Abstract
Hydrogels have been broadly studied for applications in clinically motivated fields such as tissue regeneration, drug delivery, and wound healing, as well as in a wide variety of consumer and industry uses. While the control of mechanical properties and network structures are important in all of these applications, for regenerative medicine applications in particular, matching the chemical, topographical and mechanical properties for the target use/tissue is critical. There have been multiple alternatives developed for fabricating materials with microstructures with goals of controlling the spatial location, phenotypic evolution, and signaling of cells. The commonly employed polymers such as poly(ethylene glycol) (PEG), polypeptides, and polysaccharides (as well as others) can be processed by various methods in order to control material heterogeneity and microscale structures. We review here the more commonly used polymers, chemistries, and methods for generating microstructures in biomaterials, highlighting the range of possible morphologies that can be produced, and the limitations of each method. With a focus in liquid-liquid phase separation, methods and chemistries well suited for stabilizing the interface and arresting the phase separation are covered. As the microstructures can affect cell behavior, examples of such effects are reviewed as well.
Keywords: Liquid-liquid phase separation, microstructure, tissue engineering, hydrogels, resilin
Graphical Abstract
1. Introduction
Hydrogels are formed by hydrophilic polymer networks in aqueous media and show good permeability to chemical and biological molecules, which has enabled their widespread use as a tool for tissue regeneration, drug delivery, and fundamental studies of cell behavior [1]. Engineering other hydrogel properties such as biocompatibility and biodegradability provide further handles to control cell growth and spreading and development of tissue-like structures [2]. The main function of hydrogels in tissue regeneration applications is to temporarily replace the extracellular matrix (ECM), a complex organization of macromolecules that provides the structural support to cells and the proper chemical environment for their function. Thus, it is desirable for the hydrogels to match the chemical, topographical, and mechanical properties of the ECM for the cells to attach, spread, and grow properly on the material [3].
Homogeneous hydrogels can be made of a single material [4] or can be a hybrid of two or more materials [5]. Poly(ethylene glycol) (PEG) is one of the most widely used hydrogel materials due to its biocompatibility and immuno-protective properties [4–7]. The ease with which it can be terminally functionalized enables the production materials with attractive properties such as the self-healing that is observed when PEGs functionalized with aldehyde groups are reacted with amino-functional molecules [8]. Polypeptides are also used commonly in hydrogels. Collagen like polypeptides (CLPs), based on the natural collagen amino acid motifs, Gly-Pro-X and Gly-X-Hyp, adopt a poly(proline) II helix and then further associate into triple helical structures [9–11]. The CLPs have yielded materials in which cells can be easily encapsulated with high viability, proliferation, and desired morphology [12,13], characteristics that can be enhanced in hydrogels made with other polymers such as PEG when crosslinked with CLPs [14]. In addition, combining CLPs with other polypeptides imparts new functions to the CLPs, as it has been shown with self-healing upon crosslinking with silk-like polypeptides. [15]. Elastin-like polypeptides (ELPs), inspired by the mammalian elastomeric protein, are used due to their useful mechanical properties and their characteristic inverse transition temperature[16–18]; this thermal transition has been exploited in developing injectable materials that gel at physiological temperature [19,20] with drug release properties tuned by the transition temperature [21,22] and in-situ chemical crosslinking[23,24]. Resilin-like polypeptides (RLPs), more recently developed, are based on the protein responsible for the elastic response in arthropods’ ligaments and organs [25]. Their highly resilient properties have made them suitable as materials for hydrogels with applications in the regeneration of mechanically active tissues such as cardiovascular and vocal folds [26–29]. Two sequences, GGRSPDSYGAPGGGN derived from the Drosophila melanogaster and AQTPSSQYGAP derived from Anopheles gambiae, are commonly used for such purposes [30–32]. Polysaccharides such as the anionic functional hyaluronic acid (HA), the cationic functional chitosan, and the hydroxyl functional dextran are macromolecules used in hydrogels due to their capability of interacting with structural proteins and growth factors, biodegradability [33–38], biocompatibility [39–42], and the versatility with which their surface can be modified due to their functionalities [43–46]. Among all the variety of methods to crosslink these materials so they behave as homogeneous hydrogels, Michael-type addition and photocrosslinking reactions have emerged as leading approaches [47,48].
Heterogeneous hydrogels comprising these polymers (and others) offer a reliable solution for capturing at least some of the complexity of the composition and properties of the ECM [49]. The microstructures addressed in this document comprise mainly those in which a polymeric material is encapsulated and dispersed inside a matrix of another polymeric material. Hydrogel micromechanical properties and molecules diffusivity have shown to be dependent on the microstructure [50,51], and cell behavior such as differentiation of stem cells [52,53] or migration and morphology of fibroblasts are correlated to those properties [54,55]. Furthermore, when growth factors and bioactive peptides are added to the hydrogels, biological responses such as spreading and vasculogenesis of endothelial cells on PEG hydrogels are accelerated, for example. [56]. Thus, control over the hydrogel microstructure and chemical composition continues to offer substantial opportunities in the development of model platforms and clinically focused materials.
A variety of different methods have been used to produce microstructured hydrogels. The addition of particles and fibers into a polymer matrix is a two-step technique that allows the production of organic and inorganic microstructures with extensive compositional variety, including microgels [57,58], graphene oxide platelets [59], or silver nanowires among others [60]. Photopatterning allows the generation of almost any desired 2-D pattern, but appropriate design and fabrication of the corresponding photomask are required [61]. With laser lithography, a myriad of 3-D patterns can be obtained, but such patterning is restricted to micron-size areas or the use of multiple lasers [62]. Microfluidics approaches have been used to generate microgels with different shapes that can be deposited one-by-one directly in a hydrogel precursor, and specialized microfluidic devices must be manufactured for each system [63]. Liquid-liquid phase separation (LLPS) is a one-step procedure where the microstructure (mainly limited to spherical and percolated structures) can be tuned by modifying physico-chemical parameters as temperature, concentration, or molecular weight [64]. While many of the main applications of this technique are largely focused on extraction and purification of material such as DNA [65,66], proteins [67–69], and metals [70], the opportunities are significant for producing microstructured hydrogel materials with biomedical applications such as drug delivery and tissue regeneration [71–73] or even to form synthetic membraneless organelles for modeling intracellular processes [74]. Limitations of LLPS include a loss of temporal control over the microstructure due to rapid phase separation [73,75], and biocompatible approaches to overcome this issue have been based mainly on interface stabilization by copolymers [76] and Pickering particles [77]. Besides interfacial stabilizers, there are a wide variety of chemical reactions used in hydrogel crosslinking that can arrest the phase separation, with the already mentioned Michael-type addition and photocrosslinking reactions as particularly useful options due to their fast reaction kinetics [78,79].
Some relevant reviews have been published covering methods for creating microstructured materials such as solvent casting, freeze-drying, electrospinning, gas foaming, and 3-D printing [80,81], and a report about the basics and applications of liquid-liquid phase separation for biomaterials has been reported [73]. However, they are focused mainly in the generation and control of porosities. In this contribution, we aim to cover methods for generating microstructures in which, as defined above, a polymeric material is dispersed in a continuous matrix of another polymeric material (both generally hydrogels), with a focus on LLPS and the implications on stabilizing the phase separation for controlling the target microstructure.
2. Common methods for generating microstructured materials.
2.1. Addition of particles
A common method for producing microstructured hydrogels, generally with an aim of increasing the mechanical strength of the hydrogel, involves the dispersion of micron-size particles in a polymer solution for further crosslinking, with the microparticles entrapped in the resulting hydrogel. The hydrogel polymer and the particles generally share chemical properties but differ in their mechanical rigidity. Examples of these systems include chitosan hydrogels with starch microparticles that improve both mechanical properties and swelling behavior [82] and HA-based injectable hydrogels mixed with decellularized extracellular matrix particles that help to tune the gelation kinetics and mechanical properties of the hydrogel [83]. An additional common goal of dispersing particles in hydrogels is to create new surfaces driven by the encapsulation of particles, or indirectly by using them as sacrificial templates for generating microstructures that improve cell survival, spreading, proliferation, and neovascularization. Such improvements have been observed in HA hydrogels with dextran microspheres [84], PEG hydrogels with encapsulated gelatin [85] and alginate microgels [86], among others.
The inclusion of particles in hydrogel matrices can also serve to impart new properties, such as self-healing, while maintaining biocompatibility, as has been achieved by the reversible aldimine reaction between amine-functionalized bioglass particles and aldehyde-functionalized polypeptides [87]. Shape memory triggered by near-infrared light has been enabled by the reversible physical crosslinking of polydopamine particles dispersed in poly(vinyl alcohol) based on hydrogen-bond formation [88]. Both of these reversibly-addressable, microstructured materials may find application as artificial skin materials.
2.2. Photopatterning
Unlike the addition of particles, that usually have a predetermined shape, photopatterning methods (employing photomasks and photocrosslinkable polymers) allow the creation of a wide variety of structures that will conform to the overall shape of the hydrogel (Fig. 1) [89]. A recent advance in this technology has been the expansion beyond UV-crosslinkable systems to UV-cleavable ones for generating hydrogels. In the latter case, the self-assembly of and hydrogel formation by macromolecules, such as collagen, is sterically hindered by the light-sensitive group; after UV irradiation cleaves the photolabile group, gelation by self-assembly is enabled [90]. A disadvantage of this technique is the dependence in the photomask which can only project a 2D pattern; more complicated patterns could be achieved by using different sets of photomasks applied consecutively [89].
Fig. 1.
(a) Schematic representation of the fabrication process for four‐ state thermally responsive grippers. Patterning required four photomasks that were used in sequential order to different ethylene glycol methacrylate solutions. (b) Representative images of grippers. The images show the grippers on a glass slide at different temperatures. (c) Optical images and schematic representations of four‐ state shape changes of grippers during heating and cooling. Scale bars = 2 mm. Reproduced with permission of ref [89]. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
The technique alone can be used to generate chemically bulk-crosslinked microstructured hydrogels by short-term irradiation of the entire hydrogel precursor solution to yield a low crosslink-density network, followed by complete radiation under the photomask to achieve highly crosslinked microstructures [91]. Furthermore, photopatterning is commonly used in combination with other techniques like electrospinning or 3D printing to create multilayered scaffolds and interpenetrating networks with internal microstructures, features desired for the growth of multicellular systems [92–94].
Because the design and manufacture of the photomask can be a limiting factor for this technique, the use of optical microscopes has been proposed for generating microstructures, similar to laser lithography (see below). UV light is focused on specific locations inside a hydrogel precursor, then the structure is immersed in a solution of a chemically distinct precursor and the entire sample volume exposed to light, yielding a chemically heterogenous microstructured hydrogel in which different kinds of cells can be encapsulated in distinct locations [95]. A different approach uses an electrochemical reaction triggered by visible light that releases ions capable of crosslinking ionic polymers, which provides flexibility in the encapsulation of UV-sensitive cells [96].
The use of photopatterning has been focused not only on the crosslinking of polymers, but also on the patterned functionalization of hydrogels. The directed UV radiation has been used to generate functional groups on the polymer backbone that are able to react immediately with other molecules, to graft bioactive domains or a myriad of polypeptides on specific sites, and to attach polymeric patches directly on the cell surface [97–100].
2.3. Laser lithography
The use of laser lithography methods provides significant flexibility in the production of microstructured materials, as depending on the wavelength and intensity of the laser light, photopolymerization of hydrogel precursors or photoablation of hydrogels can be performed. UV laser photopatterning is used for creating microstructures by ablation of hydrogels [101]. These approaches, however, are useful only for patterning the surface of the material or for eroding microchannels, because the laser ablates everything in its path and only 2D patterns with a projection in a third dimension are thus possible [102–104]. Turbidity in the samples will scatter the light, resulting in difficulties in maintaining the fidelity of a pattern owing to light scattering [105].
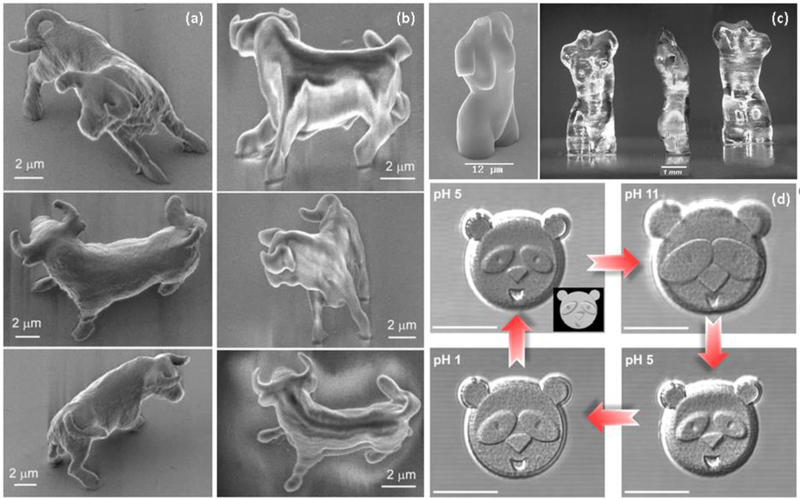
Two-photon radiation has thus been developed as an alternative to exerting high-fidelity spatial control in microstructured materials. A pulsed laser with wavelengths in the range of visible or infrared radiation is used to irradiate the sample in distinct locations; because the two-photon effect (which yields a photon in the UV-range) occurs only in a defined and small volume of the sample, patterning via photopolymerization can be achieved with no effect on the other regions of the sample [106]. Reactions commonly used for this purpose involve azobenzene isomerization, tyrosine oxidation, thiol-ene chemistry and azo-Michael addition, among others [107–110]. Furthermore, fixation of bioactive domains via these approaches has been employed to control cell growth in distinct spatial locations [111]. While these methods offer desirable precision in the fabrication of microstructures of essentially any pattern, because the two-photon effect occurs only in a small volume, the creation of large patterns or microstructured hydrogels of substantial volume is both time- and energy-consuming. For example, the fabrication of fully polymerized, micron-scale bulk shape (e.g., Figure 2) can require multiple hours for completion [112–115], although the complete polymerization of the bulk shape suffers only punctual shrinkage so the resolution is improved in comparison to structures in which only the shell is polymerized (Fig. 2a and 2b). Thus, these methods to date have been limited to the production of microstructures of some tens of square microns with thicknesses no bigger to one micron in order to have elaboration times in the range of some minutes, making them useful for fundamental studies although not yet for manufacturing.
Fig. 2.
