Abstract
Background:
The vascularized groin and submental lymph node (VGLN and VSLN) flaps are valuable options in the treatment of lymphedema. This study was to compare outcomes between VGLN and VSLN transfers for breast cancer–related lymphedema.
Methods:
Between January 2008 and December 2016, VGLN and VSLN transfers for upper limb lymphedema were compared including flap characteristics, flap elevation time, complications, and limb circumference changes.
Results:
All flaps survived. Similar vein (2.6 versus 3.2 mm; P = 0.3) and artery diameter (2.1 versus 2.8 mm; P = 0.3) and number of lymph nodes (3 versus 4; P = 0.4) were found between VGLN and VSLN groups, respectively. Circumferential reduction rate was higher in VSLN than VGLN (P = 0.04) group. Vascular complication rate with salvage rate was not statistically different between the 2 groups. Donor-site complication and total complication rates were statistically higher in VGLN than VSLN flaps (7.7% versus 0%, P = 0.004; 46.2% versus 23.3%, P = 0.002). At a mean 39.8 ± 22.4 months, the circumferential reduction rate was statistically higher in VSLN than in the VGLN group (55.5 ± 14.3% versus 48.4 ± 23.9%, P = 0.04). Both flaps were effectively decreased in the episodes of cellulitis.
Conclusions:
Both VGLN and VSLN flaps are valuable surgical options in treating breast cancer–related lymphedema. However, the VSLN flap for breast cancer–related lymphedema is better in providing more significant improvements in limb circumference, a faster flap harvest time, decreased complication rates, and minimal donor-site iatrogenic lymphedema.
INTRODUCTION
The incidence of breast cancer–related lymphedema (BCRL) has been reported to range from 4% to 62.5%.1 Several studies have found that lymphovenous anastomosis (LVA) is effective in early-stage lymphedema but less effective in advanced-stage lymphedema.2–4 This may be due to the loss of the ability of the lymphatic vessels to adequately transfer lymph fluid in advanced stages of lymphedema. Another reason could be because most of the patent lymphatic vessels suitable for lymphovenous anastomosis are deteriorated or difficult to find within the fibrotic tissues in severely lymphedematous limbs.2–4 Vascularized lymph node (VLN) transfer is typically reserved for patients with Cheng’s Lymphedema Grade II to IV lymphedema and with complete occlusion detected on lymphoscintigraphy.2–5 VLN transfer has been described in both animal and human studies.6,7
The mechanism by which VLN transfer alleviates the symptoms of lymphedema continues to be an emerging science; however, theories have been proposed.8–15 One theory is the induction of lymphangiogenesis and reconstitution of lymphatic channels with transfer of lymph nodes to the affected limb.8,11,14,15 Another theory is that VLNs behave like the motor of a pump that absorbs interstitial fluids and subsequently diverts that fluid to the venous circulation.9,10,12,13 The “catchment effect” continues to drain the lymph that when the subcutaneous interstitial pressure in the lymphedematous limb decreases, there is more lymph from the surrounding tissue that are recruited into the transferred lymph nodes.16 The “gravity effect” provides the propelling motion of swinging the arms results in fluid shifting toward distally.16 As such, by placing the pump distally where fluid accumulation is most, the drainage efficiency of the flap is maximized. This mechanism is supported by the presence of indocyanine green (ICG) dye uptake in the venous system that is detected after peripheral intradermal or intranodal injection of the dye.9,10 It had already been shown that the length of ICG latency period has an inverse relationship with the degree of circumference reduction when assessing the impact of the latency period in relation to clinical improvement.13 There was clinical evidence that transferring the VLN flap to a more distal recipient site such as the wrist results in reductions in arm circumference and more rapid movement of radiolabeled tracer on lymphoscintigraphy indicating improved lymphatic clearance.16,17 The other benefits of using a distal recipient site include an unscarred and nonoperated area with available recipient vessels and lymph fluid accumulation caused by gravity. Furthermore, the number of VLNs in the transferred flap is positively correlated with the degree of limb volume reduction.12 Therefore, choosing a VLN flap that has a high number of viable lymph nodes within it will heighten the chance of a successful outcome.
