Abstract
Large artery stiffening contributes to the pathophysiology of heart failure (HF) and associated comorbidities. Matrix Gla-Protein (MGP) is a potent inhibitor of vascular calcification. MGP activation is vitamin-K dependent. We aimed to: (1) to compare dephospho-uncarboxylated Matrix Gla-Protein (dp-ucMGP) levels between subjects with HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF), and subjects without HF; (2) to assess the relationship between dp-ucMGP levels and arterial stiffness; (3) to assess the relationship between warfarin use, dp-ucMGP levels, and arterial stiffness in HF. We enrolled 348 subjects with HFpEF (n=96), HFrEF (n=53) or no HF (n=199). Carotid-femoral pulse wave velocity (CF-PWV), a measure of large artery stiffness, was measured with arterial tonometry. Dp-ucMGP was measured with ELISA. Dp-ucMGP levels were greater in both HFrEF (582 pmol/L; 95%CI=444 to 721 pmol/L) and HFpEF (549 pmol/L; 95%CI= 455 to 643 pmol/L) compared to controls (426 pmol/L; 95%CI= 377 to 475 pmol/L; ANCOVA P=0.0067). Levels of dp-ucMGP were positively associated with CF-PWV (Standardized β=0.31; 95%CI=0.19 to 0.42; P<0.0001), which was also true in analyses restricted to patients with HF (Standardized β=0.34; 95%CI=0.16 to 0.52; P=0.0002). Warfarin use was significantly associated with CF-PWV (Standardized β=0.13; 95%CI=0.004-0.26; P=0.043), but this relationship was eliminated after adjustment for dp-ucMGP. In conclusion, levels of dp-ucMGP are increased in HFpEF and HFrEF and are independently associated with arterial stiffness. Future studies should investigate whether vitamin K supplementation represents a suitable therapeutic strategy to prevent or reduce arterial stiffness in HFpEF and HFrEF.
Keywords: heart failure, HFpEF, MGP, vitamin K, arterial stiffness, pulse wave velocity
Introduction
Large artery stiffening causes an excessive pulsatile load to the heart and to the microvasculature of various target organs. Both effects are involved in the pathophysiology of cardiac dysfunction and heart failure (HF)-associated comorbidities (such as renal dysfunction). The identification of pathways related to arterial stiffness in HF may provide novel therapeutic targets.
Matrix Gla-Protein (MGP) is a protein produced by chondrocytes and vascular smooth muscle cells.1 The inactive form of MGP (dephospho-uncarboxylated MGP, dp-ucMGP) undergoes serial post-translational γ-glutamate carboxylation and serine phosphorylation to form active MGP. The active form of MGP is a potent inhibitor of vascular calcification. Carboxylation of dp-ucMGP is vitamin K dependent and is thus reduced in vitamin K-deficient states.1 Dp-ucMGP is secreted into the circulation and an increase in its levels indicates deficient MGP activation. Recent studies in non-HF populations indicated that abnormal MGP maturation correlates with arterial stiffness in humans.2; 3 Less information is available regarding the relationship between MGP and arterial stiffness in HF. HF is associated with hemodynamic abnormalities, metabolic abnormalities, renal dysfunction and the use of multiple medications, which could impact the relationship between MGP and PWV.
A previous study demonstrated increased levels of dp-ucMGP in a HFrEF population.4 However, whether HFpEF is associated with increased dp-ucMGP levels, and more importantly, whether MGP levels correlate with arterial stiffness in subjects with HFrEF or HFpEF is unknown. This is important because HFpEF and HFrEF are different disease states, with different pathophysiology and response to therapy; furthermore, it is important to better understand HFpEF given that there are no currently available evidence-based effective pharmacologic interventions for this condition.
Patients with HF often receive warfarin, a vitamin K antagonist that may inhibit MGP activation. In animal models, warfarin administration at high doses produces profound arterial calcification5 and several lines of evidence link warfarin use to arterial calcification in humans.6;7;8;9;10 Arterial calcification is an important contributor to arterial stiffness.11 However, the relationship between warfarin use, MGP activation, and large artery stiffness in HF is unknown.
In this study, we aimed to: (1) Compare levels of plasma dp-ucMGP levels between subjects with HFpEF, HFrEF and subjects without HF; (2) Assess the relationship between dp-ucMGP levels and carotid-femoral pulse wave velocity (CF-PWV), the non-invasive gold standard index of large artery stiffness12;13, (3) Assess the relationship between warfarin use, dp-ucMGP levels and arterial stiffness in HF.
Methods
We prospectively enrolled a convenience sample of 348 adults at the Corporal Michael J. Crescenz VA Medical Center referred for a cardiac magnetic resonance imaging study. The protocol was approved by the Philadelphia VA Medical Center Institutional Review Board, and all subjects provided written informed consent. The investigation conforms with the principles outlined in the Declaration of Helsinki.
The data, analytic methods, and study materials are not publicly available for purposes of reproducing the results or replicating the procedures. Such data may be made available to other researchers for collaborative research, through the establishment of appropriate agreements.
Key exclusion criteria were as follows: (1) Claustrophobia; (2) Presence of metallic objects or implanted medical devices in body; (3) Conditions that could make the measurements of CF-PWV less accurate and/or unreliable (i.e., arrhythmia such as atrial fibrillation); (4) History of sarcoidosis or amyloidosis, or suspected infiltrative heart disease.
In order to optimize case classification according to LVEF and other cardiac parameters detailed below, we measured left ventricular ejection fraction (LVEF) and cardiac structure and function using the current gold-standard method (steady-state free precession cine cardiac MRI). HFrEF was defined as a symptomatic HF in the presence of a <50%. HFpEF was defined as (1) NYHA Class II-IV symptoms consistent with HF; (2) LVEF>50%; (3) a mitral E wave to annular (e’) ratio >14; or at least 2 of the following: (a) a mitral E wave to annular e’ ratio >8; (b) treatment with a loop diuretic for control of HF symptoms; (c) left atrial volume index >34 mL/m2 of body surface area (BSA); (d) NT-pro B-type natriuretic peptide level >200 pg/mL; and (e) LV mass index >149 g/m2 in men and 122 g/m2 in women. Subjects without HF had an LVEF >50%, and no symptoms and signs consistent with HF.