Structures obtained by laser lithography. (a) A bull completely polymerized with two-photon radiation. (b) the bull polymerized only on the surface with two-photon radiation and the bulk with UV. It is noticeable the deformation due to shrinkage. (c) Aphrodite of Milos polymerized with two-photon radiation completely (left) and only the shell followed by UV radiation (right) with noticeable deformation. (d) A panda face made with pH-responsive bovine serum albumin (BSA). The face expression changes upon pH variations. Scale bar = 20 μm. Reproduced with permission of ref [112]. Copyright 2003 SPIE. Reprinted by permission from [113]. Copyright 2004 Springer-Verlag. Reprinted with permission from [114]. Copyright 2017 American Chemical Society.
2.4. Microfluidics
In microfluidics approaches, which have been employed to generate a wide variety of shaped microparticles with myriad compositions, non-miscible liquids are injected through interconnected micron-size channels; the incompatibility drives the formation of a liquid microstructure inside the dispersing phase. Subsequent polymerization by external sources, such as light, or due to the interactions between the liquids, such as ionic polymers in the presence of multivalent ions, yields the formation of microgels [116]. This is one of the methods for generating pre-shaped microhydrogels that can be dispersed later in hydrogel precursors to form a microstructured hydrogel in which all phases are hydrogel in composition.
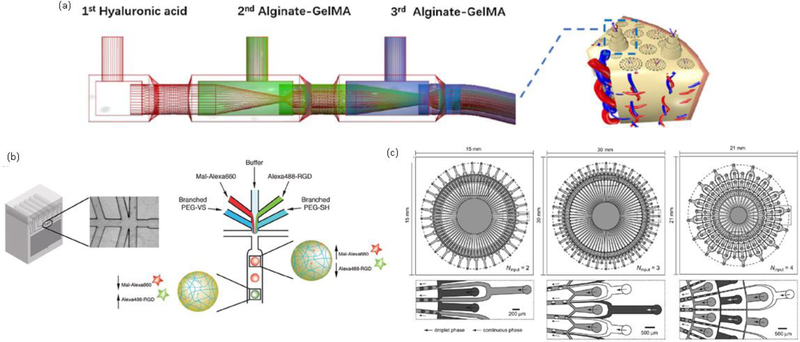
Depending on variables such as the relative position of microchannels, the rate of injection, number of liquids used, and their viscosity, different kinds of shapes can be achieved. In addition, encapsulation of cells can be achieved by incorporating them in the desired precursor solution (usually the one that will adopt a microstructure) prior to their injection into the microfluidic channel [117,118]. More complicated microfluidic devices can be used to obtain structures such as core-shell spheres, hollow tubes, osteon-like microfibers (Fig.3a), and Janus particles (particles with two defined and unique surfaces) with applications in drug delivery and scaffolds for multilayered cell systems [119–122].
Fig. 3.
(a) Microfluidic device for fabricating of osteon-like fibers. (b) A microfluidic device for fabricating multicomponent particles. (c) Complex multichannel microfluidic device for improving the yield in fabricating spheres (left), Janus particles (center), and core-shell particles (right). Reprinted from [121], Copyright 2017, with permission from Elsevier. Reproduced with permission ref [116]. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Reproduced with permission ref [125]. Copyright 2012 Royal Society of Chemistry.
One of the biggest limitations of microfluidics is the volume that can be processed over time. In the systems already described, the flow rates range from an average of 30 mL h−1 for fibers, 10 mL h−1 for solid spheres, and up to 300 μL h−1 for multicomponent spherical shapes [119–123]. Based on this flow rates, the more complicated the shape and the more components it has (Fig. 3b), the lower the flow rate must be in order to have more control. Scaled processes for spherical particles have achieved flows of 120 mL h−1 and higher with the implementation of parallelized complex multichannel devices (Fig. 3c) [124,125]. However, the addition of multiple channels for multicomponent structures will make the complete process more sensitive to another parameter: the viscosity. The differences in each component’s viscosities affect the changes in volume when the precursors come out from their respective channels, so the addition of multiple channels will potentially result in the loss of control in the final shape and composition [126].
3. Liquid-liquid phase separation (LLPS)
Liquid-liquid phase separation (LLPS) is a process with great implications in biological systems. In addition to its longstanding application in biotechnology and the purification of biological macromolecules, in recent decades, it has been proposed as the main director for the formation of membraneless organelles such as nucleoli, centrosomes, and stress granules [127,128], and it has been used for generating synthetic membraneless organelles [129]. Recent studies attribute to LLPS the formation of amyloid structures responsible for Alzheimer’s disease [128,130]. Specific internal cell processes have been proven to be driven by LLPS as well, including the formation of chromatin subcompartments [131] and the localized hyperphosphorylation of the C-terminal domain in RNA polymerase II [132]. These phenomena have inspired the construction of multiphase dynamic polymerization networks with opportunities in the amplification of peptide-like oligomers [133]. In this issue we explore the use of LLPS as a one-pot method for generating microstructured hydrogels.
In contrast to the previous methods where prefabrication of microparticles and/or the use of special equipment may be required, LLPS relies on the thermodynamic properties of polymer solutions to guide the generation of microstructure. Given the role of both enthalpic and entropic contributions, multiple variables can be tuned to alter phase separation process, including the concentration and molecular weight of the polymers, as well as the hydrophobic and charge interactions between the solution components [134].
Polymer solutions can exhibit lower or upper critical solution temperatures (LCST or UCST), depending on if they phase separate with increasing or decreasing temperature, respectively. In polypeptides, for example, the transition temperature depends on the interactions between their variety of ionic, polar, aromatic, and non-polar moieties and water molecules.
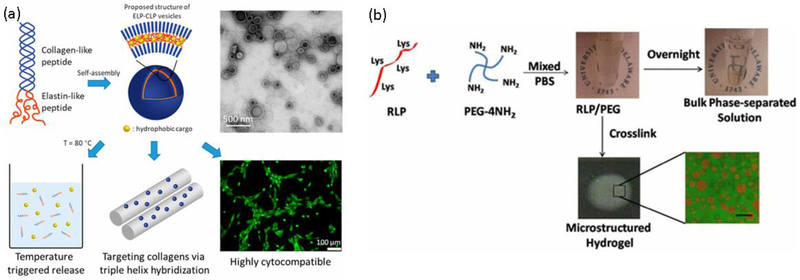
This results in UCST-like transitions in more polar sequences like RLPs and LCST-like transitions in less polar sequences such as ELPs [135,136]. The latter has been used in conjugates with CLPs taking advantage of its ability to form triple helical structures, as a result of which spherical vesicles with potential applications in drug delivery are formed (Fig. 4a) [137,138]. Other macromolecules that have shown to affect in the ELP transition temperature upon conjugation are hyaluronic acid [139] and fibronectin [140].
Fig. 4.
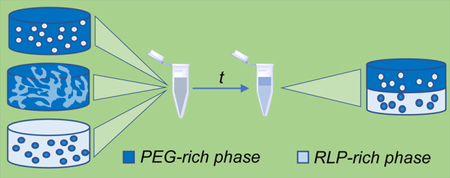
(a) CLP-ELP conjugates self-assemble into hollow spheres. The particles are used as drug delivery systems. (b) Microstructured hydrogels by LLPS of RLP and PEG. Upon formation of an emulsion, the materials are crosslinked, and the microstructure is arrested. Scale bar = 50 μm. Reproduced with permission of refs [137] and [146]. Copyright 2017 American Chemical Society.
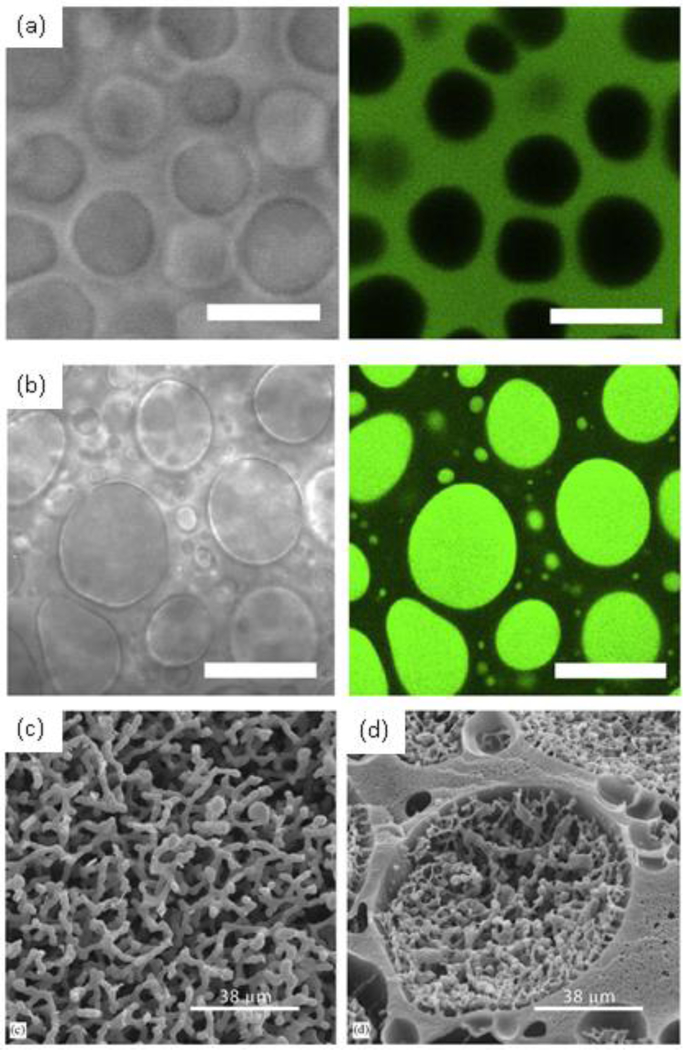
The increase of molecular weight of one or more of the components in the system is a common strategy for inducing the phase separation, and indeed, even low concentrations of PEG of high molecular weight works efficiently for segregation of proteins and other polymers such as poly(vinyl alcohol) [141–144]. This PEG-induced phase separation has been used to make heterogeneous RLP hydrogels [145] with microscale mechanical properties that vary with the composition of the phase (Fig 4b) [146]. Such behavior has not yet been reported for ELPs, which may result from the fact that (in contrast with the ELP inverse transition temperature) solutions of RLPs, in addition to LCST-like behavior, also exhibit an upper critical solution temperature (UCST) which is dependent on specific amino acid interactions [136]. Detailed studies on amino acid sequences have shown that the first nine amino acids from the sequence are responsible for the UCST behavior whereas the six remaining amino acids are more similar to ELP sequences and would be responsible for LCST-like transitions; the LCST behavior (although not UCST behavior) has been observed in modified RLP sequences synthesized in our group [136,147]. In addition to inducing LLPS in solutions of polypeptides and polysaccharides via the addition of PEG to generate microstructured hydrogels (Fig. 5a–d) [145,146,148–151], other systems including gelatin/chitosan and bacterial cellulose/poly(lactic acid) have been explored recently to obtain microstructured materials [152,153].
Fig. 5.
(a) and (b) Transmitted light (left) and fluorescence (right) images of dextran-tyramine/PEG hydrogels. Fluorescein-labeled BSA is partitioned into the dextran-rich phase (a), and PEGylated fluorescein-labeled BSA is partitioned into the PEG-rich domains. Scale bars = 5 μm. (c) and (d) Scaffolds synthesized from a system 5% PEG 10 kDa with 10% methacrylated dextran 40 kDa (c) and 25% methacrylated dextran 40 kDa (d). We can observe the formation of continuous structures and a combination of continuous inside spherical domains respectively. Reprinted from [149], Copyright 2015, with permission of Elsevier and [151], Copyright 2005, with permission of Elsevier.
The interactions between ionic groups play a key role in the phase separation and are strongly affected by the pH and the ionic strength of the solution [154]. Indeed, when sufficiently charged, proteins undergo repulsive interactions that preclude phase separation, even in the presence of PEG; this effect has been demonstrated for PEG solutions with lysozyme, papain, cytochrome c and IgG antibodies [68,155]. The type of ion also has an important effect. Based on the Hofmeister series, it has been shown that chaotropes like Br− and Cl− increase the USCT-like transition temperature of lysozyme and the LCST-like transition temperature of modified RLPs [147,156].
The use of LLPS to yield microstructured materials can provide two distinct types of structures depending on the mechanism of phase separation. Spherical domains are more commonly obtained, via nucleation and growth mechanisms, while bicontinuous structures are possible via spinodal decomposition (Fig. 6a) [64]. In the latter, fluctuations in the composition can disrupt the interconnected domains to also yield spherical domains via a percolation-to-cluster transition [157–159]. Combinations of both kinds of microstructures have been obtained by controlling the molecular weight and sequence in RLP-ELP block copolypeptides [160]. Furthermore, the diameters of the domains can be controlled as they grow over time before the system reaches equilibrium, where two separate homogeneous phases will be generated [146]. The continuous structures obtained in both cases (for spherical domains, the continuous phase is the matrix in which they are dispersed) allow cells to create their own structures by their proliferation and migration in the hydrogel continuous phase [81]. When LLPS is used for preparing emulsions that will yield microstructured hydrogel materials, the solutions must be crosslinked sufficiently rapidly to capture the desired phase/microstructure. In the following sections, we review some mechanisms that impact the kinetics of phase separation, some methods for stabilizing the emulsions without the use of conventional surfactants, and selected chemistries that can be used to prepare microstructured hydrogels from aqueous two-phase solutions.
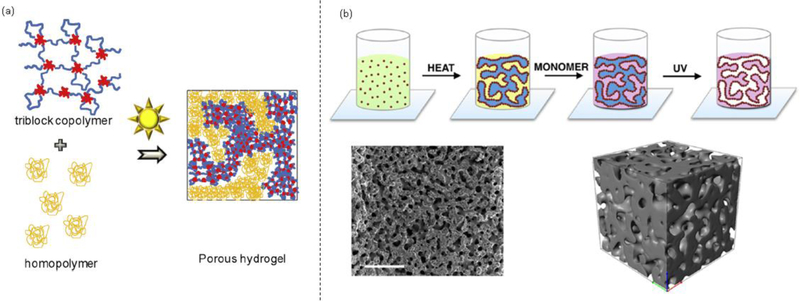
Fig. 6.
(a) Schematic of a porous hydrogel with bicontinuous structure prepared by phase separation of a triblock copolymer and a homopolymer. (b) Silica particles used as Pickering stabilizers. The bicontinuous structure is arrested by UV polymerization. Scale bar = 500 μm. Reprinted from [64], Copyright 2016, with permission from Elsevier. Reprinted with permission from [49]. Copyright 2018 American Chemical Society.