There are several donor sites available for VLN transfers; the most common include groin, submental, supraclavicular, lateral thoracic, omentum, and jejunal mesenteric.18–35 Previous studies have demonstrated the effectiveness and benefits of groin and submental flaps.19,33,34,36 While each method has been studied individually, there have not been outcomes studies to date of these 2 commonly used donor sites in direct comparison with each other accompanied with sufficient long-term follow-up. This study aims to compare the outcomes of vascularized groin lymph node (VGLN) and vascularized submental lymph node (VSLN) transfers with regard to their limb circumference improvement and complications.
PATIENTS AND METHODS
A prospectively maintained database of patients at Chang Gung Memorial Hospital between January 2008 and December 2016 who received VLN transfer for BCRL was reviewed after institutional review board approval. Before surgery, informed consent was obtained from patients. Risks, benefits, and treatment alternatives were discussed. Inclusion criteria included all patients with BCRL and had either VGLN or VSLN flap transfers. Exclusion criteria were any combined VLN transfer and LVA procedures, VLN transfer combined with liposuction or partial excision, VLN transfer and using elbow or axilla as the recipient site. All patients had preoperative clinical evaluation including lymphoscintigraphy, ICG lymphography, and radiographic work-up including computed tomography evaluation of the affected limb, duplex ultrasonography and magnetic resonance angiography of the donor sites to evaluate pedicle location, surrounding anatomical structures, and number of lymph nodes at the site.37,38 Patient circumferential measurements were obtained at the same follow-up evaluation in both submental and groin groups. Patient characteristics and demographics were collected and compared (Table 1). Harvest of the VGLN (Fig. 1) and VSLN (Fig. 2) flaps was performed as previously described.5,16,20,34 Recipient sites were at the wrist using a dorsal branch of radial artery or the ulnar artery as recipient. For the recipient vein, either cephalic or basilic vein was used. Both arterial and venous anastomoses were performed end-to-end. Superficial veins were used as recipient veins of VLN transfers since the deep veins (comitant veins of the major artery) are usually compressed by the lymphedematous tissue that results in a positive compartment pressure.
Table 1.
Comparisons of Patient Demographics, Flap Harvest Time, Number of Lymph Nodes, and Outcome between VGLN and VSLN Flaps
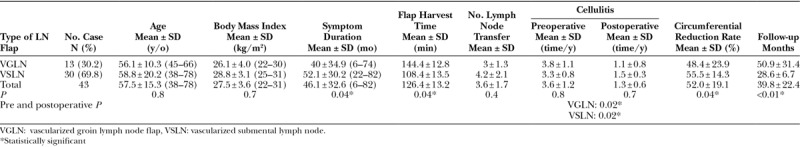
Fig. 1.
A 65-year-old woman who was a victim of right breast cancer postmastectomy, axillary lymph node dissection, and chemoradiation. She suffered from right upper limb lymphedema with 3 episodes of cellulitis per year for 2 years. She underwent right vascularized groin lymph node flap transfer to right dorsal wrist. Skin paddle 12 × 6 cm was designed on right groin below the inguinal ligament and close to common femoral vessels. One perforator was marked with pencil of medial Doppler (A). The superficial circumflex vessels were identified with vessel loop. The flap was elevated with short pedicle artery and 2 veins (C). The donor site of right groin 6 years after (D).
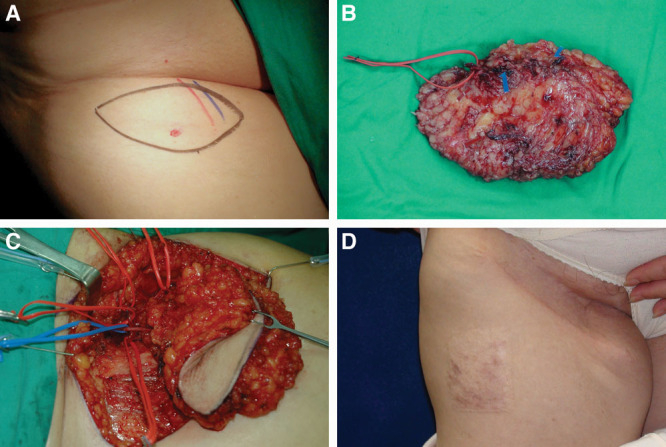
Fig. 2.
A 52-year-old woman suffered from breast cancer-related lymphedema on right upper limb for 4 years. A vascularized submental lymph node flap 8.5 × 2.2 cm was designed on right neck (A). Three sizable lymph nodes (yellow arrows) were noted on the divided flap (B). Two marginal mandibular nerves were well preserved under microscope (C). The donor site scar was inconspicuous 30 months postoperatively (D).
