Abstract
Background:
When primary repair of transected peripheral nerves is not possible due to large gaps, nerve grafts or repair using conduits are other options to bridge the gap such that the nerve is repaired without tension. When nerve gaps are repaired primarily, there is a worry about tension, failure, and poor healing. In this biomechanical study comparing nerves repaired primarily versus those repaired with conduits, we hypothesized that conduit repair provided greater mechanical breaking strength.
Methods:
We dissected fresh cadaveric sheep hooves and transacted their peripheral nerves. Subsequently, we divided these transacted nerves into 2 groups: primary repair versus repair using a nerve conduit. After repair using a standardized technique, we tensioned each of these repairs via a load tester and recorded the force required till repair failure occurred.
Results:
Six nerves using primary nerve repair and 6 nerves repaired with a nerve conduit (10 mm length × 2.5 mm diameter) were studied. The average breaking strength of the nerves repaired with the nerve conduit was 0.92 N and that using the primary nerve repair technique was 0.46 N (P = 0.001). All the nerves repaired using nerve conduit repair had an additional 5 mm added to their total length as compared with the nerves in the other group.
Conclusions:
Nerve repair using a nerve conduit ensures a higher breaking strength and potentially a greater tension-free repair as compared with primary nerve repairs in a sheep model. This study supports the use of conduits in the bridging of nerve gaps.
INTRODUCTION
The current gold standard in primary peripheral nerve repair is primary end to end coaptation of nerves.1 The avoidance of tension during repair is of utmost importance to facilitate nerve healing.2,3 However, tension-free repair is not always feasible clinically. Even if there is no nerve loss secondary to the initial injury, due to the principle of biotensegrity,4 peripheral nerves undergo a certain degree of retraction when severed, which leaves the nerve in some tension when repaired primarily. Intraoperatively, a surgeon may need to debride and shorten the ends of a transected nerve to improve its function by diminishing intraneural scarring.5 Considering the above factors, the transected nerve would be in tension when repaired primarily with the end to end coaptation technique. The drawback is that it requires postoperative immobilization of joints with a splint to maintain a tension-free state until there is adequate healing of the nerve repair site. This may produce joint stiffness and affect postoperative rehabilitation. Immobilizing a joint may even compromise the repair if postoperative contractures occur.2
Generally, surgeons do not attempt primary end to end repair when nerve gaps are more than 4 mm.6 If the gap between the transected nerves is too large to be repaired primarily without tension, autologous nerve grafting remains the most reliable repair technique currently.7,8 However, limited availability of donor tissue, sacrifice of a functional nerve, and possible neuroma formation are some problems associated with nerve grafting.1 To overcome the problems posed by autografts, nerve allografts are being increasingly used, which have shown almost similar results in terms of safety and meaningful recovery as compared with autografts.9–11
Another technique in bridging gaps during nerve repair, which overcomes the problems posed by primary repair and repair using an autograft, is by using a nerve conduit, which aims to guide axonal regrowth between the proximal and distal ends of a nerve gap via the phenomenon of neurotrophism.12 These allow for nerve debridement and tension-free nerve coaptation without grafts. Types of conduits include polyesters [eg, poly lactic acid, poly L-lactic acid, poly glycolic acid, poly lactic-co-glycolic acid (PLGA), expanded polytetrafluoroethylene, poly 3-hydroxybutyrate-co-3-hydroxyvalerate, poly 3-hydroxybutyrate, polycaprolactone, polyvinyl alcohol, polyurethane], collagen, poly glycolic acid–collagen, autologous vein grafts, fibronectin, keratin, chitin/chitosan, hyaluronic acid, and decellularized nerve allograft. Each conduit type and material has its pros and cons when used in nerve repair.1,13–15
One such synthetic bioabsorbable material used as nerve conduits is poly (DL-lactide-ε-caprolactone) (PLC). It is commercially available by the name of Neurolac (Polyganics BV, Groningen, Netherlands). They offer conduits of 1.5–10 mm inner diameters and a length of up to 3 cm and a thin-walled version Neurolac TW. Neurolac TW has a wall thickness of 40% less than Neurolac conduits, making it easier to suture. These conduits are transparent, fully synthetic, nonimmunogenic, and retain mechanical properties up to 10 weeks. Hydrolysis and natural degradation occur over 2 years.16
PLC conduits have been found to be equivalent in results to autograft nerve repair.17 In a blinded, randomized multicenter trial comparing standard treatment to PLC conduits in repair of traumatic peripheral nerve defects of the hand in 34 patients, Bertleff et al.16 found no significant difference in sensory outcomes compared with controls in the treatment of nerve gaps less than 20 mm over a 12-month period. Shin et al.18 showed that functional motor recovery was similar in rat models whose sciatic nerves had been transected and repaired using PLC conduits and autografts. However, there is lack of information available in literature on the mechanical integrity of the PLC conduits after repair.
