Summary
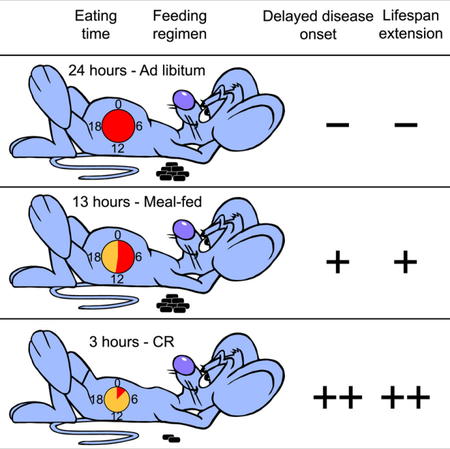
The importance of dietary composition and feeding patterns in aging remains largely unexplored, but was implicated recently in two prominent nonhuman primate studies. Here, we directly compare in mice the two diets used in the primate studies focusing on three paradigms: ad libitum (AL), 30% calorie restriction (CR), and a single meal feeding (MF) that accounts for differences in energy density and caloric intake consumed by the AL mice. MF and CR regimes enhanced longevity regardless of diet composition, which alone had no significant impact within feeding regimens. Like CR animals, MF mice ate quickly, imposing periods of extended daily fasting on themselves that produced significant improvements in morbidity and mortality compared to AL. These health and survival benefits conferred by periods of extended daily fasting, independent of dietary composition, have major implications for human health and clinical applicability.
Graphical Abstract
In Brief
Mitchell et al. show that a long daily period of fasting improves the health and survival of male mice, regardless of caloric intake, diet composition, and bodyweight.
INTRODUCTION
Calorie restriction (CR) interventions, either as a reduction of the daily caloric intake or intermittent fasting (cyclic periods of food deprivation), have been shown to mitigate age-associated declines in most pathophysiological parameters and to extend maximum lifespan in various animal species (de Cabo et al., 2014; Longo and Panda, 2016; Mattson et al., 2014). In every form of CR, prolonged fasting periods represent a consistent, but frequently overlooked, variable in energy homeostasis. Most mammals have the ability to adapt to short-term loss of energy intake without a substantial impact on vitality (individuals with impaired glycemic control or impaired lipid mobilization are exceptions). Alternating cycles of feeding and fasting trigger specific biochemical processes mediated, at least in part, by nutrient-sensing pathways (Minor et al., 2010). The length of post-absorptive periods, however, may well play an important role in the association between feeding regimens and longevity (Soty et al., 2017). These periods of fasting evoke cellular maintenance and repair mechanisms that are intrinsic to the fast and re-feed cycles (Longo and Panda, 2016; Mattson et al., 2014). Nearly every form of CR results in meal feeding, either because a reduced number of quickly-consumed calories are provided in discrete meals or because days spent without food are interspersed with days in which food is provided, almost certainly triggering fasting mechanisms. In contrast, post-absorptive periods are likely to be absent in an AL feeding paradigm. In this way, two components must be considered in the comparison of CR to AL: first, the reduction in total calorie intake and, second, the introduction of periodic fasting. This raises the possibility that the benefits of restricting the timing of food intake are separable from those associated with overt caloric intake reduction. Thus, CR-associated mechanisms are likely relevant to human health and longevity (Longo and Panda, 2016; Manoogian and Panda, 2017; Mattson et al., 2014), and meal strategies mimicking the daily fasting periods that occur with CR might be readily translated to humans if clear health benefits are observed due to such periods of fasting.
CR experiments have been conducted in nonhuman primates (NHPs) including studies at the University of Wisconsin (Colman et al., 2009) and at the National Institute on Aging (NIA) (Mattison et al., 2012). A recent comparative analysis of these studies indicated that CR and its associated mechanisms are likely to be highly relevant to human health and longevity (Mattison et al., 2017). One of the most debated differences between the studies relates to dietary composition. NIA used a naturally sourced diet while WIS used a semi-purified diet, resulting in differences in the macronutrient content of the diets (Table S1). At both locations, the diets contained ∼60% carbohydrates by weight; however, compared to the WIS diet, the NIA diet was higher in protein (17.3 vs 13.1% by weight), slightly higher in fiber (6.5–9.0 vs 5.0% by weight), lower in fat (5.0 vs 10.6% by weight), and lower in sucrose (3.9 vs 28.5% by weight). Sucrose is a disaccharide of fructose and glucose that has been part of the human diet for centuries. Excessive consumption of sucrose has been linked to metabolic disorders beyond just weight gain, where elevated fructose in the presence of glucose favors lipogenesis in the liver, leading to fatty liver and associated pathologies (Kolderup and Svihus, 2015). To shed light on the interaction between diet composition and feeding paradigms, the impact of these variables on health and longevity were tested in a genetically homogeneous mouse model. We hypothesized that consumption of the NIA naturally sourced NHP diet would confer survival advantages over the WIS-purified NHP diet. Moreover, we investigated the effect of eating patterns on morbidity and mortality regardless of diet composition in the form of 30% CR and a daily single meal feeding (MF) isocaloric to that of ad lib (AL)-fed mice. The results outlined below show that the relationship between diet and healthy lifespan is influenced by the timing of food intake, an outcome that could have major implications for human health and clinical applicability.
