Abstract
Purpose:
We sought to use non-contrast-enhanced T1 mapping to determine the native T1 values required to identify myocardial fibrosis in patients with dilated cardiomyopathy (DCM).
Methods:
A total of 25 patients with DCM and 15 healthy controls were enrolled. All subjects underwent T1 mapping using modified look–locker inversion recovery, and the patients underwent late gadolinium-enhancement (LGE) imaging. Basal and mid-ventricular levels were divided into eight segments and the T1 value was measured in each segment. The T1 values of septal segments with LGE were compared with those of the septal segments without LGE, the minimum T1 value of each patient, and the T1 values of the normal septal myocardium.
Results:
Late gadolinium-enhancement was present in 12 septal segments (24.0%) from 10 patients (40.0%). T1 values were significantly higher in septal segments with LGE than in those without (1373.7 vs. 1288.0 ms; P = 0.035) or in normal septal myocardium (1209.1 ms; P < 0.01). A receiver operating characteristic analysis revealed the appropriate cutoff value of 1349.4 ms for identifying LGE with a sensitivity of 75% and specificity of 92.1%. When the minimum T1 value + 1.2 standard deviation (SD) was used as the threshold, the sensitivity was 75% and specificity was 89.5%.
Conclusion:
Non-contrast-enhanced T1 mapping can be used for assessment of myocardial fibrosis associated with DCM by using the appropriate threshold.
Keywords: dilated cardiomyopathy, late gadolinium enhancement, myocardial fibrosis, T1 mapping
Introduction
Dilated cardiomyopathy (DCM) is characterized by left ventricular dilation and impaired cardiac function in the absence of significant coronary artery disease.1 Dilated cardiomyopathy should be differentiated from coronary artery diseases, both of which can present with heart failure (HF), because the therapeutic strategies differ between them. Patients with coronary artery diseases may benefit from revascularization, whereas DCM is treated conservatively or heart transplantation. In the worst-case scenario, DCM progresses to serious HF associated with significant morbidity and mortality.2
Cardiac (MR imaging is valuable for evaluating cardiac morphology and function. In addition, late gadolinium enhancement (LGE) cardiac MRI is able to detect myocardial damages, such as replacement fibrosis and inflammation, associated with various myocardial diseases, and the locations and patterns of LGE may suggest the etiologies of HF.3–6 Furthermore, LGE cardiac MRI assessment of myocardial damages provides additive risk stratification for various myocardial diseases.7–9 However, patients with HF often have associated (chronic kidney disease [CKD]),2,10 and gadolinium-based contrast agents cannot be used for patients with HF and end-stage CKD because of the risk of nephrogenic systemic fibrosis.11 Therefore, if non-contrast-enhanced cardiac MRI identifies myocardial fibrosis associated with DCM, it will be beneficial for the patients with DCM.
T1 mapping is another cardiac MRI technique for non-invasive characterization of myocardial tissue.12 This technique is able to detect and quantify diffuse myocardial fibrosis, edema, and inflammation,13,14 and some studies have shown that T1 mapping before and after gadolinium injection is able to measure the extracellular volume fraction of the myocardium.15,16 In recent studies, non-contrast-enhanced T1 mapping was able to identify myocardial replacement fibrosis, which is concordant with LGE, in some types of cardiomyopathy during measurement of the native T1 values of myocardium.14,17,18 However, only a few studies have investigated the correlation of LGE and non-contrast-enhanced T1 mapping in non-ischemic myocardial diseases, such as DCM and myocarditis.14,19 Acute myocarditis induces a marked increase in the tissue water content, which can change myocardial T1 values significantly,14 whereas the changes in native myocardial T1 values may be smaller in DCM. Therefore, we sought to determine whether native T1 values acquired by non-contrast-enhanced T1 mapping could identify myocardial damages (i.e., LGE) in patients with DCM.
