Abstract
The external auditory meatus (EAM) in many species of mysticete whales is filled with a waxy ear plug. Though this lamellated structure is often used to age a whale, its formation and development remain undescribed. It is thought that growth layer groups (GLGs) are laid down annually, thereby increasing the size of this structure. Since some mysticete whales are migratory and many undergo molting, we hypothesized that the cyclical production of these GLGs may be related to these processes. The epithelia of both EAM and glove finger (a part of the tympanic membrane protruding into the EAM) of one juvenile and multiple adult bowhead whales from both fall (October: non‐molting) and spring (May: molting) seasons were dissected and examined anatomically and histologically. These tissue samples were compared with the adult oral epithelia at the same time periods. These epithelia shared a similar basic broad structure, though there were differences in thickness and presence of intraepithelial structures. All epithelia in the October specimens were rich in both glycogen and lipid. The parakeratinized epithelium of the oral cavity in the juvenile and some May specimens shed via the production of several superficial epithelial fissures. Other adult May specimens exhibited deep epithelial fissures, reminiscent of pressure ulcers, which would cause the detachment of the entire epithelium from the dermis. We propose that sloughed epithelial lining is the source of the GLGs in the ear plug. Correlating a potential molting sequence with these observations explained the presence of epidermal glycogen, deep epidermal fissures and dermal glycolipid, and to some extent calls into question the origin and structure of the ear plug itself. Further morphological characterization of ear plugs in bowheads is needed to better understand cell origin and ear plug formation.
Keywords: bowhead whale, epithelium, external auditory meatus, seasonality
Introduction
In most tetrapods, the external auditory meatus (EAM) is a wide open tube connecting the tympanic membrane to the outer surface of the head. The EAM is lined with ceruminous wax glands (Gartner, 2017), the secretions of which protect the EAM by cycling out debris in the form of sloughed epithelium, with the flow of waxy secretion (Shelley & Perry, 1956). The EAM of mysticete whales is different; the surface opening of the EAM is a narrow slit (Lillie, 1910; Purves, 1955; Ichihara, 1959) that is functionally, although not anatomically closed (Fraser & Purves, 1960). Proximally, it terminates in the glove finger, a structure from the tympanic membrane which protrudes into the EAM (Purves, 1955; Ketten, 1997). As baleen whales age, there is a structured build‐up of waxy material (ear plug). The latter is presumed to consist of ceruminous substances (Purves, 1955). The EAM of odontocetes is also a closed‐off and narrow tube with no connection to the tympanic membrane; it does accumulate this waxy substance but does not form an ear plug (Ketten, 1997).
The presence of concentric layers in the mysticete ear plug has been noted (Purves, 1955; Roe, 1967; Blokhin, 1984) and has led to the hypothesis that production of the ear plug is cyclical and can be used for age estimation. Marine mammalogists describe these as growth layer groups (GLGs), a term initially defined in teeth by Perrin & Myrick (1980). Applied to ear plugs, a GLG consists of one darker (more keratinous) and one lighter (more fatty) layer, and these are arranged in a repeated pattern (Purves, 1955). Counting GLGs in the ear plug has been used specifically to age the balaenopterid and eschrichtiid whales, with both gray whales (Eschrichtius robustus: Blokhin, 1984) and numerous species of rorquals including humpback (Megaptera novaeangliae: Chittleborough, 1959; Clapham, 1992; Gabrielle et al. 2010), fin (Balaenoptera physalus: Nishiwaki et al. 1958; Ohsumi, 1964; Ichihara, 1966; Roe, 1967; Lockyer, 1972), sei (Balaenoptera borealis: Masaki, 1968; Lockyer, 1974), minke (Balaenoptera bonaerensis: Kitakado et al. 2013; Maeda et al. 2013) and blue (Balaenoptera musculus: Trumble et al. 2013). GLGs are also present in the ear plug of the right whale family (Balaenidae), but it has been stated that these ear plugs are not readable, at least not in the bowhead whale (Balaena mysticetus: Rosa et al. 2013).
Despite all these studies, very little is known about the composition or origin of the GLGs. Compositional studies focus mainly upon the lipid content (fatty acids, cholesterol and unsaponifiable material: Purves, 1955), hormones (Trumble et al. 2013) and contaminants (e.g. mercury, pesticides: Trumble et al. 2013). Though the cellular origin of the ear plug is noted (e.g. Purves, 1955; Ichihara, 1964; Roe, 1967), it has not been specifically analyzed. The few histological studies concentrate on the ear plug and discuss the laminations in some depth, but not the epithelium that forms the wax (Purves, 1955; Roe, 1967). Purves (1955), proposed that ceruminous glands in the glove finger of the tympanic membrane form the material that constitutes the GLGs in balaenopterids. Ichihara (1959) had access to balaenopterid EAM specimens and did not identify such glands, instead proposing that entire layers of the epithelium of the glove finger part of the tympanic membrane slough and form the laminations in the ear wax. Both Purves (1955) and Ichihara (1959) describe the lining epithelium as ‘keratinized’ squamous epithelium.
In general, mysticetes migrate annually between high latitude feeding grounds and low latitude wintering grounds and this migration has been proposed to result in the formation of GLGs (Nishiwaki, 1966; Braham, 1984; Dorsey et al. 1990; Rugh, 1990). Since migration occurs twice per year, Purves & Mountford (1959) proposed that two GLGs form each year. These authors also proposed that the mechanism leading to the shedding of layers is ‘inherently a molting cycle’. The view that two GLGs form each year is now largely discounted; there is agreement that a single GLG (pair of layers) forms each year (Roe, 1967). Roe (1967) identified ‘cellular degeneration’ as the source for both the light and dark layers of the GLG, but did not propose a chronology for their formation. He also discussed other factors that might play a role in the shedding of epithelium, including diet (fasting), day–night cycle and water temperature.
Bowhead whales (Balaena mysticetus) mature slowly; they are weaned approximately 1 year after birth and become sexually mature probably around 15 years for males and up to 26 years for females (Rosa et al. 2013). During the first decade of their life, their baleen is short, and they are inefficient at feeding and their axial girth is reduced (i.e. axial girth is greater in nursing bowheads than after weaning: George et al. 2016). It is not clear whether bowheads of the population studied by us (Bering–Chukchi–Beaufort population, BCB) shed their skin periodically (a process called ecdysis, following the definition of Reeb et al. 2005, or molting, following the definition of Fortune et al. 2017), or whether there is continuous epidermal shedding. However, superficial fissuring of the stratum externum and vacuolation of the stratum spinosum cells was described in the keratinized skin of bowheads of the Okhotsk population, a small, non‐migratory population (Chernova et al. 2016). It is possible that the generation of these GLGs in the external auditory meatus might be related to ecdysis.
All research on the genesis of the ear plug has been done in balaenopterid whales. Our study focuses on bowhead whales, a balaenid mysticete. First, the aim was to describe the histological structure of the epithelium of the EAM and the glove finger in adult bowhead whale at two different time points (spring and fall) and compare this with the juvenile condition. Since the nature of ‘keratinization’ of this lining epithelium is currently not clear, a comparison needs to be drawn to other thick non‐keratinized epithelia (i.e. on the roof of the mouth). The second aim was to determine how the accumulating material is formed in the EAM, and the third was to determine whether there is any connection between GLGs formation and the migratory behavior of the whales. The purpose of this paper was not to describe the ear plug in the bowhead whale, or its utility for age estimation, as our sample size is currently small.
Material and methods
In fall (September/October), bowhead whales migrate from the Beaufort to the Bering Sea, while passing through the Chukchi Sea as the ice closes off the Arctic Ocean (George et al. 2016). In spring (May), as the sea ice begins to melt, they migrate north to the Beaufort Sea (George et al. 2016). A small number of migrating bowhead whales are harvested by native Alaskans (Inupiat) in spring (May) and fall (September/October) during the whales’ seasonal migration past Utqiagvik (Barrow, Alaska, USA) and this is regulated by the AEWC (Alaska Eskimo Whaling Commission) communities. This harvest is permitted under the U.S. Endangered Species Act, as well as approved by the International Whaling Commission. This hunt takes place twice a year, and we refer to these specimens caught at these times as Spring and Fall whales. Samples of whales were collected by the Department of Wildlife Management, North Slope Borough, under NOAA‐NMFS permits, and these specimens form the basis for our study (Table 1).
