Supplemental Digital Content is available in the text.
Summary:
Mammary analogue secretory carcinoma (MASC) of salivary glands is a newly recognized tumor entity. We report a child who was initially diagnosed with lymphangioma and referred to our institute for sclerotherapy, only to find out that the tumor was in fact MASC after excision. This case of MASC is in a 7-year-old boy, the youngest case so far reported. He referred to his primary care physician with a infra-auricular swelling, and it was diagnosed as lymphatic malformation he was referred to our institution for sclerotherapy. For Doppler and ultrasound magnetic resonance imaging, there was a distinct cystic lesion with a heterogeneous solid lesion inside. Minimally invasive treatment such as sclerotherapy was thought to be more desirable due to a pediatric case, the risk of postoperative facial paralysis and scar. However, even the successful treatment of cystic lesion with sclerotherapy, solid lesion of the tumor could be remained without pathological findings. Otolaryngologist also thought the importance of pathological diagnosis, and we finally chose surgical excision. The tumor was ultimately diagnosed as MASC considering histological and genetic findings. For child case, we tend to treat patient less invasively, and it might bring a risk of MASC being incorrectly treated nonsurgically such as with sclerotherapy. This could lead to tumor progression and wider radical excision at last. We believe that histological diagnosis should become the priority in similar cases of mixed solid and cystic tumors to avoid incorrect treatment, and we need to choose surgical excision by understanding the character of salivary gland tumor occurring in childhood.
INTRODUCTION
Mammary analogue secretory carcinoma (MASC) is a rare salivary gland neoplasm first described in 2010 by Skálová et al.1 Some of the reported cases describe a slowly growing benign tumor with no occurrences of metastasis or recurrence while others describe a highly invasive tumor, which rapidly leads to death. Here, we report a child who was initially diagnosed with lymphatic malformation and referred to our institute for sclerotherapy; however, reassessment of the tumor led to the final diagnosis of MASC after complete excision.
CASE REPORT
The patient was a 7-year-old boy who referred to his primary care physician with the main complaint of left infra-auricular swelling, which slowly enlarged over a year. Imaging studies showed that the tumor was cystic in nature and originated within the parotid gland. Further cytological findings based on fine needle aspiration showed absence of atypical or abnormal cells. The tumor was first diagnosed as lymphatic malformation, and the contents were aspirated percutaneously twice. However, the tumor recurred, and the patient was referred to our institution for further treatment by sclerotherapy.
At the time of the patient’s first visit to our institution, a left cervical hemispherical mass with a size of about 5 cm was palpated (see figure, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A934, which displays a) At initial presentation to our facility: A tumor of elastic softness about 5 cm on palpation was found at the left cervical area. b) Color Doppler ultrasonography: It shows a uniform hypoechoic cystic lesion with hyperechoic parts. The lesion showed no flow or pooling. c) magnetic resonance imaging (MRI) T2WI scan images: The images show a lesion with high-intensity, there was a distinct tumorous lesion with a somewhat heterogeneous low-intensity region inside, http://links.lww.com/PRSGO/A934). The mass was rubbery and soft in nature but immobile. There was no local tenderness. Doppler ultrasound showed a thick-walled hypoechoic cystic lesion containing a small hyperechoic solid intracystic mass (Supplemental Digital Content 1). Subsequent MRI scan showed the lesion having high signal intensity in T1WI and T2WI. There was also a distinct tumorous lesion with a somewhat heterogeneous low-intensity region inside (Supplemental Digital Content 1).
Needle puncture biopsy was re-performed from the solid part of the tumor, and cytology was confirmed as class III according to the Papanicolaou classification. At first, minimally invasive treatment such as sclerotherapy was thought to be more desirable due to the patient’s age, the risk of postoperative facial paralysis, and visible scar at the neck. However, after further consideration with the otolaryngologists, we ultimately decided on complete excision of the lesion with superficial parotidectomy, because tumor including solid lesion should be made pathological diagnosis.
Intraoperative findings showed that the lesion was located below the platysma, was soft and wrapped in a fibrous capsule. Bottom part of the tumor compressed the digastric and sternocleidomastoid muscles and the facial nerve. The facial nerve branches were preserved, and the tumor was excised with the surrounding parotid gland tissue. To prevent Frey syndrome, remaining exposed parotid gland was covered with a platysma muscular flap, filling the dead space (Fig. 1).2
Fig. 1.
