-ASW 3.1 Viewer. Each sample has an average value of 10 cells and the indicated error bars were standard error of each sample. Student’s t-test was utilized to compare group means. Means with p-value below 0.05 were considered statistically different.
Detection of ROS
For detection of superoxide, 105 cells were plated and grown overnight in a glass-bottom 35mm dish (Mattek). Two fluorescent probes, MitoSox and HPF, are used according to the manufacturer’s protocol. For detection of superoxide in the mitochondria, MitoSox was added at a final concentration of 1µM and after 30 min cells were subjected to confocal microscopy. For detection of hydroxyl radical, the cells were prepared in the same way but incubated with 1µM of HPF for 30 min and subjected to confocal microscopy. Phosphorescent values of each cell were computed by measuring phosphorescence of a cell divided by an area of a whole cell using FV10-ASW 3.1 Viewer. Each sample has an average value of 10 cells and the indicated error bars were standard error of each sample. Student’s t-test was utilized to compare group means. Means with p-value below 0.05 were considered statistically different.
Results
Mitochondrial respiratory function regulates oxygen concentration
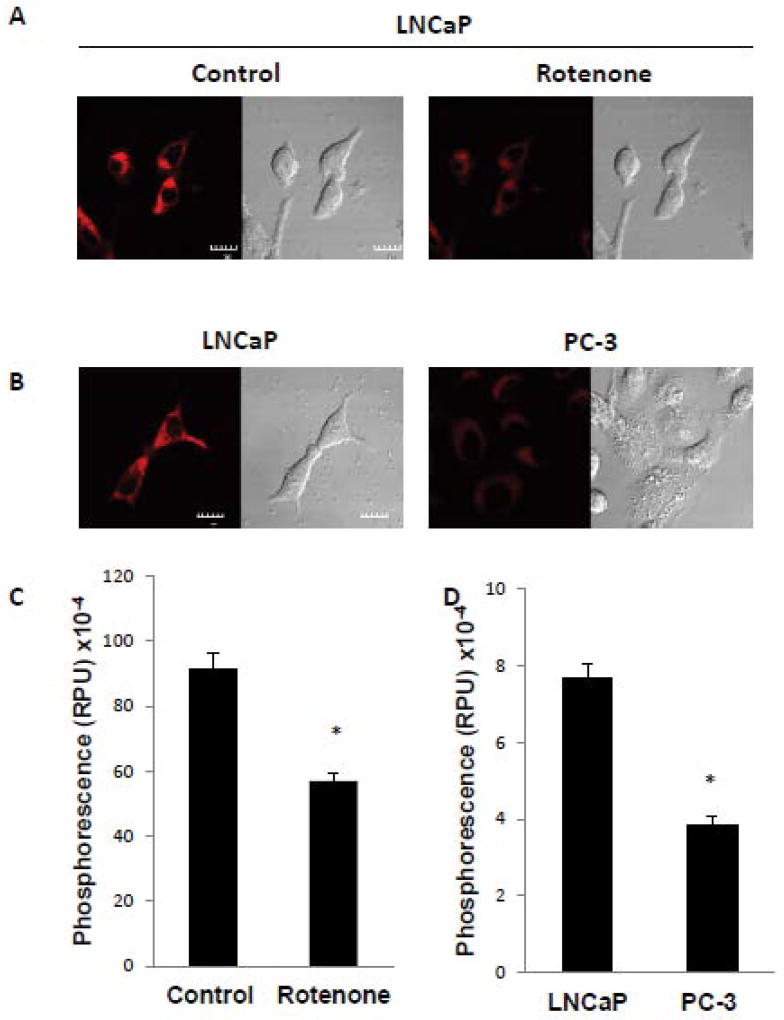
Our previous studies have indicated that PC-3 cells, a poorly-differentiated prostate cancer cell line, have low mtDNA content and reduced mitochondrial respiration resulting in high intracellular oxygen concentration [8]. Conversely, LNCaP cells, a well-differentiated prostate cancer cell line, have high mtDNA content and very low intracellular oxygen concentration (intracellular hypoxia) [8]. In order to confirm that mitochondria regulate oxygen concentration, the direct measurement of mitochondrial oxygen concentration was performed using mitochondrial BTP (mitoBTP). Previously, BTP was used to detect intracellular oxygen concentration since BTP localized in the endoplasmic reticulum (ER) [11]. MitoBTP is, however, localized in the mitochondria [12], allowing the direct measurement of mitochondrial oxygen concentration. In order to test the effectiveness of mitoBTP, we used rotenone, a mitochondrial respiratory inhibitor which blocks complex I to reduce oxygen consumption. LNCaP cells were incubated in the absence or presence