RESULTS AND DISCUSSION
A cohort of 292 male C57BL/6J (B6) mice was established, and at 4 months of age the mice were randomly divided into the two diet groups and maintained until their natural death. Mice were singly housed and fed either the low sucrose NIA NHP diet or the sucrose-richWIS NHP diet that were repelleted for mouse consumption. Lights were turned on at 6:30 AM and turned off at 6:30 PM in the vivarium. All groups of mice were fed at 3:00 PM (± 1 h) every day. Detailed descriptions of the study design (e.g., diet composition, intake measurement, feeding protocols, etc.) can be found in the Star Methods section. Briefly, groups of AL (n=90) and CR (n=121; 30% reduction) were created, and to account for differences in energy density between the two diets, two additional groups of meal-fed (MF) mice (n=81) were introduced as controls. MF mice were fed once daily either NIA or WIS diet proportioned to match the caloric intake of the other diet AL group. This strategy was used to accommodate differences in calorie density between the diets and to separate longevity effects due to lower caloric intake from those due to the once daily feeding paradigm of the CR mice. The MF mice learned quickly that food would not be continuously available, and thus, tended to gorge, spending hours each day without food. This behavior has been associated with ‘hyperactivation’ of sweet-taste and fat receptors on enteroendocrine cells in the upper gastrointestinal tract (Jang et al., 2007; Janssen and Depoortere, 2013).
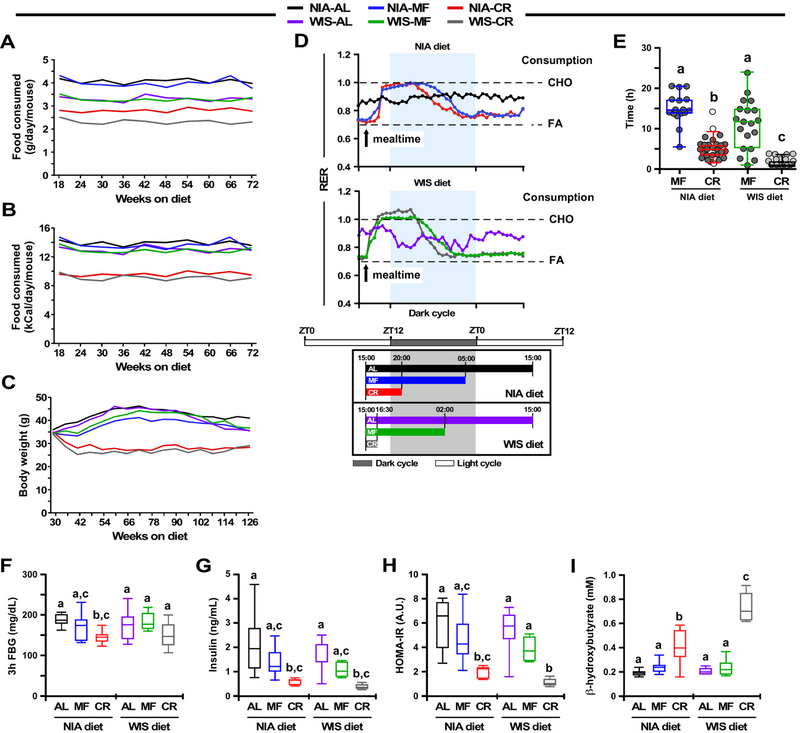
Daily food intake data under all experimental conditions were collected over a period of 54 weeks (Fig. 1A and 1B, Table 1). Overall, mice on the NIA diet consumed significantly more grams of food than those on the WIS diet, irrespective of the feeding protocol (AL, 4.1 ± 0.2 vs. 3.3 ± 0.2 g/day/mouse; MF, 3.9 ± 0.3 vs. 3.3 ± 0.2 g/day/mouse; CR, 2.8 ± 0.1 vs. 2.3 ± 0.2 g/day/mouse) (Fig. 1A). However, when the amount of food eaten was normalized by the energy content of each diet, AL and MF mice were found to consume approximately the same number of calories each day (NIA-AL, 14.0 ± 0.7; NIA-MF, 13.2 ± 1; WIS-AL, 13.1 ± 0.7; WIS-MF, 12.9 ± 0.7 kCal/day/mouse) (Fig. 1B). Although the NIA-AL group ate slightly more calories per day than any other group (p <0.002), the WIS-AL, WIS-MF, and NIA-MF mice were not different in their caloric intake. Both groups of CR mice had ~30% reduction in calorie intake compared to their AL controls, with intake equivalent for NIA and WIS animals (9.5 ± 0.5 and 9.2 ± 0.8 kcal/day/mouse, respectively) (p=0.15) (Fig. 1B). The longitudinal bodyweight trajectory was evaluated in all six groups of mice over a period of 96 weeks (Fig. 1C). The bodyweight gain trajectory of NIA-MF mice was significantly lower compared to NIA-AL controls (p=3.48E-05), while no significant difference was observed between WIS-MF and WIS-AL mice (p=0.55) (Fig. S1, upper panels). Bodyweight trajectories between diet types within each of the three feeding protocols (AL, MF and CR) were not significantly different (Fig. S1, lower panels). Mice on 30% CR weighed considerably less over the same period, regardless of diet (Fig. 1C and Fig. S1).
Figure 1. Effects of diet composition, energy density, and feeding patterns on bodyweight and metabolism.
(A-B) Trajectories of daily food intake per mouse over a period of 54 weeks expressed as grams (A) and kCal (B). N=45 mice (NIA-AL), n=44 (WIS-AL), n=40 (NIA-MF), n=41 (WIS-MF), n=59 (NIA-CR), n=62 (WIS-CR). See also Table S1.
(C) Bodyweight trajectories over a period of 96 weeks. See also Figure S1.
(D) Upper two panels, Respiratory Exchange Ratio (RER) at 41–42 weeks of age, n= 5–6. Bottom panel, Representative schematic of the study showing the estimated fasting time for each of the two diets, and three feeding paradigms across the study with respect to the Zeitgeber time, which is defined as any external clue, such as the 12:12 light:dark cycle, that synchronizes common biological rhythms in an organism. This schematic was inferred using experimental evidence from panel E. AL mice had constant access to food and so were not subjected to a daily fasting time. Arrow, start of the feeding (15:00); CHO, carbohydrate; FA, fatty acids. See also Figure S2.