Carotid-Femoral Pulse Wave Velocity Measurement
Carotid femoral pulse wave velocity (CF-PWV) was measured using the SphygmoCor system (Atcor Medical; Sydney, Australia). Briefly, carotid-to-femoral transit time (ΔT) was computed from the foot-to-foot time difference between sequentially acquired carotid and femoral waveforms, using the intersecting tangents method, and the QRS complex of the ECG as a fiducial point. The distance between the sternal notch and the carotid artery was subtracted from the distance between the sternal notch and the femoral artery (measured with rigid calipers), in order to estimate the path length (L), and CF-PWV was computed as L/ΔT. The coefficient of variation for CF-PWV measurements in our lab is <10%.
Cardiac MRI
We measured LV mass, volume and left atrial volume, using a 1.5 Tesla whole body MRI scanner (Avanto or Espree, Siemens, Malvern, PA, USA) equipped with a phase-array cardiac coil.
LV volumes (end-diastolic and end-systolic volumes) and function (ejection fraction, EF) were measured using steady-state free-precession (SSFP) cine imaging. Typical acquisition parameters were: TR=30.6 ms; TE=1.3 ms; Slice thickness=8 mm; Phases=30; Parallel image (IPAT) factor=2; and Matrix size=192×192. CMR42 software (Circle CVI, Calgary, AB, Canada) was utilized to manually trace the LV short-axis cine images at end of diastole and systole. LV mass (LVM) was calculated as the difference between epicardial and endocardial volumes, multiplied by the myocardial density. Left atrial volume was calculated by averaging the volumes measured end-systole by manually tracing the left atrial endocardial border in the apical 2-chamber and 4-chamber views.
Plasma dp-ucMGP measurement
Citrate tubes were used for collection of venous blood samples at the time of enrollment. Plasma was prepared and stored at −80 °C for batch analysis. A dual-antibody sandwich ELISA technique (VitaK; Maastricht University; The Netherlands) was used to measure dp-ucMGP. Intra-assay coefficients of variation for this assay have previously been reported at 3.1% and 5.4% for lower and upper limit of normal. Inter-assay variation coefficients are 6.9% and 13.6% for lower and upper limits of normal.2
Statistical Methods
Our study had 80% power to detect standardized effect sizes of at least 0.15 with a 2-sided alpha error rate of 0.05. Continuous and categorical variables were compared between the groups using analysis of variance (ANOVA) and chi-square tests, respectively. Given previous studies demonstrating an important influence of age, sex, ethnicity and warfarin use on dp-ucMGP,6; 7; 14 we adjusted for these factors in comparisons of dp-ucMGP levels between the groups, using ANCOVA. Post-hoc pairwise comparisons were conducted using the Bonferroni correction method. Bivariable and multivariable linear regression models were utilized to assess independent correlates of dp-ucMGP, with adjustments for multiple potential confounders. Similarly, linear regression models were used to assess the how warfarin use, dp-ucMGP levels, and various other factors relate to arterial stiffness (CF-PWV). As recommended by current guidelines12, these models were adjusted for mean arterial pressure and heart rate (which can affect CF-PWV independently of the underlying intrinsic material properties of the arterial wall). When required, Box-Cox transformation was applied to normalize regression model residuals. We present standardized regression coefficients for easier comparison of the magnitude of the effect of various independent variables on the dependent variable in regression models.
Results
Baseline characteristics of our participants with HFpEF, and HFrEF and no HF are presented and compared in Table 1. Most of the subjects were male, but the proportion of males was greater in the HFpEF group and lower in the HFrEF group. Compared to the other groups, subjects with HFpEF were significantly older, demonstrated a much greater BMI, lower estimated GFR, lower serum magnesium, and the highest prevalence of diabetes (69.79%) and hypertension (90.62%). The prevalence of coronary artery disease was highest in HFrEF (52.83%). LV mass was increased in both HFpEF and HFrEF, without significant differences between HFpEF and HFrEF, whereas LV end-diastolic volume was significant greater in HFrEF than in HFpEF. A greater percentage of HFpEF and HFrEF subjects used beta-blockers, aspirin, and furosemide as well, whereas insulin use was approximately twice as prevalent in HFpEF (32.29%) compared to either HFrEF (15.09%) or subjects without HF (14.57%).
Table 1.
General Characteristics of Study Population.
