Abstract
We evaluated the value of magnetic resonance elastography (MRE) for the prediction of response to magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eleven patients were enrolled. A fractional change of >30% in Symptoms Severity Score (SSS) was defined as a ‘substantial symptomatic improvement’ at 12 months after treatment. The fractional stiffness value reduction in the patients with a substantial improvement in SSS was significantly higher than that in those without (P = 0.0446).
Keywords: magnetic resonance-guided focused ultrasound, magnetic resonance elastography, stiffness, Symptoms Severity Score, uterine fibroid
Introduction
Uterine fibroids are common benign tumors, which occur in about one-fourth of women in their reproductive years. Although some patients are completely asymptomatic, approximately 25% are symptomatic and experience pelvic pain, menorrhagia or dysmenorrhagia, increased frequency of urination, or reproductive dysfunction.1 Historically, hysterectomy and myomectomy has been performed as the first-line treatment for uterine fibroids. In addition, less invasive treatment options are available (i.e., uterine artery embolization, magnetic resonance-guided focused ultrasound [MRgFUS], or thermal ablation therapy). Hormone-based treatment is often selected, but may cause a rebound effect in case of interruption. Magnetic resonance-guided focused ultrasound is a noninvasive method that uses focused ultrasound to ablate the tumor and reduce fibroid volume.2 However, the outcomes of symptoms can vary. To the best of our knowledge, there is no biomarker that can predict the outcome of patients’ symptoms after MRgFUS.
Magnetic resonance elastography (MRE) is a recently developed technique for measuring tissue stiffness. A previous study suggested that MRE can be used to measure fibroid stiffness, which represents the degree of fibrosis in fibroids.3 Therefore, we hypothesized that fibroid stiffness can be used as an indicator of treatment outcome in patients with fibroids treated with MRgFUS. Hence, the purpose of this study was to evaluate the usefulness of MRE for predicting the treatment outcomes of patients who receive MRgFUS treatment for uterine fibroids.
Materials and Methods
Patients
This retrospective study was performed in accordance with the principles outlined in the Declaration of Helsinki, and was approved by the relevant institutional review board. Written informed consent for MRgFUS treatment and MRE was obtained from all patients. Between February 2013 and December 2014, 11 consecutive female patients underwent MRgFUS treatment for uterine fibroids at our institution. All the patients (age range, 38 to 52 years; mean age, 45.5 ± 4.4 years) received MRgFUS treatment within 2 months of undergoing the pre-treatment MRE. They underwent the post-treatment MRE 12 months after MRgFUS treatment. There were no other inclusion or exclusion criteria. All patients had multiple fibroids (mean, 8; range, 2–17). Their chief complaint was hypermenorrhea (n = 8), abdominal tightness (n = 2), and frequent urination (n = 1). Two patients were received gonadotrophin-releasing hormone analog (GnRHa) therapies just before MRgFUS.
Patient eligibility criteria for MRgFUS were as follows; i) symptomatic fibroids, ii) without abdominal scar in the beam pathway (between ultrasound source and the targeted fibroid), iii) without intestine in the beam pathway, iv) >4 cm away from the sacrum or the lumber vertebra, v) no desire for childbearing, and vi) no contraindication for MRI.
Magnetic resonance-guided focused ultrasound
All patients underwent MRgFUS treatment on an Exablate 2000 system (INSIGHTEC Ltd., Tirat Carmel, Israel) integrated into a 1.5T MRI system (SIGNA Excite Ver.12; GE Healthcare, Milwaukee, WI, USA). The treatment procedure was as follows. After positioning the patient in the prone position, we performed localizer scan to locate the uterus and risk organs such as intestine, bladder, skin and pubic bone. The operator delineated the target fibroids and risk organs based on T2-weighted images (T2WI). The system automatically adjusted shapes, sizes, and angles of the sonication spots, and the appropriate transducer apertures to avoid the beam pathway through risk organs and created a 3D treatment plan. The size of sonication spot was 30–40 × 5–10 mm. During the treatment, we adjusted energy levels to rise over 60°C at sonication spots. After the treatment, we acquired contrast-enhanced fat-saturated T1-weighted images to evaluate the non-perfused volume (NPV) ratio of all targeted fibroids. Table 1 shows parameters of T1- and T2-weighted images.
Table 1.
