Abstract
Vasoactive intestinal peptide (VIP) has been shown to regulate corneal inflammation. Formyl peptide receptor 2 (FPR2) is a transmembrane protein belonging to the GPCR family. Ligands include pro-resolving lipids, lipoxin A4 (LXA4) and resolvin D1 (RvD1). The current study focuses on the effect of VIP regarding the FPR2 receptor axis in improving disease outcome in a mouse model of bacterial keratitis. Infection was induced in C57BL/6 (B6) mice using P. aeruginosa (PA) ATCC 19660. Mice received topical treatment (VIP or PBS) 3× daily after infection. Mean clinical scores, bacterial plate counts, Griess and myeloperoxidase (MPO) assays indicate that topical VIP effectively abrogates the disease response. Findings also reveal that VIP influences FPR2 pathway activation independent of archetypal VIP receptors. Exploring the immunoresolving role of FPR2, its ligand RvD1 and related enzymes (5-LOX, 12/15-LOX), our results suggest a mechanism by which VIP treatment influences the disease response in bacterial keratitis, which could offer a therapeutic point of intervention for enhancing this pro-resolving circuit.
Keywords: lipid mediators, ocular infection, inflammation, resolution, mouse
Introduction
Bacterial keratitis is a visually debilitating disease that compromises corneal structure and function, resulting in corneal scarring and perforation. Pseudomonas aeruginosa (P. aeruginosa/PA) is among the most common pathogen associated with bacterial keratitis [1]. The major risk factor is contact lens usage; but refractive corneal surgery, prior penetrating keratoplasty, contaminated water sources/lens care products, and immunocompromised patients increase overall risk, as well [2–4]. Pseudomonal infections of the cornea are characterized by a rapidly progressing infiltrate that extends into the stroma. It is often accompanied by purulent exudate, epithelial edema, stromal ulceration and necrosis. Secondary glaucoma and cataracts can also develop [5]. This damage is not solely due to the infectious pathogen, but also the deleterious side effects of the host response. Unfortunately, current treatments are directed toward the pathogen. For this reason, despite effective bacterial clearance with antibiotics, good visual outcome is not guaranteed. While topical corticosteroids are used to reduce the inflammatory response, there remains controversy regarding the benefits and risks associated with the use of immunosuppressive therapies [6, 7].
Effective treatments cannot merely reduce inflammation, but must activate pro-resolving circuits that are critical to restoring corneal structure and function. To this end, VIP has surfaced as an attractive therapeutic candidate for ocular infectious disease. As an endogenous neuropeptide, it is produced by parasympathetic corneal nerves [8]. It has also been shown to be expressed by immune cells, including neutrophils [9]. We have previously established VIP as an immunomodulator that can effectively prevent corneal perforation in P. aeruginosa-infected B6 mice via cytokine/chemokine modulation, innate immune cell function, and regulation of growth factors and adhesion molecules [10–12]. However, these studies were limited in clinical applicability in that VIP was administered as a prophylactic systemic treatment (intraperitoneal injection). The current study seeks to examine VIP as a topical treatment administered after infection. Furthermore, we investigate a mechanism of action whereby VIP influences specialized pro-resolving lipid mediator (SPM) pathways.
The importance of SPMs in stimulating the resolution phase of inflammation has been well established [13]. The main SPM pathways generate lipoxins, resolvins, and protectins, which are endogenous agonists biosynthesized via lipoxygenase (LOX) activity. Such enzymatic activity has been indicated in the cornea [13–16], with 15-LOX characterized as largely protective. This enzyme is fundamental for SPM biosynthesis [17] resulting in numerous pro-resolving actions, including modulation of cytokine/chemokine profiles [18, 19], reducing PMN infiltration, and enhancing efferocytosis by pro-resolving macrophages [20, 21]. In the current study, we link VIP to pro-resolving lipid mediator pathways in the eye using a murine model of Pseudomonas-induced corneal infection, thus expanding upon our understanding of VIP as an immunomodulator and enhancing its potential for therapeutic development.
Materials and Methods
Experimental animal protocol.
Female, 8-week-old C57BL/6 (B6) mice (The Jackson Laboratory, Bar Harbor, ME) had the left central cornea scarified as previously described [22]. In brief, following scarification a 5-μL aliquot containing 106 CFU/μL of Pseudomonas aeruginosa (PA) ATCC strain 19660 (Manassas, VA) was applied directly to corneal surface. All animals were treated in a manner authorized by Wayne State University Institutional Animal Care and Use committee (protocol 16-05-090) and conformed to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
VIP treatment.
Beginning at 4 hours post-infection (p.i.), mice were randomly assigned to a treatment group: experimental or control. The experimental group received 3× daily VIP treatment (5 nM in 5 μL) (Bachem, Torrance, CA, USA) administered as a topical eye drop until sacrifice. Control mice were similarly treated with sterile PBS. All mice were lightly anesthetized before each topical treatment.
Ocular response to bacterial infection.
Each day infected eyes were observed in a blinded fashion to assess disease response, which was graded using an established scale [23]. This four-point scale has been defined as follows: 0 = clear or slight opacity, partially or fully covering the pupil; +1 = slight opacity, fully covering the anterior segment; +2 = dense opacity, partially or fully covering the pupil; +3 = dense opacity, covering the entire anterior segment; and +4 = corneal perforation or phthisis. Clinical scores were calculated at 3 and 5 days p.i. for each group to express disease severity. Slit-lamp photography was used to visually document the disease response at the same time points following infection, as well.
Bacterial load.
Corneas from PBS and VIP treated mice were harvested at 3 and 5 days p.i. to quantitate bacterial load. Individual corneas were homogenized in sterile 0.9% saline containing 0.25% BSA. Serial dilutions (10-fold) of each sample was plated in triplicate on Pseudomonas isolation agar. After overnight incubation at 37°C, plates were examined for growth. Results are reported as CFU/cornea ± SEM.