Outcomes of interest were collected prospectively including flap characteristics (recipient vein and artery, vessel diameter, number of lymph nodes within the flap), operative time, intraoperative and postoperative complications, and limb circumference changes (centimeters) at the follow-up. Perioperative complications recorded were re-exploration, hematoma, seroma, infection, and skin paddle loss. Postoperative complications recorded were cellulitis episodes. Time to wound healing and the need for split-thickness skin graft were recorded. Measurement of limb circumference was performed at each clinic visit using the previously described parameters of 10 cm above and below the elbow.1 Follow-up of patients occurred initially every month until 3 months, then every 3 months.
Using SPSS 17.0 statistical software (SPSS, Inc., Chicago, Ill.), statistical analysis was performed. A chi-square test was used to analyze all complication rates. A value of P ≤ 0.05 was considered statistically significant. The nonparametric Mann-Whitney test was used for continuous variables.
RESULTS
A total of 43 patients underwent submental or groin VLN flap transfers to the wrist for upper extremity lymphedema and met inclusion and exclusion criteria during the study period. Thirteen (30.2%) underwent VGLN transfer and 30 (69.8%) had VSLN transfer; all VLN flaps survived. The mean age of all patients was 57.5 years (ranged, 38–78), with 56.1 years in the VGLN group and 58.8 years in the VSLN group, respectively (P = 0.8; Table 1). Average BMI was 27.5 for the entire study population with 26.1 in VGLN group and 28.8 in VSLN group (P = 07). The mean duration of lymphedema symptoms including swelling, heaviness, and recurrent infections was 46.1 months (ranged, 6–82 months). The VSLN group (52.1 months) had a statistically longer symptom duration than the VGLN group (40 months; P = 0.04).
In evaluating flap characteristics, similar donor vein diameter and artery diameter were found between VGLN and VSLN cohorts (Table 2). Average donor vein diameter was 2.9 mm for the entire study group with 2.6 mm in the VGLN group and 3.2 mm in the VSLN group (P = 0.3). Mean donor artery diameter was 2.5 mm for the entire study group, with 2.1 mm in the VGLN group, and 3.2 mm in the VSLN group (P = 0.3). The average number of sizable lymph nodes for the entire study group was 3.6, 3 in the VGLN group, and 4.2 in the VSLN group (P = 0.4). Harvest time of 108 minutes in the VSLN flap was statistically less than the 144 minutes in the VGLN flap (P = 0.04).
Table 2.
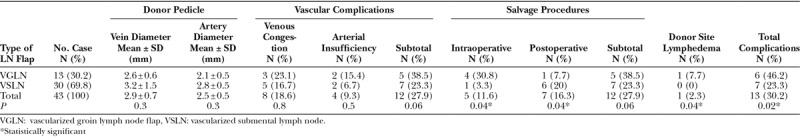
Comparisons of Vascular Complications, Salvage Procedures, and Donor-site Lymphedema between VGLN and VSLN Flaps
At a mean follow-up of 39.8 months, the mean limb circumferential reduction rate 48.4% in the VGLN group (Fig. 3) was statistically less than 55.5% for the VSLN group (Fig. 4) (P = 0.04).
Fig. 3.
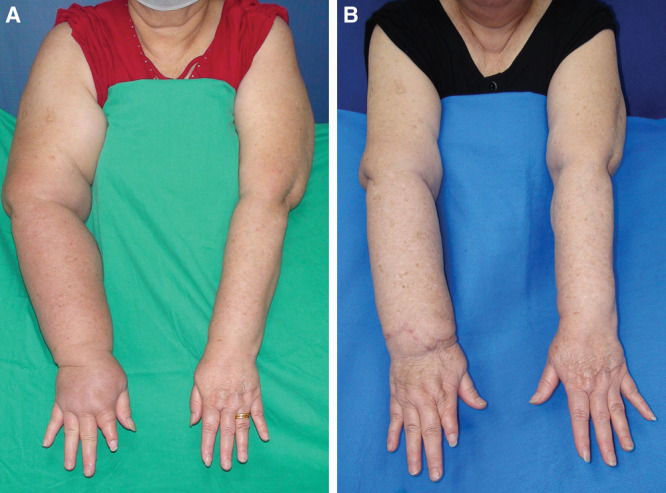
The preoperative front view of case 1 (A) and postoperative 48 months follow-up front view (B). The circumferential difference was improved from 52% to 18%.