Parameters of T1- and T2-weighted images
| Plane | T2-weighted image | Fat-saturated T1-weighted image | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Axial | Coronal | Sagittal | Axial | Coronal | Sagittal | |||||
| Sequence | Spin echo | Spin echo | Spin echo | FSPGR | FSPGR | FSPGR | ||||
| TR/TE (ms) | 4440/86.26 | 5060/82.18 | 4500/82.18 | 285.0/1.39 | 235.0/1.39 | 260.0/1.39 | ||||
| Section thickness (mm) | 5 | 5 | 5 | 5 | 5 | 5 | ||||
| Intersection gap (mm) | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Matrix size | 256 × 224 | 256 × 224 | 256 × 224 | 256 × 128 | 256 × 128 | 256 × 128 | ||||
| Echo train length | 12 | 16 | 13 | 1 | 1 | 1 | ||||
| FOV (mm) | 360 × 360 | 360 × 360 | 360 × 360 | 360 × 360 | 360 × 360 | 360 × 360 | ||||
| Flip angle (degrees) | 90 | 90 | 90 | 80 | 80 | 80 | ||||
FSPGR, fast spoiled gradient-echo dual echo.
Magnetic resonance elastography
Resonance elastography was performed using a superconducting magnet operating at 1.5 Tesla (Signa Excite Ver.12; GE Medical Systems, Waukesha, WI, USA) with an 8-channel phased-array coil. Patients were placed in the supine position, and a cylindrical passive driver was attached to the lower abdominal wall using a rubber belt. The driver then transferred the vibration to the uterus via the abdominal wall. The MR parameters were as follows: sequence, spin echo-echo planar imaging; plane, transverse; TR/TE, 500/50 ms; matrix, 96 × 96; FOV, 40 × 40 cm; section thickness/intersection gap, 5/1.5 mm; frequency of driver, 60 Hz; amplitude, 60%; number of slices, 7 slices; number of excitations, 2; parallel imaging factor, 2; phase offset, 4; and acquisition time, 38 s (2 acquisitions). Motion-sensitizing gradient pulses were concurrently applied in three directions (x, y, and z). The MR scanners automatically generated stiffness maps by processing the acquired propagating shear wave images according to a 2D inversion algorithm,4 and the shear stiffness of the tissue was translated to a pixel value (kPa). Based on the stiffness maps, two radiologists (S.I. and Y.O.), with 9 and 3 years of experience in radiology, respectively, referenced the T2WI and placed a region of interest (ROI) in the uterine fibroids of each patient. We selected one slice which has the maximum diameter on axial images and placed oval ROIs as large as possible to exclude the degeneration area (i.e., hyperintense area on T2WI). During the ROIs placement, we also paid attention to include a place where parallel waveform goes without interference on the phase images.
Statistical analysis
Fibroid volume was measured by hand, on the T2WI. For volume measurements, we used software called Medical Image Processing, Analysis, and Visualization (MIPAV) provided by National Institutes of Health (NIH). An application specialist draws a volume of interest (VOI) by manually drawing contours around the fibroid on MR images (slice-by-slice), and the software automatically calculates the total volume of that VOI. Before and after the treatment, the patients were asked to report the severity of their symptoms using a Symptoms Severity Score (SSS).5 This is a 100-point scale consists of following eight symptoms including i) heavy bleeding during menstrual period, ii) passing blood clots during menstrual period, iii) fluctuation in the duration of menstrual period compared to previous cycle, iv) fluctuation in the length of monthly cycle compared to previous cycle, v) feeling tightness or pressure in pelvic area, vi) frequent urination during the daytime hours, vii) frequent nighttime urination, and viii) feeling fatigued. We compared the pre-treatment data with the data at 12 months’ post-treatment. Two end points were applied in this study. One was substantial volume reduction, which was defined as a fractional volume reduction of more than 10%. The other was substantial symptomatic improvement, which was defined as a fractional change in SSS of more than 30%. The variables related to fibroids were analyzed as potential factors associated with treatment response. The following variables of fibroids were analyzed as potential factors related with response to treatment: GnRHa therapies before MRgFUS, number of sonication spots, signal intensity of T2WI, signal intensity ratio of fibroids-to-muscle on T2WI, location, pre-treatment volume, NPV, NPV ratio, stiffness value measured by MRE, and fractional stiffness value reduction after treatment. We defined low signal intensity on T2WI when fibroids were comparable to skeletal muscle.6 We compared these variables between the subgroups divided according to the two end points. Inter-reader agreement was assessed using the intraclass correlation coefficient (ICC). An ICC value (r) > 0.8 was considered excellent agreement, 0.6 < r ≤ 0.8 was deemed good, 0.4 < r ≤ 0.6 was moderate, 0.2 < r ≤ 0.4 was fair, and ≤ 0.2 was considered poor agreement. All statistical analyses were performed using the JMP (Ver. 10.0.2; SAS Institute, Cary, NC, USA) and IBM SPSS (Version 22.0; IBM Corp, Armonk, NY, USA) software. The statistical significance level was set at P < 0.05.