Myeloperoxidase (MPO) assay.
An MPO assay was used to estimate PMN (neutrophil) numbers in infected corneas of PBS and VIP treated mice. Whole corneas were removed at 3 and 5 days p.i. and homogenized in 1.0 mL of 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethyl-ammonium (HTAB). Samples were freeze-thawed and after centrifugation, 100 μL of supernatant was added to 2.9 mL of 50 mM phosphate buffer containing o-dianisidine dihydrochloride (16.7 mg/100 mL) and hydrogen peroxide (0.0005%). Absorbency changes (460 nm) were monitored at 30 sec intervals for 5 min. The slope of the line was determined for each sample and used to calculate units of MPO/cornea, where one unit of MPO activity is equivalent to ~2×105 PMN [24].
Griess reaction.
Levels of nitric oxide were determined using a Griess reagent (1% sulfanilamide/0.1% naphthylethylene diamine dyhydrochloride 12.5% H3PO4) to measure its stable end product, nitrite. Corneas from each treatment group at 3 and 5 days p.i. were homogenized in 500 μL of de-gassed PBS and microcentrifuged at 3500 rpm for 5 min. Next, 100 μL of supernatant was added to an equal volume of Griess reagent in triplicate on a 96-well microtiter plate and incubated at room temperature for 15 min. Absorbance (570 nm) was measured and nitrite concentrations estimated using a standard curve of sodium nitrite. Results are represented as the mean μM of nitrite/cornea ± SEM.
Real-time RT-PCR.
Total RNA was isolated from individual whole corneas using RNA-STAT 60 (Tel-Test; Friendswood, TX, USA) according to the manufacturer’s recommendations and quantified by spectrophotometric determination (260 nm). Total RNA (100 ng) was reverse transcribed and used to produce a cDNA template. cDNA was amplified using SYBR® Green Master Mix according to the manufacturer. Each 10 μL reaction mixture contained: 5 μL 2× SYBR Green PCR Master Mix, 1 μL DEPC-water, 1 μL forward and reverse primers, 2 μL cDNA (diluted 1:10). All primers for the PCR reaction were designed using Primer3 PCR primer design software and semi-quantitative real-time RT-PCR was carried out using the CFX Connect Real-Time RT-PCR Detection System (BioRad, Hercules, CA). PCR amplification conditions were determined using established methods [25]. Relative transcript levels were calculated using the relative standard curve method comparing the amount of target (gene of interest) normalized to an endogenous reference (β-actin). Data are shown as the mean fold-change ± SD compared to basal levels of uninfected controls and represent at least three individual experiments.
Protein and lipid mediator analysis.
Infected corneas from each treatment group were excised at 3 and 5 days. Corneas were homogenized in 1 mL RIPA buffer (Cell Signaling Technology, Danvers, MA) with a protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA), sonicated to promote further lysis and centrifuged at 12,000 RPM for 20 minutes. For western blot analysis, supernatants were collected and normalized for equal amounts of protein, then separated on pre-cast tris-glycine gels (Invitrogen, Carlsbad, CA) and blotted onto a nitrocellulose membrane. After blocking in TBST (10mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA, membranes were treated with the following primary antibodies: 5-LOX, 15-LOX (Abcam, San Francisco, CA), FPR2 and β-actin (Santa Cruz, Santa Cruz, CA); then followed by incubation with secondary antibodies (Santa Cruz) labeled with horseradish peroxidase. Images were collected (Azure Biosystem C500, Dublin, CA) and target protein levels were quantified (Image Studio Lite software) after normalizing to β-actin. RvD1 (Cayman Chemical, Ann Arbor, MI) levels were selectively tested by ELISA. An aliquot of each supernatant was assayed in duplicate per the manufacturer’s instruction. In addition, FPR2 levels in PMN and macrophage cell lysates were analyzed by ELISA (MyBioSource Inc., San Diego, CA) and Western blot following the in vitro stimulation assay (details below). The reported sensitives of these ELISAs are: 3.3 pg/mL for RvD1 and <18.75 pg/mL for FPR2.
Cell isolation and culture.
PMN and macrophages isolated from B6 mice were harvested as previously described [26]. Regarding PMN isolation, mice received an i.p. injection of a 9% casein solution in 1.0 mL, followed by a second injection 24 h later. Peritoneal lavage was performed 3 h after the second injection. PMNs were collected with harvest solution (0.02% EDTA in 1× PBS), washed and isolated using a 90% Percoll gradient.
Macrophages were isolated using a single i.p. injection of 3% thioglycollate solution in 1.0 mL. Peritoneal lavage was carried out 5 days post injection and cells were isolated with harvest solution (DMEM + 5% FBS). For both cell isolations, viable cells (> 95%) were counted. After resuspension in culture media (PMNs: RPMI, 10% FBS/Macrophages: DMEM, 5% FBS), cells were seeded at a specific concentration described below for the stimulation assay.
In vitro stimulation assay.
B6 PMN and macrophages were seeded separately at a concentration of 1.5 × 106 cells/well. The cells were treated +/− select receptor antagonists, WRW4 (Tocris, Minneapolis, MN) and [D-p-Cl-Phe6,Leu17]-VIP (Leu) (Tocris) (specific for FPR2 and VIPR, respectively) for 24 hours prior to VIP treatment and PA stimulation. Cells were stimulated with PA 19660 (3.75 μL) at a concentration of 106 CFU/μL (ratio of 1 cell:2.5 bacteria) +/− VIP (5 nM). Cells were stimulated for 3h (37°C, 5% CO2) and treated with gentamicin (200 μg/mL) for an additional 15 minutes to kill any extracellular bacteria present. The cells were collected by centrifugation and lysed with 500 μL ice cold lysis buffer (Cell Signaling Technology, Danvers, MA, USA). Cell lysates were sonicated on ice to promote further lysis then centrifuged for 18 minutes at 12,000 RPM. Supernatants were transferred to new tubes and the protein concentrations were normalized prior to ELISA analysis.