| Characteristics | No HF | HFrEF | HFpEF | P value |
|---|---|---|---|---|
| Age, years | 59.9 (58.2 to 61.5) | 65 (61.5 to 68.5) | 63.9 (61.3 to 66.4) | 0.0033 * # |
| Male Sex | 179 (89.95%) | 52 (98.11%) | 80 (83.33%) | 0.0182 |
| Body mass index, kg/m2 | 30 (29.1 to 30.8) | 28 (26.4 to 29.7) | 36 (34.4 to 37.5) | <0.0001 # $ |
| Hypertension | 151 (75.88%) | 43 (81.13%) | 87 (90.62%) | 0.0108 |
| Diabetes Mellitus | 82 (41.21%) | 27 (50.94%) | 67 (69.79%) | <0.0001 |
| Coronary Artery Disease | 50 (25.13%) | 28 (52.83%) | 29 (30.21%) | 0.0005 |
| Brachial SBP, mmHg | 142 (139 to 145) | 141 (136 to 147) | 148 (144 to 152) | 0.0541 |
| Carotid SBP, mmHg | 132 (129 to 136) | 136 (129 to 143) | 139 (134 to 144) | 0.0961 |
| Diastolic Blood Pressure, mmHg | 82.2 (80.4 to 84.1) | 82.4 (79.1 to 85.8) | 82.8 (80.2 to 85.4) | 0.9496 |
| Mean Artery Pressure, mmHg | 102 (100 to 104) | 103 (99 to 107) | 104 (101 to 107) | 0.5122 |
| Carotid Pulse Pressure, mmHg | 49 (46.5 to 51.6) | 52.1 (47 to 57.1) | 55.3 (51.1 to 59.5) | 0.0289 # |
| Carotid-Radial PWV, mmHg | 9.7 (9.3 to 10.1) | 10.3 (9.5 to 11.1) | 9.6 (9.1 to 10.2) | 0.3727 |
| Carotid-Femoral PWV, m/s | 9.5 (9 to 10) | 10.2 (9.1 to 11.3) | 9.9 (9.1 to 10.7) | 0.3828 |
| Serum Magnesium (mg/dL) | 1.97 (1.91 to 2.03) | 1.96 (1.84 to 2.08) | 1.84 (1.75 to 1.92) | 0.0372 # |
| Serum Phosphate (mg/dL) | 3.38 (3.28 to 3.47) | 3.31 (3.1 to 3.5) | 3.41 (3.25 to 3.56) | 0.7686 |
| Serum Calcium (mg/dL) | 8.78 (8.14 to 9.38) | 8.7 (7.41 to 9.94) | 8.42 (7.54 to 9.34) | 0.8188 |
| LV EDV index (ml/m2) | 48.9 (46.8 to 51.1) | 76.4 (70.2 to 82.7) | 56.4 (52.4 to 60.4) | <0.0001 * # $ |
| LV EF | 57.3 (55.2 to 59.4) | 31.9 (29.8 to 34.1) | 61.3 (57.6 to 64.9) | <0.0001 * $ |
| LV Mass (g) | 139 (133 to 145) | 184 (169 to 200) | 164 (153 to 176) | <0.0001 * # |
| LV Mass Index (g/height1.7) | 30.6 (29.3 to 31.8) | 38.7 (35.7 to 41.6) | 37.2 (34.7 to 39.7) | <0.0001 * # |
| Medication use | ||||
| Estimated GFR (ml/1.73 m2) | 83.2 (78.8 to 87.5) | 74.2 (66.5 to 81.9) | 68.5 (63.3 to 73.6) | <0.0001 # |
| Beta Blockers | 80 (40.20%) | 47 (88.68%) | 62 (64.58%) | <0.0001 |
| Aspirin | 103 (51.76%) | 42 (79.25%) | 69 (71.88%) | <0.0001 |
| ACE Inhibitors | 86 (43.22%) | 39 (73.58%) | 49 (51.04%) | 0.0004 |
| ARBs | 17 (8.54%) | 7 (13.21%) | 20 (20.83%) | 0.0118 |
| Furosemide | 4 (2.01%) | 37 (69.81%) | 58 (60.42%) | <0.0001 |
| Digoxin | 3 (1.51%) | 2 (3.77%) | 2 (2.08%) | 0.5787 |
| Spironolactone | 4 (2.01%) | 7 (13.21%) | 7 (7.29%) | 0.0026 |
| Hydralazine | 6 (3.02%) | 8 (15.09%) | 10 (10.42%) | 0.0024 |
| Warfarin | 14 (7.04%) | 11 (20.75%) | 3 (3.12%) | 0.0006 |
| Calcium-channel blockers | 56 (28.14%) | 11 (20.75%) | 34 (35.42%) | 0.1542 |
| Insulin | 29 (14.57%) | 8 (15.09%) | 31 (32.29%) | 0.0010 |
Numbers represent mean (95%CI) or count (percentage); GFR=glomerular filtration rate.
Post-hoc P<0.05, No HF vs. HFpEF
Post-hoc P<0.05, HFrEF vs. HFpEF
Post-hoc P<0.05 No HF vs HFpEF.
Dp-ucMGP Levels in HF
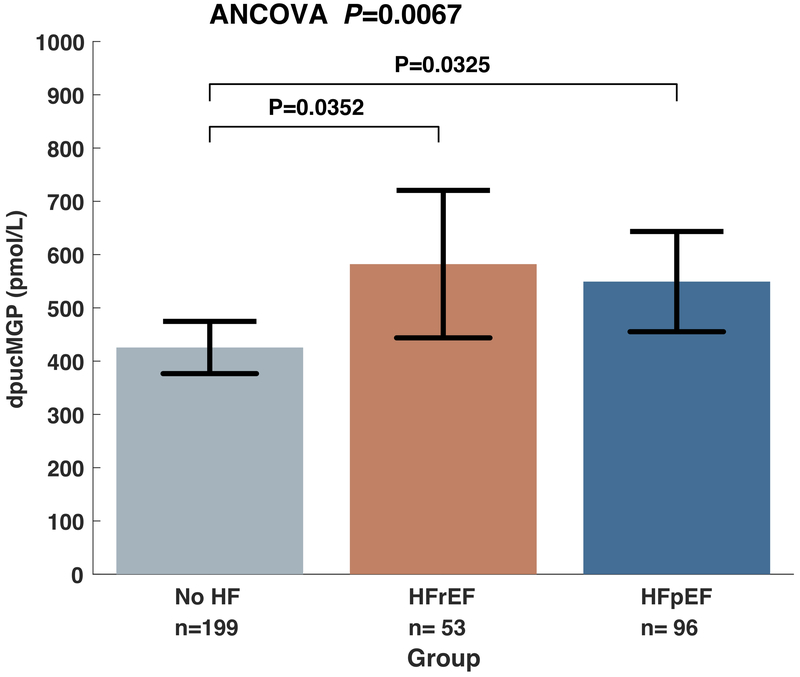
Figure 1 shows mean dp-ucMGP levels in patients without HF, HFpEF and HFrEF. There were significant between-group differences in dp-ucMGP levels (P=0.0067). In post-hoc pairwise comparisons, dp-ucMGP levels were significantly greater in HFpEF (549 pmol/L; 95%CI= 455 to 643 pmol/L) and HFrEF (582 pmol/L; 95%CI=444 to 721 pmol/L) compared to controls (426 pmol/L; 95%CI= 377 to 475 pmol/L), without significant differences between the 2 heart failure groups.
