Abstract
Objective:
To identify predictors of near dehiscence (ND) or thin rather than dehiscent bone overlying the superior semicircular canal in patients with signs and symptoms suggestive of superior semicircular canal dehiscence syndrome (SCDS), as well as post-operative outcomes.
Study Design:
Retrospective case-control study
Setting:
Tertiary referral center
Patients:
All 288 patients who underwent middle cranial fossa approach for repair of SCDS (1998-2018) were reviewed for cases of ND. Demographics, symptoms and clinical signs including nystagmus, ocular vestibular-evoked myogenic potential (oVEMP) amplitude, cervical vestibular-evoked myogenic potential (cVEMP) thresholds, and low frequency air-bone gap (ABG) were compared before and after surgery.
Main outcome measure:
Presence of pre-operative ND and post-operative symptoms and physiologic measures.
Results:
Seventeen cases of ND (16 patients, 17 ears) and 34 cases (34 ears) of frank SCDS were identified. ND cases differed from frank dehiscence cases in that they were less likely to have nystagmus in response to ear canal pressure or loud sounds, OR=0.05(95%CI 0.01-0.25) and Valsalva, OR=0.08(0.01-0.67), smaller peak-to-peak oVEMP amplitudes, OR=0.84(0.75-0.95), and higher cVEMP thresholds, OR=1.21(1.07-1.37). Patients with ND had similar symptoms to those with frank SCDS before surgery, and after surgery had outcomes similar to patients with frank SCDS.
Conclusions:
In patients with symptoms consistent with SCDS, predictors of ND include absence of nystagmus in response to pressure/loud sounds, greater cVEMP thresholds, and smaller oVEMP amplitudes. We propose ND is on a spectrum of dehiscence that partially accounts for the diversity of clinical presentations of patients with SCDS.
Introduction
Superior semicircular canal dehiscence syndrome (SCDS) is characterized by a defect in the temporal bone overlying the superior semicircular canal resulting in balance and hearing dysfunction. The prevalence of an anatomic dehiscence is estimated to be 0.5-0.6% of the population, with 1-2% having thin (<0.1 mm) bone overlying the superior semicircular canal1. A subset of patients with anatomic SCD additionally have sufficient dural compliance to transmit pressures between the otic capsule and intracranial space to complete the third mobile window syndrome known as SCDS. Vestibular symptoms of these patients include Tullio phenomenon (sound-induced dizziness), oscillopsia, and chronic unsteadiness, while auditory symptoms include autophony (hearing one’s own body’s sounds unusually loudly), pulsatile tinnitus, conductive hyperacusis, and low frequency conductive hearing loss2-4.
The diagnosis of SCDS depends on identification of an anatomic dehiscence, typically using high resolution computed tomography (CT) with reconstructions in and orthogonal to the plane of the superior semicircular canal, the presence of symptoms that characterize this disorder, and abnormal findings on physiologic testing to substantiate a third mobile window. Audiometry can help identify either an air-bone gap (ABG) or lower-than-normal bone conduction thresholds that are often present at low frequencies. Depressed cervical vestibular evoked myogenic potential thresholds (cVEMP) and elevated ocular vestibular evoked myogenic potential (oVEMP) amplitudes have also been recognized as predictive of dehiscence2-4. Treatment of patients with SCDS ranges from avoidance of triggers to surgical resurfacing or plugging of the dehiscent semicircular canal5.
In a subset of patients undergoing corrective SCDS surgery, we have found thin bone overlying their superior semicircular canal rather than frank dehiscence6. For a physiologically measurable third mobile window syndrome to have developed in these cases, we presume that the bone thinning must have exceeded the threshold for increased compliance to cause relevant lymph movements in the canal. Our previous report of patients with documented near dehiscence (ND) found that these patients did experience many of the same benefits of surgical repair but cautioned that they may be at greater risk for complications than those with SCDS and that 45% of those patients with ND had recurrence of at least one symptom of SCDS after surgery. Additionally, two patients (18%) in this case series suffered moderate-severe hearing loss, a higher rate than had been reported in previous series, and two patients (18%) developed transient facial nerve paresis postoperatively with full recovery ultimately. These findings may caution patients with suspected ND of possible increased surgical risk, with the inner ear complications potentially being related to the opening of a previously sealed semicircular canal. In this context, clinicians may be aided by the signs, symptoms or physiologic diagnostics that are predictive of ND rather than frank semicircular canal dehiscence (SCD). In the current study we use a case-control design in which patients with SCDS, but an intraoperative finding of ND are compared to those with frank dehiscence. This design offers a convenient way to ascertain factors that are associated with thinning, while adjusting for potential confounders. The purpose of this study is to identify predictors of ND and to assess whether outcomes were different among those getting surgery for ND compared to SCDS.