Fig. 4.
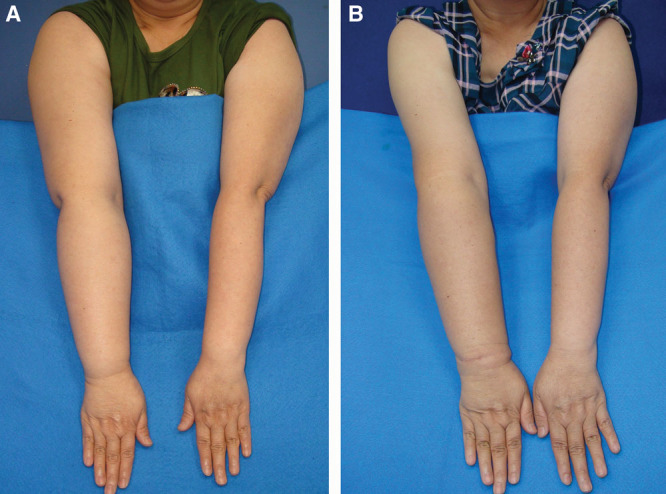
The preoperative front view of case 2 (A) and postoperative 30 months follow-up front view (B). The circumferential difference was improved from 32% to 10%.
It was found that VSLN patients had significantly fewer intraoperative salvage procedures (P = 0.04). The VGLN group also had significant fewer postoperative salvage procedures (P = 0.04). Complications in recipient site were 38.5% in the VGLN cohort as compared with the 23.3% in the VSLN cohort but the difference was not statistically significant (P = 0.06). Donor-site lymphedema was statistically significantly higher in the VGLN flap group than VSLN (7.7% versus 0%; P = 0.04). Total complication was statistically greater in the VGLN cohort than the VSLN (46.2% versus 23.3%; P = 0.02).
DISCUSSION
The reasons for the better functional recover in the VSLN group may be 2-fold: (1) greater number of lymph nodes, and (2) bigger size of donor vein in the VSLN group. Follow-up time was shorter in the VSLN group because it was used more frequently later in the study period. However, the follow-up 28.6 ± 6.7 months is ample time to see a meaningful circumferential reduction rate postoperatively within the patient group.
In severe cases of limb lymphedema, VLN transfers are commonly utilized and have been shown to be beneficial in symptom improvement.2–5,39,40 A common advantage between these VLN donor sites is that they all allow primary donor-site closure. The ideal VLN flap has the following characteristics: maximum lymphatic drainage capacity with a greater number of sizable lymph nodes, absence of donor-site lymphedema, large diameter of donor vessels, a long pedicle, and inconspicuous scar.
Each of these various donor sites has pros and cons (Table 3). Axillary VLN transfer has been described and proposed as an alternative lymph node basin.24,25 The most notable concern for its use is the creation of iatrogenic upper limb lymphedema.35 The reverse mapping technique can be employed to avoid harvesting the lymph nodes that drain the upper limb.23 Lymph nodes in this location have greater anatomic variations in relation to the thoracodorsal and lateral thoracic vessels, especially for lymph node venous drainage. Common variations in vascular anatomy can lead to requiring 2 pedicle anastomoses or the thoracodorsal nerve may be sacrificed.
Table 3.