Materials and Methods
Patients
We enrolled 25 patients with DCM who underwent cardiac MRI including non-contrast-enhanced T1 mapping between February 2011 and August 2015. They comprised 20 men and 5 women, ranging in age from 20 to 84 years (median, 64 years; interquartile range [IQR], 55.5–77.0 years). The final diagnosis of DCM was made from the combination of clinical findings, imaging findings, and histological studies (n = 12). Ischemic cardiomyopathy was excluded based on the presence of normal coronary arteries on coronary angiography. Myocarditis, stress-induced cardiomyopathy (takotsubo cardiomyopathy), severe valvular diseases, metabolic disorders, and hypertensive heart disease were excluded based on the patients’ clinical backgrounds and laboratory data. The patients with serious CKD were not included because of contraindication for gadolinium-based contrast agents. Fifteen healthy volunteers also underwent non-contrast-enhanced T1 mapping as controls. All were men, ranging in the age from 22 to 63 years (median, 39 years; IQR, 31.7–46.5 years). They did not undergo LGE because of ethical constraints on the use of contrast media. The present study followed the ethics guidelines given by our Institutional Review Board, and informed consent for the cardiac MRI examinations was given by all the subjects. Because of the retrospective study design for the patients, the requirement for additional consent for the review of medical reports and images was waived.
Magnetic resonance imaging
The MRI examinations were performed using a 3T unit (Achieva 3T, Release 3.2.3; Philips Healthcare, Best, the Netherlands). A 6-channel cardiac multicoil was used for signal reception. Electrocardiographic-gated breath-hold steady-state free-precession (SSFP) cine images in the 2- and 4-chamber views were obtained. Thereafter, short-axis SSFP images encompassing the entire left ventricle were obtained using the following imaging parameters: TR, 4.3 ms; TE, 1.96 ms; flip angle, 55°; FOV, 360 × 360 mm; imaging matrix, 224 × 218; slice thickness, 8 mm; and interslice gap, 2 mm. Non-contrast-enhanced T1 mapping was performed with a 3-(3)-5 modified look–locker inversion-recovery (MOLLI) sequence acquired with an inversion-recovery single-shot SSFP; three images were acquired after the first inversion pulse followed by three cardiac cycle interval, and five images were acquired after the second inversion pulse.20 Therefore, eight multicontrast images were acquired during 11 cardiac cycles in short-axis basal and mid-ventricular slices. The imaging parameters for MOLLI were as follows: TR, 2.4 ms; TE, 1.01 ms; flip angle, 35°; FOV, 380 × 309 mm; imaging matrix, 192 × 156; and slice thickness, 10 mm. The delay time was 159 ms with an inversion time increment of 350 ms. LGE imaging was performed approximately 10 min after a 0.15 mmol/kg gadolinium (gadopentetate dimeglumine) injection. LGE was acquired using a 2Dl T1-weighted segmented gradient-echo sequence with the following imaging parameters: TR, 6.2 ms; TE, 1.89 ms; flip angle 15°; FOV, 380 × 344 mm; imaging matrix, 256 × 175; and slice thickness, 10 mm. Look–locker T1 scout imaging was performed to determine inversion time nullifying the myocardium, and phase sensitive technique was also used for LGE imaging in our clinical routine protocol. We used a parallel imaging technique (i.e., sensitivity encoding) with a reduction factor of 1.3–2 in all sequences. The imaging planes were matched between cine, LGE, and T1 mapping.
Imaging analysis
The left ventricular (LV) functional parameters, such as LV mass, LV ejection fraction (LVEF), and LV end-diastolic and end-systolic volumes (LVEDV, LVESV), were measured on short-axis cine SSFP images using a workstation (ViewForum systems; Philips Healthcare). These functional parameters (except LVEF) were indexed to body surface area. Non-contrast-enhanced T1 mapping was generated from eight MOLLI images and T1 measurement using dedicated software (RelaxMaps, PRIDE; Philips Healthcare). Two independent observers (one with 7 years’ and the other with 4 years’ experience with cardiac MRI) manually placed ROIs on the anterior, septal, inferior, and lateral myocardium at the basal and mid-ventricular levels as large as possible, care was taken not to include LV cavity and epicardial fat within ROI, and we measured the T1 values of the myocardium. Because a previous study demonstrated the absence of susceptibility artifacts and the high prevalence of fibrosis, we mainly investigated the interventricular septum.21 We also assessed the other segment to identify the minimal average T1 value of each patient. When septal LGE was detected, the ROI was placed to include it. The observer with less experience in cardiac MRI measured the T1 value again after an interval of 18 months in the same segments. Myocardial LGE was firstly identified on visual inspection by consensus of the two observers in the clinical routine and defined quantitatively when its mean signal intensity was two standard deviations (SDs) above that of the remote myocardium.9,19 The signal intensity was measured using dedicated software (AZE VirtualPlace; AZE, Tokyo, Japan).