Table 1.
North Slope Borough, Department of Wildlife Management (NSB‐DWM) specimens used in this study. Estimated age determination via baleen length, in the form of incremental baleen growth (as per Lubetkin et al. 2008, 2013)
| Specimen number | Year | Month | Sex | Age | Total body length (m) |
|---|---|---|---|---|---|
| 2016B8a/c | 2016 | May | Female | 47 years | 15.09 |
| 2016B10a/b | 2016 | May | Male | 1 year | 8.1 |
| 2016B13a | 2016 | October | Female | 25 years | 11.6 |
| 2016B14a/b | 2016 | October | Female | 6 years | 9.72 |
| 2017B5a/b | 2017 | May | Female | 24 years | 13.35 |
| 2017B6a | 2017 | May | Male | 21 years | 10.925 |
| 2017B9a/c | 2017 | October | Male | 7 years | 9.42 |
| 2017B10b/b | 2017 | October | Male | 11 years | 8.96 |
Denotes use in histological studies for EAM and glove finger.
Denotes use in histological studies for ROM.
Denotes use in anatomical studies.
In some specimens, the lining (both epithelium and dermis) of the external auditory meatus (EAM) with the ear plug in situ was excised as a single sample from the tympanic membrane proximally to the opening of the EAM on the surface of the head. The ear plug was subsequently removed, and the subsamples (1 × 1 × 1 cm) were taken from the lining of the meatus and glove finger. Additional tissues collected from each whale included oral mucosa (as an example of parakeratinized stratified squamous epithelium) from the rostral, middle and caudal region of the roof of the mouth (ROM). Tissues were preserved in 10% phosphate‐buffered formalin and processed routinely for paraffin histology, sectioned at 7 μm, and stained with hematoxylin and eosin (HE), Mason's trichrome (MT), periodic acid‐Schiff (PAS to visualize carbohydrates) or periodic acid‐Schiff with amylase (as a negative control for glycogen) (Drury & Wallington, 1980). Not all tissues were collected for all whales.
A subset of phosphate‐buffered formalin‐fixed subsamples (EAM, glove finger, ROM and ear plug) were placed for a week in a 10% sucrose solution (to prevent tissue from crystallizing) prior to sectioning on a cryostat (Leica CM1850) at 16‐μm increments. Frozen sections were only stained with Oil Red O [ORO to visualize hydrophobic lipids and some hydrophilic lipids (Kiernan, 2015): method adapted from Lillie, 1954].
Specimens were examined, and digital images were collected using either a Zeiss Stereo Discovery.v8 dissecting microscope with an AxioCam MRC camera and AxioVision LE 64v4.9.1 software or Olympus VS120 Scanning Microscope (Model # BX61VSF). All specimens are part of the collection of the North Slope Borough, Department of Wildlife Management, Alaska (NSB‐DWM). All histological procedures took place at the Northeast Ohio Medical University.
Results
Gross anatomy
The external auditory meatus (EAM) of the bowhead whale extends laterally from the tympanic membrane (and the protruding glove finger) to the surface of the lateral side of the head. Its opening to the surface is narrow, but not anatomically fused. The EAM epithelium of these whales is black, and there is no difference in pigmentation at the point where the EAM reaches the surface, making it very difficult to see its opening. From here, the EAM crosses the entire thickness of the blubber and has a narrow lumen, although a blunt dissecting probe can easily enter it. Its cross‐section of the EAM in this area is U‐shaped, and the lining of the meatus here is black.
At the surface end of the blubber, the EAM is in contact with the caudal side of the postglenoid process of the squamosal (Fig. 1A). This bone bears a broad concave groove that extends medio‐laterally, and the meatus lies in this groove. This part of the meatus has a lumen in some bowhead whales but not in others, possibly because the ear plug, when present, forces the meatus open (Fig. 1B). This part of the meatus is pink (in unpreserved tissue) with occasional longitudinal dark streaks that extend for part of the length of the meatus. The digastric muscle crosses posterior to and in direct contact with the middle part of the EAM (Fig. 1A).
Figure 1.
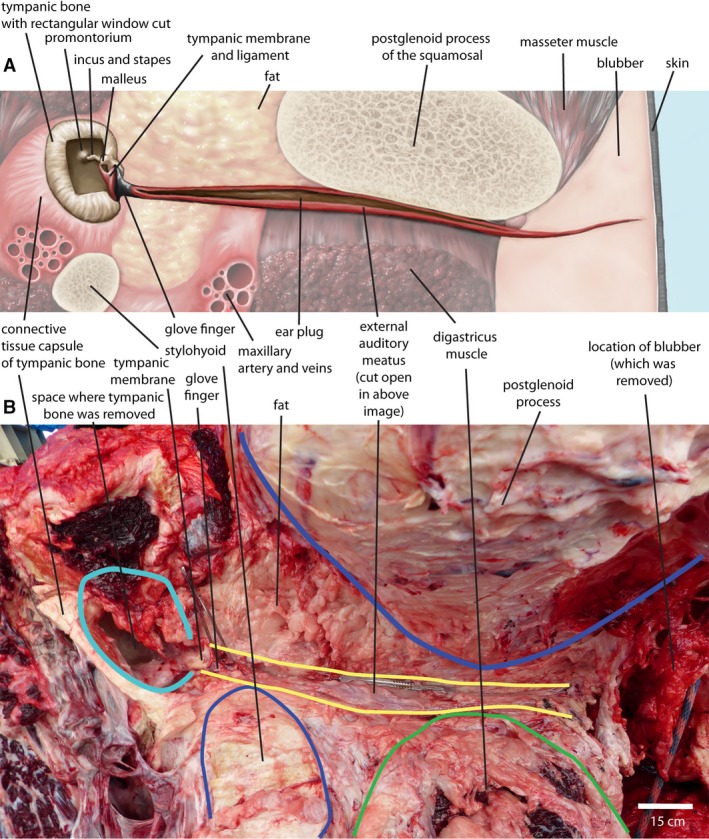
Diagram of the external acoustic meatus in the bowhead whale (A) and dissection photograph (B: NSB‐DWM 2016B8, right side, image reversed for comparability to top image). The scalpel lies along the long axis of the external auditory meatus (EAM). Structures shown in diagram are not present in a single plane or visible in a single view. Proportions of the basicranium change significantly over ontogeny. Drawing by Jacqueline Dillard.
Medial to the postglenoid process, the lumen of the EAM is always open. In the juvenile whale, the lumen is roughly triangular in cross‐section and contains an oily substance. The lining of the EAM here is pink with some pigmented streaks, as described before. In older whales, the lumen is oval or circular in cross‐section and matches the shape of the ear plug. The EAM ends at the tympanic membrane. The tympanic membrane of bowheads consists grossly of three parts, the tympanic membrane proper, the glove finger and the tympanic ligament. Of these, only the tympanic membrane proper and glove finger are in contact with the EAM. Together (EAM, glove finger and tympanic membrane proper), their outline forms a cylinder of 3–4 cm in diameter that separates them from the middle ear cavity (Fig. 1A). Most of this circular outline is taken up by the tympanic membrane proper, but the glove finger projects into the EAM from the dorso‐caudal side of the membrane proper. The glove finger has the shape of a dome, with a diameter similar in size to its height.
Histology
Our histological description of the EAM does require that we review the terminology used to describe the layers of stratified squamous epithelium (summarized in Table 2), as this varies depending upon the type of epithelium encountered. Typical mammalian stratified squamous keratinized epithelium contains four to five strata, where the superficial stratum consists of flat, dead, keratinized cells (keratins 1 and 10: Gartner, 2017). Stratified squamous nonkeratinized epithelium consists of three to four strata of cells, the outermost of which, though they contain keratin (keratins 4 and 13), are not dead (Squier & Kremer, 2001). An intermediate form is the parakeratinized epithelium. It has characteristics of both keratinized (i.e. the cells are keratinized and there is a distinct condensed superficial stratum: Skieresz‐Szewczyk et al. 2014) and nonkeratinized (has keratins 4 and 13: Squier & Kremer, 2001) epithelium. Whale skin consists of parakeratotic epithelium, which resembles parakeratinized epithelium (same strata) but differs in that the superficial stratum consists of living keratinocytes which become increasingly flatter as they approach the surface (Spearman, 1972; St Aubin et al. 1990), unlike the uniform size cells in the nonkeratinized epithelium (Gartner, 2017). The bowhead whale EAM is lined by parakeratinized epithelium, based upon the presence of three strata and flattened, uniformly shaped nucleated superficial cells.