of rotenone and then subjected to confocal microscopy to detect mitochondrial oxygen concentration using mitoBTP. Rotenone greatly reduced the red phosphorescence of mitoBTP compared to the control, indicating that the oxygen level in mitochondria was increased due to the inhibition of mitochondrial respiration (Figure 1A and 1C). We next used mitoBTP to compare mitochondrial oxygen levels in LNCaP cells to that in PC-3 cells. LNCaP cells had significantly higher levels of mitoBP phosphorescence than PC-3 cells (Figure 1B and D), showing that oxygen concentration in mitochondria was much higher in PC-3 cells than in LNCaP cells, and a trend similar to that of intracellular oxygen. These results validate the use of mitoBTP for the measurement of mitochondrial oxygen concentration.
Figure 1. Mitochondrial respiratory function regulates mitochondrial oxygen concentration.
(A) LNCaP cells were incubated in the absence or presence of 2 µM rotenone for 6 h and mitochondrial oxygen level monitored by mitoBTP, as described in Materials and Methods. Representative cells are shown with the red signal indicating mitoBTP phosphorescence and differential interference contrast microscopy showing corresponding whole cell morphology. The scale bar indicates 10 µm. (B) Measurement of mitochondrial oxygen level by mitoBTP in LNCaP and PC-3 cells. Other details as in panel A. (C, D) Quantitation of mitoBTP phosphorescence from panel A and panel B, respectively. Data were derived from the examination of 10 cells/condition and are shown as mean ± S.D. * p<0.001.
Mitochondrial respiration determines generation of superoxide
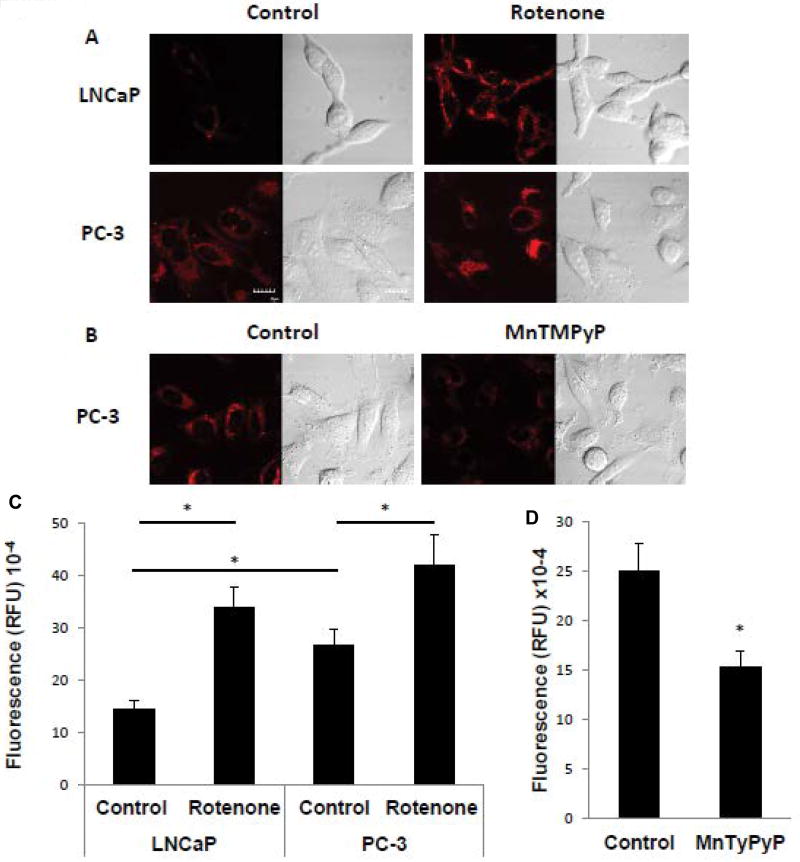
To investigate whether mitochondrial oxygen concentration plays a role in the generation of ROS, we measured superoxide content in the cell lines using MitoSox. Cells were incubated in the absence and presence of rotenone and it was found that rotenone treatment increased superoxide levels in both LNCaP and PC-3 cells (Figure 2A and C). In addition, basal superoxide levels were higher in PC-3 cells versus LNCaP cells (Figure 2A and C). These results are consistent with the notion that higher levels of intracellular and mitochondrial oxygen, whether in response to rotenone treatment or intrinsic to the cell line, result in higher superoxide levels. Importantly, the high superoxide level in PC-3 cells was decreased by an antioxidant, MnTMPyP (manganese superoxide dismutase (MnSOD) mimetic), validating the use of MitoSox as a probe for measuring superoxide (Figure 2B and D). The results suggest that the generation of superoxide parallels the degree of mitochondrial respiratory function.
