Abstract
Objective:
Individuals with temporal lobe epilepsy (TLE) often experience diminished quality of life (QoL). Although comorbid depression is one of the most recognized predictors of poor QoL in TLE, impairments in verbal memory (VM) and executive functioning (EF), have also been identified as risk factors, independent of other biological and psychosocial factors. In this study, we examine the contribution of depression, VM, and EF to QoL in 52 well-characterized medically-refractory TLE patients.
Methods:
Quality of life was assessed with the Quality of Life in Epilepsy (QOLIE-31) questionnaire and depression symptomatology was evaluated with the Beck Depression Inventory-II (BDI-II). Tests of VM included the California Verbal Learning Test-Second Edition and the Wechsler Memory Scale-Third Edition, Logical Memory and Verbal Paired Associates subtests. Tests of EF included the D-KEFS Category Switching and Color Word Interference Tests, and the Trail Making Test. Using these measures, a principal component (PC) was derived for VM and for EF. Hierarchical multiple linear regression analysis was used to evaluate the unique contributions of BDI-II Score, VM PC, and EF PC to the QOLIE-31 Total Score, while controlling for important clinical and demographic variables. Post-hoc analyses were also performed to examine the contribution of each variable to specific QOLIE subscales.
Results:
Of the clinical variables, only number of antiepileptic drugs contributed to QOLIE scores. As expected, severity of depressive symptoms was the most significant predictor of QOLIE Total Score, explaining 43.4% of the variance in total QoL. The VM PC did not contribute to the QOLIE Total Score. Rather, our EF PC emerged as an important predictor of QoL, explaining an additional 5% of the variance, after controlling for clinical variables, depression severity, and VM performance.
Significance:
These findings suggest that a combination of clinical, affective, and cognitive factors influence QoL in patients with TLE. Designing interventions with careful attention to depression and EF may be needed to optimize QoL in patients with refractory TLE and potentially other epilepsy syndromes.
Keywords: epilepsy, quality of life, temporal lobe epilepsy, verbal memory, executive functioning, depression
1.1. Introduction
Temporal lobe epilepsy (TLE) is the most common type of localization-related epilepsy in adults and is often highly refractory to antiepileptic drugs (AEDs) (Currie et al., 1971). A common finding in patients with TLE is diminished quality of life (QoL) relative to their healthy counterparts, which has been attributed to a combination of neurobiological, affective, and psychosocial factors (Lehrner et al., 1999; Pauli et al., 2017). In particular, there is evidence that poor seizure control, earlier age of seizure onset, the adverse effects of AEDs, the presence of mesial temporal sclerosis (MTS), side of seizure onset, and comorbid mood disorder may lead to diminished QoL in TLE (Alonso-Vanegas et al., 2013; Andelman et al., 2001; Aydemir et al., 2004; Kwan et al., 2009; Lehrner et al., 1999; Loring et al., 2004; McLachlan et al., 1997; Pauli et al., 2017). In fact, depressive symptoms are consistently found to be one of the strongest predictors of QoL in TLE (Boylan et al., 2004; Hermann et al., 2000; Johnson et al., 2004), often accounting for up to 50% of the variance in QoL. The aim of the present study is to add to this body of research by expanding our understanding of multivariate predictors of QoL in patients with medically-refractory TLE.
Less attention has been given to the impact of cognitive dysfunction on QoL in TLE, despite emerging evidence that cognitive impairment may contribute to poor QoL independent of the influence of depression and other seizure-related variables (Giovagnoli & Avanzini, 2000; Perrine et al., 1995). In particular, impairments in memory and/or executive functioning (EF) have been identified as potential risk factors for poor QoL (Giovagnoli & Avanzini, 2000; Giovagnoli et al., 2014), as deficits in both are commonly observed in TLE (Saling, 2009; Stretton & Thompson, 2012). Perrine et al. (1995) found that verbal memory (VM), but not EF, was associated with self-reported cognitive difficulties as well as overall QoL, independent of mood, in TLE. Similarly, Giovagnoli and Avanzini (2000) demonstrated that both objective memory performance and self-reported memory difficulties were associated with lower QoL in patients with TLE. Although Perrine et al. (1995) did not find an association between EF and QoL in TLE using a single measure of response inhibition, studies in patients with a range of epilepsy syndromes (Giovagnoli et al., 2014), as well as pediatric epilepsy (Love et al., 2016; Schraegle & Titus, 2016; Sherman et al., 2006), have found associations between EF and overall QoL using a broader scope of objective or parent-reported EF measures. Given the role of EF in adaptive functioning and social/occupational functioning (Moran, 2013; Perna et al., 2012), gaining a better appreciation of the degree to which impairments in VM and EF independently contribute to QoL in TLE beyond the contribution of depression is important.
In this study, we evaluate the impact of VM and EF on QoL in patients with TLE, taking into account the contribution of depression and other epilepsy-related variables that have been linked to QoL, including age of seizure onset, number of AEDs, seizure frequency, education, side of seizure onset, and the presence of MTS. We hypothesize that in addition to depression and important clinical variables, VM and EF will contribute to QoL in patients with TLE. To the best of our knowledge, this is the first study of patients with medically-refractory TLE evaluating relationships between cognitive variables with QoL, beyond what is accounted by depression severity.