Materials and Methods
The records of 288 patients who underwent surgical repair of SCDS at Johns Hopkins Hospital between 1998 and December 2018 were reviewed to identify cases of ND as well as cases of frank superior semicircular canal dehiscence (SCD) as confirmed at the time of surgery. Preliminary results from 10 patients with ND have previously been reported without a comparison to matched controls6. Six additional cases were ascertained in the time since the original description. Pre-operative high-resolution CT imaging (≤0.625 mm slice thickness) was performed in all cases; images were reformatted in the plane of the superior semicircular canal and orthogonal to it and were interpreted by an attending neuroradiologist. Patients were also required to have physiologic data (audiogram, cVEMP, and oVEMP measures) to substantiate a third mobile window syndrome. Similar proportions by age and sex were included in each group of ND and frank SCD with ages matched in 5-year age groups to achieve a 2:1 ratio of frank dehiscence to ND. Normative data of healthy non-SCDS patients were also used for comparison to both the groups.
Videooculography
As part of the initial clinical examination of patients for SCDS, the patient’s eyes were examined using infrared videooculography goggles (VisualEyes 505 Binocular, Micromedical, Chatham, IL USA) while provocative vestibular testing was performed. Patients were asked to perform nasal and glottic Valsalva maneuvers. Eye movements were then assessed for nystagmus in the plane of the superior semicircular canal synchronous with the application of ear canal pressure and during the application of tones. Pure tones were administered to each ear using an audiometer while the subject wears headphones. Frequencies of the applied tones ranged from 125 to 8000 Hz at intensities of at least 85 dB HL.
Audiometry
All patients underwent audiometric testing before surgery and at least 1 month postoperatively. Pure-tone hearing thresholds were obtained using both air conduction (AC) at 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz and bone conduction (BC) at 250, 500, 1,000, 2,000, and 4,000 Hz. The audiometer was calibrated to permit accurate measurement of BC thresholds to −10 dB HL. Speech discrimination was also tested for all patients using the Northwestern University word list 6 (NU-6). The air-bone gap (ABG) was calculated by subtracting the BC threshold from the AC threshold at each frequency. An average ABG was also calculated for each audiogram over the following frequencies: 250, 500, 1,000, and 2,000 Hz. Pure tone average (PTA) was recorded as the average of 500, 1000, 2000 and 4000 Hz using bone conduction values.
Vestibular-Evoked Myogenic Potentials
The cVEMP and oVEMP responses to air-conducted sound (ACS) were assessed preoperatively in all patients as previously described7,8. Patients were seated in semi-recumbent position and electromyograms were recorded with disposable silver/silver chloride electrodes. A commercial electromyographic (EMG) system was used (Medetec Synergy, software version 14.1, Care Fusion, Dublin, OH, USA). Sound stimuli delivered via calibrated TDH-49 headphones at a rate of 5 pps included: 0.1 ms positive polarity clicks at varying levels for cVEMP and 500-Hz, 125-dB sound pressure level (SPL) positive polarity tone bursts with 1 ms rise/fall time and 2 ms plateau for oVEMP. EMG signals were amplified (gain = 2,500) and band pass filtered (20-2,000 Hz for cVEMP and 3-500 Hz for oVEMP). One hundred sweeps were averaged in each trial. Threshold levels for cVEMPs (5-dB resolution) were obtained with the sternocleidomastoid muscle activity maintained at a rectified EMG value of at least 50 microvolts. Threshold level was defined as the lowest intensity level at which an elicited cVEMP could be identified. For oVEMP testing, peak-to-peak amplitude was measured from the maximum negative voltage of the n10 potential to the maximum positive voltage of the p16 potential.
Surgical Technique
The middle cranial fossa surgical approach was used in all patients, and intraoperative image guidance assisted in all procedures to locate the affected site of the superior canal. In the majority cases, the superior semicircular canal was plugged using autologous materials, and then resurfaced. When a frank dehiscence was present, the length was measured. Resurfacing alone was performed in a minority of ND patients (n=4). The canal lumen was accessed for plugging in the other ND cases by drilling open the thin bone overlying the affected canal for a length of 3 to 4 mm and gently but securely packing fascia strips, bone dust and bone chips inside the dehiscent canal to obliterate the canal lumen for 2 to 3 mm beyond either end of the opening. Careful avoidance of unnecessary force on or suction near the membranous labyrinth was observed throughout the procedure. In all procedures, the repair was covered with hydroxyapatite cement (Hydroset, Stryker, Kalamazoo, Michigan) followed by a layer of fascia and fibrin glue. Intraoperative neural monitoring was performed for the facial nerve, auditory brainstem response, electrocochleography (ECochG), and somatosensory evoked potentials. All patients received dexamethasone (6-8 mg) 3 times per day for 3 doses. Following that, if the postoperative clinical examination revealed no evidence of either sensorineural hearing loss by tuning fork examination or global labyrinthine hypofunction by horizontal head impulse testing, then steroids were tapered over 5 days. In contrast, if patients who had a tuning fork examination that lateralized to the nonsurgical ear and/or audiometric evidence of sensorineural hearing loss and had overt saccades during head impulse testing in the plane of the horizontal semicircular canal suggesting hypofunction extending beyond the superior canal on the side of surgery, then steroids were tapered over a 10- to 14-day period. Patients were assessed for benign paroxysmal positional vertigo (BPPV) by Dix-Hallpike maneuver if they reported positional vertigo. The function of each semicircular canal was assessed one month after surgery by clinical head impulse testing. Impairment of the posterior semicircular canal was determined by an overt corrective saccade after a head impulse test in the plane of the posterior semicircular canal.
