Abstract
Background:
Primary lymphedema is a debilitating disease. This study was to investigate the outcomes between vascularized lymph node transfer (VLNT) and lymphovenous anastomosis (LVA) for treating primary lymphedema.
Methods:
Between January 2010 and December 2016, 17 patients with mean age of 31.5 ± 15.5 (ranged, 2–57) years diagnosed with 19 primary limb lymphedema were recruited. Patients with patent lymphatic ducts on indocyanine green lymphography were indicated for LVA, whereas those without patent lymphatic ducts were indicated for VLNT. Circumferential limb measurements, body weight, episodes of cellulitis and Lymphedema Quality-of-Life (LYMQoL) questionnaire were compared between preoperatively and postoperatively.
Results:
Fifteen lymphedematous limbs underwent VLNT (79%) and 4 underwent LVA (21%). All VLNT flaps survived. At a mean follow-up of 19.7 ± 8.5 months, mean reduction of limb circumference, body weight, and episodes of cellulitis were 3.7 ± 2.9 cm and 1.9 ± 2.9 cm (P = 0.2); 6.6 ± 5.9 kg and 1.7 ± 0.6 kg (P < 0.05); 5.1 ± 2.8 times/y and 4.2 ± 0.5 times/y in VLNT and LVA groups, respectively (P = 0.7). Improvements in overall score (from 3.9 ± 1.2 to 6.4 ± 1.1, P < 0.05) of the LYMQoL in VLNT group had statistical significant difference than that (from 3.0 ± 1.4 to 5.0 ± 2.4, P = 0.07) in LVA group.
Conclusions:
Both VLNT and LVA can effectively treat primary lymphedema patients. The reduction of above-knee circumference, body weight, episodes of cellulitis, and the improvement of LYMQoL was significantly greater in LVNT compared with LVA.
INTRODUCTION
Primary lymphedema is the presence or development of lymphedema without relation to any underlying medical conditions. Primary lymphedema has a quoted incidence of approximately 1–3 births out of every 10,000 births,1 with a particular female preponderance to male ratio of 3.5:1.2 In North America, the incidence of primary lymphedema is approximately 1.15 births out of every 100,000 births.2 Compared with secondary lymphedema, primary lymphedema is relatively rare.
Primary lymphedema can be classified depending on the age of onset of the patients: at infancy (birth to 1 year), during childhood (1–8 or 9 years), during adolescence (9–21 years), and lastly during adulthood (after 21 years).3 Several eponymous names have been associated with primary or congenital lymphedema, but it should only be called as such with the diagnosed genetic mutations. Mutations in VEGFR3 (Milroy disease), CCBE1 (Hennekam syndrome), SOX18 (hypotrichosis-telangiectasia-lymphedema), and FOXC2 (lymphedema distichiasis) are several eponymous conditions that present at birth and involve the development of lymphedema. Familial lymphedema of the lower extremities that presents itself during adolescence is known as Meige disease. Its underlying genetic abnormality is not known yet, but its familial nature and presentation at adolescence are characteristics of it.4 Milroy disease presents with lymphedema of the lower limbs at birth and is diagnosed either with a positive family history of it or a documented mutation in VEGFR3 in patients without a positive family history of it.5
Patients with primary lymphedema often present with symptoms that require treatments later in life, often with a history of long-standing lymphedema and its associated changes. Secondary, or acquired, lymphedema is more commonly seen, with its development often related to direct trauma, surgery, radiotherapy or infectious causes, which cause a disruption in the lymphatic channels and a subsequent compromised flow of lymph from a limb. Lymphedema then presents as chronic changes and swelling of the tissue and is often associated with adipogenesis or fibrotic changes in the limb as well. Severe fibrosis occurs with long-standing lymphedema due to the accumulation of protein-rich fluid in the interstitial spaces coupled with inflammation repeated bouts of cellulitis.6
Vascularized lymph node transfers (VLNT) and lymphovenous anastomosis (LVA) are surgical treatments that have been shown to be effective in treating secondary lymphedema.7–9 VLNT involves the microsurgical transfer of lymph node-containing tissue to a lymphedematous limb, which works based on the movement of lymphatic fluid from the affected limb into the transferred lymph node and drainage via the newly anastomosed venous route.10 However, little is known about the efficacy of recent lymphatic microsurgical treatment modalities for the treatment of primary lymphedema. The purpose of this study was to investigate and compare the outcomes between VLNT and LVA in the treatment of primary lymphedema.
