INTRODUCTION
For over a century, blood pressure (BP) measurement was defined by the indelible standard of the mercury sphygmomanometer.1 Mercury was responsible for assuring uniformity of BP measurement in the first studies to identify the risks of elevated BP with regard to cardiovascular outcomes. It continued to be the standard of accuracy in BP measurement in clinical practice until recent decades. In 2013–2014, international hypertension societies ultimately concluded that the risk of toxicity superseded any potential benefit of using mercury-based BP devices.2,3 Accordingly, over the past several years, there has been a revolution in BP measurement. We have transitioned from widespread use of in-office manual aneroid sphygmomanometers (which easily lose calibration) to a broad range of options, including semi- or fully-automated (e.g. oscillometric) devices capable of measuring BP both in and out of the office.
With greater selection in BP devices came additional shortcomings. While oscillometric devices eliminate some degree of human error, many of these newer devices lack the precision afforded by mercury sphygmomanometers. Oscillometric devices, including automated office, home, and ambulatory BP measurement devices, have the potential to yield inaccurate readings, particularly if they do not undergo rigorous validation.4,5 There is no validation requirement for marketing a BP device in the Unites States.6 Moreover, publicly available information regarding validation status of widely used BP devices is very limited.7 The American Medical Association (AMA) is leading a group of well-respected individuals and organizations in the field of hypertension and BP measurement to create a transparent and easily accessible resource for identifying validated BP devices, the AMA Validated Device Listing (VDL); the proposed criteria were recently made available for public comment. Our goal in this review is to underscore the importance of this endeavor, and to call on practitioners to engage in discussion and dissemination of this important tool. Here, we provide an overview of the process of device validation, the current practice of in-office and out-of-office BP measurement, and current and future directions with regard to device listings. Of note, the AMA VDL will likely not include finger, wrist, and smartphone BP measurement devices due to generally poor validation of the models currently available;8–10 the use of these devices is reviewed elsewhere.11,12
THE VALIDATION AND CLEARANCE PROCESS
How Does an Oscillometric Device Work?
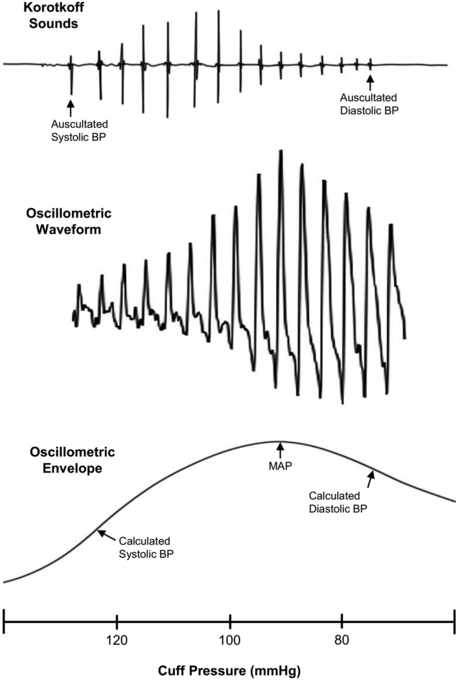
As an oscillometric device cycles from inflation to deflation, cuff-sensed arterial pulses are transduced, filtered, amplified and processed to create a mathematically-derived curve called an oscillometric waveform envelope (Figure 1). Algorithms, proprietary to each device manufacturer, are applied to the envelope to estimate systolic and diastolic BP.13 Oscillometry provides an algorithm-dependent estimate of systolic and diastolic BP, which can vary widely between different devices, algorithms and patients.14,15 Factors such as arrhythmias and medical comorbidities can result in greater error rates and inaccurate readings.16,17
Figure 1.
Depiction of manual blood pressure measurement (with auscultation of Korotkoff sounds) in contrast to automated oscillometric measurement (which derives a mathematic curve called an oscillometric waveform envelope that uses proprietary algorithms to estimate the systolic and diastolic blood pressures).
Abbreviations: BP = blood pressure; MAP = mean arterial pressure
Device Validation
BP device validation should be performed prior to marketing to assess device accuracy and precision against a reference standard, which is typically auscultation using a mercury or calibrated aneroid sphygmomanometer.4,5,18 The device should be tested in combination with the cuff(s) to be used with the device, as cuffs can be sources of variability.19,20 Auscultation is performed simultaneously by two trained observers, with careful attention paid to proper cuffing and technique. Each observer is blinded to the results of the other, and between-observer agreement of 4 mmHg or less is required to obtain an acceptable reference standard measurement. Four reference standard measurements bracketing three device measurements taken in alternating fashion are used for analysis. Criteria for subject selection and methods of analysis are protocol-specific and detailed elsewhere.4,5,18 The main features of the three main validation protocols, including expert consensus-based criteria required for a passing grade,4,5,18 are summarized in Table 1.