(E) Scatter plot depicting the duration of eating in MF and CR mice at 113 weeks of age. The duration of eating (in hours) is expressed as the median and [5–95% percentile]: NIA-MF, 14.63 [5.5–20.5]; NIA-CR, 4.875 [1.61–9.27]; WIS-MF, 11.75 [1–24]; and WIS-CR, 1 [0.5–3.57]. Every data point represents the average of two independent determinations for each mouse. Because the NIA-CR and WIS-CR datasets did not pass the Shapiro-Wilk normality test, the non-parametric Kruskal-Wallis test combined with Dunn’s multiple comparisons test was performed to establish statistical significance between groups. Variables with different letters are considered significantly different at P < 0.01.
(F) Three hour fasting blood glucose (FBG) levels in 41-week-old mice, n= 7–8 mice per cohort.
(G-I) Blood was collected from 42 week-old mice after an overnight (16 h) fasting, n=6–8/group.
(G) Serum insulin.
(H) HOMA-IR index.
(I) Serum β-hydroxybutyrate.
Data are represented as whisker plots (F-I), and analyzed using two-way ANOVA as a function of diet type (NIA and WIS diet) and feeding regimen (AL, MF, CR) with Tukey’s post hoc analysis. Variables with different letters are considered significantly different at P < 0.05. See also Table S2.
Table 1.
Significance in food intake differences between the indicated pairwise comparisons.
| Feeding paradigm | Diet composition | Caloric content | ||||
|---|---|---|---|---|---|---|
| NIA vs. WIS | NIA-AL | WIS-AL | WIS-AL | |||
| AL | 1.9E-08 | NIA-MF | 0.0015 | ------- | NIA-MF | 0.22 |
| MF | 0.33 | NIA-CR | 1.16E-33 | ------- | WIS-MF | 0.87 |
| CR | 0.15 | WIS-MF | -------- | 0.87 | ||
| WIS-CR | -------- | 2.20E-26 | ||||
AL, ad libitum feeding; MF, meal-fed; CR, 30% calorie restriction
Using a Comprehensive Lab Animal Monitoring System, we examined whether the diet type and feeding protocols were associated with differences in in vivo metabolism in 10-month old mice (Fig. 1D, upper panel, Fig. S2). AL mice had access to food 24/7 while MF and CR mice were fed daily at 3:00 PM (Fig. 1D, lower panel). For AL-fed mice, fluctuation of the respiratory exchange ratio (RER), calculated from the amount of carbon dioxide produced (VCO2) and oxygen consumed (VO2), was minimal between the light and dark cycles, irrespective of the diet composition (Fig. 1D upper and midde panels, Fig. S2). MF and CR mice on either diet exhibited large RER fluctuations (max ~1.0) upon feeding, consistent with metabolic flexibility and preferential use of carbohydrates in the fed state before slowly returning to fatty acid oxidation as primary fuel source post-prandially (RER ~0.75) (Fig. 1D, upper panel). Larger amplitudes and shorter duration of postprandial carbohydrate fuel utilization were present in WIS vs. NIA-fed mice, as might be expected considering the differences in diet composition, i.e., the sucrose-rich WIS diet that is readily absorbed and metabolized in contrast to the NIA diet composed of complex carbohydrates that, in principle, requires time to be processed and metabolized (Fig. S2).
The duration of eating varied dramatically based on diet type and feeding protocol, thus resulting in differences in length of the fasting period. A consequence of the once-daily isocaloric MF paradigm was that NIA-MF mice took ~15 h to consume their daily allotment of food while WIS-MF mice needed only ~12 h (p< 0.0001, Fig. 1D and 1E), leaving them to fast for the rest of the day. CR mice on NIA and WIS diet completed their meals within ~5 and 1 h, respectively, resulting in prolonged fasting for both CR groups (p< 0.0001, Fig. 1D and 1E).
Together, the data show that unlike in mice under AL, the MF and CR groups had extended periods of daily fasting associated with high amplitude daily rhythms in RER (~0.75 up to ~1.0) consistent with high metabolic flexibility.
To investigage the role of diet composition and feeding protocols on insulin sensitivity, circulating glucose and insulin, and the homeostatic measure of insulin resistance (HOMA-IR) were determined in fasted animals after 6 months on their respective diets. The day before blood collection, animals were fed as usual at 3:00 PM and allowed to eat for ~2 h before fasting overnight. Bleeds were then started at 9:00–10:00 AM. CR-fed mice exhibited a significant improvement in insulin sensitivity in terms of fasting glucose and insulin levels and the HOMA-IR measurement (Fig. 1F–1H) independent of diet composition, consistent with prior reports (Solon-Biet et al, 2015). In contrast, no significant differences were observed for any glucoregulatory parameters between the AL and MF mice on either diet. CR feeding was associated with a significant increase in circulating β-hydroxybutyrate (Fig. 1I), consistent with prior studies (Anson et al., 2003; Mitchell et al., 2016), but not in MF or AL where β-hydroxybutyrate levels remained unchanged.