Comparisons of Donor Sites of Vascularized Lymph Node Flap Transfer
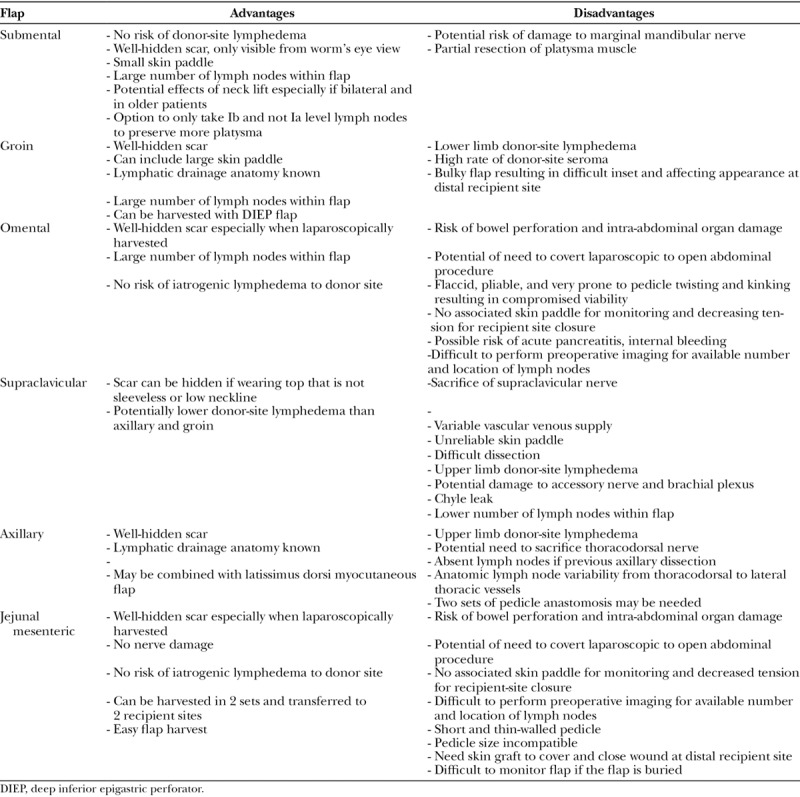
Vascularized supraclavicular lymph node (VScLN) transfer has several advantages (Table 3). Proponents of this flap argue that the scar is well-concealed; however, should a patient wear common clothing such as a tank top, strapless dress, or wide-open necklines, the scar is certainly visible. This can be a concern, given that the secondary upper limb lymphedema patient population is predominantly women.1,5,43,44 It was previously believed to bear no risk for secondary lymphedema; however, case reports have described incidences of upper limb lymphedema developing after harvest of VScLN.18,27,28 Disadvantages with the VScLN flap include damage to the accessory nerve and brachial plexus, chyle leak, and lower number of lymph nodes in anatomic studies.32,38 Furthermore, a common sequela of this flap elevation is the sacrifice of supraclavicular nerves, which is unavoidable in the course of this flap’s elevation, and causes numbness of the superior chest region.
Vascularized omentum lymph node (VOLN) flap transfer is beneficial such that the scars are almost undetectable assuming it is performed laparoscopically. It is also rich in number of lymph nodes. But possibly the most enticing for its use is that there is no risk of iatrogenic lymphedema.21,26,29 Furthermore, its immunologic potency is a characteristic only in the VOLN but not in other VLN flaps.29 There are disadvantages to the VOLN flap, which include bowel perforations, pancreatitis, internal bleeding, and damage to the intra-abdominal organs. Even when performed laparoscopically and by a very skilled surgeon, the abdominal cavity is violated and harvesting the VOLN flap results in adhesions that can make a patient more prone to small bowel obstructions in the future. As with all laparoscopic procedures, there is the possibility of conversion to an open procedure that would increase the size of the scars significantly along with an increase in associated donor-site morbidity. Due to the extremely pliable, flaccid, short, and thin nature of the gastroepiploic vessels, it is more prone to kinking of its extensive vasculature. It is necessary to take care in meticulously unraveling the omentum after it is retrieved from the laparoscopic specimen retrieval bag to ensure proper orientation to avoid ischemia and necrosis. There is also a theoretical risk of acute pancreatitis due to the close proximity of dissection to this organ.45 Finally, the VOLN has an absence of a skin paddle to provide coverage of the recipient site. Thus, the pedicle is in danger of being compressed especially with a tight primary closure of the recipient site. Additional skin graft can usually be used in VOLN flaps to close the recipient site with less tension. Should a skin graft be used instead, an unsightly appearance results as is expected with using split-thickness skin grafts, which may cause scarring and thereafter compromise the function of the lymph nodes. The skin paddle normally aids with flap monitoring, which is not an option in using VOLN flaps.
The groin flap is a procedure that most plastic surgeons are familiarized with.5,16,19,20 One of the main advantages of the VGLN flap is that it grants a substantial number of lymph nodes. The scar is also well-concealed with common everyday clothing. However, the VGLN transfer does have its drawbacks. The most concerning hazard is iatrogenic secondary lower limb lymphedema46,47 7.7% in this VGLN group in this study, but only 1 of 36 VGLN flap transfer (2.8%) patients developed donor-site lymphedema in the senior author’s experience without reverse lymphatic mapping. Many surgeons have also found that it has a high rate of donor-site seroma especially when combined with the harvest of the deep inferior epigastric perforator flap, but this was not found in this study. Studies regarding anatomical landmarks and reverse lymphatic mapping can make the use of the VGLN flap safer but cannot fully eliminate this risk.23,37,48 The VGLN flap is much bulkier than the VSLN flap, which makes the appearance of recipient site initially unsightly. Subsequent revisional and debulking procedures are usually requested by patients.
