Abstract
Glycosylation is a major post‐translational modification in which a carbohydrate known as a glycan is enzymatically attached to target proteins which regulate protein folding and stability. Glycans are strongly expressed in the developing nervous system where they play multiple roles during development. The importance of these glycan epitopes in neural development is highlighted by a group of conditions known as congenital disorders of glycosylation which lead to psychomotor difficulties, mental retardation, lissencephaly, microencephaly and epilepsy. One of these glycan epitopes, known as Lewis X, is recognised by the FORSE‐1 antibody and is regionally expressed in the developing nervous system. In this study, we report the regional and temporal expression patterns of FORSE‐1 immunolabelling during the periods of neurogenesis, gliogenesis and axonogenesis in developing mouse nervous system. We demonstrate the localisation of FORSE‐1 on subsets of neuroepithelial cells and radial glial cells, and in compartments corresponding to axon tract formation. These spatial, temporal and regional expression patterns are suggestive of roles in the determination of different cell lineages and in the patterning of white matter during development, and help provide insights into the neuroanatomical regions affected by congenital disorders of glycosylation.
Keywords: brain, development, FORSE‐1, Lewis X, mouse, spinal cord
Introduction
Glycosylation is a major post‐translational modification in which a carbohydrate known as a glycan is enzymatically attached to target conjugates important for cell–cell recognition and interactions (Ohtsubo & Marth, 2006). In the developing and adult central nervous system (CNS), glycans play roles in cell determination, migration, neurite outgrowth and fasciculation, and synapse formation and stabilisation (Kleene & Schachner, 2004; Ghazarian et al. 2011). The importance of these glycan epitopes in neural development is highlighted by a group of disorders known as congenital disorders of glycosylation (CDG) (Jaeken, 2013). These are rare inborn errors of metabolism in which protein glycosylation is deficient or defective, resulting in psychomotor difficulties, mental retardation, lissencephaly, microencephaly and epilepsy (Kleene & Schachner, 2004; Varki & Lowe, 2009). For this reason, characterising the regional and temporal expression patterns of distinct glycans during the development of the nervous system will help provide insight into the neuroanatomical basis of CDG.
Glycoconjugates including glycolipids, proteoglycans, glycoproteins, glycopeptides, peptidoglycans, lipopolysaccharides and carbohydrate epitopes play different roles depending on whether they are covalently linked to lipid or protein cores. One of these glycan epitopes, Lewis X (LeX), is recognised by the FORSE‐1 monoclonal antibody (Allendoerfer et al. 1999). LeX glycans are of considerable scientific interest due to their range of vital biological functions during development, and as neural stem cell biomarkers in medical research applications. LeX is highly expressed in the developing brain during key periods of neurogenesis and gliogenesis in the cerebellum (Allendoerfer et al. 1995; Tole et al. 1995), spinal cord (Karus et al. 2013) and later in neurogenic zones in the adult brain (Capela & Temple, 2006; Hennen et al. 2011). The FORSE‐1 antibody (which recognises LeX) has been used to isolate subsets of human neural stem cells from pluripotent cells (Pruszak et al. 2007; Elkabetz & Studer, 2008). LeX has been shown to label subsets of radial glia (Hennen et al. 2011) and radial glial cells restricted to a neuronal lineage (Capela & Temple, 2006; Mo et al. 2007) and has also been linked to axonal segregation (Mai et al. 1998). Moreover, a large number of studies have identified LeX in neural progenitor cells in which it may play important roles in regulating differentiation (Allendoerfer et al. 1995; Dasgupta et al. 1996; Capela & Temple, 2006; Pruszak et al. 2007; Kelly et al. 2009; Yagi et al. 2012). The FORSE‐1 monoclonal antibody was raised against a surface antigen expressed by retinoic acid‐induced human embryocarcinoma (NT2D1) and was shown to specifically recognise oligosaccharides containing the LeX carbohydrate epitope. This epitope is present on two unidentified glycolipid antigens and phosphacan, a chondroitan sulphate proteoglycan present on neural progenitors embryonically and postnatally, modulating neuronal adhesion and axon growth (Margolis et al. 1996; Mai et al. 1998; Allendoerfer et al. 1999). Glycolipids predominate early in embryonic development, acting as regulators of cell adhesion, signal transduction and homophilic binding partners, and as agonists for receptors (van Echten‐Deckert & Herget, 2006; Furukawa et al. 2006; Ojima et al. 2015).
Although different LeX identifying antibodies have been used to identify lineage committed neural stem cells, little is known about the roles of FORSE‐1 glycans in each compartment of the developing CNS, especially in light of incongruent LeX expression patterns and the techniques used to describe them (Hennen & Faissner, 2012). In the human, isolated embryonic stem cells containing FORSE‐1 and other precursors markers including brain lipid binding protein (BLBP), vimentin and nestin have shown region‐specific CNS fates (Elkabetz & Studer, 2008). Moreover, its alternate locations on proteoglycans and glycolipids mean the proposed roles of FORSE‐1 may vary at different stages of cell production and differentiation.
The goals of this study were to characterise the regional and temporal expression patterns of FORSE‐1 during the periods of neurogenesis, gliogenesis and axonogenesis in developing spinal cord and brain. We show the localisation of FORSE‐1 on subsets of neuroepithelial cells and in compartments corresponding to tract formation. These spatial and temporal expression patterns are suggestive of roles in the determination of different cell lineages and in the patterning of white matter during development.
Material and methods
Animals and tissue collection
All animals were sourced from the Comparative Medicine Unit at the Trinity Biomedical Sciences Institute, Trinity College Dublin. All work was carried out to the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines. Time‐mated pregnant C57BL/6 mice and later their offspring were maintained in a controlled environment on a 12‐h light/dark cycle (lights on at 07:30 h) with ad libitum access to food and water. Embryos were collected on embryonic day (E) E11, E13, E15 or E17. To do this, time‐mated C57BL/6 pregnant dams were euthanised and embryos removed by laparotomy. Postnatal tissues were collected on postnatal day (P) 0, P2 and P10. Pups were euthanised and the brains and vertebral columns were removed. All tissue samples were then processed for immunohistochemistry.
