Supplemental Digital Content is available in the text.
Abstract
Background:
Untreated surgical conditions account for one-third of the total global burden of disease, and a lack of trained providers is a significant contributor to the paucity of surgical care in low- and middle-income countries (LMICs). Wearable technology with real-time tele-proctoring has been demonstrated in high-resource settings to be an innovative method of advancing surgical education and connecting providers, but application to LMICs has not been well-described.
Methods:
Google Glass with live-stream capability was utilized to facilitate tele-proctoring between a surgeon in Mozambique and a reconstructive surgeon in the United States over a 6-month period. At the completion of the pilot period, a survey was administered regarding the acceptability of the image quality as well as the overall educational benefit of the technology in different surgical contexts.
Results:
Twelve surgical procedures were remotely proctored using the technology. No complications were experienced in any patients. Both participants reported moderate visual impairment due to image distortion and light over-exposure. Video-stream latency and connection disruption were also cited as limitations. Overall, both participants reported that the technology was highly useful as training tool in both the intraoperative and perioperative setting.
Conclusions:
Our experience in Mozambique demonstrates the feasibility of wearable technology to enhance the reach and availability of specialty surgical training in LMICs. Despite shortcomings in the technology and logistical challenges inherent to international collaborations, this educational model holds promise for connecting surgeons across the globe and introducing expanded access to education and mentorship in areas with limited opportunities for surgical trainees.
BACKGROUND
There is a paucity of trained health care providers in low- and middle-income countries (LMICs) contributing to the large unmet burden of surgical disease. Two-thirds of the world’s population are not currently able to access basic surgical and anesthetic care, and greater than 46.6% of countries have a density of skilled health professionals less than 22.8 per 10,000 population, which is categorized by the World Health Organization as a “medical workforce crisis.”1 As a result of this gap, an estimated 2.2 million additional skilled medical professionals are needed to address the current global need for surgery.2,3 Ongoing efforts are being made, by multiple organizations and institutions, to reduce this educational gap, but attempts at training additional providers are complicated by the practical and logistical constraints of volunteer travel.2 Local providers forced to seek medical training outside of their countries due to lack of domestic opportunities often fuel brain drain, a failure of the newly trained providers to return to their countries to serve the communities most in need, and strategies to build capacity and encourage in-country retention are essential. If this problem is not addressed and current educational methods are not updated, it is estimated that there will be a global deficit of about 12.9 million skilled health professionals by 2035.3
Tele-proctoring is an emerging technology where audio and video interaction facilitates the virtual presence of a teacher to provide real-time instruction and technical assistance from a different geographic location. Similar to traditional mentoring, it allows the educator to simultaneously provide training to the novice surgeon while additionally providing care to the patient. In LMICs, where training opportunities may be limited, tele-proctoring avoids many of the logistic obstacles around distance, time constraints and cost associated with volunteer educators traveling to provide in-person training.4–7 In resource-limited settings, tele-proctoring serves the additional benefit of providing access to surgical expertise for patients requiring procedures in areas where specialty care might otherwise not exist.6–9 Numerous studies have demonstrated its feasibility and efficacy as a surgical training model since the emergence of the technology in the mid 1990s,4,5,8,10 and additional studies have shown its applicability to international11 and low-resource settings.12–14
The majority of surgical tele-proctoring has developed around laparoscopic or endoscopic procedures, given the necessity of a surgical scope during operation. The vantage point of the transmitted image to the remote viewer is the same as that seen by the surgeon on the screen in the operating room, and while transmission speed and internet bandwidth raise potential concerns for image clarity, both the remote observer and operating surgeon share an identical perspective. Open surgery, on the other hand, poses a unique challenge for incorporating cameras into the surgical field and ensuring that the camera can replicate the surgeon’s point of view without interfering with his ability to use his hands. Wearable technologies, such as Google Glass (Google, Inc, Mountain View, Calif.), which was first introduced in 2012, represent an opportunity to expand tele-proctoring to a wider range of open surgeries. Sterility in the operating room is maintained by verbal control of the wearable device, allowing both hands to operate as normal, and the view of the operative field is unimpeded by the peripherally positioned camera and prism. The camera is capable of taking photographs or videos to live-stream for teaching purposes, while the prism provides a semitransparent overlay on the wearer’s visual field by projecting a computer-generated image directly onto the wearer’s retina. Sound is recorded and transmitted by means of a mastoid bone conductor and earpiece, allowing dialogue between wearer and the remote viewer. The feasibility of using Google Glass in the surgical setting was first described in Germany in 2014,15 and since then the technology has been successfully explored as a teaching tool throughout perioperative care in an array of surgical and procedural specialties in high resource settings.16–21
Though its use in educational training and mentoring in LMICs has not been described extensively, wearable technology tele-proctoring has been shown initial success as an educational tool in rural areas of Brazil, Paraguay, and Mongolia.14,22 These early examples demonstrate the potential for the application of wearable technology and live-stream tele-proctoring to other LMICs. One such country is Mozambique, where the surgical capacity is severity limited with only 0.25 surgical specialists available per 10,000 citizens and less than 1 hospital bed per 1,000 people.23 Currently, there are only 61 surgeons for a total population of 30 million.24 Specialty surgical care is even more limited, with only 3 registered reconstructive surgeons for the entire country.24 We share our 6-month experience with Google Glass in Mozambique and demonstrate the feasibility of using wearable technology with tele-proctoring to expand access to training opportunities in reconstructive surgery in this low resource setting.