Statistical analysis.
Samples sizes were determined statistically prior to experimentation. The difference in clinical score between two groups at each time point was tested by the Mann-Whitney U test (GraphPad Prism; San Diego, CA). For all other experiments, a one-way ANOVA followed by Bonferroni’s multiple comparison test (GraphPad Prism) was used for analysis of three or more groups. An unpaired Student’s t test was used for comparison between two groups. Data were considered significant at P ≤ 0.05. For all in vivo experiments, a minimum of n = 5 animals/group/time point were used. All experiments were carried out in triplicate and representative data from a typical experiment are shown unless otherwise noted.
Results and Discussion
Ocular response to topical VIP treatment
Given the encouraging findings of the systemic treatment [10–12, 27], we sought to investigate the therapeutic efficacy of VIP as a more clinically relevant topical treatment for bacterial keratitis. Furthermore, this treatment was initiated after infection instead of before as carried out in previous work. As represented by mean clinical scores shown in Figure 1, disease response was significantly improved at both time points after VIP treatment compared to PBS controls. Accompanying representative photographs taken by slit-lamp further demonstrate the observed responses. Dense opacity covering the entire anterior segment (+3) shown at 3 days p.i. progresses to corneal perforation (+4) by 5 days p.i. in PBS control mice. In contrast, VIP treatment culminates at 3 days p.i. with heavy, yet more limited opacity that does not extend throughout the peripheral cornea (+2/+3) and is maintained through 5 days after infection. This is of particular note given that corneal perforation was averted without the use of antibiotics.
Figure 1.
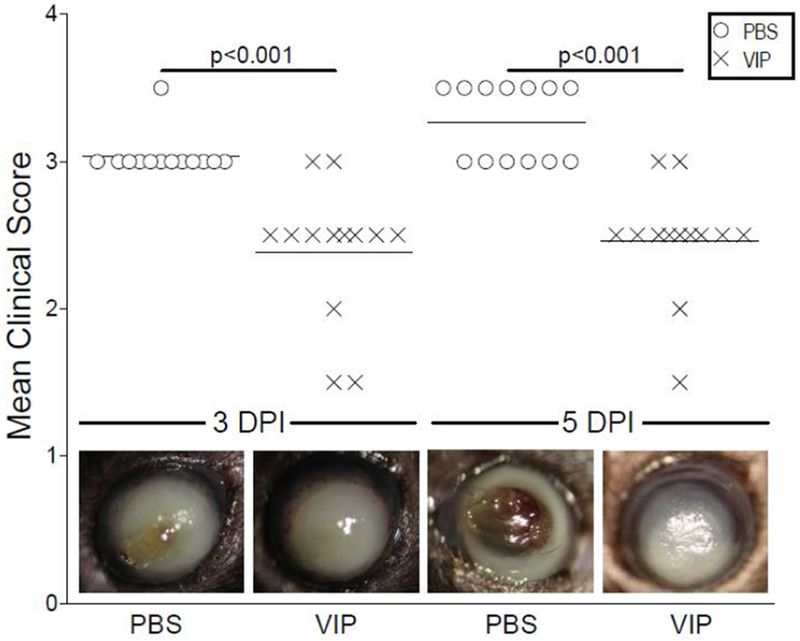
Ocular disease response of P. aeruginosa-infected B6 mice after topical treatment with PBS or VIP. Results are mean clinical scores ± SEM (n = 13 mice/group). Significant differences between the two treatment groups were observed at both 3 and 5 days p.i. Representative eyes from PBS- and VIP-treated mice were photographed at the same time points and illustrate the disease response visually. Magnification = ×35.
Host inflammatory response to topical VIP treatment
Bacterial load was assessed to determine whether topical VIP treatment enhanced bacterial killing. As shown in Figure 2A, direct plate counts at 3 and 5 days p.i. showed significant decreases in viable bacteria detected in corneas of VIP-compared to PBS-treated B6 mice. In fact, bacterial load increased over time in PBS controls and decreased with VIP treatment. These findings correlate with previous in vitro work from our lab and others indicating that VIP exerts bactericidal activity against PA [27, 28].
Figure 2.
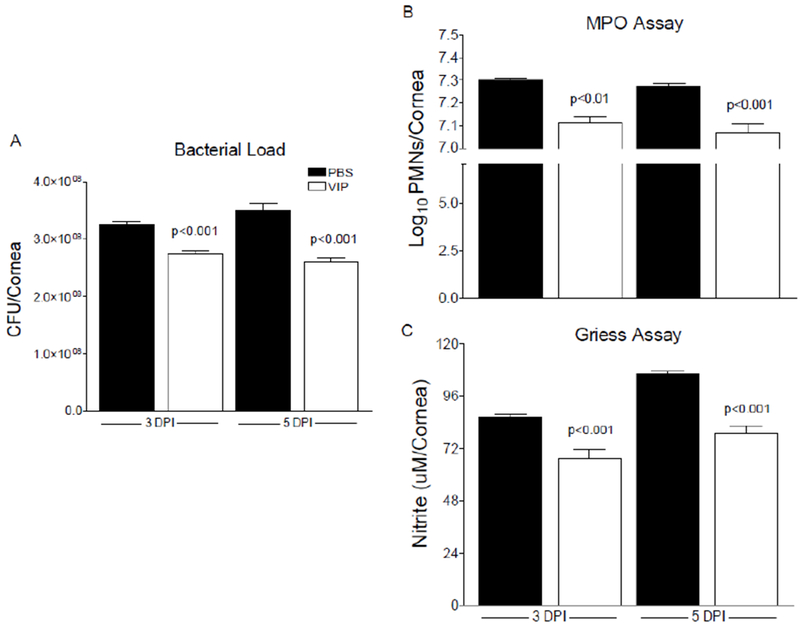
A, Viable bacteria detected by direct plate counts from corneas of PBS- and VIP-treated B6 mice at 3 and 5 days p.i. Results are reported as CFU/cornea ± SEM (n = 6 corneas/group). B, Estimation of PMN as calculated from MPO levels detected from corneas of PBS- and VIP-treated B6 mice after infection. Results are reported as log10/cornea + SEM (n = 5 corneas/group). C, Nitrite levels from infected corneas after PBS and VIP treatment. Data are reported as μM/cornea ± SEM (n = 5 corneas/group).