PATIENTS AND METHODS
Patient Demographics
This study was approved by the Institutional Review Board at Chang Gung Memorial Hospital and performed in accordance with the Helsinki Declaration ethical standards. All of the patient data were prospectively collected at Chang Gung Memorial Hospital, a tertiary academic medical center in Taoyuan, Taiwan, and all of the operations were performed by a single surgeon (M.H.C.). A total of 17 patients with 19 primary extremity lymphedema underwent lymphedema microsurgery between January 2010 and December 2016 were included. Five of these patients were children under the age of 12, and 3 patients under 2 years old. None of the patients had a medical condition that was associated with the development of lymphedema, and all of the patients reported a nonhereditary occurrence of lymphedema (Table 1).
Table 1.
Demographic Data of 19 Lymphedematous Limbs in 17 Patients Who Had Undergone Lymphatic Microsurgeries
Four patients had lower limb lymphedema with Klippel-Trenaunay syndrome, whereas the remaining 13 had idiopathic, nonhereditary lymphedema. The patients were treated with either a VLNT or LVA after a thorough evaluation of the patient’s condition and discussion of the pros and cons of each procedure. Preoperative lymphoscintigraphy and indocyanine green (ICG) lymphography were used to detect the presence of any intact lymphatic channels. If a lymphatic duct was available at ICG lymphography, the patient was offered the LVA treatment, whereas those without patent lymphatic ducts were indicated for VLNT. If the lymphoscintigraphy shown total obstruction, the patients were offered VLNT directly (Fig. 1). The patients who presented for treatment had a mean age of 31.5 ± 19.5 years, a mean body mass index of 24.2 ± 5.1 and a mean duration of symptoms of 54.2 ± 53.9 months. All of the patients were graded by using the Cheng’s Lymphedema Grading.11,12 Two patients with good compliance of compression garments who suffered from symptomatic cellulitis for many years with grade 1 lymphedema were treated with a VLNT since there were no patent lymphatic ducts available at ICG lymphography. Ten patients had grade 2 lymphedema, of whom 6 underwent a VLNT and 4 received a LVA treatments. Two patients with grade 3 lymphedema underwent VLNT treatments. Five patients had grade 4 lymphedema at the initial presentation and sought VLNT treatments (Table 1).
Fig. 1.

A 13-year-old female who was a congenital bilateral lymphedema for 12 years. Preoperative lymphoscintigraphy depicts lymphatic obstruction in the bilateral lower limb after Tc99 injected 5 minutes (A). At 2 hours after injection, a few intermediate lymph nodes were demonstrated in both knees, and right groin lymph nodes were presented from posterio- anterior view (B).
All of the patients did not use compression garments postoperatively, and a quality of life evaluation in addition to serial circumferential limb and body weight measurements, were performed in an outpatient setting. Circumferential measurements in the lower limb were performed at fixed points at 15 cm above the knee (AK) (tibial tuberosity), 15 cm below the knee (BK) (tibial tuberosity), and 10 cm above the ankle (AA) (medial malleolus) in each patient. The number of postoperative episodes of cellulitis were also noted.
A lymphedema-specific quality of life assessment Lymphedema Quality-of-Life (LYMQoL) questionnaire was performed preoperatively and 12 months postoperatively in all of the patients.12 The questionnaire that provided questions based on their function, appearance, symptoms, and mood. The functional assessment scoring was out of a total of 32 points, appearance was out of 28 points, symptoms was out of a total of 20 points and, lastly, mood was out of a score of 24 points. The lower the score was in the 4 domains, the greater the satisfaction rate was reported by the patient. Conversely, the greater the overall score (from 1 to 10) the better the quality of life was for the patient. All of questionnaires were answered by our patients themselves including the children. Their parents only help to explain the questions while they cannot understand the questions.