1.2. Methods
1.2.1. Participants
This study was approved by the Institutional Review Boards at UC San Diego and UC San Francisco, and informed consent was collected from all participants in accordance with the Declaration of Helsinki. Fifty-two patients with medically-refractory TLE were recruited for the study at one of the two centers during standard surgical workups. Inclusion criteria for patients included a unilateral TLE diagnosis by a board-certified epileptologist, in accordance with the criteria defined by the International League Against Epilepsy, based on video-EEG telemetry, seizure semiology, and neuroimaging evaluation. Patients were excluded if there was evidence on video-EEG of multifocal seizure onset, including evidence of bilateral TLE and/or a mass lesion on MRI. In 24 patients, unilateral MTS was diagnosed by a board-certified neuroradiologist, whereas the other 28 patients did not show evidence of MTS. Thirty-four patients demonstrated left temporal lobe seizure onset (LTLE) and 18 demonstrated right temporal lobe seizure onset (RTLE).
1.2.2. Quality of Life in Epilepsy
All patients received the Quality of Life in Epilepsy (QOLIE) questionnaire as part of a larger research study. This QoL scale assesses self-reported overall QoL, emotional well-being, social isolation, medication effects, work and social limitations, among other psychosocial and health-related factors that are often compromised in patients with epilepsy. The majority of patients received the QOLIE-31—an abbreviated version of the original QOLIE-89, which contains seven subscales that are most applicable to individuals with epilepsy (Cramer et al., 1998). A subset of patients received the full QOLIE-89, and for these patients, scores were converted to match the QOLIE-31 scoring procedure and create equivalent forms. The seven subscales of the QOLIE-31 include Seizure Worry, Overall Quality of Life, Emotional Well-Being, Energy/Fatigue, Cognitive Functioning, Medication Effects and Social Functioning. All 31-items across the seven subscales are aggregated to form a Total Score. Scores were then converted to T-scores, with higher T-scores indicating better QoL. This abbreviated version of the QOLIE-89 has been shown to be more practical in clinical settings, while providing similar information (Cramer et al., 1998).
1.2.3. Mood
The Beck Depression Inventory-II (BDI-II) was used to evaluate depressive symptomatology (Beck, 1996). The BDI-II is a 21-item self-report measure of common depressive symptoms. Each item has four possible responses and higher Total Scores are indicative of a greater number and severity of depressive symptoms. Scores ranging from 0–13 indicate minimal symptoms, 14–19 indicates mild symptoms, 20–28 indicates moderate symptoms, and 29–63 indicates severe symptoms of depression.
1.2.4. Verbal Memory
VM was evaluated with the delayed recall trials of the California Verbal Learning Test-Second Edition (CVLT-II) (Delis et al., 2000) and the Wechsler Memory Scale-Third Edition (WMS-III) Logical Memory (LM) and Verbal Paired Associates (VPA) subtests (Wechsler, 1997). The CVLT-II was adjusted for age and education and the WMS-III subtests were adjusted for age. These verbal memory measures were chosen because prior literature indicating that delayed recall, but not immediate recall, relates to QoL in patients with TLE (Alonso-Vanegas et al., 2013).
1.2.5. Executive Function
Verbal set-shifting and response inhibition were measured with the Delis-Kapan Executive Function System (D-KEFS) Color-Word Interference (CWI) test, Inhibition/Switching condition, and the D-KEFS Verbal Fluency test, Category Switching condition (accuracy score) (Delis et al., 2001). Visuomotor set-shifting was measured with the Trail Making Test-B (TMT-B) (Reitan, 1979). The TMT-B was adjusted for age and education and the D-KEFs subtests were adjusted for age. These EF measures were chosen based on prior studies showing their relationships with QoL, in addition to the availability of these measures in our testing battery (Giovagnoli & Avanzini, 2000; Klein et al., 2003; Noll et al., 2018).
1.2.6. Statistical Analysis
1.2.6.1. Principal component analyses
Principal component (PC) analysis was used to reduce the number of neuropsychological variables for each cognitive domain (i.e., VM and EF) in order to reduce the likelihood of Type I errors. To obtain the PCs, scaled scores were derived for each measure described above and entered into the analysis. The number of PCs was selected based on the eigenvalue > 1 rule. For the three tests of VM, only one factor was extracted with an eigenvalue of 1.843 (61.44% of variance explained), which was named the “VM PC”. For the three tests of EF, only one factor was extracted with an eigenvalue of 1.633 (54.45% of variance explained), which was named the “EF PC”.
1.2.6.2. Hierarchical linear regression analysis
Hierarchical linear regression analysis was used to evaluate the unique contributions of demographic and clinical (i.e., age of seizure onset, number of AEDs, side of seizure onset, MTS status, seizure frequency, and education), depression (i.e., BDI-II Scores), and cognitive variables (i.e., VM PC and EF PC) to the QOLIE Total Score. Post-hoc analyses were performed to examine the contribution of this set of variables to individual QOLIE subscales. An alpha level of .05 was used to determine statistical significance.
