Abstract
Background:
Hyaluronic acid is an ideal facial filler, however, although established as both safe and effective, complications do occur. Treatment recommendations that combine both expert opinions and clinical trial data are currently lacking, partly due to difficulties with diagnoses, nonspecific diagnostic investigations, and certain disorders presenting with similar symptoms, thereby confounding diagnosis and treatment.
Methods:
The purpose of this article was to provide the aesthetic clinician with practical recommendations regarding complication diagnosis arising as a consequence of hyaluronic acid filler rejuvenation treatment. It also provides recommendations for their management using step-wise treatment algorithms that are based on published expert opinions, as well as the author’s clinical experience.
Results:
Algorithms are provided for the most common categories of complication associated with hyaluronic acid filler treatment, that is, skin discoloration, edema, nodules, infection, and vascular compromise.
Conclusions:
These guidelines are not intended to be complete or exhaustive but may prove informative for aesthetic clinicians who are responsible for treating patients with hyaluronic acid fillers. It may help to guide them on recognizing potential complications and it provides clear guidance on optimum treatment pathways.
BACKGROUND
Hyaluronic acid (HA) is a common, naturally occurring linear polysaccharide found in skin and tissues where one of its functions is a lubricant and moisturizer due to its hydrophilic properties. It shows neither species nor tissue specificity and does not tend to cause immunologic reactions. These properties make HA an ideal facial filler. However, it has a short half-life, so manufacturers have altered its chemistry by crosslinking chains to retard natural degradation. The result is the creation of filler products that reduce the signs of aging by producing natural, healthy looking features with a product that is well-tolerated, nonimmunogenic and longer lasting.1
HA fillers are established as safe and effective for facial rejuvenation and they are appealing because they provide an immediate aesthetic effect. Treatment is reversible, injections can be administered as out-patients and recovery time is short. Use of HA fillers has increased markedly in recent years, reaching >3 million worldwide in 2017.2 Statistics compiled by The American Society for Aesthetic Plastic Surgery show HA fillers ranked second for nonsurgical procedures performed in 2017 in the United States (722,394 procedures). This is an increase of 2.9% compared with 2016 and an increase of 85% compared with 2012.3
Adverse effects associated with HA filler treatment are uncommon, but they do occur.4 However, providing recommendations for treating filler complications poses several problems. Although millions of filler treatments are performed every year, most recommendations regarding complications are based on expert opinions, with little data available from controlled studies. This is because clinical trials are difficult to conduct since patients are usually treated in private practices, and patients generally demand fast treatment and recovery, without the burden associated with a clinical study. Also, diagnoses are usually based solely on the clinical presentation, with further investigations (eg, biopsy, bacteriology) being difficult or nonspecific (eg, general laboratory tests). Furthermore, certain disorders can present with similar symptoms, for example, type IV delayed immune reaction versus low-grade infection associated with biofilm. Each requires different treatments, that is, immunosuppression versus antibiotics, although both may have the same medical consequences, that is, granulomatous and fibrotic processes caused by chronic immune stimulation.
The purpose of this article was to provide the aesthetic practitioner with practical recommendations regarding diagnosis of the common complications arising from HA filler treatment, as well as recommendations for their management. For practical purposes, the differential diagnoses are grouped according to the principal clinical finding and the therapeutic approaches outlined in the step-wise treatment algorithms are based on published expert opinions, as well clinical experience of the authors. These guidelines are not intended to be complete or exhaustive but should be regarded as a basis for treatment and discussion by aesthetic clinicians.
COMPLICATION MANAGEMENT
Skin Discoloration
Significant skin discoloration can occur at the site of treatment, which is usually self-limiting and usually resolves within a few weeks. The main categories of skin discoloration complications include hematoma/ecchymosis (bruising), neovascularization, hyperpigmentation, and the Tyndall effect/brightening. Ischemia will be discussed later (see Vascular Compromise section). Each of these complications has differing times to onset, clinical presentation, and differential diagnoses (Fig. 1).
Fig. 1.
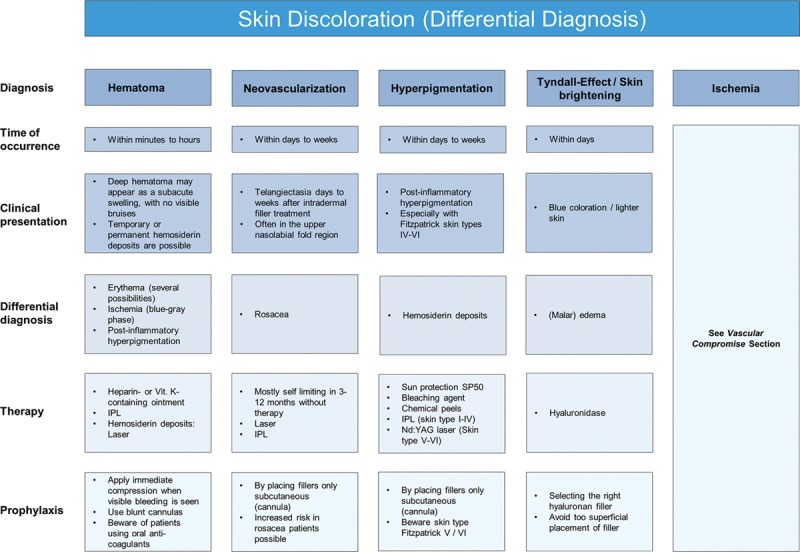
Skin discoloration (differential diagnosis).