Table 1:
Major Blood Pressure Device Validation Protocols
| Protocol | Year of Last Update |
Sample Size Required (Number of Paired Readings) |
Criteria Indicating a Valid Device |
|---|---|---|---|
| BHS | 1993 | 85 (255) | Device graded from A to D. Grade A is the highest level of accuracy and requires that the percentage of readings with a difference between the device-under-test and the reference sphygmomanometer of ≤ 5, 10, and 15 mmHg be 65, 85 and 95%, respectively. |
| ESH IP | 2010 | 33 (99) | Pass requirements are split into two phases and are based on the number of measurements with differences between the device-under-test and reference sphygmomanometer of ≤ 5, 10, and 15 mmHg. See protocol for details. This protocol is being phased out, to be replaced by a joint universal AAMI/ESH/ISO validation protocol requiring 85 subjects. |
| AAMI/ANSI/ISO | 2013 | 85 (255) | Criterion 1: When analyzed as 255 paired determinations, the mean difference between the device-under-test and reference sphygmomanometer is less than 5.0 mmHg and the standard deviation of the difference is less than 8.0 mmHg. Criterion 2: When analyzed as 85 paired determinations, the standard deviation of the difference between the device-under-test and reference sphygmomanometer is less than 4.79 to 6.95 mmHg (the actual threshold varies according to the mean difference observed – see protocol for details). |
Abbreviations: AAMI = Association for the Advancement of Medical Instrumentation; ANSI = American National Standards Institute; BHS = British Hypertension Society; ESH IP = European Society of Hypertension International Protocol; ISO = International Standards Organization;
Device Calibration
BP device calibration can be divided into two categories – static and patient-specific. Static calibration involves checking the pressure registered by the device against a reference standard (usually a mercury column or, if not available, a highly sensitive electronic manometer). A device is considered acceptable if measurements agree within 3 mmHg across the BP range.5 It may also involve assessing the device measurements against a simulator that generates sample oscillometric waveforms with known systolic and diastolic BP levels. Simulators are a convenient tool, but are not a substitute for human testing.21 The extent to which the static calibration of oscillometric devices drifts over time is unclear; aging of transducers, connectors, and cuffs may contribute to drift.
In patient-specific calibration, a comparison to auscultation is performed in a specific patient for the purposes of identifying if that device can accurately assess BP in that individual. This procedure should be done after identifying the arm with higher BP.22 The optimal, most feasible method to perform patient-specific calibration will vary from practice to practice, understanding that practical issues may exist related to patient-specific calibration, such as inadequate reference devices or time constraints. Simplified protocols have been proposed for use in the clinic.23 The same arm simultaneous (SAS) method, using a three-way connector which attaches the device to a manometer and stethoscope, is one convenient method.4 A stethoscope connected to a smartphone that records the Korotkoff sounds for playback may also be used for this method.24,25 The accuracy of any method for patient-specific calibration is dependent on the accuracy of the provider’s BP measurement. The accuracy of the SAS method can be compromised if the deflation speed of the device is faster than the recommended 2–3 mmHg per second. However, estimates from mathematical models indicate that this error is only a few mmHg, at most.26 At very rapid deflation rates, such as 10 mmHg/sec, a same arm sequential method may be more appropriate.
Patient-specific calibration is important because a device can be inaccurate in an individual patient despite achieving a passing grade in a validation protocol.14,15 Assuming the cuff is properly sized, this inaccuracy could be the result of an algorithm that performs poorly in that patient, or of altered arterial characteristics in an individual. There is no easy solution to this problem. Using a patient-specific correction factor generated using the SAS method to adjust for the degree and consistency of differences with auscultatory BP is one option, though has not been formally tested. Multiple comparisons should be performed when generating correction factors. In the authors’ experience, performing five comparisons is optimal. Switching to a device from a different manufacturer that performs better in that patient is another solution when a device performs poorly in a specific individual.