The aim of this study was to determine the mechanical strength of PLC conduit nerve repair as compared with the standard end to end coaptation repair in a sheep nerve model.
MATERIALS AND METHODS
Fresh frozen cadaveric sheep hooves were used in this experiment. The cutaneous nerves on the hooves were dissected and 14 segments of peripheral nerves were harvested, each no shorter than 5 cm.
We used permanent, nonwater soluble ink to mark a section of 0.5 cm at the midpoint of each of the dissected nerves (Fig. 1). An electronic micrometer (Draper 46599, Hampshire, United Kingdom) was used to measure the diameter of the marked segment. Under microscope magnification, the diameter of the nerve was measured when the tip of the micrometer touched the edge of the nerve. Three readings were taken and the average diameter for each nerve was calculated. Randomly selected 12 out of 14 of these nerves were divided exactly at the middle of the marked section with a surgical blade.
Fig. 1.
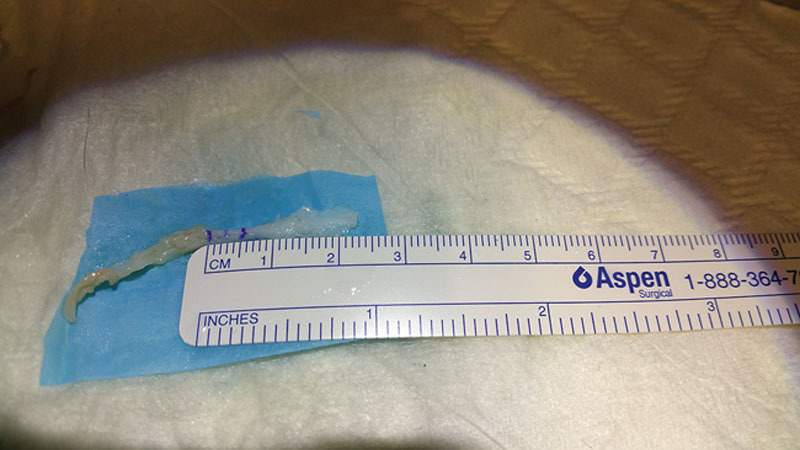
A 5 mm segment was marked, and this segment was divided in the middle such that the distance between each mark and the divided end was 2.5 mm.
Six divided nerves were repaired using primary end to end coaptation in the control group and the other 6 divided nerves were coapted using PLC conduits in the experimental group. All 12 nerves were randomly assigned to these 2 groups. The 2 remaining unsectioned nerves were used for illustration and descriptive purposes.
Using G* Power Analysis (SPSS statistical software, IBM, New York, N.Y.), two-tailed test with alpha of 0.01 and 80% power to detect meaningful difference between the maximal load or maximal displacement of 2 groups (from the experimental data), would require 3 or 1 specimen(s) per group. In our study, we had 6 specimens per group, which exceeded the requirement as mentioned above.
For standardization, all surgical coaptation in this study was conducted by the same experienced microsurgeon with the aid of loupes with 6× magnification using standard microsurgical techniques with Ethilon 8-0 round body sutures. As shown in Figure 2, 2 single throw technique was used to secure each suture and 2 sutures were used for each nerve repair in the primary repair group.
Fig. 2.
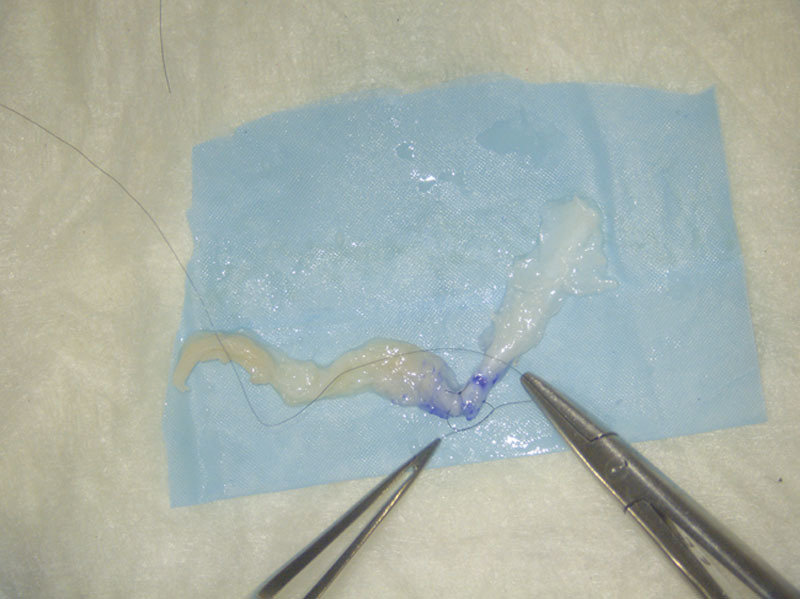
Two single throw technique was used for all repairs, and 2 such sutures were used in each nerve in the primary repair group.