CASE STUDY
Participants
A senior plastic and reconstructive surgeon based in Los Angeles, California, provided training to a Mozambican surgeon. The 2 surgeons, hereafter referred to as the mentor surgeon and field surgeon, respectively, were acquainted and worked together over the course of a 1 week visit in August 2017.
Setting
All preoperative screening, operative procedures, and postoperative care was conducted at the Provincial Hospital of Matola, in Matola, Mozambique. Mending Kids, a 501c(3) organization providing surgical care in 12 LMICs, coordinated the location and logistics in conjunction with the Mozambican Ministry of Health and faculty of Matola Hospital.
Cases
Patients were recruited from those presenting to the hospital with conditions amenable to plastic surgical intervention. Cases were presented by the field surgeon to the mentor surgeon, and after discussion, cases for tele-mentoring were selected primarily based on the difficulty of the procedure and educational value to the field surgeon. Cases were chosen to represent common presentations encountered by field surgeon, such as burn contracture, but which could utilize reconstructive approaches that would be novel to him, such as regional flaps. Operations were performed while patients were under general or local anesthesia, administered by a local anesthesiologist. All services were provided at no cost to the patients.
Video Streaming
The Google Glass wearable technology was tested during the 1-week period when both the mentor and field surgeon were in Matola, and all hardware and software requirements were ensured at that time. A portable modem “hotspot” was placed in the hospital to increase transmission speed based on this initial testing and a wi-fi hotspot from the field surgeon’s cell phone was additionally used to improve connection.
The surgeon in Mozambique transmitted video and picture data via Google Glass equipped with AMA XpertEye software suite (AMA XpertEye Inc., Woburn, Mass.) to the mentor surgeon in the United States, who accessed the live-stream via a web portal. Two-way audio was provided via a speaker, and a laptop computer in the operating room provided video feed of the mentor surgeon. The mentor surgeon conducted preoperative screening with the field surgeon via tele-proctoring, and during that session, appropriate cases were selected and the operative approach discussed. The field surgeon was then referred to literature to assist in preparation for the case. During the case, the mentor surgeon walked the field surgeon through the procedure in a step-by-step fashion (see video, Supplemental Digital Content 1, which displays an example video feed from mentor surgeon’s computer, http://links.lww.com/PRSGO/A910).
Video Graphic 1.

See video, Supplemental Digital Content 1, which displays an example video feed from mentor surgeon’s computer, http://links.lww.com/PRSGO/A910.
Tele-proctoring Software
The XpertEye software suite was equipped with 5 major functions including live streaming capability, a photo function that allowed the mentor surgeon to take a photograph with higher resolution than provided on the live stream, a drawing function that allowed the mentor surgeon to “tele-strate” or annotate images captured from the live stream and project them back onto the field surgeon’s visual field via the Google Glass prism (Fig. 1), and a zoom function that allowed the mentor surgeon to zoom into the center of the live stream video display.
Fig. 1.