Food and Drug Administration (FDA) Clearance
A common misconception is that the FDA “approves” BP devices for patient use. More accurately, the FDA ‘clears’ a device to be sold on the market. Manufacturers of non-invasive BP devices, which are categorized as Class II or moderate-risk devices, obtain clearance to market their device through a Premarket Notification application detailed in the 501(k) section of the Federal Food, Drug and Cosmetic Act.27 Importantly, manufacturers are required only to demonstrate that a new BP device is approximately as safe and effective as similar devices on the market, termed ‘substantial equivalence’.
It is important to understand the limitations of the 510(k) process.28 It does not contain explicit requirements to demonstrate accuracy and does not mandate which, if any, validation protocol should be used. A device manufacturer is also not required to perform an independent, peer-reviewed validation study. Rather, internally generated data can be submitted. These data are not readily available to the public. In addition, post-approval device modification (including new cuffs and changes to the algorithm) is common. Although the FDA provides guidance to the manufacturer when a device modification should trigger a new 510(k) application, it states that “The burden is on the 510(k) holder to decide whether or not a modification could significantly affect safety or effectiveness of the device.”29 In other words, manufacturers are allowed to decide if a new 501(k) submission is needed. The FDA also recommends that the justification for submitting or not submitting a new 510(k) be recorded in a “letter to file change”; however, submission of this documentation to the FDA is not required unless requested. Additional concerns include inadequate enforcement of false claims including ‘off-label’ use of devices beyond their intended market.6 Because of these issues, many devices are sold on the market without rigorous evaluation of the accuracy of the device.30,31 Manufacturers cannot make statements about the accuracy of devices that have not undergone appropriate validation.32 However, it is not readily apparent to patients and providers which devices on the market are appropriately validated.
THE CURRENT PRACTICE IN BP MEASUREMENT
In-Office BP Measurement
Standard Clinical vs. Research BP Measurement
For most practices, there is a major discrepancy between the careful measurement of BP in clinical research and the routine method used in clinical practice.33–35 In the typical clinical setting, often only a single measurement is obtained by a medical assistant and not by a registered nurse, mid-level provider, or physician. Most medical assistant measurement errors are related to insufficient rest time, incorrect body position, under-cuffing, and talking during the measurement,36–38 which combined result in a substantially elevated BP measurement compared to measurement by proper technique.39 The choice of equipment at large centers is often based on vendor contracts, while most smaller offices simply use what has been previously provided with often little to no attention paid to the validation status of the device. Portable aneroid BP sphygmomanometers, which are frequently used in smaller practices, are the most vulnerable to loss of calibration over time.40,41 Further, calibration of BP measuring devices is likely only performed at larger, mostly academic centers with the support of a clinical engineering department.
Automated office BP (AOBP)
Recent international guidelines encourage the use of multiple preprogrammed, observed or unobserved, fully-automated AOBP readings in routine practice.42 AOBP has the potential to improve the accuracy of in-office BP measurements by reducing the white coat effect.43 Recent findings suggest similar results in unobserved (i.e. the mean of three consecutive oscillometric readings with no clinician present) compared to observed (i.e. the mean of three consecutive oscillometric readings with a clinician present) AOBP measurements.44–46
Out-of-Office BP Measurement
Home BP Monitoring
Home BP monitoring is increasingly common and reflects the growing popularity of mobile health technologies in recent years. While it is a cornerstone of recent hypertension guideline recommendations,47 there are many unresolved issues with regard to home BP monitoring. A substantial number of home BP devices on the market are not validated, and the government has no enforcement division to prohibit selling these devices (see section on FDA Clearance).6 Although an increasing number of people in North America have arm sizes requiring a ‘large’ or ‘extra-large’ cuff, many home BP monitoring devices only come with a ‘regular’-sized cuff.38,48 Patients often are not aware of the importance of resting for five minutes, correct posture, and abstaining from talking, using a computer, or watching television during measurements.36,37 Useful tools in addressing these potential sources of error include a patient instruction handout and, more importantly, in-office individual validation of each patient’s device and periodic review of their measurement technique.14,15
Ambulatory BP Monitoring (ABPM)
ABPM performs automated BP measurements at regular intervals over a 24–48 hour period, including at least one full cycle of wakefulness and sleep. The United States Preventive Service Taskforce49 and American College of Cardiology/American Heart Association47 guidelines recommend ABPM measurement for the initial diagnosis of hypertension in many patients due to its prognostic superiority over in-office BP measurement.50–53 However, inadequate reimbursement balanced with high start-up costs for the devices and software, and the relatively time-consuming interpretation of the results make it difficult for small practices to undertake routine use of ABPM.54 Additionally, patient-level barriers include potential disruption of work or sleep, and the need to come back to the office for a second visit to return the monitor within one to two days of the initial visit.