Figure 2. Mitochondrial respiratory function regulates the generation of superoxide.
Superoxide levels were determined using MitoSox, as described in Materials and Methods. The red signal represents MitoSox fluorescence and the corresponding image of whole cells is shown by DIC microscopy. (A) LNCaP or PC-3 cells in the absence or presence of 2 µM of rotenone added for 6 h. The scale bar indicates 10 µm. (B) PC-3 cells in the absence or presence of 80 µM MnTMPyP added for 1 h. (C, D) Quantitation of MitoSox fluorescence from panel A and panel B, respectively. Data were derived from the examination of 10 cells/condition and are shown as mean ± S.D. * p<0.001.
Oxygen concentration plays a crucial role in generation of superoxide
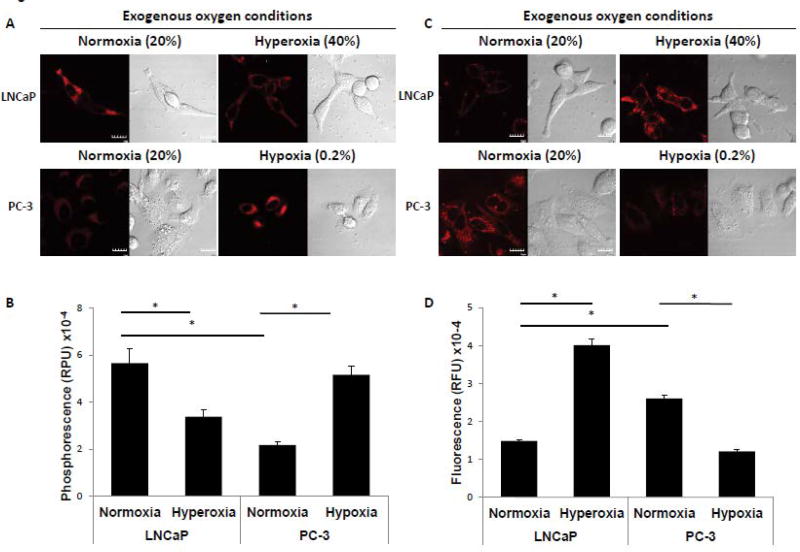
To investigate whether the generation of ROS was dependent on exogenous oxygen concentration, the cells were incubated under various oxygen conditions. MitoBTP was again used to monitor the mitochondrial oxygen level. LNCaP cells were incubated under hyperoxic conditions (40% O2/5% CO2) for 6 h in the presence of mitoBTP and examined by confocal microscopy. MitoBTP phosphorescence was significantly decreased under hyperoxic conditions, indicating an increase in mitochondrial oxygen concentration (Figure 3A and B). Next, PC-3 cells were incubated under hypoxic conditions (0.2% O2/5% CO2) for 6 h in the presence of mitoBTP and examined by confocal microscopy. As shown in Figure 3A, phosphorescence was greatly increased compared to normoxic conditions, reflecting a decrease in mitochondrial oxygen concentration. These results demonstrated that changes in external oxygen conditions yield parallel changes in mitochondrial oxygen concentration. Next, we measured the levels of superoxide under similar conditions. The generation of superoxide in LNCaP cells was greatly increased in response to exogenous hyperoxic conditions, whereas superoxide was significantly decreased under hypoxic conditions in PC-3 cells (Figure 3C and D). The results suggest that the mitochondrial oxygen concentration determines the generation of superoxide in the mitochondria