A Neurolac TW (Thin Wall) nerve connector was used in all nerve conduit repairs. A 2.5 mm diameter, 10 mm length nerve conduit was used. The nerve conduit was then sutured to the proximal and distal stumps of the dissected nerve with 2 sutures on each end as shown in Figure 3. The dimensions and layout of the entire repair are shown in Figure 4. The standard 2 throw technique was used for each suture with an Ethilon 8-0 suture.
Fig. 3.
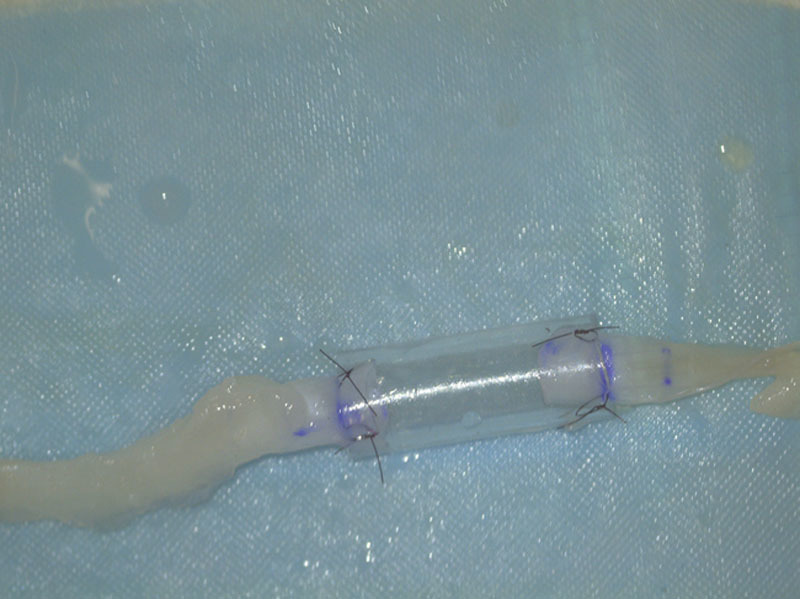
Two sutures were used to secure the dissected nerve at each end of the conduit in the conduit repair group using the same 2 single throw technique.
Fig. 4.
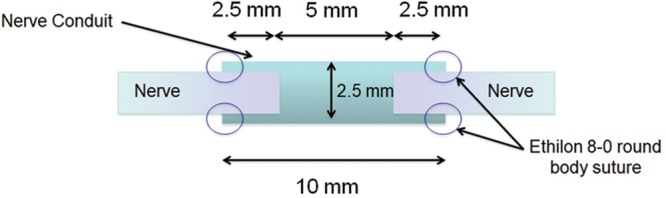
A representation of the exact dimensions and layout of the repair construct in the conduit repair group.
An E-1000 Dynamic Tester (Instron Corp., Canton, Mass.) was used in this experiment. The ends of the nerves were clamped by the grippers 1 cm away from the repair site at either end (ie, the total length from clamp to clamp in the primary repair group was 2 cm and that in the conduit repair group was 3 cm). The distance from clamp to clamp in the intact nerve group was 1 cm.
The whole set up was submerged in a saline bath at 36.9 degrees Celsius. For the biomechanical testing, the nerve was tensioned and loaded to failure at an extension rate of 1 cm/min19 and the resultant load displacement curve was obtained. A high-resolution camera was used to record the testing process and failure mechanism.
Statistical Analysis
The data were analyzed using SPSS Statistics 16.0 software (IBM, Chicago, Ill.). Descriptive statistics (means and SDs) and 1-way analysis of variance tests were used to analyze the diameters, cross-sectional area, maximal load, maximal displacement, maximal stress, maximal strain and Young’s modulus. The P value less than 0.05 was considered as a significant difference in between 2 groups.