Data Analysis
Summary statistics were computed for demographics, ABGs, VEMP amplitudes or thresholds, and presenting symptoms, as well as for postoperative symptoms and complications. Since there was a small sample size and non-normal distribution of predictor variables, nonparametric tests were used for all analyses; median and interquartile ranges (IQRs, i.e. the differences between the 25th and 75th percentiles) were tabulated. Due to the small number of cases in each group and cells with n<5, Fisher’s exact test was used to compare the pre-procedure binary categorical variables including auditory and vestibular symptoms, migraine, the prevalence of persistent/worsening symptoms and surgical complications between cases with surgically-confirmed ND versus those with frank SCD. Nonparametric Spearman correlations were used to analyze the relationships between the continuous variables of preoperative average ABG size, oVEMP amplitude, and cVEMP threshold and the outcome of finding ND or frank dehiscence at surgery. Published normative data of healthy controls are also included in the table for comparison to the cases of ND and frank SCD. Associations were considered statistically significant for 2-sided statistics with a p <0.05. For those correlations that had significant associations by the Spearman correlation, logistic regression analyses were used to provide odds ratios. All analyses were performed using Stata 12.0 (StataCorp, College Station, TX, USA).
Results
Seventeen cases of ND (16 patients, 17 ears) and 34 age- and gender-matched cases of frank SCD (34 patients, 34 ears) were identified from the 288 patients who underwent SCDS surgery that we reviewed (Table 1). All patients with true dehiscence underwent a middle cranial fossa plugging and resurfacing. The majority of ND patients underwent middle cranial fossa plugging and resurfacing (13/17) while 4 underwent middle fossa resurfacing. The mean (SD) age of cases of ND was 41.1 (12.9) years, which was no different than the mean age of cases with frank SCD, which was 42.2 (9.3) years. The cases of both ND and SCDS were 65% female. Of the 17 ND cases that were confirmed during surgery, 35% (6/17) were misdiagnosed on the radiology interpretation of preoperative CT imaging as having frank SCD and in 24% (4/17) the radiologist was noncommittal to SCD versus thinning. Of the 34 frank dehiscence cases, all cases were correctly diagnosed by preoperative CT imaging.
Table 1:
Preoperative features found in cases of near dehiscence (ND) of the superior canal vs frank superior canal dehiscence (SCD)
| ND (16 patients; 17 ears) | frank SCD (34 patients; 34 ears) | p value | ||
|---|---|---|---|---|
| Mean age (SD), years | 41.1 (12.9) | 42.2 (9.3) | 0.72 | |
| Sex | Female (%) | 11 (65) | 24 (65) | |
| Male (%) | 6 (35) | 12 (35) | ||
| Prevalence of auditory symptoms | ||||
| Pulsatile tinnitus (%) | 16 (94) | 28 (82) | 0.40 | |
| Non-pulsatile tinnitus (%) | 6 (35) | 4(12) | 0.07 | |
| *1 Any tinnitus (%) | 17 (100) | 28 (82) | 0.16 | |
| Autophony (%) | 14 (82) | 32 (94) | 0.32 | |
| Aural fullness (%) | 15 (88) | 25 (78)*2 | 0.47 | |
| Any auditory (%) | 17 (100) | 34 (100) | 0.50 | |
| Prevalence of vestibular symptoms and signs | ||||
| Vertigo to sound/pressure (%) | 12 (71) | 27 (79) | 0.50 | |
| Chronic disequilibrium (%) | 9 (53) | 13 (41) | 0.37 | |
| *3 Nystagmus (%) | 3 (19)*4 | 30 (94)*5 | 0.00001 | |
| Any vestibular (%) | 12 (71) | 32 (94) | 0.03 | |
| Other symptoms | ||||
| Any headaches (%) | 12 (71) | 22 (65) | 0.76 | |
| Cognitive/Brain Fog (%) | 4 (24) | 8 (24) | 1.0 | |
| Obstructive Sleep Apnea (%) | 2 (12) | 1 (3) | 0.25 | |
| Migraine | ||||
| Hx of migraines (%) | 7 (41) | 12 (35) | 0.76 | |
| Family Hx of migraines (%) | 6 (35) | 9 (26) | 0.53 | |
| Migraine pharma Tx (%) | 7 (41) | 6 (18) | 0.09 | |
| Migraine dietary Tx (%) | 9 (53) | 4 (12) | <0.01 | |
| Physiologic findings | ||||
| cVEMP median threshold dB | 95 (85-95, n=9) | 70 (65-70, n=30) | <0.001 | |
| nHL (IQR) | ||||
| [normal: 100 (90-105)] | ||||
| oVEMP tone median amplitude | 6.7 (1.6-20.7, n=11) | 33 (28.7-58.2, n=21) | <0.001 | |
| μV (IQR) | ||||
| [normal: 3.7 (2.8-6.9)]] | ||||
| Median low frequency ABG dB | 11.3 (9.4-16.25, n=16) | 18.1 (10-21.3), n=34) | 0.07 | |
| nHL (IQR) | ||||
SD, standard deviation, Hx, history, pharma, pharmacology, Tx, treatment, Significantly different values in cases with ND compared to those with frank dehiscence are in bold with the corresponding p values from Spearman correlations. Physiologic findings from ND and SCD patients as well as normal values included for reference are medians ± interquartile ranges (IQRs)2.