All data were checked for normality using the Kolmogorov–Smirnov test. Normally distributed data are presented as mean ± SD; non-parametric data are presented as median with IQR. First, we compared the clinical backgrounds and LV functional parameters of DCM patients with and without septal LGE. The comparison was made using an unpaired two-tailed Student’s t-test or Mann–Whitney U test. Next, the inter- or intraobserver correlation and variability for the native T1 values were assessed using intraclass-coefficient and Bland–Altman analyses, respectively. The interobserver correlation and variability were assessed in the entire 200 myocardial and the 50 septal segments of 25 DCM patients. Intraobserver correlation and variability were assessed in 80 segments of 10 patients, who were selected randomly, with an 18 months interval. Third, we compared the T1 values between the septal myocardium of DCM patients with and without LGE and the septal myocardium of the healthy volunteers. For this comparison, an analysis of variance with post hoc Bonferroni test was used. Fourth, we performed a receiver operator characteristic (ROC) analysis to determine the optimal threshold for identifying the septal LGE based on native T1 values. We also set up T1 values of the segment having minimum T1 value and its 1–2 SD of each patient to identify the optimum threshold for detecting the presence or absence of septal LGE in patients with DCM. This method might be similar to the quantitative definition of LGE: comparison with the normal-appearing remote myocardial signal in each patient. A P < 0.05 was considered significant for all statistical analyses. The area under the curve (AUC) was estimated in the ROC analysis. All statistical analyses were performed using SPSS software (IBM SPSS Statistics v23.0; IBM, Armonk, NY, USA).
Results
Patient characteristics
Cardiac MRI studies were completed and the data were analyzed successfully for all of the 25 patients and 15 controls. Late gadolinium-enhancement was present in 10 of the 25 patients (40.0%) and 12 of the 50 basal or midventricular septal segments (24.0%). Of the 150 non-septal segments, only three segments (2%) showed LGE as expected. The septal LGE showed a mid-myocardial linear pattern in six segments (50.0%), and a patchy pattern at the right ventricular insertion areas in six segments (50.0%). Table 1 summarizes the patients’ clinical characteristics and cardiac function estimated by MRI. The New York Heart Association (NYHA) functional class was significantly higher in patients with septal LGE than in those without (P < 0.05). Left ventricular ejection fraction was significantly lower in the DCM patients with septal LGE (P < 0.01). Heart rate (HR) and LVESV index was higher in patients with septal LGE than in those without (P < 0.05).
Table 1.
Patients characteristics
| LGE negative (n = 15 ) | LGE positive (n = 10 ) | P-value | |
|---|---|---|---|
| Age (years) | 66.0 (54.3–76.5) | 64.5 (52.3–79.3) | 0.88 |
| Male | 11 (73.3) | 9 (90.0) | 0.29 |
| Diabetes | 4 (26.7) | 3 (30.0) | 0.86 |
| Hypertension | 9 (60.0) | 7 (70.0) | 0.63 |
| NYHA functional score * | 1.6 ± 0.63 | 2.2 ± 0.63 | 0.029 |
| BNP (pg/ml) | 290.4 ± 348.4 | 410.5 ± 443.9 | 0.46 |
| β-blocker | 10 (66.7) | 8 (80.0) | 0.49 |
| ACE/ARB | 13 (86.7) | 8 (80.0) | 0.67 |
| Spironolactone | 7 (46.7) | 3 (30.0) | 0.43 |
| Amiodarone | 3 (20.0) | 1 (10.0) | 0.52 |
| HR (bpm) | 68.0 ± 10.1 | 81.7 ± 10.1 | 0.003 |
| LVEF (%)* | 24.7 (17.1–31.9) | 16.3 (13.7–19.4) | 0.002 |
| LVEDV index (ml/m2) * | 104.2 ± 34.2 | 131.3 ± 30.1 | 0.054 |
| LVESV index (ml/m2) * | 77.5 ± 33.3 | 108.8 ± 23.9 | 0.018 |
| LV mass index (g/m2) | 76.3 ± 24.1 | 89.6 ± 24.0 | 0.19 |
LGE negative, patients without septal late gadolinium enhancement; LGE positive, patients with septal late gadolinium enhancement; NYHA, New York Heart Association; BNP, brain natriuretic peptide; HR, heart rate; ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume. Values represent median (IQR); n (%), or mean ± SD.