RESULTS
Table 1 lists the averaged diameter, cross-sectional area, maximal load to failure, maximal displacement to failure, maximal stress, and maximal strain of all the 14 nerves in testing in all the 3 groups. Table 2 summarizes the mean results with SDs and the statistical analysis of the data. Data collected from the intact nerve group were not used for statistical analysis. There was no statistical significance between the average diameters and cross-sectional areas of nerves in the primary repair and conduit repair groups.
Table 1.
Descriptive Data of All the 14 Nerves in Testing in All the 3 Groups
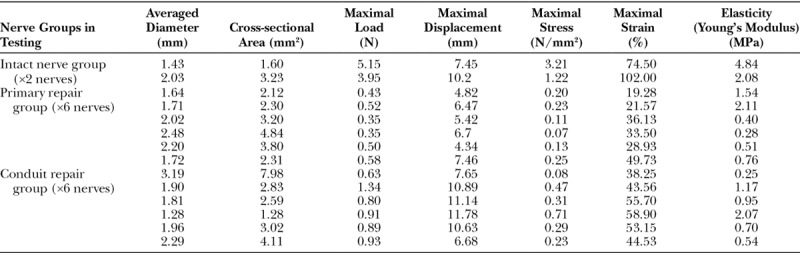
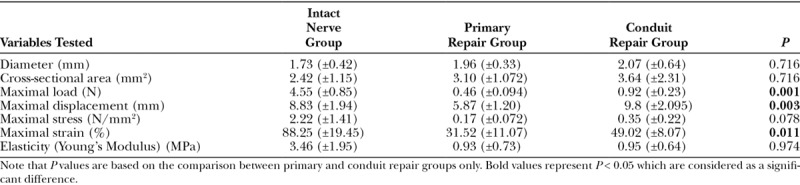
Table 2.
Experiment Mean Results
In the primary repair group, failure occurred via sutures cutting out through the nerve substance of either end of the repaired nerves. There was no breakage or unraveling of the sutures in this group.
In the conduit repair group, failure occurred via suture cut-out through the nerve substance and unraveling of the sutures (Fig. 5). There was no cut-out at the conduit-suture interface nor was there any breakage of sutures. There was also no rupture of the conduit as a cause for failure in this group. An additional 5 mm length was added in the repair construct between the 2 ends of transected nerves in the conduit repair group (Fig. 4) as compared with the primary repair group where there was end to end coaptation.
Fig. 5.
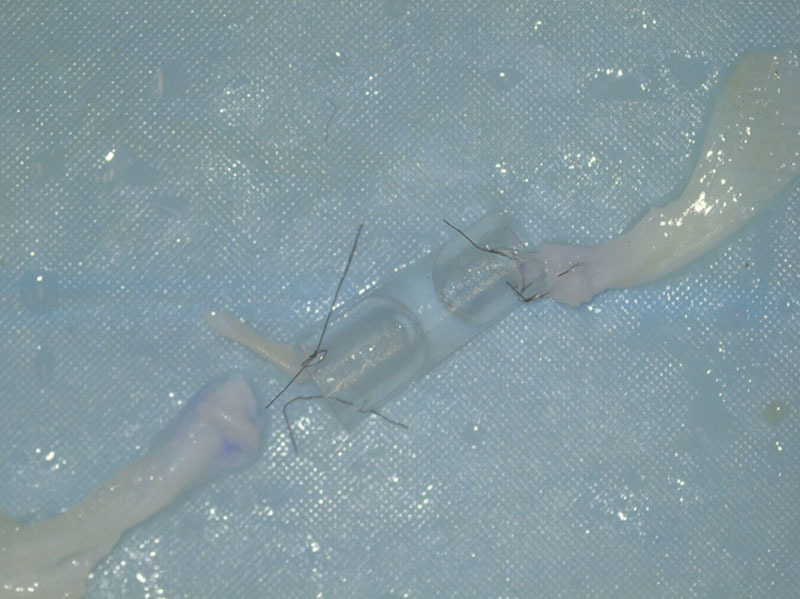
Failure in the conduit repair group occurred via cut-out through the nerve substance (top left suture) and unraveling of the sutures (bottom left suture).
Both nerves in the intact nerve group failed at the nerve clamp interface. However, there was no failure at the nerve clamp interface in both the primary and the conduit repair groups.