Results
Overall
We treated 17 fibroids (12 intramural and 5 submucosal fibroids) in 11 patients. The mean number of sonication per patient was 86.3 ± 32.8 (range, 31–135). The total treatment duration (between the first and last sonication) was 03:33 ± 00:59 h (range, 02:02–05:03).
The mean pre-treatment and post-treatment fibroid volumes were 414.8 and 370.1 mL, respectively. The mean pre-treatment and post-treatment fibroid stiffness values were 7.3 and 7.5 kPa, respectively. Six patients showed a substantial reduction in fibroid volume, while six patients showed a substantial improvement in SSS. Among these patients, three patients showed both a substantial volume reduction and substantial symptomatic improvement. No serious adverse events were documented during treatment or reported during follow-up.
Univariate analyses
In the comparison between the patients with and without a substantial reduction in fibroid volume, the pre-treatment stiffness values were significantly higher in the patients with a substantial volume reduction (P = 0.0222). The other variables showed no significant differences between the two groups (Table 1). In the comparison between the patients with and those without a substantial improvement in SSS, none of the variables showed significant differences, except for the fractional change in the fibroid stiffness values (P = 0.0446) (Table 2). In the comparison between the patients with intramural and submucosal fibroids, there was no significant difference of a substantial reduction in fibroid volume (intramural, 42.9% [3/7]; submucosal, 75.0% [3/4]) (P = 0.3031) and a substantial improvement in SSS (intramural, 42.9% [3/7]; submucosal, 75.0% [3/4]) (P = 0.3031). Figures 1 and 2 present representative clinical cases.
Table 2.
Results of univariate analyses
| Volume reduction of uterine fibroids | Improvement of SSS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No substantial reduction (n = 5 ) | Substantial reduction (n = 6 ) | P value | No substantial symptomatic improvement (n = 5 ) | Substantial symptomatic improvement (n = 6 ) | P value | |||||
| Age (years) | 46.6 (47, 44–49) | 44.7 (44.5, 38–52) | 0.7138 | 46.8 (48, 38–52) | 44.5 (44.5, 40–49) | 0.3591 | ||||
| GnRHa therapies before MRgFUS (yes:no) | 4:1 | 5:1 | 0.8865 | 4:1 | 5:1 | 0.8865 | ||||
| Number of sonication spots SI of T2WI (high:low) | 91.6 (84, 61–135) | 81.8 (82, 31–122) | 0.5839 | 79.2 (84, 31–122) | 92.2 (90.5, 60–135) | 0.7150 | ||||