The patients with septal LGE had higher NYHA score and HR, lower LVEF and higher LVESV index than those without.
Inter- and intraobserver variability for T1-mapping measurement
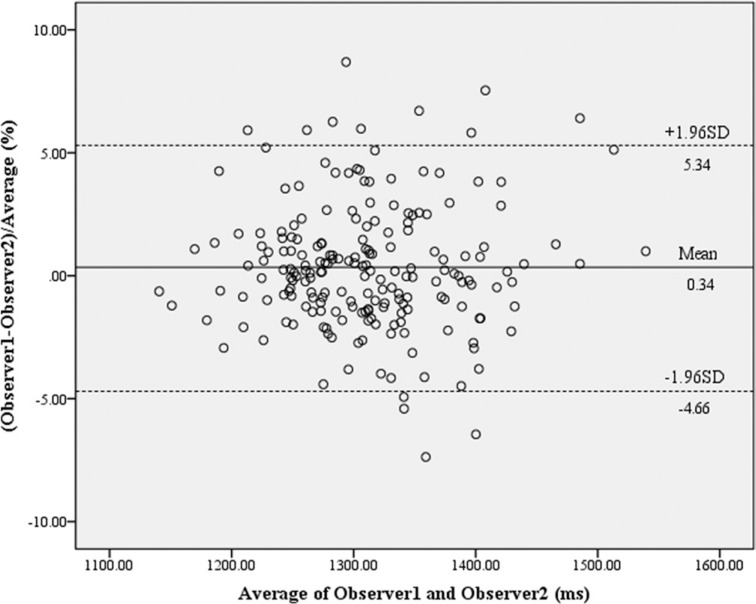
There was excellent interobserver correlation between the native T1 values of the entire myocardial segments determined by the two observers (r = 0.88, [95% CI, 0.84–0.90], P < 0.01). The intraobserver correlation was also high (r = 0.87, [95% CI, 0.80–0.91], P < 0.01). Bland–Altman analysis showed an average difference in estimates of 0.34 ± 5.0% for interobserver variability (Fig. 1) and 0.35 ± 4.8% for intraobserver variability in the entire LV myocardium. For the septal segments, the interobserver correlation was excellent (r = 0.89, P < 0.01), and an average difference was −0.04 ± −4.8%.
Fig. 1.
Bland–Altman plot demonstrates a high reproducibility of native T1 values between two observers (an average difference: 0.34 ± 5.0%). Dashed lines represent 1.96 standard deviation (SD).
Relationship between native T1 values and LGE
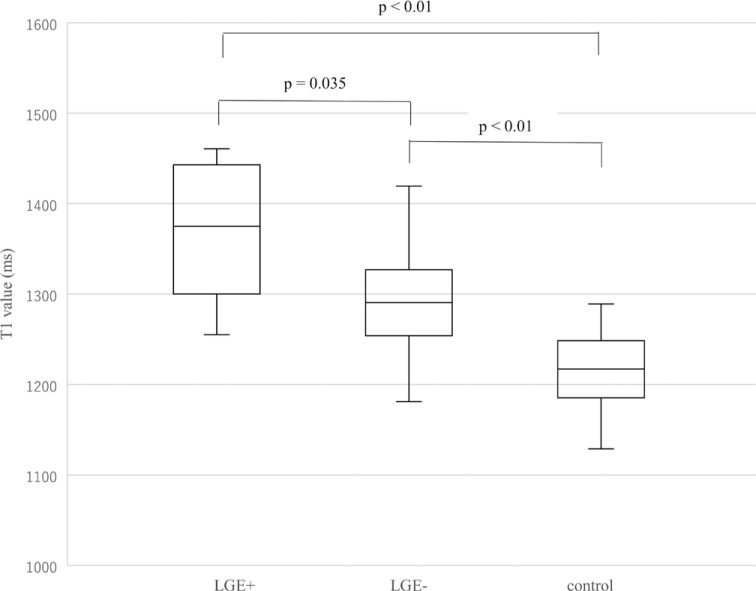
There were significant differences between the native T1 values of septal segments of DCM patients with LGE and without LGE and those of the normal myocardium from the healthy volunteers (P < 0.01). The T1 values were higher in septal segments with LGE (1373.7 ± 72.2 ms, 1255.1–1460.7 ms) than in those without (1288.0 ± 57.8 ms, 1144.2–1419.3 ms; P = 0.035) and in normal septal myocardium (1209.1 ± 56.4 ms, 1015.6–1289.2 ms; P < 0.01; Fig. 2). The T1 values in septal segments of DCM patients without LGE were significantly higher than those of healthy volunteers (P < 0.01; Fig. 2).