Table 2.
Table showing the terms used in stratified keratinized epithelia
| Type of stratified squamous epithelium | Keratinized | Parakeratotic | Parakeratinized |
|---|---|---|---|
| example | Mammal skina | Whale skinb , c | Oral mucosad , e, EAM (this study) |
| No. of strata | 4 | 3 | 3 |
| Superficial | Stratum corneum | Stratum corneum | Keratinized layer |
| Stratum granulosum | ABSENT | ABSENT | |
| Stratum spinosum | Stratum spinosum | Stratum spinosum | |
| Deepest | Stratum basale | Stratum basale | Stratum basale |
Roof of the mouth (ROM)
The roof of the mouth is characterized by a relatively thick epidermis, with long epidermal pegs that protrude up to 3 mm into the underlying dermal tissue characterizing this region (Fig. 2). The epidermis is a thick, stratified parakeratinized squamous epithelium consisting of three strata (Fig. 2A): The stratum basale consists of one or two cuboidal cells overlying the dermal pegs. These cells contain many intracellular pigment granules. No differences were observed in the structure of this zone between specimens or seasons.
Figure 2.
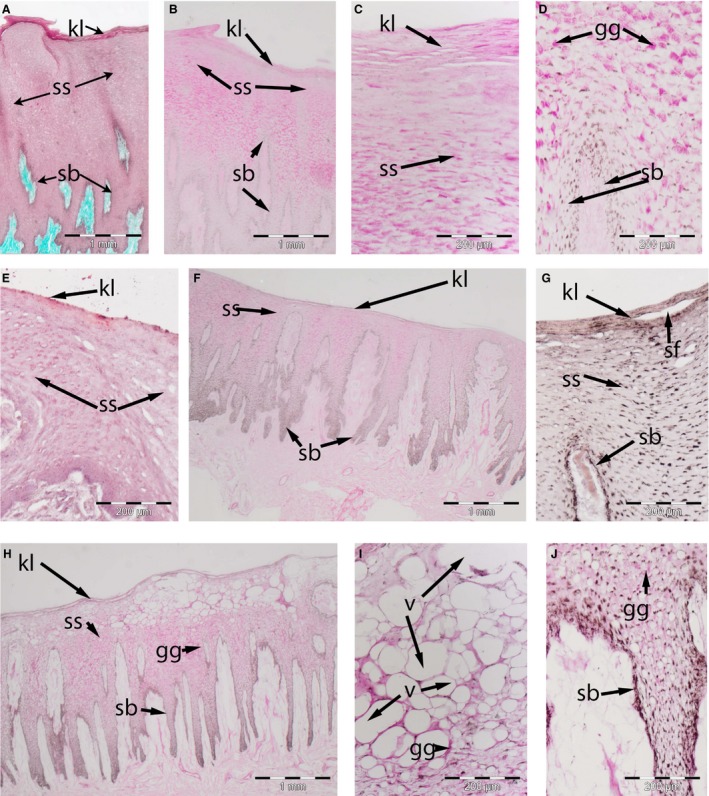
Skin of adult bowhead whale oral cavity. (A–E) October (NSB‐DWM 2016B13). (F) May (NSB‐DWM 2015B5): a low magnification showing the distribution of the glycogen (magenta) in the external auditory meatus (EAM) epithelium. (G) May (NSB‐DWM 2016B14): a low magnification view to show the distribution of lipids (orange) in the EAM epithelium. (H–J) May (NSB‐DWM 2017B5): a section of the caudal aspect of the roof of the mouth (ROM) showing the location of the intraepithelial vacuoles (v). Note the greater density of glycogen granules (gg) in the October specimen, the presence of shallow superficial fissures (SF) in the May (2016) specimen and the vacuolated spaces (v) in the May (2017) specimen. k, keratinized layer; sb, stratum basale; ss, stratum spinosum. Staining: (A) Masson's trichrome, (E,G) Oil Red O, (all remaining specimens) PAS.
The stratum spinosum is the thickest zone. Keratinocytes are stellate basally and become increasingly squamous towards to epithelial surface. In fall 2016 specimens, this stratum is at least 10 cell layers thick, at the tip of the dermal papillae, and protrudes deeply into the epidermal pegs. The keratinocytes in the stratum spinosum are filled with subnuclear glycogen as evidenced by their PAS‐positive staining (Fig. 2B), which is digested in the presence of amylase. Glycogen granules are not found in the keratinized layer (Fig. 2C), despite some PAS‐positive reaction (after amylase digestion), or in the stratum basale (Fig. 2D). There are a few perinuclear lipid ORO‐positive granules present (Fig. 2E). However, the keratinocytes closer to the oral surface (superficial area) lack the glycogen but retain the lipid droplets. In spring 2016 specimens, less glycogen and fewer lipid granules are present in the stratum spinosum (Fig. 2F,G). Large vacuolated keratinocytes are found in the superficial area in spring 2017 specimens (Fig. 2H,I), with a few vacuoles present within the epidermal pegs (Fig. 2J).
The keratinized layer is a thin, adluminal zone consisting of three to four layers of nucleated squamous keratinocytes. In Fall whales, this layer is firmly attached to the underlying stratum spinosum. Keratinocytes of the stratum spinosum contain glycogen, as evidenced by their being PAS‐positive (Fig. 2C), that is digested in the presence of amylase and contains a similar amount of lipid granules as keratinocytes in the stratum spinosum (Fig. 2E). In Spring whales, more clefts split the keratinized layer in outer and inner layers (Fig. 2G,H) than in Fall whales (Fig. 2C). Keratinocytes are devoid of glycogen, and lipid droplets are less abundant than in Fall whales.
External auditory meatus (EAM)
The epithelium of the EAM in the bowhead whale is similar to that of the ROM, in that it is a stratified squamous parakeratinized epithelium (Figs 3 and 4). However, epidermal pegs are several magnitudes smaller (both in terms of length and width) and the epidermis is much thinner (fewer cells layers). Ceruminous glands were absent. The histology of the EAM epithelium differs by season and also by ontogenetic age of the individual. We here differentiate between spring‐ and fall‐caught individuals and between the juvenile and adult individuals.
Figure 3.
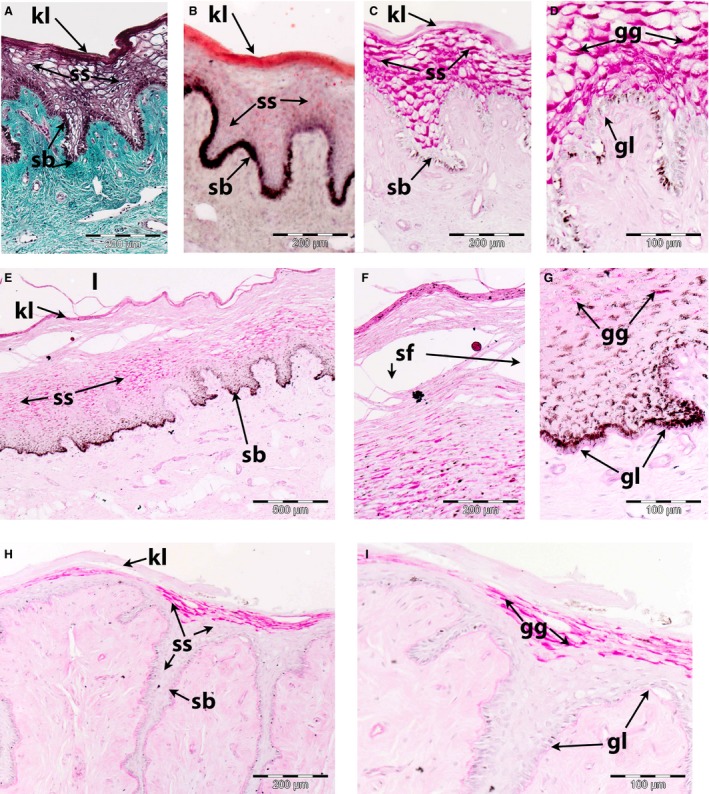
Lining (epithelium and dermis) of bowhead whale external auditory meatus. (A–D) October (A, C and D: NSB‐DWM 2016B14; B NSB‐DWM 2016B13) adult specimens. Note the density of the glycogen granules (gg) in the stratum spinosum (ss), the presence of a subepithelial glycolipid layer (gl) and lipid density in both the keratinized layer (kl) and stratum spinosum (ss). (E–G) May (NSB‐DWM 2016B10) juvenile: note the comparatively thick epithelium lining the lumen (l), the presence of the superficial fissures (sf) and the reduced amount of glycogen granules (gg) in the stratum spinosum (ss). (H–I) May (NSB‐DWM 2016B13) adult: note the reduction of the glycogen granules (gg) in the stratum spinosum (ss). sb, stratum basale. Staining: (A) Masson's trichrome, (B) Oil Red O, (all remaining specimens) PAS.