ONLINE DEVICE LISTINGS
Many automated BP devices of different varieties (home, ambulatory, clinic) are currently on the market. These devices vary widely in terms of their supporting data, from no available validation data, to full validation assessments conducted according to the most rigorous protocols.4,5 To ensure that consumers, health care providers, academic researchers, and industry have access to information summarizing which devices have been validated and which criteria were used, device validation listings have been established. These registries are typically focused on devices that have had some type of validation assessment and are funded, at least in part, by device manufacturer application fees. Validation data are typically reviewed by one or more experts in the field of BP measurement.
Dabl Educational Trust,55 established in 1993 by a group of international investigators, was the first widely used device registry. It includes manual and automated devices, with the latter category comprising home, ambulatory, and clinic devices. The Dabl registry divides devices into ‘recommended’ and ‘not recommended’ categories according to validation study results. A limitation of the Dabl registry is the classification of devices validated using the Association for the Advancement of Medical Instrumentation (AAMI)/American National Standards Institute (ANSI)/ International Organization for Standardization (ISO) protocol as ‘not recommended,’ citing ‘questionable evidence.’ In particular, the Dabl registry, although willing to accept as valid the 33-subject (with 99 paired readings) European Society of Hypertension International Protocol (ESH IP) results,18 excludes studies performed using the more rigorous 85-subject (with 255 paired readings) AAMI/ANSI/ISO protocol.4 Notably, the existing protocols are being phased out in favor of a conjoint AAMI/ESH/ISO 85-patient protocol,56,57 which requires similar high precision and large sample size as the AAMI/ANSI/ISO protocol.4 There is no longer any clear scientific oversight or regular updating of the Dabl registry.
Medaval58 is a newer device registry that, in addition to listing BP devices, also includes blood glucose meters and pulse oximeters; there is no independent scientific oversight of the registry. In addition, professional hypertension societies have sections of their websites dedicated to VDLs of devices available for purchase in their countries. Examples include Hypertension Canada,59 the British and Irish Hypertension Society (BIHS),60 and the Japanese Society of Hypertension.61 The Hypertension Canada device listing requires a manufacturer application and published validation data. It divides devices into those validated using the British Hypertension Society (BHS)5 or AAMI/ANSI/ISO protocol4 (Gold tier) and ESH IP18 (Silver tier).
The New AMA VDL
The AMA’s VDL will be established to address some of the limitations of previous and current device listings, requiring more rigorous inclusion criteria. The primary impetus for the AMA VDL was lack of any legal requirement in the United States for devices to undergo rigorous validation testing for clinical accuracy. As a result, many devices have not been tested properly and could yield inaccurate readings. There is no way to know the accuracy of a given BP measurement device without going through clinical validation protocols. Payers, health systems, physicians, and individuals can all purchase these devices in the United States. Yet there is no listing of device available in the United States that have gone through validation testing for clinical accuracy. As a result, it is very difficult for these groups to make an informed decision when choosing a device to use or recommend for self-monitoring of BP. The AMA and a group of experts who came together are seeking to create a regularly updated repository of devices that have been validated for clinical accuracy that will be publicly available online.
For a device to be listed in the AMA VDL, manufacturers will have to include in their application the details provided in Table 2. Importantly, documentation of substantial equivalence to a device already on the market is insufficient for entry into the AMA VDL if a critical component of the new device differs from the previously validated one. Critical components that may affect BP measurement include the cuff, transducer, inflation/deflation process, procedure for waveform acquisition and processing, and algorithm. Validation data must be provided that were generated externally and preferably published in peer-review format (Table 2). As with other registries, the only assurance that validation protocols were performed correctly is by peer-review.