Immunohistochemistry
All tissue samples were fixed by immersion in 4% paraformaldehyde (PFA) for 24 h, after which they were washed three times for 10 min per wash (3 × 10 min) in 10 mm phosphate‐buffered saline (PBS). All samples were then cryoprotected in 30% sucrose (Sigma, UK) in 10 mm PBS, before being snap‐frozen in liquid nitrogen‐cooled isopentane and stored at −80 °C prior to sectioning. Sections of 20 μm were cut using a cryostat (Leica), and then allowed to equilibrate to room temperature (RT) for 15 min, before being washed 3 × 10 min in 10 mm PBS. Non‐specific binding was blocked by incubating the sections in 20% normal goat serum (NGS) diluted in 10 mm PBS for 1 h at room temperature, before being incubated in the appropriate primary antibody diluted in 1% NGS in 10 mm PBS for 16 h at 4°C (see Table 1 for a list of primary antibodies). Sections were then washed 3 × 10 min in 10 mm PBS and then incubated in the appropriate Alexa Fluor 488‐ and/or 594‐conjugated secondary antibodies (1 : 500; Abcam, UK) diluted in 1% NGS in 10 mm PBS, at room temperature for 2 h in the dark. Triton‐X was added to both the blocking and antibody solutions at a final concentration of 0.05% when the target antigen was intracellular. Following secondary antibody incubation, sections were washed for 3 × 10 min in 10 mm PBS, and then counterstained with 0.001% 4’,6‐diamidino‐2‐phenylindole (DAPI) (Thermo‐Fisher Scientific, USA) in deionised water for 5 min at RT, following which they were washed for 3 × 10 min in 10 mm PBS, and coverslipped using fluoromount aqueous mounting medium (Sigma, UK). Fluorescent cryosections were captured using an Olympus BX51 upright microscope combined with a DP73 digital camera utilising the imaging software cellsens (version 1.12). Confocal micrographs were captured using a Leica TCS SB8 scanning confocal microscope using las x imaging software. Subsequent image modification was undertaken using imagej (version 1.52 g). Lower power images were captured using objective magnifications of 10 or 20×. Higher power fluorescent images were captured using objective magnifications of 40 or 60× using the 360, 488, 550 and 633 nm emission channels. Some images were cropped from lower power acquisitions and enlarged. Images were captured at an exposure time of 1 s. In all our immunohistochemical and immunocytochemical procedures, additional negative controls, in which the primary antibody was omitted, were performed to test the specificity of FORSE‐1. This confirmed that the FORSE‐1 antibody showed excellent immunoreactivity and a strong signal where present.
Table 1.
List of primary antibodies used
| Antibody | Species and clonality | Isotype | Manufacturers | Dilution |
|---|---|---|---|---|
| FORSE1 | Mouse monoclonal | IgM | Developmental Studies Hybridoma Bank | 1 : 200 |
| Nestin | Mouse monoclonal | IgG | Developmental Studies Hybridoma Bank | 1 : 200 |
| BLBP | Rabbit polyclonal | N/A | Millipore | 1 : 200 |
| GAP‐43 | Rabbit polyclonal | N/A | Thermo‐Fisher Scientific | 1 : 500 |
| TAG‐1 | Mouse monoclonal | IgM | Thermo‐Fisher Scientific | 1 : 200 |
| β‐III Tubulin | Mouse monoclonal | IgG | Thermo‐Fisher Scientific | 1 : 200 |
Cell culture
Cerebral cortex and spinal cords were dissected from E15 embryos and placed in ice‐cold calcium and magnesium‐free Hanks’ balanced salt solution (CMF HBSS) (Thermo‐Fisher Scientific, USA). CMF HBSS was aspirated and replaced with 0.25% trypsin‐ethylenediamine tetra‐acetic acid (EDTA) solution (Sigma, UK) with 0.1 mg mL−1 deoxyribonuclease. Tissue was incubated for 15–25 min at 37 °C. Trypsin solution was neutralised by adding an equal volume of Dulbecco's modified Eagle medium (DMEM; Thermo‐Fisher Scientific, USA) with 1% fetal calf serum (FCS). The mixture was triturated and centrifuged at 300 g for 5 min. Supernatant was removed and the cell pellet was resuspended in primary tissue culture medium which consisted of DMEM‐F12 glutamax (Sigma, UK) with 1% heat‐inactivated FCS (Sigma, UK), 1% penicillin streptomycin (Sigma, UK), 2% B27 supplement (Gibco, USA), 1% N2 supplement (Gibco, USA) and 30 g L−1 d‐glucose (Sigma, UK). Cells were seeded on poly‐d‐lysine (Sigma, UK)‐coated coverslips and cultured for 24 and 72 h.
Immunocytochemistry
Cells were washed with PBS and then fixed in 4% PFA for 5 min at RT. Non‐specific binding was blocked in 5% NGS in PBS for 1 h and incubated in either BLBP or FORSE‐1 with 0.05% Triton and 1% NGS for 4 h at RT. Cells were washed in PBS and incubated in appropriate fluorochrome conjugated secondary antibody in PBS with 0.05% Triton and 1% NGS for 4 h at RT. Cells nuclei were labelled using 0.001% DAPI in deionised water for 5 min at room temperature and washed in PBS.
Cell quantification
At 24 and 72 h of cell culture, the proportion of FORSE‐1‐expressing cells was quantified in spinal cord and cerebral cortex cultures. At 24 h the proportion of BLBP‐expressing cells was also determined. Images for statistical analysis were captured at an objective magnification of 20×. All cell nuclei were counterstained using DAPI labelling. Three fields of view were captured on three individual coverslips at both time points and the total number of FORSE‐1‐ and DAPI‐expressing cells were quantified in each image. The proportion of FORSE‐1‐expressing cells relative to the entire cell population was then calculated. Similarly, at 24 h the individual and co‐expressing numbers of BLBP‐ and FORSE‐1‐expressing cells were determined as a proportion of DAPI‐expressing cells. Each experiment was repeated a minimum of three times using samples obtained from separate litters. Student t‐tests were used to determine statistical significance and data were considered significant at P < 0.05.