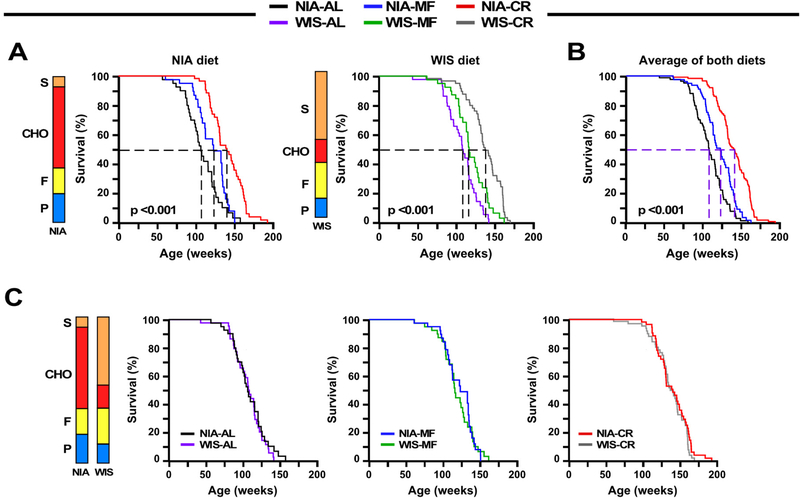
The impact of diet composition and feeding protocol on median and maximum lifespan was assessed (Fig. 2A-C, Tables S3-S5). Kaplan-Meier survival analyses were performed to determine the effects of the feeding protocol within each diet [NIA, n=45 (AL); 40 (MF); and 59 (CR) and WIS, n=45 (AL); 41 (MF); and 62 (CR)]. Survival curves were significantly different among feeding paradigms by the log-rank test for mice on NIA diet (χ2 = 45.5, P <0.001) and WIS diet (χ2 = 45.2, P <0.001) (Fig. 2A). A multiple comparison procedure (Holm-Sidak method) was used to compare longevity effects of feeding paradigms independent of diet type. As in the individual diet analysis, all comparisons were statistically significant (χ2 = 85.83, P<0.001) (Tables S3-S5), with mean lifespan extensions of 11% and 28% by MF and CR, respectively. Our original hypothesis was that diet composition would impact longevity; however, lack of differences in metabolic indices between diets for each of the feeding paradigms suggested that the outcomes might be otherwise. When survival was analyzed by diet type there were no significant differences between curves for AL, MF or CR, indicating that diet composition had no impact on survival (Fig. 2C) despite the differences in diet composition and caloric content. The results of this study reveal that eating pattern rather than diet composition is a primary determinant in longevity regulation. Although unexplored in this study, it is important to emphasize that macronutrient composition has been reported to play a role in late life health and lifespan under AL feeding conditions (Solon-Biet et al., 2014).
Figure 2. Implications of diet composition and feeding patterns on survival, tumor and nontumor pathologies.
(A) Kaplan-Meier survival curves for mice fed either NIA diet (left panel) or WIS diet (middle panel) ad libitum (AL), meal-fed (MF), or maintained on 30% calorie restriction (CR).
(B) Survival curves when the two diet groups were combined.
(C) Kaplan-Meier survival curves for mice on NIA or WIS diet fed either AL (left panel), MF (middle panel), or 30% CR (right panel).
(A-C) N = 44–45 mice each for AL, n=40–41 mice for MF, and n=59–62 mice for CR. Stacked bars depict the relative composition of the NIA and WIS diets, expressed as % kcal, P=protein; F=fat; CHO, carbohydrates other than sucrose (S). See also Tables S1 and S3-S5.
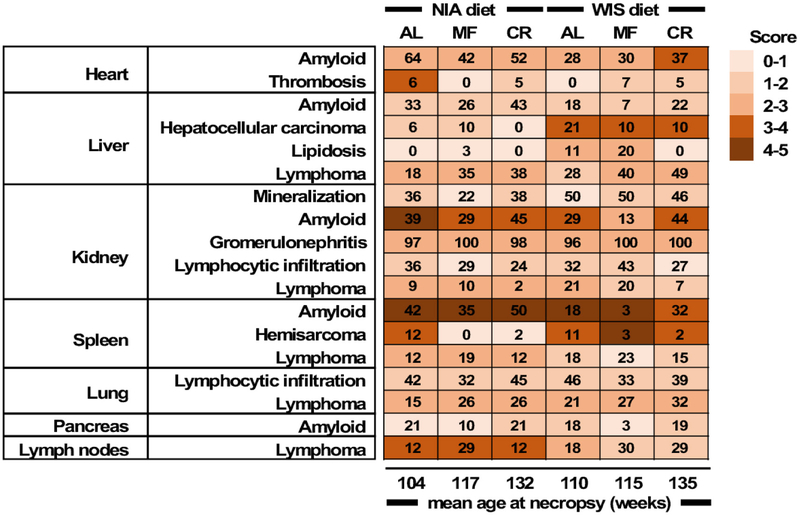
The distribution of pathologic lesions in mice that died spontaneously was examined by board certified veterinary pathologists (Table S6 and Fig. 3). AL mice died 25–28 weeks earlier than mice fed under CR, respectively. Similarly, tissues of MF mice were collected 13–15 weeks later than AL controls. As indicated here, CR and MF mice exhibited the same pathologies at death as the AL groups, but at a significantly later date. Heart, liver, kidneys, spleen, lungs and pancreas were examined. The major non-neoplastic lesion was amyloidosis, with greater incidence in the percent rate of occurrence for mice fed the NIA diet. Among the feeding paradigms, mice under MF saw a reduction in the rate of amyloidosis deposits while mice under CR had the highest occurrence of the lesion in most tissues, likely stemming from the longer lifespan of CR mice as compared with the AL controls (132–135 weeks vs. 104–110 weeks, respectively). Mineralization and glomerulonephritis of the kidneys, total lymphoid nodules of various tissues and organs, and lymphocytic infiltration of the lungs and kidneys were commonly found regardless of the diet type or eating pattern. MF and 30% CR appear to reduce and/or delay the rate of occurrence of amyloidosis deposits and other age-related pathologies at death, as previously reported (Bronson and Lipman, 1991; Lipman et al., 1993). A greater incidence of spontaneous hepatocellular carcinoma and lipidosis in the liver of mice on the WIS vs. NIA diet was found. With aging, fatty liver and hepatocellular carcinoma are two of the most common lesions occurring in B6 male mice. The increased lipidosis in WIS-fed mice under MF vs. AL is somewhat reminiscent of the study of Mustonen et al. (2013), who reported that in rodents 18 h of fasting induced fatty liver possibly by promoting hepatic fat storage.
Figure 3. Heatmap of the average score of pathologies in various tissues and organs in each experimental group of mice.
The number of animals tallied and percent to total study mice were as followed: NIA-AL, 33(73.3%); NIA-MF, 31(77.5%); NIA-CR, 42(71.2%); WIS-AL, 28(63.6%); WIS-MF, 30(73.2%); WIS-CR, 41(66.1%). For a detailed breakdown of the histopathology data, see Table S6.