Table 2:
Proposed Entry Criteria for the American Medical Association Validated Device Listing
| Criteria |
|---|
| A validation study performed according to one of the following 85-subject protocols*: |
| 1. ANSI/AAMI/ISO 81060–2:2013 |
| 2. AAMI/ISO 81060–2:2009 |
| 3. ANSI/AAMI SP10:2002 |
| 4. BHS Revised Protocol 1993 |
| One of the following methods of summarizing validation data (listed in order of preference): |
| 1. Peer-reviewed publication. |
| 2. Independent third party validation testing by a qualified entity. These may include academic institutions or credible research entities with expertise in BP measurement and knowledge of validation protocols and validation study requirements. |
The European Society of Hypertension International Protocol18 alone is not considered acceptable
Abbreviations: AAMI = Association for the Advancement of Medical Instrumentation; ANSI = American National Standards Institute; ISO = International Standards Organization; BHS = British Hypertension Society
A major difference between the AMA VDL and the Canadian and BIHS device listings is the distinction of the validation protocols deemed acceptable. The AMA VDL will not recommend devices tested in validation studies performed using only the ESH IP. This protocol has been judged inadequate for AMA VDL entry due to the low sample size and low number of paired readings; therefore, only the ANSI/AAMI/ISO or BHS protocols are considered acceptable. See Table 3 for a detailed comparison between the AMA, Canadian, and BIHS VDLs.
Table 3.
Comparison of National Society Validated Device Listings
| Validated Device Listing Criteria |
American Medical Association |
Hypertension Canada |
British and Irish Hypertension Society |
|---|---|---|---|
| Accepted validation protocols | 85-subject protocols only: • AAMI/ANSI/ISO • BHS |
85-subject protocols (Gold Tier): • AAMI/ANSI/ISO • BHS 33-subject protocols (Silver Tier): • ESH IP |
85-subject and 33-subject protocols: • BHS • ESH IP |
| Accepted methods for summarizing validation data | • Peer-reviewed publication • Unpublished independent third party validation study |
• Peer-reviewed publication | • Peer-reviewed publication • Society-performed validation study |
| Listed devices | • Only recommended devices | • Only recommended devices | • Recommended and non-recommended devices |
Abbreviations: AAMI = Association for the Advancement of Medical Instrumentation; ANSI = American National Standards Institute; ISO = International Standards Organization; BHS = British Hypertension Society; ESH IP = European Society of Hypertension International Protocol
The proposed AMA VDL criteria were open for public comment during the Summer of 2018. In the Fall of 2018, the AMA convened with a panel of experts to address the public comments. The AMA VDL is currently undergoing administrative approvals and starting production; the AMA plans to make the VDL available for public use in early to mid-2019.
CONCLUSION
The removal of mercury-based BP measurement devices resulted in a shift to aneroid and oscillometric methods to determine BP in the office and home settings. Oscillometric device validation is a critical component to ensure accurate assessment of BP and thus promote appropriate BP management. Recent hypertension guidelines emphasize lower BP treatment goals based on carefully obtained in-office readings, to be complemented by home BP monitoring.47 Accordingly, greater attention to the accuracy of the devices and methods being used to measure BP both in and out of the office is paramount. Many devices available to both healthcare providers and patients have not undergone validation prior to being placed on the market. Since BP devices are distributed worldwide, uniformity of BP accuracy standards across the world will simplify development of newer devices for manufacturers, while assuring safety for patients. Access to an updated listing of available, validated devices is an important step in improving current methods of BP measurement across healthcare systems and practices nationally. The VDL will also likely facilitate development of national standards for home BP measurement, which may in turn promote inclusion of home BP measurements in practice quality assessments and merit-based incentive programs.
We strongly encourage clinicians to actively take part in discussions around the implementation of the VDL, and to disseminate this valuable tool to the medical community and to patients involved or interested in home BP monitoring.
ACKNOWLEDGEMENTS
This is not an official AMA statement. RSP, BB, and RRT, are part of the VDL working group, and JBC served as a member of an expert panel reviewing feedback to the proposed VDL criteria. The authors would like to thank Dr. Michael Rakotz and Nar Ramkissoon with the AMA for reviewing the manuscript and assisting in responding to reviewer comments.
SOURCES OF FUNDING
Effort for this publication was supported in part by the NIH-NHLBI (K23-HL133843, PI: JBC). The interpretation and reporting of this publication is the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the NIH.
Footnotes
DISCLOSURES
Author conflicts of interest and financial disclosures include Co-Director of mmHg Inc., a start-up company focusing on innovations in BP measurement (RSP) and Consultants to Welch-Allyn (SY and RRT). The remaining authors have no disclosures.