The Any tinnitus value included data that had preoperative symptoms of pulsatile tinnitus and/or non-pulsatile tinnitus.
Information on aural fullness was unavailable for two patients with SCD producing a sample size of 32.
The Nystagmus statistic included data from any elicited nystagmus from tone or Valsalva.
Information on any nystagmus symptoms for one patient in the ND group was unavailable producing a sample size of 16.
Information on any nystagmus symptoms for two patients in the SCD group was unavailable producing a sample size of 32.
The median cVEMP threshold for ND patients was higher than in cases of frank dehiscence, whereas the median peak-to-peak oVEMP amplitude for those with ND was lower. The median value for the pre-operative average ABG for ND patients was almost, but not significantly different than for those with frank dehiscence (p=0.07). Non-parametric Spearman correlations demonstrated that the following factors predicted the presence of ND: absence of tone and Valsalva-induced nystagmus, higher cVEMP threshold, lower oVEMP amplitude, and history of migraine dietary treatment. For these significant factors, odds ratios for ND were calculated by logistic regression. ORs <1 were found for nystagmus synchronous with pressure or tones being applied to the ear (OR 0.05 (95%CI 0.01-0.25), nystagmus during Valsalva maneuvers (0.08 (95%CI 0.01-0.67), and for peak-to-peak oVEMP amplitude (OR=0.84, 95%CI: 0.75-0.95), meaning that the absence of nystagmus in response to tones or Valsalva, and smaller oVEMP amplitude predicted ND. Conversely, the OR for cVEMP threshold was 1.21 (95%CI: 1.07-1.37), meaning that greater cVEMP threshold predicted ND rather than frank dehiscence.
There were no significant differences in pre-operative symptoms between those cases with ND and those with frank SCDS (p>0.05). Despite having similar rates of history of migraine headaches, family history of migraine, and headache symptoms (p>0.05), patients with ND were more likely to have undergone treatment for migraine with dietary trigger avoidance prior to surgery than those with frank dehiscence (OR=8.44 (95%CI 2.05-34.6).
Audiometric data are shown in figures 1 and 2. After surgery, there were no significant differences in PTA or low-frequency ABG between cases of ND and frank SCDS. The prevalence of persistent or worsening auditory and vestibular symptoms was similar between the two groups (p>0.05), although there was a trend toward a higher rate of persisting auditory symptoms in the ND group (41% vs 18%, p=0.09). The prevalence of recorded complications after surgery was similar (p>0.05). Values for postoperative cVEMP threshold and oVEMP amplitude were not significantly different between the groups.
Figure 1.
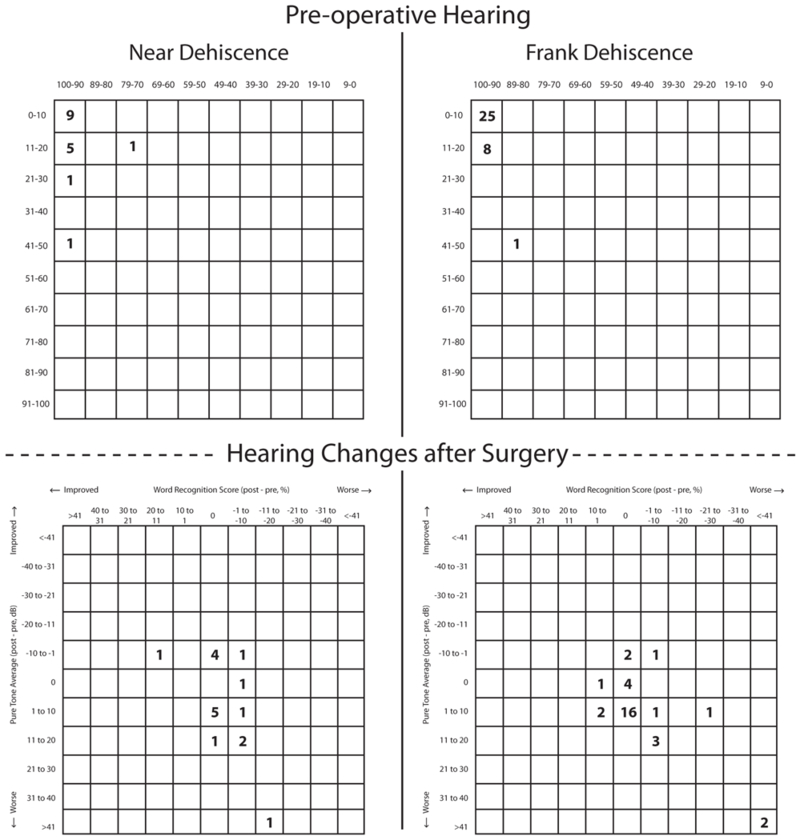
Scattergram plots of pre-operative functional hearing and post-operative changes for near dehiscence and frank dehiscence.
Figure 2.