3.1. Emulsion stabilization
Coalescence is the result of two droplets combining to form a larger droplet. Arresting the coalescence can stabilize emulsions and also can be used to yield non-spherical droplet structures [161]. The mechanism is driven mainly by two factors: the draining of the dispersing phase between the domains and the interface rupture necessary for the domains to merge. Gelation is a simple strategy to impart a yield stress to the dispersing phase, which will make it more difficult to drain and will thus slow down coalescence [162,163] and can be achieved easily in hydrogels as long as the crosslinking kinetics are fast enough. Addition of whey protein microgels and bacterial cellulose is another solution recently published to impart yield stress to the dispersing phase and overcome the emulsion coalescence [164,165]. The second factor is frequently manipulated by increasing the rigidity or elasticity of the interface to reduce the merging of droplets [166,167]. Polymers such as cellulose esters and poly(N-isopropylacrylamide) have been used for this purpose, the latter being triggerable by temperature changes due to its characteristic LCST phase transition [168,169].
The other main mechanism responsible for the instability of emulsions is Ostwald ripening, in which small particles dissolve and form larger particles over time by a molecular diffusion mechanism. Thus, the more compatible the dispersed phase is with the continuous phase, the more favored this mechanism will be [170]. These considerations are relevant to the choice of stabilizers against coalescences; if the stabilizers are too compatible with the dispersed phase, they will enhance Ostwald ripening even if delaying coalescence, so an emulsifier with low affinity for the dispersed phase is needed [171,172]. Given the competing effects of stabilizing phase separation against coalescence and Ostwald ripening, a combination of stabilizers can be used to stabilize the emulsion. For example, in the case of polymeric emulsifiers, it has been shown that using both Pluronic PE9400 and Levenol C201, in N,N dimethyl decamine, and D-limonene solutions is necessary to efficiently stabilize the emulsion [173]. Reducing temperature, if suitable for a given set of solutions and use conditions, is also a facile means to slow down Ostwald ripening via the corresponding reduction in molecular diffusion [174].
Among the most common emulsion stabilizers, low molecular weight surfactants have been broadly used for emulsion stabilization [175–177]. However, they are highly toxic for cells, limiting their use in systems with biological applications [178,179]. More cell-friendly options, such as Pickering stabilizers and block copolymers [180,181] have thus been employed. A phenomenon described more than a century ago, Pickering stabilization is based on solid and semi-solid colloidal particles that migrate to the interface of two immiscible liquids to reduce the excess interfacial energy. As the number of particles in the interface increases, they begin to cluster creating an elastic barrier that prevents the coalescence of the domains [182]. One of the most common types of Pickering stabilizers is silica nanoparticles owing to their ease of synthesis and control of particle size by means of the Stöber process and their ease of surface modification (Fig. 6b) [49,183–185]. However, due to their inherent negative surface charge, unmodified particles remain preferentially in the hydrophilic phase, so surface modifications with molecules such as chitosan, betaine, and polyethylene oxide copolymers, among others, have been performed to alter the hydropathy of the nanoparticles and permit their localization at the interface [186–188]. Janus particles have also been employed for Pickering emulsions because one half can be formed by a material with affinity to one phase and the other half with affinity to the other phase, allowing them to migrate exactly to the interface. Alginate/shellac, gliadin/chitosan, and gelatin/sodium polyacrylate are some examples of the systems recently used in the production of Janus nanoparticles for use as Pickering stabilizers [189–191]. Other types of materials such as self-assembled proteins, starch nanoparticles, and carbon quantum dots have been also used for the emulsion stabilization process [192–194]. In addition to the hydrophilic character of the particle surface, size is another factor important in their performance as stabilizers. It has been shown that polydisperse systems are more effective in stabilization, although smaller particles are more effective when the size distribution is monodisperse [195,196].
Owing to the benefit of amphipathy for stabilizers to migrate to and assemble at the phase interface, block copolymers are commonly used due to the versatility of having both hydrophilic and hydrophobic polymer chains conjugated in the same molecule. The capability of these materials to form semisolid structures and crystals impart both yield stress to the phases involved and stability against mechanical stress in the interface, so the coalescence is delayed and there is inherent steric repulsion of the polymer-covered domains [197,198]. Hydrophobic polymers such as poly(caprolactone), poly(lactide), and poly(hydroxypropyl methacrylate) and hydrophilic polymers like poly(glycerol) and poly(ethylene oxide) are the most commonly used combination for producing the copolymer emulsifiers [198–200]. Triblock copolymers involving two different hydrophobic polymers and one hydrophilic have also been used to take advantage of the crystallization of the lipophilic moieties, which reinforces both the steric and elastic effects in the stabilization process [76]. Amphiphilic polypeptides have been used as well for emulsion stabilization. For instance, phase-separated hydrophobic ELPs have been stabilized with amphiphilic block ELPs where the hydrophilic portion surrounds the hydrophobic domains and minimizes further coalescence with other domains [201]. In addition, these systems have also been explored with ELPs and amphiphilic block ELPs that employ photo-crosslinkable non-natural amino acids to impart stability to the particles upon crosslinking [202]. When it comes to comparing the effectiveness as stabilizers of both copolymers and Pickering particles, the latter has shown to be more efficient [203].
3.2. Chemistries for arresting phase separation
In addition to the stabilization of emulsions, crosslinking is necessary in most cases to generate mechanically robust, microstructured hydrogels [72,204]. Multiple chemistries have been employed for this purpose, including Michael-type addition reactions between a nucleophile and an electron-poor unsaturated compound; thiols are the most commonly used nucleophiles whereas the unsaturated group could be acrylate, maleimide, and vinyl sulfone depending on the desired reactivity that can range from the tens of minutes for acrylic compounds to a couple of minutes and even few seconds for both maleimide and vinyl sulfone [145,205]. Due to the increase in nucleophilicity of the thiol group when it is deprotonated, pH is a factor influencing the time of reaction so it can be reduced to just some minutes in acrylates when the crosslinking is conducted under basic conditions [78,206]. Higher concentrations and matched stoichiometric ratios of reactive groups can be employed to increase the rate at which the reactions are completed [47,207]. Amines are also employed in these reactions, but owing to their lower reactivity versus that of the thiol groups, the times of reaction usually range in the tens of minutes for both acrylate and vinyl sulfone groups [208,209]. When the emulsion to be crosslinked is highly unstable, the rapid Michael-type addition reactions should be employed to arrest such phase separation to produce hydrogels. This principle has been used to create microstructured hydrogels based on LLPS of cysteine-functional RLPs/vinyl sulfone functional PEG [145,210] and norbornene-functional RLPs/thiol-functional PEG with applications in cardiovascular tissue regeneration[211]. Porous materials with applications in chromatography resins and superhydrophobic sponges have also been prepared using Michael-type addition for arresting the phase separation between porogenic solvents with thiol-acrylates and poly(ethyleneimine)-acrylate systems respectively [212,213].
Tris-(hydroxymethyl phosphine) (THP) and tris-(hydroxymethyl phosphino) propionic acid (THPP), crosslinkers susceptible to nucleophilic substitution, have been employed in generating PEG-RLP microstructured hydrogels. The most common nucleophiles use in this case are amines with gelation times that begin in some minutes and reach complete conversion close to one hour [146,214,215]. Since the phosphines are used simply as a crosslinker, their increase in concentration improves the reaction times with the disadvantage that crosslinking density and mechanical properties are consequently altered, and may disrupt desired microstructures and mechanical properties [216]. While the reaction times are not as fast as the thiol-Michael addition, this reaction can be used in emulsions whose phase separation happens in some minutes, the same time frame of crosslinking [217,218].
Acrylates are the most widely employed reactive groups used in the photo-crosslinking of hydrogels by formation of free radicals with UV light in the presence of a photoinitiator, with crosslinking times lower than one minute and completed reactions in the timeframe of ten minutes [48,219,220]. This chemistry has shown to be stable at temperatures below 4°C where there is no reaction even upon UV radiation [221]. Thiol-ene reactions, in which a thiol radical reacts with electron-rich unsaturated compounds are also widely employed, offering selective and rapid reaction rates. Allyl-functionalized materials react over similar times compared to acrylates [222,223], and norbornene-functionalized materials react much more rapidly due to their strained ring structure, with complete gelation occurring in a matter of 45 seconds [79,211]. Since these reactions are triggered only in under UV radiation, they can be used to arrest emulsion phase separation quickly and on demand, something not possible in the previous chemistries in which the reaction happens immediately upon mixing. In addition, ceasing exposure to UV light permits the cessation of the reaction at any time, so the crosslinking density and mechanical properties can be spatiotemporally controlled.
Schiff base formation between aldehydes and amine or hydrazide functional groups is a reversible reaction that undergoes completion over tens of seconds and finds applications in self-healing materials [224–226] and could be employed to form responsive microstructured hydrogels. Enzyme-based reactions may be preferred for polypeptide-based materials for long-term cell studies; transglutaminase for crosslinking lysine and glutamine residues and tyrosinase for crosslinking tyramine functional materials are some examples of reactions with reaction times that range between 1 to 10 minutes [227,228]. Epoxy-functionalized crosslinkers have also been explored for the crosslinking of hydroxyl-functional biomaterials such as HA. For the reaction to be successful, 50°C and 5 hours are required, conditions that might be not favorable for arresting successfully emulsion phase separation, unless it is in systems with LCST-like behavior and low instability [229].
Although the examples of biomaterials prepared with the different chemistries shown above have resulted in cytocompatible outcomes [145,214,219,224], special precautions should be taken when working with photo-triggered reactions. For each specific case, the intensity of UV light, exposure time and photoinitiator concentration must be balanced to avoid any negative effect on cells such as cytotoxicity and genotoxicity [230–232].
4. Impact of microstructure on cell behavior
In the last pair of decades, it has been documented that cells differentiate and proliferate differently depending on the mechanical environment of the substrate. Neurites develop denser branching when the substrate matches the mechanical properties of human gray matter (0.2 kPa), for example [233]. It has been observed that human mesenchymal stem cells (hMSCs) differentiate down an osteogenic lineage in hydrogels with elastic modulus in the range of 20–40 kPa [234,235] a myogenic lineage in the rage of 8–17 kPa [235], and a neuron-like phenotype in the range of 0.1–1 kPa [235], whereas pluripotent stem cells (PSCs), also depending on the scaffold elastic modulus, could prefer mesodermal differentiation (1.5 – 6 MPa), endodermal differentiation (0.1 – 1 MPa), or ectodermal differentiation (<0.1 MPa) [52,236]. Bovine vascular smooth cells migrate and accumulate from soft (2.5 kPa) to stiff (11 kPa) regions in hydrogels with a Young’s modulus gradient [237], similar behavior as the one observed in NIH 3T3 cells in materials with a sharp soft (14 kPa) to stiff (30kPa) change in modulus[55]. Cells such as fibroblasts and hMSCs have been shown to change morphology from spheres to elongated structures as the mechanical properties of the scaffold are increased from some hundreds of Pa to thousands of Pa [54,227]. Recent cell studies have also demonstrated how the shape, mechanical properties, and size of the microstructures, among other features, affect the differentiation, migration, and morphology of cells [104,238,239].
Materials comprising a polymer encapsulated and dispersed inside hydrogel matrix, have shown an improvement in the guided growth and differentiation of cells [240]. A local increase of mechanical properties in the encapsulated material is likely to provide a mechanically desirable environment for guided adhesion and migration of cells around the microstructure [72,241], so they adhere more easily than in materials with lower modulus. Such behavior has been observed in starPEG-heparin hydrogels with collagen microstructures, where human umbilical cord vein endothelial cells (HUVECs) have been shown to grow aligned to the fibrillar collagen. Although, the bulk mechanical properties are significantly reduced when the collagen microstructures are present, the localized micromechanical properties are three to four times higher in the regions where the collagen fibrils are located [241]. Similar results have been observed in RLP-PEG hydrogels microstructured by LLPS where hMSCs show elongated morphologies and are localized only around the RLP-rich domains [72]. In addition, when smooth muscle cells (SMCs) are seeded in a devitalized ECM from fibroblasts (which is inherently microstructured in aligned patterns), they elongate and migrate following the alignment of the microstructure in the scaffold [242]. Such results suggest that, as long as the microstructures match the target morphology and mechanical properties required for specific cells and tissues, the cell growth can be guided by means of the hydrogel microstructure.
5. Conclusions
This review highlights the materials and methods commonly used in the fabrication of microstructured materials for biomedical applications with a specific emphasis on liquid-liquid phase separation. Owing to their similarity with macromolecules comprising the ECM, polypeptides and polysaccharides have been used in combination with PEG, which has been widely employed because of its biocompatibility and the ability of inducing the phase separation of other molecules. LLPS offers a fast and reliable one-pot method for producing spherical and bicontinuous microstructures in hydrogels upon gelation of precursor emulsions. Both critical temperature and concentration are the basic thermodynamic parameters required to yield an emulsion, and the selection of the correct crosslinking chemistry permits temporal control over the desired microstructure. The generation of more complex microstructures can be achieved via alternative methods such as photopatterning and laser lithography, although the need for these structures must be balanced with experimental limitations including photomask manufacture and time-consuming fabrication. The versatility of emulsion formation makes LLPS a fast and scalable method for fabricating microstructured materials. The impact on cell growth, migration and differentiation due to the size, geometry and mechanical properties of microstructures in hydrogel and non-hydrogel materials has been explored. The results show that the control over the microstructure features are relevant for guiding the cell behavior in tissue regeneration.
Statement of Significance.
Heterogeneous hydrogels with enhanced matrix complexity have been studied for a variety of biomimetic materials. A range of materials based on poly(ethylene glycol), polypeptides, proteins, and/or polysaccharides, have been employed in the studies of materials that by virtue of their microstructure, can control the behaviors of cells. Methods including microfluidics, photolithography, gelation in the presence of porogens, and liquid-liquid phase separation, are presented as possible strategies for producing materials, and their relative advantages and disadvantages are discussed. We also describe in more detail the various processes involved in LLPS, and how they can be manipulated to alter the kinetics of phase separation and to yield different microstructured materials.