1.3. Results
1.3.1. Demographic, clinical, and neuropsychological variables
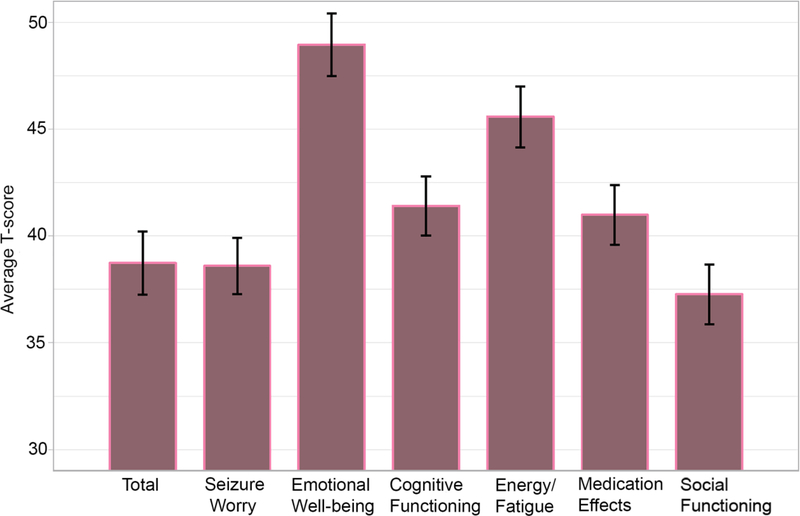
Table 1 shows clinical and demographic descriptive statistics. Table 2 displays the neuropsychological variables including the percent of our patient group with impaired performance. An “impairment score” was defined as a scale score of 5 or below. Approximately 7–20% of patients were impaired in EF measures, with TMT-B demonstrating the largest proportion of impairment. Furthermore, 15–37% of patients were impaired in VM measures, with CVLT-LDFR demonstrating the largest proportion of impairment. Figure 1 shows the means and standard errors for the QOLIE-31 Total Score and subscale scores.
Table 1:
Clinical variables and demographics
| Characteristics | Mean (SD) |
|---|---|
| Age | 35.02 (11.92) |
| Education | 13.79 (2.31) |
| Age of onset | 22.15 (14.28) |
| Duration | 12.93 (12.8) |
| Seizure frequency (# per month) | 6.98 (7.65) |
| N (percentage) | |
| Number of AEDs | |
| 1 | 10 (19.2%) |
| 2 | 19 (36.5%) |
| 3 | 17 (32.7%) |
| 4 | 5 (9.6%) |
| Sex | |
| Male | 29 (55.8%) |
| Female | 23 (44.2%) |
| Handedness | |
| Left | 4 (7.7%) |
| Right | 48 (92.3%) |
| Side of seizure onset | |
| Left | 34 (65.4%) |
| Right | 18 (34.6%) |
| MRI characteristics | |
| MTS+ | 24 (46.2%) |
| MTS− | 28 (53.8%) |
AEDs= antiepileptic drugs; MTS= mesial temporal sclerosis
Table 2:
Executive Function and Memory Performance
| Cognitive Functioning | Mean (SD) | % Impaired |
|---|---|---|
| Executive Function | ||
| TMT-B | 7.94 (3.08) | 19.2% |
| CW-Switching/Inhibition | 9.73 (2.96) | 7.7% |
| Category Switching | 9.29 (3.28) | 13.5% |
| Verbal Memory | ||
| LM-II | 8.04 (3.05) | 21.2% |
| VPA- II | 9.38 (3.23) | 15.4% |
| CVLT-LDFR | 7.25 (4.26) | 36.5% |
| Emotional Functioning | N | % |
| BDI-II | ||
| Minimal | 25 | 48.1% |
| Mild | 11 | 21.1% |
| Moderate | 11 | 21.2% |
| Severe | 5 | 9.6% |
TMT-B: trail making test-B; CW: color-word; LM: logical memory; VP: verbal paired associates; CVLT-LDFR: California verbal learning test-long delay free recall; SD: standard deviation
Fig 1.
QOLIE-31 Total Score and subscales in patients with temporal lobe epilepsy.
1.3.2. Hierarchical linear regression
1.3.2.1. Overall quality of life
Hierarchical multiple regression was utilized to evaluate the contribution of each predictor variable to the QOLIE-31 Total Score (Table 3). The first model included the following variables: age of seizure onset, seizure frequency, number of AEDs, education, MTS status, and side of seizure onset. The second model evaluated the unique contribution of BDI-II scores to the QOLIE31Total Score. Given some evidence from the literature that VM may contribute more to QoL than EF in TLE (Perrine et al., 1995), we entered the VM PC in the third model. The EF PC was entered in the fourth model.
Table 3:
Hierarchical Linear Regression
| Model | R2 | R2 change | F Change | p-value | |
|---|---|---|---|---|---|
| Total Score | 1 | .156 | .267 | ||
| 2 | .59 | .434 | 44.503 | <.001 | |
| 3 | .614 | .024 | 2.52 | .12 | |
| 4 | .664 | .05 | 5.964 | .019 | |
| Seizure Worry* | 1 | .153 | .282 | ||
| 2 | .293 | .14 | 8.347 | .006 | |
| 3 | .299 | .005 | .318 | .576 | |
| 4 | .301 | .002 | .112 | .739 | |
| Emotional Well-being* | 1 | .056 | .859 | ||
| 2 | .609 | .554 | 59.534 | <.001 | |
| 3 | .614 | .004 | .463 | .5 | |
| 4 | .634 | .02 | 2.163 | .149 | |
| Cognitive Functioning* | 1 | .119 | .458 | ||
| 2 | .3 | .181 | 10.853 | .002 | |
| 3 | .36 | .061 | 3.878 | .056 | |
| 4 | .361 | .001 | .043 | .838 | |
| Energy/ Fatigue* | 1 | .218 | .087 | ||
| 2 | .449 | .231 | 17.621 | <.001 | |
| 3 | .46 | .011 | .798 | .377 | |
| 4 | .477 | .017 | 1.32 | .257 | |
| Medication Effects* | 1 | .1 | .581 | ||
| 2 | .21 | .111 | 5.89 | .020 | |
| 3 | .21 | .000 | .005 | .944 | |
| 4 | .243 | .033 | 1.729 | .196 | |
| Social Functioning* | 1 | .253 | .042 | ||
| 2 | .413 | .16 | 11.477 | .002 | |
| 3 | .323 | .02 | .705 | .406 | |
| 4 | .451 | .027 | 1.991 | .166 | |
Model 1: Age of seizure onset, number of meds, seizure frequency, education, side of seizure onset, MTS status (covariates)
Model 2: BDI scores
Model 3: VM PC
Model 4: EF PC
Post-hoc hierarchical linear regressions; Bold italics signifies significance with a Bonferroni correction (six QOLIE subscales at an overall alpha error rate of <.05, yielding a significant p-value of .0083).