A new perspective of the relationship between diet, health, and longevity has emerged in recent years where the beneficial effects of dietary regimens, including CR, on metabolic health and survival may not be due to calories alone, but rather to a combination of total caloric intake, the length of fasting periods, and their interaction. In our study, we found that the fasting period experienced by the MF mice led to a significant increase in mean survival of 11–14% even in the absence of caloric restriction. An important observation in this study is that the difference in time spent eating (gorging vs. slower-paced intake) was dependent on diet composition and on amount of food provided, raising the possibility that time-limited food intake may also be contributing to longevity under other circumstances. The unanticipated prolonged daily fasting in MF mice was accompanied with modest increase in lifespan and delayed onset of pathologies even in the absence of differences in bodyweight or glucoregulatory responses compared to the AL-fed group and without ketosis. These observations are a departure from the CR-induced health benefits that are associated with weight loss, a reduction in HOMA-IR, and an increase in the levels of ketone bodies. One distinction between AL and MF was the daily rhythm in RER that was similarly responsive to MF and CR. A recent paper in which mice were fed a ketogenic diet did not show this daily rhythm despite having several health benefits (Roberts et al., 2017), implying that mechanisms underlying ketogenic and MF paradigms are different. Two recent studies indicated that mice on ketogenic diets exhibited either a weight gain and slight reduction in midlife mortality or a slight decrease in weight and increase in healthspan and lifespan (Newman et al., 2017; Roberts et al., 2017). Although ketogenic diets, when given in a paired feeding paradigm, extended lifespan, it is unclear whether periods of fasting may have been a contributing factor.
Even though the original NHP studies were designed to investigate CR not patterns of feeding behavior (Mattison et al., 2017, the differences in diet composition and feeding regimens between the two studies have been a point of interest. The UW NHPs had food removed overnight ensuring a period of fasting for both control and CR groups. Within the UW study the impact of CR on health and survival is not explained by differences in fasting. At NIA, NHPs had food available around the clock; however, it seems unlikely this played a role in survival since monkeys, like humans, sleep at night, and the NIA study included some of the longest lived monkeys on record. In light of our findings in mice, it would seem unlikely that differences in diet composition explain the impact of either study on survival. Apart from sheding new light on the NHP studies the current study has some important translational ramifications. Daily feeding-fasting schedules in humans that are synchronized with sleep-wake cycles have profound metabolic consequences which are highly relevant to the incidence of cancer. Recent studies in humans found that in patients with breast cancer, a fasting period greater than 13 h resulted in lower risk of breast cancer recurrence compared to those that fasted less than 13 h (Marinac et al., 2015; Marinac et al., 2016). Erratic feeding behaviors and associated lifestyles among adults, such as spreading caloric intake over 15 h or longer, can have detrimental health consequences (Gill and Panda, 2015; Gupta et al., 2017).
Data presented here suggests that extended periods of fasting, independent of diet composition or total caloric intake, might be an effective intervention to enhance healthspan and longevity and offers a tantalizing translational potential for fasting as a treatment of cardiometabolic diseases and age-related disorders (Roth et al., 2004). Aspects of the translational potential of time-restricted feeding and intermittent fasting became immensely popular among athletes during the 2000s, pointing that such paradigms had already gained recognition thus making studies on the effects of time-restricted feeding diets even more compelling. With aging, there is a progressive loss of the complex proteostasis network, characterized by an increased vulnerability of the proteome to misfolding and aggregation leading to cellular dysfunction (Kaushik and Cuervo, 2015). A number of disorders in human tissues result from amyloid deposition and other types of aggregrates, underscoring that failure to maintain protein homeostasis gives rise to disease (Chiti and Dobson, 2017) along with common age-associated disorders such as the systemic amyloidosis that worsens cardiac-related mortality in patients (Ablasser et al., 2018). Our survival analysis combined with histopathological data on tissues and organs from old B6 mice that spontaneously died has provided insights into their extent of systemic amyloidosis, with incidence consistent with previous studies (Solleveld et al., 1982). B6 mice are prone to senile amyloidosis (AApoAII) and secondary amyloidosis (Higuchi et al., 1991; Elliott-Bryant and Cathcart, 1997). Greater rate of amyloidosis occurrence was observed in NIA vs. WIS diet, irrespective of the feeding paradigm, and with increased incidence in CR than in MF, but this is likely due at least in part to the increased mean age of death. These side effects in mice should raise concern regarding the benefits of prolonged daily fasting periods for human healthspan. Observational studies relating dieting, long daily fasting periods (≥15 h) and breakfast skip with mortality and disease in humans have been reported (Sichieri et al., 1991; Williams et al., 1977; Yokoyama et al., 2016; Uzhova et al., 2017); however, some of these studies used rapid weight loss by extreme caloric restriction within a short amount of time and increased risk for gallstone among wormen. There is very little negative data associated with 12–13-hour time-restricted feeding, which is practiced by most long-lived population from around the globe (Longo and Panda, 2016).
Overall, our results highlight the importance of incorporating fasting time into a feeding protocol, as a potentially practical strategy to augment the beneficial effects attributed to CR. Increasing the fasting time may provide benefits similar to CR without the need to dramatically reduce the amount of calories, which ultimately may prove more attractive for clinical implementation. Time-restricted feeding has been reported to offer protection against diet-induced metabolic diseases, and these health benefits appear proportional to the length of the fasting period (Chaix et al., 2014). Dietary composition does seem to have an impact on indices of feeding behavior and liver health as evidenced by the tissue pathology. The gorging-like pattern of the MF and CR mice on the WIS diet, where mice ate their food quicker than those on the NIA diet, may be attributed to the increased sucrose and fat content relative to the NIA diet.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by Rafael de Cabo (decabora@mail.nih.gov).
EXPERIMENTAL MODEL DETAILS
Animals, Husbandry and Diets.