Figure 4.

Anatomy and histology of the lining of bowhead whale external auditory meatus from May 2017. (A) (NSB‐DWM 2017B6): surface view of the EAM lining, once the ear plug has been removed, showing the intact (i) epithelium, the semi‐attached slough (sl) and the exposed (e) dermal region. (B) (NSB‐DWM 2017B6): Low magnification view showing the intact epidermis on the left, and the exposed dermal papillae (dp) on the right. (C–E) NSB‐DWM 2017B5: higher magnifications of these epidermal zones, with deep fissures (df). Note the lipid distribution in the keratinized layer (kl) but not the stratum spinosum (ss), split of the stratum basal (sb) by the deep fissure (df) and the location of the glycolipid layer (gl) as the limiting structure on the newly exposed dermal papillae. (F) NSB‐DWM 2017B5: higher magnification of dermal papillae (dp). gg, glycogen granules. Staining: (C) Oil Red O; (B,D,F) PAS.
Adult – October (NSB‐DWM 2016B13, 2016B14 and 2017B9)
The epidermal pegs are up to 250 μm long and occasionally branched. The parakeratinized layer consists of three to four layers of squamous keratinocytes (Fig. 3A) which contain abundant lipid, as evidenced by their being ORO‐positive (Fig. 3B), but few carbohydrates, as they are only slightly PAS‐positive, (Fig. 3C) intracellular granules that are not glycogen, since they were not digested by amylase. The stratum spinosum is 10–15 cells thick and consists of ovoid vacuolated cells which contain much intracellular glycogen (Fig. 3C,D), and some lipid (Fig. 3B) granules. A line of extracellular glycolipids lies between the stratum basale and the underlying dermis. The stratum basale is a single layer of keratinocytes, some of which contain intracellular pigment granules (melanosomes). Pigmented streaks in the EAM were noted before, implying that not all its regions contain pigmented keratinocytes.
Juvenile – May (NSB‐DWM 2016B10)
Of the three groups, this specimen had by far the thickest epithelium (Fig. 3E). No ORO data are available. There is a relatively reduced amount of intracellular glycogen granules in the stratum spinosum (Fig. 3F) There are numerous superficial fissures in both the keratinized layer and the upper portion of the stratum spinosum (Fig. 3F,G). Some of these layers were located in the lumen of the EAM detached from its wall. There was a layer of extracellular glycolipids deep to the stratum basale (Fig. 3G).
Early molt: adult – May 2016 (NSB‐DWM 2016B8)
The epidermal pegs are long (up to 600 μm long), slender structures. The stratum spinosum is five to six cell layers thick. The degree of vacuolation of the keratinocytes and amount of intracellular glycogen varies regionally. In some regions, the cells are highly vacuolated and glycogen is present in the stellate cells within the epidermal pegs. In other regions, there are few vacuolated cells, and glycogen does not occur in the epidermal pegs (Fig. 3H). Superficial fissures were found that separate the keratinized layer from the stratum spinosum. A line of extracellular glycolipids lies between the single layer of stratum basale and the underlying dermis (Fig. 3I).
Late molt: adult – May 2017 (NSB‐DWM 2017B5 and 2017B6)
The difference between these specimens and those of NSB‐DWM2016B8 and 2016B10 is the separation of the epidermis from the dermis (Fig. 4A). This separation can be seen histologically as the presence of deep fissures (Fig. 4B). Upon closer examination, the epidermis itself has fewer lipid (Fig. 4C) and glycogen intracellular granules (Fig. 4D,E). These deep fissures split the stratum basale, such that one part is attached to the stratum spinosum and the other part remains attached to the dermal papillae (Fig. 4E). This has the effect of exposing the layer of extracellular glycolipids in the dermal papillae (Fig. 4E,F). The detached epidermis is displaced into the lumen of the EAM.
Glove finger of the tympanic membrane
Histologically, the glove finger and tympanic membrane exhibit the same characteristics as the regular EAM, with one notable exception (Fig. 5). Scattered throughout the stratum spinosum are several whorls of keratinocytes in NSB‐DWM 2016B13 and 2016B14 (Fig. 5A). The keratinocytes in these whorls are different from neighboring keratinocytes in that they possess little glycogen (Fig. 5B) but much lipid (Fig. 5C). The deep fissuring of the fall (2017B5 and 2017B6) specimens was identical in both the EAM and the glove finger (Fig. 5D and E), though the whorls were not easily located.
Figure 5.
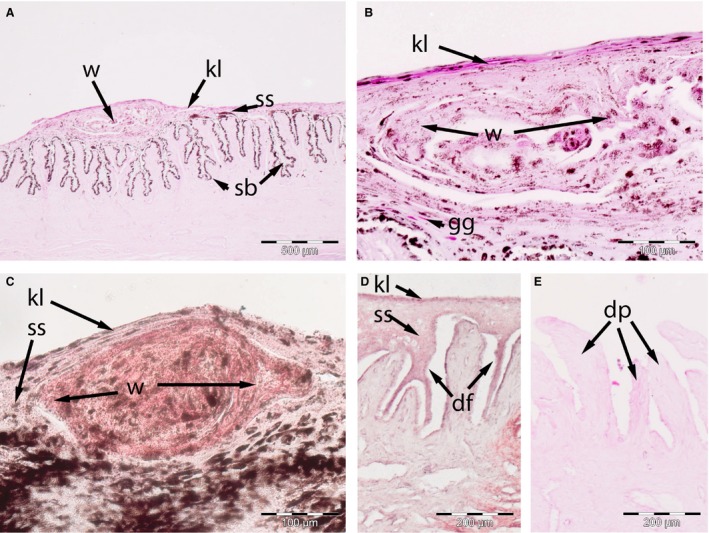
Skin of adult Bowhead whale glove finger. (A–C) October: (A,B) NSB‐DWM 2016B13, (C) NSB‐DWM 2016B13, note the presence of whorls (w) of intraepithelial tissue, which displace the glycogen granules (gg) of the stratum spinosum (ss). These whorls contain more lipid that keratinized layer (kl). (D–E) May NSB‐DWM 2017B6 specimen, note the presence of deep fissures (df) within the stratum basale (sb) in some parts and exposed dermal papillae (dp) in other. Staining: (A,B,E) PAS, (C,D) Oil Red O.
The ear plug
The ear plug is a semi‐solid, tubular structure that fills the EAM in some but not all specimens (Fig. 6). This structure tends to melt at room temperature. Upon cross‐section, in some specimens, numerous layers can be found, arranged in a roughly concentric manner, especially in the peripheral aspects (Fig. 6A). Pigmentation may be present in different areas of the same layer, but there is no evidence of consistently pigmented layers. Closer examination of the more peripheral layers reveals distinct layering of cellular material with varying levels of lipid staining in some specimens (Fig. 6B,C). However, our sample sizes are too small to determine the cause for this variation.