REFERENCES
- 1.O’Brien E, Fitzgerald D. The history of blood pressure measurement. J Hum Hypertens. 1994;8(2):73–84. [PubMed] [Google Scholar]
- 2.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–1357. 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 3.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32(1):3–15. 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 4.American National Standard. ANSI/AAMI/ISO 81060–2:2013 Non-invasive sphygmomanometers - Part 2: Clinical investigation of automated measurement type Association for the Advancement of Medical Instrumentation. Arlington, VA: 2013. [Google Scholar]
- 5.O’Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, Altman DG, Bland M, Coats A, Atkins N. The British Hypertension Society protocol for the evaluation of automated and semi-automated blood pressure measuring devices. J Hypertens. 1993;11(6):S43–S62. [DOI] [PubMed] [Google Scholar]
- 6.Alpert BS. Can ‘FDA-cleared’ blood pressure devices be trusted? A call to action. Blood Press Monit. 2017;22(4):179–181. 10.1097/MBP.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien E, Alpert BS, Stergiou GS. Accurate blood pressure measuring devices: Influencing users in the 21st century. J Clin Hypertens (Greenwich). 2018;20(7):1138–1141. 10.1111/jch.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plante TB, Urrea B, MacFarlane ZT, Blumenthal RS, Miller ER 3rd, Appel LJ, Martin SS. Validation of the Instant Blood Pressure Smartphone App. JAMA Intern Med. 2016;176(5):700–702. 10.1001/jamainternmed.2016.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elvan-Taspinar A, Uiterkamp LA, Sikkema JM, Bots ML, Koomans HA, Bruinse HW, Franx A. Validation and use of the Finometer for blood pressure measurement in normal, hypertensive and pre-eclamptic pregnancy. J Hypertens. 2003;21(11):2053–2060. 10.1097/01.hjh.0000098138.70956.68. [DOI] [PubMed] [Google Scholar]
- 10.Casiglia E, Tikhonoff V, Albertini F, Palatini P. Poor Reliability of Wrist Blood Pressure Self-Measurement at Home: A Population-Based Study. Hypertension. 2016;68(4):896–903. 10.1161/HYPERTENSIONAHA.116.07961. [DOI] [PubMed] [Google Scholar]
- 11.Kumar N, Khunger M, Gupta A, Garg N. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9(2):130–136. 10.1016/j.jash.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Ameloot K, Palmers PJ, Malbrain ML. The accuracy of noninvasive cardiac output and pressure measurements with finger cuff: a concise review. Curr Opin Crit Care. 2015;21(3):232–239. 10.1097/MCC.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 13.Forouzanfar M, Dajani HR, Groza VZ, Bolic M, Rajan S, Batkin I. Oscillometric Blood Pressure Estimation: Past, Present, and Future. IEEE Rev Biomed Eng. 2015;8:44–63. 10.1109/RBME.2015.2434215. [DOI] [PubMed] [Google Scholar]
- 14.Ringrose JS, Polley G, McLean D, Thompson A, Morales F, Padwal R. An Assessment of the Accuracy of Home Blood Pressure Monitors When Used in Device Owners. Am J Hypertens. 2017;30(7):683–689. 10.1093/ajh/hpx041. [DOI] [PubMed] [Google Scholar]
- 15.Wan Y, Heneghan C, Stevens R, McManus RJ, Ward A, Perera R, Thompson M, Tarassenko L, Mant D. Determining which automatic digital blood pressure device performs adequately: a systematic review. J Hum Hypertens. 2010;24(7):431–438. 10.1038/jhh.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stergiou GS, Kollias A, Karpettas N. Does atrial fibrillation affect the automated oscillometric blood pressure measurement? Hypertension. 2013;62(5):e37 10.1161/HYPERTENSIONAHA.113.02211. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JB, Wong TC, Alpert BS, Townsend RR. Assessing the accuracy of the OMRON HEM-907XL oscillometric blood pressure measurement device in patients with nondialytic chronic kidney disease. J Clin Hypertens (Greenwich). 2017;19(3):296–302. 10.1111/jch.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, Imai Y, Wang J, Mengden T, Shennan A, Working Group on Blood Pressure Monitoring of the European Society of H. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15(1):23–38. 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 19.Ringrose J, Millay J, Babwick SA, Neil M, Langkaas LA, Padwal R. Effect of overcuffing on the accuracy of oscillometric blood pressure measurements. J Am Soc Hypertens. 2015;9(7):563–568. 10.1016/j.jash.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Ringrose JS, McLean D, Ao P, Yousefi F, Sankaralingam S, Millay J, Padwal R. Effect of Cuff Design on Auscultatory and Oscillometric Blood Pressure Measurements. Am J Hypertens. 2016;29(9):1063–1069. 10.1093/ajh/hpw034. [DOI] [PubMed] [Google Scholar]