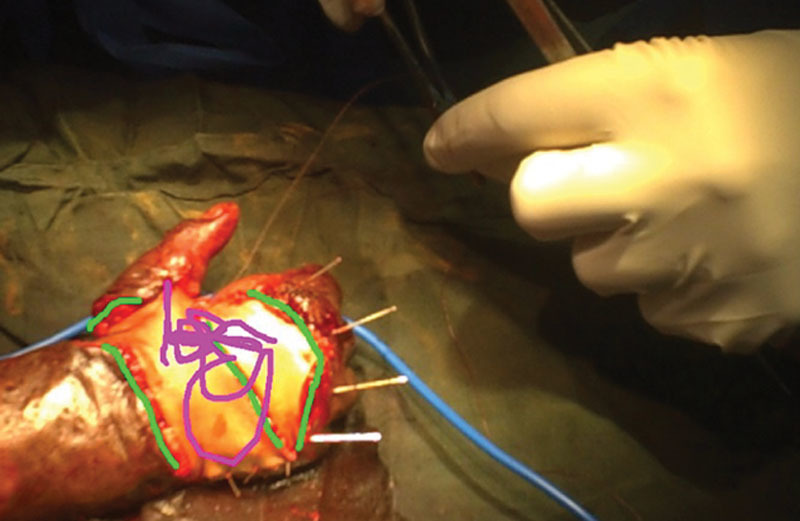
Remote “tele-stration.”
Data Collection
Twelve bimonthly surgical proctoring sessions were held over the course of a 6-month period following the initial visit during which surgeries were live streamed with 2-way audio and video communication to the mentor surgeon in the United States. Figure 2 demonstrates an example case of a patient undergoing resection of a giant cell tumor of third digit. Images were screen-captured from the mentor surgeon’s remote computer. A log was used to record all procedures performed utilizing the Google Glass technology, as well as preoperative screenings and postoperative evaluations. Notes were taken on any interruptions in the stream and any complications experienced by the patient were recorded for both the intraoperative and postoperative setting.
Fig. 2.
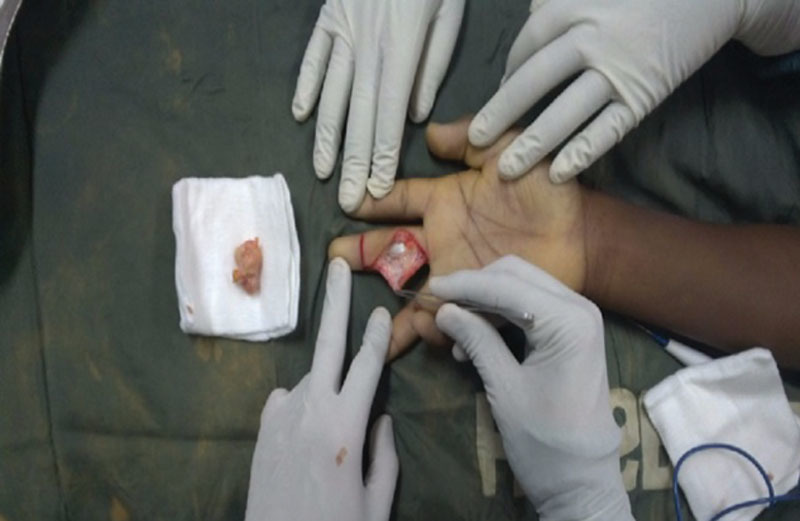
Excision of a giant cell tumor of the third digit using Google Glass tele-proctoring.
At the conclusion of the 6-month pilot phase, an online, 10-question survey was administered to both the field surgeon and mentor surgeon. The questionnaire was adapted from prior work by Hashimoto et al.25 on assessing acceptability of video platforms in surgical settings and evaluated both functionality of the Google Glass as well as the quality of video from the livestream. Additional narrative interviews were conducted with both participants to gain further insight into potential challenges and limitations of the program.