Ecchymosis (bruising) is the most common localized event and can occur in up to 68% of patients.5 Formation of a larger hematoma is less common but can occur within minutes or hours due to inadvertent laceration of facial blood vessels. Rather than forming a bruise, if blood beneath the superficial fascia becomes trapped, a firm mass can appear. To avoid hematoma formation, compression should be applied immediately when bleeding is seen. Blunt cannulas can be considered, but caution should be used when treating patients taking oral anticoagulants. A hematoma usually resolves spontaneously over several weeks, but rarely a palpable nodule due to fibrosis of the hematoma may persist.
When there is evidence of a hematoma, compression for a few minutes is advised. Recommended treatment comprises vitamin K ointment BID for 7 days, or intense pulsed light therapy.6,7 If there is persistent hemosiderin staining, pulsed-dye or potassium titanyl phosphate (KTP) laser treatment is recommended.
Neovascularization occurs when tiny new blood vessels form at the site of dermal filler injection. Potential pathophysiological mechanisms are tissue trauma, direct stimulation of angiogenesis through HA breakdown products,8 or, in the case of telangiectasia, dilatation of tiny dermal veins due to increased tissue pressure after intradermal filler injection. Neovascularization can occur days or weeks after the procedure but should fade within 3–12 months without treatment. Intense pulsed light and lasers are effective if erythema or telangiectasias do not show spontaneous improvement, with the choice of laser being dependent upon the size of the vessel.9
Postinflammatory hyperpigmentation has also been reported following HA treatment, with Fitzpatrick skin types IV-VI being more prone to such events.10,11 However, it is important to distinguish postinflammatory hyperpigmentation from hemosiderin staining following ecchymosis. Treatment comprises topical retinol, tretinoin, and hydroquinone, or intense pulsed light and laser therapy in persistent cases.
Bluish coloration or brightening effects of the skin can occur if HA is injected too superficially or in areas where the overlying skin is thin, such as the tear trough area. This phenomenon, known as the Tyndall effect, is due to partial absorption of red light and reflection of shorter wavelength light (eg, blue) due to the optic chamber that is formed by a deposition of HA. Hyaluronidase is the recommended treatment1,9 (Fig. 2).
Fig. 2.
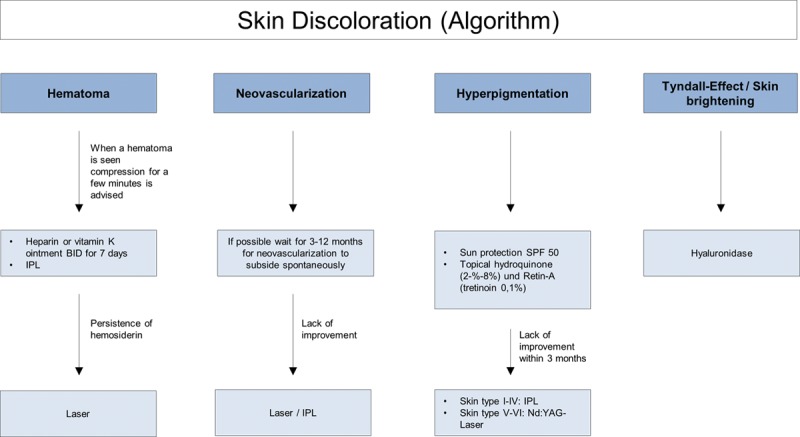
Skin discoloration (algorithm).
Edema
A variety of edema types can occur following facial filler treatment: immediate postinterventional edema, histamine-mediated edema [eg, physical urticaria, immediate type allergy (type I, IgE-mediated), malar edema, and delayed type allergy (type IV, cell-mediated)]. These vary in time to occurrence and clinical presentation, and treatment options vary depending upon category (Fig. 3).
Fig. 3.
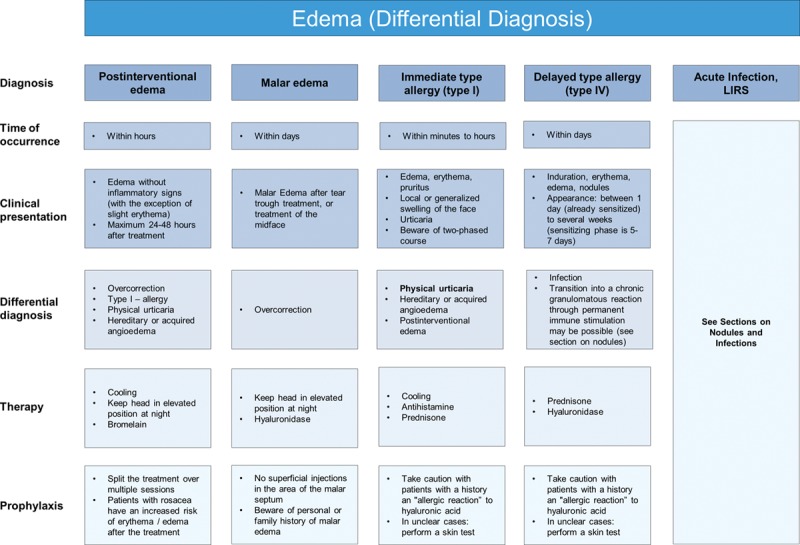
Edema (differential diagnosis).