Male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) at 6 weeks of age. Mice were single housed in duplexes (Thoren, #15 Single Housed Duplexed Cage; Dimensions 22.2 × 30.8 × 16.24 cm; Thoren Caging Systems Inc., Hazeltown, PA) from arrival with autoclaved corncob bedding and a nestlet for enrichment at the NIA Biomedical Research Center (Baltimore, MD). Low velocity HEPA filtered air is supplied through sealed shelf plenums directly into the cage through air supply orifices above the cage filter top. From arrival until they started the study at 5mo of age, mice were fed a Harlan Teklad Global 18% Protein diet (Envigo, diet #2018S, Madison, WI). Throughout, water was provided ad libitum in individual water bottles for each duplex. Baltimore City tap water treated by reverse osmosis and then hyperchlorinated to 2–3 ppm was given. Cages were changed on a biweekly basis within a biological safety cabinet or change station, with spot changes as needed. Animal rooms were maintained at 22.2 ± 1 °C and 30–70% humidity. The lights were turned off at 6:00 PM and back on at 6:00 AM each day. In March and August 2014, regular sentinel testing revealed the presence of Aspiculuris tetraptera in the animal holding room. Due to the difficulty in validating the positive test, mice received up to nine weeks of MediGel® FBZ (ClearH20®, Westbrook, ME) as they were on special study diets, which precluded the use of fenbendazole feed. Environmental decontamination of the holding room and associated procedure rooms was performed and the mice on this study were moved to a separate quarantine room in December 2014 where they remained until the last study mouse died on 9/28/2016. With the exception of a single positive sentinel test in August 2015, all other tests were negative. Of note, mice in this study never tested positive.
Starting at 4 mo of age, mice were fed either an open source diet (NIA diet) low in sucrose and fat (7.6% energy from sucrose, 17.7% from fat, 3.418 kcal/g) or a sucrose-rich diet (WIS diet) composed of purified ingredients (46% energy from sucrose, 24.4% from fat, 3.927kcal/g) for the remainder of their lives. The proportion of sucrose was 3.9% and 28.5% per gram of food for the NIA and WIS diet, respectively (Table S1). Within each diet group (NIA or WIS), mice were divided into three feeding regimens: AL, 30% CR, and meal-fed (MF). The MF group takes into account the difference in energy densities between the NIA and WIS diets, with mice on MF-NIA diet consuming the same number of calories as the AL-WIS and MF-WIS diet groups. The AL-NIA group consumed the highest amount of calories of all groups. 30% CR was selected in order to replicate the targeted CR for the NHP studies. Both diets were repelleted for mouse chow. Food consumption and bodyweight were measured every two weeks and food allotments for CR and MF groups were adjusted accordingly. CR and MF mice underwent a stepdown procedure over two weeks in order to achieve their target daily allotments. At 14 mo of age (when bodyweight and average food consumption had plateaued), MF and CR mice had their food allotment fixed for the remainder of the study. MF and CR mice were given their food in a once daily allotment at 3:00 pm ± 1 hour. AL and MF mice received food in the hopper, CR mice were fed on the floor of the cage. All animal protocols were approved by the Animal Care and Use Committee (352-TGB-2018) of the National Institute on Aging, National Institutes of Health.
Survival Study.
Animals were inspected twice daily for health issues, and death was recorded for each animal. Moribund animals were euthanized and every animal found dead or euthanized was necropsied. The criteria for euthanasia was based on an independent assessment by a veterinarian according to AAALAC guidelines and only cases, where the condition of the animal was considered incompatible with continued survival, are represented as deaths in the curves. Animals removed at sacrifice (n=9 CR per diet group; n=6 AL per diet group; n=8 MF per diet group) were considered as censored deaths. Besides the animals that were sacrificed to collect tissues and other samples, only one NIA-AL, two NIA-and WIS CR and one NIA MF mice were euthanized due to accidental death during a procedure. These mice were censored from the analysis. Tumor burden for each mouse was calculated as a sum of the number of tumors observed in all of the mouse tissues scored. If a mouse did not have a tumor in a particular tissue it was given a score of 0 for that tissue. Average tumor burden was calculated as the mean of each individual score for a particular experimental group.
METHOD DETAILS
Metabolic assessment.
Mouse metabolic rate was assessed by indirect calorimetry in open-circuit Oxymax chambers with CLAMS (Columbus Instruments) as previously described (Mitchell et al., 2018). In brief, mice were housed singly with water and food (WIS/NIA diet under AL, MF or CR feeding regimen) and maintained at ~24°C under a 12:12-h light-dark cycle (light period 0600–1800). All mice were acclimatized to monitoring cages for 3–6 h before recording began. Sample air was passed through an oxygen (O2) sensor for determination of O2 content. O2 consumption was determined by measuring oxygen concentration in air entering the chamber compared with air leaving the chamber. The sensor was calibrated against a standard gas mix containing defined quantities of O2, carbon dioxide (CO2), and nitrogen (N2). Constant airflow (0.6 L/min) was drawn through the chamber and monitored by a mass-sensitive flow meter. The concentrations of O2 and CO2 were monitored at the inlet and outlet of the sealed chambers to calculate oxygen consumption. Each chamber was measured for 30 s at 30-min intervals and data were recorded for 60 h total. Movement (both horizontal and vertical) was also monitored with beams that software transforms into counts of beam breaks by the mouse. Mice were 42 weeks of age, n = 5–6 per group.
Glucose, Insulin, and 3-Hydroxybutyrate Determination and HOMA Calculation.