Fig. 2.
Native septal T1 values of patients with dilated cardiomyopathy (DCM) and normal controls. T1 values differ significantly between the septal segments of patients with (n = 12) and without late gadolinium enhancement (late gadolinium enhancement [LGE]; n = 38), and the normal septal myocardium of the healthy volunteers (n = 30, P < 0.01). T1 values are higher in the segments with LGE than in those without (P = 0.035) and in the normal myocardium (P < 0.01). The T1 values in the septal segments of patients without LGE are also higher than those of normal controls (P < 0.01). LGE +, septal myocardium with LGE; LGE −, septal myocardium without LGE.
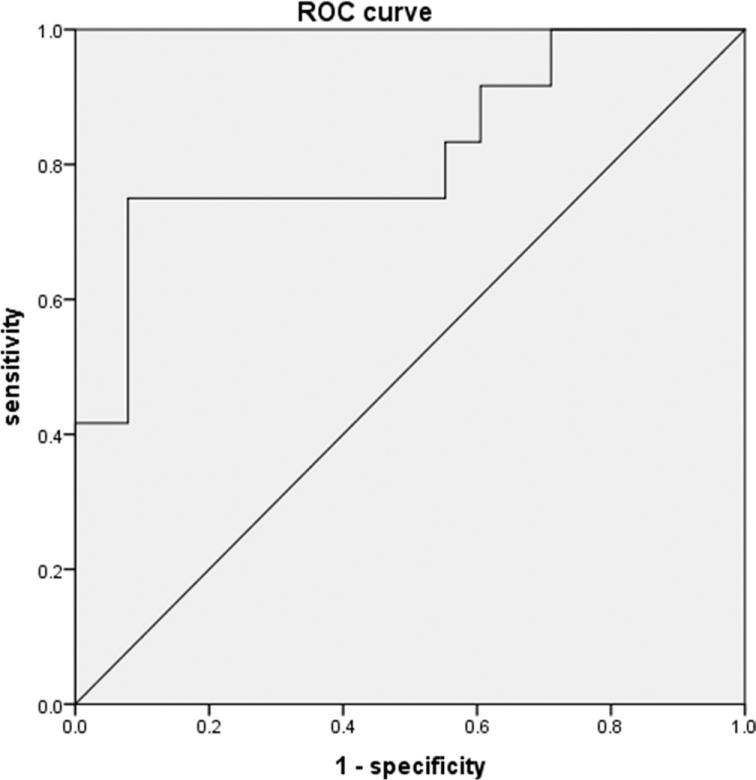
Table 2 summarizes the sensitivity, specificity, positive predictive value, and negative predictive value (NPV) of each threshold for identifying septal LGE in patients with DCM. An ROC analysis showed that the AUC of the T1 value for identifying LGE was 0.82, and the appropriate cutoff value was 1349.4 ms with sensitivity of 75.0% and specificity of 92.1% (Fig. 3). When the minimum T1 value + 1.2 SD of each patient was used as the threshold, the sensitivity and specificity were 75.0% and 89.5%, respectively. Figure 4 shows the imaging results for a typical patient with DCM and septal LGE.
Table 2.
Diagnostic performance of non-contrast-enhanced T1 mapping for identifying septal late gadolinium enhancement in patients with dilated cardiomyopathy (DCM)
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| T1 value ≥ 1349.4 ms | 75.0 | 92.1 | 75.0 | 92.1 |
| min + 1SD | 75.0 | 81.6 | 56.3 | 91.2 |
| min + 1.1SD | 75.0 | 86.8 | 64.2 | 91.7 |
| min + 1.2SD | 75.0 | 89.5 | 69.3 | 91.9 |
| min + 1.3SD | 66.7 | 92.1 | 72.7 | 89.8 |
| min + 1.5SD | 66.7 | 92.1 | 72.7 | 89.8 |
| min + 2SD | 33.3 | 97.4 | 80.2 | 82.2 |
min, minimum T1 value of the myocardium in each patient; PPV, positive predictive value; NPV, negative predictive value; SD, standard deviation.