RESULTS
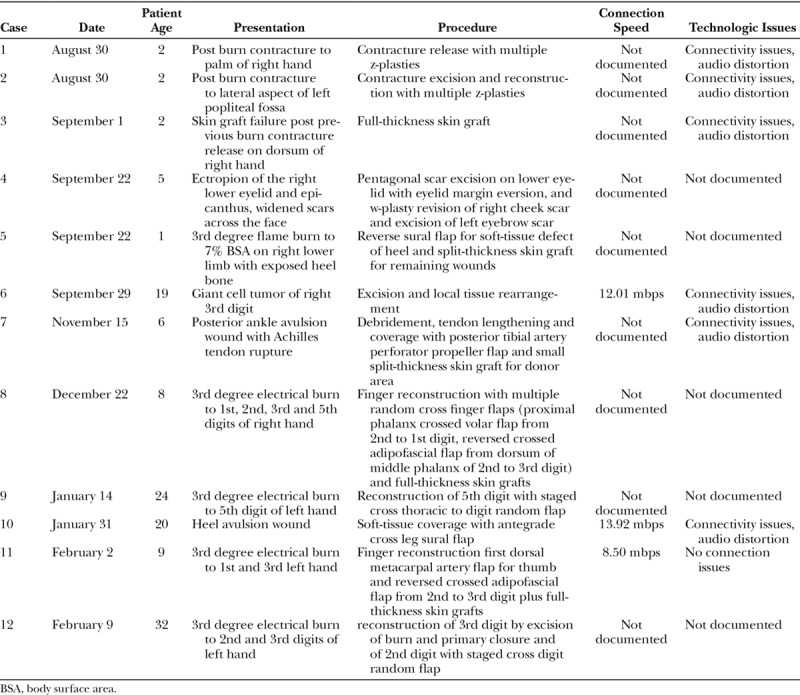
Over the course of the pilot period, 12 surgeries were completed using Google Glass and XpertEye tele-proctoring (Table 1). None of the patients experienced any complications either intraoperatively or postoperatively. Among the 12 operations, all were successfully livestreamed in real time. As previously discussed, the majority of procedures performed were unfamiliar to the field surgeon. Specifically, all rotational and pedicle flaps were new approaches for him. For techniques in which the field surgeon had previous experience, for example skin grafting and z-plasty, nuances in technique in skin marking or bolster dressings were emphasized.
Table 1.
Case Log of Tele-proctored Sessions Including Patient Information and Technical Specifications
Survey results demonstrate the biggest limitations to the experience, from the perspective of both the mentor surgeon and the field surgeon, were issues related to image distortion. Image quality was sufficient for the mentor surgeon to perceive and to comment on pertinent anatomical structures, instrument handling, positioning and technique, but distortion due to light overexposure, motion artifact, and image resolution were rated as moderate to significant impairments (Fig. 3). The field surgeon rated the overall video quality as good while the mentor surgeon rated it as fair.
Fig. 3.
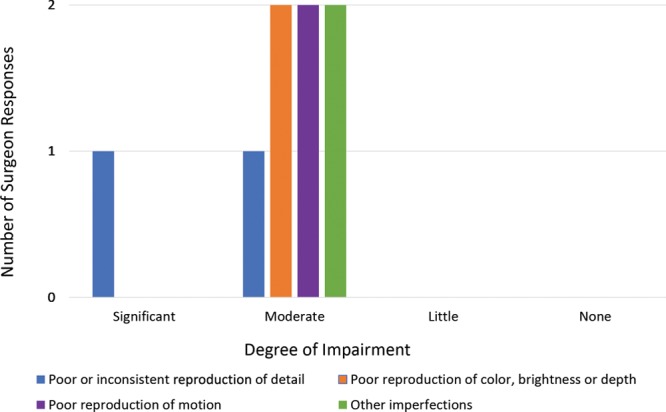
Perceived degree of impairment due to various image distortions.
Despite image distortion, both surgeons found the technology to be helpful as a teaching instrument. Figure 4 demonstrates the perceived usefulness of the technology by both the mentor surgeon and the field surgeon in various surgical contexts.
Fig. 4.
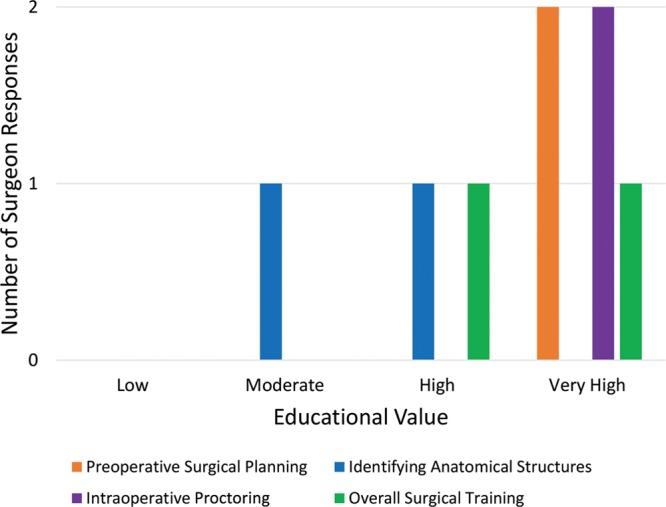
Perceived education value in various surgical contexts.