Figure 1.
Comparison of dp-ucMGP levels between subjects with no HF, HFrEF and HFpEF, adjusted for age, gender, ethnicity and warfarin use.
Multivariable correlates of dp-ucMGP
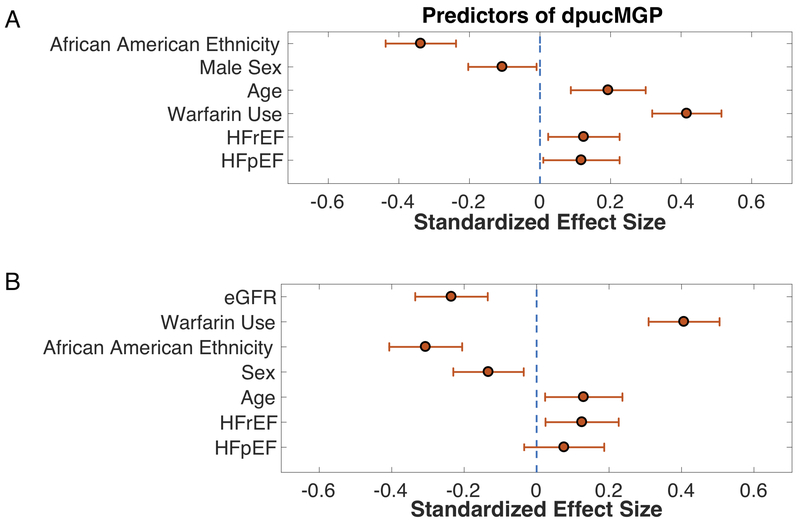
We assessed the presence of HFpEF or HFrEF and various other covariates as correlates of dp-ucMGP levels in a linear regression model (Table 2), which also included age, sex, ethnicity, BMI, systolic blood pressure, history of hypertension, coronary artery disease, diabetes, warfarin use, and serum calcium, magnesium and phosphorus. Standardized regression coefficients and 95% CIs for all independent variables are shown in Table 2. Values of standardized regression coefficients and 95% CIs for significant independent correlates of dp-ucMGP levels in this model are shown in Figure 2A.
Table 2.
Linear Regression Model Showing the Correlates of dp-ucMGP without adjustment for estimated glomerular filtration rate
| Correlates | Standardized Estimate | 95%CI, Lower Bound | 95%CI, Upper Bound | P value |
|---|---|---|---|---|
| HFpEF | 0.12 | 0.01 | 0.23 | 0.03328 |
| HFrEF | 0.12 | 0.02 | 0.23 | 0.01617 |
| Warfarin Use | 0.42 | 0.32 | 0.51 | <0.00001 |
| Age | 0.19 | 0.09 | 0.30 | 0.00039 |
| Male Sex | −0.11 | −0.20 | −0.01 | 0.03121 |
| African American Race | −0.34 | −0.44 | −0.24 | <0.00001 |
| Other Race | −0.07 | −0.16 | 0.03 | 0.16757 |
| BMI | 0.04 | −0.06 | 0.15 | 0.41162 |
| Systolic Blood Pressure | −0.02 | −0.12 | 0.08 | 0.70679 |
| Hypertension | 0.05 | −0.06 | 0.15 | 0.36387 |
| CAD | −0.05 | −0.16 | 0.05 | 0.28546 |
| DM | 0.01 | −0.10 | 0.11 | 0.89556 |
| Magnesium | −0.05 | −0.18 | 0.08 | 0.42580 |
| Phosphorus | 0.02 | −0.08 | 0.12 | 0.70950 |
| Calcium | 0.04 | −0.08 | 0.17 | 0.46720 |
Figure 2.
Multivariable model showing correlates of dp-ucMGP. 2A: without adjustment for estimated glomerular filtration rate (eGFR). 2B: with further adjustment for eGFR. Standardized regression coefficients and 95% confidence intervals are shown.
In this multivariable model, variables significantly associated with dp-ucMGP included the presence of HFpEF (Standardized β=0.12; 95%CI=0.01 to 0.23; P=0.03328), HFrEF (Standardized β=0.12; 95%CI=0.02 to 0.23; P=0.01617), warfarin use (Standardized β=0.42; 95%CI=0.32 to 0.51; P<0.00001), age (Standardized β=0.19; 95%CI=0.09 to 0.30; P=0.00039), male sex (Standardized β=−0.11; 95%CI=−0.20 to −0.01; P=0.03121) and African-American ethnicity (Standardized β=−0.34; 95%CI=−0.44 to −0.24; P<0.00001).
Since renal dysfunction has been recently shown to be independently associated with dp-ucMGP15, we also constructed a model additionally adjusted for eGFR (Table 3 and Figure 2B). In this model, eGFR was significantly associated with dp-ucMGP (Standardized β=−0.24; 95%CI=−0.34 to −0.13; P<0.0001). HFrEF remained significantly associated with dp-ucMGP (Standardized β=0.13; 95%CI=0.024 to 0.23; P=0.015), whereas HFpEF was no longer significantly associated with dp-ucMGP (Standardized β=0.08; 95%CI=−0.03 to 0.19; P=0.175). In this model, warfarin use, age, male sex, and African-American ethnicity remained significantly associated with dp-ucMGP.
Table 3.