Figure 6 and Figure 7 show the relationship of all 3 groups plotted as Load VS Displacement and Stress VS Strain curves, respectively. Elasticity or Young’s modulus was determined by taking the gradient of the linear portion of each of the Stress VS Strain curves and is represented in Tables 1, 2.
Fig. 6.
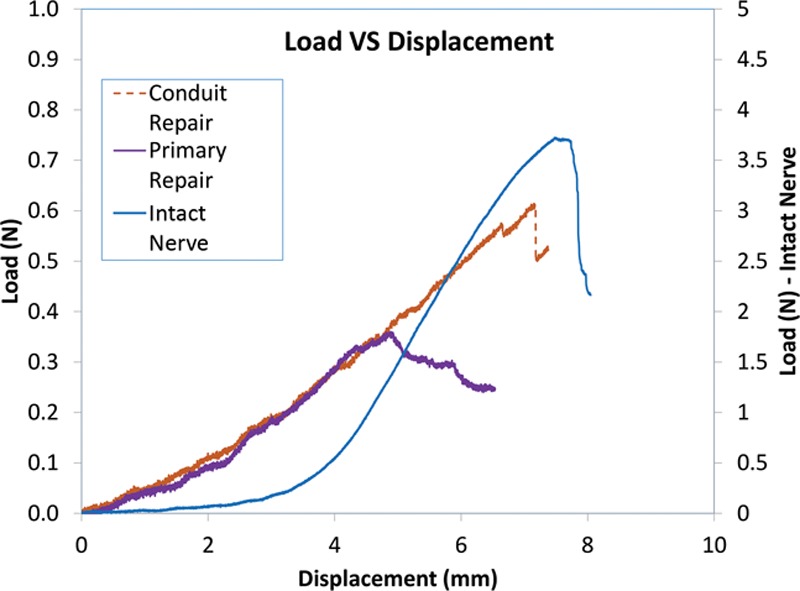
Load VS Displacement graph of the 3 groups in testing.
Fig. 7.
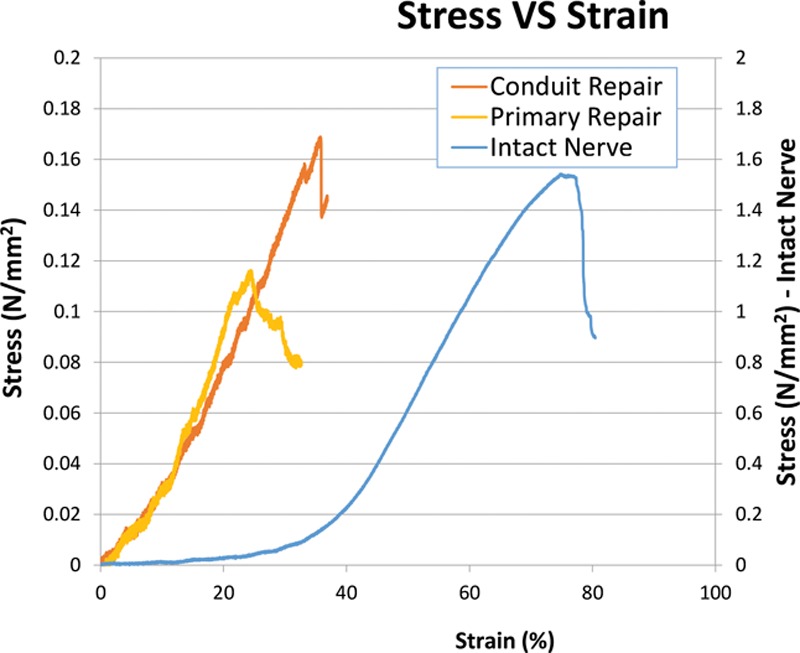
Stress VS Strain graph of the 3 groups in testing.
DISCUSSION
This study shows that there is a statistically significant increase in maximal load to failure in nerves repaired via a conduit (0.92 N) as compared with a primary end to end repair (0.46 N; P = 0.001). The nerve-conduit repair interface and the tougher PLC material of the conduit may have added to the increased breaking strength in the conduit repair group as compared with the nerve-nerve repair interface in the primary repair group. It was noted that failure never occurred due to direct cut-out at the suture-conduit interface nor breakage of the conduit itself in all the 6 repairs in the conduit group. In a study testing the tensile strength of PLGA conduits and autografts, where the number of sutures used in both repair groups were the same, Yu et al.20 found that maximal tensile load, maximum stress, elastic limit load, and elastic limit stress were increased in a fresh human cadaver sciatic nerve repair model using PLGA conduits as compared with autogenous nerve grafts. However, the author did not mention the site and size of the autogenous nerve graft harvested for the repair. Yu et al.20 concluded that PLGA conduits exhibited good intensity, elasticity and plasticity, indicating that they were fit to be used for repair in a human sciatic nerve injury.