DISCUSSION
Overall, both the mentor surgeon and field surgeon reported that the technology was very helpful for surgical training in both the preoperative and intraoperative context. The seeming discrepancy between reported rates of impairment from image distortion and the still high subjective satisfaction with the experience may be attributed to a higher tolerance for technologic shortcomings in low resource settings coupled with the strong desire for educational opportunities. While the technology was imperfect, tele-mentoring represented the only source for instruction for the field surgeon in this setting, apart from once yearly visits from the mentor surgeon and his team. In contrast to those far-spaced, in-person interactions, tele-mentoring provided a constant line of communication and a virtual presence of the mentor to provide continuity to the field surgeon’s learning experience. This benefit was felt by both the mentor and the field surgeon to far outweigh the shortcomings of the visual display.
Although objective measures of technical proficiency were not undertaken during this pilot period, the field surgeon did subjectively feel that his proficiency in the specialty had increased through the tele-mentoring. Toward the end of the pilot phase, the field surgeon passed the College of Surgeons of East, Central and Southern Africa boards after having twice previously not passed. Although causation cannot be inferred, in his narrative interview, the field surgeon did feel that the tele-mentoring experience, specifically as it facilitated conversations around surgical planning, intraoperative problem solving and critical thinking, was critical to his exam preparation.
Although both participants reported the technology to be very helpful for surgical education and desired to continuing using it, several limitations and challenges experienced during the pilot phase warrant further discussion. First, the logistical requirements for tele-proctoring were significant. The software suite required a powerful and reliable wireless internet connection in the hospital, which, given the large bandwidth required for video and audio streaming, was frequently insufficient resulting in interruptions in connectivity. Even with the addition of internet “hotspots” in the hospital to improve bandwidth and transmission speed, disruptions occurred in nearly every case. Additionally, latency in the video stream lead to poor reproducibility of motion, which was cited as a moderate impairment.