Fig. 3.
Receiver operator characteristic curve for identifying septal late gadolinium enhancement (LGE) based on native T1 values acquired by non-contrast-enhanced T1 mapping. The appropriate threshold was defined as 1349.4 ms, and the area under the curve of this T1 value for identifying LGE is 0.82. ROC, receiver operator characteristic.
Fig. 4.
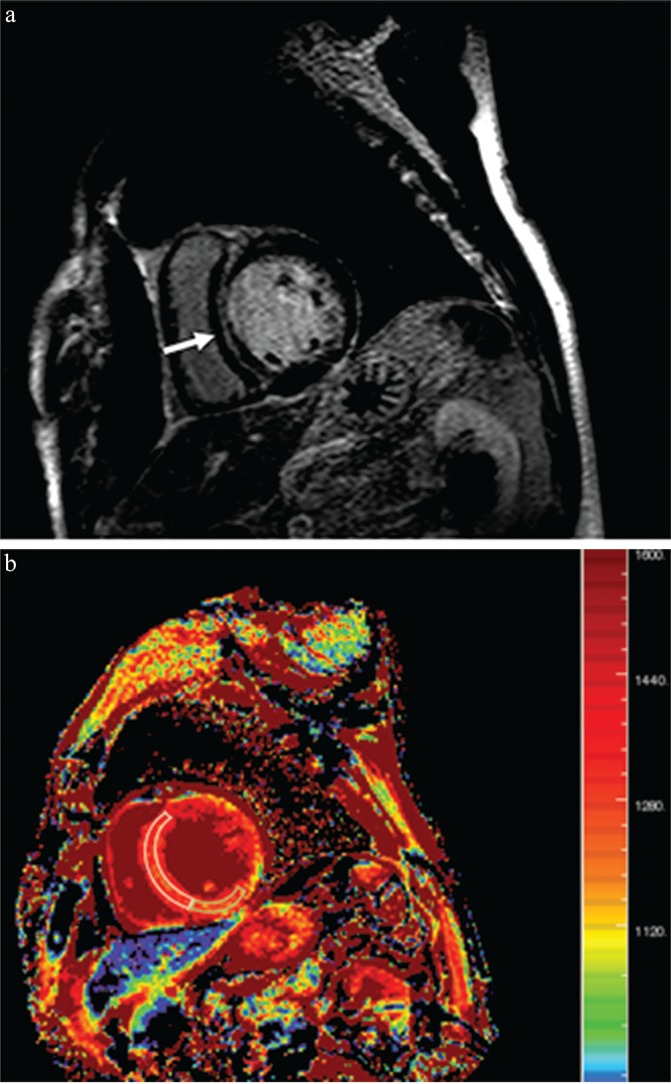
Imaging for a 60-year-old man with dilated cardiomyopathy and septal fibrosis. (a) Late gadolinium enhancement (LGE) was found in the mid-wall of the interventricular septum (arrow). (b) Non-contrast-enhanced T1 mapping shows that the native T1 value of the septal region including LGE (enclosed by a white line) is 1382.2 ms, which is more than 1349.4 ms and 1.2 standard deviation (SD) above that of the minimum T1 value (enclosed by a green line, 1262.4 ms; SD, 62.0 ms) in this patient.
Discussion
The present study demonstrated that 1) patients with DCM and septal LGE had significantly lower LVEF and higher NYHA score, HR, and LVESV index than those without septal LGE, 2) the native T1 values of the septal segments with LGE were significantly higher than those of patients without LGE and those of normal controls, and that the T1 values of the septal segments of patients without LGE were higher than those of normal controls, and 3) the threshold of 1349.4 ms can be used to assess septal fibrosis (i.e., LGE) associated with DCM on non-contrast-enhanced T1 mapping. Therefore, non-contrast-enhanced T1 mapping may be useful for evaluating the severe myocardial damages associated with DCM, when using the appropriate threshold of the native T1 values.