6. Acknowledgements
Related work in the authors’ laboratories was supported by the National Science Foundation (DMR-1609544) as well as by the National Institute of Deafness and other Communication Disorders (RO1-DC011377), the National Heart Lung and Blood Institute (RO1-HL108110), and the National Institute of General Medical Sciences (1-P30-GM110758–01 and 1-P20-RR017716 for instrument resources). The views expressed in this work are the responsibility of the authors and do not necessarily reflect the views of the NSF or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- [1].Hoffman AS, Hydrogels for biomedical applications, Adv. Drug Deliv. Rev 54 (2002) 3–12. doi: 10.1016/S0169-409X(01)00239-3. [DOI] [PubMed] [Google Scholar]
- [2].Yuk H, Zhang T, Parada GA, Liu X, Zhao X, Skin-inspired hydrogel-elastomer hybrids with robust interfaces and functional microstructures, Nat. Commun 7 (2016) 1–11. doi: 10.1038/ncomms12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jensen BEB, Edlund K, Zelikin AN, Micro-structured, spontaneously eroding hydrogels accelerate endothelialization through presentation of conjugated growth factors, Biomaterials 49 (2015) 113–124. doi: 10.1016/j.biomaterials.2015.01.036. [DOI] [PubMed] [Google Scholar]
- [4].Yao H, Xue J, Wang Q, Xie R, Li W, Liu S, Cai J, Qin D, Wang DA, Ren L, Glucosamine-modified polyethylene glycol hydrogel-mediated chondrogenic differentiation of human mesenchymal stem cells, Mater. Sci. Eng. C 79 (2017) 661–670. doi: 10.1016/j.msec.2017.05.043. [DOI] [PubMed] [Google Scholar]
- [5].Mohammadi MR, Malkovskiy AV, Jothimuthu P, Kim K-M, Parekh M, Inayathullah M, Zhuge Y, Rajadas J, PEG/Dextran Double Layer Influences Fe Ion Release and Colloidal Stability of Iron Oxide Nanoparticles, Sci. Rep 8 (2018) 1–12. doi: 10.1038/s41598-018-22644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mahoney MJ, Anseth KS, Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels, Biomaterials 27 (2006) 2265–2274. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- [7].Huang D, Zhuang Y, Shen H, Yang F, Wang X, Wu D, Acetal-linked PEGylated paclitaxel prodrugs forming free-paclitaxel-loaded pH-responsive micelles with high drug loading capacity and improved drug delivery, Mater. Sci. Eng. C 82 (2018) 60–68. doi: 10.1016/j.msec.2017.08.063. [DOI] [PubMed] [Google Scholar]
- [8].Zhang X, Xu J, Lang C, Qiao S, An G, Fan X, Zhao L, Hou C, Liu J, Enzyme-Regulated Fast Self-Healing of a Pillararene-Based Hydrogel, Biomacromolecules 18 (2017) 1885–1892. doi: 10.1021/acs.biomac.7b00321. [DOI] [PubMed] [Google Scholar]
- [9].Szpak P, Fish bone chemistry and ultrastructure: Implications for taphonomy and stable isotope analysis, J. Archaeol. Sci 38 (2011) 3358–3372. doi: 10.1016/j.jas.2011.07.022. [DOI] [Google Scholar]
- [10].Kishimoto T, Morihara Y, Osanai M, Ogata SI, Kamitakahara M, Ohtsuki C, Tanihara M, Synthesis of poly(pro-hyp-gly)n by direct polycondensation of (pro-hyp-gly)n, where n = 1, 5, and 10, and stability of the triple-helical structure, Biopolymers 79 (2005) 163–172. doi: 10.1002/bip.20348. [DOI] [PubMed] [Google Scholar]
- [11].Luo T, Kiick KL, Collagen-Like Peptide Bioconjugates, Bioconjug. Chem 28 (2017) 816–827. doi: 10.1021/acs.bioconjchem.6b00673. [DOI] [PubMed] [Google Scholar]
- [12].Nurlidar F, Kobayashi M, Terada K, Ando T, Tanihara M, Cytocompatible polyion complex gel of poly(Pro-Hyp-Gly) for simultaneous rat bone marrow stromal cell encapsulation, J. Biomater. Sci. Polym. Ed 28 (2017) 1480–1496. doi: 10.1080/09205063.2017.1331872. [DOI] [PubMed] [Google Scholar]
- [13].Nurlidar F, Yamane K, Kobayashi M, Terada K, Ando T, Tanihara M, Calcium deposition in photocrosslinked poly(Pro-Hyp-Gly) hydrogels encapsulated rat bone marrow stromal cells, J. Tissue Eng. Regen. Med 12 (2018) 1360–1369. doi: 10.1002/term.2520. [DOI] [PubMed] [Google Scholar]
- [14].Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM, Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel, Biomaterials 27 (2006) 5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- [15].Golinska MD, Włodarczyk-Biegun MK, Werten MWT, Stuart MAC, De Wolf FA, De Vries R, Dilute self-healing hydrogels of silk-collagen-like block copolypeptides at neutral pH, Biomacromolecules 15 (2014) 699–706. doi: 10.1021/bm401682n. [DOI] [PubMed] [Google Scholar]
- [16].Condon JE, Martin TB, Jayaraman A, Effect of conjugation on phase transitions in thermoresponsive polymers: an atomistic and coarse-grained simulation study, Soft Matter 13 (2017) 2907–2918. doi: 10.1039/C6SM02874H. [DOI] [PubMed] [Google Scholar]
- [17].Navon Y, Bitton R, Elastin-Like Peptides (ELPs) - Building Blocks for Stimuli-Responsive Self-Assembled Materials, Isr. J. Chem 56 (2016) 581–589. doi: 10.1002/ijch.201500016. [DOI] [Google Scholar]
- [18].Schipperus R, Eggink G, De Wolf FA, Secretion of elastin-like polypeptides with different transition temperatures by Pichia pastoris, Biotechnol. Prog 28 (2012) 242–247. doi: 10.1002/btpr.717. [DOI] [PubMed] [Google Scholar]
- [19].Anderson TR, Marquart ME, Janorkar AV, Effective release of a broad spectrum antibiotic from elastin-like polypeptide-collagen composite, J. Biomed. Mater. Res. - Part A 103 (2015) 782–790. doi: 10.1002/jbm.a.35219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Asai D, Kanamoto T, Takenaga M, Nakashima H, In situ depot formation of anti-HIV fusion-inhibitor peptide in recombinant protein polymer hydrogel, Acta Biomater 64 (2017) 116–125. doi: 10.1016/j.actbio.2017.10.024. [DOI] [PubMed] [Google Scholar]
- [21].Schaal JL, Li X, Mastria E, Bhattacharyya J, Zalutsky MR, Chilkoti A, Liu W, Injectable polypeptide micelles that form radiation crosslinked hydrogels in situ for intratumoral radiotherapy, J. Control. Release 228 (2016) 58–66. doi: 10.1016/j.jconrel.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dash BC, Thomas D, Monaghan M, Carroll O, Chen X, Woodhouse K, O’Brien T, Pandit A, An injectable elastin-based gene delivery platform for dose-dependent modulation of angiogenesis and inflammation for critical limb ischemia, Biomaterials 65 (2015) 126–139. doi: 10.1016/j.biomaterials.2015.06.037. [DOI] [PubMed] [Google Scholar]
- [23].Mukerji R, Schaal J, Li X, Bhattacharyya J, Asai D, Zalutsky MR, Chilkoti A, Liu W, Spatiotemporally photoradiation-controlled intratumoral depot for combination of brachytherapy and photodynamic therapy for solid tumor, Biomaterials 79 (2016) 79–87. doi: 10.1016/j.biomaterials.2015.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hale MKMC, a Setton L, P. D, Chilkoti A, Synthesis and in Vitro Evaluation of Enzymatically Cross-Linked Elastin-Like Polypeptide Gels for Cartilaginous Tissue Repair, Tissue Eng 11 (2005) 1768–1779. [DOI] [PubMed] [Google Scholar]
- [25].Andersen SO, The cross-links in resilin identified as dityrosine and trityrosine, Biochim. Biophys. Acta - Gen. Subj 93 (1964) 213–215. doi: 10.1016/0304-4165(64)90289-2. [DOI] [PubMed] [Google Scholar]
- [26].Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE, Synthesis and properties of crosslinked recombinant pro-resilin, Nature 437 (2005) 999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- [27].Charati MB, Ifkovits JL, Burdick JA, Linhardt JG, Kiick KL, Hydrophilic elastomeric biomaterials based on resilin-like polypeptides, Soft Matter 5 (2009) 3412. doi: 10.1039/b910980c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Su RSC, Kim Y, Liu JC, Resilin: Protein-based elastomeric biomaterials, Acta Biomater 10 (2014) 1601–1611. doi: 10.1016/j.actbio.2013.06.038. [DOI] [PubMed] [Google Scholar]
- [29].Li L, Teller S, Clifton RJ, Jia X, Kiick KL, Tunable mechanical stability and deformation response of a resilin-based elastomer, Biomacromolecules 12 (2011) 2302–2310. doi: 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lyons RE, Nairn KM, Huson MG, Kim M, Dumsday G, Elvin CM, Comparisons of recombinant resilin-like proteins: Repetitive domains are sufficient to confer resilin-like properties, Biomacromolecules 10 (2009) 3009–3014. doi: 10.1021/bm900601h. [DOI] [PubMed] [Google Scholar]
- [31].Li L, Kiick KL, Transient dynamic mechanical properties of resilin-based elastomeric hydrogels, Front. Chem 2 (2014) 1–13. doi: 10.3389/fchem.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Balu R, Dutta NK, Choudhury NR, Elvin CM, Lyons RE, Knott R, Hill AJ, An16-resilin: An advanced multi-stimuli-responsive resilin-mimetic protein polymer, Acta Biomater 10 (2014) 4768–4777. doi: 10.1016/j.actbio.2014.07.030. [DOI] [PubMed] [Google Scholar]
- [33].Fraser JRE, Laurent TC, Laurent UBG, Hyaluronan: Its nature, distribution, functions and turnover, J. Intern. Med 242 (1997) 27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- [34].Sogorkova J, Zapotocky V, Cepa M, Stepankova V, Vagnerova H, Batova J, Pospisilova M, Betak J, Nesporova K, Hermannova M, Daro D, Duffy G, Velebny V, Optimization of cell growth on palmitoyl-hyaluronan knitted scaffolds developed for tissue engineering applications, J. Biomed. Mater. Res. - Part A 106 (2018) 1488–1499. doi: 10.1002/jbm.a.36353. [DOI] [PubMed] [Google Scholar]
- [35].Galluccio F, Barskova T, Cerinic MM, Short-term effect of the combination of hyaluronic acid, chondroitin sulfate, and keratin matrix on early symptomatic knee osteoarthritis, Eur. J. Rheumatol 2 (2015) 106–108. doi: 10.5152/eurjrheum.2015.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Geissler SA, Sabin AL, Besser RR, Gooden OM, Shirk BD, Nguyen QM, Khaing ZZ, Schmidt CE, Biomimetic hydrogels direct spinal progenitor cell differentiation and promote functional recovery after spinal cord injury, J. Neural Eng 15 (2018) 025004. doi: 10.1088/1741-2552/aaa55c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yan HJ, Casalini T, Hulsart-Billström G, Wang S, Oommen OP, Salvalaglio M, Larsson S, Hilborn J, Varghese OP, Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation, Biomaterials 161 (2018) 190–202. doi: 10.1016/j.biomaterials.2018.01.041. [DOI] [PubMed] [Google Scholar]
- [38].Li Q, Niu Y, Xing P, Wang C, Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair, Chin. Med 13 (2018) 1–11. doi: 10.1186/s13020-018-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ren X, Chen C, Hou Y, Huang M, Li Y, Wang D, Zhang L, Biodegradable chitosan-based composites with dual functions acting as the bone scaffold and the inflammation inhibitor in the treatment of bone defects, Int. J. Polym. Mater. Polym. Biomater (2017) 1–8. doi: 10.1080/00914037.2017.1376196. [DOI] [Google Scholar]
- [40].Cheng F, Gao J, Wang L, Hu X, Composite chitosan/poly(ethylene oxide) electrospun nanofibrous mats as novel wound dressing matrixes for the controlled release of drugs, J. Appl. Polym. Sci 132 (2015) 1–8. doi: 10.1002/app.42060.25866416 [DOI] [Google Scholar]
- [41].Federica Chiellini CM, Puppi Dario, Piras Anna Maria, Morelli Andrea, Bartoli Cristina, Modelling of Pancreatic Ductal Adenocarcinoma in Vitro with Three-Dimensional Microstructured Hydrogels, RSC Adv 6 (2016) 54226–54235. doi: 10.1039/C6RA08420F. [DOI] [Google Scholar]
- [42].Weng L, Romanov A, Rooney J, Chen W, Non-cytotoxic, in situ gelable hydrogels composed of N-carboxyethyl chitosan and oxidized dextran, Biomaterials 29 (2008) 3905–3913. doi: 10.1016/j.biomaterials.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Burdick JA, Prestwich GD, Hyaluronic acid hydrogels for biomedical applications, Adv. Mater 23 (2011) 41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Olaru AM, Marin L, Morariu S, Pricope G, Pinteala M, Tartau-Mititelu L, Biocompatible chitosan based hydrogels for potential application in local tumour therapy, Carbohydr. Polym 179 (2018) 59–70. doi: 10.1016/j.carbpol.2017.09.066. [DOI] [PubMed] [Google Scholar]
- [45].Li Z, Yuan B, Dong X, Duan L, Tian H, He C, Chen X, Injectable polysaccharide hybrid hydrogels as scaffolds for burn wound healing, RSC Adv 5 (2015) 94248–94256. doi: 10.1039/C5RA16912G. [DOI] [Google Scholar]
- [46].Hyon SH, Nakajima N, Sugai H, Matsumura K, Low cytotoxic tissue adhesive based on oxidized dextran and epsilon-poly- l -lysine, J. Biomed. Mater. Res. - Part A 102 (2014) 2511–2520. doi: 10.1002/jbm.a.34923. [DOI] [PubMed] [Google Scholar]