| 2:3 | 0:6 | 0.0868 | 1:4 | 1:5 | 0.8865 | |||||
| SI ratio of fibroid-to-muscle on T2WI | 1.46 (1.48, 0.96–1.84) | 1.04 (0.98, 0.81–1.34) | 0.0285* | 1.26 (1.27, 0.89–1.84) | 1.21 (1.24, 0.81–1.60) | 0.8551 | ||||
| Location of fibroids (intramural: submucosal) | 4:1 | 3:3 | 0.3031 | 4:1 | 3:3 | 0.3031 | ||||
| Pre-treatment volume (cm3) | 429.8 (425.8, 152.1–727.6) | 397.4 (328.0, 28.3–864.6) | 0.7150 | 335.1 (204.7, 28.3–864.6) | 476.3 (460.4, 121.8–727.6) | 0.3613 | ||||
| NPV (cm3) | 153.2 (101.8, 88.4–251.2) | 189.2 (206.1, 12.2–314.9) | 0.3613 | 132.5 (95.1, 12.2–314.9) | 206.4 (240.3, 97.6–298.2) | 0.2012 | ||||
| NPV ratio (%) | 39.1 (31.6, 20.7–62.5) | 55.5 (50.5, 36.4–80.1) | 0.1441 | 47.4 (43.2, 20.7–74.0) | 48.6 (47.6, 27.2–80.1) | 1.0000 | ||||
| Pre-treatment SSS | 60.2 (65, 31–90) | 61.3 (65.5, 37–87) | 0.8551 | 56 (62, 37–69) | 64.8 (71.5, 31–90) | 0.3613 | ||||
| Fractional volume reduction (%) | – | – | – | −22.3 (13.2, −156.0–68.3) | 10.3 (4.0, −26.8–46.6) | 0.8551 | ||||
| Pre-treatment stiffness value (kPa) | 6.1 (5.7, 5.2–8.0) | 8.3 (8.1, 6.9–10.3) | 0.0222* | 7.4 (6.9, 5.2–10.3) | 7.3 (7.9, 5.3–8.9) | 1.0000 | ||||
| Fractional stiffness value reduction (%) | −12.0 (−20.0, −86.2–43.4) | 5.1 (22.4, −60.5–24.6) | 0.8551 | −37.7 (−60.5, −86.2–24.6) | 26.5 (24.0, 20.0–43.4) | 0.0446 * | ||||
Continuous variables were analyzed by Wilcoxon test and are expressed as the mean (median, range). Categorical variables were analyzed by the chi-squared test.
P < 0.05. GnRHa, gonadotrophin-releasing hormone analog; SI, signal intensity; T2WI, T2-weighted image; NPV, non-perfused volume; SSS, Symptoms Severity Score.
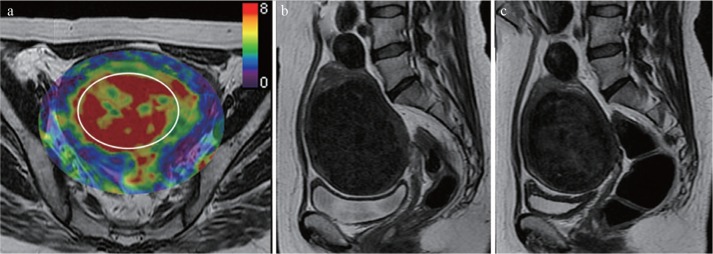
Fig. 1.
A 41-year-old woman with uterine fibroid who experienced substantial fibroid volume reduction after MR-guided focused ultrasound. (a) Pre-treatment MR elastography images that correspond with the uterus superimposed on conventional MR images; (b) pre-treatment, and (c) post-treatment sagittal T2-weighted images. Her fibroid showed a pre-treatment stiffness value of 7.8 kPa, which was higher than the average of this study. The fibroid volume was reduced by 42% after MR-guided focused ultrasound (pre-treatment volume, 451 cm 3 ; post-treatment volume, 258 cm 3). An oval region of interest (circle), as large in size as possible, was placed on the fibroid by the investigators.
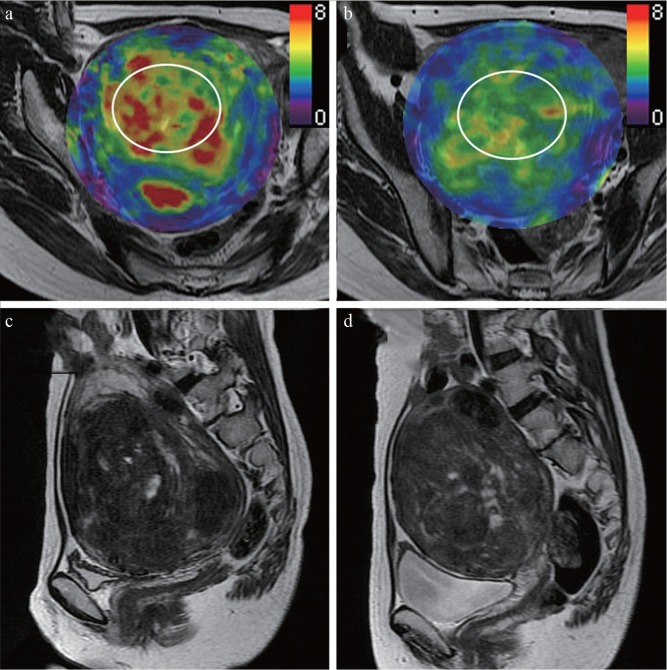
Fig. 2.