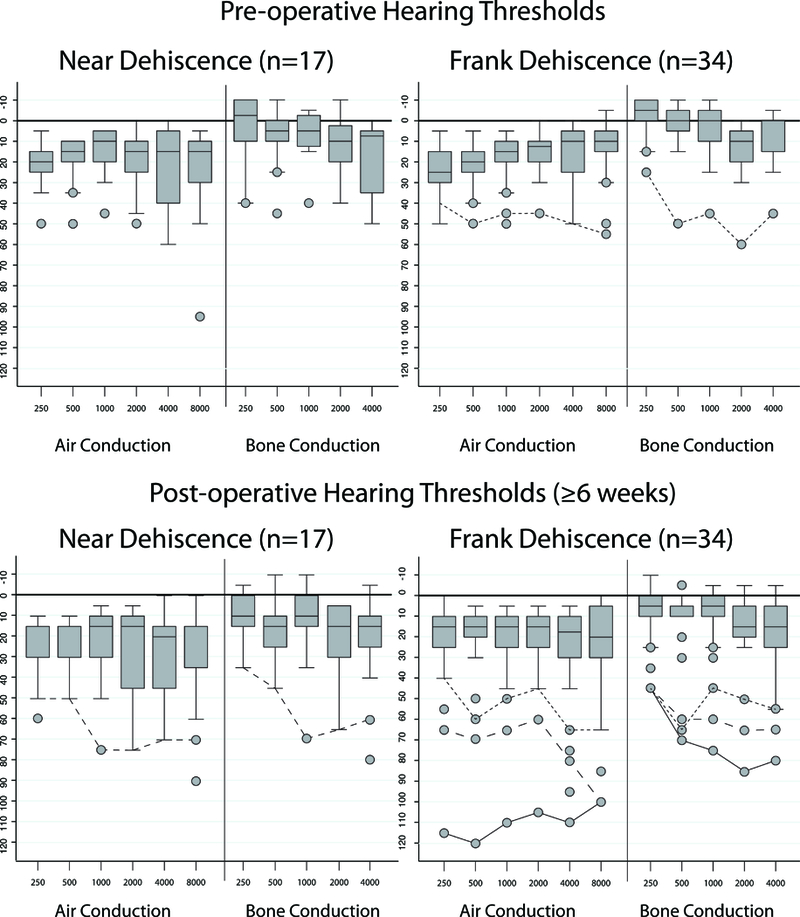
Box plots showing the median and interquartile ranges of pure tone thresholds before and at least 6 weeks after surgery for patients with near dehiscence and frank dehiscence. Outlier cases that developed a new sensorineural hearing loss after surgery were identified in figure 1 and are shown here as solid and hashed lines. One case in the frank dehiscence group had pre-operative sensorineural hearing loss that was stable after surgery.
Discussion
This case-control study was designed to identify predictors of ND in patients who presented with signs and symptoms of SCDS and to determine whether patients with ND were at increased risk for persistent symptoms or postoperative complications. A strength of the study is that the actual status of the bone over the superior canal was ultimately determined at the time of surgery in each case. The surgeries were done via the middle cranial fossa approach, which affords a direct view of the portion of the canal in question. Several physiologic findings suggested ND, including lower likelihood of having nystagmus in response to tones or Valsalva maneuvers, more modest reductions of cVEMP thresholds, and more modest elevations of oVEMP peak-to-peak amplitudes, i.e. values for these measures were between those encountered in patients with frank dehiscence and those typically found in healthy non-dehiscent volunteers.
The results of this study suggest that physiologic testing may inform the clinician of the pre-operative likelihood of finding thin rather than dehiscent bone. Janky et al.8 showed oVEMPs to be the physiologic test of greatest utility in discriminating between patients with dehiscent and non-dehiscent superior semicircular canals. It may be conceptually useful to place ND on a spectrum between these two groups. With the odds of having ND increasing for every unit decrease in oVEMP (OR = 0.84), oVEMP readings can be employed to separate frank dehiscence from ND. In practical terms, a patient with SCDS symptoms and an oVEMP amplitude of 15 μV would have 4.8 times higher odds of having ND than a patient with an oVEMP amplitude of 45 μV ((45-15) x (1-0.84)), values typical of the cases in this study. Additionally, oVEMPs have better test-retest reliability than cVEMPs9.
Mehta et al. recently showed cVEMP thresholds and ABG to be of greatest utility in distinguishing ND from frank dehiscence on CT imaging10. While compatible with their findings, this study additionally identified oVEMP amplitude lower than in frank dehiscence (but still abnormal) as predictive of ND. A possible explanation for this difference is that only those with surgically confirmed thin or dehiscent bone were included in this study, thereby eliminating crossover between groups due to limitations in the imaging modality.
Imaging is important for identifying a dehiscence of the superior semicircular canal; nevertheless, high resolution temporal bone CT scans can overestimate the presence of a dehiscence11, and rely upon a clinician’s interpretation as to whether frank dehiscence or thin bone is present over the superior semicircular canal6. Spear et al. also showed a high negative predictive value (88.2%) for thin bone using magnetic resonance imaging (MRI) but determined that it couldn’t decisively diagnose thin bone overlying the superior canal12. New imaging approaches are needed to improve the sensitivity and specificity for diagnosis of dehiscence and ND. Eibenberger et al. proposed a novel method of objectively analyzing scans with a spatial differentiation algorithm to more accurately detect a ND, achieving spatial resolution down to 0.1 mm bone thicknesses13. This or other techniques may improve the radiographic diagnosis of ND.