- [47].Chen Y, Dai X, Huang L, Sun Y, Chan HN, Shen B, Zeng X, Wu Z, Hsing IM, Guo Z, Wu H, A Universal and Facile Approach for the Formation of a Protein Hydrogel for 3D Cell Encapsulation, Adv. Funct. Mater 25 (2015) 6189–6198. doi: 10.1002/adfm.201502942. [DOI] [Google Scholar]
- [48].Correia TR, Ferreira P, Vaz R, Alves P, Figueiredo MM, Correia IJ, Coimbra P, Development of UV cross-linked gelatin coated electrospun poly(caprolactone) fibrous scaffolds for tissue engineering, Int. J. Biol. Macromol 93 (2016) 1539–1548. doi: 10.1016/j.ijbiomac.2016.05.045. [DOI] [PubMed] [Google Scholar]
- [49].Thorson TJ, Botvinick EL, Mohraz A, Composite Bijel-Templated Hydrogels for Cell Delivery, ACS Biomater. Sci. Eng 4 (2018) 587–594. doi: 10.1021/acsbiomaterials.7b00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chyasnavichyus M, Young SL, Tsukruk VV, Mapping micromechanical properties of soft polymer contact lenses, Polym. (United Kingdom) 55 (2014) 6091–6101. doi: 10.1016/j.polymer.2014.09.053. [DOI] [Google Scholar]
- [51].Moghadam MN, Kolesov V, Vogel A, Klok HA, Pioletti DP, Controlled release from a mechanically-stimulated thermosensitive self-heating composite hydrogel, Biomaterials 35 (2014) 450–455. doi: 10.1016/j.biomaterials.2013.09.065. [DOI] [PubMed] [Google Scholar]
- [52].Madl CM, Heilshorn SC, Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche, Annu. Rev. Biomed. Eng 20 (2018) 21–47. doi: 10.1146/annurev-bioeng-062117-120954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Reilly GC, Engler AJ, Intrinsic extracellular matrix properties regulate stem cell differentiation, J. Biomech 43 (2010) 55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- [54].Huang WC, Liu KH, Liu TC, Liu DM, Chen SY, Synergistic hierarchical silicone-modified polysaccharide hybrid as a soft scaffold to control cell adhesion and proliferation, Acta Biomater 10 (2014) 3546–3556. doi: 10.1016/j.actbio.2014.04.025. [DOI] [PubMed] [Google Scholar]
- [55].Lo CM, Wang HB, Dembo M, Wang YL, Cell movement is guided by the rigidity of the substrate, Biophys. J 79 (2000) 144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schweller RM, Wu ZJ, Klitzman B, West JL, Stiffness of Protease Sensitive and Cell Adhesive PEG Hydrogels Promotes Neovascularization In Vivo, Ann. Biomed. Eng 45 (2017) 1387–1398. doi: 10.1007/s10439-017-1822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hu J, Hiwatashi K, Kurokawa T, Liang SM, Wu ZL, Gong JP, Microgel-reinforced hydrogel films with high mechanical strength and their visible mesoscale fracture structure, Macromolecules 44 (2011) 7775–7781. doi: 10.1021/ma2016248. [DOI] [Google Scholar]
- [58].Hu J, Kurokawa T, Hiwatashi K, Nakajima T, Wu ZL, Liang SM, Gong JP, Structure optimization and mechanical model for microgel-reinforced hydrogels with high strength and toughness, Macromolecules 45 (2012) 5218–5228. doi: 10.1021/ma3003664. [DOI] [Google Scholar]
- [59].Sánchez-Correa F, Vidaurre-Agut C, Serrano-Aroca AJ Campillo-Fernández, Poly(2-hydroxyethyl acrylate) hydrogels reinforced with graphene oxide: Remarkable improvement of water diffusion and mechanical properties, J. Appl. Polym. Sci 135 (2018) 46158. doi: 10.1002/app.46158. [DOI] [Google Scholar]
- [60].Lee JM, Moon JY, Kim TH, Lee SW, Ahrberg CD, Chung BG, Conductive hydrogel/nanowire micropattern-based sensor for neural stem cell differentiation, Sensors Actuators, B Chem 258 (2018) 1042–1050. doi: 10.1016/j.snb.2017.11.151. [DOI] [Google Scholar]
- [61].Mosiewicz KA, Kolb L, Van Der Vlies AJ, Martino MM, Lienemann PS, Hubbell JA, Ehrbar M, Lutolf MP, In situ cell manipulation through enzymatic hydrogel photopatterning, Nat. Mater 12 (2013) 1072–1078. doi: 10.1038/nmat3766. [DOI] [PubMed] [Google Scholar]
- [62].Gandavarapu NR, Azagarsamy MA, Anseth KS, Photo-click living strategy for controlled, reversible exchange of biochemical ligands, Adv. Mater 26 (2014) 2521–2526. doi: 10.1002/adma.201304847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Matsunaga YT, Morimoto Y, Takeuchi S, Molding cell beads for rapid construction of macroscopic 3D tissue architecture, Adv. Mater 23 (2011) 90–94. doi: 10.1002/adma.201004375. [DOI] [PubMed] [Google Scholar]
- [64].Klymenko A, Nicolai T, Chassenieux C, Colombani O, Nicol E, Formation of porous hydrogels by self-assembly of photo-cross-linkable triblock copolymers in the presence of homopolymers, Polym. (United Kingdom) 106 (2016) 152–158. doi: 10.1016/j.polymer.2016.10.059. [DOI] [Google Scholar]
- [65].Nazer B, Dehghani MR, Goliaei B, Plasmid DNA affinity partitioning using polyethylene glycol – sodium sulfate aqueous two-phase systems, J. Chromatogr. B Anal. Technol. Biomed. Life Sci 1044–1045 (2017) 112–119. doi: 10.1016/j.jchromb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- [66].Stahl E, Martin TG, Praetorius F, Dietz H, Facile and scalable preparation of pure and dense DNA origami solutions, Angew. Chemie - Int. Ed 53 (2014) 12735–12740. doi: 10.1002/anie.201405991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lario LD, Malpiedi LP, Pereira JFB, Sette LD, Pessoa-Junior A, Liquid-liquid extraction of protease from cold-adapted yeast Rhodotorula mucilaginosa L7 using biocompatible and biodegradable aqueous two-phase systems, Sep. Sci. Technol 51 (2016) 57–67. doi: 10.1080/01496395.2015.1080276. [DOI] [Google Scholar]
- [68].Yao W, Wang H, Pei Y, Chen Y, Li Z, Wang J, Homogeneous capture and heterogeneous separation of proteins by PEG-functionalized ionic liquid–water systems, RSC Adv 7 (2017) 11297–11303. doi: 10.1039/C6RA28483C. [DOI] [Google Scholar]
- [69].Kalaivani S, Regupathi I, Continuous aqueous two-phase extraction of α-lactalbumin from whey in conventional rotating disc contactor, Sep. Sci. Technol 51 (2016) 2411–2419. doi: 10.1080/01496395.2016.1202278. [DOI] [Google Scholar]
- [70].Braibant B, Bourgeois D, Meyer D, Three-liquid-phase extraction in metal recovery from complex mixtures, Sep. Purif. Technol 195 (2018) 367–376. doi: 10.1016/j.seppur.2017.12.036. [DOI] [Google Scholar]
- [71].Lau HK, Kiick KL, Opportunities for multicomponent hybrid hydrogels in biomedical applications, Biomacromolecules 16 (2015) 28–42. doi: 10.1021/bm501361c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lau HK, Paul A, Sidhu I, Li L, Sabanayagam CR, Parekh SH, Kiick KL, Microstructured Elastomer-PEG Hydrogels via Kinetic Capture of Aqueous Liquid-Liquid Phase Separation, Adv. Sci 5 (2018) 1701010. doi: 10.1002/advs.201701010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Elbert DL, Acta Biomaterialia Liquid – liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications : A tutorial review, Acta Biomater 7 (2011) 31–56. doi: 10.1016/j.actbio.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dzuricky M, Roberts S, Chilkoti A, Convergence of Arti fi cial Protein Polymers and Intrinsically Disordered Proteins, Biochemistry 57 (2018) 2405–2414. doi: 10.1021/acs.biochem.8b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Firoozmand H, Rousseau D, Food Hydrocolloids Tailoring the morphology and rheology of phase-separated biopolymer gels using microbial cells as structure modi fi ers, Food Hydrocoll 42 (2014) 204–214. doi: 10.1016/j.foodhyd.2014.04.040. [DOI] [Google Scholar]
- [76].Le Kim TH, Jun H, Nam YS, Importance of crystallinity of anchoring block of semi-solid amphiphilic triblock copolymers in stabilization of silicone nanoemulsions, J. Colloid Interface Sci 503 (2017) 39–46. doi: 10.1016/j.jcis.2017.04.079. [DOI] [PubMed] [Google Scholar]
- [77].Nallamilli T, Basavaraj MG, Synergistic stabilization of Pickering emulsions by in situ modification of kaolinite with non ionic surfactant, Appl. Clay Sci 148 (2017) 68–76. doi: 10.1016/j.clay.2017.07.038. [DOI] [Google Scholar]
- [78].Darling NJ, Hung YS, Sharma S, Segura T, Controlling the kinetics of thiol-maleimide Michael-type addition gelation kinetics for the generation of homogenous poly(ethylene glycol) hydrogels, Biomaterials 101 (2016) 199–206. doi: 10.1016/j.biomaterials.2016.05.053. [DOI] [PubMed] [Google Scholar]
- [79].Roberts JJ, Bryant SJ, Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development, Biomaterials 34 (2013) 9969–9979. doi: 10.1016/j.biomaterials.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].De France KJ, Xu F, Hoare T, Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities, Adv. Healthc. Mater 7 (2018) 1–17. doi: 10.1002/adhm.201700927. [DOI] [PubMed] [Google Scholar]
- [81].Annabi N, Nichol JW, Ph D, Zhong X, Ji C, Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering, Tissue Eng. Part B 16 (2010) 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dehghan Baniani D, Bagheri R, Solouk A, Preparation and characterization of a composite biomaterial including starch micro/nano particles loaded chitosan gel, Carbohydr. Polym 174 (2017) 633–645. doi: 10.1016/j.carbpol.2017.06.095. [DOI] [PubMed] [Google Scholar]
- [83].Beachley V, Ma G, Papadimitriou C, Gibson M, Corvelli M, Elisseeff J, Extracellular matrix particle-glycosaminoglycan composite hydrogels for regenerative medicine applications, J. Biomed. Mater. Res. Part A 106 (2017) 147–159. doi: 10.1002/jbm.a.36218. [DOI] [PubMed] [Google Scholar]
- [84].Kim BS, Choi JS, Kim JD, Yeo TY, Cho YW, Improvement of stem cell viability in hyaluronic acid hydrogels using dextran microspheres, J. Biomater. Sci. Polym. Ed 21 (2010) 1701–1711. doi: 10.1163/092050609X12548957288848. [DOI] [PubMed] [Google Scholar]
- [85].Ling K, Huang G, Liu J, Zhang X, Ma Y, Lu T, Xu F, Bioprinting-Based High-Throughput Fabrication of Three-Dimensional MCF-7 Human Breast Cancer Cellular Spheroids, Engineering 1 (2015) 269–274. doi: 10.15302/J-ENG-2015062. [DOI] [Google Scholar]
- [86].Zhang W, Huang G, Ng K, Ji Y, Gao B, Huang L, Zhou J, Lu TJ, Xu F, Biomaterials Science as building blocks or sacri fi cial templates for, Biomater. Sci 6 (2018) 885–892. doi: 10.1039/c7bm01186e. [DOI] [PubMed] [Google Scholar]
- [87].Zeng Q, Desai MS, Jin HE, Lee JH, Chang J, Lee SW, Self-Healing Elastin-Bioglass Hydrogels, Biomacromolecules 17 (2016) 2619–2625. doi: 10.1021/acs.biomac.6b00621. [DOI] [PubMed] [Google Scholar]
- [88].Yang L, Wang Z, Fei G, Xia H, Polydopamine Particles Reinforced Poly(vinyl alcohol) Hydrogel with NIR Light Triggered Shape Memory and Self-Healing Capability, Macromol. Rapid Commun 38 (2017) 1700421. doi: 10.1002/marc.201700421. [DOI] [PubMed] [Google Scholar]
- [89].Kobayashi K, Oh SH, Yoon CK, Gracias DH, Multitemperature Responsive Self-Folding Soft Biomimetic Structures, Macromol. Rapid Commun 39 (2018) 1–7. doi: 10.1002/marc.201700692. [DOI] [PubMed] [Google Scholar]
- [90].Li Y, San BH, Kessler JL, Kim JH, Xu Q, Hanes J, Yu SM, Non-covalent photo-patterning of gelatin matrices using caged collagen mimetic peptides, Macromol. Biosci 15 (2015) 52–62. doi: 10.1002/mabi.201400436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gramlich WM, Kim IL, Burdick JA, Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry, Biomaterials 34 (2013) 9803–9811. doi: 10.1016/j.biomaterials.2013.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cho K, Lee HJ, Han SW, Min JH, Park H, Koh WG, Multi-Compartmental Hydrogel Microparticles Fabricated by Combination of Sequential Electrospinning and Photopatterning, Angew. Chemie - Int. Ed 54 (2015) 11511–11515. doi: 10.1002/anie.201504317. [DOI] [PubMed] [Google Scholar]
- [93].Shin HS, Kook YM, Hong HJ, Kim YM, Koh WG, Lim JY, Functional spheroid organization of human salivary gland cells cultured on hydrogel-micropatterned nanofibrous microwells, Acta Biomater 45 (2016) 121–132. doi: 10.1016/j.actbio.2016.08.058. [DOI] [PubMed] [Google Scholar]
- [94].Wang LL, Highley CB, Yeh YC, Galarraga JH, Uman S, Burdick JA, Three-dimensional extrusion bioprinting of single- and double-network hydrogels containing dynamic covalent crosslinks, J. Biomed. Mater. Res. - Part A 106 (2018) 865–875. doi: 10.1002/jbm.a.36323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Davey SK, Aung A, Agrawal G, Lim HL, Kar M, Varghese S, Embedded 3D Photopatterning of Hydrogels with Diverse and Complex Architectures for Tissue Engineering and Disease Models, Tissue Eng. Part C Methods 21 (2015) 1188–1196. doi: 10.1089/ten.tec.2015.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dai G, Wan W, Zhao Y, Wang Z, Li W, Shi P, Shen Y, Controllable 3D alginate hydrogel patterning via visible-light induced electrodeposition, Biofabrication 8 (2016) 025004. doi: 10.1088/1758-5090/8/2/025004. [DOI] [PubMed] [Google Scholar]