Time zone differences posed another logistical difficulty for live-streaming, and the 10-hour time difference between the Los Angeles, California, and Matola, Mozambique meant that the mentor surgeon often had to proctor cases in the middle of the night. Another practical consideration was the fitting of the Google Glass headset onto surgical loupes. Surgical loupes are used in the majority of reconstructive surgical procedures, which, depending on the style of the loupes, may interfere with the surgeon’s ability to wear the Google Glass headset. Our field surgeon was able to accommodate the headset due to the design of his loupes (Fig. 5), but the ability to adapt the headset to accommodate surgical loupes must be considered.
Fig. 5.
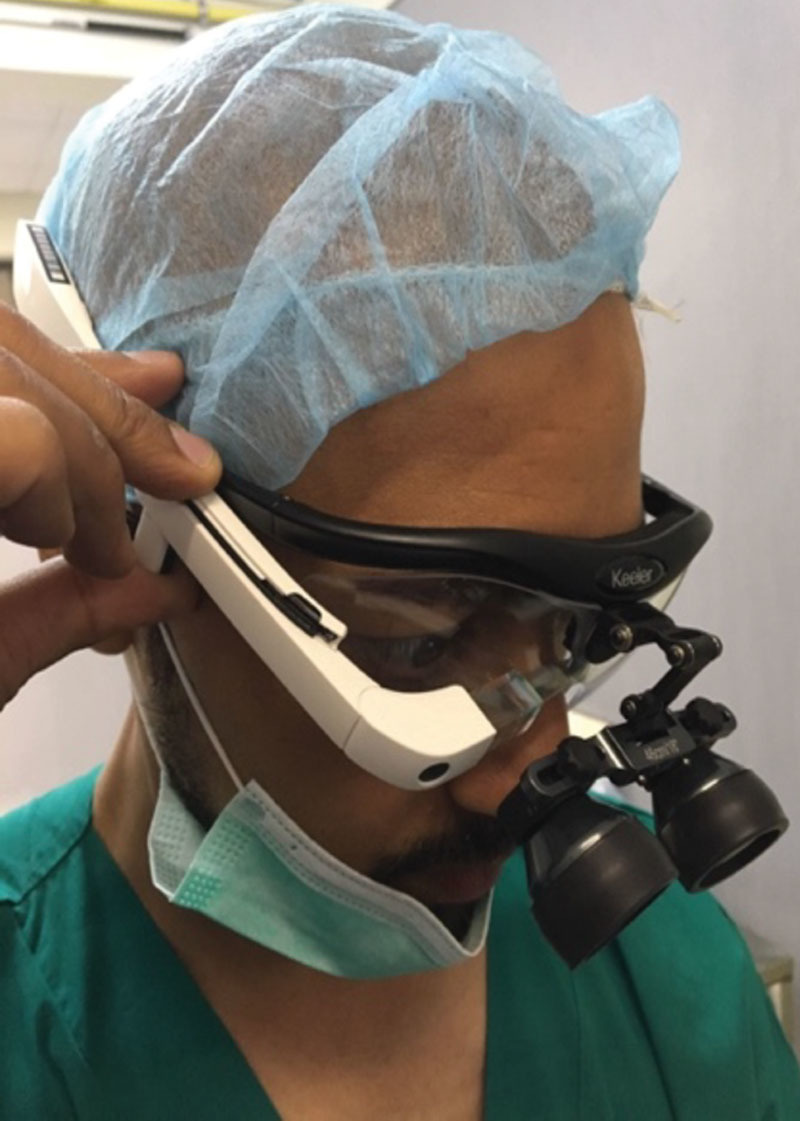
Fitting the Google Glass headset over surgical loupes.
Image quality, as cited in other studies, was also a significant limitation.21,25 One of the most common distortions was due to overexposure of the image from the operating room lights. Figure 6 demonstrates light overexposure on a case of full-thickness skin graft to the dorsal hand (A) and correction of the image after light adjustment (B). We have since trialed a neutral density gel coating (Lee International, Burbank, Calif.) over the camera to minimize glare and have found promising results in initial testing in the United States, but field testing with remote tele-proctoring has not yet been undertaken.
Fig. 6.
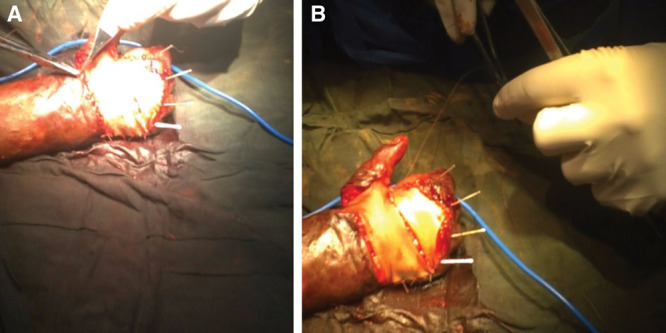
Light-over exposure (A) and correction (B).
Other challenges noted in the narrative interviews include difficulties with the zoom function of the software and the potential for parallax, or a displacement in what the surgeon sees and what the camera captures when the surgeon moves their head when operating (Fig. 7). While the zoom function could target the center of the screen, the inability to direct the zoom to other areas of the visual field led to occasional difficulty centering on the point of interest.
Fig. 7.
Image parallax and displacement of point of interest within remote viewer’s screen.
Another limitation of the platform is its cost. A yearly contract for the wearable hardware and Expert Eye operating platform is $6,990 USD. Although this is significantly less than the cost of importing a team of high-income country volunteers for a short-term surgical trip, the price is not insignificant, especially for low-resource settings. The exact costs for short-term surgical trips are varied based on the specific requirements of the setting, number of volunteers, and types of surgeries undertaken, but numerous studies in the literature have demonstrated the cost effectiveness of the model with respect to disability-adjusted life years for a variety of surgical conditions.27–29 To our knowledge, no similar studies have been undertaken on the cost-effectiveness of tele-mentoring in LMICs, but given the comparatively low cost of the hardware and software relative to the organization of an international volunteer trip, the cost-benefit ratio should be even greater.