Results
FORSE‐1 is expressed at ventricular surfaces during neurogenesis
In the spinal cord at E11, production of motor and sensory neurons has initiated in the ventral and dorsal horns respectively (Rodier, 1980). FORSE‐1 was expressed only in a discrete region in the dorsal portion of the spinal cord at the central canal surface in ependymal cells at E11 (arrow, Fig. 1A,D). It was absent from the ventral rootlets, although faint expression was evident in paraxial somites (asterisks, Fig. 1A). Nestin, a stem cell intermediate filament, was present across the thickness of the spinal cord in dorsal and ventral regions apart from the basal plates (Fig. 1B,D). Nestin was also evident in the ventral rootlets and somites (asterisks) (Fig. 1B). BLBP, an early appearing glial marker (Feng et al. 1994), was not expressed at this stage in the CNS (Fig. 1C,I). GAP‐43‐expressing axons were present in the presumptive ventral white matter and in the ventral and dorsal rootlets (Fig. 1E). FORSE‐1 radiated around the circumference of the telencephalic vesicles and the basal half of the third ventricle in proliferating ependymal cells to the emerging mantle cell layer (Fig. 1F,G,I). Radialised nestin expression was evident throughout the neuroectoderm (Fig. 1H). A rostral to caudal section at the level of the central canal and fourth ventricle showed that nestin was expressed in cells radiating from ependyma to the pial surface, in dorsal rootlets and somites (Fig. 1J,K). FORSE‐1 was expressed at cranial and caudal edges of each dermomyotome co‐localising with nestin (arrows, Fig. 1J,K). FORSE‐1 was co‐expressed with the neuronal marker β‐III tubulin in migrating neuroblasts in the emerging cortex (arrow, Fig. 1L).
Figure 1.
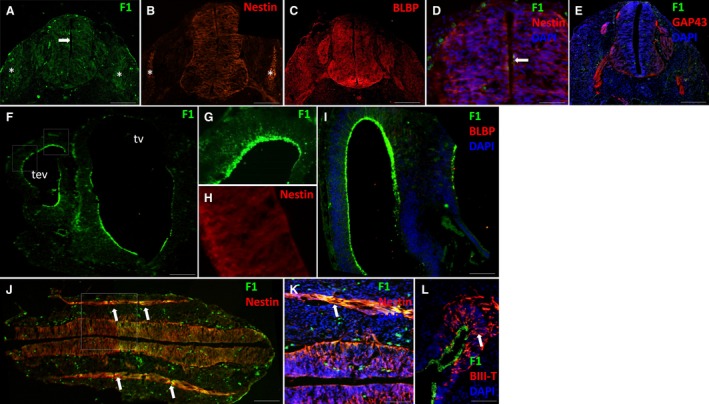
Spinal cord and forebrain at E11. (A–C) FORSE‐1, nestin and BLBP expression in the spinal cord. (D) Merged image of the dorsal spinal cord showing FORSE‐1, nestin and DAPI expression. (E) GAP43 expression in emerging axon tracts in the spinal cord, showing no localisation with FORSE‐1. (F,G) FORSE‐1 expression in the forebrain and dorsal cortex (representative region of upper box in F). (H) Nestin expression in the cortex (representative region of lower box in F). (I) Lack of BLBP expression in the brain. FORSE‐1 radiates throughout the emerging cortex. (J) Longitudinal sections showing FORSE‐1 and nestin expression in the nervous system. (K) FORSE‐1, nestin and DAPI expression in boxed region of J and in paraxial somites. (L) FORSE‐1, beta‐III tubulin and DAPI expression in the ventricular zone of the cortex. Tev, telencephalic vesicle; tv, third ventricle. Scale bar: (A‐C,E,K,L) 200 μm; (D,I) 100 μm; (F,J) 400 μm.
At E13, ventricular, mantle, marginal zones and the floor plate were distinguishable (Fig. 2A). TAG‐1 and GAP‐43, which are markers of growing axons, showed white matter patterning in Lissauer's tracts, in the ventral commissure and throughout the emerging dorsolateral and ventral marginal zone (Fig. 2A). The pseudostratified neuroectoderm was transformed to radialised nestin‐expressing cells ventrally and dorsally (Fig. 2B). FORSE‐1 was evident in the dorsal portion of the spinal cord radiating from the central canal through the emerging mantle zone and in a discrete region around the midline, potentially in the region of the sulcus limitans, on nestin‐expressing cells (arrows, Fig. 2C‐E). BLBP was now present mainly in the ventral portion of the spinal cord, where it labelled radial glial cells (Barry & McDermott, 2005), in the dorsal roots and in the dorsal root ganglia (Fig. 2F,G). FORSE‐1 was expressed only in a subpopulation of BLBP‐ and nestin‐expressing radial glial cells in the midline of the cord [Fig. 2D,E,G (arrow)].
Figure 2.
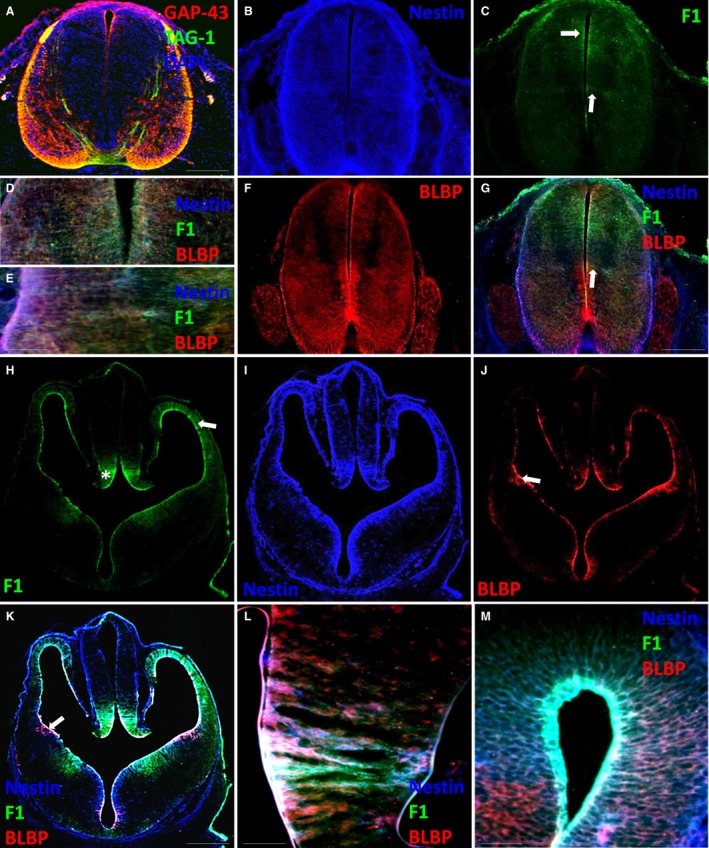
Spinal cord and forebrain at E13. (A) GAP‐43, TAG‐1 and DAPI expression in the spinal cord. (B,C) Nestin and FORSE‐1 expression in the spinal cord. (D,E) Higher magnification images showing nestin, FORSE‐1 and BLBP expression in the dorsal and lateral spinal cord. (F) BLBP expression in the spinal cord. (G) Merged image of the spinal cord showing nestin, BLBP and FORSE‐1 expression. (H‐J) FORSE‐1, nestin and BLBP expression in the forebrain. (K) Merged image showing nestin, BLBP and FORSE‐1 expression. (L,M) Higher magnification images showing nestin, BLBP and FORSE‐1 expression in the medial ganglionic eminence and dorsal cortex. Scale bar: (A,G) 200 μm; (K) 400 μm; (D,E,L,M) 50 μm.