VLNT
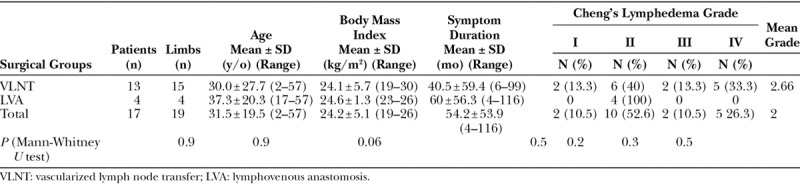
All of the patients underwent VLNT from the submental region, which was first described by Cheng et al.13 All of the flaps were transferred to the most dependent region of the extremity (ie, the ankle or wrist; Fig. 2). Basically, the submental VLNT was performed as the previous described fashions.13
Fig. 2.
She underwent right vascularized submental lymph node flap transfer to left dorsal ankle. Skin paddle 8 × 3 cm was designed on right neck (A). Three sizable lymph nodes (yellow arrows) were noted on the divided flap (B). Flap inset (C). Immediate view after delayed primary retention suture placed (D).
LVA
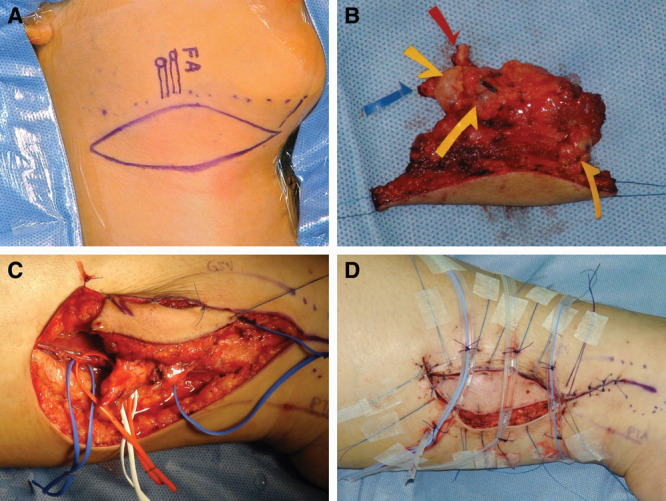
LVA was performed in each patient presented with a patent lymphatic duct with the assistance of preoperative ICG lymphography. Only 1 LVA was usually performed in an end-to-end or a side-to-end fashion of lymphatic channels to subdermal venule.14 Lymphatic flow was established and checked with an intraoperative microscopic ICG image (Fig. 3; Mitaka, Tokyo, Japan) before wound closure. The patients remained in an inpatient setting for up to 3 days to allow for an adequate stabilization of the anastomosis before being discharged.14
Fig. 3.
ICG lymphography of the right lower extremity of the lymphatic duct (yellow arrow) with an infrared light camcorder (A). A lymphatic duct with a diameter of 0.5 mm was shown and anastomosed to a subdermal venule that was 0.5 mm in diameter. The LVA was performed in an end-to-end fashion, which was patent immediately (B).
Statistical Analysis
All of the analyses were performed using SPSS software, version 17.0 (SPSS, Inc., Chicago, Ill.). Group comparisons were performed using the Mann-Whitney U test for circumference comparisons and the Wilcoxon test for preoperative and postoperative comparisons. All of the P values were 2-sided, and P values < 0.05 were considered statistically significant.