Figure 6.
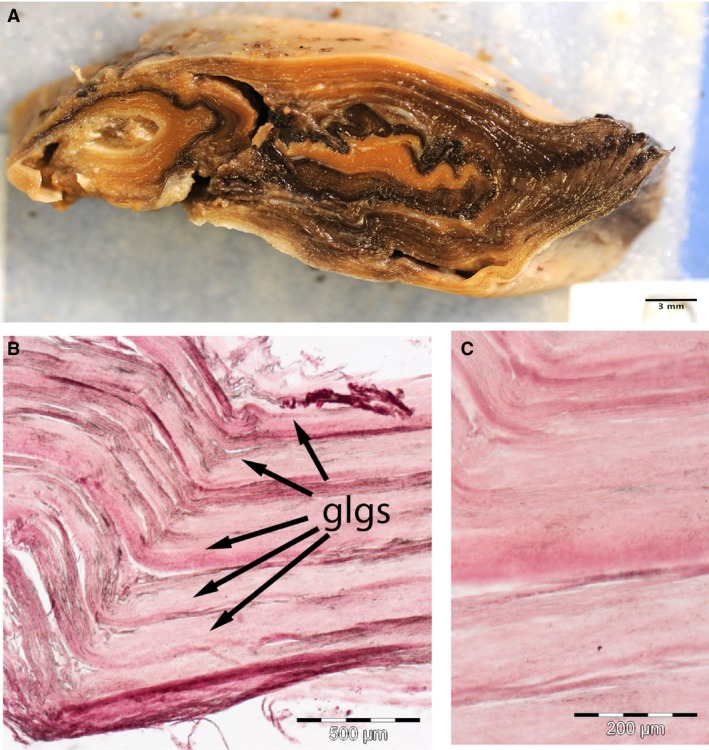
Anatomy and histology of the ear plug in an adult bowhead whale (NSB‐DWM 2017B5). (A) Gross picture of ear plug cross‐section, the long axis is in the dorsoventral plane, and the short axis is in the anteroposterior plane. Note the distinct peripheral layering and the presence of centrally located light and dark bands. (B,C) Histological frozen sections through the outer bands of the ear plug. Note the distinct layering. All histological specimens were stained with Oil Red O
Discussion
Though a number of authors has described the ear plug and its significance (Chittleborough, 1959; Purves & Mountford, 1959; Lockyer, 1972, 1974, 1984; Blokhin, 1984; Maeda et al. 2013; Trumble et al. 2013) as well as the composition of the ear plug (Purves, 1955; Ichihara, 1959, 1964; Roe, 1967) there are few publications that describe the source of this substance (Purves, 1955; Ichihara, 1959; Ichihara 1964, Roe, 1967). It is generally presumed to be ceruminous in nature, although figured specimens show keratinized material (Purves, 1955; Ichihara, 1959; 1946, Roe, 1967). To understand the composition, and thus the origin, of the ear plug, the epithelium that encases and forms it must be understood.
The epithelia of the ROM and the ear canal
The epithelia of the EAM and the glove finger consist of a stratified squamous parakeratinized epithelium, much like that of the ROM. A direct comparison of the external auditory meatus and the ROM epithelia of fall‐caught bowhead whales shows a broad similarity in structure. Both have the same three distinct cellular strata and a similar distribution both lipids and glycogen. The role of these lipid and glycogen intracellular compounds remains undetermined.
Lipids occur as large superficial accumulations within the cetacean skin (intracellular granules within the keratinocytes), for instance in southern right whales (Eubalaena australis), bottlenose dolphins (Tursiops truncatus), long‐finned pilot whales (Globicephala melaena), humpback whales (Megaptera novaeangliae) and fin whales (Balaenoptera physalus) (Pfeiffer & Jones, 1993; Reeb et al. 2007). It has been suggested that these lipids are a metabolic requirement for the skin itself (Pfeiffer & Jones, 1993).
Though glycogen has been describe in cetacean skin (Reeb et al. 2007), the extent of its distribution is unclear, although it may partially correspond to the vacuolated cells in the stratum spinosum in earlier descriptions (Severtsova & Kleinenberg, 1969). Glycogen has been described in the stratum spinosum in some mammalian fetal skin (Falin, 1961; Ling & Thomas, 1967) and is thought to play a role in skin growth and regeneration. Thus, much like the increased lipid content in the bowhead skin, the presence of glycogen may have a metabolic function (Halprin & Ohkawara, 1966).
There are two main differences between epithelia of the ROM and those of the EAM and glove finger of the external ear canal. First, the EAM epithelium is thinner (see Fig. 3B) than that of the ROM (see Fig. 2E). This could be because the ROM has to be resistant to physical abrasion during food processing. Differential regional skin thickness has been described in bowheads, ranging from 1 mm thick in the eyelid to 25 mm thick in the lower jaw (Haldiman et al. 1985). Also, in contrast to younger bowhead whales, whose EAM is not filled with the ear plug, the EAM of older individuals is stretched by the presence of the ear plug (Fig. 3B‐D). As a result, it appears that juvenile whale EAM (Fig. 3F) is thicker than that of the older adult (Fig. 3H). In addition, the dermal layer of the external auditory meatus contains a distinct extracellular layer of glycolipids, immediately deep to the stratum basale.
In addition, the epidermis of the external auditory meatus also differed from that of the glove finger of the fall‐caught specimens (NSB‐DMW 2016B13 and 2016B14) in that the latter contains intraepithelial keratinocyte whorls. The presence of these structures is undocumented. The only other description of glove finger epithelial morphology is from a comparative study of various baleen whales, specifically fin (Balaenoptera physalus), blue (Balaenoptera musculus), humpback (Megaptera novaeangliae) and sei (Balaenoptera borealis) whales (Ichihara,1959). The significance of these keratinocyte whorls is unknown and requires further investigation.
The descriptions of the glove finger epithelium in these species are similar to that of the adult bowhead EAM (NSB‐DWM 2017B5 and 2017B6) in that they are examples of a shed glove finger epithelium and exposed dermal papillae. This is the first study to have two different time points for comparison of EAM and glove finger morphology. Earlier studies on fin whales were limited to only a single time point (Purves, 1955; Ichihara, 1959) or were focused on the ear plug and only mention the dermal papillae in passing (Roe, 1967). Furthermore, only Ichihara (1959) clearly states capture time (between January and April 1956), whereas Purves (1955) only states the year. Purves (1955) only briefly mentions the epithelium, with a plate that is difficult to interpret. Ichihara (1959) provides a clear description and plates of the EAM potentially molting (i.e. dermal papillae are exposed and there is an irregular arrangement of the epidermis).
Epithelia as a dynamic structure
The lining of the external auditory canal (both EAM and glove finger) is continuous with the skin, and the skin of some marine mammals molts. This process may play a role in formation of the epithelium of the external auditory meatus, too (Purves & Mountford, 1959). Cetaceans, in general, do not undergo seasonal ecdysis (or molting) (Fortune et al. 2017); for instance, shedding of superficial epidermal cells is a year‐round process in the bottlenose dolphin (Tursiops truncatus). The turnover period for T. truncatus is estimated to be 73 days, as opposed to the 40‐ to 45‐day epidermal turnover rate of humans (Hicks et al. 1985). In contrast, in beluga whales (Delphinapterus leucas), molting is an annual process (ecdysis in the sense of Reeb et al. 2005) and it is thought that epidermal replacement takes 70–75 days postmolt (St Aubin et al. 1990). No molt has been described in the BCB population of bowhead whales that we study, the largest population of the species, which is well known to migrate with the seasons. However, superficial fissuring of the stratum externum and vacuolation of the stratum spinosum cells was described in the parakeratotic skin of bowhead of the Okhotsk population, a small, non‐migratory population of bowheads (Chernova et al. 2016). This is consistent with our finding of superficial fissuring and vacuolization in the non‐keratinized epithelium of the ROM of the bowhead whale.