By E13, the telencephalic vesicle walls are composed of the ventricular zone and the early preplate. Moreover, the ganglionic eminences and basal nuclei emerge, and the thalamic and hypothalamic nuclei continue to grow. Hindbrain components including the cerebellum and pons begin to become morphologically distinguishable. FORSE‐1 was expressed in selective regions around the subventricular and ventricular surfaces. In particular, it was evident radiating throughout the cortex surrounding the lateral telencephalic vesicles (arrow, Fig. 2H), the eminentia thalami (asterisk, Fig. 2H) and in the regions of the medial ganglionic eminence and the post optic recess (Fig. 2H,K). Nestin was expressed through each ventricular zone, where it co‐localised with FORSE‐1 and in areas where FORSE‐1 was not expressed (Fig. 2I,K‐M). BLBP co‐localised with FORSE‐1 in all regions (Fig. 2J,K) apart from the medial ganglionic eminence and the dorsal‐most portion of the telencephalic vesicles, where it appeared to be absent (Fig. 2K‐M). BLBP was, however, present in the lateral ganglionic eminence, where it did not co‐localise with FORSE‐1 (arrow, Fig. 2K,L).
FORSE‐1 expression patterns at the onset of gliogenesis
By E15, in the dorsal spinal cord, the central canal was reduced in size as the ventricular region transformed into the mantle and marginal zones. Alar and basal plates became separated by the sulcus limitans at the surface of the central canal. The cord was relatively morphologically homogeneous at this stage (data not shown). FORSE‐1 was expressed in processes extending from the lateral portion of the central canal of the spinal cord to the lateral pial surface and dorsally just above the central canal (arrows, Fig. 3A,C). Nestin was expressed in radial processes in ventral and dorsal regions and in the dorsomedial septum (Fig. 3B,C), which divides the emerging dorsal columns. White matter tracts were evident in ventral and dorsal regions of the spinal cord (Fig. 3G,H). FORSE‐1 was co‐localised with nestin in the grey and white matter laterally (Fig. 3C,D), yet was absent from the nestin‐expressing dorsomedial septum (Fig. 3C). Some FORSE‐1‐expressing processes appeared to radiate through the region of the presumptive dorsal columns (Fig. 3H). BLBP expression was found only ventrally and laterally in radial glial processes extending from the central canal to the pial surface and co‐localised with FORSE‐1 in the lateral grey and white matter (Fig. 3E), which was confirmed using confocal microscopy (Fig. 3F). BLBP was absent from the dorsomedial septum and floor plate.
Figure 3.
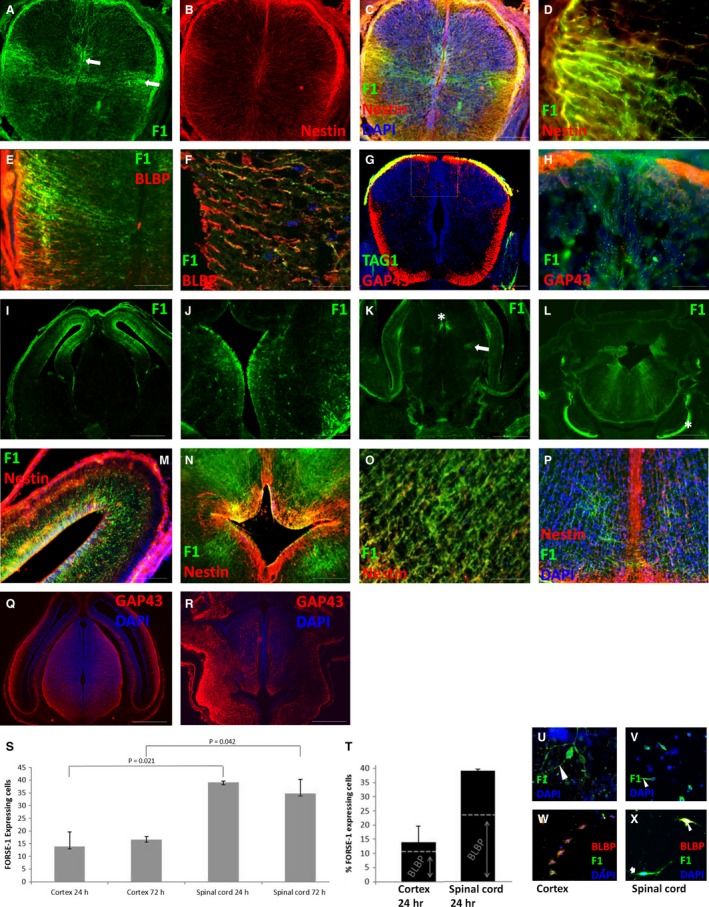
Spinal cord and brain at E15. (A,B) FORSE‐1 and nestin expression in the spinal cord. (C) Merged image showing FORSE‐1, nestin and DAPI expression. (D) FORSE‐1 and nestin co‐localisation in the lateral spinal cord. (E,F) BLBP and FORSE‐1 co‐localisation in the lateral spinal cord. (G) TAG‐1‐ and GAP43‐expressing axon tracts in the spinal cord. (H) Confocal micrograph showing FORSE‐1 and GAP43 expression in the dorsomedial spinal cord. (I‐L) FORSE‐1 expression in the forebrain, diencephalon and hindbrain. (M) Nestin and FORSE‐1 expression in the cortex. (N,O) FORSE‐1 and nestin expression around the 4th ventricle and in the midbrain. (P) FORSE‐1, nestin and DAPI expression in the midbrain. (Q,R) GAP‐43‐expressing axons in the forebrain and brainstem. (S) Percentage of FORSE‐1‐expressing cells isolated from the cortex and spinal cord at 24 and 72 h in vitro. (T) BLBP expression in FORSE‐1‐expressing cells isolated from the cortex and spinal cord at 24 h. (U‐X) FORSE‐1 and BLBP immunocytochemistry in the cortex and spinal cord. Scale bar: (C,G) 200 μm; (D‐F,M) 100 μm; (H,J,N‐P) 50 μm; (I,K,L,Q,R) 400 μm.