We previously proposed that dampening endolymph motion could explain the reduction in the gain of the superior canal’s vestibuloocular reflex seen in cases with dehiscences larger than 5 mm in length14. We speculated that ‘autoplugging’ occurred in patients with large dehiscences due to the large dehiscence allowing herniation of the dura sufficiently far into the canal to dampen lymph motions. Based on the findings in this study, we propose that symptoms of a third mobile window may exist on a spectrum, figure 3, ranging from ND to a large dehiscence with “autoplugging”, accounting for some of the variability in presenting findings in patients with SCDS. Prior studies have attempted to associate dehiscence size and symptoms; however, many of these studies used CT imaging to determine dehiscence length or to distinguish ND from frank dehiscence15-19. As noted above and observed in this study, CT imaging can over predict the presence of a dehiscence. Using intraoperative measurements Chien et al.20 determined that the only statistically significant measure that correlated positively with dehiscence length was maximum air-bone gap (p=0.046). Agrawal noted that patients with a larger dehiscence are at greater risk of having vestibular global hypofunction in the initial postoperative procedure with a 2.6-fold increase in odds with each 1 mm increase in dehiscence length21.
Figure 3.
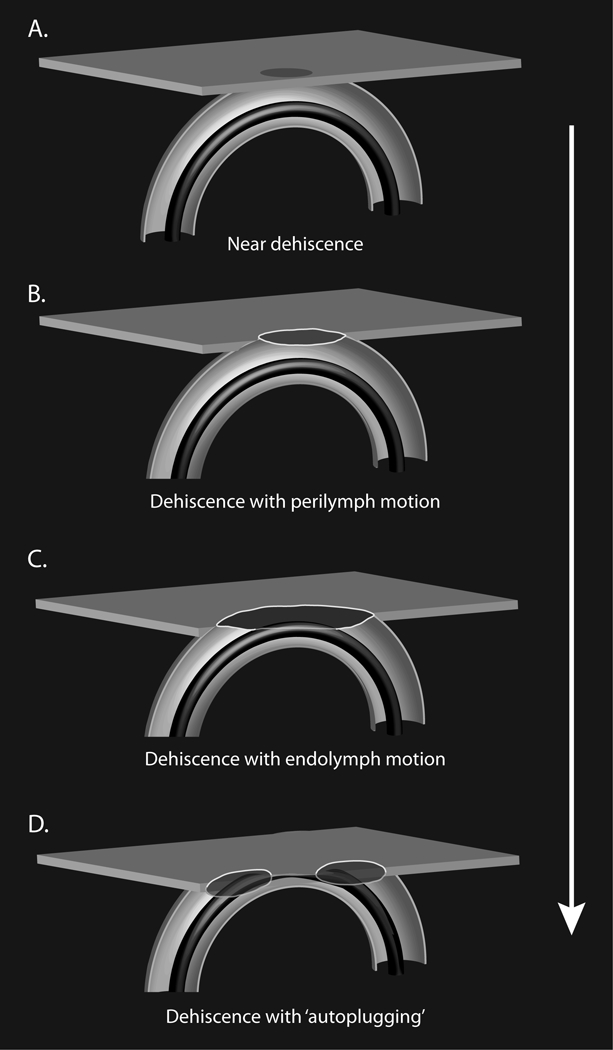
Cartoon of hypothesized spectrum of dehiscence from near dehiscence to a large dehiscence with autoplugging impairing function of the superior semicircular canal. The proposed spectrum could account for the variability in clinical presentation of patients with superior canal dehiscence syndrome.
Recently Niesten et al. sought to use anatomic differences in dehiscence location to explain variability in the clinical presentation of patients with SCDS. Their work showed larger dehiscences were associated with both auditory and vestibular symptoms, as well as larger cVEMP thresholds22. The effect of SCD size on cochlear drive from air-conducted sound has also been a subject of recent progress in cadaveric temporal bone preparations. Pisano et al. and Niesten et al. showed that for low frequency sound (below 1 kHz), increasing SCD size monotonically decreased the cochlear drive (up to 20 dB)23,24. This SCD effect saturated with dehiscence length larger than approximately 2-3 mm, although with variability between specimens23. At higher frequencies (above about 1 kHz), SCD generally had insignificant effects on cochlear drive, but surprisingly, for some ears (less than 1/3), the smallest SCD size (<0.5 mm) caused the largest decrease in intracochlear pressure in the scala vestibuli24. The authors hypothesize that cases of ND could actually have multiple micro-dehiscences due to the uneven surface of the bony semicircular canal. The sum of multiple micro-dehiscences may have the same effect as the small dehiscence observed in the temporal bone experiments24.
