The visualization of endolymphatic hydrops (EH) in a clinical setting was first achieved with MRI 24 h after intratympanic administration of gadolinium-based contrast agent (IT-GBCA). However, due to the invasiveness and instability of the IT-GBCA method, MRI 4 h after intravenous administration of a single dose of GBCA (IV-SD-GBCA) has become increasingly popular in clinical practice.1 Following IT-GBCA, 3D-real inversion recovery (IR) imaging using conventional turbo spin echo sequence is frequently utilized because 3D-real IR images allow the separation of perilymph, endolymph and surrounding bone in a single image.2 Typically, it has been difficult to apply 3D-real IR imaging after IV-SD-GBCA due to the far lower GBCA concentration in the labyrinth after IV-SD-GBCA than IT-GBCA. To overcome this, the subtraction of two image series with different inversion times has been utilized for imaging after IV-SD-GBCA; this is referred to as the HYbriD of Reversed image Of Positive endolymph signal and native image of positive perilymph Signal (HYDROPS) technique.3 The HYDROPS images are generated by subtraction of a positive endolymph image (PEI) from a heavily T2-weighted 3D-fluid attenuated IR(hT2w-3D-FLAIR) or positive perilymph image (PPI). A recently reported technique for generating improved HYDROPS (i-HYDROPS) images allows for a higher contrast to noise ratio per unit time compared to conventional HYDROPS imaging; this is accomplished by elongating the repetition time and increasing the refocusing flip angle.4 The difference between the inversion times for PPI and PEI in HYDROPS imaging is only 200 ms; however, this is doubled to 400 ms for i-HYDROPS imaging. For the separate visualization of endo- and perilymph on 3D-real IR imaging, the inversion time value is set at the middle of inversion time for PEI and that for PPI. The increased difference between the two inversion time values improves the contrast between endolymph and perilymph on 3D-real IR images by increasing the absolute size of the longitudinal magnetization of endo- and perilymph. We now routinely obtain i-HYDROPS images and 3D-real IR images for visualizing EH after IV-SD-GBCA using the elongated repetition time and the increased refocusing flip angle characteristic of the i-HYDROPS technique. So far, more than 40 patients have been examined using both the i-HYDROPS technique and 3D-real IR technique. All MRI in this report was performed using a 3T scanner (MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany) with a 32-channel array head coil. Magnetic resonance scanning was performed 4 h after IV-SD-GBCA (0.1 mmol/kg body weight) of gadobutrol (Gadovist, Bayer Pharmaceuticals, Osaka, Japan). Scan parameters are shown in Table 1.
Table 1.
Pulse sequence parameters
| Sequence name | Type | Repetition time (ms) | Echo time (ms) | Inversion time (ms) | Flip angle (degree) | Section thickness/gap (mm) | Pixel size (mm) | Number of slices | Echo train length | Field of view (mm) | Matrix size | Number of excitations | Scan time (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR cisternography (MRC) | SPACE with restore pulse | 4400 | 544 | NA | 90/ initial 180 decrease to constant 120 | 1/0 | 0.5 × 0.5 | 104 | 173 | 165 × 196 | 332 × 384 | 1.8 | 3 |
| 3D-real IR | SPACE with inversion pulse | 16000 | 661 | 2700 | 90/ constant180 | 1/0 | 0.5 × 0.5 | 104 | 173 | 165 × 196 | 332 × 384 | 2 | 10 |
| Heavily T2 weighted 3D-FLAIR (PPI) and PEI | SPACE with inversion pulse | 16000 | 544 | 2900 (2500 for PEI) | 90/ constant 180 | 1/0 | 0.5 × 0.5 | 104 | 173 | 165 × 196 | 332 × 384 | 1.4 | 7 for PPI, 7 for PEI |
GeneRalized Autocalibrating Partial Parallel Acquisition (GRAPPA) × 2 for all sequences. All sequences utilize a frequency selective fat suppression pre-pulse. Each 3D slab is set in an identical axial orientation. 3D-real IR, 3D inversion recovery with phase sensitive reconstruction (real reconstruction). PPI, positive perilymph image; PEI, positive endolymph image; SPACE, sampling perfection with application-optimized contrasts using different flip angle evolutions.
In all patients examined except one shown in Fig. 1, the size of the endolymphatic space was comparable in both the i-HYDROPS and 3D-real IR images (Fig. 2). However, we noticed a marked difference in EH visualization in one case with slightly altered endolymph fluid composition (Fig. 1). On the PPI, the endolymph should have a near zero signal, but in the case with slightly altered endolymph composition, the endolymph showed a slightly positive PPI signal. On the PEI, the altered endolymph showed a positive signal; however, this positive signal was lower than for normal endolymph on magnitude reconstruction images. Endolymph without gadolinium distribution has negative longitudinal magnetization after the inversion time to nullify the gadolinium-containing perilymph on PEI. The slight alteration in endolymph composition shortens the T1 value of the endolymph and the absolute magnitude of the negative longitudinal magnetization of endolymph on PEI would thus be smaller than usual. Thus, the subtraction of PEI from PPI results in signal cancellation of the altered endolymph on i-HYDROPS images. Using 3D-real IR images, we can differentiate positive longitudinal magnetization from negative longitudinal magnetization. The scan time to acquire i-HYDROPS images is 14 min (7 min for PPI and 7 min for PEI). The scan time for 3D-real IR images is 10 min. Furthermore, 3D-real IR does not require post-processing for subtraction and does not have the risk for the misregistration. The 3D-FLAIR imaging using conventional turbo spin echo sequence was reported to be less sensitive to low concentration gadolinium compared to heavily T2-weighted 3D-FLAIR using SPACE (sampling perfection application optimized contrasts with different flip angle evolution) sequence.5 In the present study, we used 3D-real IR-based on SPACE. This also might be the contributing factor for the successful EH imaging.