Figure 3. Exogenous oxygen conditions regulate mitochondrial oxygen concentration, which in turn regulates generation of superoxide.
(A) Detection of mitochondrial oxygen level by mitoBTP in LNCaP and PC-3 cells under various oxygen conditions for 6 h. LNCaP cells were incubated in exogenous normoxic (20% O2) or hyperoxic (40% O2) conditions. PC-3 cells were incubated in exogenous normoxic (20% O2) or hypoxic (0.2% O2) conditions. Representative cells are shown with the red signal indicating mitoBTP phosphorescence and differential interference contrast microscopy showing corresponding whole cell morphology. The scale bar indicates 10 µm. (B) Quantitation of mitoBTP phosphorescence from panel A. Data were derived from the examination of 10 cells/condition and are shown as mean ± S.D. * p<0.001. (C) Detection of superoxide level by MitoSox in LNCaP and PC-3 cells under various oxygen conditions for 6 h. LNCaP cells were incubated under exogenous normoxic (20% O2) or hyperoxic (40% O2) conditions. PC-3 cells were incubated under exogenous normoxic (20% O2) or hypoxic (0.2% O2) conditions. The red signal represents MitoSox fluorescence and the corresponding image of whole cells is shown by DIC microscopy. Scale bar indicates 10 µm. (D) Quantitation of MitoSox fluorescence from panel C. Data were derived from the examination of 10 cells/condition and are shown as mean ± S.D. * p<0.001.
Mitochondrial respiration regulates the generation of hydroxyl radical
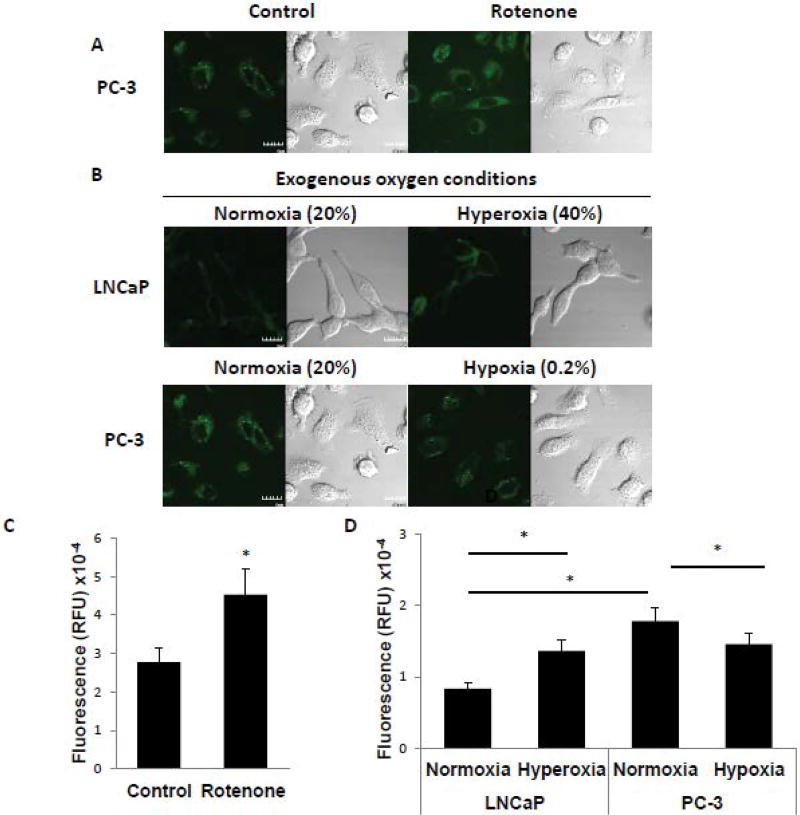
Superoxide is converted to hydroxyl radical via the Fenton reaction [13]. Hydroxyl radical is known to be more reactive than superoxide, and implicated in many human diseases [4]. Parallel studies as those described above were conducted to assess the level of hydroxyl radical using HPF, a hydroxyl radical sensing dye. Rotenone treatment induced the generation of hydroxyl radical in PC-3 cells (Figure 4A and C), and the level of hydroxyl radical was significantly higher in PC-3 cells than in LNCaP cells (Figure 4B and D). Exogenous hyperoxic conditions increased the level of hydroxyl radical in LNCaP cells (Figure 4B and D) and conversely exogenous hypoxic conditions decreased the level of hydroxyl radical in PC-3 cells (Figure 4B and D).