The composition of the bowhead ear plug
The ear plug of the bowhead whale has only received cursory treatment in published literature. Though mammalian ear wax is formed by ceruminous secretion, there are three lines of evidence that suggest that this is not the case for the bowhead whale ear plug. First, the EAM epithelium contains no ceruminous (wax) glands that could produce wax. This is consistent with observations in fin whales (Roe, 1967). Secondly, there is evidence in humans that impacted cerumen plugs consist of both ceruminous material and cells (Robinson et al. 1989), indicating that shed cells could be an important component of the ear plug. Thirdly, the presence of cellular debris arranged in sheets akin to slough in the bowhead ear plug (Fig. 6) is consistent with the findings of glove finger‐derived degenerative epidermal cells in other balaenopterid whales (Purves, 1955; Ichihara, 1959). This leads us to infer that the lamellated bowhead whale ear plug is composed of shed cells layers from the both the EAM and glove finger, and that these form the cortex and core of the ear plug, respectively.
The effect of the annual molt on the bowhead EAM
Preliminary data suggest that part of the bowhead ear plug consists of a series of concentric rings (the GLGs). In other mysticetes, these are thought to be shed annually. This occurs in fin whales (Laws & Purves, 1956; Ohsumi, 1964) and humpback whales (Chittleborough, 1959; Gabrielle et al. 2010), where these GLGs have been used for age estimation. In contrast, even though the bowhead ear plug is layered, Rosa et al. (2013) indicated that the ear plug was not readable and thus useful for age estimation of individuals. This suggestion deserves reconsideration since the bowhead ear plug consists of layers of cells. Additionally, the formation of these GLGs may coincide with the potential seasonal molting of the skin, and some records of age may be present, consistent with the view of Purves & Mountford (1959) for other mysticetes.
While annual accumulation of laminae is now widely assumed in balaenopterid ear plugs (e.g. Roe, 1967), the mechanism and timing of epithelial shedding is poorly known. Our working hypothesis is that both the EAM and the glove finger slough cell layers into the external auditory canal. This sloughing follows an annual pattern similar to the ecdysis described in both balaenids (this study) and balaenopterids (Ohsumi, 1964; Roe, 1967; Lockyer, 1972). Five observations in our bowhead specimens support this hypothesis (Fig. 7):
The variable presence of glycogen in the stratum spinosum. In dogs, glycogen is produced by keratinocytes at a constant rate (Adachi, 1961). It has been reported that epithelial glycogen content varies both seasonally in dogs (Adachi, 1961) and in response to level of nutrition in mice (Harmon & Phizackerly, 1983). Additionally, increased epidermal glycogen levels are observed under conditions of inflammation in humans (Lobitz et al. 1962) and ultraviolet irradiation in mini pigs (Ohkawara et al. 1972). In Fall bowhead whales, there is abundant intracellular accumulation of glycogen in the keratinocytes of the EAM, which we interpret as newly formed epithelium. This is consistent with a putative regenerative function of glycogen‐filled cells (Falin, 1961; Ling & Thomas, 1967; Im & Hoopes, 1970; Williams, 1972) and suggests a tremendous regenerative capacity for this epithelium. There is a reduced glycogen content in the spring bowhead epidermal EAM. Numerous hyperproliferation markers have been identified in the human ear canal (Gurgel et al. 2010) and testing for these markers in bowhead samples could test this hypothesis about their possible epithelial regenerative nature.
The presence of fissures. The extent of the separation and consistency of these epithelial tears in different specimens indicates that these are not preparation artifacts. We observed two types of epithelial fissures in our specimens: superficial intraepithelial and deep subepithelial. The superficial, intraepithelial fissures observed in NSB‐DWM 2016B8 and 2016B10 are similar in nature to the superficial fissures observed in the Spring ROM epithelium. Such superficial fissures have also been observed in molting skin of other cetaceans (Hicks et al. 1985; St Aubin et al. 1990; Reeb et al. 2005). The epithelium of the EAM in these specimens appears to be in the process of molting. The deep, subepithelial fissures observed in NSB‐DWM 2017B6 and 2017B9 split the stratum basale, ultimately separating the entire epidermis from the dermis and exposing the dermal papillae. The exact timing and nature of cell mechanism(s) underlying the formation of these subepidermal fissures in the EAM of spring whales are undetermined; however, we speculate that shear force, pressure and torsional stress exerted by the semi‐solid ear plug on the surrounding epithelium of the EAM play a role. Pressure, shear force and friction are well known risk factors for the development of pressure ulcers, where subepidermal separation occurs during stage 1 ulcer formation, in humans (Edsberg, 2007), mice (Stadler et al. 2004) and rats (Salcido et al. 1994). Similarly, the formation of subepidermal fissures is also associated with tear stress of cellophane stripping of the epidermis in molting squamate reptiles (Maderson et al. 1978). This requires further immunohistochemical (e.g. examination of agents, including desmosomes, that may be involved epidermal sloughing) and immunological (e.g. eosinophil density assays to confirm the presence of the pressure ulcer) examination.
The glycolipid layer. A glycolipid layer has not been described in the cetacean EAM literature, and there is no such layer in the ROM. No such a layer is present in squamates when the exposed dermis is partially shed during stripping (Maderson et al. 1978). We propose that it may be a specific adaptation of the bowhead EAM and glove finger. In human skin, a level 2 pressure ulcer damages the tips of the exposed dermal papillae (Edsberg, 2007). Thus, it is possible that this glycolipid layer in the whale EAM and glove finger may function to prevent further tissue loss during the molting season. Future examination of this glycolipid layer, in terms of both content and phylogenetic distribution, is necessary.
Origin of the ear plug. The adult mysticete ear plug consists of a series of concentric layers, or GLGs (Purves, 1955). Ichihara (1964) reported that there is no ear plug in juvenile individuals of balaenopterids, though some debris (possible layers) is present in fetal fin whales. This observation is consistent with the results of the EAM in the juvenile bowhead whale (this study). Thus, the EAM epithelium in juvenile balaenopterids (Ichihara, 1964) and bowheads (this study) sheds only its outermost stratum. In our model of ear plug development, as the animal grows, slough is produced during the annual molt (ecdysis) in both the glove finger and the lining of the EAM. The slough will accumulate in the lumen of the EAM. The narrow EAM compacts the slough and is expanded over time. This thus preserves the original, layered morphology of epithelial layers, and forms the GLGs in the ear plug unless disturbed by differential movement of tissues surrounding it.
Composition of the GLGs. Purves (1955) and Maeda et al. (2013) described balaenopteroid whale GLG as consisting of dark and light bands and implied that they are released at different times. Original descriptions of these GLGs also state that they consist of dark and light bands (Fin whales: Purves, 1955), implying that they are secretions released at different times (Common Minke whale: Maeda et al. 2013). However, in these bowhead specimens, distinct layers were seen peripherally with no differentiation between light and dark bands. In this study we found that slough consists of both compacted cells and their intercellular lipids (of unknown type). The presence of keratinized material has been noted (Lockyer, 1984) but a more detailed study of these layers, and their chemical composition, is needed to understand the life history records they may produce.
Figure 7.
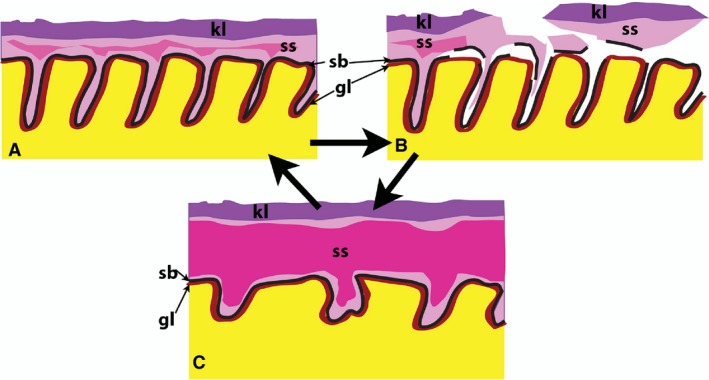
Schematic of ear plug formation in the Bowhead whale represent a continuum of the shedding cycle in the external auditory meatus (EAM) of the Bowhead whale. (A) May 2016, cf. Fig. 3H–I: all three layers are present. The superficial keratinized layer (kl) is intact, the middle stratum spinosum (ss) has a few centrally located cells containing glycogen granules (indicated by the dark pink zone) and an intact stratum basale (sb) an underlying glycolipid layer (gl) in the dermal papillae. (B) May 2017, cf. Fig. 4: intraepithelial fissures lead to the separation of the entire epithelium (keratinized layer, stratus spinosum and stratum basale) from the dermal papillae. This results in the glycolipid layer being exposed to the lumen. (C) October, 2016, cf. Fig. 3A–D: the epithelium is newly regenerated and all layers are intact. Note the thicker stratum spinosum and the abundant glycogen granule‐filled cells (in the dark pink).