- 21.Balestrieri E, Daponte P, Rapuano S. Towards accurate NIBP simulators: Manufacturers’ and researchers’ contributions. 2013 IEEE International Symposium on Medical Measurements and Applications (MeMeA). 2013. 10.1109/MeMeA.2013.6549713. [DOI] [Google Scholar]
- 22.Lane D, Beevers M, Barnes N, Bourne J, John A, Malins S, Beevers DG. Inter-arm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20(6):1089–1095. [DOI] [PubMed] [Google Scholar]
- 23.Eguchi K, Kuruvilla S, Ishikawa J, Schwartz JE, Pickering TG. A novel and simple protocol for the validation of home blood pressure monitors in clinical practice. Blood Press Monit. 2012;17(5):210–213. 10.1097/MBP.0b013e328356e196. [DOI] [PubMed] [Google Scholar]
- 24.Alpert BS. The Accutension Stetho, an automated auscultatory device to validate automated sphygmomanometer readings in individual patients. J Hum Hypertens. 2018. 10.1038/s41371-018-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu G, Zhang Z, Xu M, Huang D, Dai Q. Validation of a smartphone auscultatory blood pressure kit Accutension XYZ-110 in adults according to the ANSI/AAMI/ISO 81060–2: 2013 standard. Blood Press Monit. 2017;22(5):290–294. 10.1097/MBP.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 26.Hatsell CP. Cardiac cycle phase uncertainty: another source of error in indirect blood pressure measurement. J Med Eng Technol. 1992;16(4):157–158. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. Learn if a medical device has been cleared by FDA for marketing. https://www.fda.gov/MedicalDevices/ResourcesforYou/Consumers/ucm142523.htm. 2017. Accessed 16 May 2018.
- 28.Institute of Medicine. Medical devices and the public’s health The 510(k) clearance. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Medical-Devices-and-the-Publics-Health-The-FDA-510k-Clearance-Process-at-35-Years/510k%20Clearance%20Process%202011%20Report%20Brief.pdf. Washington, DC: 2011. Accessed 16 May 2018. [Google Scholar]
- 29.U.S. Food and Drug Administration. Is a new 510(k) required for a modification to the device? https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketNotification510k/ucm134575.htm. 2017. Accessed 30 May 2018.
- 30.Bello NA, Woolley JJ, Cleary KL, Falzon L, Alpert BS, Oparil S, Cutter G, Wapner R, Muntner P, Tita AT, Shimbo D. Accuracy of Blood Pressure Measurement Devices in Pregnancy: A Systematic Review of Validation Studies. Hypertension. 2018;71(2):326–335. 10.1161/HYPERTENSIONAHA.117.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgkinson JA, Sheppard JP, Heneghan C, Martin U, Mant J, Roberts N, McManus RJ. Accuracy of ambulatory blood pressure monitors: a systematic review of validation studies. J Hypertens. 2013;31(2):239–250. 10.1097/HJH.0b013e32835b8d8b. [DOI] [PubMed] [Google Scholar]
- 32.White JR, Schick J. Home Blood Pressure Monitoring and Diabetes. Clinical Diabetes. 2004;22(1):28–31. 10.2337/diaclin.22.1.28. [DOI] [Google Scholar]
- 33.Myers MG. The great myth of office blood pressure measurement. J Hypertens. 2012;30(10):1894–1898. 10.1097/HJH.0b013e3283577b05. [DOI] [PubMed] [Google Scholar]
- 34.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55(2):195–200. 10.1161/HYPERTENSIONAHA.109.141879. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal R Implications of Blood Pressure Measurement Technique for Implementation of Systolic Blood Pressure Intervention Trial (SPRINT). J Am Heart Assoc. 2017;6(2). 10.1161/JAHA.116.004536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terent A, Breig-Asberg E. Epidemiological perspective of body position and arm level in blood pressure measurement. Blood Press. 1994;3(3):156–163. [DOI] [PubMed] [Google Scholar]
- 37.Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4(2):97–101. [PubMed] [Google Scholar]