Short-term immediate postintervention edema comprising transient swelling is normal and common to all dermal fillers. It is related to injection volume and technique and usually disappears within 1 week.9 Generally, no treatment is required, but in severe or persistent cases, bromelain can be prescribed.12
Some patients may develop histamine-mediated edema, either through direct release of histamine in physical urticaria or through IgE-mediated antibody response from hypersensitivity to components of the filler (type I hypersensitivity reaction). Even though type I hypersensitivity reactions are commonly mentioned in literature,9,13 the authors believe that most angioedema cases seen soon after HA injections are due to physical urticaria or hereditary or acquired angioedema, respectively, triggered by mild trauma, rather than IgE-mediated histamine release. Nonetheless, in rare cases, IgE-mediated antibody response to either the disinfection agent (eg, chlorhexidine) or HA fragments or other filler components (eg, lidocaine) is possible. Reactions can be severe and long-lasting with either localized or generalized edema. Treatment depends on the severity, but if there is no spontaneous resolution, antihistamines and oral corticosteroids are recommended. If angioedema occurs after a substantial period of time from the treatment (eg, months after lip augmentation), it may be due to an acquired angioedema rather than a reaction to the filler itself.
Malar edema is a serious and long-lasting complication that has been reported with all fillers when injected into the infraorbital hollow and tear troughs. This region is particularly susceptible to adverse effects due to compromised lymphatic drainage and effects due to the impermeable barrier of the malar septum and is thus prone to fluid accumulation.14 Malar edema is difficult to treat, but initial recommendations include lymphatic drainage and massage, with the head elevated at night.14 In cases of persistent malar edema, hyaluronidase should be administered.9,14
Non–antibody-mediated (delayed, type IV) allergic reactions are mediated by T lymphocytes rather than antibodies and result in induration, erythema, and edema. Potential triggers are the injection needle in cases of nickel allergy,15–18 or possibly HA fragments and other filler components. They may occur as early as 1 day after injection but can be seen as late as several weeks after injection and may persist for many months. Delayed hypersensitivity reactions are nonresponsive to antihistamines and recommended treatment is a short course of oral corticosteroids followed by administration of hyaluronidase where there is no improvement.9,19 If the reaction commences more than 3 weeks after injection, late inflammatory response syndrome (LIRS) should be considered (Fig. 4).
Fig. 4.
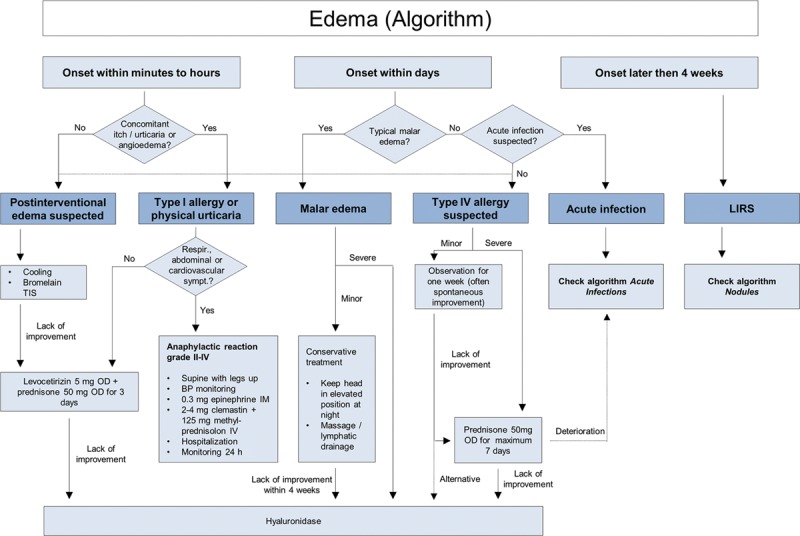
Edema (algorithm).
Nodules
Nodules, either inflammatory or noninflammatory, can arise from a number of causes, and it is important to establish a working diagnosis before treatment (Fig. 5).
Fig. 5.
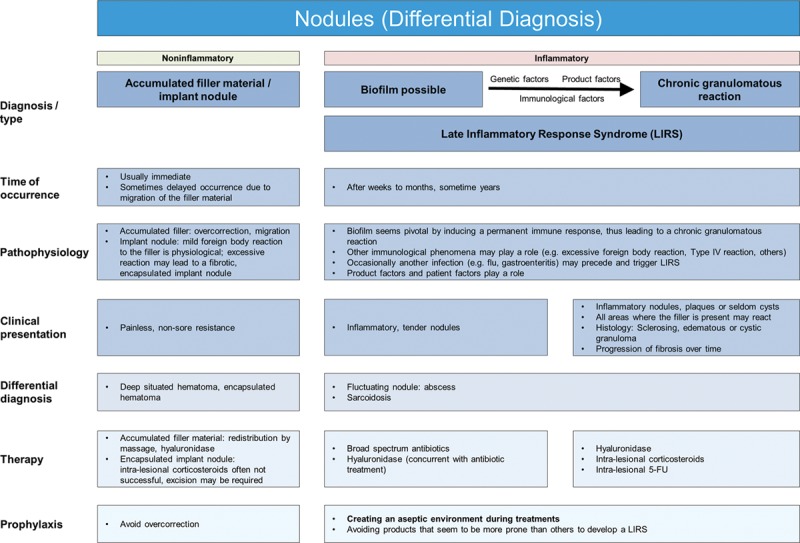
Nodules (differential diagnosis).