We found in this study that patients with ND had a similar symptomatic presentation to those patients with frank dehiscence, similar to Mehta et al9. ND cases had higher rates of treatment with migraine trigger elimination, but this likely reflects the preference of the practice to treat patients at first medically if the diagnosis of SCDS is uncertain. The decision to pursue surgical intervention should be made with a firm understanding of the third mobile window theory as it relates to the pathophysiology of SCDS. If presenting symptoms and diagnostic testing indicate abnormal transmission of pressure across a dehiscent or nearly dehiscent semicircular canal, surgical intervention may be warranted.
Pre-operative counseling for patients with likely ND should therefore include a discussion of the known risks and outcomes in patients undergoing surgical plugging or resurfacing. Prior to surgical plugging and resurfacing of a dehiscence it also may be of significant importance that the presence of thin bone/ND adjacent to a frank dehiscence be diagnosed through CT scan. Sharon et al. found that the second most common reason for revision surgery for SCDS was due to thin bone neighboring a previously plugged dehiscence 25. Lee et al. have proposed using an endoscopic-assisted middle cranial fossa approach to allow for better visualization of a ND by transilluminating thin bone 26,27. In this study, although there were not statistically significant differences in persistent or recurrent symptoms, there was a notable recurrence of at least one auditory symptom in 41% of patients operated on with ND6.
This study was primarily limited by its retrospective nature, investigation of only patients with enough suspicion and symptoms of SCDS to warrant surgery, and the small sample of patients with ND. Prospective studies are needed to estimate the rate of complications in patients undergoing surgical repair for those with ND compared to patients with frank dehiscence.
Conclusion
In the setting of a patient presenting with symptoms and diagnostic testing consistent with SCDS, smaller oVEMP amplitudes, larger cVEMP thresholds (though not within the normal range), suggest that the patient may have superior canal thinning rather than frank dehiscence. Patients with ND have similar clinical presentations to patients with SCDS and appear to have similar rates of postoperative complications and postoperative symptoms.
Table 2:
Intraoperative and Postoperative features found in cases of near dehiscence (ND) of the superior canal versus frank superior canal dehiscence (SCD)
| ND (16 patients; 17 ears) | frank SCD (34 patients; 34 ears) | p value | |
|---|---|---|---|
| Intraoperative | |||
| Length of Dehiscence (mm) | N/A | 4.2 | |
| Plugged and resurfaced (%) | 13 (76) | 34 (100) | |
| Resurfaced only (%) | 4 (24) | 0 (0) | |
| Prevalence of persistent/worse postoperative auditory symptoms | |||
| Tinnitus (%) | 5 (29) | 4 (12) | 0.14 |
| Autophony (%) | 3 (18) | 2 (6) | 0.32 |
| Aural fullness (%) | 1 (8) | 3 (9) | 1.0 |
| Any auditory symptoms (%) | 7 (41) | 6 (18) | 0.09 |
| Prevalence of persistent/worse postoperative vestibular symptoms | |||
| Vertigo (%) | 1 (6) | 4 (12) | 0.65 |
| BPPV*1 (%) | 0 (0) | 4 (12) | 0.29 |
| Vestibular hypofunction*2 (%) | 3 (18) | 13 (38) | 0.20 |
| Any vestibular symptoms (%) | 5 (29) | 16 (47) | 0.37 |
| Other Conditions | |||
| Posterior SCC impairment (%) | 2 (12) | 7 (21) | 0.70 |
| Facial nerve paresis (%) | 2 (12) | 0 (0) | 0.12 |
| Physiologic findings | |||
| cVEMP median threshold | 85 (75-97.5, n=4) | 95 (92.5-102.5, n=12) | 0.17 |
| dB nHL (IQR) | |||
| [normal: 100 (90-105)] | |||
| oVEMP tone median amplitude | 2.1 (0.9-5.7, n=6) | 2.2 (1.6-3.5, n=23) | 0.85 |
| μV (IQR) | |||
| [normal: 3.7 (2.8-6.9)]] | |||
| Median low frequency ABG | 7.5 (5-12.5, n=17) | 6.3 (3.8-12.5, n=34) | 0.73 |
| dB (IQR) | |||
| Median PTA, dB (IQR) | 12.5 (8.8-20, n=17) | 10.0 (6.2-20, n=34) | 0.41 |
Significantly different values in cases with ND compared to those with frank SCD are denoted in bold letters with the corresponding p values from the Spearman correlations. Physiologic findings from ND and SCD patients as well as normal values included for reference are medians ± interquartile ranges (IQRs)2.
(BPPV) Benign paroxysmal positional vertigo.
Temporary impairment of all semicircular canals on the side of surgery as determined by clinical head impulse testing after surgery.
Acknowledgements
Dr. Heidi Nakajima kindly provided the summary of her laboratory’s recent work on the effects of SCD size on intracochlear pressures that is included in the discussion.
Footnotes
Financial disclosures: The opinions expressed herein are those of the author(s), and are not necessarily representative of those of the Department of Defense (DOD) or the United States Navy. Medicine is a constantly changing field, and medical information is subject to frequent correction and revision. Therefore the reader is entirely responsible for verifying the accuracy and relevance of the information contained herein.