PMN are the predominant infiltrating inflammatory cell into the infected cornea. MPO, a peroxidase enzyme, is most abundantly expressed in neutrophilic granulocytes. As such, we estimated PMN numbers in the infected cornea using MPO levels (Fig 2B). VIP treatment resulted in a significant decrease in MPO levels detected compared to controls at both 3 and 5 days after infection. Although PMN are thought to contribute to a large part of the host-induced damage to the corneal tissue, these cells are still integral to the resolution phase of inflammation. It is becoming more evident that the phenotypic switch of these cells from inflammatory to pro-resolving influences the immunopathogenic response related to bacterial keratitis.
Macrophages are another major cell infiltrate known to play a role during bacterial keratitis. Although MPO has been detected in some macrophage subpopulations (Kupffer cells, peritoneal macrophages, microglia [9–11]), it has been demonstrated that MPO expression is lost during monocyte to macrophage differentiation [29]. However, these cells are known to produce nitric oxide, a free radical, during an inflammatory response. As a result, indication of macrophage activation can be estimated from the stable oxidized product of nitric oxide, nitrite. Significant decreases in nitrite levels were measured in infected corneas of VIP- versus PBS-treated mice at both 3 and 5 days, as presented in Figure 2C.
Topical VIP treatment influences pro-inflammatory mediators
As previously indicated from our systemic VIP studies, topical treatment administered post-infection also results in significant reduction of key pro-inflammatory mediators. As shown in Figure 3, mRNA levels of iNOS (A), MIP-2 (B), IL-1β (C) and TNF-α (D) were all decreased following VIP treatment compared to controls. Further, all genes were significantly downregulated at both 3 and 5 days p.i. except for MIP-2 at 5 days only. Studies have shown that VIP decreases pro-inflammatory cytokine levels through a number of signal transduction pathways and transcription factors, including cAMP, MAPK, AP-1, NF-κB and IRF-1 [30].The current study, however, expands beyond these pathways and indicates a role for VIP regarding lipid mediator pathways, as well.
Figure 3.
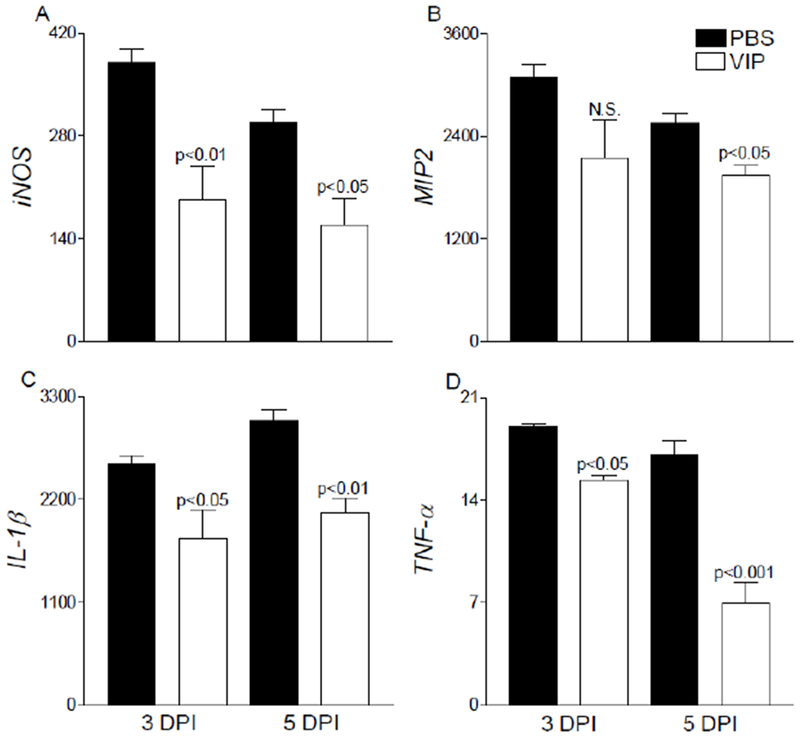
Select pro-inflammatory cytokine/chemokine transcript levels were assessed after VIP treatment in infected corneas of B6 mice. Significant decreases were observed in iNOS (A), MIP-2 (B), IL-1β (C), and TNF-α (D) mRNA levels in VIP- versus PBS-treated mice. Results are presented as relative fold change for the gene of interest normalized to β-actin ± SEM (n = 5 corneas/group).
VIP treatment influences lipid mediator pathways
We have previously indicated a signature lipid metabolite profile correlating with the susceptible phenotype of B6 mice following P. aeruginosa-induced corneal infection [19]. This profile features an early release of initiating mediators that results in an increasingly sustained production of prostaglandins, leukotrienes, and thromboxane over time. In addition, we found that activation of SPM circuits and related enzymatic activity were significantly reduced compared to the resistant phenotype observed in BALB/c mice. As a potential mechanism of action, we investigated whether VIP treatment may influence the disease response via select lipid mediator pathways.