A 49-year-old woman with uterine fibroid who experienced a substantial symptomatic improvement after treatment. (a) Pre-treatment and (b) post-treatment MR elastography images that correspond with the uterus superimposed on conventional MR images; (c) pre-treatment and (d) post-treatment sagittal T2-weighted images. Her fibroid showed a pre-treatment stiffness value of 5.7 kPa, which was lower than the average of this study. The fibroid volume was not decreased after MR-guided focused ultrasound (pre-treatment volume, 470 cm 3 ; post-treatment volume, 495 cm 3). However, her symptoms were substantially improved (pre-treatment Symptoms Severity Score, 90; post-treatment Symptoms Severity Score, 58; 37.8% decrease). The post-treatment stiffness value was decreased by 26% (from 5.7 kPa to 4.2 kPa). Oval regions of interest (circle), as large in size as possible, were placed on the fibroid.
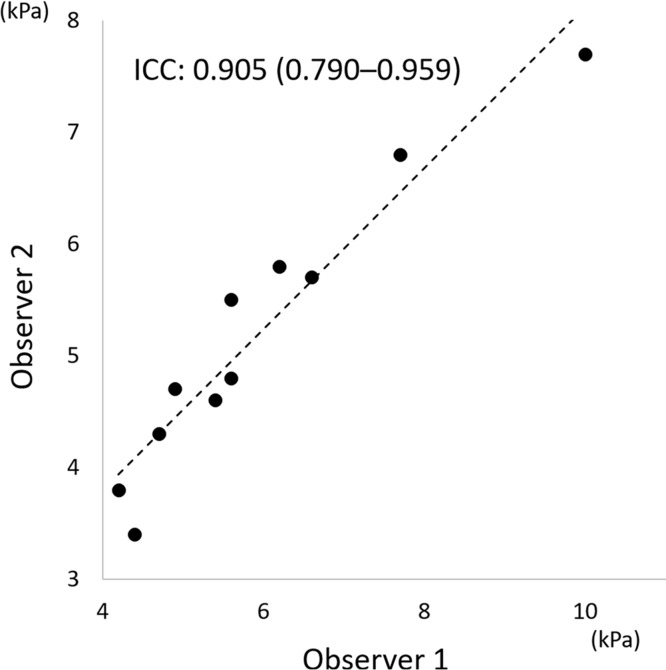
Interobserver agreement for measurement of stiffness values of uterine fibroids
The interobserver ICC for the measurement of the stiffness values of uterine fibroids was excellent (0.905; 95% confidence interval, 0.790–0.959; Fig. 3).
Fig. 3.
Scatter plot of stiffness values of uterine fibroids measured by two observers. The interobserver intraclass correlation coefficient for the measurement of stiffness values of uterine fibroids was excellent (0.905; 95% confidence interval, 0.790–0.959). Dashed line represents linear regression. ICC, intraclass correlation coefficient.
Discussion
In this study, pre-treatment fibroid volume, signal intensity ratio of fibroids-to-muscle on T2WI, and stiffness value were found to be predictive factors of volume reduction and pre-treatment fibroid volume, and the post-treatment fractional changes in fibroid stiffness value were associated with improvements in subjective symptoms.
To the best of our knowledge, there are no clinical studies evaluating the predictive value of MRE for the treatment outcomes of MRgFUS for uterine fibroids. Coagulative necrosis can be induced by heating up the tumor tissues with MRgFUS. Viable cells or fibrous tissue are damaged to become necrotic tissue. Inflammatory response also can happen, which in turn increases the local pressure and stiffness. These pathological changes happen simultaneously, and probably affect how the stiffness changes. Previous studies have showed that NPV ratio and pre-treatment fibroid volume were related to treatment response.7,8 Furthermore, low signal intensity on T2WI was reported to be an indicator of good response to MRgFUS.6,9 The relationship between the signal intensity of T2WI and the proliferative activity of the fibroid cells has been previously reported; high signal intensity fibroids have higher proliferative activity than low signal intensity fibroids.10 Fibroids of high signal intensity on T2WI may indicate fluid-rich tissues, or degeneration. In these tumors, it may be difficult to raise their core temperature to an appropriate level because of the cooling effect of blood flow.11 Typical fibroids have a denser texture than degenerative fibroids. Therefore, typical fibroids show higher stiffness values than some kinds of degenerative fibroids. In other words, it is likely that high pre-treatment fibroid stiffness values indicate dense fibrous connective tissue, which in turn predicts a good response to MRgFUS.