In our study, there were also 2 sutures on either ends of the conduit (4 in total) to secure the repair as compared with only 2 sutures in the primary repair group. The additional sutures in each conduit repair may have also offered additional breaking strength in the overall repair. This hypothesis is in line with a biomechanical cadaveric study on human digital nerves by Goldberg et al.21 that showed that nerves repaired primarily with 4 sutures had an increased load to failure as compared with nerves repaired with 2 sutures. Although the cadaveric digital nerves were able to be repaired with 4 sutures in the study by Goldberg et al.,21 it may not always be possible to add strength to a repair by using more than 2 sutures in smaller nerves. Using a conduit during nerve repair naturally enables more sutures to be placed within the construct and thus may potentiate for greater strength as compared with nerves of the same caliber repaired primarily.
Goldberg et al.21 also showed that intact digital nerves failed at a much higher force as compared with repaired nerves. Similarly, in our study, it was noted that the maximal load to failure in the intact nerve group was 4.55 N, which was far above the values in both the repair groups.
Both commercially available and autologous fibrin glue mixtures are popularly used as sealant to a primary nerve repairs. Although gapping and complete failure of repairs is often quoted as a major disadvantage when fibrin glues are used in isolation,22,23 very little evidence is available on the biomechanical advantage when fibrin glues are used in combination with suturing in primary nerve repairs. In their biomechanical study, Isaacs et al.24 concluded that when primary, 2 suture nerve repairs were augmented with commercially available nerve sealants (Tisseel, Evicel and DuraSeal), initial gapping was prevented as compared with nerve repair with suturing alone. However, they showed that none of the sealants increased the resistance to suture cut-outs nor did they increase the ultimate load to complete failure of the repair. More evidence and a direct comparison are, however, needed to evaluate if there are any differences in the cut out strength when fibrin glues are used in primary nerve repairs and in repairs with a conduit.
Nerves in the conduit repair group had an additional 5 mm added to their total length as compared with the nerves repaired using primary repair (Fig. 4). More evidence is required if this additional length contributes to potentially less tension during nerve repair and during movement of the joints in a real patient. Even though a conduit of 1 cm and a nerve gap of 0.5 cm were simulated in this experiment, most published recommendations indicate a high level of effectiveness for gaps up to 3 cm1,25–27 though repair of even longer gaps has also been reported.25 This suggests that there is a wide potential in using conduits for tension-free nerve repair.
Tension from end to end repair may result in intraneural hemorrhage, ischemia, followed by scar formation, and axoplasmic deterioration.2 Large anastomotic tension can lead to decreased axonal number and diameter, and this has also been consistent with decreased functional outcome.28 Although conduits have been described to decrease tension by adding length to the nerve repair, this has to be weighed in against the lack of robust axonal growth and decreased functional recovery when large nerve gaps greater than 3 cm are repaired with conduits.1,25–27,29 There is no evidence in literature to support at which length of nerve gap or tension a conduit repair should be performed to facilitate optimal nerve regeneration as compared with end to end repair. In a multicenter randomized prospective controlled trial comprising 98 subjects with 136 nerve transections, Weber et al.30 showed that even though there was no statistically significant difference in terms of overall functional results and speed of recovery between nerves repaired with conduits and those repaired primarily, there was improved sensation in patients when conduit repair was used for nerve gaps of 4 mm or less, compared with end-to-end repair of digital nerves. Weber et al.30 also showed that in nerve gaps of 8–30 mm, the repair using a conduit resulted in better functional recovery as compared with repair with a nerve graft. In another prospective randomized study by Lundborg et al.31 comprising 18 patients with nerve injuries, it was shown that there was no difference between sensory and motor functional recovery of the hand in the conduit and primary repair groups, with the exception of perception of touch, which showed a significant difference at the 3 months in favor of the conduit repair technique.