Lipoxygenase (LOX) and cyclooxygenase (COX) enzymes were initially assessed at the mRNA level in corneas of VIP- and PBS-treated B6 mice at 3 and 5 days p.i., as shown in Figure 4. COX-2 is quickly upregulated during inflammation, as observed in both PBS- and VIP-treated mice at 1 day p.i. (data not shown). Following VIP treatment however, transcripts of COX-2 were significantly reduced at 3 and 5 days p.i. compared to PBS controls. In addition, 5-LOX was significantly decreased in VIP- versus PBS-treated corneas at 5 days p.i. (Fig. 4B). 5-LOX activity correlates with an exacerbated inflammatory response and is a marker of inflammatory leukocytes [19]. In contrast, platelet 12-LOX (Alox12) (Fig. 4C), an enzyme associated with SPM pathways, was significantly elevated in corneas of VIP-treated mice. Surprisingly, 12/15-LOX (Fig. 4D) remained similar between the two groups. This particular enzyme is critical for SPM production (lipoxins, resolvins, protectins) and considered a marker of pathway activation in epithelial and mucosal tissue. The changes observed at the transcript level suggest less activation of pro-inflammatory lipid mediator circuits (reduced COX2 and 5-LOX) with a shift toward activation of SPM pathways We expect that with lower levels of COX2 and 5-LOX, there is less conversion of AA into PGH2 and LTA4, respectively, which are intermediates for pro-inflammatory prostaglandins and leukotrienes. This change in enzyme levels contributes to 12/15-LOX converting more AA into 15S-HETE and DHA into 17S-HpDHA with further metabolism by what 5-LOX is available into LXA4/LXB4 and RvD1.
Figure 4.
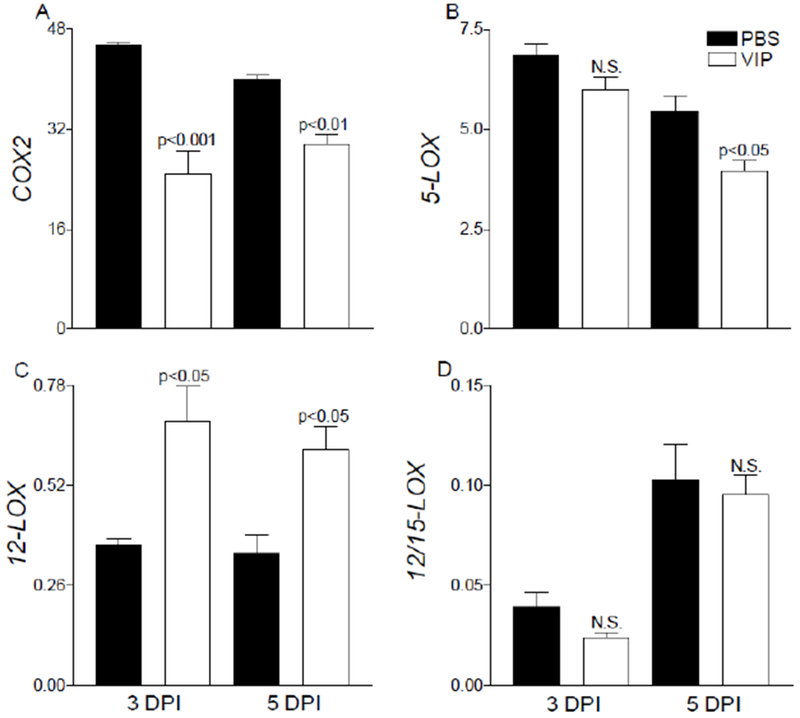
mRNA expression of lipid mediator biosynthetic enzymes, COX-2 (A), 5-LOX (B), 12-LOX (C), and 12/15-LOX (D) are provided from infected corneas of PBS- and VIP-treated B6 mice. Results are reported as relative fold change of the gene of interest normalized to β-actin ± SEM (n = 5 corneas/group).
Previous lipidomic analysis revealed that VIP treatment increased 15-LOX pathway activation as indicated by elevated 17-HDHA, a key metabolic footprint for RvD1 production [31]. Therefore, in the current study we focused on the RvD1 pathway, which requires both 15- and 5-LOX enzymes [18]. Protein levels of 5-LOX (Fig. 5A) followed similar trends observed at the mRNA level with significant downregulation at both time points after VIP treatment. 15-LOX (Fig. 5B) was increased over time in VIP- versus PBS-treated mice, however only 5 days was significant.
Figure 5.
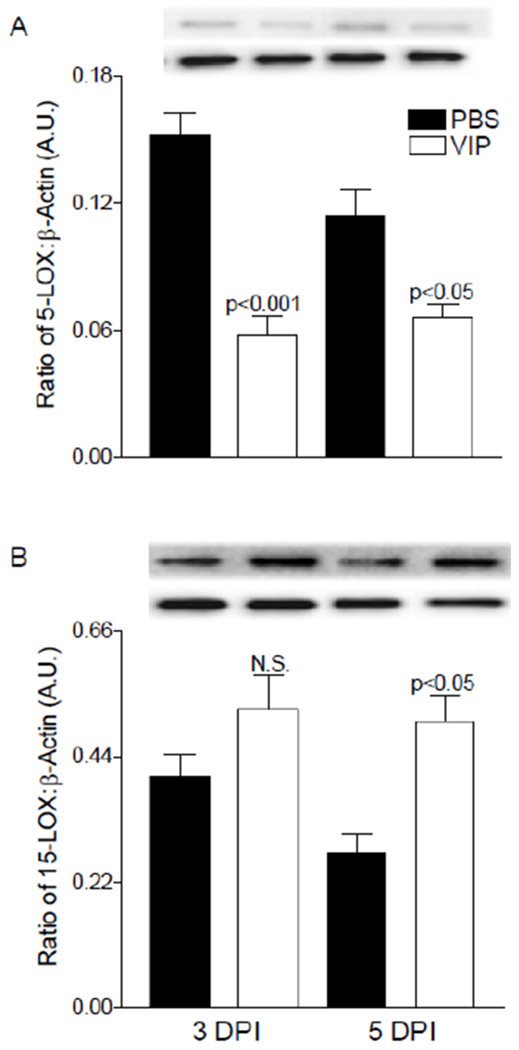
Protein levels of 5-LOX (A) and 15-LOX (B) enzymes as detected by Western blot in infected corneas of PBS- and VIP-treated mice. Data shown are representative of three independent experiments in duplicate and expressed as mean ± SEM (n = 6 corneas/group).