The VSLN flap has some disadvantages in the hands of the inexperienced surgeon.5,34,36,42 There is a risk of injury to the marginal mandibular branch of the facial nerve innervated facial muscles including depressor labii inferioris muscle, depressor anguli oris muscle, and mentalis muscle, which can be avoided with delicate dissection under microscope. Many critics state that the donor-site scar under the mandible is visible. However, the scar is not visible from the anterior or lateral front-on view of the face. It is only when the head tilted back can the scar be seen in the worm’s eye view. This is a pose that few people adopt on a day-to-day basis. Furthermore, in the more elderly patient or middle-aged patient with excess facial and neck skin laxity, VSLN has the added benefit of rejuvenating and tightening the appearance of the neck. With modifications to the earlier techniques, there is the option to not take the level Ia lymph nodes, thus allowing the anterior belly of the digastric muscle to not be disturbed while taking only level Ib lymph nodes within the flap. Recent modifications to this technique partially preserve the medial part of the platysma that may decrease the compromise of lower lip depression function.49 Finally, and most importantly, the VSLN flap is most advantageous in that it has substantial number of lymph nodes per side with greater diameter of donor facial vein while bearing no risk for postoperative iatrogenic lymphedema.
There are several limitations to this study. This was a retrospective study on prospectively collected data, which can have its inherent bias and confounding factors. Second, the sample of patients studied is relatively small; however, we chose to only focus on upper limb lymphedema to provide as homogeneous a study group as possible for comparison of the 2 VLN transfer groups. Although the study sample is small in absolute numbers, this study assessed the largest group reported so far for comparing VSLN and VGLN flaps for BCRL. Certainly, the field of lymphedema surgery continues to be an emerging science. Finally, this study compares clinical outcomes in 2 of the most commonly used donor sites for VLN transfer; however, it does not compare the VGLN and VSLN flaps to other known VLN flap donor sites. Although studies have compared LVA with VLN transfer,40,41 this is the first study to compare 2 VLN transfer flaps in a direct head-to-head comparison but certainly paves the way for future studies in comparing other donor sites.
There are several significant findings to this study. First, both VSLN and VGLN flaps are effective for BCRL, but the VSLN had significantly greater improvement in circumferential reduction rate. Second, cosmesis of VSLN is better than that of VGLN. The quality of the skin paddle has a better appearance on the wrist when it is from the submental region than from the groin. The donor site scar under the chin is well hidden, provides a thin and nice scar, and in some patients, provides an added benefit of giving an aesthetic tightening effect of the chin and neck area similar to a small neck lift especially in bilateral cases. Third, the operative time is shorter with the VSLN in comparison to the VGLN. This may in part be because the dissection required with the VSLN flap is less. Finally, total complication rate of the VSLN is less than that of the VGLN flap.
CONCLUSIONS
Vascularized groin and submental lymph node flaps are both effective surgical options in treating BCRL. The VSLN flap for BCRL provides more significant improvements in limb circumference, lesser total complication rate, a faster flap harvest time, and perhaps the most important factor: no risk of donor-site iatrogenic lymphedema.
Footnotes
Published online 13 December 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge has been paid by Ming-Huei Cheng.