Figure 4. Mitochondrial oxygen concentration regulates generation of hydroxyl radical.
(A) Measurement of hydroxyl radical level by HPF in PC-3 cells in the absence or presence of 2 µM of rotenone added for 6 h. The green signal indicates HPF fluorescence and the corresponding image of whole cells is shown by DIC microscopy. Scale bar indicates 10µm. (B) Detection of hydroxyl radical level by HPF in LNCaP and PC-3 cells under various oxygen conditions for 6 h. LNCaP cells were incubated in exogenous normoxic (20% O2) or hyperoxic (40% O2) conditions. PC-3 cells were incubated in exogenous normoxic (20% O2) or hypoxic (0.2% O2) conditions. The green signal indicates HPF fluorescence and the corresponding image of whole cells is shown by DIC microscopy. Scale bar indicates 10µm. (C, D) Quantitation of HPF fluorescence from panel A and panel B, respectively. Data were derived from the examination of 10 cells/condition and are shown as mean ± S.D. * p<0.001.
Discussion
In this study, the roles of mitochondrial respiratory function in regulation of intracellular oxygen concentration and ROS generation were investigated. The present study was greatly facilitated by the development of chemical probes for the assessment of intracellular oxygen and ROS. Zhang et al. [11] developed an oxygen-sensing phosphorescent iridium complex, known as BTP. The BTP phosphorescence is reduced by oxygen molecules, which enables qualitative detection of intracellular hypoxia. BTP phosphorescence is inversely related to intracellular oxygen concentration. Rotenone treatment and the depletion of mtDNA in LNCaP cells decreased BTP phosphorescence as oxygen concentration increased [8,10], indicating that BTP was sufficient to measure the intracellular oxygen level. Maruse et al. [12] designed mitochondrial-selective BTP, known as mitoBTP which localized specifically in the mitochondria. In order to investigate whether mitochondrial respiration regulates the generation of ROS, mitoBTP was utilized to semi-quantitatively measure mitochondrial oxygen concentration. BTP and mitoBTP were utilized to check intracellular and mitochondrial oxygen concentration during incubation of cells in exogenous hypoxic or hyperoxic conditions.