Finally, limitations to the study design include a low number of cases, the short time frame of the pilot phase and the experience of a single field surgeon and mentor surgeon. Further data will need to be collected to follow long-term patient outcomes and determine skill retention. Expanding to include additional cases and surgeons will also be essential for proving the generalizability of our experience and findings. Additionally, objectively assessing surgeon technical skill will be critical for understanding the impact of tele-proctoring with wearable technology on skill acquisition and maintenance, and future work with our collaboration will plan to utilize competency-based instruments such as the Objective Structured Assessment of Technical Skills.26
Since the pilot experience, improvements in infrastructure and equipment have been made, which should benefit future iterations of the project. Increased wireless access in the hospital, for example, should help mitigate connectivity issues. Still, disruptions in the connection were less frequently cited as concerns than image distortion and resolution. To address these hardware-specific limitations, and because active development of Google Glass hardware has been ceased, a next generation device called the Vuzix (Vuzix Corp, Rochester, N.Y.) will be utilized starting on the next trip in August 2018. Compared with Google Glass, it can run both iOS and Android operating systems, is more break resistant, and has batteries that can be exchanged without interrupting the video stream, allowing for up to 12 hours of continuous use. Additionally, increased random access memory (RAM) and video processing capabilities allow the camera to stream higher quality images while auto-focus features, image stabilization and scene illumination help to mitigate image distortion errors. Improved gyroscopes and compass systems also improve head tracking for increased fidelity to the wearer’s visual field. Finally, the headpiece can be worn without lenses to easily accommodate surgical loupes. There is no existing literature evaluating the Vuzix in the surgical setting, but its technical specifications hold promise for improving many of the technical challenges experienced during our pilot phase.
CONCLUSIONS
The global surgical community must urgently decide on how to train a vast workforce of future surgeons, how to motivate them to remain within LMICs, and how to support these surgeons to provide sustainable, high-quality care. Surgical aid to LMICs has long been dominated by short-term trips by high-income country volunteers, and creative solutions are needed to refocus efforts on surgical education and prioritize the development of local surgeons within their countries and local practice settings. Although the present tele-mentoring platform has shortcomings, constant development will continue to refine the technologic limitations and we believe will be driven, in part, by interest in and application of the technology to novel settings and problems.
Our experience in Mozambique demonstrates the feasibility of tele-proctoring with wearable technology as an educational model to enhance the reach and availability of specialty surgical training in a resource-limited setting, and its user acceptability for both trainee and educator. Despite shortcomings in the present technology and logistical challenges inherent to international collaborations, this educational model holds promise for connecting surgeons across the globe, introducing expanded access to education and mentorship in areas with limited opportunities for surgical trainees and generating discussion around the potential for innovative technologies to address needs in training and care delivery in LMICs.
Supplementary Material
Footnotes
Published online 5 December 2018.
Supported through a philanthropic donation from the Jay and Sue Roach Foundation.
Disclosure: The authors have no financial interest to declare in relation to the content of this article or products mentioned within it. The study and Article Processing Charge were funded through a philanthropic donation from the Jay and Sue Roach Foundation.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.World Health Report 2006: working together for health. Available at http://www.who.int/whr/2006/en. Accessed April 5, 2018.
- 2.Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Int J Obstet Anesth. 2016;25:75. [DOI] [PubMed] [Google Scholar]
- 3.A universal truth: no health without a workforce: Third Global Forum on Human Resources for Health Report. 2013. Available at http://www.who.int/workforcealliance/knowledge/resources/hrhreport2013/en/. Accessed April 5, 2018.