We next examined both the pro-resolving end product and its receptor, FPR2. As shown in Figure 6A, VIP significantly increased RvD1 levels in the infected cornea at both time points supporting the notion that VIP influences a pro-resolving phenotype in our bacterial keratitis model. In addition, VIP treatment resulted in a significant increase in FPR2, for which RvD1 is a ligand [32]. Pro-resolving activity of this DHA-derived autacoid has been established including attenuating inflammation and reducing PMN infiltration [18]. Specific to the eye, previous work from our lab has demonstrated that VIP restores high glucose-induced decreases in RvD1 in retinal endothelial cells [33]. Further, studies have shown that RvD1 reduces retinal angiogenesis [34] and corneal neovascularization [35], supporting a protective role within the ocular tissue.
Figure 6.
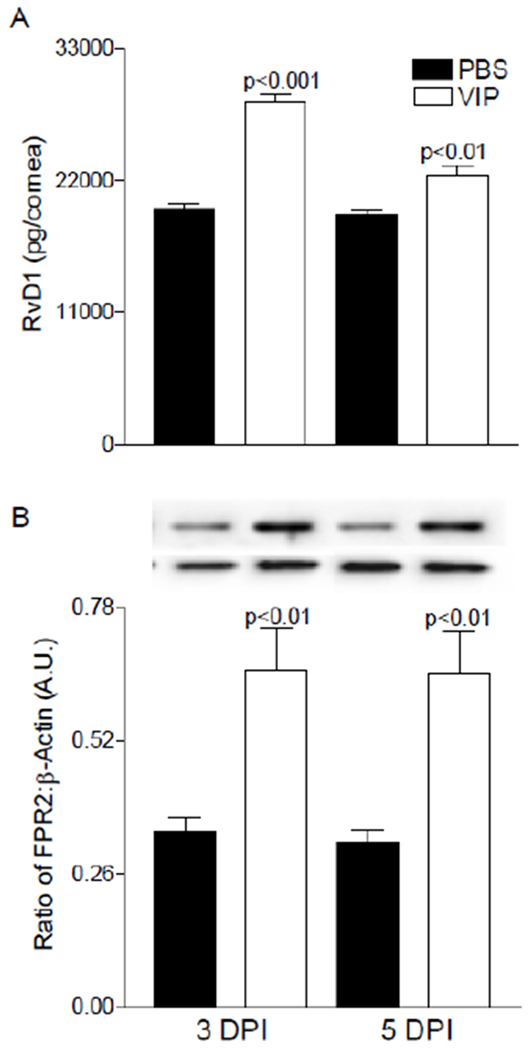
Levels of RvD1 (A) and FPR2 (B) as detected by ELISA and Western blot (respectively) in infected corneas of PBS- and VIP-treated B6 mice. Results are reported as mean (pg/cornea) ± SEM for ELISA analysis. For Western blot, a representative image is provided from three independent experiments in duplicate and expressed as mean ± SEM (n = 6 corneas/group).
VIP-induced pro-resolving effects may be FPR2 dependent
Although VPAC1/VIPR1 and VPAC2/VIPR2 are the two major VIP receptors, we next investigated whether FPR2 is involved in VIP-induced pro-resolving activity observed in two key inflammatory cells known to highly express FPR2 [36, 37]. Peritoneal-derived PMN and macrophages were treated with antagonists for VIP receptors and FPR2 prior to stimulation with PA and/or VIP. Results (Fig. 7) indicate that FPR2 is reduced in both cell types, PMN (A) and macrophages (B), when stimulated with PA only. However, levels are significantly enhanced with the addition of VIP with PA. This upregulation in FPR2 levels is abrogated when both receptor antagonists are added in combination. Levels are rescued only when the FPR2 antagonist is removed. Levels remain decreased in the presence of FPR2 antagonist, but removal of the VIP receptor antagonist. These data suggest that VIP influences FPR2 through the receptor itself and does not require either VIP receptor. El Zein et al. have showed that VIP binds FPR2 resulting in CD11b upregulation in monocytes, which was considered a pro-inflammatory event [38]. However, further work is necessary to elucidate the mechanism by which VIP influences the FPR2 receptor and the downstream signaling pathways involved.
Figure 7.
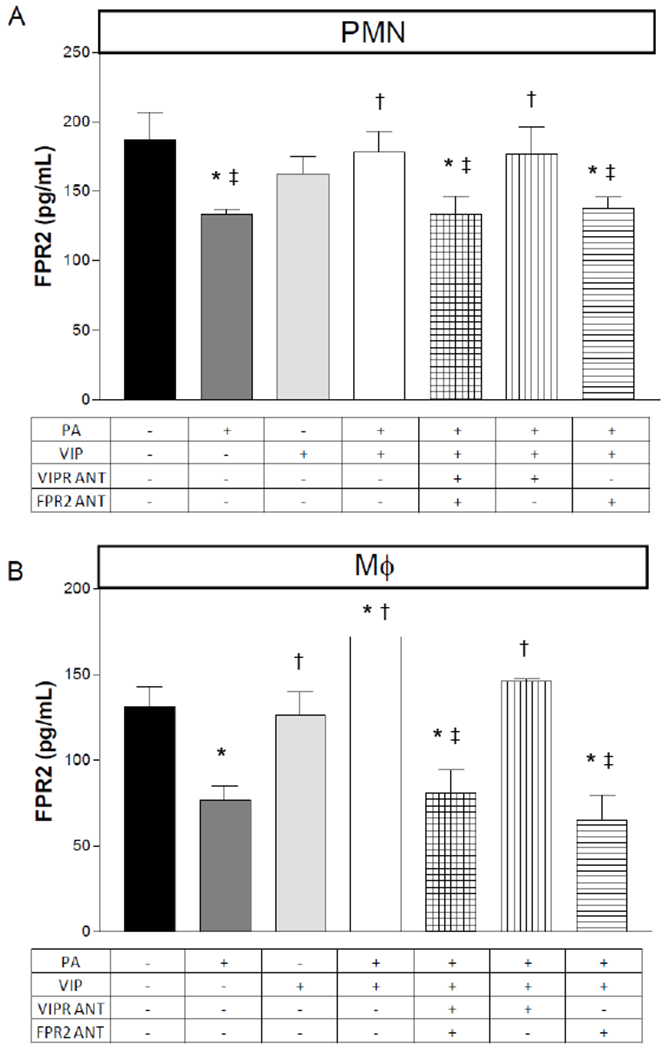
Protein levels of FPR2 as detected by ELISA in PMN and macrophages after an in vitro stimulation assay. Peritoneal-derived PMN (A) and macrophages (B) from B6 mice were exposed to P. aeruginosa (PA) +/− the presence of VIP, VIP receptor antagonist and FPR2 antagonist. Data are reported as pg/mL ± SD (n = 6). *p<0.05 vs. CTRL; †p<0.05 vs. PA; ‡p<0.05 vs. PA+VIP expressed as mean ± SEM (n = 6 corneas/group).