- [97].Ming Z, Fan J, Bao C, Xue Y, Lin Q, Zhu L, Photogenerated Aldehydes for Protein Patterns on Hydrogels and Guidance of Cell Behavior, Adv. Funct. Mater 28 (2018) 1–9. doi: 10.1002/adfm.201706918. [DOI] [Google Scholar]
- [98].Sawicki LA, Kloxin AM, Light-mediated Formation and Patterning of Hydrogels for Cell Culture Applications, J. Vis. Exp 2016 (2016) 1–10. doi: 10.3791/54462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dorsey TB, Grath A, Xu C, Hong Y, Dai G, Patterning bioactive proteins or peptides on hydrogel using photochemistry for biological applications, J. Vis. Exp 2017 (2017) 1–7. doi: 10.3791/55873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wu PJ, Lilly JL, Arreaza R, Berron BJ, Hydrogel Patches on Live Cells through Surface-Mediated Polymerization, Langmuir 33 (2017) 6778–6784. doi: 10.1021/acs.langmuir.7b01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Nawroth JC, Scudder LL, Halvorson RT, Tresback J, Ferrier JP, Sheehy SP, Cho A, Kannan S, Sunyovszki I, Goss JA, Campbell PH, Parker KK, Automated fabrication of photopatterned gelatin hydrogels for organ-on-chips applications, Biofabrication 10 (2018) 025004. doi: 10.1088/1758-5090/aa96de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ye G, Wang X, Polymer diffraction gratings on stimuli-responsive hydrogel surfaces: Soft-lithographic fabrication and optical sensing properties, Sensors Actuators, B Chem 147 (2010) 707–713. doi: 10.1016/j.snb.2010.03.052. [DOI] [Google Scholar]
- [103].Tsurkan MV, Wetzel R, Pérez-Hernández HR, Chwalek K, Kozlova A, Freudenberg U, Kempermann G, Zhang Y, Lasagni AF, Werner C, Photopatterning of Multifunctional Hydrogels to Direct Adult Neural Precursor Cells, Adv. Healthc. Mater 4 (2015) 516–521. doi: 10.1002/adhm.201400395. [DOI] [PubMed] [Google Scholar]
- [104].Berkovitch Y, Yelin D, Seliktar D, Photo-patterning PEG-based hydrogels for neuronal engineering, Eur. Polym. J 72 (2015) 473–483. doi: 10.1016/j.eurpolymj.2015.07.014. [DOI] [Google Scholar]
- [105].Khan OF, Sefton MV, Patterning collagen/poloxamine-methacrylate hydrogels for tissue-engineering-inspired microfluidic and laser lithography applications, J. Biomater. Sci. Polym. Ed 22 (2011) 2499–2514. doi: 10.1163/092050610X540693. [DOI] [PubMed] [Google Scholar]
- [106].Ustione A, Piston DW, A simple introduction to multiphoton microscopy, J. Microsc 243 (2011) 221–226. doi: 10.1111/j.1365-2818.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- [107].Loebel C, Broguiere N, Alini M, Zenobi-Wong M, Eglin D, Microfabrication of photo-cross-linked hyaluronan hydrogels by single- and two-photon tyramine oxidation, Biomacromolecules 16 (2015) 2624–2630. doi: 10.1021/acs.biomac.5b00363. [DOI] [PubMed] [Google Scholar]
- [108].Paciello A, Santonicola MG, A supramolecular two-photon-active hydrogel platform for direct bioconjugation under near-infrared radiation, J. Mater. Chem. B 3 (2015) 1313–1320. doi: 10.1039/C4TB01619J. [DOI] [PubMed] [Google Scholar]
- [109].Pennacchio FA, Fedele C, De Martino S, Cavalli S, Vecchione R, Netti PA, Pennacchio FA, 3D Microstructured Azobenzene-Containing Gelatin as Photoactuable Cell Confining System, ACS Appl. Mater. Interfaces 10 (2018) 91–97. doi: 10.1021/acsami.7b13176. [DOI] [PubMed] [Google Scholar]
- [110].Qin XH, Wang X, Rottmar M, Nelson BJ, Maniura-Weber K, Near-Infrared Light-Sensitive Polyvinyl Alcohol Hydrogel Photoresist for Spatiotemporal Control of Cell-Instructive 3D Microenvironments, Adv. Mater 30 (2018) 1–7. doi: 10.1002/adma.201705564. [DOI] [PubMed] [Google Scholar]
- [111].Hoffmann JC, West JL, Three-dimensional photolithographic micropatterning: a novel tool to probe the complexities of cell migration, Integr. Biol 5 (2013) 817–827. doi: 10.1039/c3ib20280a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Serbin J, Houbertz R, Fallnich C, Chichkov BN, Three-dimensional microfabrication with femtosecond laser pulses, Proc. SPIE - Int. Soc. Opt. Eng 4941 (2003) 73–76. doi: 10.1117/12.468235. [DOI] [Google Scholar]
- [113].Sun HB, Kawata S, Two-photon photopolymerization and 3D lithographic microfabrication, Adv. Polym. Sci 170 (2004) 169–273. doi: 10.1007/b94405. [DOI] [Google Scholar]
- [114].Wei S, Liu J, Zhao Y, Zhang T, Zheng M, Jin F, Dong X, Xing J, Duan X, Protein-Based 3D Microstructures with Controllable Morphology and pH-Responsive Properties, ACS Appl. Mater. Interfaces 9 (2017) 42247–42257. doi: 10.1021/acsami.7b14915. [DOI] [PubMed] [Google Scholar]
- [115].Yi SW, Lee SK, Cho MJ, Kong HJ, Yang D-Y, Park S, Lim T, Kim RH, Lee K-S, Fabrication of PDMS (poly-dimethyl siloxane) molding and 3D structure by two-photon absorption induced by an ultrafast laser, Proc. SPIE 5641 (2004) 227–237. doi: 10.1117/12.576633. [DOI] [Google Scholar]
- [116].Allazetta S, Negro A, Lutolf MP, Microfluidic Programming of Compositional Hydrogel Landscapes, Macromol. Rapid Commun 38 (2017) 1–10. doi: 10.1002/marc.201700255. [DOI] [PubMed] [Google Scholar]
- [117].Li F, Truong VX, Thissen H, Frith JE, Forsythe JS, Microfluidic Encapsulation of Human Mesenchymal Stem Cells for Articular Cartilage Tissue Regeneration, ACS Appl. Mater. Interfaces 9 (2017) 8589–8601. doi: 10.1021/acsami.7b00728. [DOI] [PubMed] [Google Scholar]
- [118].Liu Y, Nambu NO, Taya M, Cell-laden microgel prepared using a biocompatible aqueous two-phase strategy, Biomed. Microdevices 19 (2017) 1–3. doi: 10.1007/s10544-017-0198-8. [DOI] [PubMed] [Google Scholar]
- [119].Yu Z, Liu J, Tan CSY, Scherman OA, Abell C, Supramolecular Nested Microbeads as Building Blocks for Macroscopic Self-Healing Scaffolds, Angew. Chemie - Int. Ed 57 (2018) 3079–3083. doi: 10.1002/anie.201711522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Fujimoto K, Higashi K, Onoe H, Miki N, Development of a triple-coaxial flow device for fabricating a hydrogel microtube and its application to bioremediation, Micromachines 9 (2018) 76. doi: 10.3390/mi9020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Liu X, Zuo Y, Sun J, Guo Z, Fan H, Zhang X, Degradation regulated bioactive hydrogel as the bioink with desirable moldability for microfluidic biofabrication, Carbohydr. Polym 178 (2017) 8–17. doi: 10.1016/j.carbpol.2017.09.014. [DOI] [PubMed] [Google Scholar]
- [122].Marquis M, Davy J, Cathala B, Fang A, Renard D, Microfluidics assisted generation of innovative polysaccharide hydrogel microparticles, Carbohydr. Polym 116 (2015) 189–199. doi: 10.1016/j.carbpol.2014.01.083. [DOI] [PubMed] [Google Scholar]
- [123].Ma J, Wang Y, Liu J, Biomaterials meet microfluidics: from synthesis technologies to biological applications, Micromachines 8 (2017) 255. doi: 10.3390/mi8080255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tan W, Desai TA, Therapeutic Micro and Nanotechnology : Microfluidic Patterning of Cellular Biopolymer Matrices for Biomimetic 3-D Structures, Biomed. Microdevices 5 (2003) 235–244. [Google Scholar]
- [125].Nisisako T, Ando T, Hatsuzawa T, High-volume production of single and compound emulsions in a microfluidic parallelization arrangement coupled with coaxial annular world-to-chip interfaces, Lab Chip 12 (2012) 3426–3435. doi: 10.1039/c2lc40245a. [DOI] [PubMed] [Google Scholar]
- [126].Hatch A, Garcia E, Yager P, Diffusion-Based Analysis of Molecular Interactions in Microfluidic Devices, Proc. IEEE 92 (2004) 126–139. doi: 10.1109/JPROC.2003.820547. [DOI] [Google Scholar]
- [127].Hyman AA, Weber CA, Jülicher F, Liquid-Liquid Phase Separation in Biology, Annu. Rev. Cell Dev. Biol 30 (2014) 39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- [128].Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, Tompa P, Fuxreiter M, Protein Phase Separation: A New Phase in Cell Biology, Trends Cell Biol 28 (2018) 420–435. doi: 10.1016/j.tcb.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Schuster BS, Reed EH, Parthasarathy R, Jahnke CN, Caldwell RM, Bermudez JG, Ramage H, Good MC, Hammer DA, Controllable protein phase separation and modular recruitment to form responsive membraneless organelles, Nat. Commun 9 (2018) 1–12. doi: 10.1038/s41467-018-05403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hsieh MC, Lynn DG, Grover MA, Kinetic Model for Two-Step Nucleation of Peptide Assembly, J. Phys. Chem. B 121 (2017) 7401–7411. doi: 10.1021/acs.jpcb.7b03085. [DOI] [PubMed] [Google Scholar]
- [131].Erdel F, Rippe K, Formation of Chromatin Subcompartments by Phase Separation, Biophys. J 114 (2018) 2262–2270. doi: 10.1016/j.bpj.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lu H, Yu D, Hansen AS, Ganguly S, Liu R, Heckert A, Darzacq X, Zhou Q, Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II, Nature 558 (2018) 318–323. doi: 10.1038/s41586-018-0174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chen C, Tan J, Hsieh MC, Pan T, Goodwin JT, Mehta AK, Grover MA, Lynn DG, Design of multi-phase dynamic chemical networks, Nat. Chem 9 (2017) 799–804. doi: 10.1038/NCHEM.2737. [DOI] [PubMed] [Google Scholar]
- [134].Sariban A, Binder K, Phase Separation of Polymer Mixtures in the Presence of Solvent, Macromolecules 21 (1988) 711–726. doi: 10.1021/ma00181a028. [DOI] [Google Scholar]
- [135].Brangwynne CP, Tompa P, Pappu RV, Polymer physics of intracellular phase transitions, Nat. Phys 11 (2015) 899–904. doi: 10.1038/nphys3532. [DOI] [Google Scholar]
- [136].Quiroz FG, Chilkoti A, Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers, Nat. Mater 14 (2015) 1164–1171. doi: 10.1038/nmat4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Luo T, David MA, Dunshee LC, Scott RA, Urello MA, Price C, Kiick KL, Thermoresponsive Elastin-b-Collagen-Like Peptide Bioconjugate Nanovesicles for Targeted Drug Delivery to Collagen-Containing Matrices, Biomacromolecules 18 (2017) 2539–2551. doi: 10.1021/acs.biomac.7b00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Luo T, Kiick KL, Noncovalent Modulation of the Inverse Temperature Transition and Self-Assembly of Elastin-b-Collagen-like Peptide Bioconjugates, J. Am. Chem. Soc 137 (2015) 15362–15365. doi: 10.1021/jacs.5b09941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang H, Zhu D, Paul A, Cai L, Enejder A, Yang F, Heilshorn SC, Covalently Adaptable Elastin-Like Protein–Hyaluronic Acid (ELP–HA) Hybrid Hydrogels with Secondary Thermoresponsive Crosslinking for Injectable Stem Cell Delivery, Adv. Funct. Mater 27 (2017) 1–11. doi: 10.1002/adfm.201605609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ravi S, Caves JM, Martinez AW, Haller CA, Chaikof EL, Incorporation of fibronectin to enhance cytocompatibility in multilayer elastin-like protein scaffolds for tissue engineering, J. Biomed. Mater. Res. - Part A 101 A (2013) 1915–1925. doi: 10.1002/jbm.a.34484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].de Albuquerque Wanderley MC, Wanderley Duarte Neto JM, Campos Albuquerque WW, de D Marques Araújo Viana, de Albuquerque Lima C, da Cruz Silvério SI, de Lima Filho JL, Couto Teixeira JA, Porto ALF, Purification and characterization of a collagenase from Penicillium sp. UCP 1286 by polyethylene glycol-phosphate aqueous two-phase system, Protein Expr. Purif 133 (2017) 8–14. doi: 10.1016/j.pep.2017.02.010. [DOI] [PubMed] [Google Scholar]
- [142].Pereira MM, Cruz RAP, Almeida MR, Lima ÃS, Coutinho JAP, Freire MG, Single-step purification of ovalbumin from egg white using aqueous biphasic systems, Process Biochem 51 (2016) 781–791. doi: 10.1016/j.procbio.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yao W, Wang H, Cui G, Li Z, Wang J, Tuning phase behaviour of PEG-functionalized ionic liquids from UCST to LCST in alcohol–water mixtures, Phys. Chem. Chem. Phys 18 (2016) 29192–29198. doi: 10.1039/C6CP05668G. [DOI] [PubMed] [Google Scholar]
- [144].Murari GF, Penido JA, Machado PAL, de Lemos LR, Lemes NHT, Virtuoso LS, Rodrigues GD, Mageste AB, Phase diagrams of aqueous two-phase systems formed by polyethylene glycol+ammonium sulfate+water: Equilibrium data and thermodynamic modeling, Fluid Phase Equilib 406 (2015) 61–69. doi: 10.1016/j.fluid.2015.07.024. [DOI] [Google Scholar]