Linear Regression Model Showing the Correlates of dp-ucMGP after further adjustment for estimated glomerular filtration rate
| Correlates | Standardized Estimate | 95%CI, Lower Bound | 95%CI, Upper Bound | P value |
|---|---|---|---|---|
| HFpEF | 0.08 | −0.03 | 0.19 | 0.17573 |
| HFrEF | 0.13 | 0.02 | 0.23 | 0.01502 |
| Warfarin Use | 0.41 | 0.31 | 0.50 | <0.00001 |
| Age | 0.13 | 0.02 | 0.24 | 0.01699 |
| Male Sex | −0.13 | −0.23 | −0.04 | 0.00759 |
| African American Ethnicity | −0.31 | −0.41 | −0.21 | <0.00001 |
| eGFR | −0.24 | −0.34 | −0.13 | <0.00001 |
| Other Race/Ethnicity | −0.05 | −0.14 | 0.05 | 0.35316 |
| BMI | 0.07 | −0.04 | 0.18 | 0.21841 |
| Systolic Blood Pressure | −0.01 | −0.11 | 0.08 | 0.77311 |
| Hypertension | 0.02 | −0.09 | 0.12 | 0.74233 |
| CAD | −0.06 | −0.16 | 0.04 | 0.25006 |
| DM | 0.00 | −0.11 | 0.10 | 0.96671 |
| Magnesium | −0.10 | −0.23 | 0.03 | 0.13995 |
| Phosphorus | 0.03 | −0.07 | 0.13 | 0.57629 |
| Calcium | 0.10 | −0.02 | 0.22 | 0.11076 |
Relationship Between dp-ucMGP and CF-PWV
In unadjusted analyses, dp-ucMGP levels were positively associated with CF-PWV (Standardized β=0.31; 95%CI=0.19 to 0.42; P<0.0001). Similarly, in analyses restricted to patients with heart failure (either HFpEF or HFrEF), dp-ucMGP levels were positively associated with CF-PWV (Standardized β=0.34; 95%CI=0.16 to 0.52; P=0.0002). There was no interaction between either HFpEF (P=0.37) or HFrEF status (P=0.69) and dpuc-MGP as determinants of CF-PWV.
In a model that adjusted for age, sex, race/ethnicity, mean arterial pressure, heart rate, heart failure group membership, body mass index, history of hypertension, coronary artery disease, diabetes, warfarin use, serum calcium, magnesium and phosphorus and estimated GFR, dp-ucMGP remained significantly associated with CF-PWV (Standardized β=0.18; 95%CI=0.03-0.34; P=0.023).
In analyses restricted to subjects with HF, after adjustment for age, sex, race/ethnicity, mean arterial pressure, heart rate, heart failure group membership, body mass index, history of hypertension, coronary artery disease, diabetes, warfarin use, serum calcium, magnesium and phosphorus and estimated GFR, dp-ucMGP was significantly associated with CF-PWV (Standardized β=0.32; 95%CI=0.04-0.61; P=0.026).
Relationship Between Warfarin Use, dp-ucMGP and CF-PWV
In addition to being associated with dp-ucMGP levels, warfarin use was also independently associated with CF-PWV. In a model that adjusted for age, sex, race/ethnicity, mean arterial pressure, heart rate, heart failure group membership, body mass index, history of hypertension, coronary artery disease, diabetes, warfarin use, serum calcium, magnesium and phosphorus, and eGFR, warfarin use was significantly associated with CF-PWV (Standardized β=0.13; 95%CI=0.006-0.26; P=0.041). After further adjustment for dp-ucMGP levels, warfarin was no longer associated with CF-PWV (Standardized β=0.04; 95%CI=−0.12 to 0.19; P=0.63).
Discussion
In this study, we investigated the association between plasma levels of dp-ucMGP (a marker of vitamin-K dependent activation of MGP, a potent inhibitor of vascular calcification), HF and large artery stiffness (CF-PWV). Our study demonstrates that dp-ucMGP levels are significantly increased in subjects with both HFpEF and HFrEF in comparison to individuals without HF. We found that dp-ucMGP was significantly associated with CF-PWV after adjusting for multiple potential confounders in regression analyses, which was also true in analyses restricted to subjects with HF. Finally, we demonstrate that warfarin use is independently associated with greater large artery stiffness. Our findings are consistent with the known vitamin K-dependent activation of MGP and its inhibitory role on arterial calcification and support the paradigm that poor activation of MGP in the vascular wall is related to large artery stiffening.
Dp-ucMGP, HF and arterial stiffness
Recent reports demonstrated a positive relationship between dp-ucMGP levels and CF-PWV in a general population sample, in patients with type 2 diabetes mellitus and in patients with mild-to-moderate renal dysfunction without HF.14; 15 A recent study also demonstrated an association between E/e’ and dp-ucMGP in a general population sample without HF, suggesting that a poor vitamin K status could constitute an early risk factor for the development of diastolic dysfunction.16 To the best of our knowledge, only one previous study has assessed dp-ucMGP levels in HF patients. This study included subjects with HFrEF and demonstrated increased dp-ucMGP levels in this population.4 Our study is the first to compare levels of dp-ucMGP in HFpEF and HFrEF and to establish a relationship between dp-ucMGP and large artery stiffness in subjects with HF. We demonstrate increased dp-ucMGP levels in both HFpEF and HFrEF. Interestingly, this association was independent of multiple confounders other than renal dysfunction in both types of HF; however, in HFpEF (but not in HFrEF) it was not independent of renal function. HFpEF and HFrEF constitute different conditions with different pathophysiologic contributors and response to therapy. Comorbidities, such as renal dysfunction, are thought to play an important role in the pathophysiology of HFpEF.17 Therefore, the fact that the difference in dp-ucMGP levels was not independent of renal dysfunction may be due to the known association of HFpEF with chronic kidney disease (which was also evident in our study sample, and may primarily be responsible for increased dpucMGP levels in HFpEF), or alternatively, to the fact that patients with HFpEF who exhibit deficient MGP activation are more prone to developing progressive renal dysfunction from arterial stiffening. Although our cross-sectional study cannot assess these temporal relationships, both scenarios are plausible. A recent animal model demonstrated intrinsic abnormalities in vitamin K metabolism even in early CKD,18 and renal dysfunction has recently been shown to be strongly and independently associated with dp-ucMGP levels.15 Yet, deficient activation of MGP, with subsequent aortic calcification and stiffening, has the potential to promote the progression of renal dysfunction on one hand,19 and excessive myocardial pulsatile load (which can contribute to myocardial remodeling, dysfunction and the development of HFpEF) on the other.20; 21 Large artery stiffness and the associated pulsatile arterial hemodynamic dysfunction are implicated in the pathophysiology of HFpEF20; 21; 22; 23; 24; 25; 26; 27; 28 through effects on LV pulsatile load, the timing of wave reflections and central pressure and flow pulsatility, which in turn may promote comorbidities such as cognitive dysfunction29 and particularly renal dysfunction.12; 30; 31; 32; 33; 34 Regardless of the primary cause, our findings demonstrate that patients with HFpEF, like their HFrEF counterparts, exhibit abnormal vitamin-K dependent MGP activation, which in turn correlates with large artery stiffness. To date, effective pharmacologic interventions to improve outcomes in HFpEF are not available. Therefore, the novel identification of specific pathways that may play a role in the underlying pathophysiology of HFpEF is important, particularly if they can be targeted with available pharmacologic agents. Vitamin K2 supplementation has been shown to reduce dp-ucMGP levels, indicating that it exerts a positive effect on MGP maturation/carboxylation, consistent with its known biologic role.35; 36 Whether vitamin K2 supplementation can reduce or prevent large artery stiffening in subjects with or at risk for HFpEF or HFrEF remains to be studied in randomized trials.