When transected nerves are repaired in the upper limb, to avoid excursion and strain and reciprocal tension on the nerves, joints are kept immobilized in a position of minimal tension until the nerve heals. Wright et al.32 demonstrated that the radial nerve requires approximately 15 mm (at the elbow) and 10 mm (at the wrist) of unimpeded movement to perform full motions of all joints of the upper extremity. This created a 28% strain of the radial nerve at the elbow. In another article,33 Wright et al.32 demonstrated that 21.9 mm of ulnar nerve excursion was required at the elbow and 23.2 mm at the wrist with maximal movement of the joints of the upper limb. This created strain at the wrist and elbow of about 29%. Extension of the ulnar nerve at the wrist was similar to that of the median nerve at the wrist during wrist and finger flexion and extension.34 Although the length and diameter of the nerves in comparison are different, our experiment showed that the maximal average displacement (5.87 mm) and maximal average strain (31.52%) in the primary repair group of the sheep nerves in testing were similar to the strain and excursion experienced by nerves in the upper limbs of cadaveric specimens during unrestricted motion of joints. Although a dedicated experiment is required to prove this, it can be postulated that the forces experienced clinically on an immobilized joint may be more than adequate to cause failures in primary nerve repairs and performing a conduit repair may guard against this as the average maximal load, displacement, and strain to failure are statistically significantly higher in this experimental group.
Isaacs et al.25 commented that passing a microsuture through the rigid PLC tubes can be very difficult. This difficulty was also encountered in this study despite using the thinned wall version conduit (Neurolac TW). However, there was no bending or breaking of the suture needles.
Some limitations in this biomechanical study are that of a small sample size and the use of an animal cadaveric model. The nerves harvested from the sheep hooves were not of uniform diameter, quality, or shape and the repairs were done ex-vivo. Uniform tension stress in a single plane was applied to the repaired nerves in this experiment, which may not mimic actual stresses that occur clinically after a nerve repair.
Nerve repair using a biodegradable synthetic PLC conduit such as the Neurolac TW conduit ensures a higher breaking strength and adds length to the nerves in discontinuity in a sheep model. Our article showed that the nerve-suture interface was consistently weaker than the suture-conduit interface. More evidence is, however, needed if this has the potential for immediate postoperative mobilization of joints and enhanced recovery without a worry about nerve repair failure due to breakage or tension.
Footnotes
Published online 17 December 2018.
Podium presentation at the Singapore Orthopaedic Association 39th Annual Scientific Meeting, Singapore, 2016; Poster presentation at the 18th EFORT Conference, Vienna, Austria, 2017; Poster presentation at the Singapore Trauma and Acute Care Conference, Singapore, 2018.
The Declaration of Helsinki, statement of institutional review board approval, and clinical trial registration information are not applicable in this biomechanical laboratory study, as the authors only used cadaveric sheep models for the experiment.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Gerth DJ, Tashiro J, Thaller SR. Clinical outcomes for conduits and scaffolds in peripheral nerve repair. World J Clin Cases. 2015;3:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terzis JU, Faibisoff BU, Williams B. The nerve gap: suture under tension vs. graft. Plast Reconstr Surg. 1975;56:166. [PubMed] [Google Scholar]
- 3.Wang WZ, Crain GM, Baylis W, et al. Outcome of digital nerve injuries in adults. J Hand Surg Am. 1996;21:138. [DOI] [PubMed] [Google Scholar]
- 4.Scarr G. Biotensegrity. 2014United Kingdom: Handspring Publishing. [Google Scholar]
- 5.Lohmeyer JA, Siemers F, Machens HG, et al. The clinical use of artificial nerve conduits for digital nerve repair: a prospective cohort study and literature review. J Reconstr Microsurg. 2009;25:55. [DOI] [PubMed] [Google Scholar]
- 6.Smith KG, Robinson PP. An experimental study of three methods of lingual nerve defect repair. J Oral Maxillofac Surg. 1995;53:1052. [DOI] [PubMed] [Google Scholar]
- 7.Millesi H. Reappraisal of nerve repair. Surg Clin North Am. 1981;61:321. [DOI] [PubMed] [Google Scholar]
- 8.Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ducic I, Fu R, Iorio ML. Innovative treatment of peripheral nerve injuries: combined reconstructive concepts. Ann Plast Surg. 2012;68:180. [DOI] [PubMed] [Google Scholar]