At E15, stratification of the cerebral cortex occurs due to ongoing cell differentiation and migration from the ventricular zone, subventricular zone and the ependyma into the marginal layer. The lumen of the third ventricle narrows due to expansion of thalamic and hypothalamic nuclei. The pons and cerebellum continue to develop and limit the lateral recesses of the fourth ventricle, which populates the region with cerebellar cell types, pontine and olivary neurons, and cranial nerves are now evident along the hindbrain. In the rostral brain, FORSE‐1 expression was observed radiating from the lumen of the dorsal telencephalic vesicles to the pial surface (Fig. 3I), where it co‐localised throughout with nestin (Fig. 3M). FORSE‐1 appeared down‐regulated from the ependymal surface of the thalamic nuclei, yet dorsal expression remained at the ventricular opening radiating through the habenula (Fig. 3J). In the caudal forebrain, a discrete pattern of FORSE‐1 emerged either side of the third ventricle in regions corresponding to the emerging paraventricular thalamic nuclei (asterisk) and ventrolateral thalamic nuclei (arrow) (Fig. 3K). In the developing brainstem, there was clear FORSE‐1 expression radiating from the fourth ventricle dorsally and laterally towards the pial surface of the midbrain lateral to the growing medial lemniscus (Fig. 3L). Expression was also upregulated in the spinal nucleus of the trigeminal nerve (asterisk, Fig. 3L). FORSE‐1 and nestin were co‐localised around the fourth ventricle (Fig. 3N) and in cells radiating toward the pial surface (Fig. 3O). A nestin‐expressing septum extended from the base of the fourth ventricle to the pial surface ventrally (Fig. 3P). FORSE‐1 was not expressed in this septum, although some FORSE‐1‐expressing fibres or processes appeared to cross the midline (Fig. 3P). FORSE‐1 expression patterns did not appear to overlap with the locations of white matter tracts around the lateral or third ventricles (Fig. 3Q), although white matter compartments were established around the fourth ventricle in regions of FORSE‐1 expression (Fig. 3R). FORSE‐1 expression in the cortex and spinal cord was observed on neuroepithelial precursors. We decided to quantify the ratio of FORSE‐1‐expressing cells as a proportion of all cells isolated from the cerebral cortex and spinal cord at 24 and 72 h. Cultures from both regions showed that FORSE‐1 is expressed on bipolar cells along leading and trailing processes and in the cell body (Fig. 3U,V). At 24 and 72 h in vitro, 14 and 17% of all dissociated cortical cells express FORSE‐1. A significantly greater number of cells expressed FORSE‐1 in spinal cord cultures at 24 h (39%, P = 0.021) and 72 h (35%, P = 0.042) (Fig. 3S). As the radial glial marker BLBP was co‐expressed along a subset of FORSE‐1 processes in the spinal cord at E15, we dual‐labelled our cultures with BLBP (Fig. 3W‐X). In cortical cell cultures, 78.6% of FORSE‐1‐expressing cells contained BLBP, whereas in spinal cord, 61.5% of FORSE‐1‐expressing cells showed BLBP, indicating less fate commitment than in the cortex (Fig. 3T).
At E17, FORSE‐1 expression was mainly evident radiating from the central canal to the pial surface in the dorsal half of the spinal cord (Fig. 4A,C,E). Nestin‐expressing processes were evident both ventrally and dorsally in the white matter, but their ventricular zone attachments had largely disappeared in the grey matter (Fig. 4B). FORSE‐1 was expressed with nestin in the grey and white matter (Fig. 4C). Expression was also up‐regulated in processes surrounding the growing dorsal column pathway, but was not found on nestin‐expressing processes forming the dorsomedial septum (Fig. 4D). BLBP expression was evident ventrally and dorsally; however, at this stage its profile was no longer radialised and appeared to be expressed in isolated cells throughout the grey and white matter (Fig. 4E). FORSE‐1 was co‐localised in BLBP‐expressing cells mainly dorsally in the grey and white matter (Fig. 4F). However, occasionally FORSE‐1‐expressing cells adopting a multipolar profile were observed independent of BLBP expression (asterisk, Fig. 4F). White matter compartments were present both ventrally and dorsally at the dorsal column tracts (Fig. 4G). FORSE‐1 was expressed around this growing tract (Fig. 4D,G,H).
Figure 4.
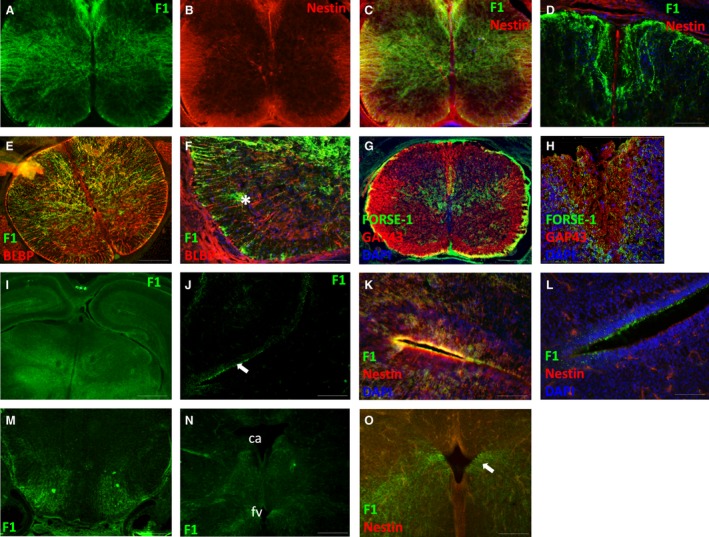
Spinal cord and brain at E17. (A‐C) FORSE‐1 and nestin expression in the spinal cord. (D) FORSE‐1 and nestin expression in the dorsal spinal cord. (E,F) BLBP and FORSE‐1 expression in the spinal cord. (G,H) GAP‐43 and FORSE‐1 expression in the spinal cord and in the emerging dorsal columns. (I) FORSE‐1 expression in the forebrain. (J) FORSE‐1 expression at inferior surface of the lateral ventricle ventricular zone. (K,L) Higher magnification view of the superior and inferior cortex showing nestin, FORSE‐1 and DAPI localisation. (M,N) FORSE‐1 expression in the emerging brainstem. (O) Nestin and FORSE‐1 expression in the region of the fourth ventricle. Ca, cerebral aqueduct; fv, fourth ventricle. Scale bar: (C,E,G,J,M) 200 μm; (D,F,O) 100 μm; (H,K,L) 50 μm; (I) 500 μm.