Our findings are summarized in the schematic provided in Figure 8. RvD1 synthesis requires both 15- and 5-LOX enzymes as illustrated. We hypothesize that less 5-LOX overall gives 15-LOX a better chance to convert DHA and AA to pro-resolving intermediates, which can then be further converted by 5-LOX into RvD1 (among other SPMs including AA-derived LXA4). Increased 15-LOX can then outcompete lower levels of 5-LOX resulting in more conversion of DHA into 17S-HpDHA (with further metabolism by 5-LOX into RvD1) and less conversion of AA into LTA4 by 5-LOX or PGH2 by COX2, both of which are intermediates for pro-inflammatory leukotrienes and prostaglandins, respectively.
Figure 8.
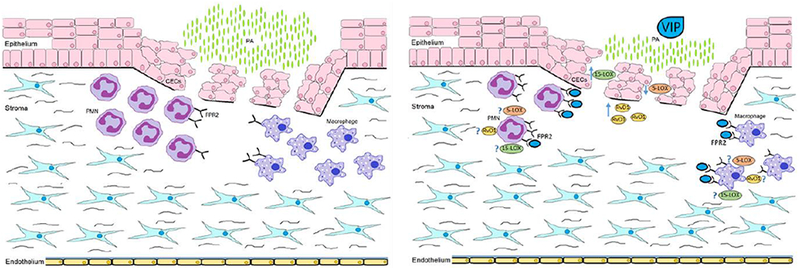
Schematic representation summarizing the influence of VIP on the cornea after P. aeruginosa-induced infection.
In conclusion, inflammation is a necessary aspect of a healthy host response to infection. Although it is essential to containing infection, resolution of inflammation and restoration of immune homeostasis is equally critical to the host. Regarding the eye, this is even more critical given that the cornea must remain clear in order to preserve visual acuity. Although current standard of care (antibiotics, fortified antibiotics) addresses the pathogen, it does not adequately induce the healing pathways essential for restoration of visual acuity. In fact, the use of fortified antibiotics can have toxic effects on the corneal epithelium, thus inhibiting the healing process [39, 40]. Concerns still exist regarding the risks of associated immunosuppressive effects with corticosteroid use, as well [6, 7]. To this end, activation of pro-resolving circuits are essential. VIP is not just anti-inflammatory but also has pro-resolving actions. It appears as though VIP’s effects are carried out, at least in part, through the FPR2 pathway. This VIP:FPR2 relationship may contribute to downregulated pro-inflammatory mediators, decreased bacterial load and the reduction in PMN and macrophage infiltration into the infected cornea; ultimately preventing corneal perforation. While the current study indicates that VIP influences the RvD1:FPR2 circuit in the infected cornea, Saban et al. have shown that RvD1 treatment reduces the ocular allergic response in an AED mouse model [41]. Furthermore, aspirin-triggered RvD1 has been shown to reduce lesion severity and corneal neovascularization in a mouse model of HSV-induced stromal keratitis [42]. These findings enhance the significance of VIP as a treatment for ocular infectious disease; a molecule that is not only characterized as anti-inflammatory without the toxicity issues of current therapies, but can also activate key SPM circuits essential for the restoration of ocular structure and function.
Highlights.