Comparative studies
Histologically, little is known regarding the structure of the external auditory canal epithelium in balaenopterid whales. EAM descriptions are largely restricted to a few short descriptions of a stratified squamous epithelium with possible keratinization at specific points in time (Purves, 1955; Ichihara, 1959, 1966) and brief notes of dermal papillae distribution (Roe, 1967). In balaenopterid whales, the ear plug may be a fibrous structure (Trumble et al. 2013) and it retains GLGs well in most individuals of some species and in individuals.
The ontogeny of the ear plug was examined in fin whales. Ohsumi (1964) found that there are no laminations in whales less than 2 years old, though Ichihara (1964) shows that the ear plug is present fetally. Fin whales presumably form two laminations per year (Nishiwaki et al. 1958), but Roe (1967) implied that this epithelium is continuously shed. Whether the skin of fin whales (as opposed to the lining of their EAM) molts annually, biannually or continuously (like dolphins) is currently unknown. The answers to these questions will help define the significance of the GLGs for balaenopterids and have implications for balaenids.
Conclusions
This is the first study to systematically examine the structure of the whale EAM and glove finger at two different times of the year (spring and fall) and compare the results with those for a juvenile. These observations may be explained if molting is used as a potential model. If the EAM and glove finger molt seasonally, then they are doing it in an unusual manner, with deep fissure formation reminiscent of stage 1 pressure ulcers. This hypothesis needs to be tested through further comparative examination of other whales at multiple time periods, immunohistochemical and immunological techniques. We used disparate histological techniques, which enabled us to uncover additional epithelial features, some of which were partially explained (e.g. glycolipid layer and glycogen accumulations) and some which were not (e.g. intraepithelial lamellar formations in the glove finger). A closer examination of these epithelia in other whales, with similar techniques, is needed to examine the unique nature of the bowhead condition. Finally, understanding the epithelial origin of the GLGs now opens a new avenue of research. Previously, studies have focused primarily on the lipid contents of the GLGs, largely ignoring the epithelial components. By focusing instead on the epithelial components (e.g. keratin), future studies can be conducted into the degradation of keratin (how and at what rate it degrades) and the possible use of this knowledge in determining the age of whales.
Author contributions
J.G.M.T. conceived the study. J.G.M.T., R.S., J.C.G. and R.S. collected the specimens. J.G.M.T. performed the gross dissections. S.J.R. and D.M.M. performed the histological and histochemical analyses. S.J.R. drafted the final manuscript. All authors gave final approval for publication.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
Collection of material was carried out under an NOAA‐NMFS permit (Nos 17350‐01 and 17350‐02) to J.C.G. This study was partially funded by qualified outer continental shelf oil and gas revenues by a substantial grant from the Coastal Impact Assistance program, Fish and Wildlife Service, US. Department of the Interior and the North Slope Borough.
References
- Adachi K (1961) Metabolism of glycogen in the skin and the effect of X‐rays. J Invest Dermatol 37, 381–395. [PubMed] [Google Scholar]
- Blokhin SA (1984) Investigations of gray whales taken in the Chukchi coastal waters, U.S.S.R. In: The Gray Whale Eschrichtius Robustus. (eds Jones ML, Swartz SL, Letherwood S.), pp. 487–509. London: Academic Press, Inc. [Google Scholar]
- Braham HW (1984) Distribution and migration of gray whales in Alaska In: The Gray Whale ESCHRICHTIUS ROBUSTUS (eds Jones ML, Swartz SL, Letherwood S.), pp. 249–266. London: Academic Press, Inc. [Google Scholar]
- Chernova OF, Shpak OV, Kildaze AB, et al. (2016) Summer molting of bowhead whales Balaena mysticetus Linnaeus, 1958, of the Okhotsk sea population. Dokl Biol Sci 471, 261–265. [DOI] [PubMed] [Google Scholar]
- Chittleborough RG (1959) Determination of age in the humpback whale, Megaptera nodosa (Bonnaterre). Aus J Mar Freshwater Res 10, 125–143. [Google Scholar]
- Clapham PJ (1992) Age at attainment of sexual maturity in humpback whales, Megaptera novaeangliae . Can J Zool 70, 1470–1472. [Google Scholar]
- Dorsey EM, Stern J, Hoelzel AR, et al. (1990) Minke whales (Balaenoptera acutorostrata) from the west coast of North America: individual recognition and small‐scale site fidelity. Rep Manage Int Whaling Commission(Special issue 12), 357–368. [Google Scholar]
- Drury RAB, Wallington EA (1980) Carelton's Histological Technique. 5th edn Oxford: Oxford University Press. [Google Scholar]
- Edsberg LE (2007) Pressure ulcer tissue histology: an appraisal of current knowledge. Ostomy Wound Manage 53(10), 40–49. [PubMed] [Google Scholar]
- Falin LI (1961) Glycogen in the epithelium if mucous membranes and skin and its significance. Acta Anat 46, 244–276. [DOI] [PubMed] [Google Scholar]
- Fortune SME, Koski WR, Higdon JW, et al. (2017) Evidence of molting and the function of ‘rock nosing’ behavior in bowhead whales in the eastern Canadian Arctic. PLoS ONE 12, e0186156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser FC, Purves PE (1960) Anatomy and function of the cetacean ear. Proc R Soc Lond Ser B Biol. Sci 152(946), 62–77. [DOI] [PubMed] [Google Scholar]
- Gabrielle CM, Lockyer C, Straley JM, et al. (2010) Sighting history of a naturally marked humpback whale (Megaptera novaeangliae) suggests earplug growth layer groups are deposited annually. Mar Mamm. Sci 26(2), 443–450. [Google Scholar]
- Gartner LP (2017) Textbook of Histology. 4th edn Philadelphia: Elsevier. [Google Scholar]
- George JC, Stimmelmayr R, Suydam R, et al. (2016) Severe bone loss as part of the life history strategy of bowhead whales. PLoS ONE 11(6), e0156753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel JDC, Pereira CSB, Alves AL, et al. (2010) Hyperproliferation markers in ear canalepidermis. Braz J Otorhinolaryngol 76(5), 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldiman JT, Hank WG, Henry RW, et al. (1985) Epidermal and papillary dermal characteristics of the bowhead whale (Balaena mysticetus). Anat Rec. 211, 391–402. [DOI] [PubMed] [Google Scholar]
- Halprin KM, Ohkawara A (1966) Glucose and glycogen metabolism in the human epidermis. J Invest Dermatol 46(1), 43050. [DOI] [PubMed] [Google Scholar]
- Harmon CS, Phizackerly PJR (1983) The effects of starvation and re‐feeding on glycogen metabolism in mouse tail skin. Biochem J 212, 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BD, St. Aubin DJ, Geraci JR, et al. (1985) Epidermal growth in the bottlenose dolphin, Tursiops truncatus . J Invest Dermatol 85, 60–63. [DOI] [PubMed] [Google Scholar]
- Ichihara T (1959) Formation mechanism of ear plug in baleen whales in relation to glove‐finger. Sci Rep Whales Res Inst 14, 107–135. [Google Scholar]
- Ichihara T (1964) Prenatal development of ear plug in baleen whales. Sci Rep Whales Res Inst Tokyo 18, 29–48. [Google Scholar]