- 4.Moore RG, Adams JB, Partin AW, et al. Telementoring of laparoscopic procedures: initial clinical experience. Surg Endosc. 1996;10:107. [DOI] [PubMed] [Google Scholar]
- 5.Challacombe B, Kandaswamy R, Dasgupta P, et al. Telementoring facilitates independent hand-assisted laparoscopic living donor nephrectomy. Transplant Proc. 2005;37:613. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Sharma V, Patel P, et al. Telementoring: an overview and our preliminary experience in the setting up of a cost-effective telementoring facility. Indian J Surg. 2016;78:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ereso AQ, Garcia P, Tseng E, et al. Live transference of surgical subspecialty skills using telerobotic proctoring to remote general surgeons. J Am Coll Surg. 2010;211:400. [DOI] [PubMed] [Google Scholar]
- 8.Rosser JC, Wood M, Payne JH, et al. Telementoring. A practical option in surgical training. Surg Endosc. 1997;11:852. [DOI] [PubMed] [Google Scholar]
- 9.Sebajang H, Trudeau P, Dougall A, et al. Telementoring: an important enabling tool for the community surgeon. Surg Innov. 2005;12:327. [DOI] [PubMed] [Google Scholar]
- 10.Schulam PG, Docimo SG, Saleh W, et al. Telesurgical mentoring. Initial clinical experience. Surg Endosc. 1997;11:1001. [DOI] [PubMed] [Google Scholar]
- 11.Lee BR, Bishoff JT, Janetschek G, et al. A novel method of surgical instruction: international telementoring. World J Urol. 1998;16:367. [DOI] [PubMed] [Google Scholar]
- 12.Camara JG, Rodriguez RE. Real-time telementoring in ophthalmology. Telemed J. 1998;4:375. [DOI] [PubMed] [Google Scholar]
- 13.Okrainec A, Smith L, Azzie G. Surgical simulation in Africa: the feasibility and impact of a 3-day fundamentals of laparoscopic surgery course. Surg Endosc. 2009;23:2493. [DOI] [PubMed] [Google Scholar]
- 14.Datta N, MacQueen IT, Schroeder AD, et al. Wearable technology for global surgical teleproctoring. J Surg Educ. 2015;72:1290. [DOI] [PubMed] [Google Scholar]
- 15.Muensterer OJ, Lacher M, Zoeller C, et al. Google Glass in pediatric surgery: an exploratory study. Int J Surg. 2014;12:281. [DOI] [PubMed] [Google Scholar]
- 16.Mitrasinovic S, Camacho E, Trivedi N, et al. Clinical and surgical applications of smart glasses. Technol Health Care. 2015;23:381. [DOI] [PubMed] [Google Scholar]
- 17.Chang JY, Tsui LY, Yeung KS, et al. Surgical vision: Google Glass and surgery. Surg Innov. 2016;23:422. [DOI] [PubMed] [Google Scholar]
- 18.García-Cruz E, Bretonnet A, Alcaraz A. Testing smart glasses in urology: clinical and surgical potential applications. Actas Urol Esp. 2018;42:207. [DOI] [PubMed] [Google Scholar]
- 19.Wei NJ, Dougherty B, Myers A, et al. Using Google Glass in surgical settings: systematic review. JMIR Mhealth Uhealth. 2018;6:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiranaka T, Nakanishi Y, Fujishiro T, et al. The use of smart glasses for surgical video streaming. Surg Innov. 2017;24:151. [DOI] [PubMed] [Google Scholar]
- 21.Sinkin JC, Rahman OF, Nahabedian MY. Google Glass in the operating room: the plastic surgeon’s perspective. Plast Reconstr Surg. 2016;138:298. [DOI] [PubMed] [Google Scholar]
- 22.Nakhla J, Kobets A, De la Garza Ramos R, et al. Use of Google Glass to enhance surgical education of neurosurgery residents: “Proof-of-Concept” study. World Neurosurg. 2017;98:711. [DOI] [PubMed] [Google Scholar]
- 23.World bank. Available at https://data.worldbank.org/indicator/SH.MED.SAOP.P5?end=2016&locations=MZ-US&start=2011&view=chart. Accessed May 2, 2018.
- 24.Interactive Global Surgery Map. COSECA.org. Available at http://www.cosecsa.org/global-surgery-map. Accessed May 2, 2018.
- 25.Hashimoto DA, Phitayakorn R, Fernandez-del Castillo C, et al. A blinded assessment of video quality in wearable technology for telementoring in open surgery: the Google Glass experience. Surg Endosc. 2016;30:372. [DOI] [PubMed] [Google Scholar]
- 26.Martin JA, Regehr G, Reznick R, et al. Objective structured assessment of technical skill (OSATS) for surgical residents. Br J Surg. 1997;84:273. [DOI] [PubMed] [Google Scholar]
- 27.Hackenberg B, Ramos MS, Campbell A, et al. Measuring and comparing the cost-effectiveness of surgical care delivery in low-resource settings: cleft lip and palate as a model. J Craniofac Surg. 2015;26:1121. [DOI] [PubMed] [Google Scholar]
- 28.Davis MC, Than KD, Garton HJ. Cost effectiveness of a short-term pediatric neurosurgical brigade to Guatemala. World Neurosurg. 2014;82:974. [DOI] [PubMed] [Google Scholar]
- 29.Egle JP, McKendrick A, Mittal VK, et al. Short-term surgical mission to the Dominican Republic: a cost-benefit analysis. Int J Surg. 2014;12:1045. [DOI] [PubMed] [Google Scholar]