We found a relationship between warfarin use and large artery stiffness (CF-PWV). It is known that warfarin interferes with vitamin K epoxide reductase in the vitamin K cycle, thereby interfering with the activation of vitamin K dependent proteins (VKDPs) such as MGP, which leads to increased levels of inactive MGP (dp-ucMGP).37; 38 We report an association between warfarin with increased dp-ucMGP levels in HF and demonstrate that warfarin use is associated with increased large artery stiffness (CF-PWV) in heart failure. This relationship was eliminated after adjustment for dp-ucMGP levels. These findings support the importance of poor activation of MGP in the vascular wall as an underlying factor in large artery stiffening. It is possible that warfarin use may potentiate this sequence of deleterious events by interfering with MGP activation. This off-target effect of warfarin may have important clinical implications, given the millions of patients with HF and other conditions current being treated with warfarin, and the availability of Direct Oral Anticoagulants (DOACs). Not only could the use of DOACs avoid warfarin-induced effects on vitamin-K dependent arterial calcification and stiffening, but the potential benefits of vitamin K2 supplementation could also be extended to patients with heart failure who require chronic anticoagulation. These important clinical issues need to be addressed in future prospective studies.
Strengths and limitations
Our study should be interpreted in the context of its strengths and limitations. Strengths of our study include the inclusion of a multiethnic population of patients and the inclusion of both HFpEF and HFrEF. We used high-fidelity carotid and femoral tonometry for measurements of CF-PWV, which is considered the gold-standard non-invasive approach for the non-invasive assessment of large artery stiffness.12 We utilized cardiac magnetic resonance imaging to accurately assess cardiac structure and function, and precisely quantify LVEF, which is critical for HF case classification. Our study also has some limitations. Consistent with the demographics of patients in a VA Medical Center, our sample was composed predominantly of males. Finally, this was a cross-sectional investigation, which provides evidence of associations, rather than direct causal inferences. However, our observations and hypotheses were based on, and should be interpreted in the context of the biologic effects of dp-ucMGP that impact arterial stiffness, and the well-established effects of warfarin on vitamin K-dependent protein carboxylation. However, future prospective longitudinal studies in larger cohorts are needed to demonstrate the causal relationship between dp-ucMGP and HF.
Perspectives
In summary, we demonstrate that both HFpEF and HFrEF are associated with greater dp-ucMGP levels, indicating a deficient vitamin K-dependent activation of MGP, an inhibitor of arterial calcification. We further demonstrate that dp-ucMGP levels are independently associated with large artery stiffening in HF and that warfarin use is associated with arterial stiffness. Interpreted in the context of the biologic role of MGP, our observations support a role for deficient vitamin-K dependent MGP activation in large artery stiffening in human HF. Warfarin use may exert deleterious effects on arterial stiffness via inhibition of vitamin-K-dependent MGP activation, which has important clinical implications. Future studies (including randomized trials) should examine the potential therapeutic implications of avoidance of warfarin on arterial calcification and stiffening, and of vitamin K2 supplementation in patients with or at risk for HF.
Novelty and Significance.
1. What is New?
Our study is the first to demonstrate increased dp-ucMGP levels in both HFpEF and HFrEF.
Warfarin use is significantly associated with large artery stiffness in HF, which could relate to its effects on the MGP-dependent pathway.
2. What is Relevant?
Large artery stiffness plays an important role in the development of cardiac dysfunction and target organ damage (such as the kidney), thus contributing both to heart failure and to associated comorbidities.
Warfarin, a widely used anticoagulant agent, is associated with large artery stiffness in HF patients, which may relate to its inhibition of vitamin K-dependent MGP activation.
Given the increasing use of DOACs, future studies should examine whether warfarin use leads to large artery stiffening in HF patients and whether vitamin K2 supplementation could represent a novel therapy to reduce or prevent the progression of large artery calcification and stiffness in patients with or at risk for heart failure.
3. Summary
We demonstrate increased levels of dp-ucMGP in HFpEF and HFrEF subjects in comparison to controls without HF. We also demonstrate that warfarin is associated with large artery stiffness, which may be due to the known vitamin K-dependent activation of MGP and its inhibitory role on arterial calcification. This relationship has important implications regarding the potential deleterious effects of warfarin (and potential benefits of vitamin K supplementation) on large artery stiffness in human HF, which should be tested in future trials.
Acknowledgments
Sources of Funding: Dr. Chirinos is supported by NIH grants R01 HL-121510-01A1 and R56 HL-124073-01A.
Disclosures: J.A.C. has received consulting honoraria from Bristol-Myers-Squibb, OPKO Healthcare, Fukuda-Denshi, Pfizer, Microsoft, Ironwood Pharmaceuticals, Sanifit, Bayer and Merck. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol Myers Squibb, Microsoft, and device loans from Atcor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. Other authors have no disclosures.