For QOLIE-31 Total Score, the first model was not significant (F(6, 43) = 1.423, p = .267, R2 = .156). However, number of AEDs was a significant individual predictor in this first model (β = −.345, p = .026). After introducing BDI-II scores into the model, the model was significant (F(7, 42) = 8.641, p < .001, R2 = .434). BDI-II scores explained 43.4% of the variance in QOLIE Total Scores (R2 change = .434; F(1, 42) = 44.503, p < .001). Introducing the VM PC (Model 3) did not significantly improve the model, (R2 change = .024; F(1, 41) = 2.52, p = .12). However, adding the EF PC (Model 4) explained an additional 5% of the variance in QOLIE Total Score (R2 change = .05; F(1, 40) = 5.964, p = .019). The final model was significant (F(9, 40) = 8.785, p < .001, R2 = .664). In this final adjusted model, number of AEDs (β = −.252, p = .017), BDI-II Scores (β = −.682, p < .001) and the EF PC (β = .266, p = .019) were significant predictors of Total QOLIE score.
Given the above findings demonstrating that the EF PC had a significant contribution to QOLIE Total score after controlling for depression and other clinical variables, we conducted a post-hoc causal mediation analyses to explore whether EF mediates the relationship between BDI-II scores and QOLIE Total score. To increase statistical power, we used a nonparametric bootstrap sampling procedure to test significance of the mediation effect (Tingley, 2014). Unstandardized mediation effects and the 95% confidence intervals were computed for each of the 1,000 bootstrapped samples. These results showed that EF did not mediate the relationship between BDI-II scores and QOLIE Total (ACME: β = 0.0079; 95% CI [−0.1003, 0.13]; p = 0.78). Furthermore, EF did not mediate the relationship between BDI scores and QOLIE Total when controlling for the clinical variable included in the hierarchical linear regression (ACME: β= −0.0093; 95% CI [−0.2328, 0.19]; p=0.87).
1.3.2.2. QOLIE Subscales
In order to evaluate the relationship between the predictor variables and the QOLIE subscales, we ran post-hoc regressions using the same models as in the omnibus analysis (see Table 3). The following subscales were evaluated: Seizure Worry, Emotional Well-being, Cognitive Functioning, Energy/Fatigue, Medication Effects, and Social Functioning. The Overall Quality of Life subscale was not evaluated due its low number of items. Bonferroni correction was used to control for multiple comparisons (six QOLIE subscales at an overall alpha error rate of < .05, yielding a significant p-value of .0083) for both the overall models and the individual predictors.
For the individual subscale analyses, BDI-II scores contributed to all subscales except for the Medication Effects subscale (see Table 3). Neither the VM PC nor the EF PC had a significant contribution to the subscales scores.
1.4. Discussion
There is a growing interest in understanding the unique clinical, mood, and cognitive influences associated with QoL in different epilepsy syndromes. In this study, we evaluate the contribution of two important cognitive domains—VM and EF—to QoL in patients with medically-refractory TLE, beyond the contributions of depression severity and other seizure-related variables. As expected, we replicate previous findings revealing that depression is the strongest predictor of QoL (Boylan et al., 2004; Cramer et al., 2003; Kanner, 2003; Perrine et al., 1995). Contrary to our hypotheses, we did not find VM to contribute to QoL. Rather, our data reveal a unique relationship between EF and QoL in patients with TLE. Thus, while depression may lead to poor QoL, EF appears to play an important role beyond what is accounted for by depression. However, EF does not appear to mediate the relationship between depression and QoL. These data add to the literature by demonstrating that both emotional and specific cognitive comorbidities may lower QoL in patients with medically-refractory TLE.
Depression severity has consistently predicted poor QoL in patients with epilepsy and other medical conditions (Blakemore et al., 2014; Sturm et al., 2004; Verma et al., 2010). Kanner (2011) described how underlying biological processes common to depression and epilepsy may potentiate one another, leading to a bidirectional relationship between depression and epilepsy. In particular, patients with TLE and primary depressive disorders have abnormalities of serotonin and norepinephrine neurotransmission in addition to structural and functional abnormalities affecting medial temporal lobe structures (Kanner, 2017; Kemmotsu et al., 2014). In addition, there are many psychosocial limitations imposed by a seizure disorder which can increase depression (Baker, 2002), including restrictions to driving and under- or unemployment (Baker, 2002; Elwes et al., 1991). These limitations are associated with reduced QoL (Elsharkawy et al., 2009) and are related to increased depression severity (Dooley et al., 2000; Ragland et al., 2005). Similar to other studies of patients with epilepsy (Boylan et al., 2004; Hermann et al., 2000; Johnson et al., 2004), depression explained the greatest amount of variance (43.4%) in total QoL and across a majority of subscales. However, it is important to note that both the BDI-II and the QOLIE are self-report measures, which may explain some of the shared variance between these measures. Nonetheless, these findings demonstrate the pervasive influence of depression on many aspects of a patient’s QoL, and highlight the critical need for identifying and implementing evidence-based treatments that target the complex nature of depression in TLE.