RESULTS
Limb Circumference
Fifteen lymphedematous limbs underwent VLNT (79%) and 4 underwent LVA (21%), based on patent lymphatic channels were preoperatively evaluated on ICG lymphography. A 100% flap of VLNT success rate was observed in this series. At an average follow-up of 18.2 ± 8.9 months, 15 lymphedematous limbs underwent VLNT had a 3.7 ± 2.9 cm average limb circumference reduction; 3.8 ± 3.0 cm at AK level, 3.6 ± 3.6 cm at BK level, and 4 ± 2.5 cm at AA level, respectively. At an average follow-up of 25.5 ± 0.6 months, 4 lymphedematous limbs with preoperative patent lymphatic ducts on ICG lymphography received LVA treatment had a 1.9 ± 2.9 cm average circumferential reduction; 1.3 ± 2.0 cm at AK level, 3.0 ± 4.0 cm at BK level, and 1.5 ± 4.4 cm at AA level, respectively (Table 2). The most significant reduction in limb circumference was seen above the knee between VLNT and LVA groups (P < 0.05), which may be attributed by the effect of gravity (Table 2). Patients with long-standing primary lymphedema also presented with a large amount of fatty deposition, which was not addressed in this initial transfer.11,15
Table 2.
Functional Outcomes Assessed in 19 Primary Lymphedematous Limbs in 17 Patients Receiving VLNT or Lymphovenous Anastomosis Treatment both Preoperatively and Postoperatively

Body Weight
Patients were also weighed preoperatively and every 3 months postoperatively in the clinic. A significant reduction in body weight 6.6 ± 5.9 kg (from 62.8 ± 28.9 to 56.2 ± 22.8 kg, P < 0.05) was seen in patients who received VLNT. Patients in the LVA group also had a weight reduction of 1.7 ± 0.6 kg (from 57.7 ± 3.6 to 55.9 ± 4.3 kg, P < 0.5). The mean reduction of body weight in VLNT group had greater significance than that in LVA group (P < 0.05). The most significant body weight reduction was in VLNT group between pre- and postoperative (P < 0.05; Table 2). All of the patients reported to the best of their knowledge a return to normal lifestyle postoperatively with no reported change in diet or deliberate weight loss. It can be inferred that the majority of the weight loss recorded here is due to the reduction in the fluid collection in the lower limb (Table 2).
Episodes of Cellulitis
Patients in the VLNT group had statistical difference 5.1 ± 2.8 times/y of the episodes of cellulitis between 5.2 ± 3.5 times/y preoperatively and 0.1 ± 0.3 times/y postoperatively (P < 0.05; Table 2). Patients in the LVA group reported an average reduction 4.2 ± 0.5 times/y in the episodes of cellulitis from 5 ± 2 times/y preoperatively to 0.8 ± 1.5 times/y postoperatively, P = 0.07 (Table 2). There was no statistical difference between VLNT and LVA groups in the episodes of cellulitis (P = 0.7).
Quality of Life
Patients in the VLNT group reported statistical improvements in the LYMQoL in overall score (from 3.9 ± 1.2 to 6.4 ± 1.1) and 4 domains of Function (from 26.9 ± 6.3 to 21.8 ± 4.1), Appearance (from 27.7 ± 0.7 to 21.6 ± 3.6), Symptoms (from 19.1 ± 1.3 to 15.1 ± 1.6), and Mood (from 22.4 ± 1.5 to 13.5 ± 4.1), respectively (All 5 P < 0.05) (Table 3).
Table 3.
Quality of Life Outcomes Preoperatively and Postoperatively in Patients undergoing Vascularized Lymph Node Transfer or Lymphovenous Anastomosis Treatment
Patients in the LVA group reported improvements in the LYMQoL in overall score (from 3.0 ± 1.4 to 5.0 ± 2.4) and 4 domains of Function (from 28.5 ± 5.7 to 26.3 ± 6.2), Appearance (from 28.0 ± 0.1 to 26.8 ± 7.0), Symptoms (from 19.5 ± 1 to 17.8 ± 3.5), and Mood (from 23 ± 1.2 to 21 ± 5.7), respectively (P = 0.07, 0.1, 0.1, 0.07, 0.07, respectively) (Table 3).
DISCUSSION
To the best of our knowledge, this study reports the first surgical outcomes using VLNT and LVA to treat patients with long-standing primary lymphedema. From the results of this study, we found that VLNT does appear to provide greater functional improvements, as represented by a circumference reduction, which were most markedly observed above the knee in our patients with primary lymphedema. There was also a greater reduction in body weight in patients receiving VLNT compared with those receiving LVA. Both VLNT and LVA appear to reduce the number of episodes of cellulitis. In the quality of life assessment, VLNT appears to significantly improve the quality of life for patients with primary lymphedema compared with LVA.