References
- 1.DALMEIJER GW et al. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem, v. 24, n. 4, p. 624–8, April 2013. ISSN 1873-4847 (Electronic) 0955-2863 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/22819559 >. [DOI] [PubMed] [Google Scholar]
- 2.PIVIN E et al. Inactive Matrix Gla-Protein Is Associated With Arterial Stiffness in an Adult Population-Based Study. Hypertension, v. 66, n. 1, p. 85–92, July 2015. ISSN 1524-4563 (Electronic) 0194-911X (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/25987667 >. [DOI] [PubMed] [Google Scholar]
- 3.MAYER O JR. et al. Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens, v. 30, n. 7, p. 418–23, July 2016. ISSN 1476-5527 (Electronic) 0950-9240 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/26016598 >. [DOI] [PubMed] [Google Scholar]
- 4.UELAND T et al. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin Sci (Lond), v. 121, n. 3, p. 119–27, August 2011. ISSN 1470-8736 (Electronic) 0143–5221 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/21294711 >. [DOI] [PubMed] [Google Scholar]
- 5.PRICE PA; FAUS SA; WILLIAMSON MK Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol, v. 18, n. 9, p. 1400–7, September 1998. ISSN 1079-5642 (Print) 1079-5642 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/9743228 >. [DOI] [PubMed] [Google Scholar]
- 6.CHATROU ML et al. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev, v. 26, n. 4, p. 155–66, July 2012. ISSN 1532-1681 (Electronic) 0268-960X (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/22520397 >. [DOI] [PubMed] [Google Scholar]
- 7.POTERUCHA TJ; GOLDHABER SZ Warfarin and Vascular Calcification. Am J Med, v. 129, n. 6, p. 635 e1–4, June 2016. ISSN 1555-7162 (Electronic) 0002-9343 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/26714212 >. [DOI] [PubMed] [Google Scholar]
- 8.DEMER LL; BOSTROM KI Conflicting forces of warfarin and matrix gla protein in the artery wall. Arterioscler Thromb Vasc Biol, v. 35, n. 1, p. 9–10, January 2015. ISSN 1524-4636 (Electronic) 1079-5642 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/25520520 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.TANTISATTAMO E; HAN KH; O’NEILL WC Increased vascular calcification in patients receiving warfarin. Arterioscler Thromb Vasc Biol, v. 35, n. 1, p. 237–42, January 2015. ISSN 1524-4636 (Electronic) 1079-5642 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/25324574 >. [DOI] [PubMed] [Google Scholar]
- 10.MAC-WAY F et al. The impact of warfarin on the rate of progression of aortic stiffness in hemodialysis patients: a longitudinal study. Nephrol Dial Transplant, v. 29, n. 11, p. 2113–20, November 2014. ISSN 1460-2385 (Electronic) 0931–0509 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/24944209 >. [DOI] [PubMed] [Google Scholar]
- 11.MCENIERY CM et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension, v. 53, n. 3, p. 524–31, March 2009. ISSN 1524-4563 (Electronic) 0194–911X (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/19171791 >. [DOI] [PubMed] [Google Scholar]
- 12.TOWNSEND RR et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension, v. 66, n. 3, p. 698–722, September 2015. ISSN 1524-4563 (Electronic) 0194-911X (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/26160955 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LAURENT S et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J, v. 27, n. 21, p. 2588–605, November 2006. ISSN 0195-668X (Print) 0195-668X (Linking). Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/17000623 >. [DOI] [PubMed] [Google Scholar]
- 14.SARDANA M et al. Inactive Matrix Gla-Protein and Arterial Stiffness in Type 2 Diabetes Mellitus. Am J Hypertens, v. 30, n. 2, p. 196–201, February 2017. ISSN 1941-7225 (Electronic) 0895-7061 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/27927630 >. Disponível em: < http://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ajh/30/2/10.1093_ajh_hpw146/3/hpw146.pdf?Expires=1485470684&Signature=PRwDoSqoqvDMdxLOOJX9BJClBh0Yv2M~KVNLlyUcFJ086fV2ATmCSAxVFgANWJzPV-HoyCuqXBQycIoniqR738ouLvePAbT4fZ9pK~idqhH8xpakh1jwkm~iefQ8ARL6p5MXGabfP0JFZLdQvWgh1vm8tgNYg1xnn-B0GO0hdavmTuJ-eZdFn5oXZeYlRchfhSL2NyXjHoDQlKiWtB1QFsoTBi6zoBtnM0Q6AETIAHtDgIqc8beHzxuQdFLiki6IzZ4dYQctbZwhdOk9CBl1iw99uqx0r-~UvG20cJ9ZtB60yrkWANMUplST8ThIlLtkMFIKWG0ekFLLHU2Lc70fcg__&Key-Pair-Id=APKAIUCZBIA4LVPAVW >. [DOI] [PubMed] [Google Scholar]
- 15.PUZANTIAN H et al. Circulating dp-ucMGP is Associated with Kidney Dysfunction and Arterial Stiffness. Am J Hypertens, May 17 2018. ISSN 1941-7225 (Electronic) 0895-7061 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/29788226 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WEI FF et al. Epidemiological and histological findings implicate matrix Gla protein in diastolic left ventricular dysfunction. PLoS One, v. 13, n. 3, p. e0193967, 2018. ISSN 1932-6203 (Electronic) 1932-6203 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/29529056 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SHAH SJ et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation, v. 134, n. 1, p. 73–90, July 05 2016. ISSN 1524-4539 (Electronic) 0009-7322 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/27358439 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MCCABE KM et al. Vitamin K Metabolism in a Rat Model of Chronic Kidney Disease. Am J Nephrol, v. 45, n. 1, p. 4–13, 2017. ISSN 1421-9670 (Electronic) 0250-8095 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/27846632 >. [DOI] [PubMed] [Google Scholar]
- 19.TOWNSEND RR et al. Association of Pulse Wave Velocity With Chronic Kidney Disease Progression and Mortality: Findings From the CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension, v. 71, n. 6, p. 1101–1107, June 2018. ISSN 1524-4563 (Electronic) 0194-911X (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/29712736 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WEBER T et al. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens, v. 21, n. 11, p. 1194–202, November 2008. ISSN 1941-7225 (Electronic) 0895-7061 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/18787521 >. [DOI] [PubMed] [Google Scholar]