Non-invasive measurement of diffuse myocardial fibrosis using non-contrast-enhanced T1 mapping is predictive of mortality and hospitalization induced by HF in patients with DCM.22 Tachi et al.23 demonstrated that the post-contrast T1 value of the myocardium is correlated with LV dysfunction, while LGE is related to serious ventricular tachycardia in patients with DCM and LVEF < 35%. Other studies suggest that the presence of LGE is an important marker for adverse LV remodeling and the severity of cardiac dysfunction9,10 In our study, the patients with DCM and septal LGE had significantly lower LVEF and higher NYHA scores than those without LGE. Thus, the identification of myocardial LGE using non-contrast-enhanced T1 mapping may be beneficial for patients with DCM and CKD, for whom gadolinium-based contrast agents are contraindicated.
In the present study, the native T1 values of the septal segments with LGE were significantly higher than those of segments without LGE and those of normal controls. The threshold of 1349.4 ms provided the high specificity and NPV for septal LGE in DCM. These results indicate that non-contrast-enhanced T1 mapping can presume the septal LGE, and rather it can identify the DCM patients without septal fibrosis, who may have better prognosis.8,9 We used the 3-(3)-5 MOLLI sequence,20,23 and it could be affected by HR.24 The native T1 values of the myocardium may be underestimated in the subjects with high HR. Nonetheless, the patients with septal LGE and higher HR showed a greater native T1 value of the septum than those without LGE. In addition, we also compared the T1 value of septal LGE with minimum T1 value used as an internal reference in each patient, which might minimize the influence of HR. Consequently, the minimum T1 +1.2 SD provided the sensitivity, specificity, and NPV comparable to those obtained with the threshold of 1349.4 ms. This finding may be related to the mechanism of LGE, which reflects T1 or signal difference between the serious myocardial damages, including replacement fibrosis, and normal or less damaged myocardial tissues, but not the myocardial T1 value itself. In our study, the difference in T1 values appeared small between septal segments with LGE and segments with a minimum T1 value (i.e., above 1.2 SD), compared to the previous studies of myocardial infraction and myocarditis.25,26 Because even the myocardium without LGE could include diffuse or interstitial fibrosis in DCM, the difference in native T1 values may be smaller between septal segments with and without LGE. Indeed, the native T1 values of septal myocardium without LGE in patients with DCM were significantly higher than those of the normal controls.
There are some limitations to this study. First, the sample size was relatively small. However, we analyzed 320 ROIs including those of patients and healthy volunteers. Second, because myocardial T1 values are largely dependent on the MRI scanners and protocols, the thresholds shown herein cannot be necessarily extrapolated to other institutions.12,15,27 Third, the observers’ experience in placing ROIs on the T1 mapping might lead to slightly higher intra- and interobserver variability of native T1 values in the present study (i.e., about 5.0%), compared to the previous studies (i.e., 2.0–4.8%).17,19,21 However, the mean differences in native T1 values between septal LGE, septal myocardium without LGE of DCM, and normal septal myocardium were approximately 80–90 ms, which was larger than the 5.0% bias, approximately 60–70 ms. In addition, the minimum T1 +1.2 SD could be used as the threshold for identifying septal LGE in each patient with DCM. Therefore, the present intra- and interobserver variability of native T1 values might be tolerable for clinical non-contrast-enhanced T1 mapping in patients with DCM. Fourth, the control subjects were all men and younger than the patients with DCM, although myocardial T1 values do not vary with age in men.28 Fifth, we did not evaluate the prognosis of patients with DCM. However, the patients with septal LGE had more serious clinical symptoms and LV dysfunction than those without. Gulati A, et al.8 demonstrate that LGE is an independent predictor of the prognosis in the patients with DCM. Finally, we did not compare the MRI and histological findings in approximately half of our patients. In addition, the histological specimens were acquired from the endocardial myocardium of the basal inferior wall and not from the mid-wall of the interventricular septum, which we intensively investigated using non-contrast-enhanced T1 mapping and LGE cardiac MRI.
Conclusion
Non-contrast-enhanced T1 mapping can be used for assessment of septal myocardial damages (i.e., LGE) associated with DCM. In the present study, the T1 threshold value of 1349.4 ms or the minimum T1 value +1.2 SD provided high specificity and NPV for evaluating septal LGE in patients with DCM.
Acknowledgments
The authors appreciate Yoshio Matsumura, RT (Nippon Medical School) and Makoto Obara, RT (Philips Electronics Japan) for their technical support.
Footnotes
Conflicts of Interest
The authors declared no conflict of interest related to this article.