In the cortex, FORSE‐1 expression was largely down‐regulated from ventricular zones, apart from discrete regions lining the lumen of the lateral ventricles (Fig. 4I–N). Faint radialised nestin expression was observed at the lateral ventricular surfaces (Fig. 4K,L). Further caudally, FORSE‐1 appeared to radiate through the emerging lateral hypothalamus from the pial surface (Fig. 4M). In the brainstem, FORSE‐1 expression was present, extending from the infero‐lateral margins of the cerebral aqueduct passing through the periaqueductal grey and from the infero‐lateral margins of the fourth ventricle passing through the reticular formation (Fig. 4N). Nestin was down‐regulated from the brainstem, apart from septal regions superior and inferior to the fourth ventricle (Fig. 4O).
FORSE‐1 expression patterns postnatally
At P0 and P2, FORSE‐1 was mainly found throughout the white matter and in the lateral regions of the grey matter in the spinal cord (Fig. 5A,B). By P2, nestin expression was lost from the spinal cord apart from faint expression in the dorsomedial septum (Fig. 5C). At P10, FORSE‐1 was diffusely expressed in white matter dorsally and ventrally (Fig. 5D). In the brain at P0 and P2, FORSE‐1 was down‐regulated throughout the ventricular system and forebrain (Fig. 5E,G); however, expression was evident in the region of the lateral thalamus, in particular in the lateral geniculate nucleus (arrow, Fig. 5E). In the brainstem, expression was lost from all regions of the brain except for the basal region portion of the brainstem, where FORSE‐1 radiated from the pial surface either side of the cerebral aqueduct and the fourth ventricle (Fig 5F,H). At P10, FORSE‐1 was no longer expressed in the forebrain and could not be detected in the brainstem (Fig. 5I).
Figure 5.
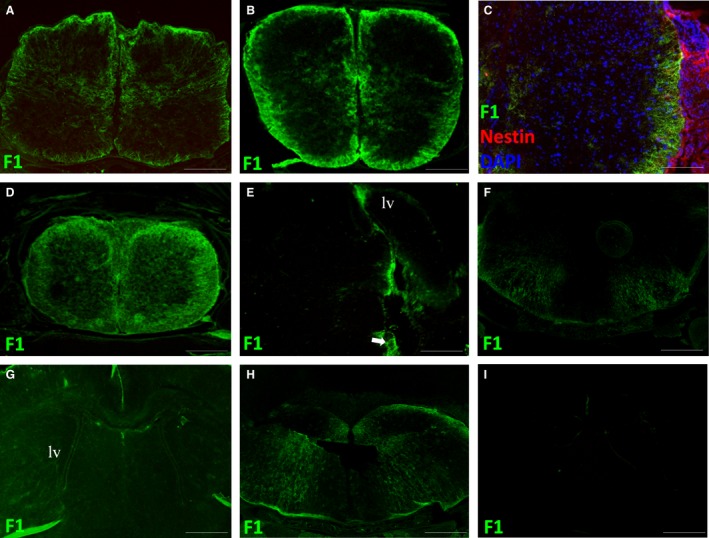
P0, P2 and P10 spinal cord and brain. (A,B,D) FORSE‐1 expression in the spinal cord at P0, P2 and P10 respectively. (C) Nestin, FORSE‐1 and DAPI expression at P2. (E,G,I) FORSE‐1 expression in the forebrain at P0, P2 and P10, respectively. (F,H) FORSE‐1 expression in the brainstem. lv, lateral ventricle. Scale bar: (A,B,D‐I) 400 μm; (C) 200 μm.
Discussion
Over the past 40 years, antibodies have been generated to recognise LeX‐containing glycans including SSEA‐1 (Solter & Knowles, 1978), MC‐480 (Fukui et al. 2002), 487/L5 (Streit et al. 1990), AK97 (Yanagisawa et al. 1999), MMA (Hanjan et al. 1982), 5750 (Hennen et al. 2011) and the present clone FORSE‐1 (Tole et al. 1995). Their reported expression patterns in the CNS vary and they differentially label neural progenitors, glial and neuronal populations, showing diverse biological functions (Hennen et al. 2011; Hennen & Faissner, 2012). In light of reported inconsistencies in FORSE‐1 expression in the rat and mouse CNS, we set out to characterise and update known FORSE‐1 expression in the developing and postnatal mouse spinal cord, brainstem and brain using immunohistochemical techniques in vivo. To gain insight into the lineage potentials of FORSE‐1‐expressing cells, we dual‐labelled with the neuroepithelial marker nestin and the radial glial cell marker BLBP. For the first time, we describe clear labelling patterns in somites, in the presumptive diencephalon and developing spinal cord early in development and in specific nuclei and dorsal pathways later in development.