NonInflammatory Nodules
Noninflammatory nodules may be visible and palpable, particularly in areas where there is thin soft-tissue coverage (eg, eyelids, nasojugal region and lips).20 They occur when too much filler accumulates due to poor technique caused by overcorrection, superficial filler placement, facial area with significant muscular activity (eg, around the modiolus) or incorrect filler selection for the anatomical area. These nodules form isolated lumps at the injection site and are well defined from surrounding tissue.9 Recommended initial treatment is massage to redistribute the filler material, but expression after puncturing the skin with a large gauge needle (22-18G) or hyaluronidase may be required. Approximately 30 units of hyaluronidase have been shown to effectively dissolve 0.1 cc of various HA-based fillers; however, the degree of dissolution is based on the 3-dimensional structure and cross-linking properties of the specific filler substance, as well as the time period postinjection.21–23
Inflammatory Nodules
All injected implants cause an initial influx of neutrophils and mononuclear cells24 with phagocytosis by aggregated macrophages, and activation of fibroblasts with subsequent collagen deposition. A certain foreign body reaction to HA implants is not only physiological but also desired, since it stimulates the production of several extracellular matrix components.25 However, if there is a failure of effective phagocytosis or inadequate stimulation of immune cells with ongoing macrophage activation, this may lead to a granulomatous and fibrotic process.26 Clinical findings may present as multiple red, tender, inflammatory nodules with swelling, erythema, and occasional suppuration.27 Inflammatory granulomatous processes are characterized by later onset, appearing weeks to months after treatment.
The pathophysiology behind the transition of physiological foreign body reactions into severe inflammatory granulomatous processes is complex and not fully understood, with numerous factors playing a potential role. Different chain lengths of HA components have different effects on macrophage activation28 and bioimplants seem to act as T-cell specific activators in individuals with a predisposing genetic background.26 Occasionally, a trivial infection (influenza or gastroenteritis) may precede a late inflammatory reaction29–33 and it is well-known that IFN-therapy (eg, in hepatitis C virus [HCV] therapy) may trigger a granulomatous process at sites of filler implants.34,35 These findings suggest that reactivity levels of an individual’s immune system play a crucial role.
Finally, there is abundant evidence that biofilms may be pivotal in triggering late inflammatory reactions. Biofilms that may be preceded by infection are widespread and comprise densely packed communities of common skin colonizing bacteria (eg, S. epidermidis, P. acnes)36 that self-encapsulate with a complex protective adhesive matrix. This allows them to irreversibly adhere to living structures or inert surface, so when a filler is injected, it can become coated with bacteria and forms a biofilm, inducing a permanent immune response that may eventually lead to a chronic granulomatous reaction.27,37
Recommended treatment is systemic antibiotic therapy, but filler removal is important, so hyaluronidase should be used 24–48 hours after commencing antibiotic treatment. In cases of biofilm formation, hyaluronidase will help break down the matrix, thus increasing the efficacy of antibiotic therapy, and in direct immunological reaction to the filler, hyaluronidase will remove the substrate. If inflammation and edema are severe, a short course of oral corticosteroids may prove beneficial. If lesions are unresponsive to antibiotic and hyaluronidase treatment, intralesional corticosteroids should be used.9 If lesions are still unresponsive, the addition of 5-FU is recommended.9 Schelke et al.38 recently demonstrated that material removal with intralesional laser treatment was effective and so may be considered as another treatment option (Fig. 6).
Fig. 6.
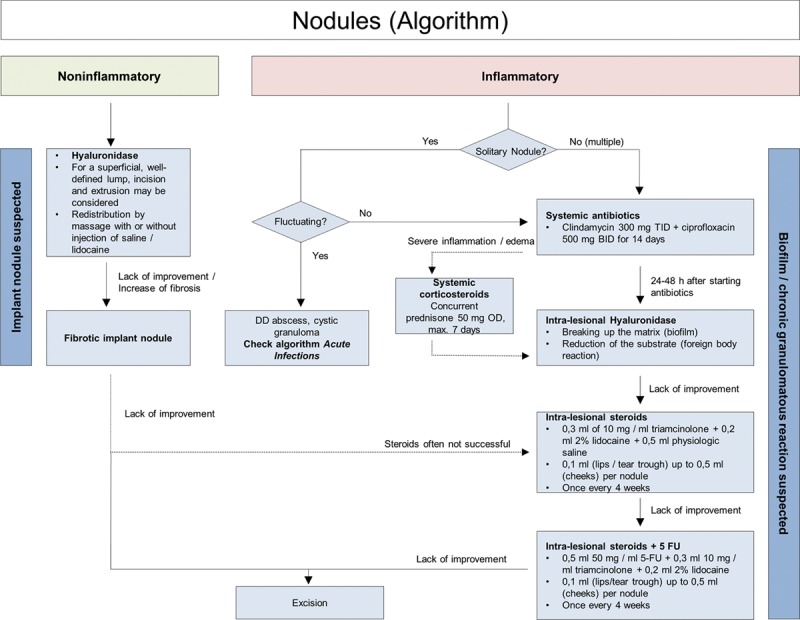
Nodules (algorithm).
Infections
As with any procedure that breaks the surface of the skin, dermal filler injections are associated with a risk of infection.20 It is important to minimize this risk by using thorough antisepsis throughout the procedure. Although rare, acute resistant postfiller infections can occur, and may be bacterial or viral in nature.9 This article focuses on the following potential consequences of infections: erysipelas/cellulitis, abscess, herpes simplex, and biofilms (Fig. 7).
Fig. 7.