Mice were fasted for 3 h (from 6:00 AM-9:00 AM) for the measure of fasting blood glucose (FBG) in whole blood using the Bayer Breeze2 handheld glucometer (Bayer, Mishawaka, IN). For the measure of insulin and 3-hydroxybutyrate levels, mice were fasted overnight for 16h as followed: The day before blood collection, animals were fed at 3:00 PM and allowed to eat for 2 h after which they fasted overnight. Bleeds started at 9:00–10:00 AM in the morning. Serum insulin was measured using a mouse ultra-sensitive enzyme-linked immunosorbent assay (Catalog #90080; Crystal Chem, Inc., Downers Grove, IL) while the 3-hydroxybutyrate levels were determined with a commercially available kit (Catalog #700190; Cayman Chemicals, Ann Arbor, MI) according to the manufacturer’s instructions. Insulin resistance was calculated from fasted glucose and insulin values using the HOMA2 Calculator software available from the Oxford Centre for Diabetes, Endocrinology and Metabolism, Diabetes Trials Unit website (www.dtu.ox.ac.uk). mice were 41–42 weeks of age, n=6–8 per group.
Time Needed to Eat their Food Allotment (MF and CR groups).
Mice were fed their usual daily allotment of food at the regular time and monitored every hour until they consumed their meal. Time taken to eat a meal was the number of hours that it took for each individual mouse to consume its meal. This experiment was repeated twice on two consecutive weeks to ensure consistency between recordings. Data are represented as an average of the two recordings (n=17 NIA-MF; n=49 NIA-CR; n=18 WIS-MF; n=44 WIS-CR; 113 weeks of age).
Necropsy and Histopathology at Death.
All mice that died as part of the study were subject to gross histopathological analysis (n=28–47 per study group) and organs were collected and fixed in 4% paraformaldehyde for further analysis. Within these tissues, those that were excised and fixed within 24 h of death were selected for further histopathology. These tissues were embedded in paraffin and stained with hematoxylin and eosin according to standardized protocols by a commercial service (Histoserv, Germantown, MD). Each slide was scored by two board certified veterinary pathologists blinded to the treatment group (n=28–42 per study group). Tissues and organs included were heart, liver, kidney, spleen, lung, pancreas, and lymph nodes. Each criterion was given an arbitrary score where 0= no pathology present, and 5=presence of very severe pathology.
QUANTIFICATION AND STATISTICAL ANALYSIS
Mortality during the survival study was assessed using the log rank test to compare the differences in Kaplan-Meier survival curves. Maximal lifespan was defined as the 10th percentile of mice still alive. Comparisons between groups were performed using Student’s t-Test or two-way ANOVA with post-hoc tests as specified. Data are presented as mean ± SEM unless otherwise specified, with p value of ≤ 0.05 considered statistically significant. The statistical parameters (n, mean, SEM) can be found within the figure legends. No statistical method was used to determine whether the data met assumptions of the statistical approach. Analyses were performed using Graph Pad Prism 6.0 (San Diego, CA), Excel 2010 (Microsoft Corp., Redmond, WA), IBM SPSS Statistics (v24.0 Amonk, NY), and SigmaStat 3.0 (Aspire Software International, Ashburn, VA).
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Critical Commercial Assays | ||
| Mouse insulin ELISA | Crystal Chem, Inc. | 90080 |
| Beta-hydroxybutyrate colorimetric assay kit | Cayman Chemicals | 700190 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX 000664 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| Prism 6.0 | GraphPad | http://www.graphpad.com/scientific-software/prism; RRID:SCR_015807 |
| Excel 2010 | Microsoft Corp. | |
| SigmaStat 3.0 | Aspire Software Int. |
http://www.sigmaplot.com/products/sigmaplot/sigmaplot-details.php RRID:SCR_010285 |
| Other | ||
| Oxymax Open Circuit Indirect Calorimeters | Columbus Instruments | http://www.colinst.com/docs/OxymaxBrochure.pdf |
| Breeze2 Glucometer | Bayer | personalcare.manualsonline.com › … › Bayer HealthCare Blood Glucose Meter |
Limitations of the Study.
The basis for this work is an extension of the NHP Aging and CR studies that were carried out at NIA and the University of Wisconsin. Here, we show that the effects of MF were similar on the two diets tested and were not due to reduced caloric intake. MF animals given one meal a day were metabolically healthier and lived significantly longer than AL mice, even though they were consuming a similar amount of calories regardless of diet composition. The lack of survival advantage in the dilution-based reduction in calorie intake under AL feeding (Solon-Biet et al., 2014) could have been offset by the large bulk of non-digestable food consumed. The modest, but significant extension of longevity in MF animals, associated with delayed onset of fatty liver disease and hepatocellular carcinoma, re-opens the issue of the relative importance of reduced caloric intake per se in the beneficial effects of CR as opposed to periods of fasting that occur as a result of lower food intake (Acosta-Rodriguez et al., 2017; Masoro, 2004). This work extends previous important findings from our laboratory (Anson et al., 2003) and others (Chaix et al., 2014; Xie et al., 2017). Because most humans already have about a 8 to 12h night fast built into their day, a longer daily fasting period may be required to confer health and survival benefits.
A potential limitation of our study is that it was carried out in male mice only, and further studies should be performed in the future to evaluate the response in other animal species and female mammals. Morever, the effect of the length of fasting time on various health parameters should be more rigorously examined. Nevertheless, given the multiple links between fasting periods and disease (e.g., obesity, cardiometabolic syndrome, cancer) and, ultimately, longevity, we propose that periods of daily fasting may be as important as diet quality, and could be a viable means by which to promote health and survival.
Highlights.
The duration of eating/fasting varies based on diet type and feeding protocol
Meal feeding and CR, unlike AL, show high metabolic flexibility in male mice
Eating patterns rather than diet composition influences longevity regulation
A prolonged daily fasting is associated with delayed onset of liver pathologies
ACKNOWLEDGMENTS
We are grateful to the Comparative Medicine Section of the National Institute on Aging, NIH for their exceptional animal care and particularly highlight Dawn Nines, Dawn Boyer, Kristan Gavin and Kevin Jenkins for their assistance with the study. We thank Marissa Louis and Devin Wahl for their assistance with data entry. We thank Ed Tilmont at the NIA Nonhuman Primate Core for procuring food for the study. This work was supported by the Intramural Research Program of the National Institute on Aging, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION of INTERESTS
The authors declare no competing interests.