REFERENCES
- 1.Ugur S, Arici C, Yaprak M, et al. Risk factors of breast cancer-related lymphedema. Lymphat Res Biol. 2013;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campisi C, Bellini C, Accogli S, et al. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery. 2010;30:256. [DOI] [PubMed] [Google Scholar]
- 3.Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010;126:752. [DOI] [PubMed] [Google Scholar]
- 4.Koshima I, Nanba Y, Tsutsui T, et al. Minimal invasive lymphaticovenular anastomosis under local anesthesia for leg lymphedema: is it effective for stage III and IV? Ann Plast Surg. 2004;53:261. [DOI] [PubMed] [Google Scholar]
- 5.Cheng MH, Chang DW, Patel KM. Principles and Practice of Lymphedema Surgery. 2016Oxford, UK: Elsevier. [Google Scholar]
- 6.Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HC, O’Brien BM, Rogers IW, et al. Lymph node transfer for the treatment of obstructive lymphoedema in the canine model. Br J Plast Surg. 1990;43:578. [DOI] [PubMed] [Google Scholar]
- 8.Can J, Cai R, Li S, et al. Experimental study of lymph node auto-transplantation in rats. Chin Med J (Engl). 1998;111:239. [PubMed] [Google Scholar]
- 9.Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg. 2014;133:192e. [DOI] [PubMed] [Google Scholar]
- 10.Ito R, Zelken J, Yang CY, et al. Proposed pathway and mechanism of vascularized lymph node flaps. Gynecol Oncol. 2016;141:182. [DOI] [PubMed] [Google Scholar]
- 11.Lähteenvuo M, Honkonen K, Tervala T, et al. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation. 2011;123:613. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen DH, Chou PY, Hsieh YH, et al. Quantity of lymph nodes correlates with improvement in lymphatic drainage in treatment of hind limb lymphedema with lymph node flap transfer in rats. Microsurgery. 2016;36:239. [DOI] [PubMed] [Google Scholar]
- 13.Patel KM, Lin CY, Cheng MH. From theory to evidence: long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J Reconstr Microsurg. 2015;31:26. [DOI] [PubMed] [Google Scholar]
- 14.Rabson JA, Geyer SJ, Levine G, et al. Tumor immunity in rat lymph nodes following transplantation. Ann Surg. 1982;196:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shesol BF, Nakashima R, Alavi A, et al. Successful lymph node transplantation in rats, with restoration of lymphatic function. Plast Reconstr Surg. 1979;63:817. [PubMed] [Google Scholar]
- 16.Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg. 2009;123:1265. [DOI] [PubMed] [Google Scholar]
- 17.Gharb BB, Rampazzo A, Spanio di Spilimbergo S, et al. Vascularized lymph node transfer based on the hilar perforators improves the outcome in upper limb lymphedema. Ann Plast Surg. 2011;67:589. [DOI] [PubMed] [Google Scholar]
- 18.Althubaiti GA, Crosby MA, Chang DW. Vascularized supraclavicular lymph node transfer for lower extremity lymphedema treatment. Plast Reconstr Surg. 2013;131:133e. [DOI] [PubMed] [Google Scholar]
- 19.Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2013;131:1286. [DOI] [PubMed] [Google Scholar]
- 20.Cheng MH, Chen SC, Henry SL, et al. Reply: vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: the flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2014;133:428e. [DOI] [PubMed] [Google Scholar]
- 21.Ciuce C, Seddiq F, Fodor M, et al. Omental free-tissue transfer: indications and results from personal experience. Microsurgery. 2003;23:198. [DOI] [PubMed] [Google Scholar]
- 22.Coriddi M, Wee C, Meyerson J, et al. Vascularized jejunal mesenteric lymph node transfer: a novel surgical treatment for extremity lymphedema. J Am Coll Surg. 2017;225:650. [DOI] [PubMed] [Google Scholar]
- 23.Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg. 2015;135:277. [DOI] [PubMed] [Google Scholar]
- 24.Gerety PA, Pannucci CJ, Basta MN, et al. Lymph node content of supraclavicular and thoracodorsal-based axillary flaps for vascularized lymph node transfer. J Vasc Surg Venous Lymphat Disord. 2016;4:80. [DOI] [PubMed] [Google Scholar]
- 25.Kwiecien GJ, Uygur S, Korn J, et al. Vascularized axillary lymph node transfer: a novel model in the rat. Microsurgery. 2015;35:662. [DOI] [PubMed] [Google Scholar]