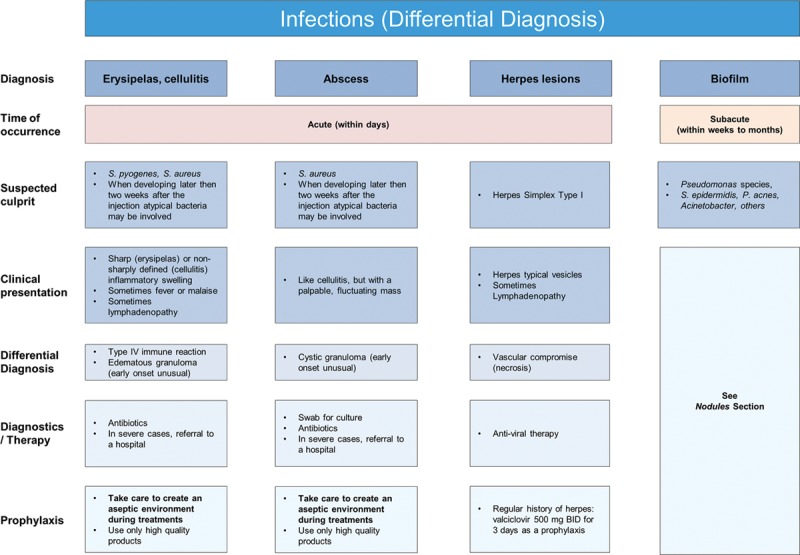
Infections (differential diagnosis).
Erysipelas and cellulitis can be considered manifestations of the same condition and the terms are often used interchangeably. They are acute, painful, and potentially serious infections of the skin and subcutaneous tissues. The most common causative organisms are Streptococcus or Staphylococcus spp. Erysipelas is almost always caused by β-hemolytic streptococci and consists of a superficial cutaneous process in the dermis, with prominent lymphatic involvement. It has 3 distinguishing features: lesions are prominent, bright red, with clear demarcation between involved and uninvolved tissue. Streptococcal cellulitis is an acute spreading inflammation of large areas of skin and subcutaneous tissues. Clinical findings include local pain, tenderness, swelling, and erythema, whereas systemic features include fever, chills, malaise, and lymphangitis, and/or bacteremia.39
Mild forms may be treated with oral antibiotics, while more serious cases require intravenous antibiotics and hospitalization. Amoxicillin/clavulanate 625 mg TID or clindamycin 600mg TID for 7–10 days is recommended. In unresolved cases, specialist infectious disease advice should be sought.
Abscess formation is rare but can occur any time from weeks to months following treatment. Abscesses should be treated with antibiotics, incision, and drainage, with cultures being obtained and treatment tailored to sensitivity reports.1,9,20 The most common organism is S. aureus.40 If the abscess emerges late (weeks to months postinjection) and cultures are negative, the abscess may represent a cystic granuloma24 and should be treated as a late inflammatory reaction (Fig. 5).
Reactivation of Herpes simplex is the most common viral infection to occur after filler injection into the lips. Patients with a history of cold sores or fever blisters may be treated prophylactically with valacyclovir 500 mg BID for 3 days. If the patient has not received prophylactic treatment, but infection is recognized within 24 hours, valaciclovir at a dose of 500 mg BID for 5 days is recommended1,9 (Fig. 8).
Fig. 8.
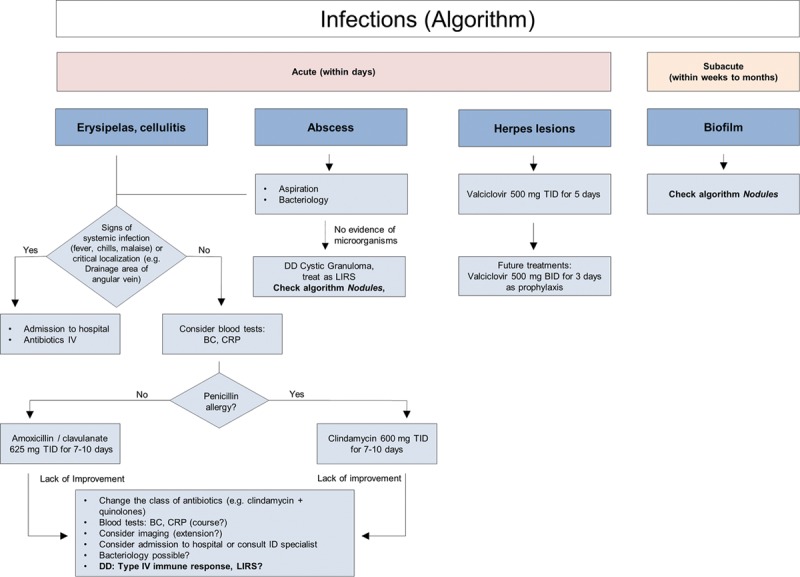
Infections (algorithm).
Vascular Compromise
Vascular complications are rare following dermal fillers, but numbers are rising due to increases in dermal filler use. The risk increases when bolus technique and smaller needles instead of cannulas are used.41 For this article, the discussion is focused on peripheral ischemia with impending tissue necrosis and retinal ischemia (Fig. 9).
Fig. 9.
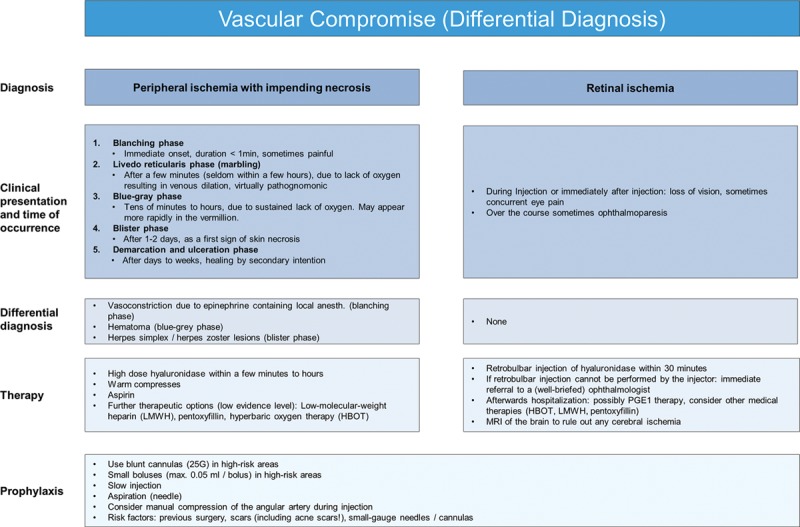
Vascular compromise (differential diagnosis).