Primary lymphedema often occurs at birth and for causes or by mechanisms that are unknown. When primary lymphedema becomes symptomatic in adulthood, these patients often have a long-standing history of lymphedema that is associated with the destruction of lymphatic channels. Adipogenesis or proliferation of adipose tissue coupled with dense fibrosis often results in severe lymphedema of the limb and a more severe presentation. As such, in this series, VLNT (79%) was often preferred over LVA (21%), especially if no patent lymphatic channels were preoperatively evaluated on ICG lymphography. From the patients with primary lymphedema in this series, only 4 limbs (21%) were found to have intact lymphatic channels and were suitable for LVA. As such, our treatment population dictated that the rest of the affected limbs (79%) without intact lymphatic channels should be clinically treated using VLNT as part of our established treatment algorithm.9 This forms a limitation of our study due to the fewer primary lymphedema patients who were suitable for LVA treatment.
One possible reason as to why VLNTs appear to be more beneficial in treating primary lymphedema is that the absence of normal lymphatic channels requires bypassing the congested lymph through the transferred lymph nodes to the affected limb.10,16 Due to the positive interstitial pressure and the provision of a novel route for lymphaticovenous connections, the stagnant lymph can be effectively drained into the newly anastomosed pedicle vein.10,16 The hydrostatic pressure results in the preferential removal of lymphatic fluid via the transferred lymph node flap into the draining venous system, which has been shown in prior studies.10,16,17 One precluding factor is that venous outflow of the limb has to be unaffected to obtain the maximum benefit. Further improvement using adjunctive treatments, such as the use of liposuction if lipodystrophy of the proximal part of the limb presented, could be combined in the treatment of severe primary lymphedema in second stage. This not only decreases the burden on the transferred lymph node flap but also helps with the removal of limb weight and increases both the functional and quality of life outcomes.
Three children with the age of 2 years who were treated with VLNT also reported a good improvement in their limb lymphedema with no evidence of a worsening or progression of the disease (Figs. 4, 5). Children with a stemming of the disease progression and who underwent VLNT also appeared to benefit from an early intervention. These 3 patients in our series had safely undergone VLNT treatment for lower limb lymphedema and produced remarkable results. This preliminary evidence shows promise, and a longer follow-up in our series would provide further conclusive evidence.
Fig. 4.

She suffered from bilateral lower limb lymphedema with 3 episodes of cellulitis per year. The preoperative front view (A). After 2 years follow-up, the right lower limb circumferential difference was improved 1 cm on above knee, 2 cm on below knee. Left lower limb, both of the circumferential difference was improved 2 cm on above and below knee without compression garment (B). After 4-year follow-up, both limbs improved 2.5 cm on above knee and 3.5 cm on below knee without compression garments (C).
Fig. 5.
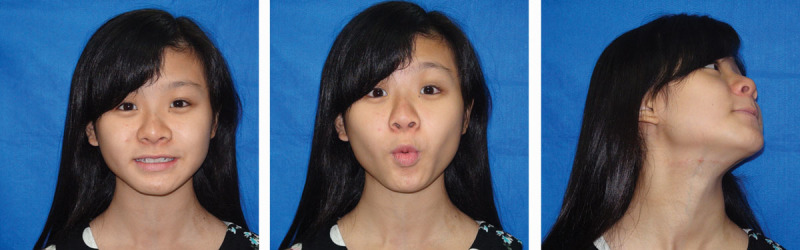
Minimal donor-site morbidity with an inconspicuous scar after the vascularized submental lymph node flap transfer at the 4-year follow-up.
CONCLUSIONS
Primary lymphedema can be effectively treated by using VLNT in 79% or LVA in 21% for better functional and quality of life outcomes. The reduction of above-knee circumference, body weight, episodes of cellulitis, and the improvement of LYMQoL was significantly greater in LVNT compared with LVA.
ACKNOWLEDGMENTS
The English grammar in this article was polished by the American Journal Experts 7CA0-750E-54E7-DE2C-0E95.