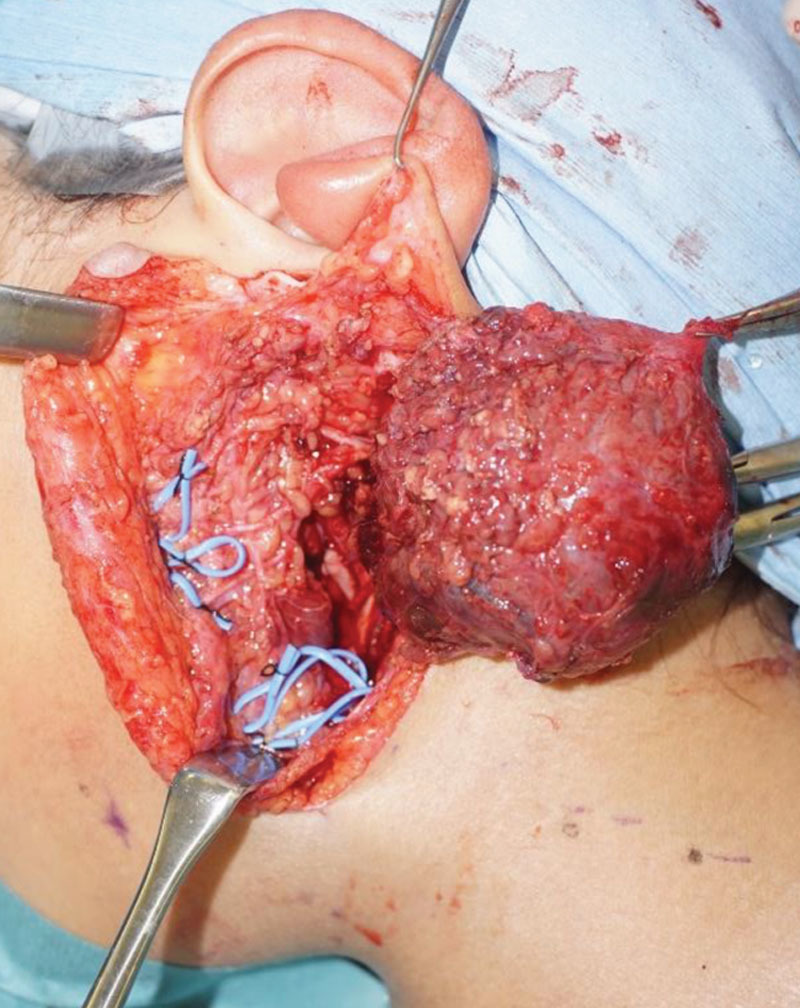
Intraoperative findings after excision: The facial nerve was preserved and the lesion was totally removed.
Hematoxylin and Eosin staining of the tumor showed a vascular fibrotic stroma with proliferating cells rising from the cyst wall. Tumor cells showed similar circular nuclei and slightly basophilic cytoplasm. The cells showed proliferation into a microcystic structure, cells of distinct nucleoli were also observed (Fig. 2A). The tumor cells were positive for mammaglobin, GCDFP-15, S-100, vimentin, and CAM 5.2. ETV6-NTRK3 gene fusion was demonstrated with Reverse Transcription Polymerase Chain Reaction (Fig. 2B). The tumor was ultimately diagnosed as mammary analogue secretory carcinoma considering histological and genetic findings. Tumor diameter was 3 cm and surgical margins were negative. There were no lymph node or distant metastasis (T2N0M0, stage II), ruling out the necessity of any additional treatment.
Fig. 2.
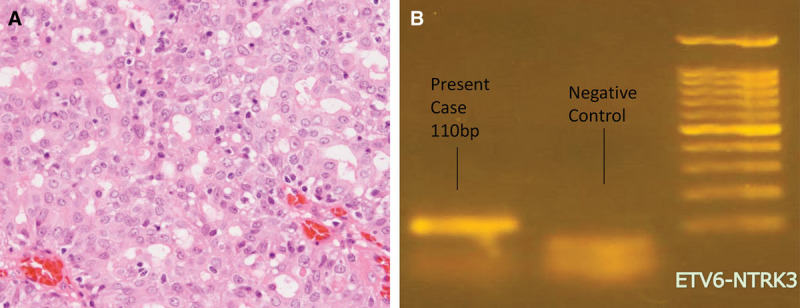
A, Histopathological image of the surgical specimens (HE staining ×100). From the cyst wall, tumor cells grow within the vascular fibrotic stroma forming a microcystic structure. B, Genetic analysis by PCR revealed ETV6-NTRK3 gene fusion. PCR, polymerase chain reaction.
Early postoperative course was good without any complications (Fig. 3). At 20 months after surgery, the patient had no long-term complications including Frey syndrome.
Fig. 3.