Vascular-related events are major, immediate complications that result from intravascular injection into an artery, causing an embolism that obstructs blood flow. This potentially leads to tissue anoxia, ischemia and possible progression to necrosis (both anterograde and retrograde).9,41 Localized color changes should increase suspicion of vascular compromise. An immediate onset blanching, followed by livedo reticularis (marbling) usually occurs within hours after injection due to lack of oxygen resulting in venous dilation. The blue-gray phase follows later due to sustained lack of oxygen, and 1–2 days later the blister phase indicates the first signs of skin necrosis. Clinicians should have detailed knowledge of facial anatomy since the rich vascular network of the face provides numerous potential target sites. Recognition of a vascular event must be swift, followed by aggressive treatment to avoid serious, potentially irreversible complications, such as tissue necrosis and permanent vision loss.41
Treatment recommendations for peripheral ischemia are prompt administration of high-dose hyaluronidase41,42 and warm compresses. Half-life time of hyaluronidase is short, so injections should be repeated hourly until capillary refill improves.42 If blanching persists, lidocaine without epinephrine can be added to promote vasodilation. Low-dose acetylsalicylic acid (ASA) may be given for 7 days; however, evidence for its benefit is low. Topical nitropaste is no longer recommended, as studies in animal models have shown no benefit.43
Retinal ischemia is a rare but serious complication that occurs when the dermal filler inadvertently enters the ocular circulation through retrograde arterial flow into the ophthalmic artery.44 Immediate blurring and potential visual blindness can result due to the injected material moving distally to the retina and blocking blood supply.9 As the ophthalmic artery communicates directly with the circle of Willis, intracerebral infarctions are possible and associated symptoms like loss of consciousness or vertigo may be observed.45
Treatment recommendations are primarily based on expert opinions and therefore have low level of evidence. One drop of topical timolol 0.5% can be applied to the impaired eye to reduce intraocular pressure.46 The next intervention step is rapid clearance of retinal HA emboli. Carruthers et al.47,48 suggested retro- or parabulbar injections of hyaluronidase; however, this procedure requires specialized training, so immediate referral to a ophthalmologist may be needed. The speed of response is essential because of the extreme intolerance of the retina to hypoxia. There are only a few reports on outcomes after retrobulbar hyaluronidase injections; Chesnut49 reported on one case of visual loss restoration, but Zhu et al.50 were unable to clear retinal artery occlusion in 4 patients treated with high doses of retrobulbar hyaluronidase injected after 4 hours or longer. Fast vascular reperfusion has been demonstrated in animal models using both hyaluronidase and urokinase, and this may become a standard treatment option in the future.51 In this animal study, the retinal embolus had to be removed within 45 minutes to prevent permanent blindness but more research is needed to provide an estimate for the time limits of the human eye.
After hyaluronidase treatment, the patient should be hospitalized, evaluated for retrobulbar hematoma, and a brain magnetic resonance imaging obtained to rule out any cerebral ischemia. Further therapeutic options for retinal or severe peripheral ischemia include prostaglandin E1 (PGE1) IV, pentoxifylline, low molecular weight heparins (LMWH) and hyperbaric oxygen treatment and for peripheral ischemia, injection of platelet rich plasma. However, evidence levels for all these treatments are low (Fig. 10).
Fig. 10.
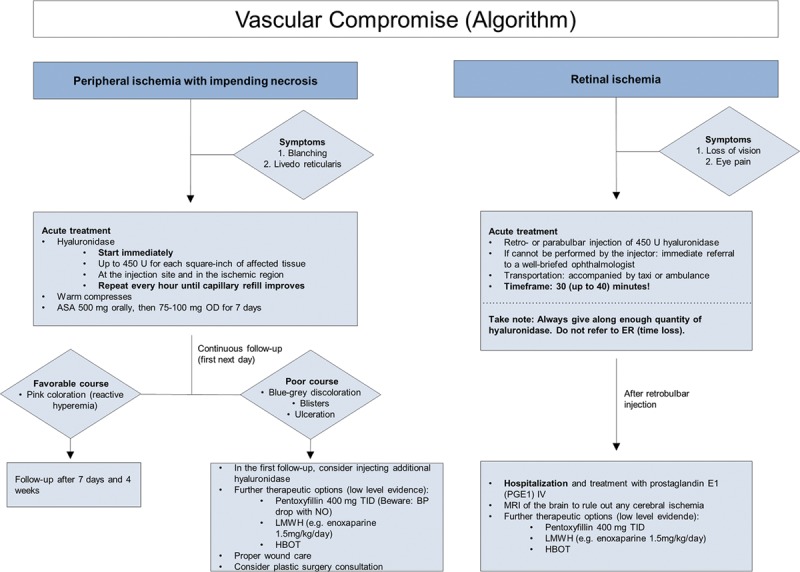
Vascular compromise (algorithm).