Footnotes
Published online 20 December 2018.
This article was podium presented and won the award of the best paper at the 2016 Annual Meeting of the American Society of Plastic Surgeons, September 23–27, 2016, Los Angeles, Calif.; this article was podium presented at ninth Congress of World Society for Reconstructive Microsurgery (WSRM) meeting, June 14–17, 2017, South Korea.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Kurland LT, Molgaard CA. The patient record in epidemiology. Scientific American, 1981;24:554. [DOI] [PubMed] [Google Scholar]
- 2.Smeltzer DM, Stickler GB, Schirger A. Primary lymphedema in children and adolescents: a follow-up study and review. Pediatrics. 1985;76:206. [PubMed] [Google Scholar]
- 3.Maclellan RA, Greene AK. Lymphedema. Semin Pediatr Surg. 2014;23:191. [DOI] [PubMed] [Google Scholar]
- 4.Rezaie T, Ghoroghchian R, Bell R, et al. Primary non-syndromic lymphoedema (Meige disease) is not caused by mutations in FOXC2. Eur J Hum Genet. 2008;16:300. [DOI] [PubMed] [Google Scholar]
- 5.Connell FC, Ostergaard P, Carver C, et al. ; Lymphoedema Consortium. Analysis of the coding regions of VEGFR3 and VEGFC in Milroy disease and other primary lymphoedemas. Hum Genet. 2009;124:625. [DOI] [PubMed] [Google Scholar]
- 6.Brorson H. Adipose tissue in lymphedema: the ignorance of adipose tissue in lymphedema. Lymphology. 2004;37:175. [PubMed] [Google Scholar]
- 7.Loh CY, Wu JC, Nguyen A, et al. The 5th world symposium for lymphedema surgery—recent updates in lymphedema surgery and setting up of a global knowledge exchange platform. J Surg Oncol. 2017;115:6. [DOI] [PubMed] [Google Scholar]
- 8.Allen RJ, Jr, Cheng MH. Lymphedema surgery: patient selection and an overview of surgical techniques. J Surg Oncol. 2016;113:923. [DOI] [PubMed] [Google Scholar]
- 9.Qiu SS, Chen HY, Cheng MH. Vascularized lymph node flap transfer and lymphovenous anastomosis for Klippel-Trenaunay syndrome with congenital lymphedema. Plast Reconstr Surg Glob Open. 2014;2:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito R, Zelken J, Yang CY, et al. Proposed pathway and mechanism of vascularized lymph node flaps. Gynecol Oncol. 2016;141:182. [DOI] [PubMed] [Google Scholar]
- 11.Patel KM, Lin CY, Cheng MH. A prospective evaluation of lymphedema-specific quality-of-life outcomes following vascularized lymph node transfer. Ann Surg Oncol. 2015;22:2424. [DOI] [PubMed] [Google Scholar]
- 12.Cheng MH, et al. Chapter 6: Definition, incidence and pathophysiology of lymphedema. Principles and Practice of Lymphedema Surgery. 20161st ed Oxford, United Kingdom.; N.p.: Elsevier Inc.. [Google Scholar]
- 13.Cheng MH, Nguyen DH, Huang JJ, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol. 2012;126:93. [DOI] [PubMed] [Google Scholar]
- 14.Ito R, Wu CT, Lin MC, et al. Successful treatment of early-stage lower extremity lymphedema with side-to-end lymphovenous anastomosis with indocyanine green lymphography assisted. Microsurgery. 2016;36:310. [DOI] [PubMed] [Google Scholar]
- 15.Granzow JW, Soderberg JM, Dauphine C. A novel two-stage surgical approach to treat chronic lymphedema. Breast J; 2014; 20: 420. [DOI] [PubMed] [Google Scholar]
- 16.Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg. 2014;133:192e. [DOI] [PubMed] [Google Scholar]
- 17.Patel KM, Lin CY, Cheng MH. From theory to evidence: long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J Reconstr Microsurg. 2015;31:26. [DOI] [PubMed] [Google Scholar]