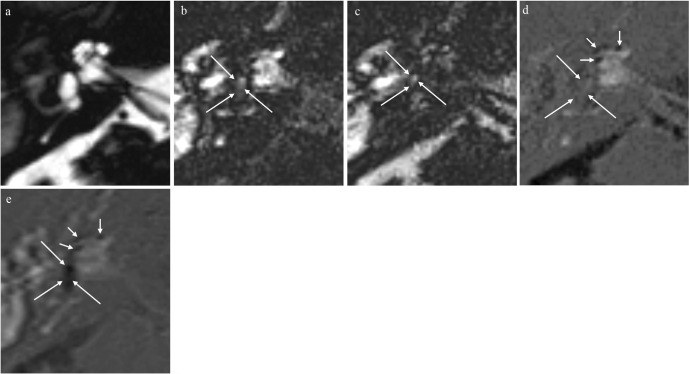
Fig. 1.
A 77-year-old man with Meniere’s disease of the right ear, with otitis media. (a) Magnetic resonance cisternography shows the entire fluid-filled space of the right inner ear. (b) Positive perilymph image (positive perilymph image [PPI], TR/TE/inversion time [TI]:16,000/544/2900). An area of slightly elevated signal (long arrows) is seen in the center of the vestibule. (c) Positive endolymph image (positive endolymph image [PEI], TR/TE/TI: 16,000/544/2500). An area of slightly elevated signal (long arrows) is seen in the center of the vestibule. (d) Improved HYbriD of Reversed image of Positive endolymph signal and native image of positive perilymph Signal (i-HYDROPS) images are generated by the subtraction of PEI from PPI. Endolymphatic hydrops in the vestibule is not visible, and appears as a gray region (long arrows). This is due to the signal cancelation of endolymph on PPI and PEI resulting from the subtraction. Endolymphatic hydrops (EH) in the cochlea is clearly visualized (arrows). (e) Three-dimensional-real IR image (TR/TE/TI: 16000/538/2700). Endolymphatic hydrops in the cochlea (arrows) appears very similar to i-HYDROPS image. Endolymphatic hydrops in the vestibule can be visualized in this image (long arrows).
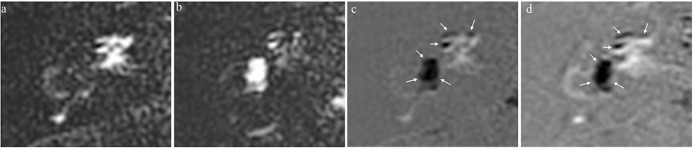
Fig. 2.
A 37-year-old woman with Meniere’s disease in the right ear. Images were obtained 4 h after intravenous administration of a single dose of gadobutrol. (a) Heavily T2-weighted 3D-fluid-attenuated inversion recovery (FLAIR) image; positive perilymph image (positive perilymph image [PPI], TR/TE/inversion time [TI]:16,000/544/2900). The perilymph shows a bright signal from the distribution of gadobutrol. (b) Positive endolymph image (positive endolymph image [PEI], TR/TE/TI: 16,000/544/2500). Endolymph without gadobutrol distribution shows a bright signal. (c) Improved HYbriD of Reversed image of Positive endolymph signal and native image of positive perilymph Signal (i-HYDROPS) images are generated by the subtraction of PEI from PPI. Separation between bright perilymph, black endolymph, and gray bone is possible with just a single image. Significant endolymphatic hydrops (EH) is observed in the cochlea and the vestibule (arrows). (d) 3D-real IR image (TR/TE/TI: 16,000/538/2700). The visualization of EH in the cochlea and the vestibule (arrows) is very similar to i-HYDROPS image.
In conclusion, 3D-real IR-imaging-based on phase-sensitive reconstruction might be more robust toward slight compositional alterations in endolymph than i-HYDROPS imaging-based on magnitude reconstruction.
Acknowledgments
This study was partly supported by Grants-in-Aid for scientific research from the Japanese Society for the Promotion of Science (JSPS KAKENHI, numbers 17H04259) to S.N.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1. Nakashima T, Pyykkö I, Arroll MA, et al. Meniere’s disease. Nat Rev Dis Primers 2016; 2: 16028. [DOI] [PubMed] [Google Scholar]
- 2. Naganawa S, Satake H, Kawamura M, Fukatsu H, Sone M, Nakashima T. Separate visualization of endolymphatic space, perilymphatic space and bone by a single pulse sequence; 3D-inversion recovery imaging utilizing real reconstruction after intratympanic Gd-DTPA administration at 3 Tesla. Eur Radiol 2008; 18: 920– 924. [DOI] [PubMed] [Google Scholar]
- 3. Naganawa S, Yamazaki M, Kawai H, Bokura K, Sone M, Nakashima T. Imaging of Ménière’s disease after intravenous administration of single-dose gadodiamide: utility of subtraction images with different inversion time. Magn Reson Med Sci 2012; 11: 213– 219. [DOI] [PubMed] [Google Scholar]
- 4. Naganawa S, Kawai H, Taoka T, Sone M. Improved HYDROPS: Imaging of endolymphatic hydrops after intravenous administration of gadolinium. Magn Reson Med Sci 2017; 16: 357– 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naganawa S, Kawai H, Sone M, Nakashima T. Increased sensitivity to low concentration gadolinium contrast by optimized heavily T2-weighted 3D-FLAIR to visualize endolymphatic space. Magn Reson Med Sci 2010; 9: 73– 80. [DOI] [PubMed] [Google Scholar]