Another interesting result in our study was that the symptoms were improved when the fibroid stiffness was decreased, even if the size of the fibroid did not decrease (Fig. 2). We speculate that this may be due to diminished compression of the urinary bladder or endometrium. The aim of MRgFUS treatment is not necessarily to decrease the size of the treated fibroids, but to improve symptoms. Therefore, fibroid stiffness reduction could be a good indicator of the efficacy of MRgFUS. A similar result was reported in a previous study using real-time elastography by transvaginal ultrasound (US).12 The advantages of MRE compared to US elastography are as follows. First, the 2D or 3D displacement vector was assessed in MRE, whereas only one directional measurement was performed in US elastography, which might be vulnerable to complex waves including reflection and refraction. Second, the area measured in the fibroid was larger in MRE than in US elastography. Third, in MRE, it can be measured any time later if the data remains. On the other hand, the weakness of MRE compared to US elastography are high cost and some contraindications of MRI such as metallic device in the body, restlessness, or claustrophobia.
Our study was mainly limited by its retrospective design and small sample size. A prospective study with a larger cohort would be necessary to confirm the usefulness of MRE for the assessment of the efficacy of MRgFUS.
In conclusion, the fractional change in stiffness value of uterine fibroids measured by MRE would be related with the treatment outcomes after MRgFUS.
Acknowledgments
We thank Yair Bauer from INSIGHTEC Ltd. Japan for the technical support.
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1. Stewart EA. Uterine fibroids. Lancet 2001; 357: 293– 298. [DOI] [PubMed] [Google Scholar]
- 2. Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol 2003; 189: 48– 54. [DOI] [PubMed] [Google Scholar]
- 3. Stewart EA, Taran FA, Chen J, et al. Magnetic resonance elastography of uterine leiomyomas: a feasibility study. Fertil Steril 2011; 95: 281– 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal 2001; 5: 237– 254. [DOI] [PubMed] [Google Scholar]
- 5. Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002; 99: 290– 300. [DOI] [PubMed] [Google Scholar]
- 6. Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol 2007; 196: 184.e1– e6. [DOI] [PubMed] [Google Scholar]
- 7. Okada A, Morita Y, Fukunishi H, Takeichi K, Murakami T. Non-invasive magnetic resonance-guided focused ultrasound treatment of uterine fibroids in a large Japanese population: impact of the learning curve on patient outcome. Ultrasound Obstet Gynecol 2009; 34: 579– 583. [DOI] [PubMed] [Google Scholar]
- 8. Mindjuk I, Trumm CG, Herzog P, Stahl R, Matzko M. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol 2015; 25: 1317– 1328. [DOI] [PubMed] [Google Scholar]
- 9. Funaki K, Sawada K, Maeda F, Nagai S. Subjective effect of magnetic resonance-guided focused ultrasound surgery for uterine fibroids. J Obstet Gynecol Res 2007; 33: 834– 839. [DOI] [PubMed] [Google Scholar]
- 10. Oguchi O, Mori A, Kobayashi Y, Horiuchi A, Nikaido T, Fujii S. Prediction of histopathologic features and proliferative activity of uterine leiomyoma by magnetic resonance imaging prior to GnRH analogue therapy: correlation between T2-weighted images and effect of GnRH analogue. J Obstet Gynecol (Tokyo 1995) 1995; 21: 107– 117. [DOI] [PubMed] [Google Scholar]
- 11. Billard BE, Hynynen K, Roemer RB. Effects of physical parameters on high temperature ultrasound hyperthermia. Ultrasound Med Biol 1990; 16: 409– 420. [DOI] [PubMed] [Google Scholar]
- 12. Marigliano C, Panzironi G, Molisso L, et al. First experience of real-time elastography with transvaginal approach in assessing response to MRgFUS treatment of uterine fibroids. Radiol Med 2016; 121: 926– 934. [DOI] [PubMed] [Google Scholar]