- [145].McGann CL, Akins RE, Kiick KL, Resilin-PEG hybrid hydrogels yield degradable elastomeric scaffolds with heterogeneous microstructure, Biomacromolecules 17 (2016) 128–140. doi: 10.1021/acs.biomac.5b01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Lau HK, Li L, Jurusik AK, Sabanayagam CR, Kiick KL, Aqueous Liquid-Liquid Phase Separation of Resilin-Like Polypeptide/Polyethylene Glycol Solutions for the Formation of Microstructured Hydrogels, ACS Biomater. Sci. Eng 3 (2017) 757–766. doi: 10.1021/acsbiomaterials.6b00076. [DOI] [PubMed] [Google Scholar]
- [147].Li L, Luo T, Kiick KL, Temperature-triggered phase separation of a hydrophilic resilin-like polypeptide, Macromol. Rapid Commun 36 (2015) 90–95. doi: 10.1002/marc.201400521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Aydın D, Kızılel S, Water-in-Water Emulsion Based Synthesis of Hydrogel Nanospheres with Tunable Release Kinetics, Jom 69 (2017) 1185–1194. doi: 10.1007/s11837-016-1969-z. [DOI] [Google Scholar]
- [149].Bae KH, Lee F, Xu K, Keng CT, Tan SY, Tan YJ, Chen Q, Kurisawa M, Microstructured dextran hydrogels for burst-free sustained release of PEGylated protein drugs, Biomaterials 63 (2015) 146–157. doi: 10.1016/j.biomaterials.2015.06.008. [DOI] [PubMed] [Google Scholar]
- [150].Shimanovich U, Song Y, Brujic J, Shum HC, Knowles TPJ, Multiphase protein microgels, Macromol. Biosci 15 (2015) 501–508. doi: 10.1002/mabi.201400366. [DOI] [PubMed] [Google Scholar]
- [151].Lévesque SG, Lim RM, Shoichet MS, Macroporous interconnected dextran scaffolds of controlled porosity for tissue-engineering applications, Biomaterials 26 (2005) 7436–7446. doi: 10.1016/j.biomaterials.2005.05.054. [DOI] [PubMed] [Google Scholar]
- [152].Wang CS, Virgilio N, Wood-Adams PM, Heuzey MC, A gelation mechanism for gelatin/polysaccharide aqueous mixtures, Food Hydrocoll 79 (2018) 462–472. doi: 10.1016/j.foodhyd.2018.01.016. [DOI] [Google Scholar]
- [153].Kanno T, Uyama H, Unique Ivy-Like Morphology Composed of Poly(lactic acid) and Bacterial Cellulose Cryogel, ACS Omega 3 (2018) 631–635. doi: 10.1021/acsomega.7b01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Tsaih ML, Chen RH, Effects of Ionic Strength and pH on the Diffusion Coefficients and Conformation of Chitosans, J App Polym Sci 73 (1999) 2041–2050. doi:10.1002/(SICI)1097–4628(19990906)73:10<2041::AID-APP22>3.0.CO;2-T. [Google Scholar]
- [155].Thompson RW, Latypov RF, Wang Y, Lomakin A, Meyer JA, Vunnum S, Benedek GB, Evaluation of effects of pH and ionic strength on colloidal stability of IgG solutions by PEG-induced liquid-liquid phase separation, J. Chem. Phys 145 (2016) 185101. doi: 10.1063/1.4966708. [DOI] [PubMed] [Google Scholar]
- [156].Broide ML, Tominc TM, Saxowsky MD, Using phase transitions to investigate the effect of salts on protein interactions, Phys. Rev. E 53 (1996) 6325–6335. doi: 10.1103/PhysRevE.53.6325. [DOI] [PubMed] [Google Scholar]
- [157].Zhang W, Phase Behavior and Phase Separation Kinetics in Polymer Solutions under High Pressure Phase Behavior and Phase Separation Kinetics in Polymer Solutions under High Pressure, Virginia Polytechnic Institute and State University, 2005. https://vtechworks.lib.vt.edu/bitstream/handle/10919/26963/Wei_Zhang_PhD_Dissertation.pdf?sequence=1&isAll owed=y. [Google Scholar]
- [158].Läuger J, Lay R, Gronski W, The percolation-to-cluster transition during spinodal decomposition of an off-critical polymer mixture. Observation by light scattering and optical microscopy, J. Chem. Phys 101 (1994) 7181–7184. doi: 10.1063/1.468304. [DOI] [Google Scholar]
- [159].Takeno H, Iwata M, Takenaka M, Hashimoto T, Combined light scattering and laser scanning confocal microscopy studies of a polymer mixture involving a percolation-to-cluster transition, Macromolecules 33 (2000) 9657–9665. doi: 10.1021/ma001316u. [DOI] [Google Scholar]
- [160].Weitzhandler I, Dzuricky M, Hoffmann I, Garcia Quiroz F, Gradzielski M, Chilkoti A, Micellar Self-Assembly of Recombinant Resilin-/Elastin-Like Block Copolypeptides, Biomacromolecules 18 (2017) 2419–2426. doi: 10.1021/acs.biomac.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Dahiya P, DeBenedictis A, Atherton TJ, Caggioni M, Prescott SW, Hartel RW, Spicer PT, Arrested coalescence of viscoelastic droplets: triplet shape and restructuring, Soft Matter 13 (2017) 2686–2697. doi: 10.1039/C6SM02830F. [DOI] [PubMed] [Google Scholar]
- [162].Goel S, Ramachandran A, The suppression of droplet-droplet coalescence in a sheared yield stress fluid, J. Colloid Interface Sci 492 (2017) 199–206. doi: 10.1016/j.jcis.2016.12.055. [DOI] [PubMed] [Google Scholar]
- [163].Andrade J, Wright AJ, Corredig M, In vitro digestion behavior of water-in-oil-in-water emulsions with gelled oil-water inner phases, Food Res. Int 105 (2018) 41–51. doi: 10.1016/j.foodres.2017.10.070. [DOI] [PubMed] [Google Scholar]
- [164].Zamani S, Malchione N, Selig MJ, Abbaspourrad A, Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels, Food Funct 9 (2018) 982–990. doi: 10.1039/c7fo01800b. [DOI] [PubMed] [Google Scholar]
- [165].Paximada P, Koutinas AA, Scholten E, Mandala IG, Effect of bacterial cellulose addition on physical properties of WPI emulsions. Comparison with common thickeners, Food Hydrocoll 54 (2016) 245–254. doi: 10.1016/j.foodhyd.2015.10.014. [DOI] [Google Scholar]
- [166].Thiel AE, Hartel RW, Spicer PT, Hendrickson KJ, Coalescence Behavior of Pure and Natural Fat Droplets Characterized via Micromanipulation, JAOCS, J. Am. Oil Chem. Soc 93 (2016) 1467–1477. doi: 10.1007/s11746-016-2896-4. [DOI] [Google Scholar]
- [167].Chen H, McClements DJ, Chen E, Liu S, Li B, Li Y, In situ interfacial conjugation of chitosan with cinnamaldehyde during homogenization improves the formation and stability of chitosan-stabilized emulsions, Langmuir 33 (2017) 14608–14617. doi: 10.1021/acs.langmuir.7b03852. [DOI] [PubMed] [Google Scholar]
- [168].Ren K, Lamsal BP, Synthesis of some glucose-fatty acid esters by lipase from Candida antarctica and their emulsion functions, Food Chem 214 (2017) 556–563. doi: 10.1016/j.foodchem.2016.07.031. [DOI] [PubMed] [Google Scholar]
- [169].Sun J, Wang W, He F, Chen Z-H, Xie R, Ju X-J, Liu Z, Chu L-Y, On-chip thermo-triggered coalescence of controllable Pickering emulsion droplet pairs, RSC Adv 6 (2016) 64182–64192. doi: 10.1039/C6RA12594H. [DOI] [Google Scholar]
- [170].Bidita BS, Suraya AR, Shazed MA, Mohd Salleh MA, Idris A, Influence of fuel additive in the formulation and combustion characteristics of water-in-diesel nanoemulsion fuel, Energy and Fuels 28 (2014) 4149–4161. doi: 10.1021/ef5002259. [DOI] [Google Scholar]
- [171].Han SW, Song HY, Moon TW, Choi SJ, Influence of emulsion interfacial membrane characteristics on Ostwald ripening in a model emulsion, Food Chem 242 (2018) 91–97. doi: 10.1016/j.foodchem.2017.09.018. [DOI] [PubMed] [Google Scholar]
- [172].Pérez-Mosqueda LM, Ramírez P, Trujillo-Cayado LA, Santos J, Muñoz J, Development of eco-friendly submicron emulsions stabilized by a bio-derived gum, Colloids Surfaces B Biointerfaces 123 (2014) 797–802. doi: 10.1016/j.colsurfb.2014.10.022. [DOI] [PubMed] [Google Scholar]
- [173].Santos J, Calero N, Trujillo-Cayado LA, Garcia MC, Muñoz J, Assessing differences between Ostwald ripening and coalescence by rheology, laser diffraction and multiple light scattering, Colloids Surfaces B Biointerfaces 159 (2017) 405–411. doi: 10.1016/j.colsurfb.2017.08.015. [DOI] [PubMed] [Google Scholar]
- [174].Biswas DP, Tran PA, Tallon C, O’Connor AJ, Combining mechanical foaming and thermally induced phase separation to generate chitosan scaffolds for soft tissue engineering, J. Biomater. Sci. Polym. Ed 28 (2017) 207–226. doi: 10.1080/09205063.2016.1262164. [DOI] [PubMed] [Google Scholar]
- [175].Kumar A, Li S, Cheng CM, Lee D, Recent Developments in Phase Inversion Emulsification, Ind. Eng. Chem. Res 54 (2015) 8375–8396. doi: 10.1021/acs.iecr.5b01122. [DOI] [Google Scholar]
- [176].Mallikarjunachari G, Nallamilli T, Ravindran P, Basavaraj MG, Nanoindentation of clay colloidosomes, Colloids Surfaces A Physicochem. Eng. Asp 550 (2018) 167–175. doi: 10.1016/j.colsurfa.2018.04.041. [DOI] [Google Scholar]
- [177].Pradilla D, Barrera A, Sætran MG, Sørland G, Alvarez O, Mechanisms of Physical Stabilization of Concentrated Water-In-Oil Emulsions Probed by Pulse Field Gradient Nuclear Magnetic Resonance and Rheology through a Multiscale Approach, Langmuir 34 (2018) 9489–9499. doi: 10.1021/acs.langmuir.8b01393. [DOI] [PubMed] [Google Scholar]
- [178].Pan F, Li Z, Gong H, Petkov JT, Lu JR, Membrane-lytic actions of sulphonated methyl ester surfactants and implications to bactericidal effect and cytotoxicity, J. Colloid Interface Sci 531 (2018) 18–27. doi: 10.1016/j.jcis.2018.07.031. [DOI] [PubMed] [Google Scholar]
- [179].Seweryn A, Interactions between surfactants and the skin – Theory and practice, Adv. Colloid Interface Sci 256 (2018) 242–255. doi: 10.1016/j.cis.2018.04.002. [DOI] [PubMed] [Google Scholar]
- [180].Chen H, Mcfaul C, Titushkin I, Cho M, Lee R, Lee R, Mcfaul C, Surfactant Copolymer Annealing of Chemically Permeabilized Cell Membranes, Regen. Eng. Transl. Med 4 (2018) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Khoee S, Karimi MR, Dual-drug loaded Janus graphene oxide-based thermoresponsive nanoparticles for targeted therapy, Polym. (United Kingdom) 142 (2018) 80–98. doi: 10.1016/j.polymer.2018.03.022. [DOI] [Google Scholar]
- [182].Pickering SU, CXCVI.-Emulsions, J. Chem. Soc., Trans 91 (1907) 2001–2021. [Google Scholar]
- [183].Sawiak L, Bailes K, Harbottle D, Clegg PS, Mixing Time, Inversion and Multiple Emulsion Formation in a Limonene and Water Pickering Emulsion, Front. Chem 6 (2018) 132. doi: 10.3389/fchem.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Young S, Basiana E, Nitin N, Effects of interfacial composition on the stability of emulsion and encapsulated bioactives after thermal and high pressure processing, J. Food Eng 231 (2018) 22–29. doi: 10.1016/j.jfoodeng.2018.02.022. [DOI] [Google Scholar]
- [185].Glorani G, Marin R, Canton P, Pinto M, Conti G, Fracasso G, Riello P, Pegylated silica nanoparticles: cytotoxicity and macrophage uptake, J. Nanoparticle Res 19 (2017) 294. doi: 10.1007/s11051-017-3964-x. [DOI] [Google Scholar]
- [186].Alison L, Rühs PA, Tervoort E, Teleki A, Zanini M, Isa L, Studart AR, Pickering and Network Stabilization of Biocompatible Emulsions Using Chitosan-Modified Silica Nanoparticles, Langmuir 32 (2016) 13446–13457. doi: 10.1021/acs.langmuir.6b03439. [DOI] [PubMed] [Google Scholar]
- [187].Lin Q, Liu KH, Cui ZG, Pei XM, Jiang JZ, Song BL, pH-Responsive Pickering foams stabilized by silica nanoparticles in combination with trace amount of dodecyl dimethyl carboxyl betaine, Colloids Surfaces A Physicochem. Eng. Asp 544 (2018) 44–52. doi: 10.1016/j.colsurfa.2018.02.027. [DOI] [Google Scholar]
- [188].Lebdioua K, Aimable A, Cerbelaud M, Videcoq A, Peyratout C, Influence of different surfactants on Pickering emulsions stabilized by submicronic silica particles, J. Colloid Interface Sci 520 (2018) 127–133. doi: 10.1016/j.jcis.2018.03.019. [DOI] [PubMed] [Google Scholar]
- [189].Chen D, Amstad E, Zhao CX, Cai L, Fan J, Chen Q, Hai M, Koehler S, Zhang H, Liang F, Yang Z, Weitz DA, Biocompatible Amphiphilic Hydrogel-Solid Dimer Particles as Colloidal Surfactants, ACS Nano 11 (2017) 11978–11985. doi: 10.1021/acsnano.7b03110. [DOI] [PubMed] [Google Scholar]
- [190].Yuan DB, Hu YQ, Zeng T, Yin SW, Tang CH, Yang XQ, Development of stable Pickering emulsions/oil powders and Pickering HIPEs stabilized by gliadin/chitosan complex particles, Food Funct 8 (2017) 2220–2230. doi: 10.1039/C7FO00418D. [DOI] [PubMed] [Google Scholar]
- [191].Raju RR, Kosmella S, Friberg SE, Koetz J, Pickering Janus emulsions and polyelectrolyte complex-stabilized Janus gels, Colloids Surfaces A Physicochem. Eng. Asp 533 (2017) 241–248. doi: 10.1016/j.colsurfa.2017.08.022. [DOI] [Google Scholar]
- [192].Sarker M, Tomczak N, Lim S, Protein Nanocage as a pH-Switchable Pickering Emulsifier, ACS Appl. Mater. Interfaces 9 (2017) 11193–11201. doi: 10.1021/acsami.6b14349. [DOI] [PubMed] [Google Scholar]
- [193].Ogunlaja SB, Pal R, Sarikhani K, Effects of starch nanoparticles on phase inversion of Pickering emulsions, Can. J. Chem. Eng 96 (2018) 1089–1097. doi: 10.1002/cjce.23078. [DOI] [Google Scholar]
- [194].Zhai X, Gao J, Wang X, Mei S, Zhao R, Wu Y, Hao C, Yang J, Liu Y, Inverse Pickering emulsions stabilized by carbon quantum dots: Influencing factors and their application as templates, Chem. Eng. J 345 (2018) 209–220. doi: 10.1016/j.cej.2018.03.075. [DOI] [Google Scholar]