The most interesting finding in our study is that EF, but not VM, contributed to QoL in patients with refractory TLE beyond the contribution of depression. EF has been shown to contribute to QoL in both children and adults with a variety of epilepsy syndromes (Giovagnoli et al., 2014; Love et al., 2016; Sherman et al., 2006). In fact, children with focal and generalized epilepsy who had poor EF, per parent report, were found to be twice as likely to have poor QoL than the participants with intact EF (Sherman et al., 2006). In adults with a broad range of epilepsy syndromes, impairment in attention/EF was shown to be related to poor QoL (Giovagnoli et al., 2014). However, not all studies have found a relationship between EF and QoL (Alonso-Vanegas et al., 2013), nor has EF been found to predict QoL in medically-refractory TLE specifically. One reason for this may be that many previous studies in TLE have used a single measure of EF, which may not be comprehensive enough to capture the relationship between different EF abilities and QoL. In our study, we utilized principal component analysis to extract the common variance across different EF tests measuring verbal set-shifting, response inhibition and visuomotor set-shifting. Thus, our approach may have provided greater sensitivity for capturing the relationship between EF and QoL. Furthermore, studies demonstrating an association between EF and QoL in the pediatric literature (Love et al., 2016; Schraegle & Titus, 2016) have used parent/teacher report measures of EF. It is possible that parent-report measures of EF share greater variance with self-reported QoL than objective measures of EF. Nonetheless, our results demonstrate the importance of evaluating EF performance across a range of tests in order to fully appreciate EF impairment, and to determine how EF may be impacting patients in their day-to-day functioning and well-being.
In addition to depression, only one clinical variable, number of AEDs, contributed to QoL in our patients. These findings align with prior studies showing that a greater number of AEDs is associated with poorer QoL in patient with epilepsy, potentially due to the increased risk of negative side effects with each additional AED (Gilliam et al., 1999; Kwan et al., 2009; Pirio Richardson et al., 2004). In our sample of medically-refractory TLE, most patients were on polytherapy (88% with 2 or more AEDs), making the contribution of AEDs and untoward side effects particularly salient. However, polytherapy is typically associated with a worse clinical profile, including higher seizure frequency, longer duration of epilepsy, and severity of the underlying brain pathology. Although a post-hoc analysis in our sample revealed that number of AEDs was not associated with seizure frequency or duration of epilepsy and that patients with MTS did not differ in number of AEDs relative to patients with normal MRI (all p-values >.05), it is possible that other aspects of clinical severity contributed to this relationship. Given that our sample included only patients with medically-refractory TLE who were undergoing surgical workup, it is also possible that adverse effects from AEDs were an important factor in their decision to consider surgery. The use of newer AEDs with improved side effect profiles will hopefully lessen the contribution of AEDs to poor QoL in the future.
In contrast to prior studies, the majority of the clinical variables in our analyses were not significant predictors of QoL. This may indicate that no single variable, but rather, a combination of variables associated with clinical severity (i.e., total “seizure burden,” including seizure type, frequency, and duration) leads to poorer QoL. In addition, the VM PC did not contribute to the QoL Total Score or subscales, which is in contrast to some prior research that has shown VM to be an important predictor (Giovagnoli & Avanzini, 2000; Perrine et al., 1995). One possibitliy is that VM deficits alone are not sufficient to significantly affect QoL. Rather, patients with VM deficits plus global cognitive dysfunction or other co-morbidities may be at high risk for poor QoL since they would be less able to compensate for VM loss with other cognitive skills. Although our sample size and the available data did not permit us to thoroughly test this possibility, future studies with large sample sizes would be well-positioned to address whether there are other factors that influence whether VM deficits contribute to poor QoL. Furthermore, the relationship between objective cognitive measures and subjective reports of cognitive dysfunction has been inconsistent in the literature, with some studies demonstrating a relationship between performance on neuropsychological measures and the QOLIE cognitive subscale (Giovagnoli & Avanzini, 2000; Perrine et al., 1995), while other studies fail to find an association (Giovagnoli et al., 2014). Noteworthy, Banos et al. (2004) demonstrated that in TLE specifically, objective cognitive performance did not predict self-reported cognitive functioning, but instead self-report was highly predicted by emotional and psychosocial factors. Thus, our results provide additional evidence that depression and other emotional factors play a more central role in patients’ self-perceptions of their deficits than their actual deficits.