- 21.CHIRINOS JA Deep Phenotyping of Systemic Arterial Hemodynamics in HFpEF (Part 2): Clinical and Therapeutic Considerations. J Cardiovasc Transl Res, April 11 2017. ISSN 1937-5395 (Electronic) 1937-5387 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/28401511 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CHIRINOS JA; SWEITZER N Ventricular-Arterial Coupling in Chronic Heart Failure. Card Fail Rev, v. 3, n. 1, p. 12–18, April 2017. ISSN 2057-7540 (Print) 2057–7540 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/28785470 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CHIRINOS JA Deciphering Systolic-Diastolic Coupling in the Intact Heart. Hypertension, v. 69, n. 4, p. 575–577, April 2017. ISSN 1524-4563 (Electronic) 0194-911X (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/28223470 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ZAMANI P et al. Pulsatile Load Components, Resistive Load and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis (MESA). J Card Fail, v. 22, n. 12, p. 988–995, December 2016. ISSN 1532-8414 (Electronic) 1071-9164 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/27109621 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WEBER T et al. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. Journal of the American College of Cardiology, v. 61, n. 18, p. 1874–83, May 7 2013. ISSN 1558-3597 (Electronic) 0735-1097 (Linking). Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/23500307 >. [DOI] [PubMed] [Google Scholar]
- 26.GILLEBERT TC; LEITE-MOREIRA AF; DE HERT SG Load dependent diastolic dysfunction in heart failure. Heart Fail Rev, v. 5, n. 4, p. 345–55, December 2000. ISSN 1382-4147 (Print) 1382-4147 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/11498648 >. [DOI] [PubMed] [Google Scholar]
- 27.TARTIERE-KESRI L et al. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol, v. 59, n. 5, p. 455–61, January 31 2012. ISSN 1558-3597 (Electronic) 0735-1097 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/22281248 >. [DOI] [PubMed] [Google Scholar]
- 28.CHIRINOS JA et al. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail, v. 7, n. 5, p. 709–16, September 2014. ISSN 1941-3297 (Electronic) 1941-3289 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/25059422 >. Disponível em: < http://circheartfailure.ahajournals.org/content/circhf/7/5/709.full.pdf >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DE ROOS A et al. Magnetic Resonance Imaging of Cardiovascular Function and the Brain: Is Dementia a Cardiovascular-Driven Disease? Circulation, v. 135, n. 22, p. 2178–2195, May 30 2017. ISSN 1524-4539 (Electronic) 0009-7322 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/28559496 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WOODARD T et al. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol, v. 26, n. 5, p. 1181–7, May 2015. ISSN 1533-3450 (Electronic) 1046-6673 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/25294231 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’ROURKE MF; SAFAR ME Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension, v. 46, n. 1, p. 200–4, July 2005. ISSN 1524-4563 (Electronic) 0194-911X (Linking). Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15911742 >. Disponível em: < http://hyper.ahajournals.org/content/hypertensionaha/46/1/200.full.pdf >. [DOI] [PubMed] [Google Scholar]
- 32.BLACHER J et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation, v. 99, n. 18, p. 2434–9, May 11 1999. ISSN 1524-4539 (Electronic) 0009-7322 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/10318666 >. [DOI] [PubMed] [Google Scholar]
- 33.TOWNSEND RR et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens, v. 23, n. 3, p. 282–9, March 2010. ISSN 1941-7225 (Electronic) 0895-7061 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/20019670 >. Disponível em: < http://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ajh/23/3/10.1038_ajh.2009.240/3/23_3_282.pdf?Expires=1485470657&Signature=JP6aARwka0AUDgzNaGhjxUR~gER4rLkyzZEx8w3eX9ZPGq8cqd1Vea6oMbpG-ALnmVVL5Wnv9D25iDADwzaaKIgJ52KB~cPiIaI6YxIjTEbALBNMcjW3wvMECcqa4KJu5CnEpRwair0rdfaT0utHbgcziKbXJY7toyqJw7z3td1LhisYTB2Ca3d7Tg7GrpERgEjiVcl2Z7SjopUArxsaNWZRbOrNhr7HeycOf0SA5fAxpwXQ9VZxSMJOqk2A2iWV8hMMICManj0Sqaz9DQlBMGRm7SXumoJqSGObaZCw0Bdj3rev18cKRFs6Vvu1PnTVBd-QLWJOYSsHL2CBif1CHQ__&Key-Pair-Id=APKAIUCZBIA4LV >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SAFAR ME et al. Pulse pressure, arterial stiffness, and end-organ damage. Curr Hypertens Rep, v. 14, n. 4, p. 339–44, August 2012. ISSN 1534-3111 (Electronic) 1522-6417 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/22555981 >. [DOI] [PubMed] [Google Scholar]
- 35.SCHLIEPER G et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol, v. 22, n. 2, p. 387–95, February 2011. ISSN 1533-3450 (Electronic) 1046-6673 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/21289218 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.KURNATOWSKA I et al. Plasma Desphospho-Uncarboxylated Matrix Gla Protein as a Marker of Kidney Damage and Cardiovascular Risk in Advanced Stage of Chronic Kidney Disease. Kidney Blood Press Res, v. 41, n. 3, p. 231–9, 2016. ISSN 1423-0143 (Electronic) 1420-4096 (Linking). Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/27100101 >. [DOI] [PubMed] [Google Scholar]
- 37.DANZIGER J Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol, v. 3, n. 5, p. 1504–10, September 2008. ISSN 1555-9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.UELAND T et al. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med, v. 268, n. 5, p. 483–92, November 2010. ISSN 0954-6820. [DOI] [PubMed] [Google Scholar]