REFERENCES
- Ablasser K, Verheyen N, Glantschnig T, Agnetti G, and Rainer PP (2018). Unfolding Cardiac Amyloidosis - From Pathophysiology to Cure. Curr Med Chem doi: 10.2174/0929867325666180104153338. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, and Takahashi JS (2017). Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab 26, 267–277 e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, and Mattson MP (2003). Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A 100, 6216–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson RT and Lipman RD (1991). Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth Dev Aging 55, 169–184. [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, and Panda S (2014). Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, and Dobson CM (2017). Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu Rev Biochem 86, 27–68. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, and Madeo F (2014). The search for antiaging interventions: from elixirs to fasting regimens. Cell 157, 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott-Bryant R, and Cathcart ES (1997). Apolipoprotein E and apolipoprotein A-1 knock-out mice readily develop amyloid A protein amyloidosis. Clin Immunol Immunopathol 85, 104–108. [DOI] [PubMed] [Google Scholar]
- Gill S, and Panda S (2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta NJ, Kumar V, and Panda S (2017). A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS One 12, e0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K, Kitagawa K, Naiki H, Hanada K, Hosokawa M, and Takeda T (1991). Polymorphism of apolipoprotein A-II (apoA-II) among inbred strains of mice. Relationship between the molecular type of apoA-II and mouse senile amyloidosis. Biochem J 279, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. (2007). Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 104, 15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S, and Depoortere I (2013). Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol Metab 24, 92–100. [DOI] [PubMed] [Google Scholar]
- Kaushik S, and Cuervo AM (2015). Proteostasis and aging. Nat Med 21, 1406–1415. [DOI] [PubMed] [Google Scholar]
- Kolderup A, and Svihus B (2015). Fructose Metabolism and Relation to Atherosclerosis, Type 2 Diabetes, and Obesity. J Nutr Metab 2015, 823081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman RD, Gaillard ET, Harrison DE, and Bronson RT (1993). Husbandry factors and the prevalence of age-related amyloidosis in mice. Lab Anim Sci 43, 439–444. [PubMed] [Google Scholar]
- Longo VD, and Panda S (2016). Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab 23, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoogian ENC, and Panda S (2017). Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev 39, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E, and Patterson RE (2015). Prolonged Nightly Fasting and Breast Cancer Risk: Findings from NHANES (2009–2010). Cancer Epidemiol Biomarkers Prev 24, 783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinac CR, Nelson SH, Breen CI, Hartman SJ, Natarajan L, Pierce JP, Flatt SW, Sears DD, and Patterson RE (2016). Prolonged Nightly Fasting and Breast Cancer Prognosis. JAMA Oncol 2, 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ (2004). Caloric intake versus temporal pattern of food intake. Aging Clin Exp Res 16, 423–424. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, Cabo R.d., and Anderson RM (2017). Caloric restriction improves health and survival of rhesus monkeys. Nat Commun 8, 14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, et al. (2014). Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A 111, 16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Allard JS, Younts CM, Ward TM, and de Cabo R (2010). Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci 65, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, et al. (2016). Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab 23, 1093–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, Palacios HH, Ali A, Navas-Enamorado I, Di Francesco, et al. (2018). Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab 27, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen AM, Kärjä V, Kilpiö M, Tammi R, Tammi M, Rouvinen-Watt K, Halonen T, and Nieminen P (2013). Manifestations of fasting-induced fatty liver and rapid recovery from steatosis in voles fed lard or flaxseed oil lipids. Nutrients 5, 4211–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, and Verdin E (2017). Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab 26, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, et al. (2017). A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab 26, 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, and Ingram DK (2004). Aging in rhesus monkeys: relevance to human health interventions. Science 305, 1423–1426. [DOI] [PubMed] [Google Scholar]
- Sichieri R, Everhart JE, and Roth H (1991). A prospective study of hospitalization with gallstone disease among women: role of dietary factors, fasting period, and dieting. Am J Public Health 81, 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solleveld HA, van Zwieten MJ, Zurcher C, and Hollander CF (1982). A histopathological survey of aged Praomys (mastomys) natalensis. J Gerontol 37, 656–665. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, de Cabo R, Simpson SJ, and Le Couteur DG (2015). Dietary Protein to Carbohydrate Ratio and Caloric Restriction: Comparing Metabolic Outcomes in Mice. Cell Rep 11, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soty M, Gautier-Stein A, Rajas F, and Mithieux G (2017). Gut-Brain Glucose Signaling in Energy Homeostasis. Cell Metab 25, 1231–1242. [DOI] [PubMed] [Google Scholar]
- Uzhova I, Fuster V, Fernández-Ortiz A, Ordovás JM, Sanz J, Fernández-Friera L, López-Melgar B, Mendiguren JM, Ibáñez B, Bueno H, et al. (2017). The Importance of Breakfast in Atherosclerosis Disease: Insights From the PESA Study. J Am Coll Cardiol 70, 1833–1842. [DOI] [PubMed] [Google Scholar]
- Williams CN, Morse JW, MacDonald IA, Kotoor R, and Riding MD (1977). Increased lithogenicity of bile on fasting in normal subjects. Dig Dis Sci 22, 189–194. [DOI] [PubMed] [Google Scholar]
- Xie K, Neff F, Markert A, Rozman J, Aguilar-Pimentel JA, Amarie OV, Becker L, Brommage R, Garrett L, Henzel KS, et al. (2017). Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat Commun 8, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama Y, Onishi K, Hosoda T, Amano H, Otani S, Kurozawa Y, and Tamakoshi A (2016). Skipping Breakfast and Risk of Mortality from Cancer, Circulatory Diseases and All Causes: Findings from the Japan Collaborative Cohort Study. Yonago Acta Med 59, 55–60. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.