To date, most of the research on QoL in TLE has focused on changes in QoL associated with epilepsy surgery or other interventions (Seiam et al., 2011; Taylor et al., 2011). Many of these studies have found seizure freedom to be the strongest predictor of improved QoL following surgery, regardless of the presence of post-operative cognitive decline (Helmstaedter et al., 2003; Langfitt et al., 2007). However, in patients with persistent seizures, memory decline, in particular, has been found to further decrease QoL (Langfitt et al., 2007). Although EF has been shown to remain stable or improve after surgery in many TLE patients (Sherman et al., 2011), very little is known about the impact of changes in EF on QoL in patients with poor post-operative seizure outcome and in the subset of patients who do experience EF decline. Our findings suggest that EF contributes to QoL in presurgical patients, and it is also possible that EF may have a strong impact on QoL following surgery, especially for patients with persisting seizures. Thus, a comprehensive analysis of factors that predict EF outcomes following surgery may help to increase our understanding of how to facilitate improvements in QoL in patients with TLE. Furthermore, psychotherapy and psychopharmacological treatment of patients with TLE and comorbid depression is shown to improve QoL (Orjuela-Rojas et al., 2015), and adding a cognitive rehabilitation intervention that targets EF may help to further improve QoL in pre- or post-operative patients.
There are several limitations in our study that should be noted. First, our study includes a rather modest sample size compared to previous studies that have evaluated QoL across epilepsy syndromes. However, we choose to focus on medically-refractory TLE specifically to examine unique predictors of QoL within the syndrome of TLE. Although neither side of seizure onset nor MTS status contributed to QoL in our study, a larger sample size would permit the sample to be broken down into LTLE and RTLE or MTS-positive and MTS-negative patients. Given that these TLE subtypes show distinct cognitive profiles (Saling, 2009; Stretton & Thompson, 2012) and patients with MTS often report a greater number of depressive symptoms compared to MTS-patients (Quiske et al., 2000), future studies evaluating the contribution of cognitive impairment to QoL across different TLE subtypes are warranted. Furthermore, we only included patients with unilateral TLE in our study. Given the large proportion of patients with refractory TLE who have bilateral/multifocal seizure onset, understanding the impact of cognitive impairment on QoL in the context of long-term management and care of seizures via AEDs or other non-resection procedures (i.e., responsive neurostimulation) would be valuable. Second, we only evaluated VM and EF. We selected these two domains due to evidence in the literature that both are important to QoL in epilepsy, and the availability of sufficient measures in our test battery to create a PC for each. However, it is possible that other aspects of cognition may contribute to QoL. In addition, we utilized three commonly-used measures of EF that capture response inhibition, verbal set-shifting, and visuomotor set-shifting to create an EF composite. However, other components of EF that were not assessed (i.e., planning, problem-solving) may have strong ecological validity and be particularly salient for QoL in patients with TLE. Additionally, the use of self- and informant-reported EF may increase our ability to explain QoL in patients with TLE. As is common in most studies, we were unable to capture the full diversity of EF, VM, psychosocial, and mood characteristics that may contribute to QoL. Finally, our study is cross-sectional. Longitudinal studies that follow the trajectory of QoL from childhood through adulthood may provide valuable information as to how different clinical, cognitive, and psychosocial factors influence QoL across the lifespan in patients with TLE and other epilepsy syndromes.
1.5. Conclusion
We demonstrate that beyond the overall level of depression, EF contributes to QoL in patients with medically-refractory TLE. The impact of EF on total QoL in TLE provides important idiographic information that can lead to targeted patient interventions to improve QoL, which may require a different approach for patients with different levels of depression severity and EF impairment.
Supplementary Material
Highlights.
Depression was the strongest predictor of QoL in TLE patients
Verbal memory did not contribute to QoL
Executive function was a significant predictor of QoL
Acknowledgments
Funding
This work was supported by the National Institute of Health (R01 NS065838 to C.R.M.).
Footnotes
Disclosure of Conflicts of Interest/Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso-Vanegas MA, Cisneros-Franco JM, Castillo-Montoya C, Martinez-Rosas AR, Gomez-Perez ME, & Rubio-Donnadieu F (2013). Self-reported quality of life in pharmacoresistant temporal lobe epilepsy: correlation with clinical variables and memory evaluation. Epileptic Disord, 15(3), 263–271. doi: 10.1684/epd.2013.0590 [DOI] [PubMed] [Google Scholar]
- Andelman F, Fried I, & Neufeld MY (2001). Quality of life self-assessment as a function of lateralization of lesion in candidates for epilepsy surgery. Epilepsia, 42(4), 549–555. [DOI] [PubMed] [Google Scholar]
- Aydemir N, Ozkara C, Canbeyli R, & Tekcan A (2004). Changes in quality of life and self-perspective related to surgery in patients with temporal lobe epilepsy. Epilepsy Behav, 5(5), 735–742. doi: 10.1016/j.yebeh.2004.06.022 [DOI] [PubMed] [Google Scholar]
- Baker GA (2002). The psychosocial burden of epilepsy. Epilepsia, 43 Suppl 6, 26–30. [DOI] [PubMed] [Google Scholar]
- Banos JH, LaGory J, Sawrie S, Faught E, Knowlton R, Prasad A, … Martin RC (2004). Self-report of cognitive abilities in temporal lobe epilepsy: cognitive, psychosocial, and emotional factors. Epilepsy Behav, 5(4), 575–579. doi: 10.1016/j.yebeh.2004.04.010 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown G (1996). Beck Depression Inventory–II.