- 10.Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1. [DOI] [PubMed] [Google Scholar]
- 11.Giusti G, Willems WF, Kremer T, et al. Return of motor function after segmental nerve loss in a rat model: comparison of autogenous nerve graft, collagen conduit, and processed allograft (AxoGen). J Bone Joint Surg Am. 2012;94:410. [DOI] [PubMed] [Google Scholar]
- 12.Mackinnon SE, Dellon AL, Lundborg G, et al. A study of neurotrophism in a primate model. J Hand Surg Am. 1986;11:888. [DOI] [PubMed] [Google Scholar]
- 13.Arslantunali D, Dursun T, Yucel D, et al. Peripheral nerve conduits: technology update. Med Devices (Auckl). 2014;7:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichihara S, Inada Y, Nakamura T. Artificial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts. Injury. 2008;39:29. [DOI] [PubMed] [Google Scholar]
- 15.Colen KL, Choi M, Chiu DT. Nerve grafts and conduits. Plast Reconstr Surg. 2009;124:e386. [DOI] [PubMed] [Google Scholar]
- 16.Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable Neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg Am. 2005;30:513. [DOI] [PubMed] [Google Scholar]
- 17.Deal DN, Griffin JW, Hogan MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20:63. [DOI] [PubMed] [Google Scholar]
- 18.Shin RH, Friedrich PF, Crum BA, et al. Treatment of a segmental nerve defect in the rat with use of bioabsorbable synthetic nerve conduits: a comparison of commercially available conduits. J Bone Joint Surg Am. 2009;91:2194. [DOI] [PubMed] [Google Scholar]
- 19.Kwan MK, Wall EJ, Massie J, et al. Strain, stress and stretch of peripheral nerve. Rabbit experiments in vitro and in vivo. Acta Orthop Scand. 1992;63:267. [DOI] [PubMed] [Google Scholar]
- 20.Yu T, Zhao C, Li P, et al. Poly(lactic-co-glycolic acid) conduit for repair of injured sciatic nerve: a mechanical analysis. Neural Regen Res. 2013;8:1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg SH, Jobin CM, Hayes AG, et al. Biomechanics and histology of intact and repaired digital nerves: an in vitro study. J Hand Surg Am. 2007;32:474. [DOI] [PubMed] [Google Scholar]
- 22.Cruz NI, Debs N, Fiol RE. Evaluation of fibrin glue in rat sciatic nerve repairs. Plast Reconstr Surg. 1986;78:369. [DOI] [PubMed] [Google Scholar]
- 23.Temple CL, Ross DC, Dunning CE, et al. Resistance to disruption and gapping of peripheral nerve repairs: an in vitro biomechanical assessment of techniques. J Reconstr Microsurg. 2004;20:645. [DOI] [PubMed] [Google Scholar]
- 24.Isaacs JE, McDaniel CO, Owen JR, et al. Comparative analysis of biomechanical performance of available “nerve glues”. J Hand Surg Am. 2008;33:893. [DOI] [PubMed] [Google Scholar]
- 25.Isaacs J, Browne T. Overcoming short gaps in peripheral nerve repair: conduits and human acellular nerve allograft. Hand (N Y). 2014;9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houschyar KS, Momeni A, Pyles MN, et al. The role of current techniques and concepts in peripheral nerve repair. Plast Surg Int. 2016;2016:4175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pabari A, Lloyd-Hughes H, Seifalian AM, et al. Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg. 2014;133:1420. [DOI] [PubMed] [Google Scholar]
- 28.Zhang F, Inserra M, Richards L, et al. Quantification of nerve tension after nerve repair: correlations with nerve defects and nerve regeneration. J Reconstr Microsurg. 2001;17:445. [DOI] [PubMed] [Google Scholar]
- 29.Stanec S, Stanec Z, Budi S. Reconstruction of upper extremity peripheral nerve injuries with an ePTFE conduit. J Reconstr Microsurg. 1998;14:227. [DOI] [PubMed] [Google Scholar]
- 30.Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036; discussion 1046. [DOI] [PubMed] [Google Scholar]
- 31.Lundborg G, Rosén B, Dahlin L, et al. Tubular versus conventional repair of median and ulnar nerves in the human forearm: early results from a prospective, randomized, clinical study. J Hand Surg Am. 1997;22:99. [DOI] [PubMed] [Google Scholar]
- 32.Wright TW, Glowczewskie F, Jr, Cowin D, et al. Radial nerve excursion and strain at the elbow and wrist associated with upper-extremity motion. J Hand Surg Am. 2005;30:990. [DOI] [PubMed] [Google Scholar]
- 33.Wright TW, Glowczewskie F, Jr, Cowin D, et al. Ulnar nerve excursion and strain at the elbow and wrist associated with upper extremity motion. J Hand Surg Am. 2001;26:655. [DOI] [PubMed] [Google Scholar]
- 34.Wright TW, Glowczewskie F, Wheeler D, et al. Excursion and strain of the median nerve. J Bone Joint Surg Am. 1996;78:1897. [DOI] [PubMed] [Google Scholar]