CONCLUSIONS
HA fillers are safe and effective for facial rejuvenation and, although adverse effects associated with their use are uncommon, they do occur. Currently, there is a lack of standardized guidance on how to treat HA-related complications that combine expert opinion with evidence from clinical trials. In this article, the authors have provided practical recommendations for concise treatment algorithms based on clinical presentation of the complications rather than the final diagnosis, using published expert opinions together with the authors’ experience. The focus is on the common categories of complication associated with HA filler treatment: skin discoloration, edema, nodules, infection, and vascular compromise. These guidelines are not intended to be complete or exhaustive but may prove informative for aesthetic clinicians as how best to recognize potential complications and provide clear guidance on optimum treatment pathways.
ACKNOWLEDGMENTS
The authors thank Wolfgang Philipp-Dormston and Nabila Azib for their review of the treatment algorithms. Medical writing support for this article was provided by Debbie Jordan Ltd, with funding from Pharm-Allergan GmbH.
Footnotes
Published online 17 December 2018.
Disclosure: Dr. Snozzi is a faculty member of the Allergan Medical Institute and reports personal fees and nonfinancial support from Allergan, Merz, and Galderma outside of this submitted work. He declares no conflict of interest or influence regarding this publication. Dr. van Loghem is a member of the Global Faculty of the Merz Institute of Advanced Aesthetics and has received payment and nonfinancial support from Merz, Allergan, and Ipsen outside of this submitted work and has no conflicts of interest to declare regarding this publication. Allergan was responsible for paying the Article Processing Charge.
REFERENCES
- 1.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561. [DOI] [PubMed] [Google Scholar]
- 2.International Society of Aesthetic Plastic Surgeons (ISAPS). International study on aesthetic/cosmetic procedures performed in 2017 worldwide. Available at https://na01.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.isaps.org%2Fwp-content%2Fuploads%2F2018%2F10%2FISAPS_2017_International_Study_Cosmetic_Procedures.pdf&data=02%7C01%7Cshannon.funk%40wolterskluwer.com%7C563e366c67074ea957f708d644d92c59%7C8ac76c91e7f141ffa89c3553b2da2c17%7C0%7C0%7C636772096879923892&sdata=QB8xSYfyiGxO0Z8pN9Ug10ZK6HeLv6B1drCJ%2BoN2GHo%3D&reserved=0. Accessed 7 November 2018.
- 3.American Society for Aesthetic Plastic Surgery (ASAPS). Cosmetic surgery national data bank statistics. Available at media@surgery.org. Accessed May 2018. [DOI] [PubMed]
- 4.Philipp-Dormston WG, Bergfeld D, Sommer BM, et al. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers. J Eur Acad Dermatol Venereol. 2017;31:1088. [DOI] [PubMed] [Google Scholar]
- 5.King M. The management of bruising following nonsurgical cosmetic treatment. J Clin Aesthet Dermatol. 2017;10:E1. [PMC free article] [PubMed] [Google Scholar]
- 6.Shah NS, Lazarus MC, Bugdodel R, et al. The effects of topical vitamin K on bruising after laser treatment. J Am Acad Dermatol. 2002;47:241. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JL, Bhatia AC. The role of topical vitamin K oxide gel in the resolution of postprocedural purpura. J Drugs Dermatol. 2009;8:1020. [PubMed] [Google Scholar]
- 8.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528. [DOI] [PubMed] [Google Scholar]
- 9.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35:1653. [DOI] [PubMed] [Google Scholar]
- 11.Heath CR, Taylor SC. Fillers in the skin of color population. J Drugs Dermatol. 2011;10:494. [PubMed] [Google Scholar]
- 12.Orsini RA; Plastic Surgery Educational Foundation Technology Assessment Committee. Bromelain. Plast Reconstr Surg. 2006;118:1640. [DOI] [PubMed] [Google Scholar]
- 13.Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Derm Surg. 2016;20:100. [Google Scholar]
- 14.Funt DK. Avoiding malar edema during midface/cheek augmentation with dermal fillers. J Clin Aesthet Dermatol. 2011;4:32. [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav S, Dogra S. A cutaneous reaction to microneedling for postacne scarring caused by nickel hypersensitivity. Aesthet Surg J. 2016;36:NP168. [DOI] [PubMed] [Google Scholar]
- 16.Corazza M, Borghi A, Sarno O, et al. Antecubital allergic contact dermatitis from intravenous iron infusion: a possible etiological role of nickel. Eur J Dermatol. 2011;21:284. [DOI] [PubMed] [Google Scholar]
- 17.Corazza M, Maranini C, Aleotti A, et al. Nickel contact dermatitis due to the needle of an infusion pump, confirmed by microanalysis. Contact Dermatitis. 1998;39:144. [DOI] [PubMed] [Google Scholar]
- 18.Lachapelle JM, Tennstedt D. An anatomo-clinical study of delayed skin allergic reactions to nickel following intradermal injections of lidocaïne with a Dermo-jet. Contact Dermatitis. 1982;8:193. [DOI] [PubMed] [Google Scholar]
- 19.Bitterman-Deutsch O, Kogan L, Nasser F. Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Reports. 2015;7:5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Ahn DK, Jeong HS, et al. Treatment algorithm of complications after filler injection: based on wound healing process. J Korean Med Sci. 2014;29:S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones D, Tezel A, Borrell M. In vitro resistance to degradation of hyaluronic acid dermal fillers by ovine testicular hyaluronidase. Dermatol Surg. 2010;36:804 [Google Scholar]