- Blakemore A, Dickens C, Guthrie E, Bower P, Kontopantelis E, Afzal C, & Coventry PA (2014). Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis, 9, 501–512. doi: 10.2147/COPD.S58136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K, & Devinsky O (2004). Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology, 62(2), 258–261. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Blum D, Reed M, Fanning K, & Epilepsy Impact Project G (2003). The influence of comorbid depression on quality of life for people with epilepsy. Epilepsy Behav, 4(5), 515–521. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, & Hermann B (1998). Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia, 39(1), 81–88. [DOI] [PubMed] [Google Scholar]
- Currie S, Heathfield KW, Henson RA, & Scott DF (1971). Clinical course and prognosis of temporal lobe epilepsy. A survey of 666 patients. Brain, 94(1), 173–190. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan executive function system. [DOI] [PubMed]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). CVLT-II: California verbal learning test: adult version. : Psychological Corporation. [Google Scholar]
- Dooley D, Prause J, & Ham-Rowbottom KA (2000). Underemployment and depression: longitudinal relationships. J Health Soc Behav, 41(4), 421–436. [PubMed] [Google Scholar]
- Elsharkawy AE, May T, Thorbecke R, Koch-Stoecker S, Villagran A, Urak L, … Ebner A (2009). Long-term outcome and determinants of quality of life after temporal lobe epilepsy surgery in adults. Epilepsy Res, 86(2–3), 191–199. doi: 10.1016/j.eplepsyres.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Elwes RD, Marshall J, Beattie A, & Newman PK (1991). Epilepsy and employment. A community based survey in an area of high unemployment. J Neurol Neurosurg Psychiatry, 54(3), 200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam F, Kuzniecky R, Meador K, Martin R, Sawrie S, Viikinsalo M, … Faught E (1999). Patient-oriented outcome assessment after temporal lobectomy for refractory epilepsy. Neurology, 53(4), 687–694. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, & Avanzini G (2000). Quality of life and memory performance in patients with temporal lobe epilepsy. Acta Neurol Scand, 101(5), 295–300. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Parente A, Tarallo A, Casazza M, Franceschetti S, & Avanzini G (2014). Self-rated and assessed cognitive functions in epilepsy: impact on quality of life. Epilepsy Res, 108(8), 1461–1468. doi: 10.1016/j.eplepsyres.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Lux S, Reuber M, & Elger CE (2003). Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol, 54(4), 425–432. doi: 10.1002/ana.10692 [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Bell B, Woodard A, Rutecki P, & Sheth R (2000). Comorbid psychiatric symptoms in temporal lobe epilepsy: association with chronicity of epilepsy and impact on quality of life. Epilepsy Behav, 1(3), 184–190. doi: 10.1006/ebeh.2000.0066 [DOI] [PubMed] [Google Scholar]
- Johnson EK, Jones JE, Seidenberg M, & Hermann BP (2004). The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia, 45(5), 544–550. doi: 10.1111/j.0013-9580.2004.47003.x [DOI] [PubMed] [Google Scholar]
- Kanner AM (2003). Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry, 54(3), 388–398. [DOI] [PubMed] [Google Scholar]
- Kanner AM (2011). Depression and epilepsy: A bidirectional relation? Epilepsia, 52 Suppl 1, 21–27. doi: 10.1111/j.1528-1167.2010.02907.x [DOI] [PubMed] [Google Scholar]
- Kanner AM (2017). Can Neurochemical Changes of Mood Disorders Explain the Increase Risk of Epilepsy or its Worse Seizure Control? Neurochem Res, 42(7), 2071–2076. doi: 10.1007/s11064-017-2331-8 [DOI] [PubMed] [Google Scholar]
- Kemmotsu N, Kucukboyaci NE, Leyden KM, Cheng CE, Girard HM, Iragui VJ, … McDonald CR (2014). Frontolimbic brain networks predict depressive symptoms in temporal lobe epilepsy. Epilepsy Res, 108(9), 1554–1563. doi: 10.1016/j.eplepsyres.2014.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Engelberts NH, van der Ploeg HM, Kasteleijn-Nolst Trenite DG, Aaronson NK, Taphoorn MJ, … Heimans JJ (2003). Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol, 54(4), 514–520. doi: 10.1002/ana.10712 [DOI] [PubMed] [Google Scholar]
- Kwan P, Yu E, Leung H, Leon T, & Mychaskiw MA (2009). Association of subjective anxiety, depression, and sleep disturbance with quality-of-life ratings in adults with epilepsy. Epilepsia, 50(5), 1059–1066. doi: 10.1111/j.1528-1167.2008.01938.x [DOI] [PubMed] [Google Scholar]
- Langfitt JT, Westerveld M, Hamberger MJ, Walczak TS, Cicchetti DV, Berg AT, … Spencer SS (2007). Worsening of quality of life after epilepsy surgery: effect of seizures and memory decline. Neurology, 68(23), 1988–1994. doi: 10.1212/01.wnl.0000264000.11511.30 [DOI] [PubMed] [Google Scholar]
- Lehrner J, Kalchmayr R, Serles W, Olbrich A, Pataraia E, Aull S, … Baumgartner C (1999). Health-related quality of life (HRQOL), activity of daily living (ADL) and depressive mood disorder in temporal lobe epilepsy patients. Seizure, 8(2), 88–92. doi: 10.1053/seiz.1999.0272 [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, & Lee GP (2004). Determinants of quality of life in epilepsy. Epilepsy Behav, 5(6), 976–980. doi: 10.1016/j.yebeh.2004.08.019 [DOI] [PubMed] [Google Scholar]