Previous studies from our laboratory have shown that more aggressive prostate cancer cell lines harbor a reduction in mtDNA content which in turn inhibited mitochondrial respiration and thus increased intracellular oxygen concentration [8,9]. The results presented in this study showed that such an increase in cellular oxygen level exhibited by cells with reduced mtDNA also resulted in an increase in ROS generation. Further, manipulating the external oxygen concentration, or inhibiting the mitochondrial respiratory chain, induced ROS generation as intracellular oxygen concentration increased. In turn, these results suggest that increased ROS generation is an important factor in development of prostate cancer cells to more aggressive forms. Our results add to a growing body of evidence implicating ROS in cancer progression. For example, ROS have been strongly implicated in the regulation of angiogenesis which is key to the growth of solid tumors [14], and in lipid peroxidation which produces signaling molecules important for cancer growth and survival [15]. Conversely, natural antioxidants have anti-tumor activity and are an important focus for cancer prevention strategies [16,17]. In this respect, the results of the present study emphasize the important role mitochondria play as antioxidants, consuming intracellular oxygen and minimizing toxicity resulting from ROS generation.
Acknowledgments
We thank Dr. Zhang for kindly providing mitoBTP. This work is dedicated to the memory of Dr. Masahiro Higuchi, senior author of this paper, who passed away unexpectedly on November 25, 2014. This work was supported by National Institutes of Health grant CA100846 from the National Cancer Institute.
Abbreviations
- ROS
reactive oxygen species
- mtDNA
Mitochondrial DNA
- DIC
Differential interference contrast
- HPF
3’-(p-hydroxyphenyl) fluorescein
- MnTMPyP
Manganese (III) tetrakis (1-methyl-4-pyridyl)porphyrin
References
- 1.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–86. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 2.Weisiger RA, Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]
- 3.Frazão C, Silva G, Gomes CM, Matias P, Coelho R, Sieker L, et al. Structure of a dioxygen reduction enzyme from Desulfovibrio gigas. Nat Struct Mol Biol. 2000;7:1041–1045. doi: 10.1038/80961. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nohl H, Gille L, Staniek K. Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol. 2005;69:719–723. doi: 10.1016/j.bcp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, et al. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. Journal of Clinical Investigation. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–4. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 8.Cook CC, Kim A, Terao S, Gotoh A, Higuchi M. Consumption of oxygen: a mitochondrial-generated progression signal of advanced cancer. Cell Death Dis. 2012;3:e258. doi: 10.1038/cddis.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prior S, Kim A, Yoshihara T, Tobita S, Takeuchi T, Higuchi M. Mitochondrial Respiratory Function Induces Endogenous Hypoxia. PLoS ONE. 2014;9:e88911. doi: 10.1371/journal.pone.0088911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim A, Davis R, Higuchi M. Intracellular oxygen determined by respiration regulates localization of Ras and prenylated proteins. Cell Death Dis. 2015;16:64. doi: 10.1038/cddis.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Hosaka M, Yoshihara T, Negishi K, Iida Y, Tobita S, et al. Phosphorescent light-emitting iridium complexes serve as a hypoxia-sensing probe for tumor imaging in living animals. Cancer Research. 2010;70:4490–4498. doi: 10.1158/0008-5472.CAN-09-3948. [DOI] [PubMed] [Google Scholar]
- 12.Murase T, Yoshihara T, Tobita S. Mitochondria-specific oxygen probe based on iridium complexes bearing triphenylphosphonium cation. Chemistry Letters. 2012;41:262–263. [Google Scholar]
- 13.Gutteridge JM. Superoxide dismutase inhibits the superoxide-driven Fenton reaction at two different levels. Implications for a wider protective role. FEBS Lett. 1985;185:19–23. doi: 10.1016/0014-5793(85)80732-8. [DOI] [PubMed] [Google Scholar]
- 14.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–30. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 15.Zhong H, Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 17.Yang CS, Hong J, Hou Z, Sang S. Green tea polyphenols: antioxidative and prooxidative effects. J Nutr. 2004;134:11. doi: 10.1093/jn/134.11.3181S. [DOI] [PubMed] [Google Scholar]