- 22.Rao V, Chi S, Woodward J. Reversing facial fillers: interactions between hyaluronidase and commercially available hyaluronic-acid based fillers. J Drugs Dermatol. 2014;13:1053. [PubMed] [Google Scholar]
- 23.Kim DW, Yoon ES, Ji YH, et al. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg. 2011;64:1590. [DOI] [PubMed] [Google Scholar]
- 24.Lemperle G, Gauthier-Hazan N, Wolters M, et al. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123:1842. [DOI] [PubMed] [Google Scholar]
- 25.Paliwal S, Fagien S, Sun X, et al. Skin extracellular matrix stimulation following injection of a hyaluronic acid-based dermal filler in a rat model. Plast Reconstr Surg. 2014;134:1224. [DOI] [PubMed] [Google Scholar]
- 26.Alijotas-Reig J, Fernández-Figueras MT, Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45:97. [DOI] [PubMed] [Google Scholar]
- 27.Daines SM, Williams EF. Complications associated with injectable soft-tissue fillers: a 5-year retrospective review. JAMA Facial Plast Surg. 2013;15:226. [DOI] [PubMed] [Google Scholar]
- 28.Rayahin JE, Buhrman JS, Zhang Y, et al. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. 2015;1:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemperle G, Romano JJ, Busso M. Soft tissue augmentation with artecoll: 10-year history, indications, techniques, and complications. Dermatol Surg. 2003;29:573; discussion 587. [DOI] [PubMed] [Google Scholar]
- 30.Orentreich DS. Liquid injectable silicone: techniques for soft tissue augmentation. Clin Plast Surg. 2000;27:595. [PubMed] [Google Scholar]
- 31.Achauer BM. A serious complication following medical-grade silicone injection of the face. Plast Reconstr Surg. 1983;71:251. [DOI] [PubMed] [Google Scholar]
- 32.Duffy DM. The silicone conundrum: a battle of anecdotes. Dermatol Surg. 2002;28:590. [DOI] [PubMed] [Google Scholar]
- 33.Wolters M, Lampe H. Nicht—operative Faltenbehandlung im Gesicht. Plast Chir. 2003;2:69. [Google Scholar]
- 34.Fischer J, Metzler G, Schaller M. Cosmetic permanent fillers for soft tissue augmentation: a new contraindication for interferon therapies. Arch Dermatol. 2007;143:507. [DOI] [PubMed] [Google Scholar]
- 35.López-Pestaña A, Tuneu A, Lobo C, et al. Granulomas sarcoideos en material de relleno facial inducidos por interferon y ribavirina en pacientes con hepatitis C. Actas Dermosifiliogr. 2011;102:746. [DOI] [PubMed] [Google Scholar]
- 36.Saththianathan M, Johani K, Taylor A, et al. The role of bacterial biofilm in adverse soft-tissue filler reactions: a combined laboratory and clinical study. Plast Reconstr Surg. 2017;139:613. [DOI] [PubMed] [Google Scholar]
- 37.Alhede M, Er Ö, Eickhardt S, et al. Bacterial biofilm formation and treatment in soft tissue fillers. Pathog Dis. 2014;70:339. [DOI] [PubMed] [Google Scholar]
- 38.Schelke LW, Decates TS, van der Lugt CIM, et al. Intralesional laser treatment for dermal filler complications. Plast Reconstr Surg. 2018;141:1361. [DOI] [PubMed] [Google Scholar]
- 39.Stevens DL, Bryant AE. Ferretti JJ, Stevens DL, Fischetti VA. Impetigo, erysipelas and cellulitis. In: Streptococcus Pyogenes: Basic Biology to Clinical Manifestations [Internet]. 2016. 2016Oklahoma City, Okla.: University of Oklahoma Health Sciences Center. [Google Scholar]
- 40.Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34:584. [DOI] [PubMed] [Google Scholar]
- 42.DeLorenzi C. New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthet Surg J. 2017;37:814. [DOI] [PubMed] [Google Scholar]
- 43.Hwang CJ, Morgan PV, Pimentel A, et al. Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: an animal model with ICG imaging. Ophthalmic Plast Reconstr Surg. 2016;32:118. [DOI] [PubMed] [Google Scholar]
- 44.Hayreh SS. Orbital vascular anatomy. Eye (Lond). 2006;20:1130. [DOI] [PubMed] [Google Scholar]
- 45.Lazzeri D, Agostini T, Figus M, et al. Blindness following cosmetic injections of the face. Plast Reconstr Surg. 2012;129:995. [DOI] [PubMed] [Google Scholar]
- 46.Loh KT, Chua JJ, Lee HM, et al. Prevention and management of vision loss relating to facial filler injections. Singapore Med J. 2016;57:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carruthers J, Fagien S, Dolman P. Retro or PeriBulbar injection techniques to reverse visual loss after filler injections. Dermatol Surg. 2015;41:S354. [DOI] [PubMed] [Google Scholar]
- 48.Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197. [DOI] [PubMed] [Google Scholar]
- 49.Chesnut C. Restoration of visual loss with retrobulbar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018. Mar;44(3):435. [DOI] [PubMed] [Google Scholar]
- 50.Zhu GZ, Sun ZS, Liao WX, et al. Efficacy of retrobulbar hyaluronidase injection for vision loss resulting from hyaluronic acid filler embolization. Aesthet Surg J. 2017. doi: 10.1093/asj/sjw216 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51.Chiang C, Zhou S, Chen C, et al. Intravenous hyaluronidase with urokinase as treatment for rabbit retinal artery hyaluronic acid embolism. Plast Reconstr Surg. 2016;138:1221. [DOI] [PubMed] [Google Scholar]