- Ichihara T (1966) Criterion for determining age of fin whale with reference to ear plug and baleen plate. Sci Rep Whales Res Inst Tokyo 20, 17–82. [Google Scholar]
- Im MJ, Hoopes JE (1970) Energy metabolism in healing skin wounds. J Surg Res 10(10), 459–464. [DOI] [PubMed] [Google Scholar]
- Ketten DR (1997) Structure and function in whale ears. Bioacoustics 8, 103–135. [Google Scholar]
- Kiernan JA (2015) Histological and Histochemcial Methods: Theory and Practice. 5th edn Banbury: Scion Publishing Ltd. [Google Scholar]
- Kitakado T, Lockyer C, Punt AE (2013) A statistical model for quantifying age‐reading errors and its application to the Antarctic minke whales. J Cetacean Res Manage 13(3), 181–190. [Google Scholar]
- Laws RM, Purves PE (1956) The ear plug of the Mysticeti as an indication of age with special reference to the North Atlantic Fin Whale (Balaenoptera physalus Linn.). Norsk Hvalfangst‐Tidende 45(8), 413–425. [Google Scholar]
- Lillie BA (1910) Observations on the anatomy and general biology of some members of the larger cetacean. J Zool 80(3), 769–792. [Google Scholar]
- Lillie RD (1954) Histopathologic Technic and Practical Histochemistry. New York: Blakiston Co. Inc. [Google Scholar]
- Ling JK, Thomas CDB (1967) The skin and hair of the southern elephant seal, (Mirounga Leonina (L.) II. Pre‐natal and early post‐natal development and moulting. Aus J Zool 15(2), 349–365. [Google Scholar]
- Lobitz WC, Brophy D, Larner AE, et al. (1962) Glycogen response in human epidermal basal cell. Arch Derm 86(2), 207–211. [DOI] [PubMed] [Google Scholar]
- Lockyer C (1972) The age at sexual maturity of the southern fin whale (Balaenoptera physalus) using annual layer counts in the ear plug. J Cons Int Explor Mer 34(2), 276–294. [Google Scholar]
- Lockyer C (1974) Investigation of the ear plug of the southern sei whale, Balaenoptera borealis, as a valid means of determining age. J Con Int Explor Mer 36(10), 71–81. [Google Scholar]
- Lockyer C (1984) Age determination by means of the earplug in baleen whales. Rep Manage Int Whaling Commission 34, 692–696. [Google Scholar]
- Lubetkin SC, Zeh JE, Rosa C, et al. (2008) Age estimation for young bowhead whales (Balaena mysticetus) using annual baleen growth increments. Can J Zool 86(6), 525–538. [Google Scholar]
- Lubetkin SC, Zeh JE, George JC (2013) Statistical modeling of baleen and body length at age in bowhead whales (Balaena mysticetus). Can J Zool 90(8), 15–931. [Google Scholar]
- Maderson PFA, Zucker AH, Roth SI (1978) Epidermal regeneration and percutaneous water loss following cellophane stripping of reptile epidermis. J Exp Zool 204, 11–32. [DOI] [PubMed] [Google Scholar]
- Maeda H, Kawamoto T, Kato H (2013) A study on the improvement of age estimation in common minke whales using the method of gelatinized extraction of earplug. NAMMCO Sci Pub. 10. [Google Scholar]
- Masaki Y (1968) A Trial for Reading of Earplug Lamination of the Sei Whale by Means of Soft X‐ray, Vol. 26 Oslo: International Whaling Commission on Age Determination in Whales. [Google Scholar]
- Nishiwaki M (1966) Distribution and migration of the larger cetaceans in the North Pacific as shown by Japanese whaling results In: Whales, Dolphins and Porpoises (ed. Norris KS.), pp. 171–191. Berkeley: University of California Press. [Google Scholar]
- Nishiwaki M, Ichihara T, Osumi S (1958) Age studies of fin whale based on ear plug. Sci Rep Whales Res Inst 13, 155–169. [Google Scholar]
- Ohkawara A, Halprin KM, Levine V (1972) Glycogen metabolism following ultraviolet irradiation. J Invest Dermatol 59(3), 264–268. [DOI] [PubMed] [Google Scholar]
- Ohsumi S (1964) Examination on age determination on the fin whale. Sci Rep Whales Res Inst 18, 49–88. [Google Scholar]
- Perrin WF, Myrick AC (1980) Age Determination of Toothed Whales and Sirenians. No. 3. International Whaling Commission. [Google Scholar]
- Pfeiffer CJ, Jones FM (1993) Epidermal lipid in several cetacean species: ultrastructural observations. Anat Embryol 188, 209–218. [DOI] [PubMed] [Google Scholar]
- Purves PE (1955) The wax plug in the external auditory meatus on the mysticeti. Disc Rep 27, 293–302 + plates XIV‐XVIII. [Google Scholar]
- Purves PE, Mountford MD (1959) Ear plug laminations in relation to the age composition of a population of fin whales (Balaenoptera physalus). Bull Br Mus Nat Hist Zool 5(6), 125–161. [Google Scholar]
- Reeb D, Duffield M, Best PB (2005) Evidence of postnatal ecdysis in southern right whales. Eubalaena australis . J Mamm 86(1), 131–138. [Google Scholar]
- Reeb D, Best PB, Kidson SH (2007) Structure of the integument of Southern Right Whales, Eublalaena australis . Anat Rec 290, 596–613. [DOI] [PubMed] [Google Scholar]
- Robinson AC, Hawke M, MacKay A, et al. (1989) The mechanism of ceruminolysis. J Otolaryngol 18(6), 268–273. [PubMed] [Google Scholar]
- Roe HSJ (1967) Seasonal formation of laminae in the ear plug of the fin whale. Disc Rep 35, 1–30 + plates I‐IV. [Google Scholar]
- Rosa C, Zeh J, George JC, et al. (2013) Age estimates based on aspartic acid racemization for bowhead whales (Balaena mysticetus) harvested in 1998‐2000 and the relationship between racemization rate and body temperature. Mar Mamm Sci 29(3), 424–445. [Google Scholar]
- Rugh D(1990) Bowhead whales reidentified through aerial photography near Point Barrow, Alaska. Rep Manage Int Whaling Commission 289–294. [Google Scholar]
- Salcido R, Donofrio JC, Fisher SB, et al. (1994) Histopathology of pressure ulcers as a result of sequential computer‐controlled pressure sessions in a fuzzy rat model. Adv Wound Care 7(5), 23–40. [PubMed] [Google Scholar]
- Severt͡sova AN, Kleinenberg SE(1969) Beluga (Delphinapterus leucas): Investigation of the species. Jersulsalem: Israel Program for Scientific Translations. Akademiia nauk SSSR. Moscow: Institut morfologii Zhivotnykh im.
- Shelley WB, Perry ET (1956) The physiology of the apocrine (ceruminous) gland of the human ear canal. J Invet Derm 26(1), 13–22. [DOI] [PubMed] [Google Scholar]
- Skieresz‐Szewczyk K, Jackowiak H, Ratajczak M (2014) LM and TEM study of the orthokeratinized and parakeratinized epithelium of the tongue in the domestic duck (Anas platyrhynchos f. domestica). Micron 67, 117–124. [DOI] [PubMed] [Google Scholar]
- Spearman RIC (1972) The epidermal stratus corneum of the whale. J Anat 11393, 373–381. [PMC free article] [PubMed] [Google Scholar]
- Squier CA, Kremer MJ (2001) Biology of oral mucosa and esophagus. J Natl Cancer Inst Monographs 29, 7–15. [DOI] [PubMed] [Google Scholar]
- St Aubin DJ, Smith TG, Geraci JR (1990) Seasonal epidermal molt in beluga whales, Delphinapterus leucas . Can J Zool 68, 359–367. [Google Scholar]
- Stadler I, Zhang R‐Y, Whittaker MS, et al. (2004) Development of a simple, noninvasive, clinically relevant model of pressure ulcers in the mouse. J Invest Surg 17, 221–227. [DOI] [PubMed] [Google Scholar]
- Trumble SJ, Robinson EM, Berman‐Kowalewski M, et al. (2013) Blue whale earplug reveals lifetime contaminant exposure and hormone profiles. PNAS 110(42), 16922–16926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JPG (1972) Interrelation of epithelial glycogen, cell proliferation and cellular migration with cyclic adenosine monophosphate in epithelial wound healing. Cell Diff 1(5–6), 317–323. [DOI] [PubMed] [Google Scholar]