VIP topical treatment improves disease outcome
Pro-resolution pathways are augmented by VIP treatment
FPR2 signaling contributes to disease pathogenesis
VIP:FPR2 relationship may influence pro-resolving circuits
Acknowledgments
Funding: This work was supported by the National Institutes of Health [NEI R01 EY023226, P30EY004068] and Research to Prevent Blindness
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lichtinger A, et al. , Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology, 2012. 119(9): p. 1785–90. [DOI] [PubMed] [Google Scholar]
- 2.Keay L, Stapleton F, and Schein O, Epidemiology of contact lens-related inflammation and microbial keratitis: a 20-year perspective. Eye Contact Lens, 2007. 33(6 Pt 2): p. 346–53, discussion 362–3. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, et al. , The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology, 2008. 115(10): p. 1655–62. [DOI] [PubMed] [Google Scholar]
- 4.Yildiz EH, et al. , Trends in contact lens-related corneal ulcers at a tertiary referral center. Cornea, 2012. 31(10): p. 1097–102. [DOI] [PubMed] [Google Scholar]
- 5.Lotti R and Dart JK, Cataract as a complication of severe microbial keratitis. Eye (Lond), 1992. 6 (Pt 4): p. 400–3. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelmus KR, Indecision about corticosteroids for bacterial keratitis: an evidence-based update. Ophthalmology, 2002. 109(5): p. 835–42; quiz 843. [DOI] [PubMed] [Google Scholar]
- 7.Tallab RT and Stone DU, Corticosteroids as a therapy for bacterial keratitis: an evidence-based review of ‘who, when and why’. Br J Ophthalmol, 2016. 100(6): p. 731–5. [DOI] [PubMed] [Google Scholar]
- 8.Muller LJ, et al. , Corneal nerves: structure, contents and function. Exp Eye Res, 2003. 76(5): p. 521–42. [DOI] [PubMed] [Google Scholar]
- 9.Pozo D, et al. , Immunobiology of vasoactive intestinal peptide (VIP) (vol 21, pg 7, 2000). Vol. 21 2000. 7–11. [DOI] [PubMed] [Google Scholar]
- 10.Szliter EA, et al. , Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J Immunol, 2007. 178(2): p. 1105–14. [DOI] [PubMed] [Google Scholar]
- 11.Berger EA, et al. , VIP promotes resistance in the Pseudomonas aeruginosa-infected cornea by modulating adhesion molecule expression. Invest Ophthalmol Vis Sci, 2010. 51(11): p. 5776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger EA, et al. , Effects of VIP on corneal reconstitution and homeostasis following Pseudomonas aeruginosa induced keratitis. Invest Ophthalmol Vis Sci, 2012. 53(12): p. 7432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN, et al. , Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med, 2000. 192(8): p. 1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J and Gronert K, The role of pro-resolving lipid mediators in ocular diseases. Mol Aspects Med, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liclican EL and Gronert K, Molecular circuits of resolution in the eye. Scientific World Journal, 2010. 10: p. 1029–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, et al. , Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther, 2010. 26(5): p. 431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serhan CN, A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol, 2004. 122(4): p. 305–21. [DOI] [PubMed] [Google Scholar]
- 18.Serhan CN, et al. , Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med, 2002. 196(8): p. 1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carion TW, et al. , Immunoregulatory role of 15-lipoxygenase in the pathogenesis of bacterial keratitis. The FASEB Journal, 2018. 32(9): p. 5026–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalli J and Serhan CN, Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood, 2012. 120(15): p. e60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab JM, et al. , Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature, 2007. 447(7146): p. 869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudner XL, et al. , Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol, 2000. 164(12): p. 6576–82. [DOI] [PubMed] [Google Scholar]
- 23.Hazlett LD, et al. , Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci, 1987. 28(12): p. 1978–85. [PubMed] [Google Scholar]
- 24.Williams RN, et al. , Quantification of ocular inflammation: evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Curr Eye Res, 1982. 2(7): p. 465–70. [DOI] [PubMed] [Google Scholar]
- 25.Roux KH, Optimization and troubleshooting in PCR. PCR Methods Appl, 1995. 4(5): p. S185–94. [DOI] [PubMed] [Google Scholar]
- 26.Luo Y and Dorf ME, Isolation of mouse neutrophils. Curr Protoc Immunol, 2001. Chapter 3: p. Unit 3.20. [DOI] [PubMed] [Google Scholar]
- 27.Carion TW, et al. , Efficacy of VIP as Treatment for Bacteria-Induced Keratitis Against Multiple Pseudomonas aeruginosa Strains. Invest Ophthalmol Vis Sci, 2015. 56(11): p. 6932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Karim IA, et al. , Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol, 2008. 200(1-2): p. 11–6. [DOI] [PubMed] [Google Scholar]
- 29.Brown KE, Brunt EM, and Heinecke JW, Immunohistochemical Detection of Myeloperoxidase and Its Oxidation Products in Kupffer Cells of Human Liver. The American Journal of Pathology, 2001. 159(6): p. 2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado M, Pozo D, and Ganea D, The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev, 2004. 56(2): p. 249–90. [DOI] [PubMed] [Google Scholar]
- 31.Carion TW, et al. , The role of FPR2 signaling in the pathogenesis of bacterial keratitis, in Invest Ophthalmol Vis Sci. 2016. p. E-Abstract 1831. [Google Scholar]
- 32.Krishnamoorthy S, et al. , Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol, 2012. 180(5): p. 2018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi H, et al. , VIP protects human retinal microvascular endothelial cells against high glucose-induced increases in TNF-alpha and enhances RvD1. Prostaglandins Other Lipid Mediat, 2016. 123: p. 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connor KM, et al. , Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med, 2007. 13(7): p. 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Y, et al. , Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci, 2009. 50(10): p. 4743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takano T, et al. , Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med, 1997. 185(9): p. 1693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiore S, et al. , Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med, 1994. 180(1): p. 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Zein N, Badran B, and Sariban E, VIP differentially activates beta2 integrins, CR1, and matrix metalloproteinase-9 in human monocytes through cAMP/PKA, EPAC, and PI-3K signaling pathways via VIP receptor type 1 and FPRL1. J Leukoc Biol, 2008. 83(4): p. 972–81. [DOI] [PubMed] [Google Scholar]
- 39.Sosa AB, Epstein SP, and Asbell PA, Evaluation of toxicity of commercial ophthalmic fluoroquinolone antibiotics as assessed on immortalized corneal and conjunctival epithelial cells. Cornea, 2008. 27(8): p. 930–4. [DOI] [PubMed] [Google Scholar]
- 40.Oum BS, et al. , Effects of fluoroquinolone eye solutions without preservatives on human corneal epithelial cells in vitro. Ophthalmic Res, 2014. 51(4): p. 216–23. [DOI] [PubMed] [Google Scholar]
- 41.Saban DR, et al. , Resolvin D1 treatment on goblet cell mucin and immune responses in the chronic allergic eye disease (AED) model. Mucosal Immunol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajasagi NK, et al. , Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology. J Leukoc Biol, 2017. 102(5): p. 1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]