Conflict of Interest: None
References
- 1.Carey JP, Minor LB, Nager GT. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol Head Neck Surg 2000; 126:137–147. [DOI] [PubMed] [Google Scholar]
- 2.Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology 2008; 70:464–472. [DOI] [PubMed] [Google Scholar]
- 3.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope 2005; 115:1717–1727. [DOI] [PubMed] [Google Scholar]
- 4.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg 1998; 124:249–258. [DOI] [PubMed] [Google Scholar]
- 5.Ward BK, Carey JP, Minor LB. Superior Canal Dehiscence Syndrome: Lessons from the First 20 Years. Front Neurol 2017; 8:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward BK, Wenzel A, Ritzl EK et al. Near-dehiscence: clinical findings in patients with thin bone over the superior semicircular canal. Otol Neurotol 2013; 34:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 1994; 57:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janky KL, Nguyen KD, Welgampola M, Zuniga MG, Carey JP. Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Otol Neurotol 2013; 34:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol 2010; 31:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta R, Klumpp ML, Spear SA, Bowen MA, Arriaga MA, Ying YL. Subjective and objective findings in patients with true dehiscence versus thin bone over the superior semicircular canal. Otol Neurotol 2015; 36:289–294. [DOI] [PubMed] [Google Scholar]
- 11.Sequeira SM, Whiting BR, Shimony JS, Vo KD, Hullar TE. Accuracy of computed tomography detection of superior canal dehiscence. Otol Neurotol 2011; 32:1500–1505. [DOI] [PubMed] [Google Scholar]
- 12.Spear SA, Jackson NM, Mehta R et al. Is MRI Equal to CT in the Evaluation of Thin and Dehiscent Superior Semicircular Canals? Otol Neurotol 2016; 37:167–170. [DOI] [PubMed] [Google Scholar]
- 13.Eibenberger K, Carey J, Ehtiati T, Trevino C, Dolberg J, Haslwanter T. A novel method of 3D image analysis of high-resolution cone beam CT and multi slice CT for the detection of semicircular canal dehiscence. Otol Neurotol 2014; 35:329–337. [DOI] [PubMed] [Google Scholar]
- 14.Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology 2000; 55:1833–1841. [DOI] [PubMed] [Google Scholar]
- 15.Noij KS, Duarte MJ, Wong K et al. Toward Optimizing Cervical Vestibular Evoked Myogenic Potentials (cVEMP): Combining Air-Bone Gap and cVEMP Thresholds to Improve Diagnosis of Superior Canal Dehiscence. Otol Neurotol 2018; 39:212–220. [DOI] [PubMed] [Google Scholar]
- 16.Lookabaugh S, Kelly HR, Carter MS et al. Radiologic classification of superior canal dehiscence: implications for surgical repair. Otol Neurotol 2015; 36:118–125. [DOI] [PubMed] [Google Scholar]
- 17.Brantberg K, Bergenius J, Mendel L, Witt H, Tribukait A, Ygge J. Symptoms, findings and treatment in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol 2001; 121:68–75. [DOI] [PubMed] [Google Scholar]
- 18.Pfammatter A, Darrouzet V, Gartner M et al. A superior semicircular canal dehiscence syndrome multicenter study: is there an association between size and symptoms? Otol Neurotol 2010; 31:447–454. [DOI] [PubMed] [Google Scholar]
- 19.Mikulec AA, McKenna MJ, Ramsey MJ et al. Superior semicircular canal dehiscence presenting as conductive hearing loss without vertigo. Otol Neurotol 2004; 25:121–129. [DOI] [PubMed] [Google Scholar]
- 20.Chien WW, Janky K, Minor LB, Carey JP. Superior canal dehiscence size: multivariate assessment of clinical impact. Otol Neurotol 2012; 33:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal Y, Migliaccio AA, Minor LB, Carey JP. Vestibular hypofunction in the initial postoperative period after surgical treatment of superior semicircular canal dehiscence. Otol Neurotol 2009; 30:502–506. [DOI] [PubMed] [Google Scholar]
- 22.Niesten ME, Hamberg LM, Silverman JB et al. Superior canal dehiscence length and location influences clinical presentation and audiometric and cervical vestibular-evoked myogenic potential testing. Audiol Neurootol 2014; 19:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niesten ME, Stieger C, Lee DJ et al. Assessment of the effects of superior canal dehiscence location and size on intracochlear sound pressures. Audiol Neurootol 2015; 20:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisano DV, Niesten ME, Merchant SN, Nakajima HH. The effect of superior semicircular canal dehiscence on intracochlear sound pressures. Audiol Neurootol 2012; 17:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharon JD, Pross SE, Ward BK, Carey JP. Revision Surgery for Superior Canal Dehiscence Syndrome. Otol Neurotol 2016; 37:1096–1103. [DOI] [PubMed] [Google Scholar]
- 26.Cheng YS, Kozin ED, Lee DJ. Endoscopic-Assisted Repair of Superior Canal Dehiscence. Otolaryngol Clin North Am 2016; 49:1189–1204. [DOI] [PubMed] [Google Scholar]
- 27.Carter MS, Lookabaugh S, Lee DJ. Endoscopic-assisted repair of superior canal dehiscence syndrome. Laryngoscope 2014; 124:1464–1468. [DOI] [PubMed] [Google Scholar]