During neurogenesis, FORSE‐1 expression was observed at the dorsal spinal cord and in regions of the telencephalic neuroectoderm, spanning the thickness of the emerging cortical wall, the caudal portion of the third vesicle and the dorsal half of the spinal cord at the central canal. At this stage of development, neuroepithelial cells are differentiating into radialised neuroepithelial cells, neurons and radial glial cells (Huttner & Brand, 1997; Malatesta et al. 2000; Barry & McDermott, 2005). FORSE‐1 expression localised with nestin and BLBP in most subventricular and ventricular proliferative regions of the forebrain and spinal cord, except in the medial ganglionic eminence and ventral spinal cord, which were BLBP/nestin positive, FORSE‐1 negative. Our labelling patterns largely align with the original descriptions of FORSE‐1 expression in the rat telencephalon, despite alternative immunochemical techniques and what appears to be poorer antibody sensitivity (our DSHB sourced antibody consistently showed excellent signal using standard immunohistochemical techniques and microscopy (Tole et al. 1995; Tole & Patterson, 1995; Allendoerfer et al. 1999). These authors showed labelling throughout the lateral and ventral regions around the telencephalic vesicles, the ventral thalamus and preoptic area, but no expression in subregions of the medial (Tole & Patterson, 1995) and lateral ganglionic eminence (Allendoerfer et al. 1999). FORSE‐1 then became absent from the diencephalon or was clustered to midline regions, and was absent or weak in the spinal cord (Tole et al. 1995; Allendoerfer et al. 1999). Unlike the brain, we report significant differences in FORSE‐1 expression in the diencephalon and spinal cord. However, analogous nestin localisation expression patterns have been demonstrated around the central canal of the spinal cord during gliogenesis using LeX clones 487 and 5750 with diffuse white matter labelling from E15.5 using 5750 and earlier with 487 (Karus et al. 2013; Safina et al. 2016). Diffuse white matter labelling was not present with FORSE‐1 until postnatal ages, as shown with 487 and 5750 at embryonic ages, likely reflecting the delayed diffusion of FORSE‐1 LeX carriers (possibly phosphacan) into the extracellular space. Furthermore, 487 and 5750 showed reduced expression in the medial and lateral ganglionic eminences of the embryonic cortex at a similar age (Hennen et al. 2011).
The selective expression of FORSE‐1 in discrete regions of the brain and spinal cord in the absence of BLBP was suggestive of a cell phenotype identifiable by FORSE‐1 that perhaps can not be described as radial glial, remaining nestin‐expressing radial neuroepithelial cells, and as such uncommitted. Not all progenitor cells express LeX, as 5750‐negative and SSEA‐1‐negative cells isolated from the embryonic ganglionic eminence (Hennen et al. 2011) and spinal cord (Kelly et al. 2009) can generate neurospheres in vitro. Indeed, not all LeX carriers are neurogenic; astrocyte and oligodendrocyte precursor cells are recognised by LeX clone 487/L5 (Streit et al. 1993; Hennen et al. 2011). At least in the spinal cord, FORSE‐1‐expressing radial glia are spatiotemporally suggestive of a neuronal lineage early in development and a subsequent glial lineage with continued expression into postnatal life (Barry et al. 2014). A functional discrepancy for same‐age LeX epitopes in the brain and spinal cord has been hinted at before. LeX clone 487 did not fully deplete neurosphere‐forming cultures in the spinal cord, unlike the cortex, which showed a subpopulation of LeX‐negative progenitors in the spinal cord (Kelly et al. 2009; Hennen et al. 2011; Karus et al. 2013). These are perhaps analogous to the BLBP/nestin‐positive and FORSE‐1‐negative cells in dorsolateral and ventral regions of the spinal cord evident in this study. Furthermore, FORSE‐1 expression in discrete regions of the dorsal diencephalon, the trigeminal nucleus, paraxial somites and surrounding axon pathways such as the medial lemniscus in the brainstem and dorsal columns in the spinal cord, suggest alternate roles in cell adhesion and axon growth. These patterns have rarely been reported, although previous studies have referenced unpublished reports of clustered diencephalic nuclei in the rat (Allendoerfer et al. 1999). From late embryogenesis, FORSE‐1 expression was down‐regulated in the cortex, which considering the known roles of LeX as a marker of neural stem cells is not surprising, yet expression remained high in the spinal cord and the ventral brainstem regions of the periaqueductal grey and the reticular formation. In the adult, LeX glycans have been shown in the corpus callosum, the striatum and on some cells in the cortex (Capela & Temple, 2002; Nishihara et al. 2003).
Conclusions
The potentials of LeX glycans in diagnostics and in restorative medicine as predictors and manipulators of cell fates in each CNS region are being revealed (Hennen & Faissner, 2012). Current stem and radial glial cell differentiation procedures do not yield pure cell populations for transplantation and often become cancerous after scientific/medical application (White & Barry, 2015). Therefore, carefully isolating region‐specific stem populations may be critical in selecting purpose specific cells for transplantation. In this respect, FORSE‐1 is especially significant, as surface biomarkers capable of sorting human stem cell subtypes are rarely reported or described in each CNS compartment during development. We present the regional and temporal expression patterns of FORSE‐1 in the emerging brain, brainstem and spinal cord, and consider its early expression in the context of nestin‐ and BLBP‐expressing precursors, which will contribute to the potentials of FORSE‐1 as neural stem cell biomarkers. Gain and loss of function experiments underlying FORSE‐1 activity will provide deeper insight into its cell differentiation and axon guidance roles during neurogenesis and gliogenesis. However, our data suggest that FORSE‐1 labels subsets of radial glial cells during neurogenesis and gliogenesis across the brain and spinal cord that exist alongside FORSE‐1 negative precursors and as such represent a separate progenitor pool, which are likely uncommitted, based on BLBP expression. Furthermore, the pattern of FORSE‐1 expression in regions of white matter formation such as the dorsal columns is suggestive of additional roles in axon guidance. Moreover, by contributing to the literature concerning the localisation of LeX in the developing brain, we hope to support future functional studies to gain further insight into the migration and lineage abnormalities underlying CBG.
Conflict of interest
None.
Acknowledgements
Funding for this research was supported by Trinity College Dublin.