- Love CE, Webbe F, Kim G, Lee KH, Westerveld M, & Salinas CM (2016). The role of executive functioning in quality of life in pediatric intractable epilepsy. Epilepsy Behav, 64(Pt A), 37–43. doi: 10.1016/j.yebeh.2016.08.018 [DOI] [PubMed] [Google Scholar]
- McLachlan RS, Rose KJ, Derry PA, Bonnar C, Blume WT, & Girvin JP (1997). Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol, 41(4), 482–489. doi: 10.1002/ana.410410411 [DOI] [PubMed] [Google Scholar]
- Moran JM (2013). Lifespan development: the effects of typical aging on theory of mind. Behav Brain Res, 237, 32–40. doi: 10.1016/j.bbr.2012.09.020 [DOI] [PubMed] [Google Scholar]
- Noll KR, Bradshaw ME, Weinberg JS, & Wefel JS (2018). Neurocognitive functioning is associated with functional independence in newly diagnosed patients with temporal lobe glioma. Neurooncol Pract, 5(3), 184–193. doi: 10.1093/nop/npx028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjuela-Rojas JM, Martinez-Juarez IE, Ruiz-Chow A, & Crail-Melendez D (2015). Treatment of depression in patients with temporal lobe epilepsy: A pilot study of cognitive behavioral therapy vs. selective serotonin reuptake inhibitors. Epilepsy Behav, 51, 176–181. doi: 10.1016/j.yebeh.2015.07.033 [DOI] [PubMed] [Google Scholar]
- Pauli C, Schwarzbold ML, Diaz AP, de Oliveira Thais MER, Kondageski C, Linhares MN, … Walz R (2017). Predictors of meaningful improvement in quality of life after temporal lobe epilepsy surgery: A prospective study. Epilepsia, 58(5), 755–763. doi: 10.1111/epi.13721 [DOI] [PubMed] [Google Scholar]
- Perna R, Loughan AR, & Talka K (2012). Executive functioning and adaptive living skills after acquired brain injury. Appl Neuropsychol Adult, 19(4), 263–271. doi: 10.1080/09084282.2012.670147 [DOI] [PubMed] [Google Scholar]
- Perrine K, Hermann BP, Meador KJ, Vickrey BG, Cramer JA, Hays RD, & Devinsky O (1995). The relationship of neuropsychological functioning to quality of life in epilepsy. Arch Neurol, 52(10), 997–1003. [DOI] [PubMed] [Google Scholar]
- Pirio Richardson S, Farias ST, Lima AR 3rd, & Alsaadi TM (2004). Improvement in seizure control and quality of life in medically refractory epilepsy patients converted from polypharmacy to monotherapy. Epilepsy Behav, 5(3), 343–347. doi: 10.1016/j.yebeh.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Quiske A, Helmstaedter C, Lux S, & Elger CE (2000). Depression in patients with temporal lobe epilepsy is related to mesial temporal sclerosis. Epilepsy Res, 39(2), 121–125. [DOI] [PubMed] [Google Scholar]
- Ragland DR, Satariano WA, & MacLeod KE (2005). Driving cessation and increased depressive symptoms. J Gerontol A Biol Sci Med Sci, 60(3), 399–403. [DOI] [PubMed] [Google Scholar]
- Reitan RM (1979). Manual for administration of neuropsychological test batteries for adults and children. Tucson, AZ: Neuropsychology Laboratory. [Google Scholar]
- Saling MM (2009). Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain, 132(Pt 3), 570–582. doi: 10.1093/brain/awp012 [DOI] [PubMed] [Google Scholar]
- Schraegle WA, & Titus JB (2016). Executive function and health-related quality of life in pediatric epilepsy. Epilepsy Behav, 62, 20–26. doi: 10.1016/j.yebeh.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Seiam AH, Dhaliwal H, & Wiebe S (2011). Determinants of quality of life after epilepsy surgery: systematic review and evidence summary. Epilepsy Behav, 21(4), 441–445. doi: 10.1016/j.yebeh.2011.05.005 [DOI] [PubMed] [Google Scholar]
- Sherman EM, Slick DJ, & Eyrl KL (2006). Executive dysfunction is a significant predictor of poor quality of life in children with epilepsy. Epilepsia, 47(11), 1936–1942. doi: 10.1111/j.1528-1167.2006.00816.x [DOI] [PubMed] [Google Scholar]
- Sherman EM, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, … Jette N (2011). Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia, 52(5), 857–869. doi: 10.1111/j.15281167.2011.03022.x [DOI] [PubMed] [Google Scholar]
- Stretton J, & Thompson PJ (2012). Frontal lobe function in temporal lobe epilepsy. Epilepsy Res, 98(1), 1–13. doi: 10.1016/j.eplepsyres.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm JW, Donnan GA, Dewey HM, Macdonell RA, Gilligan AK, Srikanth V, & Thrift AG (2004). Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke, 35(10), 2340–2345. doi: 10.1161/01.STR.0000141977.18520.3b [DOI] [PubMed] [Google Scholar]
- Taylor RS, Sander JW, Taylor RJ, & Baker GA (2011). Predictors of health-related quality of life and costs in adults with epilepsy: a systematic review. Epilepsia, 52(12), 2168–2180. doi: 10.1111/j.1528-1167.2011.03213.x [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, & Imai K. (2014). Mediation: R package for causal mediation analysis.
- Verma SK, Luo N, Subramaniam M, Sum CF, Stahl D, Liow PH, & Chong SA (2010). Impact of depression on health related quality of life in patients with diabetes. Ann Acad Med Singapore, 39(12), 913–917. [PubMed] [Google Scholar]
- Wechsler D (1997). WMS-III: Wechsler memory scale administration and scoring manual. : Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.