- [195].Matos M, Marefati A, Bordes R, Gutiérrez G, Rayner M, Combined emulsifying capacity of polysaccharide particles of different size and shape, Carbohydr. Polym 169 (2017) 127–138. doi: 10.1016/j.carbpol.2017.04.006. [DOI] [PubMed] [Google Scholar]
- [196].Kim I, Worthen AJ, Johnston KP, DiCarlo DA, Huh C, Size-dependent properties of silica nanoparticles for Pickering stabilization of emulsions and foams, J. Nanoparticle Res 18 (2016) 1–12. doi: 10.1007/s11051-016-3395-0. [DOI] [Google Scholar]
- [197].Jun H, Le Kim TH, Han SW, Seo M, Kim JW, Nam YS, Polyglycerol-poly(ε-caprolactone) block copolymer as a new semi-solid polymeric emulsifier to stabilize O/W nanoemulsions, Colloid Polym. Sci 293 (2015) 2949–2956. doi: 10.1007/s00396-015-3659-8. [DOI] [Google Scholar]
- [198].Shin K, Kim JW, Park H, Choi HS, Chae PS, Nam YS, Kim JW, Fabrication and stabilization of nanoscale emulsions by formation of a thin polymer membrane at the oil–water interface, RSC Adv 5 (2015) 46276–46281. doi: 10.1039/C5RA03872C. [DOI] [Google Scholar]
- [199].Le Kim TH, Jun H, Kim JH, Park K, Kim JS, Nam YS, Lipiodol nanoemulsions stabilized with polyglycerol-polycaprolactone block copolymers for theranostic applications, Biomater. Res 21 (2017) 1–10. doi: 10.1186/s40824-017-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Thompson KL, Mable CJ, Cockram A, Warren NJ, Cunningham VJ, Jones ER, Verber R, Armes SP, Are block copolymer worms more effective Pickering emulsifiers than block copolymer spheres?, Soft Matter 10 (2014) 8615–8626. doi: 10.1039/C4SM01724B. [DOI] [PubMed] [Google Scholar]
- [201].Simon JR, Carroll NJ, Rubinstein M, Chilkoti A, López GP, Programming molecular self-assembly of intrinsically disordered proteins containing sequences of low complexity, Nat. Chem 9 (2017) 509–515. doi: 10.1038/nchem.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Costa SA, Simon JR, Amiram M, Tang L, Zauscher S, Brustad EM, Isaacs FJ, Chilkoti A, Photo-Crosslinkable Unnatural Amino Acids Enable Facile Synthesis of Thermoresponsive Nano- to Microgels of Intrinsically Disordered Polypeptides, 1704878 (2018) 1–9. doi: 10.1002/adma.201704878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Yeganeh JK, Goharpey F, Moghimi E, Petekidis G, Foudazi R, Controlling the kinetics of viscoelastic phase separation through self-assembly of spherical nanoparticles or block copolymers, Soft Matter 10 (2014) 9270–9280. doi: 10.1039/C4SM01499E. [DOI] [PubMed] [Google Scholar]
- [204].Wang H, Paul A, Nguyen D, Enejder A, Heilshorn SC, Tunable Control of Hydrogel Microstructure by Kinetic Competition between Self-Assembly and Crosslinking of Elastin-like Proteins, ACS Appl. Mater. Interfaces 10 (2018) 21808–21815. doi: 10.1021/acsami.8b02461. [DOI] [PubMed] [Google Scholar]
- [205].Bi X, Liang A, Tan Y, Maturavongsadit P, Higginbothem A, Gado T, Gramling A, Bahn H, Wang Q, Thiol-ene crosslinking polyamidoamine dendrimer-hyaluronic acid hydrogel system for biomedical applications, J. Biomater. Sci. Polym. Ed 27 (2016) 743–757. doi: 10.1080/09205063.2016.1159473. [DOI] [PubMed] [Google Scholar]
- [206].Jain E, Sheth S, Dunn A, Zustiak SP, Sell SA, Sustained release of multicomponent platelet-rich plasma proteins from hydrolytically degradable PEG hydrogels, J. Biomed. Mater. Res. - Part A 105 (2017) 3304–3314. doi: 10.1002/jbm.a.36187. [DOI] [PubMed] [Google Scholar]
- [207].Kim J, Kong YP, Niedzielski SM, Singh RK, Putnam AJ, Shikanov A, Characterization of the crosslinking kinetics of multi-arm poly(ethylene glycol) hydrogels formed via Michael-type addition, Soft Matter 12 (2016) 2076–2085. doi: 10.1039/C5SM02668G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Palumbo FS, Fiorica C, Di Stefano M, Pitarresi G, Gulino A, Agnello S, Giammona G, In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration, Carbohydr. Polym 122 (2015) 408–416. doi: 10.1016/j.carbpol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- [209].Wang J, He H, Cooper RC, Yang H, In Situ-Forming Polyamidoamine Dendrimer Hydrogels with Tunable Properties Prepared via Aza-Michael Addition Reaction, ACS Appl. Mater. Interfaces 9 (2017) 10494–10503. doi: 10.1021/acsami.7b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].McGann CL, Levenson EA, Kiick KL, Resilin-based hybrid hydrogels for cardiovascular tissue engineering, Macromol. Chem. Phys 214 (2013) 203–213. doi: 10.1002/macp.201200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].McGann CL, Dumm RE, Jurusik AK, Sidhu I, Kiick KL, Thiol-ene Photocrosslinking of Cytocompatible Resilin-Like Polypeptide-PEG Hydrogels, Macromol. Biosci 16 (2016) 129–138. doi: 10.1002/mabi.201500305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Lin H, Ou J, Liu Z, Wang H, Dong J, Zou H, Facile construction of macroporous hybrid monoliths via thiol-methacrylate Michael addition click reaction for capillary liquid chromatography, J. Chromatogr. A 1379 (2015) 34–42. doi: 10.1016/j.chroma.2014.12.031. [DOI] [PubMed] [Google Scholar]
- [213].Rather AM, Manna U, Facile Synthesis of Tunable and Durable Bulk Superhydrophobic Material from Amine “ Reactive “ Polymeric Gel, Chem. Mater 28 (2016) 8689–8699. doi: 10.1021/acs.chemmater.6b03862. [DOI] [Google Scholar]
- [214].Li L, Tong Z, Jia X, Kiick KL, Resilin-like polypeptide hydrogels engineered for versatile biological function, Soft Matter 9 (2013) 665–673. doi: 10.1039/C2SM26812D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Lim DW, Nettles DL, Setton LA, Chilkoti A, Rapid cross-linking of elastin-like polypeptides with (hydroxymethyl)phosphines in aqueous solution, Biomacromolecules 8 (2007) 1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Wang H, Cai L, Paul A, Enejder A, Heilshorn SC, Hybrid elastin-like polypeptide-polyethylene glycol (ELP-PEG) hydrogels with improved transparency and independent control of matrix mechanics and cell ligand density, Biomacromolecules 15 (2014) 3421–3428. doi: 10.1021/bm500969d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Mahara A, Kiick KL, Yamaoka T, In vivo guided vascular regeneration with a non-porous elastin-like polypeptide hydrogel tubular scaffold, J. Biomed. Mater. Res. - Part A 105 (2017) 1746–1755. doi: 10.1002/jbm.a.36018. [DOI] [PubMed] [Google Scholar]
- [218].Renner JN, Cherry KM, Su RSC, Liu JC, Characterization of resilin-based materials for tissue engineering applications, Biomacromolecules 13 (2012) 3678–3685. doi: 10.1021/bm301129b. [DOI] [PubMed] [Google Scholar]
- [219].Eke G, Mangir N, Hasirci N, MacNeil S, Hasirci V, Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering, Biomaterials 129 (2017) 188–198. doi: 10.1016/j.biomaterials.2017.03.021. [DOI] [PubMed] [Google Scholar]
- [220].Rizwan M, Peh GSL, Ang HP, Lwin NC, Adnan K, Mehta JS, Tan WS, Yim EKF, Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications, Biomaterials 120 (2017) 139–154. doi: 10.1016/j.biomaterials.2016.12.026. [DOI] [PubMed] [Google Scholar]
- [221].Kim YM, Potta T, Park KH, Song SC, Temperature responsive chemical crosslinkable UV pretreated hydrogel for application to injectable tissue regeneration system via differentiations of encapsulated hMSCs, Biomaterials 112 (2017) 248–256. doi: 10.1016/j.biomaterials.2016.10.025. [DOI] [PubMed] [Google Scholar]
- [222].Stichler S, Jungst T, Schamel M, Zilkowski I, Kuhlmann M, Böck T, Blunk T, Teßmar J, Groll J, Thiol-ene Clickable Poly(glycidol) Hydrogels for Biofabrication, Ann. Biomed. Eng 45 (2017) 273–285. doi: 10.1007/s10439-016-1633-3. [DOI] [PubMed] [Google Scholar]
- [223].Liyanage W, Ardoña HAM, Mao HQ, Tovar JD, Cross-Linking Approaches to Tuning the Mechanical Properties of Peptide π-Electron Hydrogels, Bioconjug. Chem 28 (2017) 751–759. doi: 10.1021/acs.bioconjchem.6b00593. [DOI] [PubMed] [Google Scholar]
- [224].Hozumi T, Kageyama T, Ohta S, Fukuda J, Ito T, Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation, Biomacromolecules 19 (2018) 288–297. doi: 10.1021/acs.biomac.7b01133. [DOI] [PubMed] [Google Scholar]
- [225].Cui N, Qian J, Zhao N, Wang H, Functional hyaluronic acid hydrogels prepared by a novel method, Mater. Sci. Eng. C 45 (2014) 573–577. doi: 10.1016/j.msec.2014.10.001. [DOI] [PubMed] [Google Scholar]
- [226].Li L, Wang N, Jin X, Deng R, Nie S, Sun L, Wu Q, Wei Y, Gong C, Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention, Biomaterials 35 (2014) 3903–3917. doi: 10.1016/j.biomaterials.2014.01.050. [DOI] [PubMed] [Google Scholar]
- [227].Kim Y, Gill EE, Liu JC, Enzymatic cross-linking of resilin-based proteins for vascular tissue engineering applications, Biomacromolecules 17 (2016) 2530–2539. doi: 10.1021/acs.biomac.6b00500. [DOI] [PubMed] [Google Scholar]
- [228].kim SH, An YH, Kim HD, Kim K, Lee SH, Yim HG, Kim BG, Hwang NS, Enzyme-mediated tissue adhesive hydrogels for meniscus repair, Int. J. Biol. Macromol 110 (2018) 479–487. doi: 10.1016/j.ijbiomac.2017.12.053. [DOI] [PubMed] [Google Scholar]
- [229].Li Z, Ma X, Fan D, Zhu C, Zhao L, Liu Y, Chang L, Cross-linking of hyaluronic acid with 1, 2, 7, 8-diepoxyoctane, Mater. Res. Innov 19 (2015) S9–268-S9–272. doi: 10.1179/1432891715Z.0000000001983. [DOI] [Google Scholar]
- [230].Wollensak G, Spoerl E, Reber F, Seiler T, Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro, Eye 18 (2004) 718–722. doi: 10.1038/sj.eye.6700751. [DOI] [PubMed] [Google Scholar]
- [231].Sugiyama M, Tsuzuki K, Matsumoto K, Ogura R, Effect of Vitamin E on Cytotoxicity, Dna Single Strand Breaks, Chromosomal Aberrations, and Mutation in Chinese Hamster V‐ 79 Cells Exposed To Ultraviolet‐ B Light, Photochem. Photobiol 56 (1992) 31–34. doi: 10.1111/j.1751-1097.1992.tb09598.x. [DOI] [PubMed] [Google Scholar]
- [232].Bryant SJ, Nuttelman CR, Anseth KS, Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro, J. Biomater. Sci. Polym. Ed 11 (2000) 439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- [233].Flanagan LA, El Ju Y, Marg B, Osterfield M, Janmey PA, Neurite branching on deformable substrates, Neuroreport 13 (2002) 2411–2415. doi: 10.1097/00001756-200212200-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ, Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate, Nat. Mater 9 (2010) 518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix Elasticity Directs Stem Cell Lineage Specification, Cell 126 (2006) 677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [236].Zoldan J, Karagiannis ED, Lee CY, Anderson DG, Langer R, Levenberg S, The influence of scaffold elasticity on germ layer specification of human embryonic stem cells, Biomaterials 32 (2011) 9612–9621. doi: 10.1016/j.biomaterials.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Wong JY, Velasco A, Rajagopalan P, Pham Q, Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels, Langmuir 19 (2003) 1908–1913. doi: 10.1021/la026403p. [DOI] [Google Scholar]
- [238].Floren M, Bonani W, Dharmarajan A, Motta A, Migliaresi C, Tan W, Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype, Acta Biomater 31 (2016) 156–166. doi: 10.1016/j.actbio.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Joly P, Duda GN, Schöne M, Welzel PB, Freudenberg U, Werner C, Petersen A, Geometry-Driven Cell Organization Determines Tissue Growths in Scaffold Pores: Consequences for Fibronectin Organization, PLoS One 8 (2013) 1–12. doi: 10.1371/journal.pone.0073545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Omidinia-Anarkoli A, Boesveld S, Tuvshindorj U, Rose JC, Haraszti T, De Laporte L, An Injectable Hybrid Hydrogel with Oriented Short Fibers Induces Unidirectional Growth of Functional Nerve Cells, Small 13 (2017) 1–8. doi: 10.1002/smll.201702207. [DOI] [PubMed] [Google Scholar]
- [241].Binner M, Bray LJ, Friedrichs J, Freudenberg U, Tsurkan MV, Werner C, Cell-instructive starPEG-heparin-collagen composite matrices, Acta Biomater 53 (2017) 70–80. doi: 10.1016/j.actbio.2017.01.086. [DOI] [PubMed] [Google Scholar]
- [242].Bourget JM, Laterreur V, Gauvin R, Guillemette MD, Miville-Godin C, Mounier M, Tondreau MY, Tremblay C, Labbé R, Ruel J, Auger FA, Veres T, Germain L, Microstructured human fibroblast-derived extracellular matrix scaffold for vascular media fabrication, J. Tissue Eng. Regen. Med 11 (2017) 2479–2489. doi: 10.1002/term.2146. [DOI] [PubMed] [Google Scholar]