- 38.Palatini P, Asmar R. Cuff challenges in blood pressure measurement. J Clin Hypertens (Greenwich). 2018;20(7):1100–1103. 10.1111/jch.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatt H, Siddiqui M, Judd E, Oparil S, Calhoun D. Prevalence of pseudoresistant hypertension due to inaccurate blood pressure measurement. J Am Soc Hypertens. 2016;10(6):493–499. 10.1016/j.jash.2016.03.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarows SA, Qian K. Accuracy of aneroid sphygmomanometers in clinical usage: University of Michigan experience. Blood Press Monit. 2001;6(2):101–106. [DOI] [PubMed] [Google Scholar]
- 41.Canzanello VJ, Jensen PL, Schwartz GL. Are aneroid sphygmomanometers accurate in hospital and clinic settings? Arch Intern Med. 2001;161(5):729–731. [DOI] [PubMed] [Google Scholar]
- 42.Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32(5):569–588. 10.1016/j.cjca.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 43.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Grant FC, Kaczorowski J. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. British Medical Journal. 2011;342 ARTN d286 10.1136/bmj.d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreadis EA, Geladari CV, Angelopoulos ET, Savva FS, Georgantoni AI, Papademetriou V. Attended and Unattended Automated Office Blood Pressure Measurements Have Better Agreement With Ambulatory Monitoring Than Conventional Office Readings. J Am Heart Assoc. 2018;7(8). 10.1161/JAHA.118.008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stergiou G, Kollias A, Parati G, O’Brien E. Office Blood Pressure Measurement: The Weak Cornerstone of Hypertension Diagnosis. Hypertension. 2018;71(5):813–815. 10.1161/HYPERTENSIONAHA.118.10850. [DOI] [PubMed] [Google Scholar]
- 46.Johnson KC, Whelton PK, Cushman WC, et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71(5):848–857. 10.1161/HYPERTENSIONAHA.117.10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 48.Mourad JJ, Lopez-Sublet M, Aoun-Bahous S, Villeneuve F, Jaboureck O, Dourmap-Collas C, Denolle T, Fourcade J, Baguet JP. Impact of miscuffing during home blood pressure measurement on the prevalence of masked hypertension. Am J Hypertens. 2013;26(10):1205–1209. 10.1093/ajh/hpt084. [DOI] [PubMed] [Google Scholar]
- 49.Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(3):192–204. 10.7326/M14-1539. [DOI] [PubMed] [Google Scholar]
- 50.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46(3):508–515. 10.1016/j.jacc.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 51.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodriguez-Artalejo F, Williams B. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. N Engl J Med. 2018;378(16):1509–1520. 10.1056/NEJMoa1712231. [DOI] [PubMed] [Google Scholar]
- 52.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46(1):156–161. 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Thijs L, Hansen TW, et al. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55(4):1040–1048. 10.1161/HYPERTENSIONAHA.109.137273. [DOI] [PubMed] [Google Scholar]
- 54.Cohen JB, Cohen DL. Integrating Out-of-Office Blood Pressure in the Diagnosis and Management of Hypertension. Curr Cardiol Rep. 2016;18(11):112 10.1007/s11886-016-0780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dabl Educational Trust. Blood Pressure Monitors - Validations, Papers and Reviews. http://www.dableducational.org/. 2018. Access 18 Jun 2018.
- 56.Stergiou GS, Alpert B, Mieke S, et al. A Universal Standard for the Validation of Blood Pressure Measuring Devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71(3):368–374. 10.1161/HYPERTENSIONAHA.117.10237. [DOI] [PubMed] [Google Scholar]
- 57.Stergiou GS, Alpert BS, Mieke S, Wang J, O’Brien E. Validation protocols for blood pressure measuring devices in the 21st century. J Clin Hypertens (Greenwich). 2018;20(7):1096–1099. 10.1111/jch.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medaval Certified Accuracy. http://medaval.ie/. 2018. Accessed 18 Jun 2018.
- 59.Blood Pressure Devices Recommended by Hypertension Canada. https://hypertension.ca/hypertension-and-you/managing-hypertension/measuring-blood-pressure/devices/. 2018. Accessed 20 Jun 2018.
- 60.British and Irish Hypertension Society. BP Monitors. https://bihsoc.org/bp-monitors/. 2018. Accessed 20 Jun 2018.
- 61.Japanese Society of Hypertension Device Listing. http://www.jpnsh.jp/com_ac_wg1.html 2018. Accessed 13 Nov 2018.