References
- 1. Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet 2010; 375: 752– 762. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147– e239. [DOI] [PubMed] [Google Scholar]
- 3. McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 2003; 108: 54– 59. [DOI] [PubMed] [Google Scholar]
- 4. Yoshida A, Ishibashi-Ueda H, Yamada N, et al. Direct comparison of the diagnostic capability of cardiac magnetic resonance and endomyocardial biopsy in patients with heart failure. Eur J Heart Fail 2013; 15: 166– 175. [DOI] [PubMed] [Google Scholar]
- 5. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005; 26: 1461– 1474. [DOI] [PubMed] [Google Scholar]
- 6. Vogel-Claussen J, Rochitte CE, Wu KC, et al. Delayed enhancement MR imaging: utility in myocardial assessment. Radiographics 2006; 26: 795– 810. [DOI] [PubMed] [Google Scholar]
- 7. Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol 2009; 54: 1407– 1424. [DOI] [PubMed] [Google Scholar]
- 8. Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013; 309: 896– 908. [DOI] [PubMed] [Google Scholar]
- 9. Neilan TG, Coelho-Filho OR, Danik SB, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging 2013; 6: 944– 954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A. The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens 2004; 13: 163– 170. [DOI] [PubMed] [Google Scholar]
- 11. Thomsen HS, Morcos SK, Almén T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 2013; 23: 307– 318. [DOI] [PubMed] [Google Scholar]
- 12. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004; 52: 141– 146. [DOI] [PubMed] [Google Scholar]
- 13. Bull S, White SK, Piechnik SK, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013; 99: 932– 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferreira VM, Piechnik SK, Dall’Armellina E, et al. T1 mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013; 6: 1048– 1058. [DOI] [PubMed] [Google Scholar]
- 15. Moon JC, Messroghli DR, Kellman P, et al. Society for Cardiovascular Magnetic Resonance Imaging; Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013; 15: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inui K, Tachi M, Saito T, et al. Superiority of the extracellular volume fraction over the myocardial T1 value for the assessment of myocardial fibrosis in patients with non-ischemic cardiomyopathy. Magn Reson Imaging 2016; 34: 1141– 1145. [DOI] [PubMed] [Google Scholar]
- 17. Małek ŁA, Werys K, Kłopotowski M, et al. Native T1-mapping for non-contrast assessment of myocardial fibrosis in patients with hypertrophic cardiomyopathy—comparison with late enhancement quantification. Magn Reson Imaging 2015; 33: 718– 724. [DOI] [PubMed] [Google Scholar]
- 18. Kali A, Cokic I, Tang RL, et al. Determination of location, size, and transmurality of chronic myocardial infarction without exogenous contrast media by using cardiac magnetic resonance imaging at 3 T. Circ Cardiovasc Imaging 2014; 7: 471– 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging 2012; 5: 726– 733. [DOI] [PubMed] [Google Scholar]
- 20. Lee JJ, Liu S, Nacif MS, et al. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson 2011; 13: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhandiwad AR, Cummings KW, Crowley M, Woodard PK. Cardiovascular magnetic resonance with an MR compatible pacemaker. J Cardiovasc Magn Reson 2013; 15: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Puntmann VO, Carr-White G, Jabbour A, et al. ; International T1 Multicentre CMR Outcome Study. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. JACC Cardiovasc Imaging 2016; 9: 40– 50. [DOI] [PubMed] [Google Scholar]
- 23. Tachi M, Amano Y, Inui K, et al. Relationship of postcontrast myocardial T1 value and delayed enhancement to reduced cardiac function and serious arrhythmia in dilated cardiomyopathy with left ventricular ejection fraction less than 35. Acta Radiol 2016; 57: 430– 436. [DOI] [PubMed] [Google Scholar]
- 24. Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014; 16: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Messroghli DR, Niendrof T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. J Cariovasc Magn Reson 2003; 5: 353– 359. [DOI] [PubMed] [Google Scholar]
- 26. Hinojar R, Foote L, Arroyo Ucar E, et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 2015; 8: 37– 46. [DOI] [PubMed] [Google Scholar]
- 27. Roujol S, Weingärtner S, Foppa M, et al. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 2014; 272: 683– 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rauhalammi SM, Mangion K, Barrientos PH, et al. Native myocardial longitudinal (T1) relaxation time: regional, age, and sex associations in the healthy adult heart. J Magn Reson Imaging 2016; 44: 541– 548. [DOI] [PMC free article] [PubMed] [Google Scholar]