- 26.Lasso JM, Pinilla C, Castellano M. New refinements in greater omentum free flap transfer for severe secondary lymphedema surgical treatment. Plast Reconstr Surg Glob Open. 2015;3:e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, McClure E, Reinertsen E, et al. Lymphedema of the upper extremity following supraclavicular lymph node harvest. Plast Reconstr Surg. 2015;135:1079e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mardonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: a prospective study of 100 consecutive cases. J Surg Oncol. 2017;115:68. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen AT, Suami H. Laparoscopic free omental lymphatic flap for the treatment of lymphedema. Plast Reconstr Surg. 2015;136:114. [DOI] [PubMed] [Google Scholar]
- 30.Pannucci C, Gerety PA, Basta MN, et al. Vascularized lymph node transfer for lymphedema: anatomic comparison of the supraclavicular and thoracodorsal lymph node flaps. J Vasc Surg Venous Lymphat Disord. 2015;3:124. [DOI] [PubMed] [Google Scholar]
- 31.Scaglioni MF, Arvanitakis M, Chen YC, et al. Comprehensive review of vascularized lymph node transfers for lymphedema: outcomes and complications. Microsurgery. 2016;38:222. [DOI] [PubMed] [Google Scholar]
- 32.Steinbacher J, Tinhofer IE, Meng S, et al. The surgical anatomy of the supraclavicular lymph node flap: a basis for the free vascularized lymph node transfer. J Surg Oncol. 2017;115:60. [DOI] [PubMed] [Google Scholar]
- 33.Tan PW, Goh T, Nonomura H, et al. Hilar vessels of the submandibular and upper jugular neck lymph nodes: anatomical study for vascularized lymph node transfer to extremity lymphedema. Ann Plast Surg. 2016;76:117. [DOI] [PubMed] [Google Scholar]
- 34.Tzou CH, Meng S, Ines T, et al. Surgical anatomy of the vascularized submental lymph node flap: anatomic study of correlation of submental artery perforators and quantity of submental lymph node. J Surg Oncol. 2017;115:54. [DOI] [PubMed] [Google Scholar]
- 35.Vignes S, Blanchard M, Yannoutsos A, et al. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg. 2013;45:516. [DOI] [PubMed] [Google Scholar]
- 36.Cheng MH, Huang JJ, Huang JJ, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol. 2012;126:93. [DOI] [PubMed] [Google Scholar]
- 37.Dayan JH, Dayan E, Kagen A, et al. The use of magnetic resonance angiography in vascularized groin lymph node transfer: an anatomic study. J Reconstr Microsurg. 2014;30:41. [DOI] [PubMed] [Google Scholar]
- 38.Patel KM, Chu SY, Huang JJ, et al. Preplanning vascularized lymph node transfer with duplex ultrasonography: an evaluation of 3 donor sites. Plast Reconstr Surg Glob Open. 2014;2:e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mardonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: a prospective study of 100 consecutive cases. J Surg Oncol. 2017;115:68. [DOI] [PubMed] [Google Scholar]
- 40.Akita S, Mitsukawa N, Kuriyama M, et al. Comparison of vascularized supraclavicular lymph node transfer and lymphaticovenular anastomosis for advanced stage lower extremity lymphedema. Ann Plast Surg. 2015;74:573. [DOI] [PubMed] [Google Scholar]
- 41.Engel H, Lin CY, Huang JJ, et al. Outcomes of lymphedema microsurgery for breast cancer-related lymphedema with or without microvascular breast reconstruction. Ann Surg. 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Allen RJ, Jr, Cheng MH. Lymphedema surgery: patient selection and an overview of surgical techniques. J Surg Oncol. 2016;113:923. [DOI] [PubMed] [Google Scholar]
- 43.Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest. 2005;23:76. [PubMed] [Google Scholar]
- 44.Nesvold IL, Dahl AA, Løkkevik E, et al. Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol. 2008;47:835. [DOI] [PubMed] [Google Scholar]
- 45.Dayan JH. Lymphedema treatment: non-surgical and surgical approaches. Annual Meeting The American Society of Plastic Surgeons. 2016Los Angeles, Calif.: Olivia A. Ho. [Google Scholar]
- 46.Pons G, Masia J, Loschi P, et al. A case of donor-site lymphoedema after lymph node-superficial circumflex iliac artery perforator flap transfer. J Plast Reconstr Aesthet Surg. 2014;67:119. [DOI] [PubMed] [Google Scholar]
- 47.Viitanen TP, Mäki MT, Seppänen MP, et al. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg. 2012;130:1246. [DOI] [PubMed] [Google Scholar]
- 48.Patel KM, Manrique O, Sosin M, et al. Lymphatic mapping and lymphedema surgery in the breast cancer patient. Gland Surg. 2015;4:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poccia I, Lin CY, Cheng MH. Platysma-sparing vascularized submental lymph node flap transfer for extremity lymphedema. J Surg Oncol. 2017;115:48. [DOI] [PubMed] [Google Scholar]