References
- Allendoerfer KL, Magnani JL, Patterson PH (1995) FORSE‐1, an antibody that labels regionally restricted subpopulations of progenitor cells in the embryonic central nervous system, recognizes the lex carbohydrate on a proteoglycan and two glycolipid antigens. Mol Cell Neurosci 6, 381–395. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Durairaj A, Matthews GA, et al. (1999) Morphological domains of Lewis‐X/FORSE‐1 immunolabeling in the embryonic neural tube are due to developmental regulation of cell surface carbohydrate expression. Dev Biol 211, 208–219. [DOI] [PubMed] [Google Scholar]
- Barry D, McDermott K (2005) Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia 50, 187–197. [DOI] [PubMed] [Google Scholar]
- Barry DS, Pakan JM, McDermott KW (2014) Radial glial cells: key organisers in CNS development. Int J Biochem Cell Biol 46, 76–79. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S (2002) LeX/ssea‐1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35, 865–875. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S (2006) LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt‐1. Dev Biol 291, 300–313. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Hogan EL, Spicer SS (1996) Stage‐specific expression of fuco‐neolacto‐ (Lewis X) and ganglio‐series neutral glycosphingolipids during brain development: characterization of Lewis X and related glycosphingolipids in bovine, human and rat brain. Glycoconj J 13, 367–375. [DOI] [PubMed] [Google Scholar]
- van Echten‐Deckert G, Herget T (2006) Sphingolipid metabolism in neural cells. Biochim Biophys Acta 1758, 1978–1994. [DOI] [PubMed] [Google Scholar]
- Elkabetz Y, Studer L (2008) Human ESC‐derived neural rosettes and neural stem cell progression. Cold Spring Harb Symp Quant Biol 73, 377–387. [DOI] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N (1994) Brain lipid‐binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron 12, 895–908. [DOI] [PubMed] [Google Scholar]
- Fukui S, Feizi T, Galustian C, et al. (2002) Oligosaccharide microarrays for high‐throughput detection and specificity assignments of carbohydrate‐protein interactions. Nat Biotechnol 20, 1011–1017. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Okuda T, Furukawa K (2006) Roles of glycolipids in the development and maintenance of nervous tissues. Methods Enzymol 417, 37–52. [DOI] [PubMed] [Google Scholar]
- Ghazarian H, Idoni B, Oppenheimer SB (2011) A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem 113, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanjan SN, Kearney JF, Cooper MD (1982) A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol 23, 172–188. [DOI] [PubMed] [Google Scholar]
- Hennen E, Faissner A (2012) LewisX: a neural stem cell specific glycan? Int J Biochem Cell Biol 44, 830–833. [DOI] [PubMed] [Google Scholar]
- Hennen E, Czopka T, Faissner A (2011) Structurally distinct LewisX glycans distinguish subpopulations of neural stem/progenitor cells. J Biol Chem 286, 16321–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Brand M (1997) Asymmetric division and polarity of neuroepithelial cells. Curr Opin Neurobiol 7, 29–39. [DOI] [PubMed] [Google Scholar]
- Jaeken J (2013) Congenital disorders of glycosylation. Handb Clin Neurol 113, 1737–1743. [DOI] [PubMed] [Google Scholar]
- Karus M, Hennen E, Safina D, et al. (2013) Differential expression of micro‐heterogeneous LewisX‐type glycans in the stem cell compartment of the developing mouse spinal cord. Neurochem Res 38, 1285–1294. [DOI] [PubMed] [Google Scholar]
- Kelly TK, Karsten SL, Geschwind DH, et al. (2009) Cell lineage and regional identity of cultured spinal cord neural stem cells and comparison to brain‐derived neural stem cells. PLoS ONE 4, e4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R, Schachner M (2004) Glycans and neural cell interactions. Nat Rev Neurosci 5, 195–208. [DOI] [PubMed] [Google Scholar]
- Mai JK, Andressen C, Ashwell KW (1998) Demarcation of prosencephalic regions by CD15‐positive radial glia. Eur J Neurosci 10, 746–751. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M (2000) Isolation of radial glial cells by fluorescent‐activated cell sorting reveals a neuronal lineage. Development 127, 5253–5263. [DOI] [PubMed] [Google Scholar]
- Margolis RK, Rauch U, Maurel P, et al. (1996) Neurocan and phosphacan: two major nervous tissue‐specific chondroitin sulfate proteoglycans. Perspect Dev Neurobiol 3, 273–290. [PubMed] [Google Scholar]
- Mo Z, Moore AR, Filipovic R, et al. (2007) Human cortical neurons originate from radial glia and neuron‐restricted progenitors. J Neurosci 27, 4132–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara S, Iwasaki H, Nakajima K, et al. (2003) Alpha 1,3‐fucosyltransferase IX (Fut9) determines Lewis X expression in brain. Glycobiology 13, 445–455. [DOI] [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867. [DOI] [PubMed] [Google Scholar]
- Ojima T, Shibata E, Saito S, et al. (2015) Glycolipid dynamics in generation and differentiation of induced pluripotent stem cells. Sci Rep 5, 14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszak J, Sonntag K‐C, Aung MH, et al. (2007) Markers and methods for cell sorting of human embryonic stem cell‐derived neural cell populations. Stem Cells (Dayton, OH) 25, 2257–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM (1980) Chronology of neuron development: animal studies and their clinical implications. Dev Med Child Neurol 22, 525–545. [DOI] [PubMed] [Google Scholar]
- Safina D, Schlitt F, Romeo R, et al. (2016) Low‐density lipoprotein receptor‐related protein 1 is a novel modulator of radial glia stem cell proliferation, survival, and differentiation. Glia 64, 1363–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D, Knowles BB (1978) Monoclonal antibody defining a stage‐specific mouse embryonic antigen (SSEA‐1). Proc Natl Acad Sci U S A 75, 5565–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Faissner A, Gehrig B, et al. (1990) Isolation and biochemical characterization of a neural proteoglycan expressing the L5 carbohydrate epitope. J Neurochem 55, 1494–1506. [DOI] [PubMed] [Google Scholar]
- Streit A, Nolte C, Rasony T, et al. (1993) Interaction of astrochondrin with extracellular matrix components and its involvement in astrocyte process formation and cerebellar granule cell migration. J Cell Biol 120, 799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tole S, Patterson PH (1995) Regionalization of the developing forebrain: a comparison of FORSE‐1, Dlx‐2, and BF‐1. J Neurosci 15, 970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tole S, Kaprielian Z, Ou SK, et al. (1995) FORSE‐1: a positionally regulated epitope in the developing rat central nervous system. J Neurosci 15, 957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Lowe JB (2009) Biological roles of glycans In Essentials of Glycobiology (eds Varki A, Cummings RD, Esko JD, et al.), pp. 75–88. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- White RE, Barry DS (2015) The emerging roles of transplanted radial glial cells in regenerating the central nervous system. Neural Regen Res 10, 1548–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Saito T, Yanagisawa M, et al. (2012) Lewis X‐carrying N‐glycans regulate the proliferation of mouse embryonic neural stem cells via the notch signaling pathway. J Biol Chem 287, 24356–24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kojima H, Kawakami Y, et al. (1999) A monoclonal antibody against a glycolipid SEGLx from Spirometra erinaceieuropaei plerocercoid. Mol Biochem Parasitol 102, 225–235. [DOI] [PubMed] [Google Scholar]