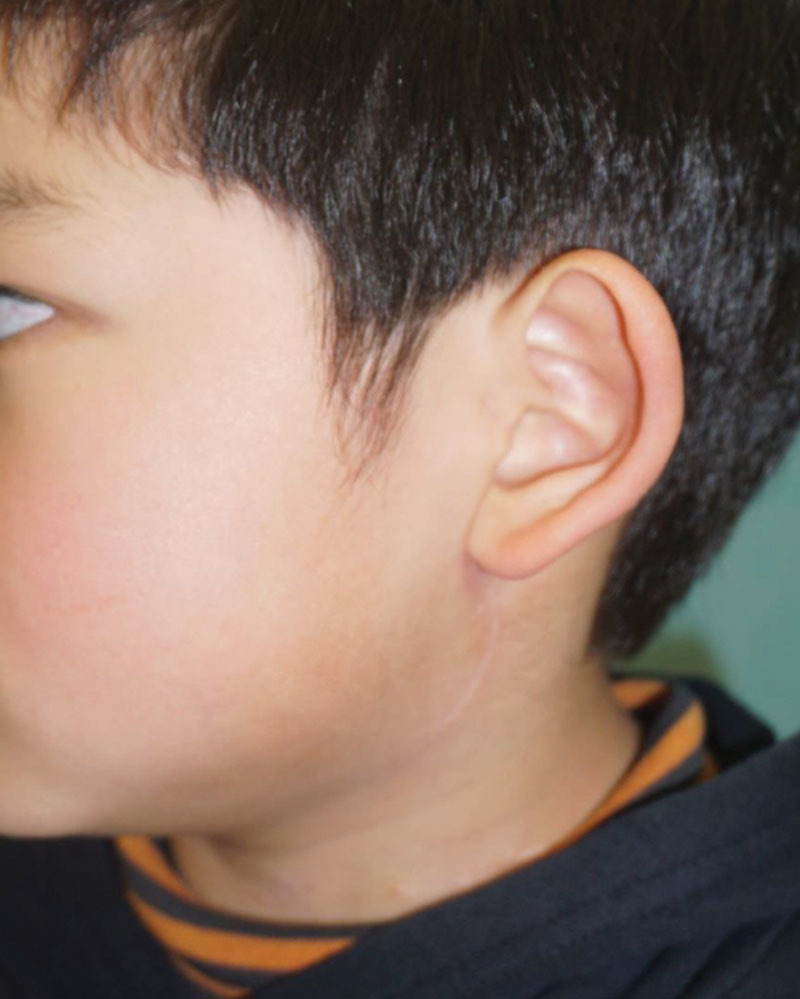
At 1 postoperative year: good cosmetic results were achieved, and there was no recurrence of the primary tumor.
DISCUSSION
MASC of salivary glands is a newly recognized tumor entity. In the initially reported 16 cases, mean patient age was 46 years, mean tumor diameter was 2 cm, and there was no significant gender difference.1 Among the past reports, the youngest example of this disease was 13 years old.3 We report a case of MASC in a 7-year-old boy, the youngest case so far reported. The past reports demonstrate MASC as a slowly growing, painless solid tumor, which sometimes includes large cystic cavities.1 It is also reported that approximately 70% of the tumors defined as MASC develop in the parotid gland.4 Surgical resection is the main choice of treatment and early treatment is recommended. The prognosis is usually good; however, several deaths due to the condition are reported as well.1,4
The rate of childhood salivary gland tumors was reported as 1–5% of the overall of salivary gland tumors, and half of these are malignant neoplasms such as mucoepidermoid carcinoma, acinar cell carcinoma, and undifferentiated carcinoma. Common benign salivary gland neoplasms seen in children are also different from adults, with pleomorphic adenoma being the main neoplasms occurring in pediatric case, and adenolymphoma being rare.5
Cyst forming tumors such as hemangiomas or lymphatic malformation are usually benign. However, malignant tumors such as mucoepidermoid carcinoma, acinar cell carcinoma, and MASC might also form cysts especially in the pediatric population. In addition, MASC is considered to have a higher cyst formation tendency, which might lead to the incorrect diagnosis of a benign tumor.1,4
The main treatment option for venous malformations or lymphatic malformation is sclerotherapy. For pediatric cases, the intention of less invasive treatment might bring the risk of MASC being incorrectly treated nonsurgically such as with sclerotherapy. This could lead to further tumor progression and wider radical excision ultimately. We believe that histological diagnosis should become the priority in similar cases of mixed solid and cystic tumors to avoid incorrect treatment.
CONCLUSIONS
In the present case, we report a child who was initially diagnosed with lymphatic malformation and referred to our institute for sclerotherapy, only to find out that the tumor was in fact MASC after excision. This case of MASC is in a 7-year-old boy, the youngest case so far reported. For pediatric cases, we tend to treat patient less invasively, and it might bring a risk of MASC being incorrectly treated nonsurgically, which could lead to tumor progression and wider radical excision ultimately. Cystic lesions of the parotid lesion all require imaging by MRI and should be assessed by a specialist vascular anomalies team. Where doubt exists about the diagnosis biopsy should be performed.
Supplementary Material
Footnotes
Published online 13 December 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi A, Mochizuki M, Suda S, et al. Effectiveness of platysma muscle flap in preventing Frey syndrome and depressive deformities after parotidectomy. J Plast Reconstr Aesthet Surg. 2016;69:663. [DOI] [PubMed] [Google Scholar]
- 3.Hwang MJ, Wu PR, Chen CM, et al. A rare malignancy of the parotid gland in a 13-year-old Taiwanese boy: case report of a mammary analogue secretory carcinoma of the salivary gland with molecular study. Med Mol Morphol. 2014;47:57. [DOI] [PubMed] [Google Scholar]
- 4.Sethi R, Kozin E, Remenschneider A, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope. 2014;124:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nito T, Ichimura K, Naito R, et al. Salivary gland tumor in children—a 20-year experience. Pediatr Otolaryngol. 1998;9:40. [Google Scholar]


